Precursor B cell acute lymphoblastic leukemia (preB ALL) is the most common childhood cancer [1,2]. Despite improved overall progress in treatment, patients with certain types of preB ALL have a poor prognosis with a 10-year survival rate of 41±3% [3]. In addition, life-long irreversible late effects from chemo- and radiation therapy, including secondary malignancies, are growing problems for leukemia survivors [4]. Targeted therapy holds enormous promise for leukemia treatment that is more effective and has fewer side effects than conventional therapies.
In B-cell type ALL, including preB ALL, the majority of leukemia cells express several surface antigens, including CD19, CD20 and CD22, which are available for targeted therapeutics. Although the expression levels of CD19 and CD22 are similar, it has been reported that CD22 can internalize more effectively and rapidly than CD19 upon antibody binding [5]. The expression levels of CD22 are higher than those of CD20 in preB ALL and CD22 internalization is faster than CD20 [6]. Therefore, when compared to other potential targets the CD22 antigen can serve as an ideal target for antibody-mediated delivery to B-cell ALL.
Monoclonal antibodies (mAbs) that target CD22 have been developed and used in the clinic. The anti-CD22 (αCD22) mAb, epratuzumab, was first studied in children with relapsed preB ALL in 2008 and the rate of molecular response was increased in combination with chemotherapy [7]. An international Phase III study of chemotherapy with or without epratuzumab for standard risk childhood relapsed ALL is currently ongoing (ClinicalTrials.gov Identifier: NCT01802814). Furthermore, antibody drug conjugates (ADCs) provide a method to deliver a chemotherapeutic agent to the antigen-positive tumor cells using mAbs as a vehicle for targeted drug delivery. Currently, at least four different αCD22 ADCs are in Phase I or II clinical trials, including inotuzumab ozogamicin, moxetumomab pasudotox, combotox, and DCDT2980S (αCD22-MC-vc-PAB-monomethyl auristatin E (MMAE)) for B-cell lymphoid malignancies [8]. Of these, inotuzumab ozogamicin and combotox are in clinical trials for B-cell type ALL. Inotuzumab ozogamicin is a humanized IgG4 αCD22 antibody (Ab) linked to calicheamicin, which has shown encouraging activity in indolent and aggressive non-Hodgkin’s lymphoma (NHL) and relapsed/refractory B-cell type ALL in Phase I and II studies [8]. Combotox is a mixture of two immunotoxins prepared from a deglycosylated ricin A (dgRTA) chain conjugated to mAbs directed against CD22 and CD19, which has demonstrated single-agent activity in heavily pretreated patients with B-cell type ALL and NHL patients in Phase I studies [8].
MMAE is a very potent anti-mitotic drug that inhibits cell division and induces apoptosis by binding to microtubules and blocking the polymerization of tubulin. Several mAb-MMAE ADCs, including CD19, CD22, CD79b and CD30, have demonstrated efficacy [9,10]. The efficacy of αCD19-vcMMAE was reported in a preclinical preB ALL xenograft mouse model, and the other mAb-MMAE ADCs, including CD22 and CD79b, were reported only for lymphoma xenograft models or patients. In this study, we evaluated αCD22 Ab-MMAE using a unique αCD22 ligand blocking mAb that our group developed as previously described [11] and maleimide-functionalized valine-citrulline cleavable peptide linker MMAE (mal-vcMMAE) [12]. Our study is the first to demonstrate the therapeutic efficacy of the CD22 ligand blocking αCD22 Ab-MMAE in a preclinical patient-derived preB ALL xenograft mouse model.
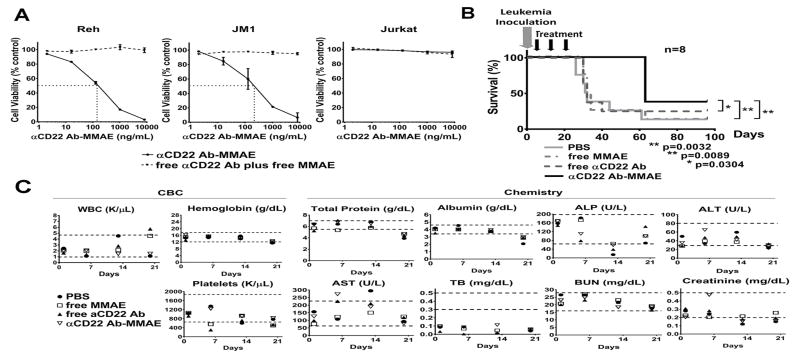
The αCD22 Ab, free MMAE and αCD22 Ab-MMAE were provided by Dr. Joseph Tuscano at UC Davis, and 4.6 molecules of MMAE were covalently conjugated to the Ab [12]. First we demonstrated in vitro cytotoxicities of the αCD22 Ab-MMAE in a preB ALL cell line (Reh), a preB lymphoma and leukemia cell line (JM1) and a T-ALL cell line (Jurkat) (Figure 1A). Reh and JM1 cells were confirmed to be positive and Jurkat cells were negative for CD22 expression by flow cytometry [13]. Using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay, IC50 (50% inhibitory concentration) of the αCD22 Ab-MMAE was determined to be 143.3 and 211.0 ng/mL for Reh and JM1, respectively (calculated using the Prism 6 statistical program). Free αCD22 plus free MMAE had no cytotoxic effect in Reh or JM1 cells at tested concentrations. As expected, the αCD22 Ab-MMAE showed no cytotoxicity in Jurkat cells at tested concentrations. These findings suggest that our αCD22 Ab-MMAE is effective only in CD22 positive cells and the cytotoxicity is mediated by αCD22 Ab-facilitated MMAE delivery in vitro.
Figure 1. αCD22 Ab-MMAE shows cytotoxicity in human preB ALL both in vitro and in vivo.
(A) αCD22 Ab-MMAE shows cytotoxicity in B-cell type ALL cell lines (Reh and JM1), but not in a T-ALL cell line (Jurkat), in vitro. Reh, JM1 and Jurkat cells were treated continuously for 72 hours with αCD22 Ab-MMAE or equivalent doses of free αCD22 Ab plus free MMAE. Cell viability was assessed by MTS assay. The mean ± SD of 2 independent experiments in triplicate is shown. The IC50 value of αCD22 Ab-MMAE was 143.3 ng/mL for Reh and 211.0 ng/mL for JM1. Free αCD22 Ab plus free MMAE showed no cytotoxic effect in Reh and JM1 cells. No cytotoxicity was observed at tested concentrations of αCD22 Ab-MMAE in Jurkat cells.
(B) αCD22 Ab-MMAE shows significant in vivo therapeutic efficacy in a primary human leukemia mouse model. Treatment groups: 1) PBS, 2) free MMAE (0.165mg/kg), 3) free αCD22 Ab (7.335mg/kg), and 4) αCD22 Ab-MMAE (7.5mg/kg). n=8 per group. Gray arrow shows the leukemia inoculation. Black arrows show treatments by intravenous injection once a week for three weeks. When compared to controls (PBS, free αCD22 Ab or free MMAE treatments), the αCD22 Ab-MMAE treatment increased the median survival time of the mice by two fold. There were leukemia engraftment failure mice: one to three mice each from the PBS and free MMAE groups, two mice from the free aCD22 Ab group, and three mice from the aCD22 Ab-MMAE group. In the survival time analysis, we defined the event as death only due to leukemia. Therefore, these mice were included in the survival curve; however, the data of these mice were censored. The median survival time of each group was the median of only leukemia mice.
(C) αCD22 Ab-MMAE treatment does not show toxicities in the primary human leukemia mouse model. CBC, WBC, ALP, ALT, AST, TB and BUN are abbreviations for complete blood counts, white blood cells, alkaline phosphatase, alanine transaminase, aspartate transaminase, total bilirubin and blood urea nitrogen, respectively. Results of weekly CBC and chemistry panels are shown. The reference range of blood examination for NOD/SCID mice (female, age 8–10 weeks) is shown as dotted lines in each graph: WBC 960–4,680/μL, hemoglobin 12.1–17.6 g/dL, platelet 651–1871 × 103/μL, total protein 5.5–7.0 g/dL, albumin 3.4–4.6 g/dL, ALP 64–198 U/L, ALT 29–80 U/L, AST 63–227 U/L, TB 0.3–0.5 mg/dL, BUN 16–28 mg/dL, creatinine 0.2–0.5 mg/dL.
Next we assessed in vivo therapeutic efficacy of the αCD22 Ab-MMAE in a human preB ALL xenograft mouse model. All procedures were performed in compliance with our institutionally-approved animal care protocol in the barrier facility vivarium at the Institute for Regenerative Cures at UC Davis in accordance with AALAC. The dose of αCD22 Ab-MMAE was determined based on our previous study for non-Hodgkin lymphoma [12]. A xenograft model was created using primary preB ALL cells which were 92% CD22 positive and NOD/SCID/IL2Rg−/− (NSG) mice. Primary leukemia cells were collected from a patient with informed consent based on our institutionally-approved IRB protocol. Characteristics of the primary leukemia sample are summarized in Table I. Passage 3 of serially transplanted mice were used for efficacy studies. Five million leukemia cells were inoculated via intra-bone marrow injection to each mouse. These mice were randomly assigned to each treatment group (n=8 per group): 1) PBS, 2) free MMAE (0.165mg/kg), 3) free αCD22 Ab (7.335mg/kg) and 4) αCD22 Ab-MMAE (7.5mg/kg). The dose of free αCD22 Ab and free MMAE was equivalent to the dose of each component in the αCD22 Ab-MMAE conjugate. Treatment was initiated 24 hours after leukemia inoculation, with a weekly intravenous injection for 3 weeks. The mice were monitored daily until they developed signs of sickness, such as scruffy coats, poor activity and splenomegaly. They were then sacrificed and confirmed to have developed leukemia. The leukemia cells were harvested from bone marrow, and confirmed to be positive for HLA and CD22. The end point was survival time. Gehan-Breslow-Wilcoxon test was used to compare the survival times (Prism 6 statistical program). The mice in each of the control groups were euthanized due to signs of leukemia and confirmed to have leukemia at approximately the same time: between days 26 and 61 (median survival time 31.5 days) for the PBS group, between days 30 and 63 (median survival time 33 days) for the free MMAE group, and between days 30 and 40 (median survival time 32 days) for the free αCD22 Ab group. The mice that received αCD22 Ab-MMAE were euthanized and confirmed to have leukemia on day 63 (median survival time 63 days). Therefore, the mice that received αCD22 Ab-MMAE survived significantly longer than any of the control groups (vs. PBS p < 0.005, vs. free MMAE p < 0.05, and vs. free αCD22 Ab p < 0.05) (Figure 1B).
Table I.
Characteristics of the primary leukemia sample used in the study
| initial WBC (x 10e3/uL) | 439.6 |
| age (year old) / sex | 4 / male |
| cytogenetics | 47, XY, +mar[13]/46, XY[7] |
| morphology | lymphoblast |
| phenotype | CD10, 19, 20, TdT, cCD79a positive |
| CD22 expression % | 92 |
WBC: white blood cells. CD22 expression was the % relative to HLA expression on the cells at inoculation for the xenograft models.
Toxicity was assessed with complete blood counts (CBC) and chemistry panels weekly during treatment. White blood cell and platelet count, total protein, albumin, alkaline phosphatase, aspartate transaminase and creatinine showed some variability. Total bilirubin was lower than the reference range from day 1 in all mice; therefore, these depressions were not due to treatments. More importantly, these results were not significantly different among each treatment group at each time point (Figure 1C). During treatment, the mice in all the treatment groups remained healthy and active, and did not lose weight. CBC and chemistry panels were analyzed by one way ANOVA (Prism 6 statistical program).
Over the last few years, several αCD22 Ab ADCs have been successfully developed and shown to improve the potency of chemotherapy by increasing the target specificity with reduced off target effects [8]. Although adverse effects were observed during the trials, such as neutropenia, thrombocytopenia, infections, neuropathy, hepatic transaminase elevation, hypoalbuminemia and infusion reactions, these effects were managed through standard supportive care. More encouraging data with αCD22 Ab ADCs such as αCD22 Ab-saporin [13] and αCD22 Ab-anthracycline analogue [14] have been reported in in vivo animal studies. αCD22-targeted ADCs are a promising and less toxic approach than conventional chemotherapy for B-cell malignancies.
In our study, we demonstrated the therapeutic efficacy and safety profile of the αCD22 Ab-MMAE in a preclinical preB ALL xenograft mouse model. There are previously-reported studies showing the efficacy of αCD22 Ab-MMAEs (DCDT2980S and our αCD22 Ab-MMAE conjugate) in lymphomas (NHL, follicular lymphoma and diffuse large B-cell lymphoma) [12,15]. Our study, however, is the first to demonstrate the efficacy of αCD22 Ab-MMAE in preB ALL. More importantly, this is the first pilot study to demonstrate the therapeutic efficacy of αCD22 Ab-MMAE in a human patient-derived preB ALL xenograft model which has direct clinical translational applications. Our αCD22 Ab-MMAE, using only three doses, doubled survival times in a primary preB ALL xenograft model with no adverse events. These results suggest that the αCD22 Ab-MMAE can be a new candidate drug, as a single agent or as part of combination therapy for preB ALL patients. Future studies, including larger studies with more patient samples, and pharmacokinetics studies in larger animals, are necessary to confirm the efficacy, and determine appropriate treatment doses and schedules. We also plan to assess the potential for improved cytotoxicities by combining the αCD22 Ab-MMAE with standard chemotherapeutics that have demonstrated efficacy for preB ALL.
Footnotes
Disclosure
The authors have no conflicts of interest.
References
- 1.Hunger SP, Mullighan CG. Acute Lymphoblastic Leukemia in Children. N Engl J Med. 2015;373:1541–1552. doi: 10.1056/NEJMra1400972. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 3.Schrappe M, Hunger SP, Pui CH, et al. Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med. 2012;366:1371–1381. doi: 10.1056/NEJMoa1110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmiegelow K, Levinsen MF, Attarbaschi A, et al. Second malignant neoplasms after treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2013;31:2469–2476. doi: 10.1200/JCO.2012.47.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du X, Beers R, Fitzgerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carnahan J, Wang P, Kendall R, et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res. 2003;9:3982s–3990s. [PubMed] [Google Scholar]
- 7.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robak T, Robak E. Current Phase II antibody-drug conjugates for the treatment of lymphoid malignancies. Expert Opin Investig Drugs. 2014;23:911–924. doi: 10.1517/13543784.2014.908184. [DOI] [PubMed] [Google Scholar]
- 9.de Goeij BE, Lambert JM. New developments for antibody-drug conjugate-based therapeutic approaches. Curr Opin Immunol. 2016;40:14–23. doi: 10.1016/j.coi.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Gerber HP, Kung-Sutherland M, Stone I, et al. Potent antitumor activity of the anti-CD19 auristatin antibody drug conjugate hBU12-vcMMAE against rituximab-sensitive and -resistant lymphomas. Blood. 2009;113:4352–4361. doi: 10.1182/blood-2008-09-179143. [DOI] [PubMed] [Google Scholar]
- 11.Engel P, Nojima Y, Rothstein D, et al. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J Immunol. 1993;150:4719–4732. [PubMed] [Google Scholar]
- 12.Abuhay M, Kato J, Tuscano E, et al. The HB22.7-vcMMAE antibody-drug conjugate has efficacy against non-Hodgkin lymphoma mouse xenografts with minimal systemic toxicity. Cancer Immunol Immunother. 2016 doi: 10.1007/s00262-016-1873-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato J, Satake N, O’Donnell RT, Abuhay M, Lewis C, Tuscano JM. Efficacy of a CD22-targeted antibody-saporin conjugate in a xenograft model of precursor-B cell acute lymphoblastic leukemia. Leuk Res. 2013;37:83–88. doi: 10.1016/j.leukres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 14.Yu SF, Zheng B, Go M, et al. A Novel Anti-CD22 Anthracycline-Based Antibody-Drug Conjugate (ADC) That Overcomes Resistance to Auristatin-Based ADCs. Clin Cancer Res. 2015;21:3298–3306. doi: 10.1158/1078-0432.CCR-14-2035. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Poon KA, Yu SF, et al. DCDT2980S, an anti-CD22-monomethyl auristatin E antibody-drug conjugate, is a potential treatment for non-Hodgkin lymphoma. Mol Cancer Ther. 2013;12:1255–1265. doi: 10.1158/1535-7163.MCT-12-1173. [DOI] [PubMed] [Google Scholar]