Abstract
Background:
We performed a meta-analysis of randomized controlled trials (RCTs) to compare the efficacy and safety of combined intravenous (IV) and topical tranexamic acid (TXA) with IV-TXA alone for controlling blood loss in patients following primary total hip arthroplasty (THA).
Methods:
PubMed, EMBASE, the Cochrane Central Register of Controlled Trials, the Google database, the Chinese Wanfang database, and the China National Knowledge Infrastructure database were searched to identify studies comparing combined IV and topical TXA with IV-TXA alone in patients who were prepared for THA. The weighed mean differences for total blood loss, hemoglobin drop, intraoperative blood loss, and the length of hospital stay were calculated. We calculated risk ratios for the need for transfusion and the occurrence of deep venous thrombosis (DVT) in the combined TXA and IV-TXA alone groups. Relevant data were analyzed using Reviewer Manager 5.3.0.
Results:
Eight RCTs with a total of 850 patients (combined TXA: n = 471; IV-TXA: n = 479) were included in this meta-analysis. Pooled results indicated that compared with the IV-TXA alone group, the combined TXA group was associated with a lesser need for transfusion, total blood loss, intraoperative blood loss, and hemoglobin drop (P < .05). There was no significant difference between the 2 groups for the length of hospital stay and the occurrence of DVT (P > .05).
Conclusions:
The current meta-analysis indicated that combined topical and IV-TXA was a relatively effective hemostasis method compared with IV-TXA alone. The number of studies included in this meta-analysis is limited, and more studies are needed to verify the effects of combined IV and topical TXA in THA patients.
Keywords: combined, intravenous, topical, total hip arthroplasty, tranexamic acid
1. Introduction
Total hip arthroplasty (THA) is the most common surgical procedure for patients with end-stage hip diseases, including femoral head necrosis and osteoarthritis.[1] The utilization of THA has increased to >700,000 cases annually.[2] One of the common risks of THA is bleeding, which can subsequently lead to blood transfusion.[3,4] It is reported that the percentage of THA patients needing postoperative blood transfusion ranges from 16% to 37%.[5,6] Although autologous and allogeneic blood transfusions can keep the blood pressure and vital signs of patients at normal levels, the potential risk of infection with the hepatitis C virus, and other infectious diseases limit the widespread use of blood transfusion.[7] To reduce total blood loss and, subsequently, the need for blood transfusion after THA, a multimodal blood management protocol has been administered. Tranexamic acid (TXA) is the most potentially cost-effective alternative agent.[8]
The pharmacological methods of TXA include oral, intra-articular, intravenous (IV), and combined intra-articular and IV-TXA. Among the above methods, IV-TXA has been identified as the most potent method for maximizing anti-fibrinolysis effects.[9] Combined topical and IV-TXA is an emerging method for enhancing the hemostasis effects of TXA.[10] However, whether combined topical and IV-TXA is superior to IV-TXA alone remains unknown. Thus, this systematic review and meta-analysis aimed to determine whether combined topical and IV-TXA was associated with lower total blood loss, lower intraoperative blood loss, a lower hemoglobin drop, less need for transfusion, and a shorter length of hospital stay than was IV-TXA alone in patients following THA.
2. Materials and methods
This meta-analysis was carried out in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines for the meta-analysis of intervention trials.[11]
2.1. Search strategies
PubMed, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), the Google database, the Chinese Wanfang database, and the China National Knowledge Infrastructure (CNKI) database were searched by 2 reviewers (XW and CZ) from inception until March 2017. Randomized controlled trials (RCTs) that compared combined TXA and IV-TXA alone for reducing blood loss in patients undergoing THA were included. The search terms in the PubMed database were as follows: ((((((“Arthroplasty, Replacement, Hip”[Mesh]) OR THR) OR THA) OR total hip arthroplasty) OR total hip replacement)) AND tranexamic acid OR TXA. The language of publications was not limited. Reference lists of all eligible studies and relevant reviews were manually searched for additional studies. This is a meta-analysis and thus no ethical approval was need for this paper.
2.2. Inclusion criteria and exclusion criteria
2.2.1. Inclusion criteria
Participants: patients undergoing primary THA. Intervention: combined topical and IV-TXA. Comparison: IV-TXA alone. Outcomes: the primary outcomes included the need for transfusion, total blood loss, intraoperative blood loss, hemoglobin drop, hidden blood loss, the occurrence of deep venous thrombosis (DVT), and the length of hospital stay; study types: only RCTs. Articles that fulfilled the inclusion criteria and reported at least 1 outcome were included, whereas those without the outcome measures of interest were excluded.
2.2.2. Exclusion criteria
Quasi-RCT or non-RCT, retrospective studies, letters, comments, editorials, and practice guidelines.
2.3. Data extraction and quality assessment
Two reviewers (CZ and BX) independently reviewed all titles and abstracts of studies. If studies met the inclusion criteria, full text of articles that were reviewed thoroughly. Disagreements were resolved by consultation of a third reviewer (HZ). A specific extraction was conducted to collect relevant data in a pregenerated standard Microsoft Excel (Microsoft Corporation, Redmond, WA) file. We extracted the information of the studies according to the “PICOS” principle, and other factors that may influence blood loss. The data in other forms were converted to the mean ± standard deviation (SD), according to the Cochrane Handbook.[12] If data were not reported numerically, we extracted them by the “Get data” software (Get data, Suzhou, China) from published figures.
Two authors (CZ and BX) independently appraise the risk of bias and summarized the risk of bias and risk of bias graph following the established criteria.[12] A total of 7 fields (random sequence generation, allocation concealment, participant blinding, blinding of the outcome assessment, incomplete outcome data, incomplete outcome data, selective outcome reporting, and other potential sources bias) were evaluated. Each of the fields was describe as “low risk of bias,” “unclear risk of bias,” or “high risk of bias.” The reliability of the study selection was determined by the Cohen Kappa test and the acceptable threshold was set at 0.61.[13]
2.4. Statistical analysis
We conducted all analyses using Review Manager 5.3.0 software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Risk ratios (RRs) with 95% confidence interval (CI) were calculated for dichotomous data (the need for transfusion and the occurrence of DVT). The weighted mean difference (WMD) with a 95% CI was calculated for continuous outcomes (total blood loss, hemoglobin drop, intraoperative blood loss, and length of hospital stay). A 2-tailed P value of <.05 was used to determine statistical significance. Statistical heterogeneity was assessed and quantified by the I2 index, with substantial heterogeneity being represented by I2 > 50%. “Leave-one-out” sensitivity analysis was conducted by omitting 1 study at a time and examining the influence of each individual study on the pooled effect size.[14] The sensitivity analysis was conducted using the Stata 12.0 software (Stata Corp, College Station, TX).
3. Results
3.1. Search results and general characteristics
The flow diagram for the included studies is shown in Fig. 1. In the initial search, a total of 516 studies were identified from the electronic databases and 3 additional records were obtained from other sources. Then, all papers were entered into Endnote X7 software (Thomson Reuters Corp, CA, USA) to remove duplicate papers. A total of 355 papers were reviewed and 345 papers were removed according to the exclusion criteria at the abstract and title levels. Three studies were then excluded when the full-length of these articles were read. Finally, 8 studies[3,15–21] including a total of 850 patients (combined TX group: n = 471; IV-TXA alone group: n = 479) were eligible for meta-analysis.
Figure 1.
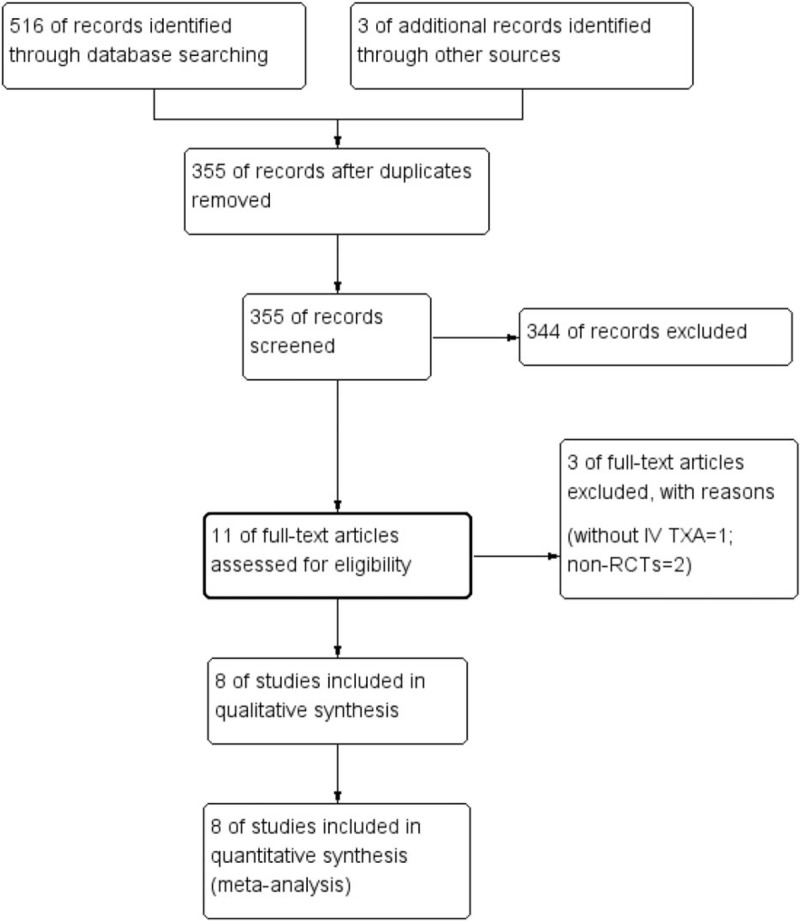
Flow diagram of the included studies.
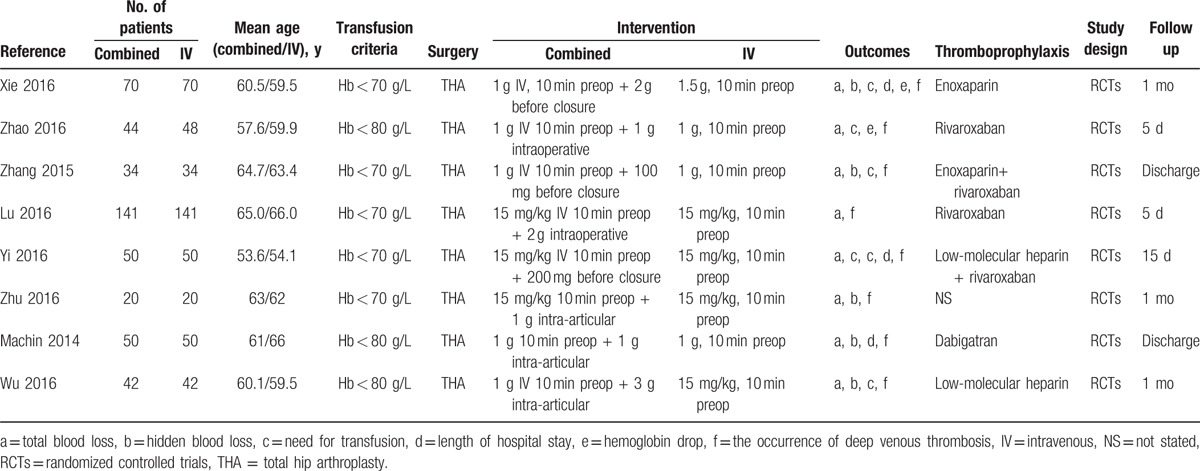
Table 1 summarizes the characteristics of the included RCTs. The sample size of the studies ranged from 20 to 141 and the mean age of the patients ranged from 53.6 to 66 years. All of the trials state the transfusion criteria and 5 studies set the transfusion trigger at <70 g/L, while the other 3 studies set the transfusion trigger at <80 g/L. The dose of IV-TXA in these 3 studies was 15 mg/kg, while the dose of IV-TXA in the other 5 studies ranged from 1 to 1.5 g and was taken 10 min before THA. Administration of IV-TXA was 10 min before THA. The time of topical TXA is set before the wound closure. The follow-up period ranged from 5 days to 1 month.
Table 1.
General characteristics of the included studies.
3.2. Study quality
Risk of bias summary and risk of bias graph are shown in Figs. 2 and 3, respectively. Among the included studies, 5 studies were randomized by computer-generated numbers,[15,16,19] and the remaining 3[17,18] did not report the method of random sequence generation. One study[17] did not report allocation concealment, 2 studies[16] presented an unclear risk, and the rest[15,18,19] used a sealed envelope or box. Two studies[15,19] were double-blind to participants and outcome assessors, except one in which this information was not reported. The overall kappa value regarding the evaluation of the risk of bias of the included RCTs was 0.811, indicating that the agreement between the 2 reviewers was acceptable.
Figure 2.
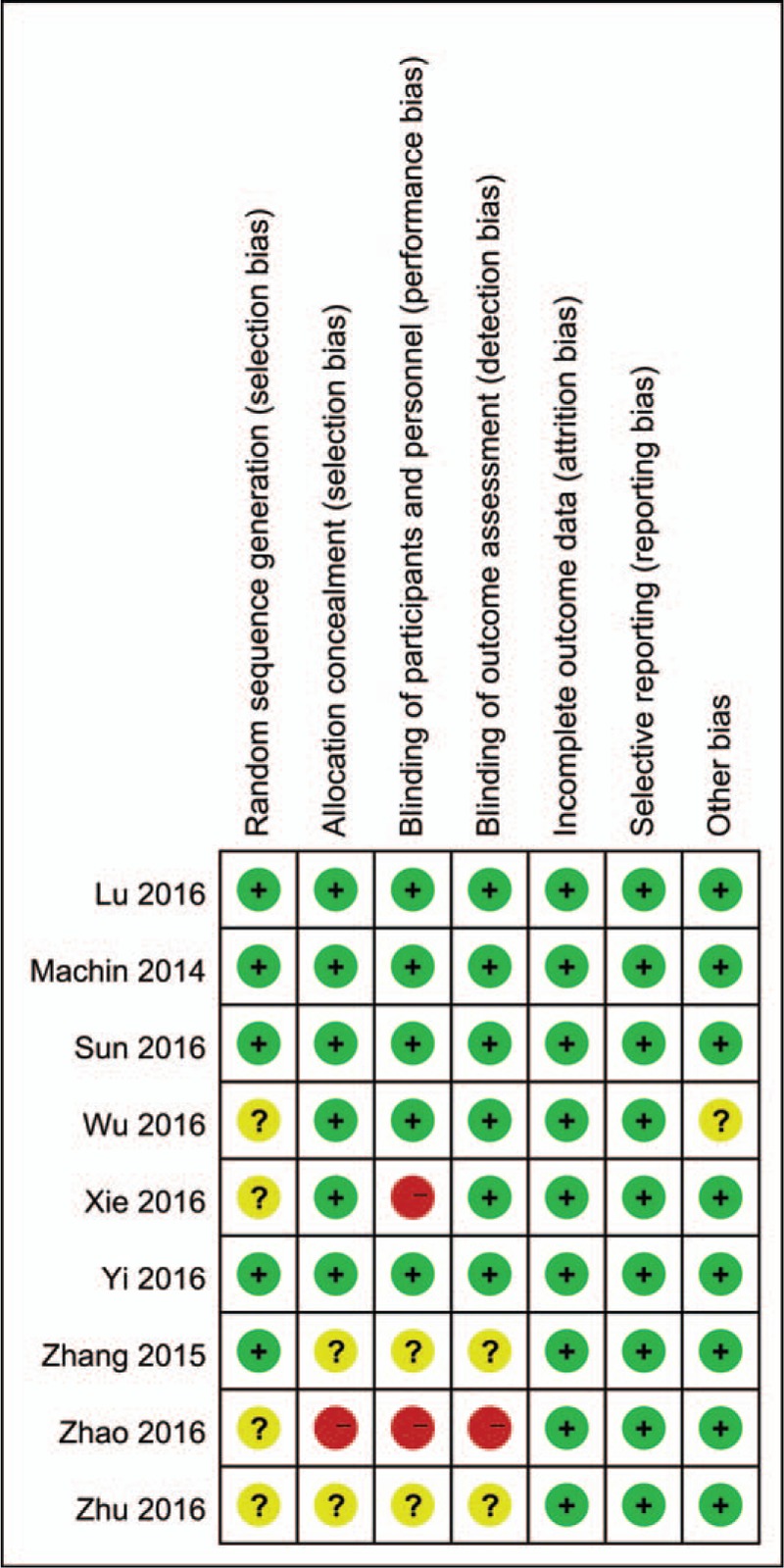
Risk of bias summary of the included studies.
Figure 3.
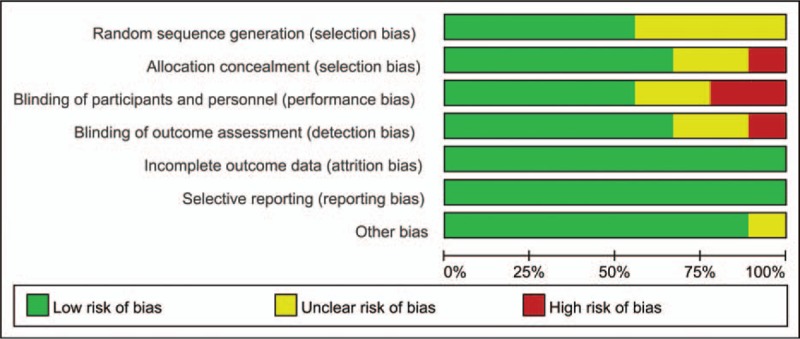
Risk of bias graph of the included studies.
3.3. Results of the meta-analysis
3.3.1. Need for transfusion
Seven studies[17–19,21–23] (668 total THAs) assessed the need for transfusion between the combined TXA group and the IV-TXA alone group. There was no significant heterogeneity between the included studies (χ2 = 2.74, df = 6, I2 = 0%, P = .84, Fig. 4). Compared with the IV-TXA alone group, the combined TXA group had a significantly lower need for transfusion (7.3% vs. 18.3%, RR = 0.41; 95% CI: 0.27–0.63; P < .0001). We then performed a funnel plot to identify whether publication bias existed. The results of this are presented in Fig. 5 and the effect size was symmetrical and indicated that there was no publication bias among the included studies.
Figure 4.
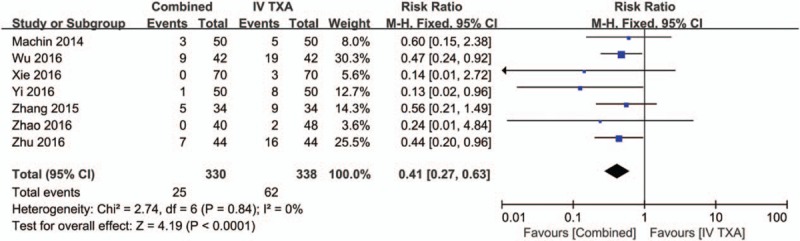
Forest plot of the need for transfusion between the 2 groups.
Figure 5.
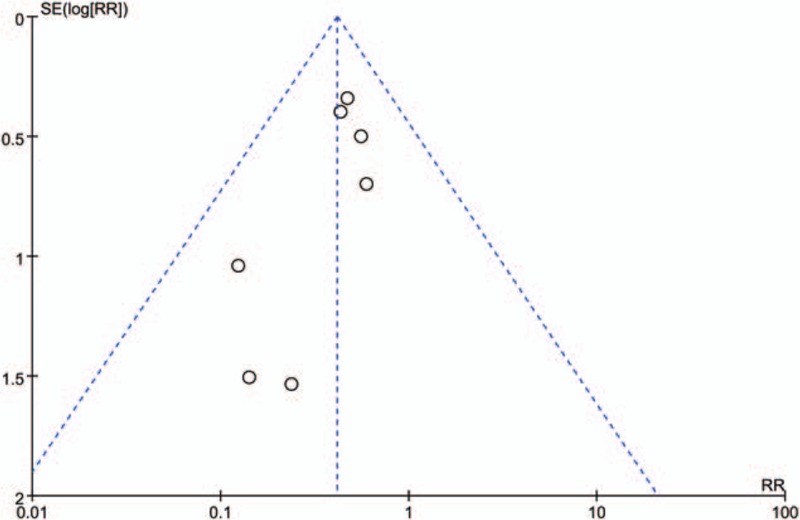
Funnel plot of the need for transfusion between the 2 groups.
3.3.2. Total blood loss
Data from 7 studies,[15–19,21] involving a total of 668 patients, reported the total blood loss, which was assessed by the Gross formula. There was large heterogeneity between the included studies (χ2 = 69.97, df = 13, I2 = 81%, P < .00001, Fig. 6) and a random-effects model was used to pool the relevant data. Compared to the IV-TXA alone group, the combined TXA group had a 145.44 mL lower amount of total blood loss (WMD = −145.44; 95% CI: −128.46 to −105.02, P < .0001, Fig. 6).
Figure 6.
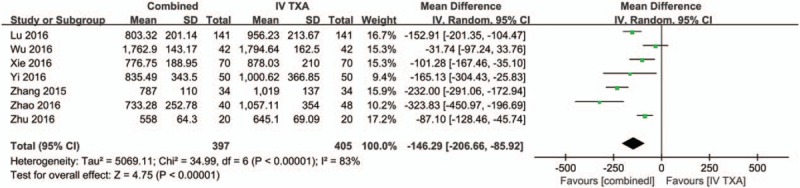
Forest plot of the total blood loss between the 2 groups.
To minimize the large heterogeneity between the included studies, sensitivity analysis was conducted to try to determine the source of heterogeneity. The results are shown in Fig. 7, and none of the included studies affected the heterogeneity between studies.
Figure 7.
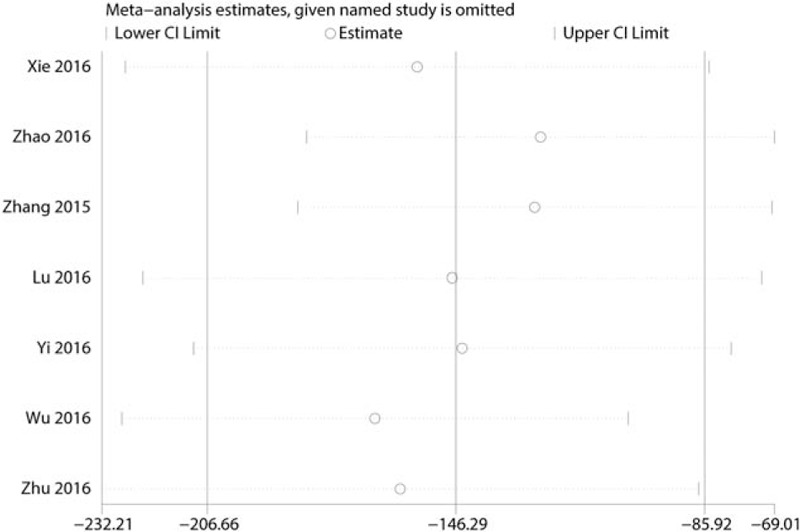
Sensitivity analysis of the total blood loss.
3.3.3. Hidden blood loss
Three studies[16–18] with a total of 248 patients reported the hidden blood loss after THA. There was large heterogeneity between the included studies (χ2 = 7.12, df = 2, I2 = 72%, P = .03, Fig. 8). Compared with the IV-TXA alone group, the combined TXA group had a significantly lower amount of hidden blood loss (WMD = −88.37; 95% CI: −114.89 to −61.85, P < .00001, Fig. 8).
Figure 8.
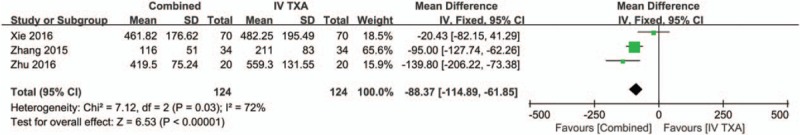
Forest plot of the hidden blood loss between the 2 groups.
3.3.4. Intraoperative blood loss
Five studies[15,17,18,24] with a total of 654 patients reported the intraoperative blood loss in THA. There was large heterogeneity between the included studies (χ2 = 21.81, df = 4, I2 = 82%, P = .00002, Fig. 9). Compared with the IV-TXA alone group, the combined TXA group had a 64.65 mL lower amount of intraoperative blood loss (WMD = −64.65; 95% CI: −74.75 to −54.55, P < .00001, Fig. 9).
Figure 9.
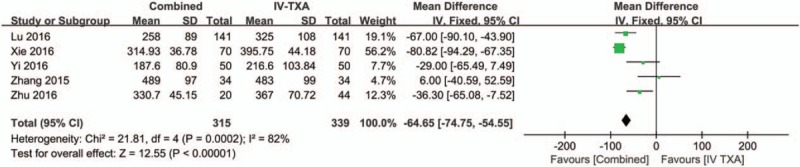
Forest plot of the intraoperative blood loss between the 2 groups.
3.3.5. Hemoglobin drop
Four studies[16–18] with a total of 412 patients reported the hemoglobin drop after THA. There was large heterogeneity between the included studies (χ2 = 0.07, df = 3, I2 = 66%, P = .03, Fig. 10). Compared with the IV-TXA alone group, the combined TXA group had a lower hemoglobin drop (WMD = −0.43; 95% CI: −0.75 to −0.10, P = .01, Fig. 10).
Figure 10.
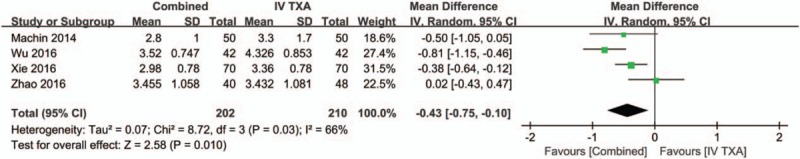
Forest plot of the hemoglobin drop between the 2 groups.
3.3.6. Length of hospital stay
Four studies[16,17,23] with a total of 424 patients reported the length of hospital stay after THA, and there was no heterogeneity between the included studies (χ2 = 1.56, df = 3, I2 = 0%, P = .67, Fig. 11). Meta-analysis showed that the combined TXA group had a shorter length of hospital stay (WMD = −0.15, 95% CI −0.40 to 0.11, P = .25, Fig. 11) than the IV-TXA alone group, but with no statistical significance.
Figure 11.
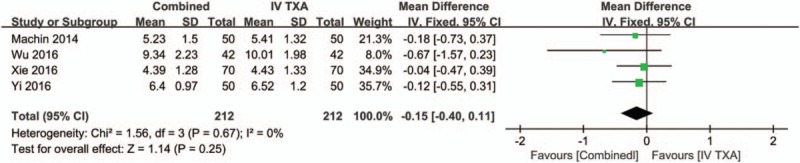
Forest plot of the length of hospital stay between the 2 groups.
3.3.7. Occurrence of DVT
Eight studies[3,15–21] with a total of 960 patients reported the occurrence of DVT, and there was no heterogeneity between the included studies (χ2 = 2.01, df = 5, I2 = 0%, P = .85, Fig. 12). There were no significant differences in the occurrence of DVT between the combined TXA group and the IV-TXA alone group (RR = 1.22, 95% CI: 0.52–2.89, P = .65, Fig. 12).
Figure 12.
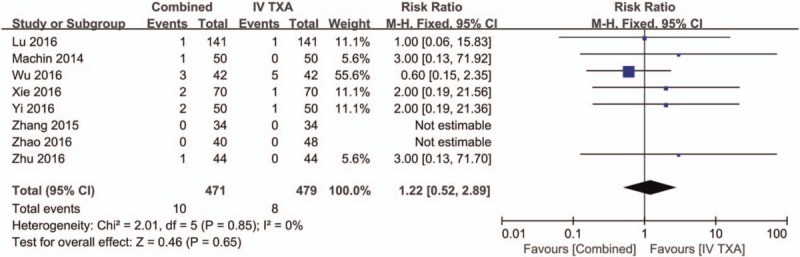
Forest plot of the deep venous thrombosis between the 2 groups.
4. Discussion
This meta-analysis of RCTs aimed to compare the efficacy and safety of combined topical and IV-TXA with those of IV-TXA alone for controlling blood loss after THA. A total of 8 RCTs were included in the final meta-analysis. Pooled results indicated that combined topical and IV-TXA was associated with lower amounts of total blood loss, hidden blood loss, intraoperative blood loss, hemoglobin drop, and a lower transfusion rate compared with IV-TXA alone. Furthermore, there was no significant difference between the occurrence of DVT between the combined TXA group and the IV-TXA alone group. The quality of the included studies was relatively high and their baseline characteristics were comparable. A major strength of current meta-analysis was that we performed a comprehensive search from both English databases (PubMed, EMBASE, the CENTRAL, and the Google database) and Chinese databases (the Chinese Wanfang database and the CNKI database).
IV-TXA is an effective protocol for reducing perioperative blood loss and the need for transfusion in THA patients.[24] Many RCTs and meta-analyses have identified the effects of IV-TXA on reducing perioperative blood loss and the need for transfusion in THA patients.[25] Combined topical and IV-TXA was another alternative hemostasis protocol for mitigating blood loss. The current meta-analysis indicated that the combined TXA group had a significantly lower amount of blood loss, by 145.44 mL, than the IV-TXA alone group (WMD = −145.44; 95% CI: −128.46 to −105.02, P < .0001). In addition, subsequent blood transfusion was reduced by 11.0% for the combined TXA group compared with the IV-TXA alone group (7.3% vs. 18.3%, RR = 0.41; 95% CI: 0.27 to 0.63; P < .0001). A previous meta-analysis by Lin et al[26] indicated that combined TXA decreased total blood loss volume by 554.03 mL compared with IV-TXA alone (WMD = −554.03, 95% CI: −1066.21 to −41.85, P = .034) in total knee arthroplasty (TKA). Zhang et al[9] conducted a meta-analysis and compared combined TXA with topical TXA and IV-TXA in THA. However, one of the major limitations of this study was that it included non-RCTs. As we know, non-RCTs have a large selection bias and may cause large heterogeneity to exist among the included studies.
We also compared the hemoglobin drop, intraoperative blood loss, and hidden blood loss between the combined TXA group and IV-TXA alone group. Pooled results indicated that, compared with the IV-TXA alone group, the combined TXA group had lower amounts of hemoglobin drop, intraoperative blood loss, and hidden blood loss. Liu et al[27] conducted a meta-analysis that compared combined TXA with IV-TXA alone, and its major shortcoming was that 3 studies were neglected to be included, and thus, a selection bias existed. As far as we know, none of the published meta-analyses compare this outcome. The IV-TXA dose in THA ranged from 10 to 15 mg/kg and was always infused at 5 to 10 min before TKA to reach the highest plasma concentration. When TKA surgery finished, a supplement topical TXA stabilized the plasma concentration of TXA and thus the hemostasis effects were maintained.
For the length of hospital stay and the occurrence of DVT, there were no significant differences between the combined TXA group and the IV-TXA alone group. As we know, the length of hospital stay for TKA patients was affected by many factors, such as the type of prosthesis, approach, and postoperative rehabilitation. Astedt et al[28] reported that TXA did not suppress the fibrinolytic activity in normal vessel walls, which is the most important link in the fibrinolytic defense system against thrombosis. This is the most potent reason why IV-TXA will not increase the occurrence of DVT after THA.
There are a total of 5 main limitations of our meta-analysis. First, only 8 studies were included, and the sample size of each study was relatively small. Second, the variation in doses of the combined TXA and IV-TXA alone between the studies might cause heterogeneity. Third, differences in transfusion criteria, surgical time, approaches, and postoperative blood saving measures may have influenced the results. Fourth, the follow-up was relatively short to estimate the occurrence of DVT. Finally, drain output, range of motion of knee, costs per patient, and postoperative swelling were not evaluated owing to insufficient data.
5. Conclusion
The combined IV and topical TXA is more effective in decreasing total blood loss, intraoperative blood loss, hemoglobin drop, and transfusion rate compared with IV-TXA alone in patients following THA. No significant differences were seen in the length of hospital stay and the occurrence of DVT between the combined TXA group and the IV-TXA alone group. However, the large heterogeneity existing between the included studies was the main limitation in the current meta-analysis. Large numbers of patients and RCTs with multiple centers are needed to illustrate the efficacy and safety of combined TXA in patients following THA.
Footnotes
Abbreviations: CI = confidence interval, DVT = deep venous thrombosis, IV = intravenous, RCTs = randomized controlled trials, RR = risk ratio, THA = total hip arthroplasty, TXA = tranexamic acid, WMD = weighted mean difference.
This work was funded by National Natural Science Foundation of Hainan Province (Grant No. 20168271) and Funds for the Associated Committee of Hainan Social Science (Grant No. JD 16-14).
The authors have no conflicts of interest to disclose.
References
- [1].Min JK, Zhang QH, Li HD, et al. The efficacy of bipolar sealer on blood loss in primary total hip arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Molloy IB, Martin BI, Moschetti WE, et al. Effects of the length of stay on the cost of total knee and total hip arthroplasty from 2002 to 2013. J Bone Joint Surg Am 2017;99:402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wu YG, Zeng Y, Yang TM, et al. The efficacy and safety of combination of intravenous and topical tranexamic acid in revision hip arthroplasty: a randomized, controlled trial. J Arthroplasty 2016;31:2548–53. [DOI] [PubMed] [Google Scholar]
- [4].Hallstrom B, Singal B, Cowen ME, et al. The Michigan experience with safety and effectiveness of tranexamic acid use in hip and knee arthroplasty. J Bone Joint Surg Am 2016;98:1646–55. [DOI] [PubMed] [Google Scholar]
- [5].Kim JL, Park JH, Han SB, et al. Allogeneic blood transfusion is a significant risk factor for surgical-site infection following total hip and knee arthroplasty: a meta-analysis. J Arthroplasty 2017;32:320–5. [DOI] [PubMed] [Google Scholar]
- [6].Li J, Li HB, Zhao XC, et al. A systematic review and meta-analysis of the topical administration of fibrin sealant in total hip and knee arthroplasty. Int J Surg 2016;36:127–37. [DOI] [PubMed] [Google Scholar]
- [7].Ramos Flores C, Echeagaray E, Castaneda G, et al. Linking hepatitis C virus infection to pre-1994 blood transfusions in female patients. Medwave 2017;17:e6886. [DOI] [PubMed] [Google Scholar]
- [8].Sun X, Dong Q, Zhang YG. Intravenous versus topical tranexamic acid in primary total hip replacement: a systemic review and meta-analysis. Int J Surg 2016;32:10–8. [DOI] [PubMed] [Google Scholar]
- [9].Zhang P, Liang Y, Chen P, et al. Combined application versus topical and intravenous application of tranexamic acid following primary total hip arthroplasty: a meta-analysis. BMC Musculoskelet Disord 2017;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lee SY, Chong S, Balasubramanian D, et al. What is the ideal route of administration of tranexamic acid in TKA? A randomized controlled trial. Clin Orthop Relat Res 2017;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].GS HJ. Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at: [http://handbook.cochrane.org/]. Accessed 2011. [Google Scholar]
- [13].Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ 2001;323:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lu C, Guo H, Hao YQ, et al. A prospective randomized controlled study of tranexamic acid used in different ways to reduce blood loss in total hip arthroplasty. Chinese J Bone Joint Surg 2016;9:25–31. [Google Scholar]
- [16].Zhang L, Wang D, Zhao GP, et al. The efficacy of combined topical with intravenous tranexamic acid in primary total hip arthroplasty. J Pract Med 2015;31:3358–60. [Google Scholar]
- [17].Zhao QB, Ren JD, Zhang XG, et al. Comparison of perioperative blood loss and transfusion rate in primary unilateral total hip arthroplasty by topical, intravenous application or combined application of tranexamic acid. Chinese J Tissue Eng Res 2016;20:459–64. [Google Scholar]
- [18].Xie J, Ma J, Yue C, et al. Combined use of intravenous and topical tranexamic acid following cementless total hip arthroplasty: a randomised clinical trial. Hip Int 2016;26:36–42. [DOI] [PubMed] [Google Scholar]
- [19].Yi Z, Bin S, Jing Y, et al. Tranexamic acid administration in primary total hip arthroplasty: a randomized controlled trial of intravenous combined with topical versus single-dose intravenous administration. J Bone Joint Surg Am 2016;98:983–91. [DOI] [PubMed] [Google Scholar]
- [20].Machin JT, Batta V, Soler JA, et al. Comparison of intra-operative regimes of tranexamic acid administration in primary total hip replacement. Acta Orthop Belg 2014;80:228–33. [PubMed] [Google Scholar]
- [21].Zhu C, Chu XB, Zhang JH. Different methods of tranexamic acid administration on reducing blood loss during total hip arthroplasty. J Jiangxi Univ Tradit Chinese Med 2016;28:47–9. [Google Scholar]
- [22].Zhang CH, Liu Y, Zhao JN, et al. Intravenous and topical application using tranexamic acid decrease hidden blood loss after total hip arthroplasty. J Clin Rehab Tissue Eng Res 2015;44:7071–6. [Google Scholar]
- [23].Sun S, Yang L, Xie S, et al. Combined use of intraarticular and intravenous tranexamic acid in total hip arthroplasty. Chinese J Tissue Eng Res 2016;20:7149–55. [Google Scholar]
- [24].Moskal JT, Capps SG. Meta-analysis of intravenous tranexamic acid in primary total hip arthroplasty. Orthopedics 2016;39:e883–92. [DOI] [PubMed] [Google Scholar]
- [25].DiBlasi JF, Smith RP, Garavaglia J, et al. Comparing cost, efficacy, and safety of intravenous and topical tranexamic acid in total hip and knee arthroplasty. Am J Orthop (Belle Mead NJ) 2016;45:e439–43. [PMC free article] [PubMed] [Google Scholar]
- [26].Lin C, Qi Y, Jie L, et al. Is combined topical with intravenous tranexamic acid superior than topical, intravenous tranexamic acid alone and control groups for blood loss controlling after total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu X, Liu J, Sun G. A comparison of combined intravenous and topical administration of tranexamic acid with intravenous tranexamic acid alone for blood loss reduction after total hip arthroplasty: a meta-analysis. Int J Surg 2017;41:34–43. [DOI] [PubMed] [Google Scholar]
- [28].Astedt B, Liedholm P, Wingerup L. The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chir Gynaecol 1978;67:203–5. [PubMed] [Google Scholar]


