INTRODUCTION
Frey syndrome is a postoperative phenomenon following salivary gland surgery and less commonly neck dissection, facelift procedures, and trauma that is characterized by gustatory sweating and flushing. Frey syndrome was first described by Lucie Frey in 1923 and was termed auriculotemporal syndrome. It described sweating and flushing in the preauricular area in response to mastication or a salivary stimulus.1 Initially thought to be rare, it was later recognized as a common occurrence after salivary gland surgery, occurring in 4% to 62% of postparotidectomy patients 6 to 18 months after surgery.2–5
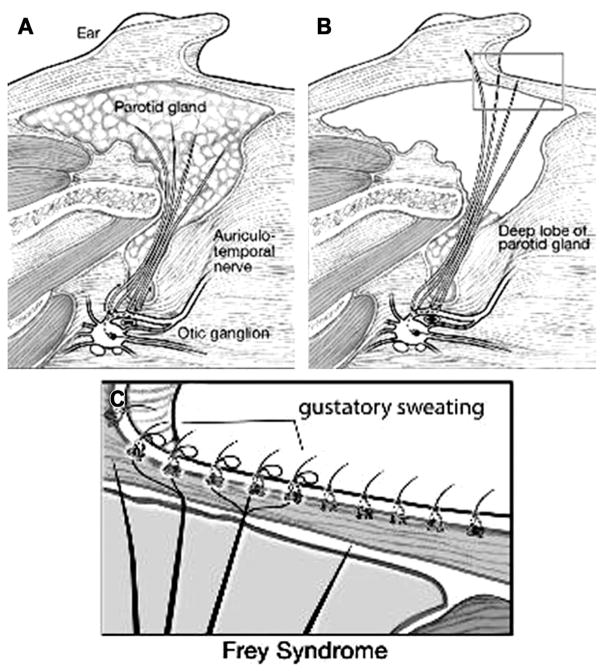
The synkinetic mechanism for Frey syndrome is aberrant reinnervation of postganglionic parasympathetic neurons to nearby denervated sweat glands and cutaneous blood vessels.6 Consequently, this results in flushing and sweating in the sympathetically void skin in response to mastication and salivation. The previous sympathetic responses of sweating and flushing are now controlled by postganglionic parasympathetic fibers. Mastication, which releases acetylcholine from the parasympathetic nerve endings,3,6 now induces sweating and flushing, which was a sympathetic cholinergic response before synkinesis of parasympathetic nerve fibers (Fig. 1).
Fig. 1.
(A) Normal innervation of the parotid gland by the postganglionic parasympathetic nerve fibers from the auriculotemporal nerve. (B) Postoperative diagram depicting the regenerated postganglionic parasympathetic nerve fibers extending to the overlying cutaneous tissue. (C) Postganglionic parasympathetic nerve fibers innervating the cutaneous sweat gland that results in gustatory sweating. (From Hoff S, Mohyuddin N, Yao M. Complications of parotid surgery. Operative Techniques in Otolaryngology–Head and Neck Surgery 2009;20:129; with permission.)
The symptoms of Frey syndrome can include flushing, sweating, burning, neuralgia, and itching. Generally, the symptoms are mild but can result in discomfort as well as social anxiety and avoidance. A survey conducted by Baek and colleagues7 revealed that Frey syndrome was the most commonly self-perceived consequence of parotidectomy in a group undergoing parotidectomy for benign disease. With significant psychosocial morbidity resulting from Frey syndrome,8 interventions to prevent the development and to treat this sequela of parotid surgery have been the topic of focus in the literature.
Diagnosis of Frey syndrome is based on clinical history, but confirmatory testing can be done with a Minor starch-iodine test. The starch-iodine test consists of painting the patient’s postsurgical affected region with iodine. Once dry, dry starch is then applied to the painted area, and a salivary stimulus is given. The starch turns blue/brown in the presence of iodine and sweat (Fig. 2). Patients who underwent parotidectomy had a positive Minor starch-iodine test in 62% of cases, whereas the self-reported incidence of symptoms was only 23% in the same group.5 These numbers attest both to the high incidence of the synkinesis and to the subclinical nature of Frey syndrome.
Fig. 2.
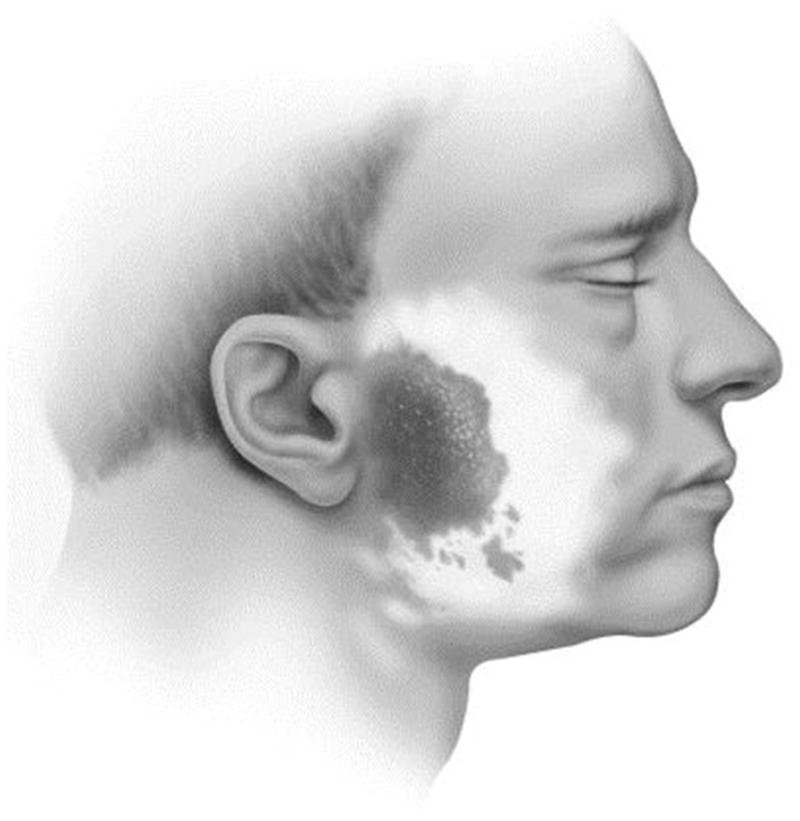
Demonstration of a Minor starch-iodine test: iodine is painted on the area of interest and allowed to dry. This area is then coated with starch powder. When sweat reacts with iodine, it turns the starch brown. The dark pretragal area demonstrates a positive Minor starch-iodine test, whereas the other lighter areas are considered negative. (From Arad A, Blitzer A. Botulinum toxin in the treatment of autonomic nervous system disorders. Operative Techniques in Otolaryngology–Head and Neck Surgery 2004;15:119; with permission.)
SURGICAL METHODS FOR THE PREVENTION OF FREY SYNDROME
Prevention of Frey syndrome has been guided by the alteration of surgical techniques or the addition of procedures focused on preventing synkinesis. The overarching theme for the surgical prevention of Frey syndrome has been the incorporation and maintenance of a barrier between the underlying postganglionic parasympathetic nerve endings within the transected parotid and the overlying cutaneous tissue. Many techniques aimed at accomplishing this have been described and include increased skin flap thickness, local fascia or muscle flaps, and the use of acellular dermal matrix (ADM) or free fat grafts.
Increased Skin Flap Thickness
Within the facial skin, the sweat glands are positioned at the same level or slightly deeper than the base of the hair follicles. Based on this, it has been presumed that increasing the thickness of the elevated skin flap, to keep the sweat glands from being exposed, affords protection from the aberrant parasympathetic nerve regeneration that results in Frey syndrome. Early studies suggested that there was a significant increase in the rate of Frey syndrome when a thin flap was elevated compared with that of a thick flap (12.5 vs 2.6%, P<.05).9 Although this study did lay a foundation for surgical technique, its measurements for flap thickness were crude, and the diagnosis of Frey syndrome lacked objective measurements.9
More recent studies, which objectively measured flap thickness and assessed Frey syndrome by clinical symptoms as well as starch-iodine testing, have failed to demonstrate a reduction in the incidence of Frey syndrome with increased flap thickness.10,11 Although flap thickness did not decrease the incidence of Frey syndrome in these studies, it did show a decrease in the total skin surface area affected and perhaps the overall severity of the disease.11
Transposition Muscle or Fascia Flaps
Similar to increasing the thickness of the elevated skin flap to shield the facial sweat glands from aberrant reinnervation, pedicled muscle and fascia flaps have been used to cover the resected parotid gland in an attempt to create a physical barrier between the overlying dermis and the transected nerve fibers within the parotid.
Temporoparietal fascia flap
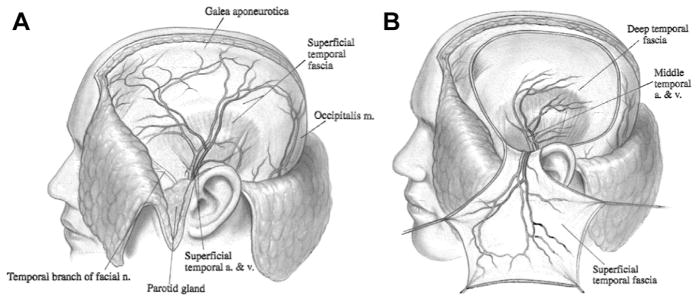
The temporoparietal fascia flap (TPFF) is a broad, vascularized fascia flap that is based off the superficial temporal artery (Fig. 3). It was first described as a composite flap for ear reconstruction in 1976 by Fox and Edgerton12 and was later adapted to a fascia flap. Given its accessibility, predictable vasculature, and low donor site morbidity, it became a common method for reconstruction of the cheek, ear, nasal cavity, and orbit.
Fig. 3.
(A) An anatomic drawing of the superficial temporal artery and vein supplying the superficial temporal fascia demonstrating the proximity to the temporal branch of the facial nerve. (B) An elevated TPFF. (From Larrabee A, Reynolds B, Long C. The temporoparietal fascia flap. Operative Techniques in Otolaryngology–Head and Neck Surgery 1993;4:19; with permission.)
The use of a TPFF was first described in a series of 7 patients undergoing parotidectomy for the prevention of Frey syndrome in 1995 by Sultan and colleagues.13 The results of this series showed that prophylactic inlay of a TPFF to the parotidectomy defect prevented Frey syndrome as assessed by both clinical history and starch-iodine testing in all 7 patients. In addition, the TPFF allowed for improved cosmetic outcome because it reduced the contour defect.13 Since its initial description, multiple retrospective studies have confirmed the utility of TPFF for the prevention of Frey syndrome. In these studies, the use of a TPFF decreased the rate of Frey syndrome after parotid surgery, as determined by positive starch-iodine test, to 4% to 17%, and reduced the clinical incidence of gustatory sweating to 4% to 8%.13,14 This reduced clinical incidence compared with 39% to 57% and 34% to 43%, respectively, for patients who did not undergo TPFF reconstruction.13,14
Although this represents an effective technique for the prevention of Frey syndrome, it does require an extended incision, which can generally be hidden in the hairline. In addition, the course of the temporal branch of the facial nerve is at potential risk for injury. Moreover, local flaps increase operative time and can require a second reconstructive team, which may increase overall cost of the procedure.
Sternocleidomastoid muscle flap
The sternocleidomastoid (SCM) muscles flap is a muscular flap with a tripartite blood supply. The occipital artery, which enters the posterior surface of the muscle in the upper third, is the predominant blood supply to the superior SCM flap. This technique has been favored for reconstruction secondary to its proximity and ability to easily rotate into a parotidectomy defect. Although the SCM flap is easy to harvest and can provide adequate cosmetic reconstruction, its ability to prevent Frey syndrome after parotidectomy is unclear. Reports in the literature have supported that the use of an SCM muscle flap decreases the incidence of Frey syndrome. In a retrospective study of 43 patients, Filho and colleagues15 demonstrated that SCM muscle flap reconstruction following parotidectomy resulted in no cases of Frey syndrome in 24 patients, when assessed clinically and by starch-iodine testing, comparing with 52.6% and 63.2%, respectively, in a control group of patients not undergoing SCM flap reconstruction.15 In addition, a meta-analysis published in 2009 by Curry and colleagues16 concluded that SCM flaps decrease the rate of Frey syndrome after parotidectomy. However, other studies have shown that the muscle flaps are ineffective at preventing the sequela of Frey syndrome.17,18 Unfortunately, given the relatively small number of patients evaluated in the studies and the immense heterogeneity between studies, it is difficult to draw conclusions based on the data available.18
Superficial musculoaponeurotic system flap
Another technique, focused on creating a physical barrier between the underlying regenerating auriculotemporal nerve fibers and the overlying dermis, is a superficial musculoaponeurotic system (SMAS) flap. This SMAS flap can be harvested using a standard modified Blair incision or the facelift incision, and the SMAS can be easily separated from the overlying skin and the parotid tissue to be tightly plicated to the ear perichondrium and the SCM muscle, creating a tight surface that prevents the retromandibular collapse for improved contouring. Similar to the other types of local reconstruction, the efficacy data are mixed. Bonanno and colleagues19 found this technique overwhelmingly effective in preventing the development of Frey syndrome, while other groups failed to demonstrate its ability to do so.10,20–23 However, although the incidence of postparotidectomy Frey syndrome was not significantly changed in these studies, the severity and overall surface area affected were significantly less.23 Although this technique does serve to isolate the underlying regenerating nerve fibers, it is more commonly used in clinical practice for cosmetic reasons rather the prevention of Frey syndrome.
Biomaterial and Autologous Implantation
Autologous and biosynthetic material have been used to create the physical barrier between the transected parotid and the overlying cutaneous tissues. Numerous products have been reported, but the most commonly cited are acellular dermal matrix implantation and autologous fat grafting.
Acellular dermal matrix
ADM is a soft tissue matrix graft that is generated by decellularization of tissue that results an intact extracellular matrix. It is commonly used in wound healing and reconstructive surgery because it provides a scaffold for regenerating tissues. Since its development, it has been used in parotidectomy for the prevention of Frey syndrome. As with muscle or fascia flaps, the goal of this graft is to create a biologic barrier between the facial skin flap and the transected parotid gland. In a limited number of studies that have investigated its effectiveness at preventing Frey syndrome, there are limited data that suggest it is effective in reducing both objective and clinical measures of Frey syndrome.24–26
Abdominal fat grafting
Abdominal fat implantation to the parotidectomy defect is a commonly used technique for decreasing the postsurgical defect and improving cosmesis. In very limited studies, there have been reports that abdominal fat implantation decreases the occurrence of Frey syndrome. However, this has failed to be substantiated. In addition, abdominal fat harvest requires an additional incision on the abdomen and can frequently be complicated by donor site hematoma and surgical site seroma.
POSTSURGICAL TREATMENT OF FREY SYNDROME
Medical Management
Although intraoperative techniques try to reduce severity and incidence of Frey syndrome, postoperative interventions have been focused on ameliorating symptoms once they develop. Most of the therapies used are given via injection therapy or by topical application. Previous agents have included topical antiperspirants as well as injection with alcohol, scopolamine, glycopyrrolate, or botulinum toxin A (BTA). Currently, BTA is the most widely used agent for intradermal injection. Previous studies have demonstrated that patients undergoing BTA injection demonstrate improvement in symptoms of gustatory sweating and flushing.27,28 In addition, it has been shown to improve patient quality of life.28 Unfortunately, with BTA injection, symptomatic recurrence has been demonstrated in up to 27% and 92% of patients at 1 and 3 years, respectively.29 However, despite a high rate of return symptoms after BTA injection, repeat BTA injection has been shown to be effective.29 For the studies investigating BTA, the injection dose was between 1.9 and 2.5 U/cm2 in the involved area.27–29 Unfortunately, no randomized control studies have been documented, and based on a Cochrane Review of the literature, no conclusions can be made on its efficacy.30
Surgical Management
Historically, surgical treatment of Frey syndrome has not been used. Reports of surgical transection of the auriculotemporal nerve, tympanic nerve, and greater auricular nerve have been described for the management of Frey syndrome, but they are not commonly practiced. Recently, a cohort of 17 patients with postparotidectomy Frey syndrome who underwent both SCM and temporalis fascia transposition was reported by Dia and colleagues.31 This report demonstrated that greater than 50% (9/17) of patients who underwent the transposition procedure had complete resolution by starch-iodine testing.31 In addition, there was a significant reduction in the average surface area of gustatory-sweating–positive skin from 12.80 to 1.32 cm2 in all patients postoperatively.31 Although this method is compelling and does appear to be a feasible option for surgical management of Frey syndrome, it does have an increased risk for facial nerve injury. Given the limited number of studies on transposition procedures, no recommendations can be made on its evidence-based efficacy. However, if surgery for Frey syndrome is to be attempted, it should be only be used in cases that are refractory to conservative nonsurgical measures.
SUMMARY
Despite the uncertainty of effectiveness, the above-mentioned intraoperative preventative techniques are low risk and often can also be used to improve cosmetic outcomes. Despite the lack of level 1 evidence-based data, there is likely to be benefit in using an SMAS, SCM, or TPFF reconstruction for both cosmesis and the prevention of Frey syndrome. In addition, ADM represents a suitable alternative to local fascia and muscle flaps for the prevention of Frey syndrome. Of note, local reconstruction with the above techniques is not advised in the presence of malignant disease or gross spillage of benign tumors due to the concern for local recurrence.
In managing symptomatic complaints of Frey syndrome, BTA injection, although not definitive therapy, can significantly decrease the severity and thus morbidity of Frey syndrome. Surgical management of postparotidectomy Frey syndrome should be reserved for severe and refractory cases, as there are limited data to support its use.
KEY POINTS.
Frey syndrome is a common sequela of parotid gland surgery, affecting up to 64% of patients with varying degrees of severity.
Frey syndrome is secondary to synkinesis of postganglionic parasympathetic nerve fibers within the transected parotid gland reinnervating the overlying sweat glands.
Many surgical techniques, which are aimed at creating a barrier between the transected parotid gland and the overlying skin, have been used with varying degrees of success to prevent the development of Frey syndrome.
Subcutaneous injection of botulinum toxin A into affected areas can be used to treat the postoperative symptoms of Frey syndrome.
Surgical treatment for Frey syndrome refractory to medical management has been described, but clinical data to support its utilization are limited.
References
- 1.Frey L. Le syndrome du nerf auriculo-temporal. Revue Neurologique. 1923;2:92–104. [Google Scholar]
- 2.Freedberg A, Shaw R, McManus J. The auriculotemporal syndrome. A clinical and pharmacologic study. J Clin Invest. 1948;27(5):669–76. doi: 10.1172/JCI102015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drummond PD. Mechanisms of gustatory flushing in Frey’s syndrome. Clin Auton Res. 2002;12:144–6. doi: 10.1007/s10286-002-0042-x. [DOI] [PubMed] [Google Scholar]
- 4.Rustemeyer J, Eufinger H, Bremerich A. The incidence of Frey’s syndrome. J Craniomaxillofac Surg. 2008;36:34–7. doi: 10.1016/j.jcms.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Neumann A, Rosenberger D, Vorsprach O, et al. The incidence of Frey syndrome following parotidectomy: results of a survey and follow-up. HNO. 2011;2:173–8. doi: 10.1007/s00106-010-2223-6. [DOI] [PubMed] [Google Scholar]
- 6.Gardner WJ, McCubbin JW. Auriculotemporal syndrome: gustatory sweating due to misdirection of regenerated nerve fibers. J Am Med Assoc. 1956;160:272–7. doi: 10.1001/jama.1956.02960390022007. [DOI] [PubMed] [Google Scholar]
- 7.Baek C, Chung M, Jeong H, et al. Questionnaire evaluation of sequelae over 5 years after parotidectomy for benign diseases. J Plast Reconstr Aesthet Surg. 2009;62(5):633–8. doi: 10.1016/j.bjps.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Hartl D, Julieron M, LeRidant A, et al. Botulinum toxin A for quality of life improvement in post-parotidectomy gustatory sweating (Frey’s syndrome) J Laryngol Otol. 2008;122(10):1100–4. doi: 10.1017/S0022215108001771. [DOI] [PubMed] [Google Scholar]
- 9.Singleton G, Cassisi N. Frey’s syndrome: incidence related to skin flap thickness in parotidectomy. Laryngoscope. 1980;90:1636–40. [PubMed] [Google Scholar]
- 10.Taylor SM, Yoo J, Matthews TW, et al. Frey’s syndrome and parotidectomy flaps: a retrospective cohort study. Otolaryngol Head Neck Surg. 2000;122(2):201–3. doi: 10.1016/S0194-5998(00)70239-7. [DOI] [PubMed] [Google Scholar]
- 11.Durgut O, Basut O, Demir U, et al. Association between skin flap thickness and Frey’s syndrome in parotid surgery. Head Neck. 2012;35(12):1781–6. doi: 10.1002/hed.23233. [DOI] [PubMed] [Google Scholar]
- 12.Fox J, Edgerton M. The fan flap: an adjunct to ear reconstruction. Plast Reconstr Surg. 1976;58:663–7. doi: 10.1097/00006534-197612000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Sultan M, Wider T, Hugo N. Frey’s syndrome: prevention with the temporopartietal fascial flap interposition. Ann Plast Surg. 1995;34:292–7. doi: 10.1097/00000637-199503000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Ahmen O, Kolhe P. Prevention of Frey’s syndrome and volume deficit after parotidectomy using the superficial temporal artery fascial flap. Br J Plast Surg. 1999;52:256–60. doi: 10.1054/bjps.1998.0137. [DOI] [PubMed] [Google Scholar]
- 15.Filho W, Dedivitis R, Rapoport A, et al. Sternocleidomastoid muscle flap preventing Frey syndrome following parotidectomy. World J Surg. 2004;28:361–4. doi: 10.1007/s00268-003-7304-1. [DOI] [PubMed] [Google Scholar]
- 16.Curry J, King N, Reiter D, et al. Meta-analysis of surgical techniques for preventing parotidectomy sequelae. Arch Facial Plast Surg. 2009;11(5):327–31. doi: 10.1001/archfacial.2009.62. [DOI] [PubMed] [Google Scholar]
- 17.Kornblut A. Sternocleidomastoid muscle transfer in the prevention of Frey’s syndrome. Laryngoscope. 1991;101:571–2. doi: 10.1288/00005537-199105000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Sanabria A, Kowalski L, Bradley P, et al. Sternocleidomastoid muscle flap in preventing Frey’s syndrome after parotidectomy: a systematic review. Head Neck. 2012;34(4):589–98. doi: 10.1002/hed.21722. [DOI] [PubMed] [Google Scholar]
- 19.Bonanno P, Palaia D, Rosenberg M, et al. Prophylaxis against Frey’s syndrome in parotid surgery. Ann Plast Surg. 2000;44:498–550. doi: 10.1097/00000637-200044050-00006. [DOI] [PubMed] [Google Scholar]
- 20.Wille-Bischofberger A, Rajan G, Linder T, et al. Impact of the SMAS on Frey’s syndrome after parotid surgery: a prospective, long-term study. Plast Reconstr Surg. 2007;120:1519–23. doi: 10.1097/01.prs.0000282036.04717.1d. [DOI] [PubMed] [Google Scholar]
- 21.Yu L, Hamilton R. Frey’s syndrome: prevention with conservative parotidectomy and superficial musculoaponeurotic system preservation. Ann Plast Surg. 1992;29:217–22. doi: 10.1097/00000637-199209000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Allison G, Rappaport I. Prevention of Frey’s syndrome with superficial musculoaponeurotic system interposition. Am J Surg. 1993;166:407–10. doi: 10.1016/s0002-9610(05)80343-5. [DOI] [PubMed] [Google Scholar]
- 23.Barbera R, Castillo F, D’oleo C, et al. Superficial musculoaponeurotic system flap in partial parotidectomy and clinical and subclinical Frey’s syndrome. Cosmesis and quality of life. Head Neck. 2014;36(1):130–6. doi: 10.1002/hed.23215. [DOI] [PubMed] [Google Scholar]
- 24.Govindaraj S, Cohen M, Genden EM, et al. The use of acellular dermis in the prevention of Frey’s syndrome. Laryngoscope. 2001;111:1993–8. doi: 10.1097/00005537-200111000-00024. [DOI] [PubMed] [Google Scholar]
- 25.Ye W, Zhu H, Zheng J, et al. Use of allogenic acellular dermal matrix in prevention of Frey’s syndrome after parotidectomy. Br J Oral Maxillofac Surg. 2008;46:649–52. doi: 10.1016/j.bjoms.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Fan J, Sun C, et al. Systemic evaluation on the use of acellular matrix graft in prevention of Frey syndrome after parotid neoplasm surgery. J Craniofac Surg. 2013;24:1526–9. doi: 10.1097/SCS.0b013e31828dcdb3. [DOI] [PubMed] [Google Scholar]
- 27.Beerens A, Snow G. Botulinum toxin A in the treatment of patients with Frey syndrome. Br J Surg. 2002;89(1):116–9. doi: 10.1046/j.0007-1323.2001.01982.x. [DOI] [PubMed] [Google Scholar]
- 28.Tugnoli V, Marchese Ragona R, Eleopra R, et al. The role of gustatory flushing in Frey’s syndrome and its treatment with botulinum toxin type A. Clin Auton Res. 2002;12(3):174–8. doi: 10.1007/s10286-002-0026-x. [DOI] [PubMed] [Google Scholar]
- 29.Laccourreye O, Akl E, Gutierrez-Fonseca R, et al. Recurrent gustatory sweating (Frey’s syndrome) after intracutaneous injection of botulinum toxin type A: incidence, management, and outcome. Arch Otolaryngol Head Neck Surg. 1999;125(3):283–6. doi: 10.1001/archotol.125.3.283. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Wu F, Zhang Q, et al. Interventions for the treatment of Frey’s syndrome. Co-chrane Database Syst Rev. 2015;(3):CD009959. doi: 10.1002/14651858.CD009959.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dia X, Liu H, He J, et al. Treatment of postparotidectomy Frey syndrome with the interposition of temporalis fascia and sternocleidomastoid flaps. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119(5):514–21. doi: 10.1016/j.oooo.2014.12.025. [DOI] [PubMed] [Google Scholar]