Abstract
Low serum levels of vitamin D have been associated with fatigue in both healthy and clinical populations. Our aim was to evaluate the effect of vitamin D supplementation on fatigue in kidney transplant recipients (KTRs). In total, 137 patients after kidney transplant and 119 age- and sex-matched healthy volunteers were recruited. Serum levels of 25-hydroxyvitamin D (25(OH)D) were measured by competitive protein-binding assay. Fatigue was assessed using the subscale fatigue of the Checklist Individual Strength (CIS). Of all KTRs, 60 patients without initial vitamin D3 supplementation were started on vitamin D3 supplementation (cholecalciferol) 800 IU/d, with a follow-up examination after 3.0 to 9.0 months (mean, 6 months). Fatigue was found in 40.1% of KTRs. Serum 25(OH)D levels were inversely and independently associated with CIS scores in KTRs (P = .002). In the 60 patients who received vitamin D3 supplementation, 25(OH)D was overall increased at follow-up with 18.5% (P = .004) and CIS scores improved with 10.0% (P = .007). As vitamin D has beneficial effects on fatigue scores in KTRs, we suggest monitoring this parameter in KTRs and supplementation with vitamin D3 when vitamin D levels are low.
Keywords: fatigue, kidney, transplant, vitamin D
1. Introduction
Fatigue is an extremely common symptom among kidney transplant recipients (KTRs), with prevalence ranging from 39% to 59%.[1,2] Fatigue can be described as an awareness of negative balance between available energy and the cost in physical, cognitive, emotional, or/and functional components. Patients with fatigue have more functional impairments and poorer quality of life.[2–4] However, it is often medically unexplained, clinically underestimated, and usually undertreated.
Vitamin D is a critical fat-soluble hormone maintaining mineral homeostasis. The formation of active vitamin D requires 2-step hydroxylation; the first step occurring in the liver where vitamin D is converted into 25-hydroxyvitamin D (25(OH)D), and the second step in the kidney where 25(OH)D is transformed into 1,25-dihydroxyvitamin D. Its synthesis is regulated by serum parathyroid hormone (PTH), fibroblast growth factor 23, and calcium and phosphate levels.
Recent evidences sustain a wide range of nonclassical vitamin D action on maintaining the integrity of structure and function in muscle cells,[5] the modulation of cell growth, neuromuscular and immunological functions, and in reducing inflammation.[6] More recently, an important association between low vitamin D levels and fatigue has been observed in both healthy and clinical populations.[7–9] In addition, 1 randomized, double-blind, placebo-controlled trial with vitamin D supplementation in patients with juvenile systemic lupus erythematous showed a positive effect in improving fatigue.[10]
Reduced levels of vitamin D are highly frequent in KTRs, with an extensive prevalence of insufficiency and deficiency up to 81% and 30%, respectively.[11] Whether serum vitamin D levels are associated with fatigue in KTRs is unknown. Low vitamin D levels have been associated with increased risk of all-cause mortality and glomerular filtration rate decline in KTRs,[12,13] suggesting that vitamin D supplementation could be beneficial for KTRs. Considering the involvement of vitamin D in fatigue and the high prevalence of low vitamin D levels among KTRs, we examined whether vitamin D supplementation has positive effects on fatigue post kidney transplantation.
2. Subjects and methods
Stable KTRs with a functioning graft for greater than 1 year were invited to the study from the 2 outpatient clinics at First Affiliated Hospital of Jiaxing University and First Affiliated Hospital of Wenzhou Medical University during the course of the year, between February 2013 and February 2015. The donors for all patients were procured from volunteers participating in the Pilot Program of Organ Donation after Cardiac Death in China.[14] No donor organs were derived from executed prisoners. Exclusion criteria included episodes of acute rejection within the last 6 months, evidence of sepsis in the last 6 weeks, known active malignancy or chronic infection, history of psychiatric disorder or chronic fatigue syndrome, and history of thyroid disease or adrenal insufficiency. Meanwhile, 119 age- and sex-matched healthy volunteers, without prescribed vitamin D3 medication, were recruited from a health survey. The study was approved by the Ethics Committee of the First Affiliated Hospital of Jiaxing University and was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consents were obtained from all subjects or their relatives.
Age, gender, marital status, donor type, time after transplantation, and current medication were taken from patients’ medical records. Drinking status, smoking status, and body mass index (BMI) were collected by a self-report questionnaire. BMI was calculated according to the standard formula (kg/m2). Normal weight was defined as BMI of 19 to 25. Overweight was defined as BMI of 25 to 30, and obesity was defined as BMI of 30 to 35. Further, severe obesity was defined as BMI > 35.[15]
Fatigue was assessed using the subscale fatigue of the Checklist Individual Strength (CIS), which is well validated,[16,17] sensitive to detect change, and often used in study on diverse patients.[18–20] The subscale consists of 8 items. Each item is scored on a 7-point scale. The total score ranging from 8 to 56 is calculated as the sum of the responses to the 8 items. Significant fatigue is defined as a score of 35 or higher on the CIS fatigue. Trained researchers who performed the CIS status were blinded to serum levels of vitamin D at the time of examination and to previous CIS scores. Depression and anxiety were assessed by the Hospital Anxiety and Depression Scale.[21] Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI).[22]
Blood samples were obtained after an 8 to 12-hour overnight fasting period. EDTA serum samples were stored at −80 °C until measurement of biochemical variables for the study. All control samples were collected at the end of November 2014. Vitamin D status was assessed by measuring 25(OH)D using a competitive protein-binding assay. PTH was determined using an immunocheminomatric assay. Serum creatinine, calcium, and phosphate were measured by routine laboratory measurements.
Demographic and clinical parameters of all subjects were compared using the Student t test, one-way analysis of variance, Mann–Whitney U test, χ2 test, and Fisher exact test, as appropriate. Correlations between serum vitamin D and demographic and clinical parameters were tested by bivariate correlation (Pearson or Spearman rank correlation). Multivariable linear regression, including variables significantly different in the univariate analysis, was performed to identify independent determinants of CIS scores. Related samples Wilcoxon signed rank test was used to calculate differences in 25(OH)D level and CIS before and after therapeutic supplementation of vitamin D3. All statistical tests were performed with SPSS for Windows (Release 17.0; SPSS, Chicago, IL). Values of P < .05 were considered to indicate statistical significance.
3. Results
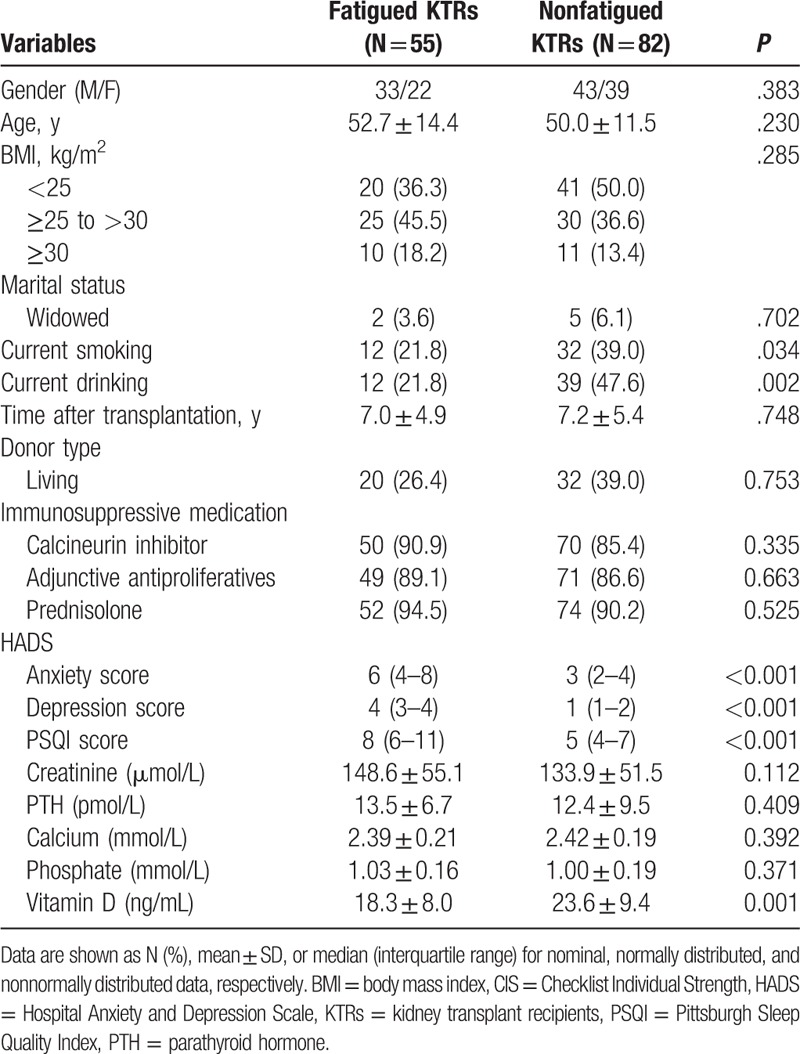
Of 150 eligible KTRs approached, 8 refused to participate and 5 did not attend the research visit. A total of 137 KTRs (76 men and 61 women) with a mean age of 51 years were included in this study, and 55 (40.1%) with CIS scores over 35 were clinically fatigued. Fatigued recipients had lower vitamin D level (P = .001), higher depression and anxiety score (both P < .001), more current smoking (P = .03), and more current drinking (P = .002) (Table 1). The mean level of 25(OH)D was 21.4 ± 9.2 ng/mL in KTRs and 26.1 ± 7.8 ng/mL in controls (P < .001).
Table 1.
Difference between fatigued and nonfatigued KTRs in clinical variables.
Of all KTRs, 56 (40.8%) were vitamin D deficient (25(OH)D < 20 ng/mL), 71 (51.8%) were insufficient (20–30 ng/mL), and 10 (7.3%) were sufficient (30 > ng/mL). The vitamin D levels correlated with PTH (r = −0.55, P < .001). No correlation was found between age and vitamin D (r = −0.04, P = .68). Further, no between-season sampling differences were found for the 25(OH)D levels in KTRs: winter (N = 41), 20.7 ± 9.4 ng/mL; spring (N = 47), 17.6 ± 9.2 ng/mL; summer (N = 15), 13.5 ± 8.9 ng/mL; and fall (N = 34), 18.5 ± 8.9 ng/mL (P = .20).
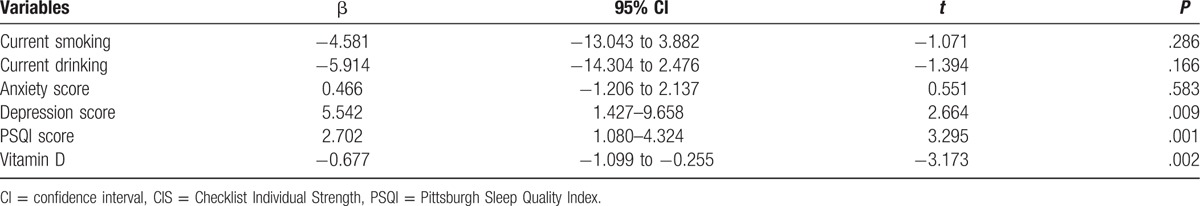
With all KTRs taken as a whole, CIS scores taken as a dependent variable and all factors (current smoking, current drinking, anxiety score, depression score, PSQI score, and vitamin D) significantly different in the univariate analysis taken as independent variables in the multivariable linear regression analysis, 25(OH)D levels were inversely and independently associated with CIS scores in KTRs (P = .002). Moreover, HAMD scores and PSQI scores were negatively and significantly associated with CIS scores in KTRs (P = .009 and .001, respectively) (Table 2).
Table 2.
Multivariable linear regression models of CIS score.
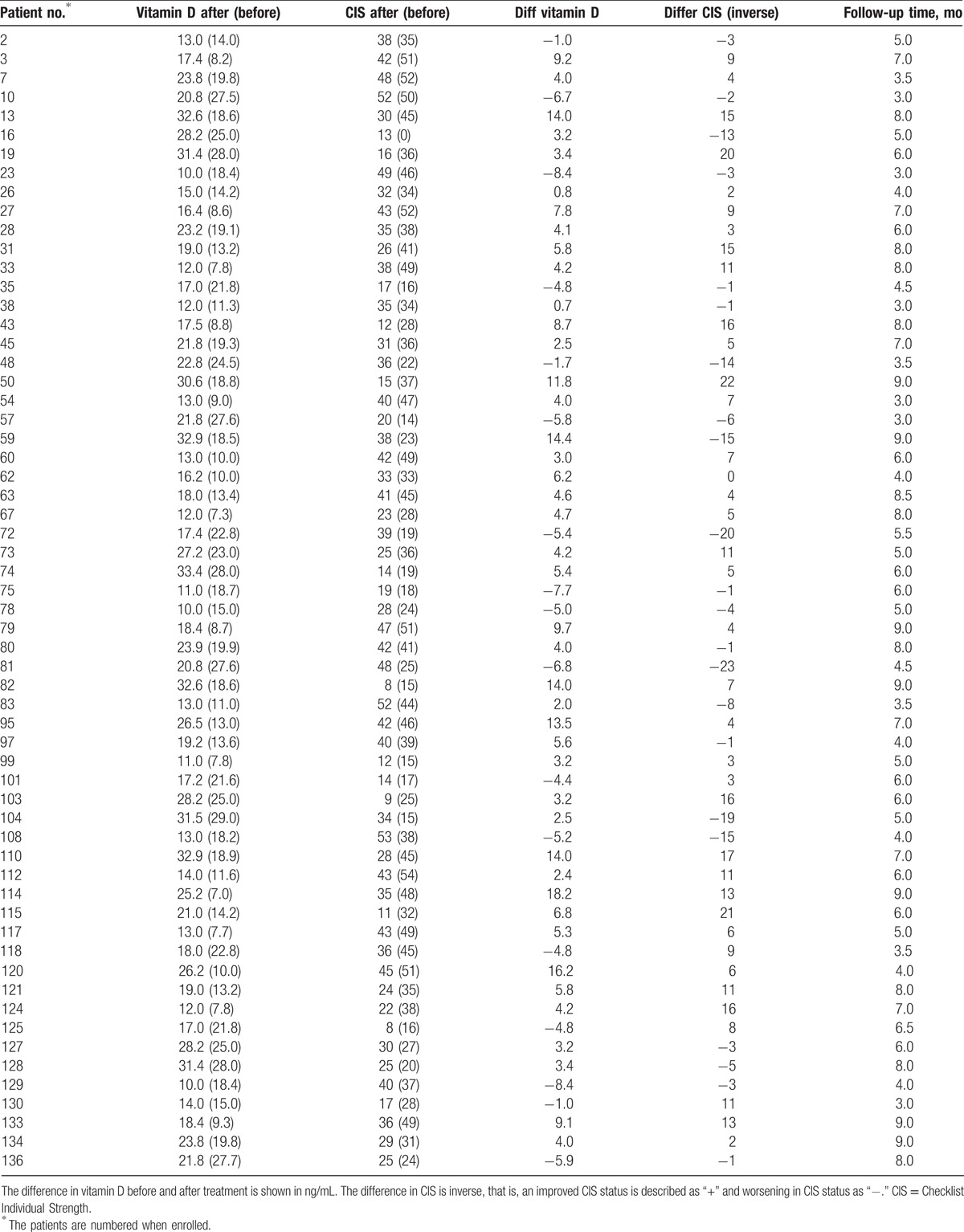
Of the 137 KTRs who formed the study sample, 60 without previous vitamin D3 treatment, was initiated supplementation with cholecalciferol 800 IU/d. The 25(OH)D level before supplement in the group was 17.0 (95% confidence interval [CI] 15.3–18.8) ng/mL, and at follow-up, 25(OH)D level had a mean of 20.2 (95% CI 18.4–22.0) ng/mL. Serum levels of 25(OH)D were markedly increased in 43 of 60 KTRs (Table 3), with an overall increase of 18.5% (P = .004). The CIS score before vitamin D3 supplement was 34 (95% CI 31–38), and after supplement, was 31 (95% CI 28–34). CIS score was markedly reduced in 37 of 60 KTRs (Table 3), with an overall reduction of 10.0% (P = .007).
Table 3.
Vitamin D levels and clinical CIS score before and after treatment with vitamin D3.
4. Discussion
This is, to the best of our knowledge, the first study to examine the effect of vitamin D supplementation on fatigue in KTRs. Our results suggest that vitamin D supplementation improves fatigue in most KTRs, which may provide novel proposal for the prevention and treatment of fatigue in KTRs.
In the present study, we found that 40.1% of KTRs presented with clinical significant fatigue, which is similar to previous study.[1] Our results also demonstrated that depression and inferior sleep quality were risk factors for fatigue in KTRs, which broadly agrees with the findings of earlier researches.[1,2,23] No significant association between fatigue and other controversial variables was found, including being female, body mass index, and immunosuppressive medication. Further studies are needed.
We found an important association between vitamin D and fatigue in KTRs, which is supported by recent researches.[7–9] The exact role of vitamin D in the pathophysiology of fatigue remains unknown so far. Vitamin D exerts action as that required to maintain muscle function through its effect on the vitamin D receptor in muscle cells.[24] Vitamin D deficiency causes muscle weakness and myalgia.[25] Furthermore, 2 recently published studies demonstrated an association between low levels of vitamin D and rotator cuff muscles and fatty degeneration of thigh.[26,27] These potential effects of vitamin D on muscle biology could be involved in fatigue among KTRs. Another possible explanation is the involvement of vitamin D in reducing inflammation. Experimental studies have suggested that vitamin D is able to skew the T-cell compartment into a more anti-inflammatory.[6] Chronic inflammation is common in KTRs and comparable to that of patients with chronic kidney disease.[28,29] Various dimensions of fatigue assessed by Multidimensional Fatigue Inventory-20 in KTRs have been shown to be independently associated with chronic inflammation.[1] Taken together, these results seem to suggest that vitamin D has an important role in the pathophysiology of fatigue in KTRs.
A striking finding in our study is the significant effect of vitamin D supplementation on fatigue in most KTRs. A recent cross-sectional study on patients with multiple sclerosis showed reduced odds of fatigue and supplementation with vitamin D.[30] Askmark et al[31] found a decrease of fatigue score after vitamin D supplementation in patients with myasthenia gravis. More recently, 1 randomized, double-blind, placebo-controlled trial with vitamin D supplementation in patients with juvenile systemic lupus erythematous showed an improvement in many aspects of fatigue scores.[10] These results are similar to our findings.
Several limitations of this study should be acknowledged. First, the limited number of KTRs in our sample reduced the statistical power of the study. Second, in the present study, fatigue, depressive symptoms, and sleep quality can only be assessed by self-report. There is, therefore, a possibility of measurement error due to self-report bias. Third, other effective factors affecting serum levels of vitamin D, such as nutritional habits and lifestyle,[32] were not analyzed in our study. Forth, the main issue with the intervention study is the lack of a placebo-control group. Fifth, the results of alteration in vitamin D levels and fatigue score in Table 2 should be presented at the group level, not individual level. Lastly, the study subjects were recruited from only 1 clinic. Therefore, our results may not be readily generalized to other patients.
Despite these limitations, our study demonstrates an important association between vitamin D levels and fatigue in KTRs. A therapeutic intervention may be recommended in KTRs with low levels of vitamin D. However, large multicenter trials should be encouraged to confirm this finding.
Acknowledgments
The authors thank all participants and staff members for their time and efforts and also thank Dr Dong-jie Zhou for data analysis support.
Footnotes
Abbreviations: 25(OH)D = 25-hydroxyvitamin D, BMI = body mass index, CIS = Checklist Individual Strength, KTR = kidney transplant recipient, PSQI = Pittsburgh Sleep Quality Index, PTH = parathyroid hormone.
BH and XW cofirst author.
Funding/support: The study is funded by the grant from the Jiaxing Municipal Sci-Tech Bureau Program (2016BY28005) and the Medical and Health Research Foundation of Zhejiang Province (2017203489).
The authors have no conflicts of interest to disclose.
References
- [1].Goedendorp MM, Hoitsma AJ, Bloot L, et al. Severe fatigue after kidney transplantation: a highly prevalent, disabling and multifactorial symptom. Transpl Int 2013;26:1007–15. [DOI] [PubMed] [Google Scholar]
- [2].Chan W, Bosch JA, Jones D, et al. Predictors and consequences of fatigue in prevalent kidney transplant recipients. Transplantation 2013;96:987–94. [DOI] [PubMed] [Google Scholar]
- [3].Procopio FO, Cruz VP, Scavonec CM, et al. Fatigue effects in daily life activities of kidney transplant recipients. Transplant Proc 2014;46:1745–9. [DOI] [PubMed] [Google Scholar]
- [4].Chan W, Jones D, Bosch JA, et al. Cardiovascular, muscular and perceptual contributions to physical fatigue in prevalent kidney transplant recipients. Transpl Int 2016;29:338–51. [DOI] [PubMed] [Google Scholar]
- [5].Montero-Odasso M, Duque G. Vitamin D in the aging musculoskeletal system: an authentic strength preserving hormone. Mol Aspects Med 2005;26:203–19. [DOI] [PubMed] [Google Scholar]
- [6].Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of vitamin D in multiple sclerosis. Brain 2009;132(Pt 5):1146–60. [DOI] [PubMed] [Google Scholar]
- [7].Masoudi Alavi N, Madani M, Sadat Z, et al. Fatigue and vitamin D status in Iranian female nurses. Glob J Health Sci 2015;8:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Martinez-Alonso M, Dusso A, Ariza G, et al. Vitamin D deficiency and its association with fatigue and quality of life in advanced cancer patients under palliative care: a cross-sectional study. Palliat Med 2016;30:89–96. [DOI] [PubMed] [Google Scholar]
- [9].Schnieders J, Willemsen D, de Boer H. Factors contributing to chronic fatigue after traumatic brain injury. J Head Trauma Rehabil 2012;27:404–12. [DOI] [PubMed] [Google Scholar]
- [10].Lima GL, Paupitz J, Aikawa NE, et al. Vitamin D supplementation in adolescents and young adults with juvenile systemic lupus erythematosus for improvement in disease activity and fatigue scores: a randomized, double-blind, placebo-controlled trial. Arthritis Care Res 2016;68:91–8. [DOI] [PubMed] [Google Scholar]
- [11].Boudville NC, Hodsman AB. Renal function and 25-hydroxyvitamin D concentrations predict parathyroid hormone levels in renal transplant patients. Nephrol Dial Transplant 2006;21:2621–4. [DOI] [PubMed] [Google Scholar]
- [12].Bienaime F, Girard D, Anglicheau D, et al. Vitamin D status and outcomes after renal transplantation. J Am Soc Nephrol 2013;24:831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Keyzer CA, Riphagen IJ, Joosten MM, et al. Associations of 25(OH) and 1,25(OH)2 vitamin D with long-term outcomes in stable renal transplant recipients. J Clin Endocrinol Metab 2015;100:81–9. [DOI] [PubMed] [Google Scholar]
- [14].Huang J, Millis JM, Mao Y, et al. A pilot programme of organ donation after cardiac death in China. Lancet 2012;379:862–5. [DOI] [PubMed] [Google Scholar]
- [15].The World Health Organization, World Health Organization. BMI classification. 2015. [Google Scholar]
- [16].Dittner AJ, Wessely SC, Brown RG. The assessment of fatigue: a practical guide for clinicians and researchers. J Psychosom Res 2004;56:157–70. [DOI] [PubMed] [Google Scholar]
- [17].Ergin G, Yildirim Y. A validity and reliability study of the Turkish Checklist Individual Strength (CIS) questionnaire in musculoskeletal physical therapy patients. Physiother Theory Pract 2012;28:624–32. [DOI] [PubMed] [Google Scholar]
- [18].Goedendorp MM, Gielissen MF, Verhagen CA, et al. Development of fatigue in cancer survivors: a prospective follow-up study from diagnosis into the year after treatment. J Pain Symptom Manage 2013;45:213–22. [DOI] [PubMed] [Google Scholar]
- [19].Snaphaan L, van der Werf S, de Leeuw FE. Time course and risk factors of post-stroke fatigue: a prospective cohort study. Eur J Neurol 2011;18:611–7. [DOI] [PubMed] [Google Scholar]
- [20].Nijhof SL, Bleijenberg G, Uiterwaal CS, et al. Effectiveness of internet-based cognitive behavioural treatment for adolescents with chronic fatigue syndrome (FITNET): a randomised controlled trial. Lancet 2012;379:1412–8. [DOI] [PubMed] [Google Scholar]
- [21].Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- [22].Backhaus J, Junghanns K, Broocks A, et al. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res 2002;53:737–40. [DOI] [PubMed] [Google Scholar]
- [23].Bossola M, Pepe G, Vulpio C. Fatigue in kidney transplant recipients. Clin Transplant 2016;30:1387–93. [DOI] [PubMed] [Google Scholar]
- [24].Bischoff-Ferrari HA, Borchers M, Gudat F, et al. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res 2004;19:265–9. [DOI] [PubMed] [Google Scholar]
- [25].Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- [26].Oh JH, Kim SH, Kim JH, et al. The level of vitamin D in the serum correlates with fatty degeneration of the muscles of the rotator cuff. J Bone Joint Surg Br 2009;91:1587–93. [DOI] [PubMed] [Google Scholar]
- [27].Tagliafico AS, Ameri P, Bovio M, et al. Relationship between fatty degeneration of thigh muscles and vitamin D status in the elderly: a preliminary MRI study. AJR Am J Roentgenol 2010;194:728–34. [DOI] [PubMed] [Google Scholar]
- [28].Azancot MA, Ramos N, Torres IB, et al. Inflammation and atherosclerosis are associated with hypertension in kidney transplant recipients. J Clin Hypertens 2015;17:963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Molnar MZ, Carrero JJ, Mucsi I, et al. Comparison of the malnutrition-inflammation score in chronic kidney disease patients and kidney transplant recipients. Int Urol Nephrol 2015;47:1025–33. [DOI] [PubMed] [Google Scholar]
- [30].Weiland TJ, Jelinek GA, Marck CH, et al. Clinically significant fatigue: prevalence and associated factors in an international sample of adults with multiple sclerosis recruited via the internet. PLoS One 2015;10:e0115541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Askmark H, Haggard L, Nygren I, et al. Vitamin D deficiency in patients with myasthenia gravis and improvement of fatigue after supplementation of vitamin D3: a pilot study. Eur J Neurol 2012;19:1554–60. [DOI] [PubMed] [Google Scholar]
- [32].Vu LH, Whiteman DC, van der Pols JC, et al. Serum vitamin D levels in office workers in a subtropical climate. Photochem Photobiol 2011;87:714–20. [DOI] [PubMed] [Google Scholar]


