Supplemental Digital Content is available in the text
Keywords: efficacy, food additives, necrotizing enterocolitis, network meta-analysis, premature infants
Abstract
Background:
Necrotizing enterocolitis (NEC) is a serious multifactorial gastrointestinal disease which is often discovered in premature infants. Various additives have been used to prevent NEC; yet, their relative efficacy and safety remain disputed. This study aims to compare the efficacy and safety of 5 food additives, namely, probiotics, probiotics + fructo-oligosaccharides, pentoxifylline, arginine, and lactoferrin in preventing NEC in neonates.
Methods:
Embase, PubMed, and Cochrane Library had been searched for all eligible randomized control trials. Odds ratios (ORs) were estimated for dichotomous data and mean differences with 95% credible intervals (CrIs) were estimated for continuous data. Surface under the cumulative ranking curve was used to rank efficacy and safety of the prevention methods on each endpoint.
Results:
A total of 27 eligible studies with 4649 preterm infants were included in this network meta-analysis (NMA), and the efficacy and safety of 5 food additives were evaluated. Probiotic and arginine exhibited better preventive efficacy compared with placebo (OR = 0.50, 95% CrIs: 0.32–0.73; OR = 0.30, 95% CrIs: 0.12–0.73, respectively). Only probiotic achieved a considerable decrease in the risk of mortality compared to placebo (OR = 0.68, 95% CrIs: 0.46–0.98). NEC patients with lactoferrin appeared to have lower incidence of sepsis than those of placebo (OR = 0.13, 95% CrIs: 0.03–0.61) or probiotic (OR = 0.18, 95% CrIs: 0.03–0.83).
Conclusion:
Based on this NMA, probiotics had the potential to be the most preferable additive, since it exhibited a significant superiority for NEC and mortality as well as a relatively balanced performance in safety.
1. Introduction
Necrotizing enterocolitis (NEC) is a severe multifactorial gastrointestinal disease discovered in premature infants. Intensive care, surgery intervention, and potent antimicrobial agents have been introduced in order to reduce its morbidity and mortality.[1,2] Over 85% of the NEC cases occur in newborns with very low birth weight (VLBW, birth weight <1500 g).[3] The average prevalence rate of NEC in VLBW preterm infants is approximately 7%, and 20% to 30% VLBW infants with NEC eventually experience fatal outcomes.[4,5] Besides that, NEC may cause long-term adverse effects on infants, including short bowel syndrome, intestinal stricture, and neuro-developmental retardation.[6] Moreover, there are several risk factors linked with NEC as well, including pathologic bacteria, gastrointestinal immaturity, excessive protein substrate in the intestinal cavity, and enteral feeding (especially formula feeding).
Probiotics are believed to be particularly beneficial to preterm infants, because microorganisms are able to regulate immune response, host metabolism, and produce antimicrobial substances.[2] Moreover, some microorganisms can reduce the potential growth of pathogenic bacteria, enhance antibiotic activities, increase the barrier function of the intestinal barrier, and promote the production of anti-inflammatory cytokines.[7] Besides, both lactoferrin and l-arginine are resistant to a wide range of antibiotics, which can help prevent intestinal infection.[8,9] Some studies also suggested that lactoferrin was able to prevent NEC. There were articles which studied immunoglobulin as an intervention for prevention of neonatal NEC, but nowadays, it seems to be replaced.[10,11] However, the current literature has not conclusively recommended an optimum prevention treatment for NEC in premature infants.
Since there is an increasing demand for reviewing and disclosing the relative efficacy and safety of the above therapies, a thorough review and network meta-analysis (NMA) may help clinicians achieve the objectives. Therefore, we designed this study in order to discover whether these additives exhibited equivalent efficacy and safety with respect to NEC prevention in neonatology. A total of 6 additives were researched in the current literature, including probiotics, pentoxifylline, lactoferrin, probiotics + fructo-oligosaccharides, and arginine. The relative efficacy and safety of the above therapies were evaluated by using the following endpoints: NEC incidence, all-cause mortality, NEC related mortality, sepsis, and hospitalization days.
2. Material and methods
2.1. Study design and selection strategy
Embase, PubMed, and Cochrane Library had been searched for all eligible randomized control trials (RCTs). Besides that, trial databases of the main regulatory agencies were also searched to identify relevant studies published before June 5, 2016. The following key terms and their synonyms were used to find relevant studies: “necrotizing enterocolitis,” “infants,” “newborn,” “probiotics,” “anti-bacterial agents,” “pentoxifylline,” “laparotomy,” “arginine,” “lactoferrin,” “fructo-oligose,” and “randomized control trials.”
2.2. Inclusion criteria
We have considered a large scale of studies and all the relevant researches have to meet the following conditions for inclusion: all the trials should be designed as RCT; research subjects in our study must be newborns; all trials should include at least one of the following endpoint: NEC incidence, all-cause mortality, mortality related to NEC, sepsis, and hospitalization days; primary trials should contain enough information or data for NMA.
2.3. Exclusion criteria
Studies should be eliminated if they had any of the following situations: studies focusing on feeding rate or studies without additives; duplicated studies from the same cohort; meeting abstract, meta-analysis, and case reports.
2.4. Outcome measures and data extraction
In our study, NEC incidence was considered as the primary outcome since it was investigated by the majority of trials. Secondary outcomes included all-cause mortality, sepsis, NEC-related mortality and hospitalization days. Two investigators extracted the corresponding data independently. Once there were disagreements, a further discussion was implemented. We extracted the following data from eligible studies: the first author's name, publication year, study design, sample size, intervention method of addictive, gestational age, birth weight, delivery pattern, Apgar score, as well as necessary data about 5 outcomes. The statistics of outcomes, including NEC, all-cause mortality, NEC-related mortality, sepsis, and hospitalization were also extracted from eligible studies. The Jadad scale system was used to assess the risk of bias in included studies.
2.5. Statistical analysis
A Bayesian NMA was performed to obtain estimates for primary and secondary outcomes in order to compare their efficacy and safety for preventing NEC. STATA version 13.1 (Stata Corp, College Station, TX) and WinBUGS 1.4.3 (MRC Bio-statistics Unit, Cambridge, UK) were used to perform statistical analysis. For continuous outcomes, mean differences with their 95% credible intervals (CrIs) were estimated. For dichotomous outcomes, odds ratios (ORs) with their 95% CrIs were calculated. Surface under the cumulative ranking curve (SUCRA) was applied to rank all of the above therapies with respect to each endpoint. The larger the SUCRA value was, the better the performance of treatment presented. Finally, potential publication bias was assessed by comparison-adjusted funnel plots.
3. Results
3.1. Study selection
The process of study selection is displayed in Fig. 1. First, a total of 1531 records were identified using the searching strategy as mentioned, among which 563 came from PubMed and 968 were retrieved from Embase. Then 1184 studies were removed after reviewing the titles and abstracts and another 320 studies were also excluded because of insufficient information or irrelevant comparisons. Finally, we included 27 RCTs which were subject to full-text review and data extraction.[12–38] All the 27 eligible studies were published between 1999 and 2016.
Figure 1.
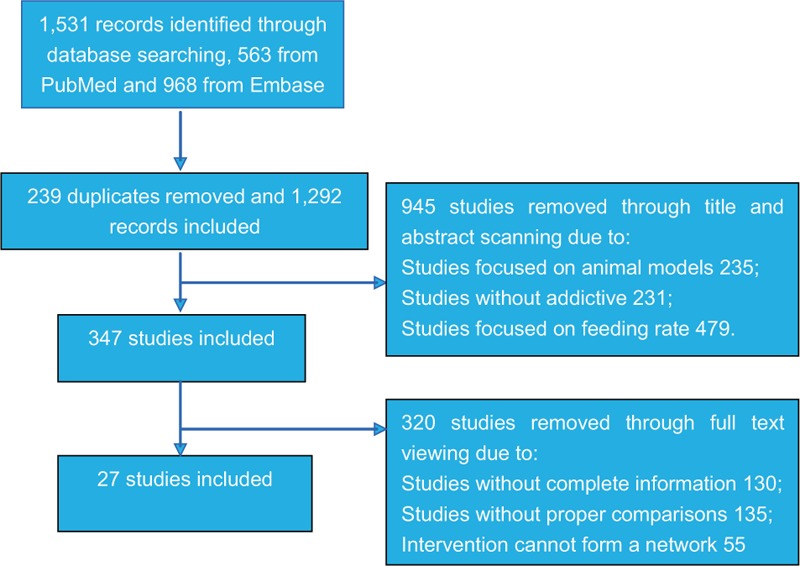
Flowchart of study selection.
3.2. Characteristics of included studies
The baseline characteristics of RCTs included in the NMA are summarized in Table 1 . A total of 4649 preterm infants from 27 studies were involved (the sample size of trials ranged from 37 to 585 participants), and the outcome of NEC incidence was assessed by all included studies. The majority of included studies were designed as double blinding RCTs while only 1 study was single-blinded.[25] All of the included studies compared study additive with placebo in order to determine their relative efficacy or safety. The network structure of evidence with respect to each endpoint can be illustrated in Fig. 2. Moreover, the quality of included studies was overall medium-high using the Jadad Scale that incorporates whether the design of each study used any appropriate randomization techniques, whether an appropriate blinding procedure was introduced, and whether the study disclosed any information about withdrawals.
Table 1.
Characteristics of included studies.
Figure 2.

Network plot of randomized controlled trials comparing different addictive agents for necrotizing enterocolitis prevention. The width of the lines is proportional to the number of trials comparing each pair of treatments with numbers on the lines illustrating the exact number. The size of circles represents the cumulative number of patients for each intervention.
3.3. Network meta-analysis results for NEC incidence and mortality
As shown in Table 2 and Fig. 3, premature infants fed with probiotics or arginine exhibited significantly lower risk of NEC incidence compared to those with placebo (probiotics: OR = 0.50, 95% CrIs: 0.32–0.73; arginine: OR = 0.30, 95% CrIs: 0.12–0.73). Besides that, preterm infants with probiotics also appeared to have significantly reduced risk of mortality compared to those with placebo (OR = 0.68, 95% CrIs: 0.46–0.98).
Table 1 (Continued).
Characteristics of included studies.
Figure 3.
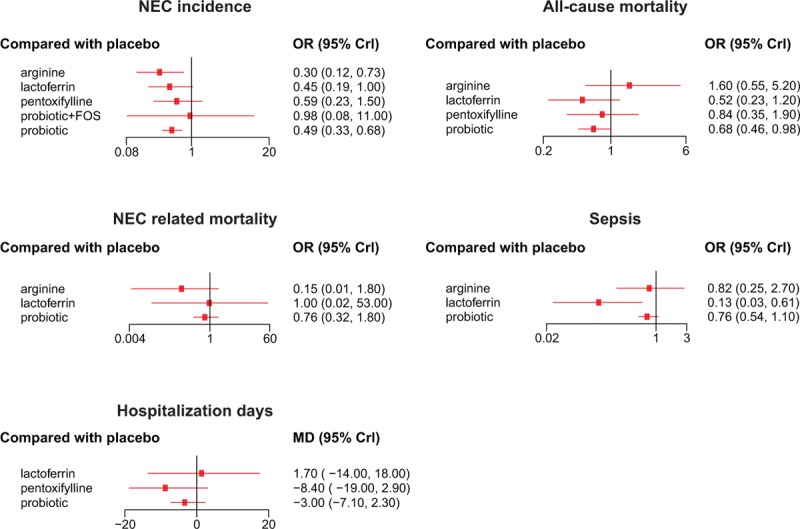
Forest plots for network comparison of necrotizing enterocolitis prevention under 5 endpoints. 95% CrI = 95% credible interval, OR = odds ratio.
3.4. Network meta-analysis results for NEC-related mortality, sepsis, and hospitalization days
As for the endpoint of NEC-related mortality, no significant difference was found between food additives and placebo, as well as among different additives (Table 3). Lactoferrin was associated with a decrease in the risk of sepsis compared to placebo (OR = 0.13, 95% CrIs: 0.03–0.61), as well as probiotics (OR = 0.18, 95% CrIs: 0.03–0.83). Similar to the results of NEC-related mortality, food additives revealed no remarkable difference in hospitalization days mutually or compared to placebo (Table 4).
Table 2.
Network meta-analysis results for NEC incidence and all-cause mortality.

Table 3.
Network meta-analysis results for sepsis and NEC related mortality.

3.5. Ranking of 6 food additives and cluster analysis
The SUCRA value for each food additive implied their potential rankings for each outcome (Table 5, and Fig. S1). Arginine and lactoferrin exhibited the highest SUCRA values with respect to NEC incidence (SUCRA = 0.850 and 0.640, respectively). However, the performance of arginine was compromised by its worst SUCRA ranking under the outcome of mortality (SUCRA = 0.118), while lactoferrin appeared to have the highest SUCRA value (SUCRA = 0.855). Cluster analysis was performed in order to categorize the above 6 food additives into distinctive groups (Fig. 4). The 2-dimensional graph indicated that lactoferrin had relatively stable performance with respect to almost all of the outcomes. Since there were no substantial asymmetry patterns in the funnel plots (Fig. 5), we concluded that no significant publication bias was presented in our study.
Table 4.
Network meta-analysis results for hospitalization days.

Figure 4.
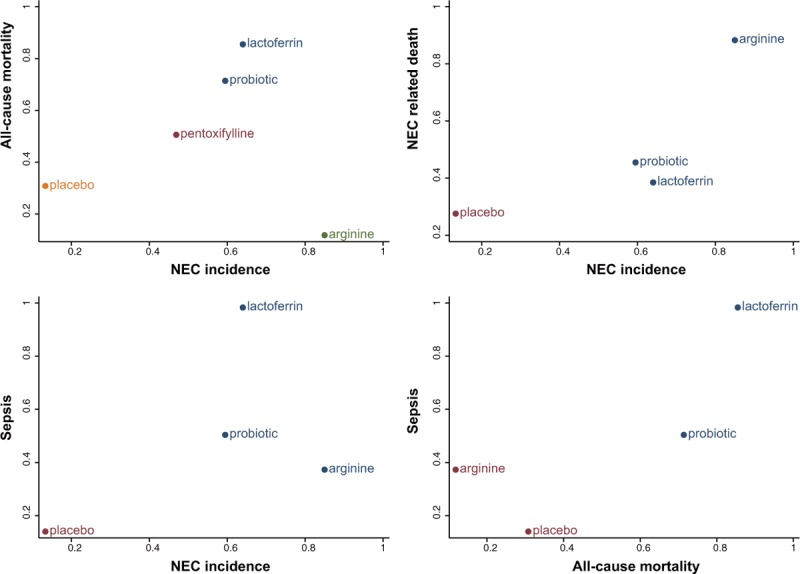
Two-dimensional cluster analysis for the combination of 5 endpoints. The same color of a group of treatments represents that they display a similar performance under specific 2 dimensional aspects.
Figure 5.
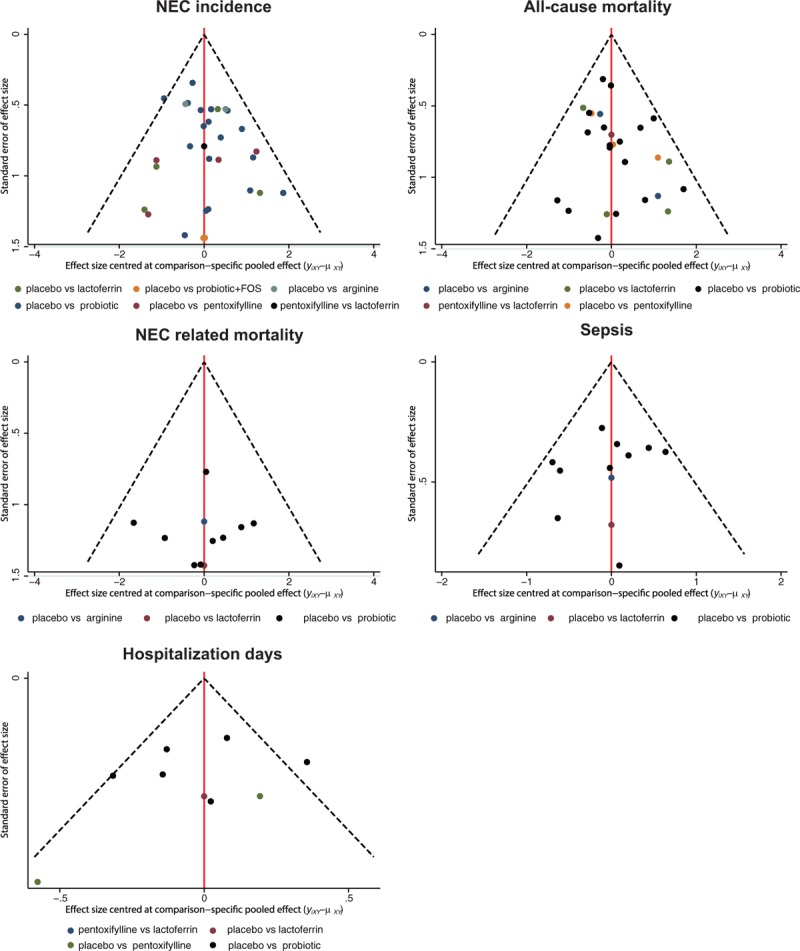
Comparison adjusted funnel plots for publication bias analysis.
Table 5.
SUCRA values for the treatments under 5 endpoints.
4. Discussion
Since NEC is a major challenge in neonatology, which has ongoing adverse effects on preterm infants, preventing this disease in preterm infants has been strongly advocated, and thus far enormous efforts have been made to unfold its pathogenesis. Several food additives have been introduced to reduce the incidence and mortality of NEC.[39,40] For the aim of understanding relative efficacy and safety of different food additives, our researchers conducted the first and most comprehensive NMA in this area. In this NMA, we involved 27 eligible studies with 4649 preterm infants.
In our study, arginine and probiotic were more favorable than others since preterm infants with these 2 food additives exhibited a significantly reduced risk of NEC. However, the performance of arginine was compromised by its performance under the outcome of mortality, while probiotic was still superior to other additives under this endpoint. Platelet-activating factor and nitric oxide played major roles in the etiopathogenesis of NEC. Nitric oxide was synthesized from the amino acid arginine through nitric oxide synthases.[9] Many animal models suggested that suppressing nitric oxide might increase the area of intestinal damage significantly.[41,42] For this mechanism, arginine might function effectively in preventing NEC in preterm infants. The gastrointestinal tract of preterm infants often exhibited abnormal bacterial colonization and inadequate immune defenses.[4] This kind of deficiency could be offset by probiotics, and our conclusions appear to support this hypothesis.
However, selecting an appropriate food additive merely based on its efficacy for preventing NEC may lead to biased results. Therefore, we included several safety outcomes in order to provide clinicians with more informative conclusions. Arginine for infants should be used with caution because excessive arginine ingestion may generate more nitric oxide, and this may cause adverse effects on infants.[9] The potential harm of arginine to infants appeared to be supported by our SUCRA results in which arginine exhibited worse performance than placebo with respect to the outcome of mortality. Although some studies proposed that the adverse effects of arginine could be reduced if the corresponding dose was reduced,[43] more evidence should be disclosed to verify its safety in infants. On the other hand, preterm infants with probiotics exhibited a significantly decreased risk of NEC and mortality, and probiotics also performed relatively well for NEC-related mortality, sepsis, and hospitalization days. Thus, probiotics may be a better option for preterm infants.
Nevertheless, several limitations were likely to affect the validity of our conclusions. First, the nature of systematic review and NMA did not enable us to adjust for a few confounding factors. In this study, there were several inevitable confounding factors, including gestational age, birth weight, various ways of using probiotics, umbilical channeling, and stage of NEC. The specific type, dose, start time, and treatment duration of probiotics were not unified, which could affect the results. As the ways of taking drugs, umbilical vein catheterization and umbilical artery catheterization may also have some influence. The stage of NEC involved in our study was generally II or above. It was difficult to establish more detailed stage subgroups, because some original trials didn’t report certain numbers of each stage. Another important confounding factor can be feeding method. Mother's milk, which is regarded as a fundamental nutritional source for neonates, can reduce the risk of NEC, but donor breast milk may not work this way, in which the pasteurization affects the composition of bioactive compounds. We did consider about this factor before, but the percentage of different breast milk source was only reported in 1 study,[35] some studies didn’t even specify the source type. It was plausible that the above confounding factors had influence on the summary effects in a way. Second, some direct comparisons could not be achieved due to the lack of evidence and significant inconsistency may exist within the network structure. Therefore, approaches that were able to assess the risk of heterogeneity and inconsistency should be included in order to ensure that the statistical assumption of our NMA was valid. Third, the corresponding evidence in the network appeared to be substantially unbalanced, resulting in some unexpectedly unreliable estimates. Specifically speaking, the group at greatest risk of NEC, that is, those with a birth weight of less than 1000 g, though was involved in some studies,[17,18,26,33,34,38] was not considered in a separate way in our study. Moreover, the number of probiotic-related studies was obviously more than others. It's possible that the premature infants involved was underrepresented, as a result, it's a pity that we were not able to have adequate evidence of either efficacy or safety to recommend probiotics as universal prophylactic administration to all premature infants. In summary, our study indicated that probiotics had the potential to be the most preferable additive, since it exhibited a relatively balanced performance in the efficacy and safety. The use of arginine in preterm infants should be further justified, considering its high risk of resulting in mortality. More advanced research methodologies should be included in future studies to determine the most appropriate additive in preterm infants who are at great risk of NEC.
Supplementary Material
Footnotes
Abbreviations: CrI = credible interval, NEC = necrotizing enterocolitis, NMA = network meta-analysis, OR = odds ratio, RCT = randomized control trial, SUCRA = surface under the cumulative ranking curve, VLBW = very low birth weight.
WY and WS are first co-authors.
Ethics statement: There work involves no human related experiments, patient consent, and ethical approval is not necessary.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Yang G, Wang Y, Jiang X. Diagnostic value of intestinal fatty-acid-binding protein in necrotizing enterocolitis: a systematic review and meta-analysis. Indian J Pediatr 2016;83:1410–9. [DOI] [PubMed] [Google Scholar]
- [2].Yang Y, Guo Y, Kan Q, et al. A meta-analysis of probiotics for preventing necrotizing enterocolitis in preterm neonates. Braz J Med Biol Res 2014;47:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hunter C, Dimaguila MA, Gal P, et al. Effect of routine probiotic, Lactobacillus reuteri DSM 17938, use on rates of necrotizing enterocolitis in neonates with birthweight <1000 grams: a sequential analysis. BMC Pediatr 2012;12:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rubin RT, Poland RE, Lesser IM, et al. Neuroendocrine aspects of primary endogenous depression. III. Cortisol secretion in relation to diagnosis and symptom patterns. Psychol Med 1987;17:609–19. [DOI] [PubMed] [Google Scholar]
- [5].Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet 2006;368:1271–83. [DOI] [PubMed] [Google Scholar]
- [6].Lau CS, Chamberlain RS. Probiotic administration can prevent necrotizing enterocolitis in preterm infants: a meta-analysis. J Pediatr Surg 2015;50:1405–12. [DOI] [PubMed] [Google Scholar]
- [7].Bernardo WM, Aires FT, Carneiro RM, et al. Effectiveness of probiotics in the prophylaxis of necrotizing enterocolitis in preterm neonates: a systematic review and meta-analysis. J Pediatr (Rio J) 2013;89:18–24. [DOI] [PubMed] [Google Scholar]
- [8].Pammi M, Haque KN. Pentoxifylline for treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev 2015;CD004205. [DOI] [PubMed] [Google Scholar]
- [9].Mitchell K, Lyttle A, Amin H, et al. Arginine supplementation in prevention of necrotizing enterocolitis in the premature infant: an updated systematic review. BMC Pediatr 2014;14:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lawrence G, Tudehope D, Baumann K, et al. Enteral human IgG for prevention of necrotising enterocolitis: a placebo-controlled, randomised trial. Lancet 2001;357:2090–4. [DOI] [PubMed] [Google Scholar]
- [11].Eibl MM, Wolf HM, Furnkranz H, et al. Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA–IgG feeding. N Engl J Med 1988;319:1–7. [DOI] [PubMed] [Google Scholar]
- [12].Lauterbach R, Pawlik D, Kowalczyk D, et al. Effect of the immunomodulating agent, pentoxifylline, in the treatment of sepsis in prematurely delivered infants: a placebo-controlled, double-blind trial. Crit Care Med 1999;27:807–14. [DOI] [PubMed] [Google Scholar]
- [13].Amin HJ, Zamora SA, McMillan DD, et al. Arginine supplementation prevents necrotizing enterocolitis in the premature infant. J Pediatr 2002;140:425–31. [DOI] [PubMed] [Google Scholar]
- [14].Dani C, Biadaioli R, Bertini G, et al. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002;82:103–8. [DOI] [PubMed] [Google Scholar]
- [15].Costalos C, Skouteri V, Gounaris A, et al. Enteral feeding of premature infants with Saccharomyces boulardii. Early Hum Dev 2003;74:89–96. [DOI] [PubMed] [Google Scholar]
- [16].Bin-Nun A, Bromiker R, Wilschanski M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005;147:192–6. [DOI] [PubMed] [Google Scholar]
- [17].Manzoni P, Mostert M, Leonessa ML, et al. Oral supplementation with Lactobacillus casei subspecies rhamnosus prevents enteric colonization by Candida species in preterm neonates: a randomized study. Clin Infect Dis 2006;42:1735–42. [DOI] [PubMed] [Google Scholar]
- [18].Rouge C, Piloquet H, Butel MJ, et al. Oral supplementation with probiotics in very-low-birth-weight preterm infants: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr 2009;89:1828–35. [DOI] [PubMed] [Google Scholar]
- [19].Samanta M, Sarkar M, Ghosh P, et al. Prophylactic probiotics for prevention of necrotizing enterocolitis in very low birth weight newborns. J Trop Pediatr 2009;55:128–31. [DOI] [PubMed] [Google Scholar]
- [20].Underwood MA, Salzman NH, Bennett SH, et al. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J Pediatr Gastroenterol Nutr 2009;48:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Adel M, Awad HA, Abdel-Naim AB, et al. Effects of pentoxifylline on coagulation profile and disseminated intravascular coagulation incidence in Egyptian septic neonates. J Clin Pharm Ther 2010;35:257–65. [DOI] [PubMed] [Google Scholar]
- [22].Awad H, Mokhtar H, Imam SS, et al. Comparison between killed and living probiotic usage versus placebo for the prevention of necrotizing enterocolitis and sepsis in neonates. Pak J Biol Sci 2010;13:253–62. [DOI] [PubMed] [Google Scholar]
- [23].Mihatsch WA, Vossbeck S, Eikmanns B, et al. Effect of Bifidobacterium lactis on the incidence of nosocomial infections in very-low-birth-weight infants: a randomized controlled trial. Neonatology 2010;98:156–63. [DOI] [PubMed] [Google Scholar]
- [24].Braga TD, da Silva GA, de Lira PI, et al. Efficacy of Bifidobacterium breve and Lactobacillus casei oral supplementation on necrotizing enterocolitis in very-low-birth-weight preterm infants: a double-blind, randomized, controlled trial. Am J Clin Nutr 2011;93:81–6. [DOI] [PubMed] [Google Scholar]
- [25].Sari FN, Dizdar EA, Oguz S, et al. Oral probiotics: Lactobacillus sporogenes for prevention of necrotizing enterocolitis in very low-birth weight infants: a randomized, controlled trial. Eur J Clin Nutr 2011;65:434–9. [DOI] [PubMed] [Google Scholar]
- [26].Al-Hosni M, Duenas M, Hawk M, et al. Probiotics-supplemented feeding in extremely low-birth-weight infants. J Perinatol 2012;32:253–9. [DOI] [PubMed] [Google Scholar]
- [27].Fernandez-Carrocera LA, Solis-Herrera A, Cabanillas-Ayon M, et al. Double-blind, randomised clinical assay to evaluate the efficacy of probiotics in preterm newborns weighing less than 1500 g in the prevention of necrotising enterocolitis. Arch Dis Child Fetal Neonatal Ed 2013;98:F5–9. [DOI] [PubMed] [Google Scholar]
- [28].Polycarpou E, Zachaki S, Tsolia M, et al. Enteral l-arginine supplementation for prevention of necrotizing enterocolitis in very low birth weight neonates: a double-blind randomized pilot study of efficacy and safety. JPEN J Parenter Enteral Nutr 2013;37:617–22. [DOI] [PubMed] [Google Scholar]
- [29].Serce O, Benzer D, Gursoy T, et al. Efficacy of Saccharomyces boulardii on necrotizing enterocolitis or sepsis in very low birth weight infants: a randomised controlled trial. Early Hum Dev 2013;89:1033–6. [DOI] [PubMed] [Google Scholar]
- [30].Akdag A, Dilmen U, Haque K, et al. Role of pentoxifylline and/or IgM-enriched intravenous immunoglobulin in the management of neonatal sepsis. Am J Perinatol 2014;31:905–12. [DOI] [PubMed] [Google Scholar]
- [31].Akin IM, Atasay B, Dogu F, et al. Oral lactoferrin to prevent nosocomial sepsis and necrotizing enterocolitis of premature neonates and effect on T-regulatory cells. Am J Perinatol 2014;31:1111–20. [DOI] [PubMed] [Google Scholar]
- [32].Benor S, Marom R, Ben Tov A, et al. Probiotic supplementation in mothers of very low birth weight infants. Am J Perinatol 2014;31:497–504. [DOI] [PubMed] [Google Scholar]
- [33].Manzoni P, Meyer M, Stolfi I, et al. Bovine lactoferrin supplementation for prevention of necrotizing enterocolitis in very-low-birth-weight neonates: a randomized clinical trial. Early Hum Dev 2014;90(suppl 1):S60–5. [DOI] [PubMed] [Google Scholar]
- [34].Oncel MY, Sari FN, Arayici S, et al. Lactobacillus reuteri for the prevention of necrotising enterocolitis in very low birthweight infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2014;99:F110–5. [DOI] [PubMed] [Google Scholar]
- [35].Patole S, Keil AD, Chang A, et al. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates—a randomised double blind placebo controlled trial. PLoS One 2014;9:e89511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Totsu S, Yamasaki C, Terahara M, et al. Bifidobacterium and enteral feeding in preterm infants: cluster-randomized trial. Pediatr Int 2014;56:714–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shabaan AE, Nasef N, Shouman B, et al. Pentoxifylline therapy for late-onset sepsis in preterm infants: a randomized controlled trial. Pediatr Infect Dis J 2015;34:e143–8. [DOI] [PubMed] [Google Scholar]
- [38].Sherman MP, Adamkin DH, Niklas V, et al. Randomized controlled trial of talactoferrin oral solution in preterm infants. J Pediatr 2016;175:68-73.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Torrazza RM, Li N, Neu J. Decoding the enigma of necrotizing enterocolitis in premature infants. Pathophysiology 2014;21:21–7. [DOI] [PubMed] [Google Scholar]
- [40].Fitzgibbons SC, Ching Y, Yu D, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg 2009;44:1072–5. discussion 1075–6. [DOI] [PubMed] [Google Scholar]
- [41].Payne D, Kubes P. Nitric oxide donors reduce the rise in reperfusion-induced intestinal mucosal permeability. Am J Physiol 1993;265:G189–95. [DOI] [PubMed] [Google Scholar]
- [42].Caplan MS, Hedlund E, Hill N, et al. The role of endogenous nitric oxide and platelet-activating factor in hypoxia-induced intestinal injury in rats. Gastroenterology 1994;106:346–52. [DOI] [PubMed] [Google Scholar]
- [43].Shah P, Shah V. Arginine supplementation for prevention of necrotising enterocolitis in preterm infants. Cochrane Database Syst Rev 2007;CD004339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


