Abstract
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is regarded as effective surgical treatments in patients with peritoneal metastasis. This study aimed to evaluate the clinical outcomes of CRS and HIPEC in patients with appendiceal or colorectal cancer with peritoneal carcinomatosis.
A total of 66 patients who underwent CRS with HIPEC for appendiceal or colorectal cancer with peritoneal metastasis at 2 tertiary referral centers in Korea were evaluated between July 2014 and March 2016. The perioperative outcomes and postoperative complications were evaluated prospectively.
The mean peritoneal cancer index (PCI) was 15.3 ± 10.5. The distributions thereof were as follows: PCI < 10, 33.3%; PCI 10–19, 36.4%; and PCI≥20, 30.3%. Regarding completeness of cytoreduction (CC), 59.1% of patients achieved CC-0, with 18.2% showing CC-1 and 22.7% showing CC-2. The mean operation time was 9.4 hours, and the mean hospital stay was 20.2 days. The overall rate of short-term complications was 74.2%; the rate of long-term complications was 10.6%. In the short-term period, most complications were grades I-II complications (62.1%), compared to grades III-V (12.1%). All long-term complications, occurring in 10.6% of patients, were grades III-V.
In this study, CRS with HIPEC was deemed feasible and safe for treating stage IV appendiceal or colorectal cancer with peritoneal carcinomatosis in Koreans.
Keywords: appendiceal neoplasms, colorectal neoplasms, cytoreduction surgical procedures, injections, intraperitoneal, peritoneal neoplasms
1. Introduction
Peritoneal carcinomatosis is the second most common metastatic lesion in patients with stage IV colorectal cancer, with a cancer recurrence incidence of 10% to 35%.[1,2] When free intraperitoneal cancer cells are exfoliated from a primary cancer, they can be disseminated and lead to peritoneal metastasis or local recurrence.[3] Meanwhile, cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is based on the concept that microscopic residual tumors can be eradicated by direct penetration of anticancer drugs into tumor cells at 42°C to 43°C after removal of the macroscopic tumor.[4] According to the randomized controlled trial by Verwaal et al,[5,6] the median survival after CRS with HIPEC in patients with peritoneal metastasis of colorectal origin was improved above that achieved with standard systemic chemotherapy (22.3 vs 12.6 months, P = .032).[5,6] In a phase II multicentric French study, Elias et al[7] reported that patients who underwent surgery with perioperative intraperitoneal chemotherapy experienced a median survival of 30.1 months with 27% of 5-year overall survival rates.
Although CRS with HIPEC offers advantages in treating peritoneal metastasis, the procedure is technically demanding and must be performed by a highly experienced surgical team. Moreover, a high rate of postoperative complications and a steep learning curve hinder wider performance of CRS with HIPEC.[8–12] Since July 2014, our institutions began offering CRS with HIPEC for patients with colorectal cancer with peritoneal carcinomatosis. In this study, we aimed to evaluate clinical outcomes of CRS with HIPEC and its feasibility in treating Korean patients with appendiceal or colorectal cancer with peritoneal carcinomatosis.
2. Methods
2.1. Study population and data collection
A colorectal surgeon (SH Baik) started CRC with HIPEC at Severance Hospital in July 2014, and since then, a total of 66 patients with peritoneal metastasis originating from colorectal or appendiceal cancer have underwent CRS with HIPEC at 2 tertiary referral hospitals (Severance Hospital and Gangnam Severance Hospital) of the Yonsei University Health System as of March 2016. Patients were diagnosed with peritoneal metastases preoperatively via abdomino-pelvic computed tomography (CT), positron emission tomography-CT, or diagnostic laparoscopy. Among patients diagnosed with peritoneal carcinomatosis, patients younger than 80 years with an Eastern Cooperative Oncologic Group (ECOG) score less than 2 were included in this study. Patients who exhibited massive involvement of metastatic lesions, which are unresectable or agglomerated in the small bowel or its mesentery, on preoperative imaging studies or intraoperative findings were contraindicated for HIPEC. Patients who underwent emergent operations or only early postoperative intraperitoneal chemotherapy without HIPEC were excluded.
Before surgery, eligibility for CRS with HIPEC was discussed in a multidisciplinary colorectal team conference using preoperative radiologic examinations. Clinical outcomes and postoperative complications were collected prospectively in an electronic database (Yonsei Colorectal Database). This study was approved by the Institutional Review Board of our institution (IRB No. 3-2016-0196).
2.2. Evaluation parameters
Preoperatively, patients were evaluated for combined morbidities and the American Society of Anesthesiologists (ASA) classification, along with assessment of previous chemotherapy history or abdominal surgery. Peritoneal Surface Disease Severity Score (PSDSS) was determined from stages I to IV, which were assessed according to 3 categories based on clinical symptoms, extent of carcinomatosis (peritoneal cancer index [PCI] on preoperative CT scan or first exploration), and histopathology of the primary tumor.[13,14]
PCI was assessed through the 13 abdomino-pelvic regions. Each region was graded using the following scale: 0 points, absence of tumor; 1 point, tumor less than 0.5 cm; 2 points, tumor from 0.5 cm to 5 cm; and 3 points, tumor larger than 5 cm. Completeness of cytoreduction (CC) was assessed according to the extent of the remnant tumor: CC-0, complete removal of visible tumor; CC-1, remnant tumor less than 0.25 cm; CC-2, residual tumor between 0.25 cm and 2.5 cm; and CC-3, visible tumor larger than 2.5 cm in diameter.
Synchronous operations were performed with resection of the primary tumor followed by a peritonectomy. The length of stay in the intensive care unit (ICU) was assessed from the day of the operation to admittance in the general ward. The length of hospital stay was calculated from the date of admission to discharge. Patients received adjuvant chemotherapy based on the regimens of FOLFOX, FOLFIRI, or capecitabine with targeted cancer therapeutics, such as bevacizumab or cetuximab. The interval of the first postoperative chemotherapy was calculated as the time elapsed from the date of the operation to the date the patient received the first cycle. Readmission was evaluated for complication-related admission after discharge.
Postoperative complications were classified by the Clavien–Dindo classification of surgical complications.[15,16] Short-term complications were considered as those that developed within the first 30 postoperative days, while long-term complications were evaluated when more than 30 days had elapsed.
2.3. Surgical technique
Before CRS, bowel preparation was performed, except in patients who had intestinal obstructions. Prophylactic antibiotics with a third-generation cephalosporin were administered. CRS was performed by resection of the metastatic organs from the primary cancer with a peritonectomy. Parietal peritonectomy and visceral resections were performed by following the Sugarbaker techniques.[17,18] Anterior peritonectomy, upper quadrant peritonectomy, pelvic peritonectomy, subphrenic peritonectomy, and omental bursectomy were performed selectively, depending on the site of peritoneal metastasis. HIPEC was performed by circulating the mixed solution of 35 mg/m2 of mitomycin-C and 3L of hypertonic solution (Dianeal, 1.5% Dextrose Peritoneal Dialysis Solution). Mitomycin-C was inserted 17.5 mg/m2 initially and 8.8 mg/m2 at 30 and 60 minutes. The mixed solution was circulated 800–1000 mL/min in a HIPEC pump (The Belmont Hyperthermic Pump) to maintain 42°C to 43°C for 90 minutes. The temperatures of both the inflow and outflow of the HIPEC solution, as well as the patient's body temperature, were recorded every 5 minutes. Anastomosis of resected bowels was performed after HIPEC. A diverting ostomy was performed depending on the surgeon's decision in consideration the risk of anastomotic leakage or the extent of CRS. All patients were sent to the ICU after surgery.
3. Results
3.1. Patient characteristics
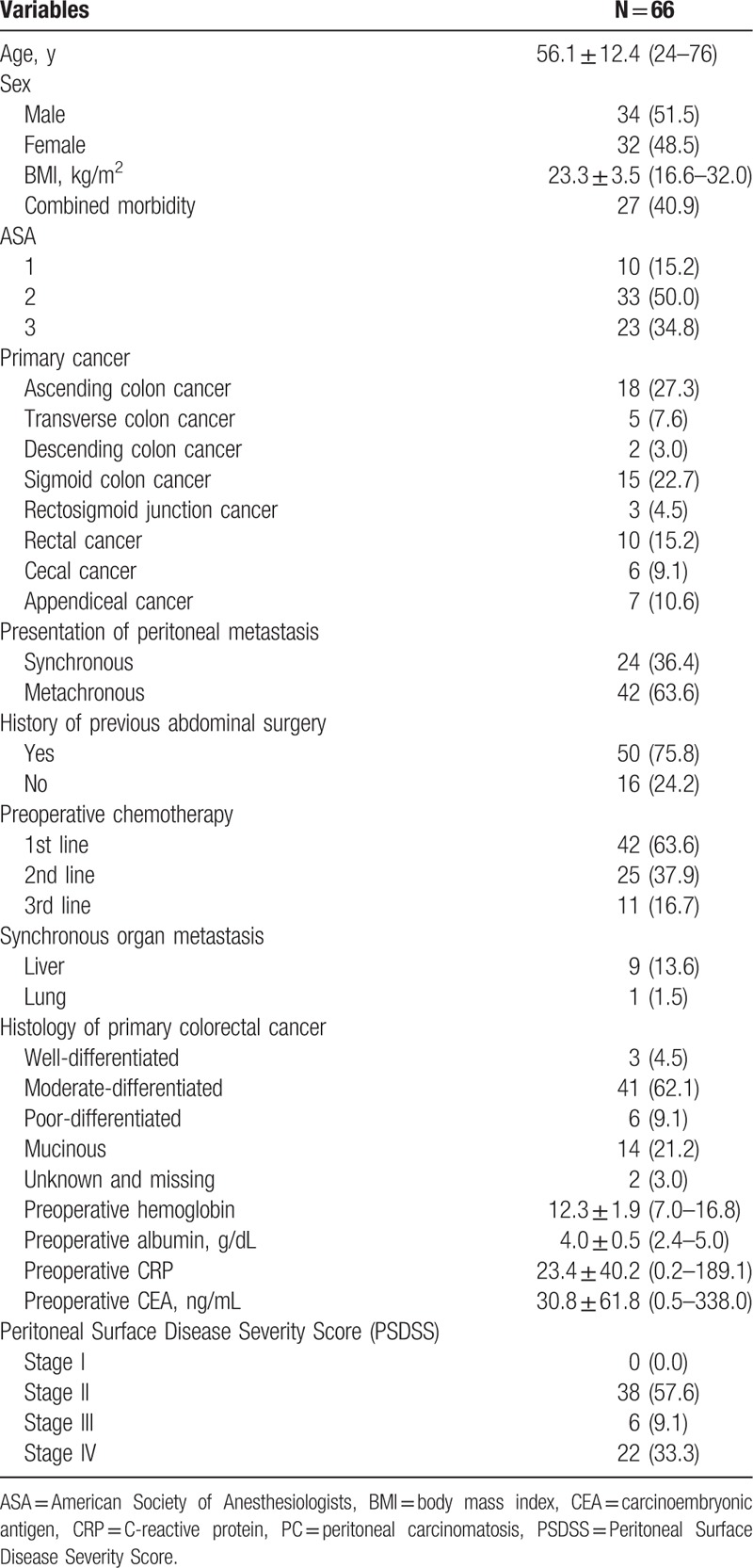
The mean age of the patients was 56.1 years old, with 51.5% males and 48.5% females. Body mass index (BMI) of the patients was 23.3 ± 3.5 (range, 16.6–32.0). Overall, 40.9% of the patients had co-morbidities, with 34.8% of the patients having an ASA score of 3. The most frequent site of primary tumor was the ascending colon. Before CRS with HIPEC, 24 patients (36.4%) had a primary tumor with synchronous peritoneal metastasis, whereas 42 patients (63.6%) had metachronous peritoneal metastasis. In total, 50 patients had previously underwent abdominal surgeries. Among them, 42 patients underwent colorectal cancer surgery, and 3 patients had palliative surgery before CRS plus HIPEC. All 3 cases of palliative surgery were ileostomy formations to treat intestinal obstruction from peritoneal carcinomatosis.
There were 63.6% of the patients who received systemic chemotherapy before CRS with HIPEC (first-line systemic chemotherapy, 63.6%; second-line, 37.9%; third-line, 16.7%). Synchronous organ metastases in addition to peritoneal carcinomatosis were noted in 9 patients with liver metastasis and 1 patient with lung metastasis. Regarding the histologic differentiation of the primary colorectal cancer, moderate differentiation was most common (62.1%). The mean value of preoperative carcinoembryonic antigen was 30.8 ng/mL. In the assessment of PSDSS, 57.6% of the patients were stage II, which was a higher rate than the 9.1% of stage III and 33.3% of stage IV (Table 1).
Table 1.
Baseline characteristics of patients.
3.2. Intraoperative outcomes
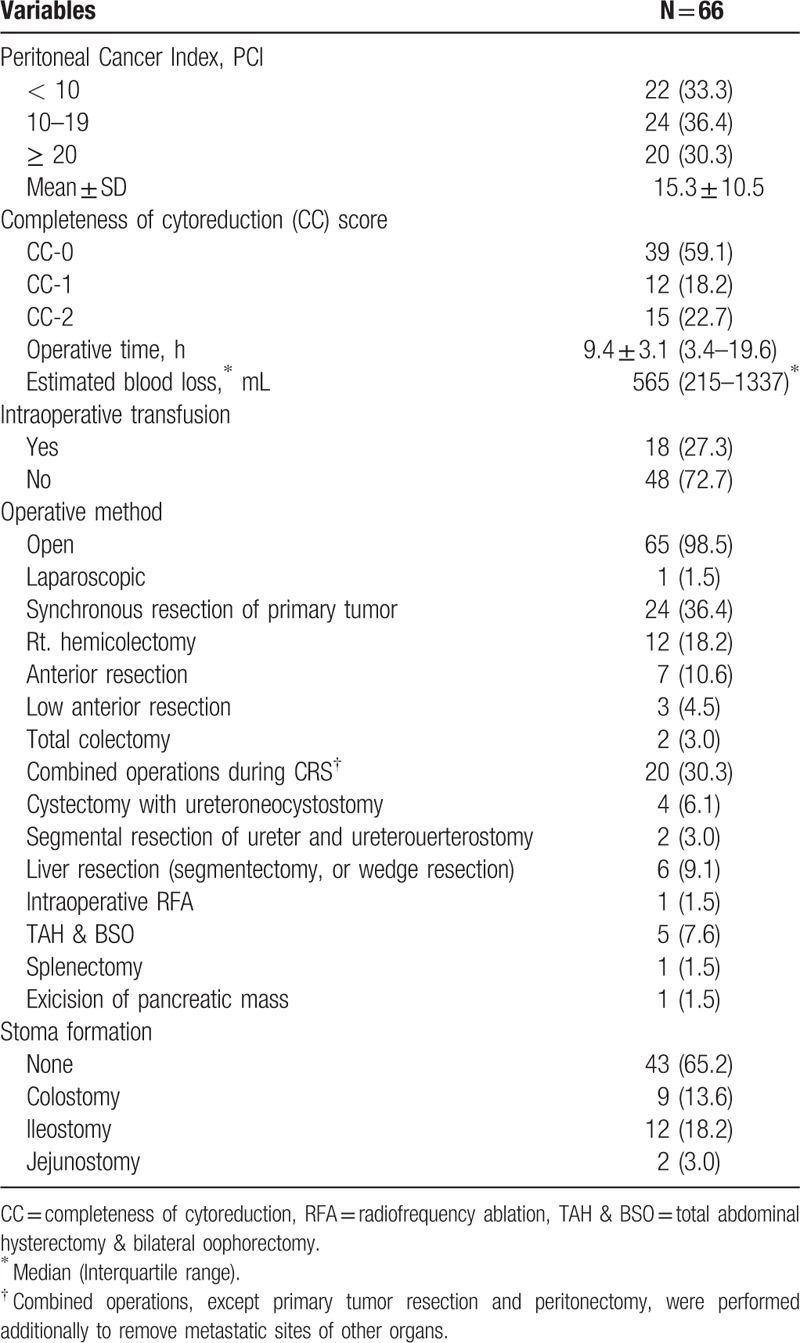
In assessment of extent of peritoneal carcinomatosis, the mean PCI was 15.3 ± 10.5 (range, 1–39). 33.3% of the patients had a PCI less than 10, 36.4% with a PCI of 10 to 19, and 30.3% greater than PCI 20. CC-0 was accomplished in 59.1% of the patients, and CC-1 was achieved in 18.2%. The mean operation time was 9.4 hours. Intraoperative transfusion was performed in 27.3% of the patients, and the mean amount of intraoperative blood loss was 565 mL. HIPEC was performed by the open method with the Coliseum technique, except in 1 case where the laparoscopic approach was used. Synchronous resection of the primary tumor during CRS plus HIPEC was performed in 24 patients (36.4%), whereas resection of the recurrent tumor was performed in 42 patients (63.6%). Among all patients, 20 patients additionally underwent combined operations to remove metastatic lesions of other organs during CRS. Total abdominal hysterectomy with bilateral oophorectomy was performed in 7.6% of the patients. In addition, liver metastasis was treated by surgical resection in 6 patients, and intraoperative radiofrequency ablation in 1 patient. Two other patients with synchronous liver metastasis were treated by systemic chemotherapy with targeted anticancer agents to induce resectable conversion of metastatic liver lesions. Bladder invasion from carcinomatosis was treated by cystectomy in 6.1% of the patients. Stoma formation was performed in 34.8% of the patients: colostomy (13.6%), ileostomy (18.2%), and jejunostomy (3.0%) (Table 2).
Table 2.
Intraoperative outcomes.
3.3. Postoperative clinical outcomes
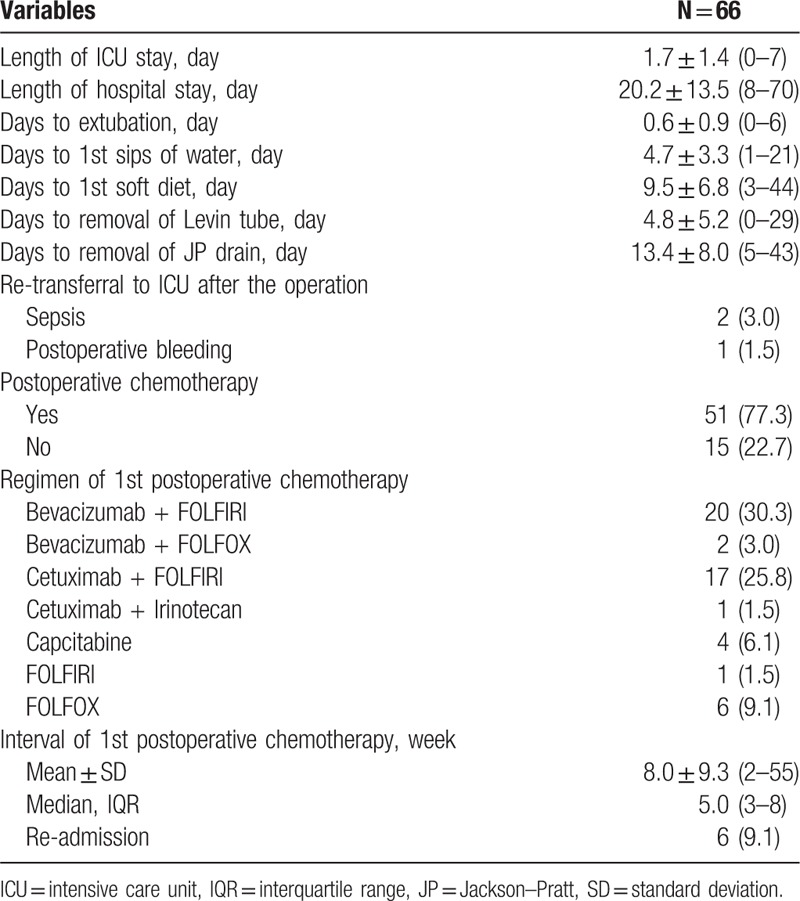
After surgery, the average lengths of stay in the ICU and the hospital were 1.7 days and 20.2 days, respectively. Levin tubes inserted before the operation were removed at 4.8 postoperative days on average. In addition, patients began to eat a soft diet at 9.5 postoperative days. Closed suction drains were removed at 13.4 days. After CRS with HIPEC, 2 patients were re-admitted to the ICU due to sepsis and postoperative bleeding.
After CRS with HIPEC, 77.3% of the patients received postoperative chemotherapy. Among them, 39 patients (59.1%) were treated with adjuvant chemotherapy within the first 2 months. Targeted chemotherapeutic agents, such as bevacizumab or cetuximab, were used in 60.6% of the patients, and FOLFIRI with bevacizumab was used most commonly as the first line adjuvant chemotherapy after HIPEC. Re-admission related to postoperative complications occurred in 9.1% of the patients (Table 3).
Table 3.
Postoperative clinical outcomes.
3.4. Postoperative complications according to the clavien-dindo classification
The overall rate of short-term complications was 74.2%, whereas the rate of long-term complications was 10.6%. Among short-term complications, 62.1% were grade I-II complications, and 12.1% were grade III-V complications. Long-term complications consisted of grade III-V complications: grade III, 7.6%; grade IV, 1.5%; and grade V, 1.5%.
Among short-term complications, hematologic abnormalities were the most common grade II complications: neutropenia (15.2%) and thrombocytopenia (6.1%). In addition, 7.6% of the patients required transfusions postoperatively. Among patients with grade IIIa complications, 3 received interventional therapies for pleural effusion, ureteral stricture, and postoperative bleeding. In addition, there were 4 patients who underwent a re-operation due to postoperative leak, intestinal obstruction, and wound evisceration as grade IIIb complications.
Among long-term complications, grade III complications were recorded in 5 patients (7.6%). In addition, 1 patient with a grade IV long-term complication suffered from septic shock, which developed from bile leakage after hepatectomy following CRS with HIPEC. The main cause of grade V complications was sepsis, which postoperatively occurred with pneumonia. Detailed descriptions are shown in Table 4, and the proportions of postoperative complications according to the follow-up period are demonstrated in Fig. 1.
Table 4.
Postoperative complications according to the Clavien–Dindo classification.
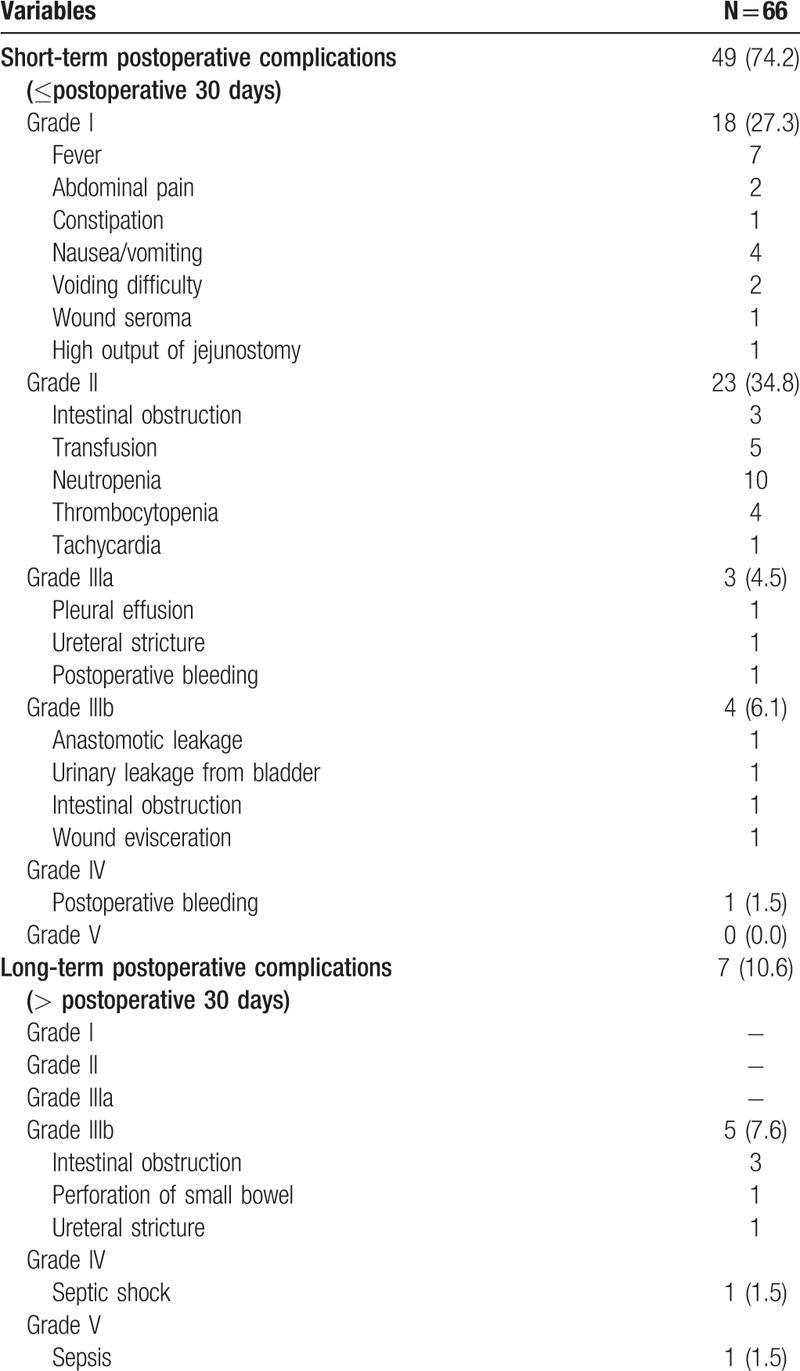
Figure 1.
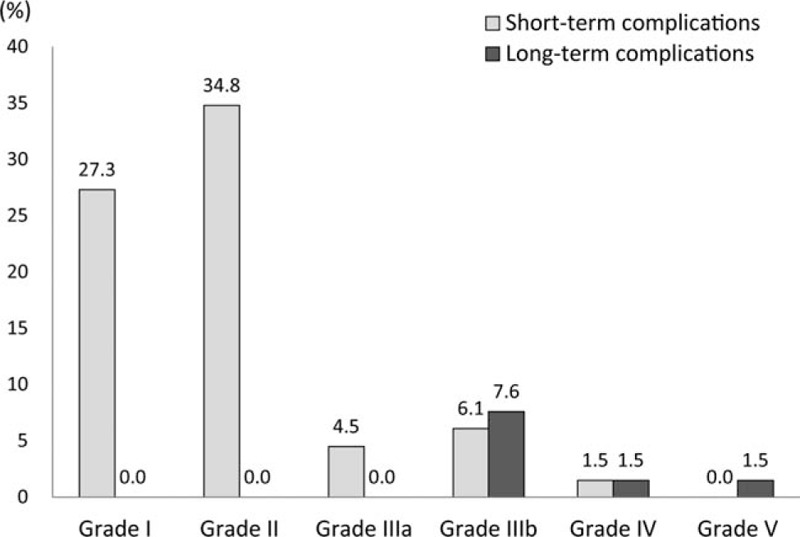
Postoperative complications according to Clavien–Dindo classification.
4. Discussion
CRS and HIPEC have been applied to treat patients with peritoneal carcinomatosis originating from appendiceal cancer, colorectal cancer, ovarian cancer, and pseudomyxoma peritonei. According to the tumor cell entrapment hypothesis, intraoperative manipulation of the primary cancer and cancer-contaminated fluid can result in local-regional dissemination of tumor cells. In addition, spillage of free cancer cells in the abdominal cavity can be implanted in the peritoneal surface and progress to peritoneal metastasis.[19] In addressing these issues, HIPEC has advantages of eradicating residual tumor cells in the peritoneal space, allowing anticancer agents to penetrate into the tumor core and increase cytotoxic effects. Since Sugarbaker et al first began a prospective randomized trial for peritoneal surface malignancy in 1985, CRS with HIPEC has been regarded as an effective treatment to prolong survival in patients with colorectal cancer with peritoneal carcinomatosis.[5,6,20,21]
The perioperative results in this study were comparable to those in previous reports for CRS with HIPEC. The mean operation time was 9.4 hours (range 3.4–19.6), and the length of hospital stay was 20.2 days (8–70). In addition, 59.1% of the patients began postoperative chemotherapy within 2 postoperative months. According to Kusamura et al,[10] 420 patients who underwent CRS with HIPEC showed a mean operation time of 563 minutes and a hospital stay of 22 days. In addition, Polanco et al[22] reported that the median operation time was 430 minutes and the length of hospital stay was 15.86 days in an analysis of 370 cases. Although the days to begin the first soft diet and removal of inserted drains were delayed more than 4 to 5 days, compared with conventional colorectal surgeries, the postoperative outcomes of this study were acceptable in terms of the extensive surgical procedures of CRS, as well as HIPEC.[23,24]
HIPEC seeks to increase cytotoxic effects in tumor cells and to reduce systemic toxicities.[25] To satisfy these purposes, it is crucial to select proper anticancer drugs directed for HIPEC. In this study, we used 35 mg/m2 of mitomycin-C mixed in 3L of 1.5% dextrose peritoneal dialysis solution, which was a similar protocol of Verwaal et al.[5,6] However, mitomycin-C has adverse effects of myelosuppression and renal toxicity. Predisposing factors that decrease excretion of mitomycin-C from the kidney and increase plasma concentration of mitomycin-C might affect postoperative myelosuppression after HIPEC. In addition, immunologic suppression from postoperative surgical stress might aggravate its negative impact on postoperative recovery in hematopoietic systems.[26] Thus, it is important to conduct proper patient selection and to consider dose reductions of anticancer agents for HIPEC in patients who have a very low BMI, long period of prior chemotherapies, decreased renal function, and are older than 60 years old.
Postoperative complications occurred in 74.2% of the patients in the short-term period and in 10.6% in the long-term period. In the short-term period, grade I-II complications developed in 62.1% of the patients. The most common complications of grade II were hematologic abnormalities: neutropenia (15.2%) and thrombocytopenia (6.1%). These developed within 5–7 postoperative days and were treated using hematopoietic agents. In previous studies, the rates of postoperative major complications after CRS with HIPEC were approximately 30–40%.[8,27] However, the rate of postoperative major complications in this study was 12.1% in the short-term period and 10.6% in the long-term period. Although CRS and HIPEC were performed in just 66 cases as an early experience, our results for postoperative complications are acceptable, compared to previous reports. On the other hand, while the majority of short-term complications were grades I-II, all long-term complications were grades III-V as shown in Fig. 1. This suggests that long-term complications require more attention, because they can result in more severe morbidities, compared to short-term complications. It is known that CRS with HIPEC requires a steep learning curve (100–140 cases) to ensure proficient performance of the techniques.[10,27] Because of the aggressive and high-skilled procedures of CRS with HIPEC, postoperative morbidities have been found to differ among institutions, from 10% to 60%.[28] However, it is remarkable that the rate of perioperative mortality is nearly zero at high-volume centers with greater experience with CRS with HIPEC.[28] In these aspects, intensive training and sustained efforts to accumulate surgical experience are key to achieving successful postoperative outcomes after CRS with HIPEC.[29]
Both PCI and CC scores are regarded as key prognostic factors that affect the clinical and oncologic outcomes after CRS with HIPEC.[3,7,30] Because PCI assesses tumor distribution quantitatively, it can predict the likelihood of complete cytoreduction.[30] As shown in Fig. 2, patients with low PCI had high rates of CC-0. On the other hand, patients with high PCI had low rates of CC-0 and high rates of CC-2. These correlations suggest that extensive peritoneal seeding can result in incomplete resections and a poor prognosis.[25] In this study, CC-2 occurred in 22.7% of the patients. Although preoperative imaging studies were performed to examine the extent of peritoneal carcinomatosis before CRS with HIPEC, intraoperative PCI was usually higher than preoperatively predicted values, because it is still difficult to find small peritoneal seeding nodules in the current CT scans. Preoperatively, improved diagnosis of the extent of peritoneal seeding is necessary to determine a patient's eligibility for CRS with HIPEC and to enhance both postoperative and oncologic outcomes.
Figure 2.
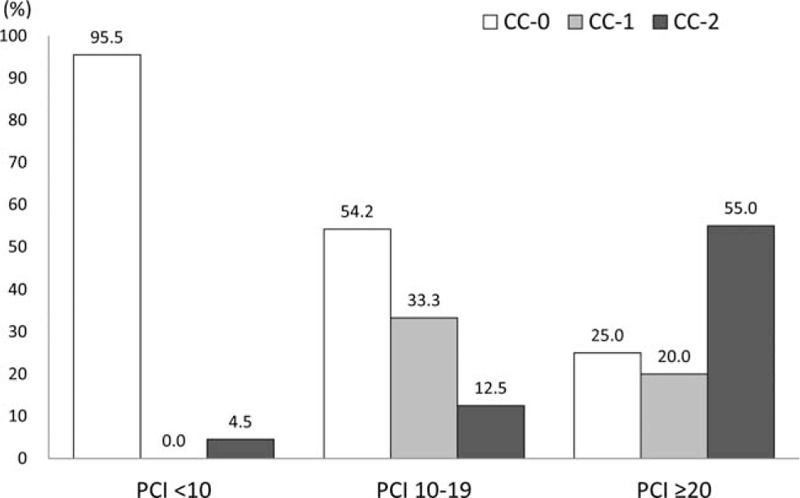
Completeness of cytoreduction (CC) score according to the peritoneal cancer index (PCI). PCI = peritoneal cancer index.
This study has limitations of using a small study population to assess short-term outcomes after CRS plus HIPEC. Although our prospective data collection offers advantages of recording detailed postoperative outcomes, assessment with larger numbers of patients is needed to confirm our clinical outcomes after CRS with HIPEC. However, this study is still meaningful as we demonstrated acceptable short-term clinical outcomes of CRS with HIPEC, which can be used as effective treatment strategies to treat peritoneal metastasis from colorectal cancer.
In conclusion, CRS with HIPEC was deemed feasible for treating appendiceal and colorectal cancer with peritoneal metastasis. We expect that this method will be more widely accepted in treating peritoneal carcinomatosis in patients with stage IV appendiceal and colorectal cancer.
Acknowledgments
The authors thank MiSun Park for the English language editing of this manuscript and Dong-Su Jang, MFA, (Medical Illustrator, Seoul, Korea) for his help with the illustrations.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = body mass index, CC = completeness of cytoreduction, CRS = cytoreductive surgery, CT = computed tomography, ECOG = Eastern Cooperative Oncologic Group, HIPEC = hyperthermic intraperitoneal chemotherapy, ICU = intensive care unit, PCI = peritoneal cancer index, PSDSS = Peritoneal Surface Disease Severity Score.
This article was presented at the 10th International Congress on Peritoneal Surface Malignancy (PSOGI) 2016 Biannual Meeting, Washington DC, USA.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284–92. [DOI] [PubMed] [Google Scholar]
- [2].Brodsky JT, Cohen AM. Peritoneal seeding following potentially curative resection of colonic carcinoma: implications for adjuvant therapy. Dis Colon Rectum 1991;34:723–7. [DOI] [PubMed] [Google Scholar]
- [3].Sugarbaker PH. Management of peritoneal-surface malignancy: the surgeon's role. Langenbecks Arch Surg 1999;384:576–87. [DOI] [PubMed] [Google Scholar]
- [4].Sugarbaker PH, Van der Speeten K, Stuart OA. Pharmacologic rationale for treatments of peritoneal surface malignancy from colorectal cancer. World J Gastrointest Oncol 2010;2:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737–43. [DOI] [PubMed] [Google Scholar]
- [6].Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426–32. [DOI] [PubMed] [Google Scholar]
- [7].Elias D, Gilly F, Boutitie F, et al. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol 2010;28:63–8. [DOI] [PubMed] [Google Scholar]
- [8].Canda AE, Sokmen S, Terzi C, et al. Complications and toxicities after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2013;20:1082–7. [DOI] [PubMed] [Google Scholar]
- [9].Kuijpers AM, Hauptmann M, Aalbers AG, et al. Cytoreduction and hyperthermic intraperitoneal chemotherapy: the learning curve reassessed. Eur J Surg Oncol 2016;42:244–50. [DOI] [PubMed] [Google Scholar]
- [10].Kusamura S, Baratti D, Deraco M. Multidimensional analysis of the learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies. Ann Surg 2012;255:348–56. [DOI] [PubMed] [Google Scholar]
- [11].Chua TC, Yan TD, Saxena A, et al. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg 2009;249:900–7. [DOI] [PubMed] [Google Scholar]
- [12].Kusamura S, Baratti D, Virzi S, et al. Learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies: analysis of two centres. J Surg Oncol 2013;107:312–9. [DOI] [PubMed] [Google Scholar]
- [13].Chua TC, Morris DL, Esquivel J. Impact of the peritoneal surface disease severity score on survival in patients with colorectal cancer peritoneal carcinomatosis undergoing complete cytoreduction and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2010;17:1330–6. [DOI] [PubMed] [Google Scholar]
- [14].Pelz JO, Stojadinovic A, Nissan A, et al. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol 2009;99:9–15. [DOI] [PubMed] [Google Scholar]
- [15].Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187–96. [DOI] [PubMed] [Google Scholar]
- [16].Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sugarbaker PH. Management of peritoneal metastases—basic concepts. J BUON 2015;20(suppl 1):S2–11. [PubMed] [Google Scholar]
- [19].Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev 2016;48:42–9. [DOI] [PubMed] [Google Scholar]
- [20].Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681–5. [DOI] [PubMed] [Google Scholar]
- [21].Sugarbaker PH, Gianola FJ, Speyer JC, et al. Prospective, randomized trial of intravenous versus intraperitoneal 5-fluorouracil in patients with advanced primary colon or rectal cancer. Surgery 1985;98:414–22. [PubMed] [Google Scholar]
- [22].Polanco PM, Ding Y, Knox JM, et al. Institutional learning curve of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for peritoneal malignancies. Ann Surg Oncol 2015;22:1673–9. [DOI] [PubMed] [Google Scholar]
- [23].Cho MS, Kim CW, Baek SJ, et al. Minimally invasive versus open total mesorectal excision for rectal cancer: long-term results from a case-matched study of 633 patients. Surgery 2015;157:1121–9. [DOI] [PubMed] [Google Scholar]
- [24].Park EJ, Cho MS, Baek SJ, et al. Long-term oncologic outcomes of robotic low anterior resection for rectal cancer: a comparative study with laparoscopic surgery. Ann Surg 2015;261:129–37. [DOI] [PubMed] [Google Scholar]
- [25].Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol 2010;2:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baratti D, Kusamura S, Iusco D, et al. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 2014;57:858–68. [DOI] [PubMed] [Google Scholar]
- [27].Moradi BN, 3rd, Esquivel J. Learning curve in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2009;100:293–6. [DOI] [PubMed] [Google Scholar]
- [28].Gusani NJ, Cho SW, Colovos C, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 2008;15:754–63. [DOI] [PubMed] [Google Scholar]
- [29].Moran BJ. Decision-making and technical factors account for the learning curve in complex surgery. J Public Health (Oxf) 2006;28:375–8. [DOI] [PubMed] [Google Scholar]
- [30].Sugarbaker PH, Ryan DP. Cytoreductive surgery plus hyperthermic perioperative chemotherapy to treat peritoneal metastases from colorectal cancer: standard of care or an experimental approach? Lancet Oncol 2012;13:e362–9. [DOI] [PubMed] [Google Scholar]


