An increase in ventricular volume induces an immediate increase in pressure development or stroke volume, a phenomenon that is known as the Frank-Starling Law of the heart. At the myocardial level, conveniently studied by using an isolated cardiac muscle preparation in an experimental tissue bath, this cardiac property manifests as an immediate increase in twitch force upon muscle stretch [1]. At the cellular level, it is now well established that modulation of the responsiveness of the cardiac contractile apparatus to activating Ca2+ is largely responsible for the Frank-Starling property, a regulatory mechanism that is termed myofilament length dependent activation [2]. However, despite intense investigation, the molecular mechanisms underlying myofilament length dependent activation are still not resolved [3].
In muscle, both the number of strongly attached cross-bridges and the unitary force that is generated by each of these cross-bridges, determine force development. Hence, the increase in force development that is seen upon muscle stretch could be caused by modulation of either parameter. One potential mechanism whereby the number of strongly bound cross-bridges and the force per cross-bridge is modulated, is an alteration in cross-bridge cycling rate in response to stretch. Indeed, some reports have shown a reduction in the rate of force development (Ktr) upon muscle stretch, e.g. [4–6]. Those data, derived from muscle length isometric studies, suggest that muscle stretch is associated with a decrease in cross-bridge cycling rate. This would be expected to increase the number of strongly bound cross-bridges, possibly concomitant with an increase in unitary cross-bridge force (force per cross-bridge) due to a reduction in the rate of cross-bridge detachment. Other reports, however, employing sarcomere isometric stress(σ), force corrected for cross-sectional area, tension-cost [7], sinusoidal sarcomere length perturbation frequency analysis [8], or segment length isometric contractions [9], have indicated that muscle stretch is not associated with alterations in cross-bridge cycle kinetics. Recent studies by Pavlov and Landesberg published in this journal [10] studying force responses to sarcomere shortening ramps in intact muscle during a steady state maximum tetanic contraction derive a similar conclusion, that is, cross-bridge cycling kinetics is not affected by sarcomere length per se.
One potentially important difference between studies that do show modulation of cross-bridge kinetics upon stretch and those that do not, is whether the experiments employed measurement and/or control of sarcomere length, as was pointed out by Pavlov and Landesberg [10]. At this point, therefore, it is useful to review the reasons that measurement of sarcomere length is crucial in the study of cardiac muscle mechanics. Fig. 1 shows representative recordings of force, muscle length (ML), and laser diffraction linear scanning diode array derived real-time sarcomere length (SL) obtained from an isolated cat right ventricular cardiac trabecula (data modified from [11]). The left panel shows, at an expanded time scale, twitch force and SL in response to electrical stimulation (cf. arrow head). ML was kept isometric at slack length, which was ~2.8 mm for this particular preparation. Despite constant ML, SL shortens from ~1.9 μm in diastole to ~1.7 μm at peak systole. This “internal SL shortening” is caused by stretch of visco-elastic structures external to the cardiac sarcomere that are formed by damage inflicted upon the ends of the preparation during the isolation procedure [1]. Regardless of the level of care or the species employed, this damage is unavoidable. Moreover, it affects all cardiac muscle preparations, be it a cardiac trabecula or a small papillary muscle. One immediate and obvious consequence of internal SL shortening is that peak twitch force occurs at a SL that is significantly shorter than the SL in diastole. The right panel shows the impact of a slow, ~45% ML stretch to ~4 mm over the course of ~20 twitches. Several observations are apparent in this record. First, diastolic SL initially increases roughly in direct proportion to ML. Second, when diastolic SL reaches ~2.3 μm, ML stretch no longer induces a proportional increase in diastolic SL, and diastolic (passive) force increases steeply. Third, upon such large ML stretch, the magnitude of internal SL shortening during the contraction diminishes substantially.
Fig. 1. Contractile response to increase in length.
Stress (σ), force corrected for cross-sectional area, and real-time laser diffraction derived sarcomere length (SL) was recorded from an isolated cat cardiac trabecula that was electrically stimulated at 0.2 Hz (arrow head); the middle panel shows muscle length (ML). The left panel shows a single twitch at an expanded time-scale at slack length. The right panel shows the contractile response to a slow ML ramp stretch to ~45%. The left scales depict absolute calibrations, while the right scales depict relative calibrations in percent normalized to slack length (ML% and SL%). Modified from [11].
What is causing the disparate behavior of ML and SL upon stretch? It is the mechanical consequence of an arrangement where a “healthy” contractile central myocardial segment is placed in series with visco-elastic damaged ends on either side of the muscle preparation. The damaged ends were introduced during dissection of the muscle from the ventricular wall. When the “healthy” central sarcomeres contract, the damaged ends are stretched, thereby allowing for systolic SL shortening. Upon initial stretch from slack SL (~1.9 μm), sarcomere passive stiffness is relatively low and, consequently, diastolic passive force development, due to the combined impacts of inter-sarcomeric titin and an extra-cellular perimysial collagen [3,12], is low and diastolic SL increases in rough proportion to overall muscle length. However, upon further stretch, when SL reaches ~2.3 μm, sarcomere stiffness increases precipitously due to collagen bundles in parallel with cardiac fascicles [12], causing a sharp rise in diastolic passive force development. This passive force is carried by both the central “healthy” sarcomeres and the damaged-end visco-elastic structures, since both elements are arranged in series. Moreover, the elastic properties of the damaged-end compliance are, as is common for biological materials, non-linear, such that overall elastic stiffness increases with length. As a consequence, upon extended ML stretch, the extent of SL shortening decreases, despite the increase in active twitch force development.
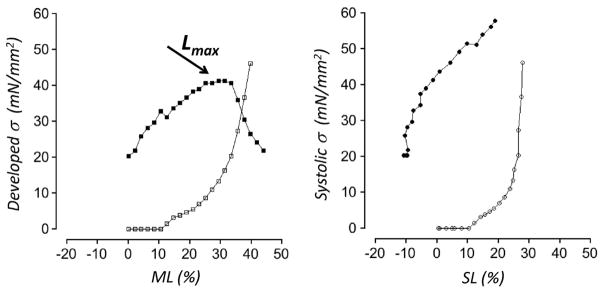
The impact of damage-end compliance on the calculated force-length relation, which represents the Frank-Starling relationship at the muscle level, derived from the data displayed in Fig. 1, is illustrated in Fig. 2. To allow for comparison between ML and SL based analyses, SL was normalized to diastolic slack SL (~1.9 μm), while ML was normalized to slack muscle length (~2.8 mm). Force was divided by average cross-sectional area so as to derive normalized stress. The left panel shows systolic active developed stress (closed symbols) and passive diastolic stress (open symbols) as function of normalized ML. This is the “traditional” length-stress relationship, as found in many text books, where passive stress is subtracted from total peak twitch stress to derive the active stress that is developed by the contractile apparatus at each ML. This “traditional” length-stress relationship exhibits a maximum where developed stress is optimal; the ML at which this occurs is termed Lmax (arrow). However, as discussed above and illustrated in Fig. 1, even though the muscle contracts isometrically, the central sarcomeres are far from isometric. Hence, peak systolic twitch stress occurs at a lower SL than that existed prior to stimulation in diastole. Moreover, the passive stress carried by the centrally located “healthy” sarcomeres at the peak of the contraction will be less than the passive stress observed in diastole. In effect, the passive element parallel to the sarcomeres is ‘unloaded’ during the contraction due to central sarcomere internal shortening. The extent of passive element unloading can be calculated directly from the diastolic stress-SL relationship. The right panel of Fig. 2 illustrates the stress-SL relationship as is calculated when the unloading phenomenon is accounted for. Here, passive stress at the equivalent systolic SL (open symbols), not the diastolic stress prior to stimulation, is subtracted from total twitch stress at each ML to derive active systolic twitch stress, which is then plotted as function of systolic SL (closed symbols). Several observations for the cardiac length-stress relationship are apparent when real-time measurement of sarcomere length is taken into account, in contrast to measurements based solely on ML. First, maximum active stress developed by the centrally located “healthy” sarcomere is significantly higher (here ~50%!). Second, no optimum SL for stress development is observed, in contrast to the prominent Lmax that is seen in the left panel of Fig. 2; thus, cardiac contractile activity occurs exclusively on the ascending limb of the length-stress relationship, and the cardiac Lmax concept is an experimental artifact. Third, the cardiac sarcomere operates over a range of SL that is smaller than can be appreciated from the traditional ML based measurements. Finally, the slope of the length-stress relationship at the level of the cardiac sarcomere level is significantly steeper. In addition, the overall time-course of the cardiac contraction is substantially shorter in ML isometric contractions as compared to SL isometric contraction (not shown here, but see [1]). Of note, at first sight it may appear possible to estimate the stiffness of the damaged-end series elasticity throughout the twitch without actually directly measuring real-time SL (for example, by imposing rapid ML releases in test contractions at various times during the twitch). However, the applicability of such an approach is greatly diminished by the fact that the damaged ends are in reality not purely visco-elastic but, rather, are composed of a mixture of cardiac myocytes that are either in contracture, depolarized and relaxed, or partially contracting. The relative distribution of these myocyte populations is likely highly variable, depending on factors such as SL, bathing [Ca2+], inotropic agents that may be applied in the experiment, after-load, and stimulus frequency, to name but a few. Thus, unambiguous analysis of cardiac muscle mechanics, requires, at a minimum measurement of real-time sarcomere length throughout the entire twitch. Even further control can be accomplished by application of either SL feed-back [1] or iterative SL feed-forward [13] approaches.
Fig. 2. Stress-Sarcomere Length versus Stress-Muscle length relationships.
Analysis of the data shown in Fig. 1. The left panel shows the “traditional” ML based relationship between developed-stress (peak twitch stress minus passive stress; closed squares) and passive stress (open squares), as function of normalized ML; maximum developed stress occurs at the optimal ML, Lmax (arrow). The right panel shows SL based approach, that is, the relationship between systolic-stress (peak twitch stress minus the passive stress that is recorded at the systolic SL; active- (closed circles) and passive stress (open circles), as function of normalized-SL. Unambiguous analysis of cardiac muscle mechanics requires measurement of real-time sarcomere length.
The study by Pavlov and Landesberg [10] concluded that at the level of the cardiac sarcomere, myofilament length dependent activation is not caused by a modulation of cross-bridge cycle kinetics. Moreover, their data indicate that the immediate change in twitch force upon a change in sarcomere length is solely due to recruitment, that is variation of the number of strongly attached force generating cross-bridges, and that force per cross-bridge for a given shortening velocity is constant. The mechanisms underlying sarcomere length modulation of cross-bridge recruitment could not be determined from their study. From our own studies, we believe that we have conclusively excluded variation of inter-filament spacing with SL as mechanism [3]. Rather, sarcomere stretch appears to induce an immediate [14] alteration in the ordering of diastolic myosin heads that is directly correlated with the increase in force development for the subsequent twitch, in support of the cross-bridge recruitment hypothesis [15]. Of interest, a recent x-ray diffraction structural study that employed frog skeletal undergoing transient shortening ramps suggests another, until now unappreciated, mode of cross-bridge recruitment modulation. Here, the authors propose that muscle load, possibly via a direct strain sensing mechanism within the thick-filament, induces recruitment of myosin heads from a dormant “OFF” state conformation towards an active “ON” state [16]. This mechanism may very well also contribute to the myofilament length dependent activation phenomenon discussed here.
Acknowledgments
Supported, in part, by NIH HL75494 and HL 62426 (PdT), and grants Alberta H&SF (HtK). We would like to dedicate this editorial to the memory of Dr. William C. Little (5/1/1950 - 7/9/2015).
Footnotes
Disclosures
none.
Contributor Information
Pieter P. de Tombe, Cell and Molecular Physiology, Loyola University Chicago, Maywood IL, USA
Henk E.D.J. ter Keurs, Medicine and Medical Physiology, University of Calgary, Calgary, Alberta, Canada
References
- 1.ter Keurs HEDJ, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae: evidence of length-dependent activation. Circ Res. 1980;46:703–714. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- 2.Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986;58:755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- 3.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. http://dx.doi.org/10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stelzer JE, Moss RL. Contributions of stretch activation to length-dependent contraction in murine myocardium. J Gen Physiol. 2006;128:461–471. doi: 10.1085/jgp.200609634. http://dx.doi.org/10.1085/jgp.200609634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J. 2004;87:1784–1794. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milani-Nejad N, Xu Y, Davis JP, Campbell KS, Janssen PML. Effect of muscle length on cross-bridge kinetics in intact cardiac trabeculae at body temperature. J Gen Physiol. 2013;141:133–139. doi: 10.1085/jgp.201210894. http://dx.doi.org/10.1085/jgp.201210894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wannenburg T, Janssen PM, Fan D, de Tombe PP. The frank-starling mechanism is not mediated by changes in rate of cross-bridge detachment. Am J Physiol. 1997;273:H2428–H2435. doi: 10.1152/ajpheart.1997.273.5.H2428. [DOI] [PubMed] [Google Scholar]
- 8.Wannenburg T, Heijne GH, Geerdink JH, Van Den Dool HW, Janssen PM, de Tombe PP. Cross-bridge kinetics in rat myocardium: effect of sarcomere length and calcium activation. Am J Physiol Heart Circ Physiol. 2000;279:H779–H790. doi: 10.1152/ajpheart.2000.279.2.H779. [DOI] [PubMed] [Google Scholar]
- 9.Hancock WO, Martyn DA, Huntsman LL. Ca2 + and segment length dependence of isometric force kinetics in intact ferret cardiac muscle. Circ Res. 1993;73:603–611. doi: 10.1161/01.res.73.4.603. [DOI] [PubMed] [Google Scholar]
- 10.Pavlov DA, Landesberg A. The Cross-Bridge Dynamics is Determined by two Length Independent Kinetics; Implications on Muscle Economy and Frank-Starling Law. J Mol Cell …. 2016 doi: 10.1016/j.yjmcc.2015.11.007. http://dx.doi.org/10.1016/j.yjmcc.2015.11.007. [DOI] [PubMed]
- 11.de Tombe PP, ter Keurs HE. Sarcomere dynamics in cat cardiac trabeculae. Circ Res. 1991;68:588–596. doi: 10.1161/01.res.68.2.588. [DOI] [PubMed] [Google Scholar]
- 12.Hanley PJ, Young AA, LeGrice IJ, Edgar SG, Loiselle DS. 3-dimensional configuration of perimysial collagen fibres in rat cardiac muscle at resting and extended sarcomere lengths. J Physiol. 1999;517(Pt 3):831–837. doi: 10.1111/j.1469-7793.1999.0831s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Tombe PP, Little WC. Inotropic effects of ejection are myocardial properties. Am J Physiol. 1994;266:H1202–H1213. doi: 10.1152/ajpheart.1994.266.3.H1202. [DOI] [PubMed] [Google Scholar]
- 14.Mateja RD, de Tombe PP. Myofilament length-dependent activation develops within 5 ms in guinea-pig myocardium. Biophys J. 2012;103:L13–L15. doi: 10.1016/j.bpj.2012.05.034. http://dx.doi.org/10.1016/j.bpj.2012.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farman GP, Gore D, Allen E, Schoenfelt K, Irving TC, de Tombe PP. Myosin head orientation: a structural determinant for the frank-starling relationship. Am J Physiol Heart Circ Physiol. 2011;300:H2155–H2160. doi: 10.1152/ajpheart.01221.2010. http://dx.doi.org/10.1152/ajpheart.01221.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Linari M, Brunello E, Reconditi M, Fusi L, Caremani M, Narayanan T, et al. Force Generation by Skeletal Muscle is Controlled by Mechanosensing in Myosin Filaments. Nature. 2015 doi: 10.1038/nature15727. http://dx.doi.org/10.1038/nature15727. [DOI] [PubMed]