Abstract
Recent studies have revealed a link between estradiol (E2) and glucose homeostasis. We aimed to assess the association between cord blood hormone levels and the risk of gestational diabetes mellitus (GDM).
A total of 204 pregnant women with GDM and 204 pregnant women without GDM (control) were included in the study. Maternal GDM were diagnosed using a 75 g oral glucose tolerance test at 24 to 26 weeks of gestation. Cord blood samples from neonates were collected immediately post delivery. Controls, which were randomly selected from the study population, were matched (cases to controls ratio: 1:1) to cases by age, sex of fetus, and gestational week.
Pregravid body mass index (BMI) (mean ± standard deviation) was (GDM vs. control): 24.5 ± 2.1 versus 22.8 ± 2.4 (P = .001). Cord blood estradiol in the GDM group was significantly lower than in the control group (P < .05). Pregravid BMI in the GDM group was significantly higher than in the control group (P < .05). Estradiol concentrations in cord blood were negatively correlated with birth weight (r = −0.121, P < .05). Conditional logistic regressions showed pregravid BMI, cord blood estradiol, and parity independently and positively predicted GDM. Multivariable regression splines characterize a nonlinear relationship between cord blood estradiol and GDM risk.
These results demonstrate a relationship between cord blood estradiol levels and GDM. Estradiol might be involved in the pathophysiology of GDM. Further studies are needed to explore potential mechanism.
Keywords: cord blood, estradiol, gestational diabetes mellitus, pregnancy
1. Introduction
Women with GDM are at high risk for pregnancy and delivery complications. It affects about 9.2% of all pregnancies.[1] GDM prevalence has been gradually increasing with the rise of obesity.[2] The molecular mechanism underlying metabolic effect of GDM remains uncertain. Pregnancy-induced insulin resistance has been linked to gestational hormones, abnormal adipokine secretion, and insulin target tissues.[3] Clinical and experimental studies proved that estradiol intervenes in glucose homeostasis and insulin signaling.
Some physiological and pathological states such as the menstrual cycle,[4] gestation,[5] and polycystic ovarian syndrome,[6] which accompanied with insulin resistance, were characterized by variability in estradiol levels.
And Montelongo et al[7] found plasma estradiol levels during gestation were always lower in diabetic women than in normal women, and propose that the development of a dyslipidemic condition in diabetic pregnancy depends on the balance between the metabolic state and the level of sex hormones.
Previous studies in animal model found estradiol is an important mediator of glucose homeostas. Insulin-resistant state can be reversed by estradiol treatment in male aromatase knockout (ArKO) mice.[8] And another study found[9] ArKO mice have increased fat mass and insulin which was prone to developing obesity and type 2 diabetes. The mechanisms by which estradiol modulates glycemia are still not fully understood. And estradiol can improve the expression of insulin signaling molecules in skeletal muscle and it prevents the onset of insulin resistance as a result of estradiol deficiency in adult female rats.[10]
We hypothesized that cord blood hormones may be involved in the pathophysiology of GDM. We investigated the alteration of cord blood hormones levels in offspring exposed to GDM, and explored the correlation between hormones and birthweight.
2. Methods
Shanghai Changning District Maternity and Infant Health Hospital located in Shanghai, China. The hospital delivered >11,000 babies every year. It contains almost all pregnant women resident in Changning District. Pregnant women were consecutively invited to participate in at their first visit to the hospital from June 2015 to December 2015. The study was approved by the Local Ethics Committee of the hospital and conducted according to the guidelines in the declaration of Helsinki. Written informed consent was obtained from all subjects before participation in the study.
All subjects routinely underwent an oral glucose test (OGTT) at 24 to 28 weeks. Pregnant women with GDM were recruited to participate in the study, and women with a singleton pregnancy were eligible. Women with preexisting diabetes, hypertension, known thyroid dysfunction, twin pregnancy, any pregnancy-related disease, assisted pregnancy, acute or chronic infections, or any other condition affecting fetomaternal well-being were excluded (Fig. 1).
Figure 1.
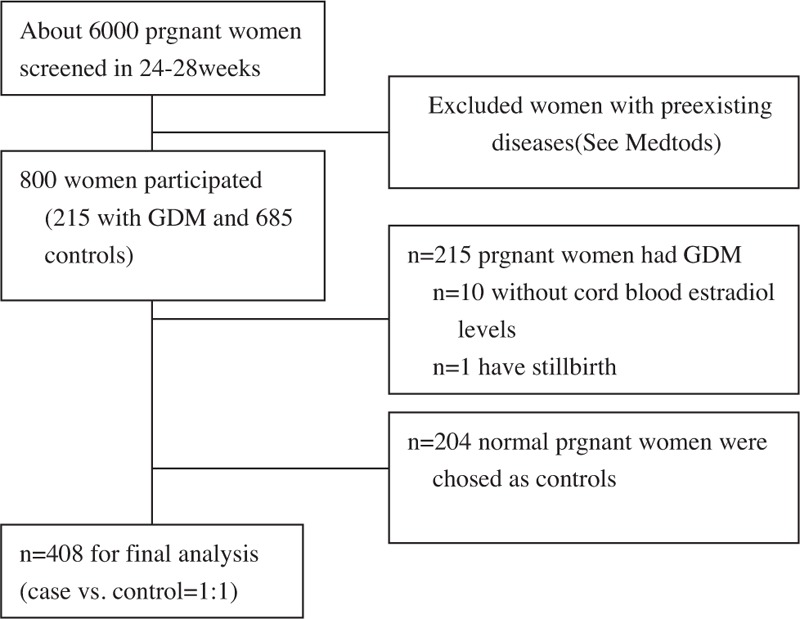
Flow chat diagram for study population. GDM = gestational diabetes mellitus.
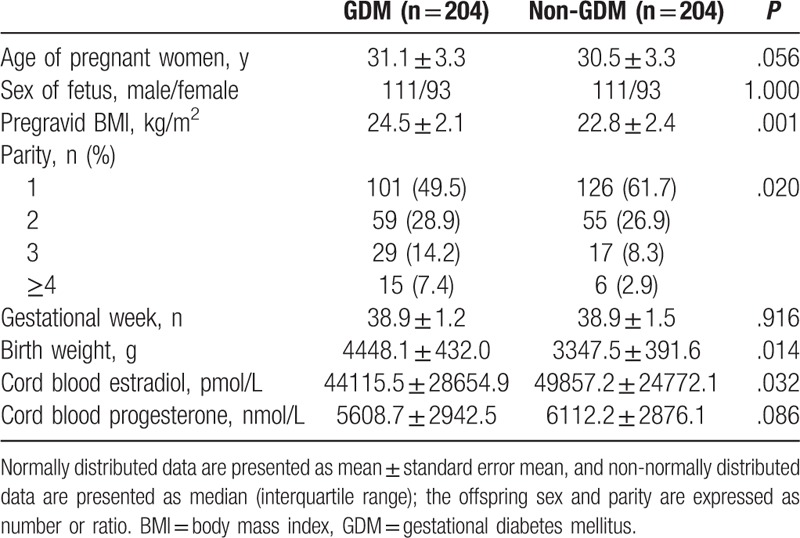
There were no significant differences in the age, sex of fetus, and gestational week between cases and controls (Table 1). A written informed consent was obtained from all participants. Weight at booking was recorded from the patient record cards. Body mass index (BMI) was calculated (weight in kg/height in m2).
Table 1.
Baseline characteristics of women with and without gestational diabetes mellitus.
The diagnosis of GDM was based on the guidelines of International Association of the Diabetes and Pregnancy Study (IADPSG) diagnostic criteria. For example, a fasting glucose ≥92 mg/dL (5.1 mmol/L) and/or 1 hour: ≥180 mg/dL (10.0 mmol/L) and/or 2 hours: ≥153 mg/dL (8.5 mmol/L) (when any of the following plasma glucose values are exceeded, the subject is considered as having GDM).[11] A total of 204 females were diagnosed as GDM cases, while normal glucose tolerant.
2.1. Cases and controls
Patients with GDM were compared with the remaining non-GDM patients during the study period. A matched case-control study was conducted to analyze risk factors for GDM and clinical characteristics of GDM. Cases were pregnant women with GDM. Controls were pregnant women without GDM. Controls, which were randomly selected from the study population, were matched (cases to controls ratio: 1:1) to cases by age, sex of fetus, and gestational week.
2.2. Biochemical analyses
After delivery, cord blood samples were collected immediately from the umbilical vein following standard protocols. Thereafter, the cord blood was centrifuged at 4°C. Estradiol and progesterone were measured within 2 hours. Cord blood levels for estradiol and progesterone were determined by chemiluminescent immunoassay (ADVIA Centaur; Siemens, Tarrytown, NY). The intra- and interassay coefficients of variation for estradiol kit were 4.8% and 4.4%, respectively. And the intra- and interassay coefficients of variation for progesterone kit were 4.1% and 5.0%, respectively.
2.3. Statistical analysis
Data are presented as means ± standard deviation for normally distributed variables and as median (interquartile range) for variables with a skewed distribution. Parity and sex were analyzed by χ2 distribution in Table 1. Differences between groups were examined by independent-sample t tests for normally distributed variables and Mann-Whitney U test for non-normally distributed data. Differences in offspring parity and sex distribution were examined by χ2 test. Correlations were examined by Spearman rank correlation coefficients in Table 2. Conditional logistic regressions were used to estimate the odds ratio of GDM in each tertile using the lowest tertile as the reference category adjusting for other covariates. All of the covariates were introduced using the enter method. The covariates of models in Conditional logistic regressions were: age, sex, BMI, gestational weeks, parity, birth weight, and estradiol.
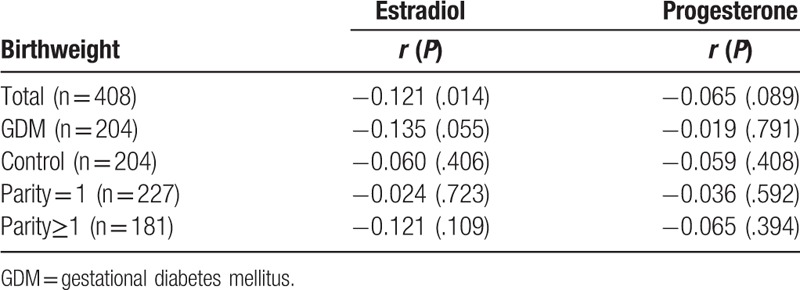
Table 2.
Spearman correlation between cord blood hormone and birthweight.
Considering that the association between estradiol levels and GDM risk might be nonlinear, restricted cubic spline (RCS) analysis was used to describe nonlinear relationships between the continuous estradiol levels and the GDM risk. The RCS analysis uses piecewise cubic polynomials that are connected across different intervals of a continuous variable. We chose 3 knots at quantiles 0.050, 0.500, and 0.950.[12] In RCS analysis, the median value of estradiol levels was used as the referent, and the odds ratio (ORs) of all other estradiol levels versus the referent value were calculated and plotted against their respective estradiol levels.
All statistical analyses were performed using Stata software (version 10.2) and using SAS system software version 9.2 (SAS Institute, Cary, NC). A 2-sided P < .05 was considered statistically significant.
3. Results
Subject characteristics are presented in Table 1. A total of 408 women enrolled in the study including 204 patients with GDM and 204 controls. Higher pregravid BMI, birthweight, and parity number were observed in patients with GDM than in control group (P < .05). There was no significant difference about age and sex of the offspring between GDM and control groups. Women with GDM had a significantly lower cord blood estradiol concentration than controls (P = .032).
Cord blood estradiol levels were also positively correlated with progesterone levels (spearman correlation, r = 0.449, P = .000). Cord blood estradiol levels were negatively correlated with birthweight (P < .05); moreover, the cord blood progesterone also revealed a similar trend (r = −0.065, P = .089). The correlation between cord blood estradiol levels and birthweight was weak (r = −0.135, P = .055) and no relationship (r = −0.019, P = .791) was found between cord blood estradiol levels and birthweight in control group (Table 2).
In multivariable analysis (Table 3), after adjustment for covariates, compared to the reference group (parity = 1), 2nd group (parity = 2) (adjusted OR: 1.27 [0.77–2.12]), and 3rd group levels (parity = 3) (adjusted OR: 2.31 [1.18–4.53]) were associated with GDM in a graded manner. After adjustment for age, sex, pregravid BMI, gestational weeks, parity, and birthweight (Fig. 2), significant negative relationships were observed between cord blood estradiol and the risk of GDM (tertile 2 vs. tertile 1: OR 0.49, 95% confidence interval [CI] 0.28–0.85; tertile 3 vs. tertile 1: OR 0.50, 95% CI 0.29–0.87).
Table 3.
Multiple conditional logistic regressions results for the relationship between estradiol with variables studied.

Figure 2.
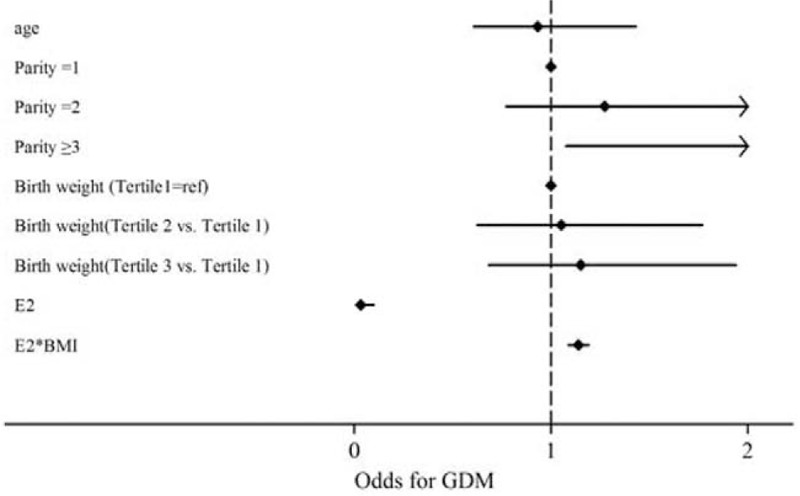
Adjusted odds and 95% confidence interval of GDM in multivariate conditional logistic regression. GDM = gestational diabetes mellitus.
A dose-response association between cord blood estradiol levels and GDM risk was found. On spline analysis, the estradiol level was nonlinearly associated with OR of GDM starting at level well <41,947.5 pmol/L, and OR of GDM rapidly decelerated reaching a plateau of 42,000 pmol/L (Fig. 3).
Figure 3.
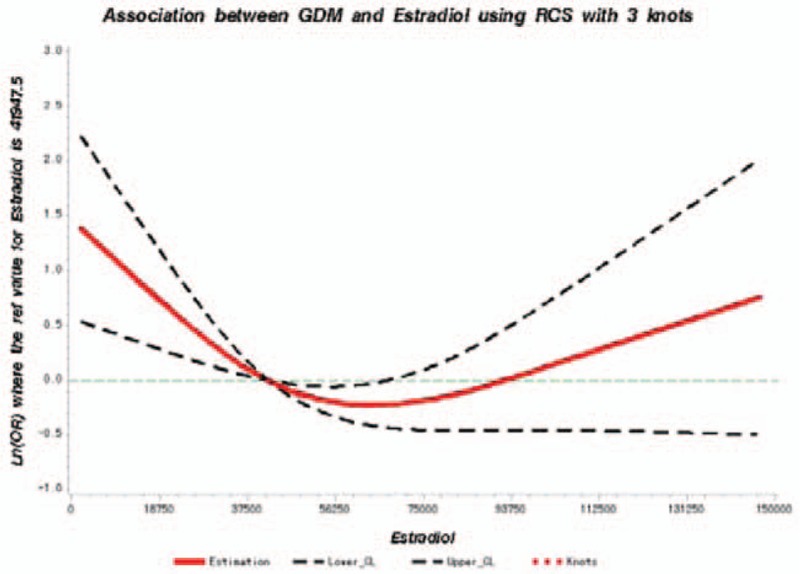
Adjusted dose-response association between estradiol (pg/mL) and the presence of GDM with 3 knots located at the 5th, 50th, and 95th percentiles. Y-axis represents the Ln (odds ratio) to present GDM for any value of cord blood estradiol compared to individuals with 41,947.5 pmol/L. The red line is the adjusted curve and dashed lines are 95% confidence intervals. GDM = gestational diabetes mellitus.
4. Discussion
There are consistent epidemiological data showing the associations between high pregravid BMI and future risk of GDM. For example, and pregravid obesity is a strong risk factor for GDM in different racial groups.[13] Although there was significant variation in the association between GDM and BMI by racial group. The study found Asians women have a greater risk of GDM at a lower BMI than other racial and ethnic groups.[13]. Moreover, cord blood estradiol was significantly correlated with birthweight in total women. There is a tread of positive relationship between estradiol and birthweight in women with GDM. We thought cord blood estradiol is a kind of early-life environment which plays a substantial role in offspring energy metabolism.
We showed that low estradiol levels in cord blood were associated with GDM. We speculated values of estradiol levels during gestation were always lower in diabetic women than in normal women. Other authors also have reported decreased plasma estradiol levels in pregnant diabetic women.[7] The author found the decreased level of these hormones in diabetic women during gestation may have restrained the development of an overtly hyperlipidemic condition in our diabetic pregnant patients. Here we found decreased level of cord blood estradiol in diabetic women.
Pregnancy is characterized by a dramatic increase in hormones, including prolactin, progesterone, and estradiol. Estradiol was mainly produced by placenta.[14,15] Aromatizing enzyme activity was correlated with estrogen production in placenta during pregnancy. Recent data have revealed a surprising role for estradiol in regulating energy metabolism and glucose homeostasis. Aromatase knockout mice have reduced estradiol levels accompanying reduced glucose oxidation, increased adiposity, and insulin levels.[16] And the glucose intolerance and insulin resistance state can be reversed by estradiol treatment.[8]
Previous studies have found estradiol can increase replication and improve survival and function of insulin-secreting cells in vitro and in vivo[17,18,19,20] during pregnancy. A recent study found estradiol can induced pregnancy-associated β cell mass expansion through repression of miR-338–3p.[21]
The placenta plays a crucial role in fetal growth and is also recognized as an endocrine organ as it produces essential hormones for gestation.
In healthy women, there was a nonlinear association between body fat and estradiol levels. Both very low and high body fat were associated with decreased estradiol levels.[22] In this study, we found women with GDM were associated with decreased estradiol levels. Study found E2 can decrease the release of resistin from placenta.[23] This may be one of the mechanisms of GDM. Correlations have been also found among plasma leptin level, BMI, and adipose tissue mass in health women.[24] Decreased leptin release from placenta from GDM-complicated pregnancies was found,[23] and 17β-estradiol increased leptin production.[25] Consistent with our study, we speculated that decreased estradiol levels may modulate the release of leptin from GDM placentas, therefore accounting for the mechanisms of GDM. Therefore, we speculated that cord blood estradiol is a protective effect during pregency.
Although pregnancy is also characterized by a plasmatic increase in maternal progesterone, in this study, no significant change in cord blood progesterone was noted between GDM and normal pregnancy, and no relationship between cord blood progesterone and birthweight.
Limitations of our study also deserve comment. First, this study was performed at a single center and uses a very small sample population. Selective bias may be inevitable. Second, it is possible that unknown risk factors may not have been identified. Third, this study was also limited by its cross-sectional design and does not infer a causal relationship between decreased estradiol levels and the development of GDM. Serial changes in cord blood estradiol also need to be measured at different time points during pregency. And glucose, insulin, and peptide-C in umbilical cord blood were not measured. We believe that these data are informative; additional studies in multiple centers are necessary.
In summary, our results indicate that cord blood estradiol concentrations were significantly lower in GDM group than non-GDM group. This finding indicates that estradiol could participate in glucose homeostasis and involved in insulin sensitivity. The regulatory mechanism involved still requires further investigation.
Footnotes
Abbreviations: ArKO = aromatase knockout, BMI = body mass index, CI = confidence interval, E2 = estradiol, GDM = gestational diabetes mellitus, IADPSG = International Association of the Diabetes and Pregnancy Study, P = progesterone, RCS = restricted cubic spline, SD = standard deviation.
This work has been financially supported by research grants ID: 20114Y05001 from Changning Health and Family Planning Commission.
The authors report no conflicts of interest.
References
- [1].DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007-2010. Prev Chronic Dis 2014;19:E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Albrecht SS, Kuklina EV, Bansil P, et al. Diabetes trends among delivery hospitalizations in the U.S., 1994-2004. Diabetes Care 2010;33:768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med 2006;12:425–31. [DOI] [PubMed] [Google Scholar]
- [4].Solomon CG, Hu FB, Dunaif A, et al. Long or highly irregular menstrual cycles as a marker for risk of type 2 diabetes mellitus. JAMA 2001;286:2421–6. [DOI] [PubMed] [Google Scholar]
- [5].Buchanan TA, Metzger BE, Freinkel N, et al. Insulin sensitivity and B-cell responsiveness to glucose during late pregnancy in lean and moderately obese women with normal glucose tolerance or mild gestational diabetes. Am J Obstet Gynecol 1990;162:1008–14. [DOI] [PubMed] [Google Scholar]
- [6].Dunaif A, Segal KR, Futterweit W, et al. Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 1989;38:1165–74. [DOI] [PubMed] [Google Scholar]
- [7].Montelongo A, Lasunción MA, Pallardo LF, et al. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes 1992;41:1651–9. [DOI] [PubMed] [Google Scholar]
- [8].Takeda K, Toda K, Saibara T, et al. Progressive development of insulin resistance phenotype in male mice with complete aromatase (CYP19) deficiency. J Endocrinol 2003;176:237–46. [DOI] [PubMed] [Google Scholar]
- [9].Fisher CR, Graves KH, Parlow AF, et al. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 1998;95:6965–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Narasimhan A, Sampath S, Jayaraman S, et al. Estradiol favors glucose oxidation in gastrocnemius muscle through modulation of insulin signaling molecules in adult female rats. Endocr Res 2013;38:251–62. [DOI] [PubMed] [Google Scholar]
- [11].Benhalima K, Devlieger R, Van Assche A. Screening and management of gestational diabetes. Best Pract Res Clin Obstet Gynaecol 2015;29:339–49. [DOI] [PubMed] [Google Scholar]
- [12].Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–57. [DOI] [PubMed] [Google Scholar]
- [13].Hedderson M, Ehrlich S, Sridhar S, et al. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012;35:1492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ishida T, Seo F, Hirato K, et al. Changes in placental enzymatic activities in relation to estrogen production during pregnancy. Nihon Sanka Fujinka Gakkai Zasshi 1985;37:547–54. [PubMed] [Google Scholar]
- [15].Knight JW. Aspects of placental estrogen synthesis in the pig. Exp Clin Endocrinol 1994;102:175–84. [DOI] [PubMed] [Google Scholar]
- [16].Jones ME, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A 2000;97:12735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sorenson RL, Brelje TC, Roth C. Effects of steroid and lactogenic hormones on islets of Langerhans: a new hypothesis for the role of pregnancy steroids in the adaptation of islets to pregnancy. Endocrinology 1993;133:2227–34. [DOI] [PubMed] [Google Scholar]
- [18].Le May C, Chu K, Hu M, et al. Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci U S A 2006;103:9232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu S, Mauvais-Jarvis F. Minireview: Estrogenic protection of beta-cell failure in metabolic diseases. Endocrinology 2010;151:859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nadal A, Alonso-Magdalena P, Soriano S, et al. The role of oestrogens in the adaptation of islets to insulin resistance. J Physiol 2009;587:5031–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jacovetti C, Abderrahmani A, Parnaud G, et al. MicroRNAs contribute to compensatory cell expansion during pregnancy and obesity. J Clin Invest 2012;122:3541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ziomkiewicz A, Ellison PT, Lipson SF, et al. Body fat, energy balance and estradiol levels: a study based on hormonal profiles from complete menstrual cycles. Human Reproduction 2008;23:2555–63. [DOI] [PubMed] [Google Scholar]
- [23].Lappas M, Yee K, Permezel M, et al. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol 2005;186:457–65. [DOI] [PubMed] [Google Scholar]
- [24].Cseh K, Baranyi E, Melczer Z, et al. The pathophysiological influence of leptin and the tumor necrosis factor system on maternal insulin resistance: negative correlation with anthropometric parameters of neonates in gestational diabetes. Gynecol Endocrinol 2002;16:453–60. [PubMed] [Google Scholar]
- [25].Chardonnens D, Cameo P, Aubert ML, et al. Modulation of human cytotrophoblastic leptin secretion by interleukin-1alpha and 17beta-oestradiol and its effect on HCG secretion. Mol Human Reprod 1999;5:1077–82. [DOI] [PubMed] [Google Scholar]


