Abstract
We evaluated the clinical usefulness of the combined use of 123I-ioflupane brain single photon emission computed tomography (SPECT) and 123I-metaiodobenzylguanidine (MIBG) cardiac scintigraphy in discriminating uncertain parkinsonism with vascular lesions in striatal nuclei at magnetic resonance imaging (MRI). Forty-three consecutive patients with uncertain parkinsonism and vascular lesions at MRI in striatal nuclei were retrospectively evaluated; the uncertain differential diagnosis was between Parkinson's disease and vascular parkinsonism (PD/VP) in 22 patients, between PD and other neurodegenerative parkinsonism (PD/PS) in 11 patients and between Lewy body dementia and Alzheimer disease (LBD/AD) in the remaining 10 cases. All patients underwent 123I-ioflupane SPECT with striatal dopaminergic activity determination as binding potentials (BP; cut-off: 3.3). 123I-MIBG cardiac planar scintigraphy was performed 2 weeks later, in early (15 minutes) and delayed (240 minutes) phases also calculating heart to mediastinum (H/M) ratio (cut-off: 1.56). 123I-Ioflupane uptake was normal in 9 patients with BP values >3.3, while it was reduced in 34/43 cases with BP values <3.3 at least in one of the striatal nuclei. 123I-MIBG uptake was normal in 21/43 patients (5 of whom with normal and 16 with 123I-ioflupane striatal defects) showing the H/M ratio >1.56 in all cases; the uptake was reduced in 22/43 cases, (4 of whom were normal and 18 were with 123I-ioflupane striatal defects) with the H/M ratio <1.56 in all cases. No statistical differences were found when early and delayed H/M ratios were mutually compared. Combining the 2 radioisotopic procedures, a more reliable diagnosis was achieved in 39/43 cases properly classifying 13 PD, 10 VP, 7 PS, 5 LBD, and 4 AD. However, the diagnosis remained uncertain in four patients with normal 123I-ioflupane and reduced 123I-MIBG uptake. The results of the present study confirmed that in uncertain parkinsonian syndromes associated with vascular lesions in striatal nuclei, brain 123I-ioflupane SPECT alone did not prove able to discriminate between the different forms of disease. Only the association with 123I-MIBG cardiac scintigraphy, also with the early acquisition alone, allowed the most appropriate diagnosis in 90.7% of our cases. However, patients with normal 123I-ioflupane and reduced 123I-I-MIBG uptakes need a close clinical and instrumental follow-up as sympathetic damage could precede striatal disorders in the early stage of PD and LBD.
Keywords: 123I-ioflupane SPECT, 123I-MIBG cardiac scintigraphy, uncertain parkinsonism
1. Introduction
The differential diagnosis of parkinsonian disorders represents a clinical dilemma especially in early phase, as clinical symptoms (slow movements, tremor, difficulty with walking and balance, stiffness, and rigidity) could be related to different conditions other than idiopathic Parkinson's disease (PD) such as progressive supranuclear palsy (PSP), multiple system atrophy (MSA), Lewy body dementia (LBD), cerebrovascular disorders, and iatrogenic drug effects.[1,2] In particular, vascular parkinsonism (VP) due to vascular lesions mainly in the striatal nuclei, but also in white matter, is considered a distinct clinical form[1,3] and the differential diagnosis between VP and PD is a crucial clinical point because of the difference in their progression, correct treatment strategy, potential supportive therapy, and prognosis.[4,5]
In the diagnostic criteria for suspected VP, there are included the clinical symptoms, the exclusion of Parkinson-plus syndromes, and other causes of secondary parkinsonism, while hypertensive cerebral vascular disease or previous history of transient ischemic attack or stroke, even if expected, are not considered as required parameters.[6]
Brain structural magnetic resonance imaging (MRI), evidencing vascular lesions, could be considered a supportive but not conclusive tool for confirming clinical diagnosis, as a specific pattern for VP has not yet been identified and some authors have underlined that ischemic brain lesions could be evidenced both in VP and PD cases[3,7,8] as well as in Alzheimer dementia[9] or in normal aged people with cardiovascular diseases or hypertension.[10]
Functional neuroimaging with 123I-ioflupane single photon emission computed tomography (SPECT), evaluating presynaptic striatal dopaminergic transporter with both qualitative and semiquantitative analyses,[11] usually represents a useful tool both in PD initial diagnosis[12,13] and in the differential diagnosis of uncertain parkinsonism.[14,15] However, some authors evidenced that 123I-ioflupane SPECT was not able to differentiate PD from VP, being observed in some VP patients with vascular damage and unilateral disease an asymmetrical tracer striatal uptake similar to that evidenced in PD cases probably due to the limited tracer possibility of arriving to the binding sites.[16] Therefore, PD diagnosis could be excluded when 123I-ioflupane SPECT was normal, but it remains unclear when a reduced tracer uptake occurs, thus leading to a diagnostic overlap.
123I-Metaiodobenzylguanidine (MIBG) cardiac scintigraphy represents a noninvasive method to evaluate postganglionic presynaptic cardiac sympathetic innervation.[17,18] MIBG is an analog of the adrenergic-blocking agent guanethidine and has the same chemical structure that of norepinephrine; the chemical structure of the tracer allows its detection with an active mechanism by postganglionic presynaptic fibers, the storage in synaptic vesicles, and the release during nerve excitement with the same mechanism that of noradrenaline.
On the other hand, unlike noradrenaline, MIBG is not bound to cardiac receptors and is not degraded by the enzymatic systems catechol-O-methyl transferase and monoamine oxidase , but it is reabsorbed in the presynaptic side and accumulated in the vesicles nerve ending for a long time.
These features mean that 123I-MIBG represents the ideal radiotracer for the “in vivo” cardiac sympathetic system evaluation.
Moreover, the early 123I-MIBG cardiac uptake well correlates with denervation damage, whereas the evaluation of delayed phase, representing the tracer wash out, directly expresses the degree of sympatheticotonia.[18]
123I-MIBG cardiac scintigraphy was originally used to evaluate the presynaptic postganglionic endings of sympathetic system in a large number of cardiac diseases, such as congestive heart failure, ischemic heart disease, and cardiomyopathy.[19–23] Afterwards, the procedure was applied in the diagnosis of PD[24] and in the differential diagnosis of this disease from other neurodegenerative disorders, such as MSA, PSP, corticobasal degeneration,[22,25] and VP.[26,27]
In this study we further evaluated the usefulness of 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy combined use in clinical practice for differentiating clinically uncertain parkinsonian conditions associated with vascular cerebral lesions ascertained at MRI.
2. Material and methods
2.1. Patients
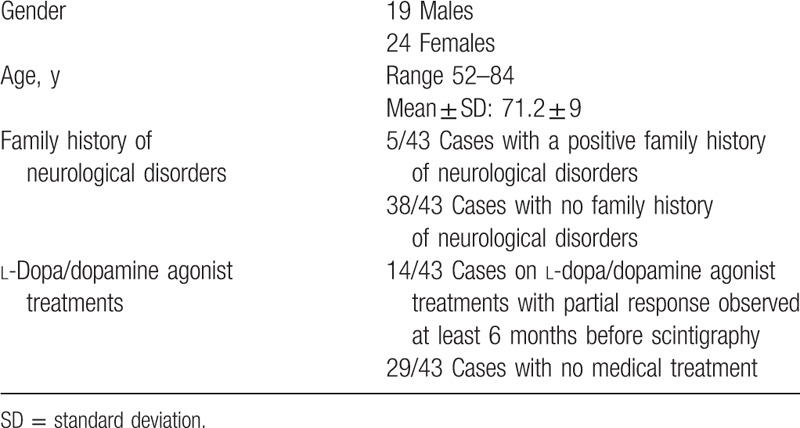
We evaluated retrospectively 43 consecutive patients, 19 males and 24 females aged 52 to 84 years (mean±SD value: 71.2 ± 9). All patients showed symptoms attributable to parkinsonian disorders associated with vascular cerebral lesions mainly in the striatal nuclei, but also in white matter ascertained at MRI; they were clinically classified as uncertain parkinsonism evidencing the presence of at least 1 of the following criteria: only 1 of the cardinal symptoms of PD, 2 clinical signs excluding bradykinesia, signs of mild intensity, atypical signs, poor response to l-dopa/dopamine agonist treatments, and lack of disease progression. The patients were investigated about neurological family history, about the presence of other related neurological diseases as well as about medical treatments such as antidepressants and corticosteroids, some of which can cause iatrogenic forms of parkinsonism. Exclusion criteria: metabolic disorders, such as renal and hepatic diseases, diabetes, and treatments affecting both 123I-ioflupane and 123I-MIBG uptakes. All patients had medical therapies for hypertension and hypercholesterolemia.
Table 1 illustrates the demographic, clinical, and treatment characteristics of the 43 patients.
Table 1.
Demographic, clinical, and treatment characteristics of the whole group of 43 patients.
All patients underwent brain 123I-ioflupane SPECT and afterwards 123I-MIBG cardiac scintigraphy within 2 weeks from the former examination.
Before scintigraphic procedures, all patients were subdivided into 3 groups according to possible differential diagnostic hypotheses formulated on clinical and MRI data:
Group 1: with uncertain diagnosis between PD and VP (22 cases; 51.2%)
Group 2: with uncertain diagnosis between PD and other neurodegenerative parkinsonian syndromes (PS) (11 cases; 25.5%)
Group 3: with uncertain diagnosis between LBD and AD (10 cases; 23.3%)
2.2. Brain 123I-ioflupane SPECT
According to European Association Nuclear Medicine procedure guidelines,[28] all patients were previously treated with 1000 mg of potassium perchlorate to minimize radiation exposure to the thyroid gland 30 minutes before the intravenous injection of 148 MBq of 123I-ioflupane (DaTSCAN, Amersham Health). Brain SPECT was performed 3 to 4 hours after the tracer injection by a dual-head gamma camera equipped with fan beam collimators (VG millennium; GE Healthcare). Acquisition and processing protocols had been previously prepared using a phantom for striatal imaging. The gamma camera was calibrated using the 159 keV photo peak ± 10% energy window. SPECT acquisition standardized parameters: rotation 180° for each head; frame size 128×128; zoom factor 1; frame time 30 seconds; and angular step 3°. SPECT images were acquired with the patients in the supine position and the head fixed in a holder.
SPECT data were normalized, processed by the back projection filter method applying a Butterworth filter (cut off frequency 0.5; order 10), and finally, a report of images, evaluated considering transaxial, coronal, and sagittal orbitomeatal-oriented slices (slice thickness: 2.23 mm), was obtained.
The images were qualitatively classified as normal (with striatal homogeneous and symmetrical intense tracer uptake in both caudate and putamen nuclei) or pathological (with striatal symmetrical or asymmetrical uptake defects);
In a postprocessing phase, a semiquantitative evaluation of the striatal 123I-ioflupane uptake was also performed by a dedicated software program (NEUROTRANS 3D; Segami Corp.), which defines striatal dopaminergic activity as binding potentials (BP) applying attenuation and partial volume effect corrections using a deformable 3D model segmentation generated by the degrading Talairach atlas. The BP cut-off of 3.3 for caudate and putamen was determined in 20 sex- and age-matched normal controls (mean values: 4.9 ± 0.71 and 4.6 ± 0.67 for caudate and putamen, respectively).
2.3. Cardiac 123I-MIBG scintigraphy
The EANM Cardiovascular Committee and the European Council of Nuclear Cardiology[19] protocol was used to standardize both the acquisition and processing procedures.
Twenty-four hours before tracer injection and imaging, all patients were warned to stop taking drugs that could potentially interfere with catecholamine uptake, such as antidepressants, antipsychotics, and calcium channel blockers.[19]
In accordance with safety administration procedures, 123I-MIBG (111 MBq) was slowly intravenously injected with patients at rest in the supine position, after a weekly blood pressure monitoring, an overnight fast, and a thyroid blockade with oral administration of potassium perchlorate (1000 mg).
Cardiac planar imaging, in anterior–posterior and anterior-left oblique views, was acquired in all cases, 15 minutes (early) and 240 minutes (delayed) after tracer i.v. injection, by a dual-head gamma camera (INFINIA; GE Healthcare) equipped with low-energy, high-resolution, parallel hole collimators; the acquisition parameters were: matrix 128 × 128, zoom 1.4, time 600 seconds; the photopeak energy was centered at 159 keV with a window of 10%; the patients were in the same supine position in both acquisition phases.
Early and delayed images were evaluated by qualitative method and were considered normal with homogeneous tracer cardiac uptake and pathological with irregular inhomogeneous one. Qualitative analysis was supported by the semiquantitative data obtained calculating the heart to mediastinum (H/M) ratio by dividing the mean count density of left ventricle to those of the upper mediastinum (excluding the thyroid gland) deduced by regions of interest (ROIs) manually drawn in the anterior view both in early and delayed phases. The H/M ratio cut-off was set applying 2 different artificial classifiers, random forest and classification tree, on a large number of clinically ascertained cases;[29,30] both statistical algorithms defined a cut-off of 1.56 in early and delayed phases.
Both 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy were interpreted separately by 3 nuclear medicine physicians (SN, AS, and GM) who were informed of the clinical reason pertinent to the scintigraphy, but were unaware of the results of any investigations. Scintigraphic data were classified as normal with physiologic tracer distribution or pathological with scans evidenced of abnormal decreased tracer uptake in striatal nuclei and heart, respectively. Disagreements were resolved by consensus.
All clinical and instrumental examinations were performed in a University Hospital setting as part of the clinical care of neurodegenerative disease patients. This retrospective study was performed in accordance with the regulations of the Institutional Review Board and in accordance with the Declaration of Helsinki. Routinely, written informed consent had been obtained by all patients whose data were treated in accordance with the local privacy rules and regulations.
2.4. Statistical analysis
Student's t test for independent variables was utilized to compare early and delayed H/M ratios in normal and pathological cases. Statistical significance was considered as P <.05
3. Results
3.1. 123I-Ioflupane SPECT
Brain 123I-ioflupane qualitative analysis showed normal bilateral caudate and putamen tracer uptake in 9/43 cases (20.9%), 5 with PD/VP, 1 with PD/PS, and 3 with LBD/AD uncertain differential diagnosis, while it was evidenced pathological, slight to severe irregular reduced tracer uptake, in 34/43 (79.1%), 17 with PD/VP, 10 with PD/PS, and 7 with LBD/AD uncertain differential diagnosis.
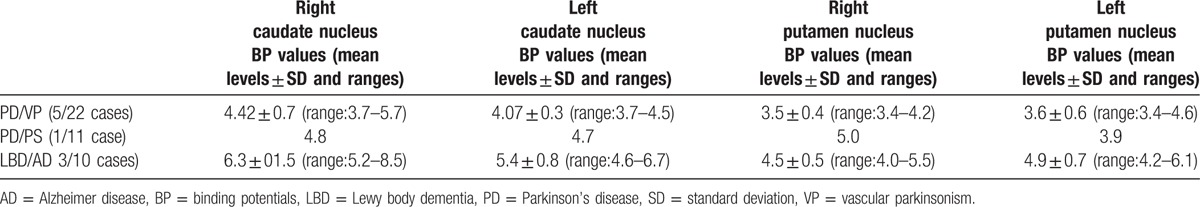
In all the 9 normal cases, BP values were above cut-off value in both caudate and putamen nuclei, as illustrated in Table 2.
Table 2.
BP values (mean levels ± SD and ranges) obtained separately for each of caudate and putamen nuclei according to the diagnostic hypotheses in 9 normal 123I-ioflupane uptake cases.
In all the 34 pathological cases, BP values were under cut-off value at least in one of striatal nuclei when BP values obtained for each of caudate and putamen nuclei were considered subdividing these on the basis of different diagnostic hypotheses, as illustrated in Table 3.
Table 3.
BP values (mean levels ± SD and ranges) obtained separately for each of caudate and putamen nuclei considered on the basis of the different diagnostic hypotheses in pathological 123I-ioflupane uptake cases.
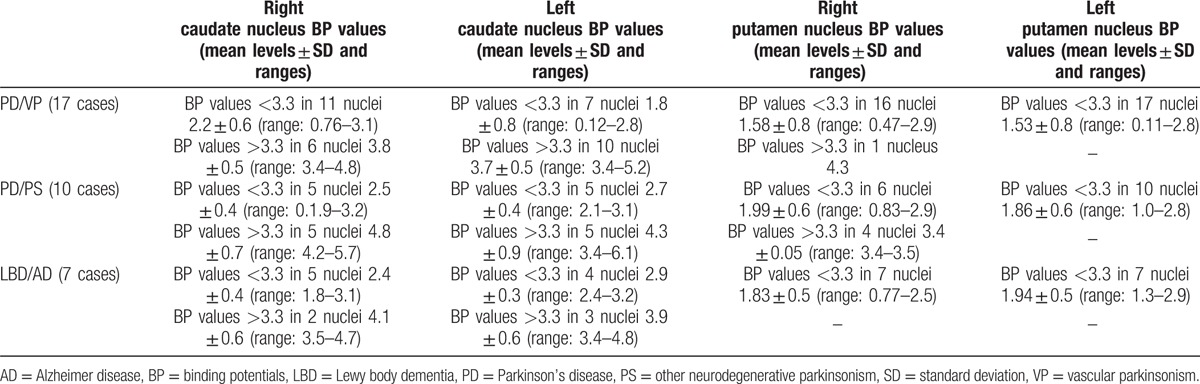
In particular, considering separately each nucleus, it was observed that in right caudate BP values were low in 22/34 nuclei and they were included between 0 and 1 in 1 nucleus, between 1 and 2 in 4, and between 2 and 3.3 in remaining 17 nuclei; BP values were above cut-off value in 12/34 nuclei. In the left caudate, BP values were low in 16/34 nuclei and they were included between 0 and 1 in 1 nucleus, between 1 and 2 in 3, and between 2 and 3.3 in remaining 12 nuclei; BP values were above cut-off value in 18/34 nuclei.
In right putamen, BP values were low in 29/34 nuclei and these were included between 0 and 1 in 7 nuclei, between 1 and 2 in 15, and between 2 and 3.3 in remaining 10 nuclei; BP values were above cut-off value in 5/34 nuclei. In the left putamen, BP values were low in all 34 nuclei and these were included between 0 and 1 in 6 nuclei, between 1 and 2 in 15, and between 2 and 3.3 in remaining 13 nuclei.
3.2. 123I-MIBG cardiac scintigraphy
123I-MIBG cardiac scintigraphy qualitative evaluation showed, both in early and delayed phases, a homogenous tracer uptake in 21/43 patients (48.8%), 5 of whom had normal and 16 pathological 123I-ioflupane SPECT.
The H/M ratio values were >1.56, in both phases, in all 21 cases; no statistical difference was observed when early (1.71 ± 0.09) and delayed (1.76 ± 0.09) H/M ratio mean values were mutually compared, even when the patients were classified on the basis of 123I-ioflupane SPECT results (Table 4).
Table 4.
Qualitative analysis of 123I-MIBG cardiac scintigraphy results with early and delayed H/M ratio calculation in the 43 patients also subdivided on the basis of normal and pathological 123I-ioflupane striatal uptake.
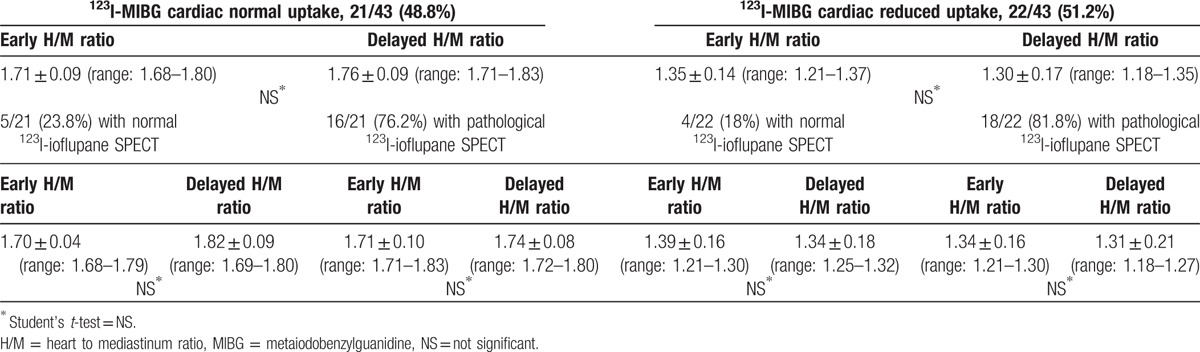
123I-MIBG cardiac distribution showed slight to severe reduction in 22/43 cases (51.2%), 4 with normal and 18 with pathological 123I-ioflupane SPECT.
The H/M ratio values were <1.56, in both phases, in all 22 cases; no statistical difference was observed in the comparison between early (1.35 ± 0.14) and delayed (1.30 ± 0.17) H/M ratio mean values, even when patients were classified on the basis of 123I-ioflupane SPECT results (Table 4).
3.3. 123I-Ioflupane SPECT and 123I-MIBG cardiac scintigraphy combined use
The results obtained combining the 2 procedures have permitted to identify 4 different conditions:
-
A.
Normal 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy (5/43 cases; 11.6%)
-
B.
Normal 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy (4/43 cases; 9.3%)
-
C.
Pathological 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy (16/43 cases; 37.2%)
-
D.
Pathological 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy (18/43; 41.8%).
After 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy results, a second clinical evaluation was performed to better define diagnosis.
Globally, when the data of the combined scintigraphic procedures were suggestive of normal or pathological conditions, the diagnosis was considered more reliable in 39/43 cases, whereas the diagnosis remained doubtful in those cases (4/43) with discordant scintigraphic results.
In particular, as shown in Table 5, among the 22 initially considered PD/VP uncertain cases, 19 (86.4%) were more properly classified: 9/22 cases with pathological 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy as PD (a case is illustrated in Fig. 1); and 10/22 cases with normal 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy or pathological 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy, as VP (a case is illustrated in Fig. 2). In the remaining 3/22 cases (13.6%), with normal 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy, the diagnosis was considered doubtful (a case is illustrated in Fig. 3).
Table 5.
Initial differential diagnostic hypothesis and variation of clinical approach after 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy combined use.
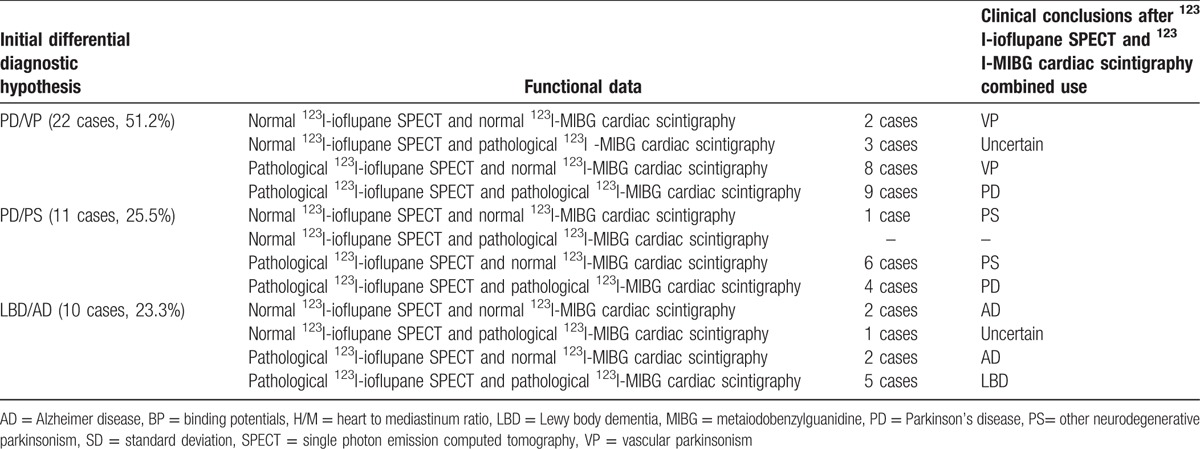
Figure 1.
A 78-year-old female patient with uncertain parkinsonism and vascular lesions in subcortical areas, semioval centers, basal ganglia, and left temporal lobe at MRI. 123I-Ioflupane SPECT was pathological in both putamen nuclei (A) with also reduced BP values (1.7 and 1.8 in right and left putamen, respectively); 123I-MIBG cardiac scintigraphy (B) was pathological with reduced H/M values both in early (1.2) and delayed (1.10) phases. The patient was finally classified as PD. MRI= magnetic resonance imaging, SPECT = single photon emission computed tomography.
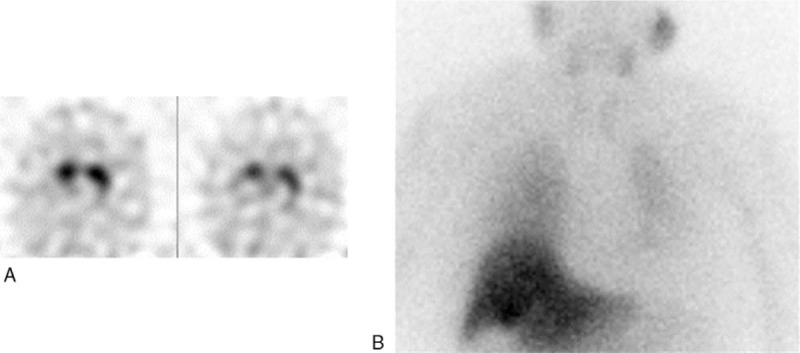
Figure 2.
A 70-year-old male patient with uncertain parkinsonism, vascular lesions in basal ganglia at MRI, and partial response to dopamine agonist treatment. 123I-Ioflupane SPECT was pathological in left putamen nucleus (A) with also reduced BP value (1.9); 123I-MIBG cardiac scintigraphy was normal (B) with H/M values above cut-off both in early (1.8) and delayed (1.8) phases. The patient was finally classified as VP. H/M = heart to mediastinum ratio, MIBG = metaiodobenzylguanidine, SPECT = single photon emission computed tomography, VP = vascular parkinsonism.
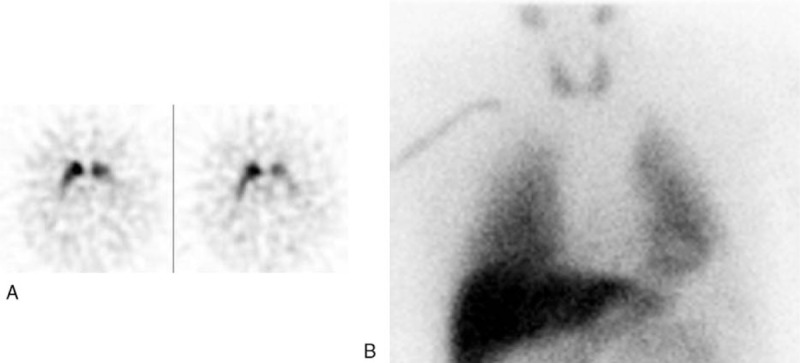
Figure 3.
A 55-year-old male patient with uncertain parkinsonism, vascular lesions in left basal ganglia and midbrain at MRI, and partial response to dopamine agonist treatment. 123I-Ioflupane SPECT was normal (A) with also BP values above cut-off in caudate and putamen nuclei, bilaterally; 123I-MIBG cardiac scintigraphy was pathological (B) with reduced H/M values both in early (1.43) and delayed (1.38) phases. The patient was finally confirmed with uncertain parkinsonism and monitored in a close follow up. H/M = heart to mediastinum ratio, MRI= magnetic resonance imaging, MIBG = metaiodobenzylguanidine, SPECT = single photon emission computed tomography.
Of the 11 initially uncertain PD/PS patients, 4/11 with pathological 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy had finally a clinical diagnosis of PD. The remaining 7/11 with normal 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy or pathological 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy were classified as PS.
Among the 10 initially doubtful LBD/AD cases, 5/10 with pathological 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy were finally considered as LBD and 4/10 with normal 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy or pathological 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy as AD. The remaining 1 patient with normal 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy was confirmed as uncertain diagnosis.
All 4 patients classified as doubtful diagnosis are still monitored in a close follow-up.
4. Discussion
Parkinsonian syndromes often represent a clinical dilemma especially in the early stage and neuroimaging techniques are usually employed as complementary diagnostic tools in the initial differential diagnosis[31] of disease.
123I-Ioflupane SPECT is a useful functional imaging in the diagnosis of PD and its high specificity suggests that the combined use of this procedure with the neurological examination in the initial evaluation of the disease could reduce the overdiagnosis of PD, thus limiting the use of inappropriate medical treatments.[32] However, controversial data have been reported applying 123I-ioflupane SPECT in uncertain parkinsonian syndromes when multiple vascular lesions occur, especially in the few studies focalized on the differential diagnosis between PD and VP, the 2 most frequent clinical forms that need to be distinguished. In comparative studies between VP and PD patients, some authors[33] pointed that 123I-ioflupane uptake may be useful in the differential diagnosis between PD and VP, observing significant statistical differences in striatal tracer binding in these 2 conditions. However, the same authors observed an overlap of the whole striatal binding in some PD and VP patients, thus reducing 123I-ioflupane SPECT accuracy. In addition, other authors[34,35] have suggested caution in the use of 123I-ioflupane SPECT in the differential diagnosis between VP and PD, not being clearly yet determined, in their opinion, the patterns of tracer uptake for each disease. Furthermore, although 123I-ioflupane SPECT may represent a potential reliable tool also in the differential diagnosis between PD and PS and between AD and LBD, there are no data demonstrating its use in these disorders when vascular damage occurs.
The data of the present study, in respect of the previous mentioned studies that only included ascertained PD and VP cases, came from different groups of patients with uncertain parkinsonian syndromes and with vascular lesions mainly in the striatal nuclei, but also in white matter at MRI. Moreover, cases with differential diagnosis between PD and VP (PD/VP) were also included as well as patients with differential diagnosis between PD and PS (PD/PS) and between LBD and AD (LBD/AD). The results evidenced that 123I-ioflupane SPECT alone provided additional information, but not useful to effectively support the clinical differential diagnosis when cerebrovascular lesions occurred at MRI, as approximately 20% of our cases had normal scintigraphic data, whereas the remaining 80% showed pathological results, but not useful to clarify the differential diagnosis between the different forms of disease. These data emphasized the overlap of 123I-ioflupane uptake in the different clinical forms not only in PD/VP cases, in accordance with the results of other authors,[33,34] but also in a wider number of other clinical forms, as reported in the present study probably in relation to the inability of the tracer to reach the presynaptic receptor level due to vascular disruption.
It is well known that 123I-MIBG cardiac scintigraphy, expressing the cardiac sympathetic postganglionic function, could be used both in the initial diagnosis of PD[24] and in the differential diagnosis between PD and other neurodegenerative parkinsonism[22,36] as well as in distinguishing LBD from AD.[37] However, only a few studies,[38–43] with different approaches, have evaluated the clinical utility of the combined use of the 2 procedures in the differential diagnosis of uncertain parkinsonian disorders; only one of these studies[43] have reported the complementary role of 123I-MIBG cardiac scintigraphy in the patients with vascular lesions at MRI and, in particular, considering the differential diagnosis between VP and PD, as brain vascular lesions often can be responsible of uncertain results of 123I-ioflupane SPECT.
A contemporary damage in dopaminergic nigrostriatal system and in sympathetic cardiac neurons in PD was hypothesized[38] obtaining a significant positive correlation between reduced 123I-ioflupane SPECT uptake and cardiac decreased uptake of 123I-MIBG in early PD cases with still normal MRI data. These results seem to suggest that the combined use of both 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy in early diagnosis of PD may be clinically useful, especially when only slight symptoms occur; the clinical “in vivo” diagnosis is difficult to achieve in this conditions. An overlap between patients with PD and other parkinsonian syndromes, such as MSA, can also appear, as postmortem studies have ascertained.[44,45]
Some authors[39] evaluated a multidimensional statistical approach of both 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy; they also studied 123I-labeled dopamine D2 receptor ligands (IBZM) in the differential diagnosis of both PD and other atypical parkinsonism. The results of this study confirmed that there is a low diagnostic accuracy when the single procedures were used alone, evidencing an overlap between PD and PS. The same authors observed that the best diagnostic accuracy in distinguishing PD from PS was achieved combining the 3 procedures. However, in order to minimize cost and radiation exposure, the combined use of 123I-IBZM and 123I-MIBG scintigraphy should be suggested as the better choice as these 2 procedures evidence the striatal D2 receptor expression and the cardiac MIBG accumulation, respectively; the employment of 123I-ioflupane SPECT could, however, additionally be associated in borderline or contradictory results.
Other authors[40–42] evaluated the role of the combined use of 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy in patients with mixed tremors or in those cases in whom is necessary to differentiate LBD from other dementias. They suggested that the higher sensitivity of 123I-ioflupane SPECT and the higher specificity of 123I-MIBG cardiac scintigraphy could lead, when combined, to more accurate differential diagnosis, thus permitting the most appropriate treatments.
Our data, thought including a more complex series of patients in respect of the other mentioned casuistries, seem to confirm the results obtained by other authors[43] in the only study that consider the combined use of the 2 procedures. In particular, in respect of the initial clinical evaluation and of the evaluation performed after 123I-ioflupane SPECT, the clinical diagnosis based on the results of the combined use of the 2 scintigraphic procedures was more correct in 90.7% of our cases. This result seems to confirm that 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy combined use could represent a more valuable tool in the diagnosis of uncertain parkinsonian syndromes, also considering the easy availability of the radiopharmaceuticals and the rather limited radioprotection problems.
Considering the patients with normal 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy as well as those with pathological 123I-ioflupane SPECT and normal 123I-MIBG cardiac scintigraphy, a final diagnosis was achieved as VP in 10 cases, PS in 7 cases, and AD in 4 cases. Moreover, pathological 123I-ioflupane SPECT and pathological 123I-MIBG cardiac scintigraphy enforced the diagnosis of PD in 13 patients and LBD in 5 cases.
Therefore, the combined use of the 2 radioisotopic procedures, supporting clinical diagnosis, have contributed to modify the treatment in those cases already treated, but with poor results, at the moment of scintigraphic exams and, at the same time, to guide to a more specific therapy those cases not yet treated.
In 4 cases (3 PD/VP and 1 LBD/AD), the 2 scintigraphic methods were inconclusive as 123I-MIBG cardiac scintigraphy was pathological despite normal ioflupane SPECT. These controversial results were also reported by other authors[46,47] who hypothesized an early PD stage with no evident motor signs and with a striatal damage not yet manifest, but with cardiac sympathetic disorder, thus speculating an earliest involvement of autonomic system. We think that a similar mechanism could also be possible in the 3 our PD/VP cases and in the 1 LBD/AD case, having been excluded a priori all interfering factors in the 2 tracer uptakes. However, only a close follow up of the patients could confirm this hypothesis, and they are still monitored and are waiting of treatment. However, as often it happens, only autopsy could be determinant for final diagnosis.
Moreover, with regard to semiquantitative analysis of 123I-MIBG, our results have not evidenced statistically significant differences in the comparison between early and delayed H/M ratio mean values, regardless of patients classified on the basis of 123I-ioflupane SPECT results. For clinical neurological purposes, these data seem to suggest that the early phase alone could contribute to the diagnosis permitting also an easier patient management, according to the study of other authors[48] who, evaluating a large series of LBD patients, achieved an accurate differential diagnosis between LBD and non-LBD cases only applying an early H/M ratio.
5. Conclusions
In conclusion, our results proved that in uncertain parkinsonian syndromes with vascular lesions in striatal nuclei and in white matter ascertained at MRI, 123I-ioflupane SPECT alone is not able to clearly differentiate PD from VP and from other parkinsonism as well as LBD from AD often showing an overlap in tracer uptake. Only the combined use of 123I-ioflupane SPECT and 123I-MIBG cardiac scintigraphy, also with the early acquisition of this latter alone, seems to give a more appropriate diagnosis in association with neurological examination, as in our cases, the results of these associated neuroimaging procedures gave useful information in more than 90% of cases. Moreover, in the presence of doubtful results with pathological 123I-MIBG uptake and normal 123I-ioflupane uptake, the patients must be monitored with a close follow up as sympathetic damage could precede striatal disorders in an early stage of Parkinson's or LBD diseases.
Thus, a wider employment of these 2 combined procedures is suggested when striatal vascular damage is associated with uncertain Parkinsonian syndromes.
Footnotes
Abbreviations: AD = Alzheimer disease, BP = binding potentials, CBD = corticobasal degeneration, DaT = striatal dopaminergic transporter, H/M = heart to mediastinum ratio, LBD = Lewy body dementia, MIBG = metaiodobenzylguanidine, MSA = multiple system atrophy, PD = Parkinson's disease, PS= other neurodegenerative parkinsonism, PSP = progressive supranuclear palsy, SPECT = single photon emission computed tomography, VP = vascular parkinsonism.
The authors have no conflicts of interest to disclose.
References
- [1].Quinn N. Parkinsonism recognition and differential diagnosis. BMJ 1995;310:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Catafau AM, Tolosa E. Impact of dopamine transporter SPECT using 123I-ioflupane on diagnosis and management of patients with clinically uncertain parkinsonian syndromes. Mov Disord 2004;19:1175–82. [DOI] [PubMed] [Google Scholar]
- [3].Demirkiran M, Bozdemir H, Sarica Y. Vascular parkinsonism: a distinct, heterogeneous clinical entity. Acta Neurol Scand 2001;104:63–7. [DOI] [PubMed] [Google Scholar]
- [4].Thanvi B, Lo N, Robinson T. Vascular parkinsonism an important cause of parkinsonism in older people. Age Ageing 2005;34:114–9. [DOI] [PubMed] [Google Scholar]
- [5].Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson's disease in the community? J Neurol Neurosurg Psychiatry 2002;73:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zijlmans JCM, Daniel SE, Hughes AJ, et al. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord 2004;19:630–40. [DOI] [PubMed] [Google Scholar]
- [7].Piccini P, Pavese N, Canapicchi R, et al. White matter hyperintensities in Parkinson's disease: clinical correlations. Arch Neurol 1995;52:191–4. [DOI] [PubMed] [Google Scholar]
- [8].Rampello L, Alvano A, Battaglia G, et al. Different clinical and evolutional patterns in late idiopathic and vascular parkinsonism. J Neurol 2005;252:1045–9. [DOI] [PubMed] [Google Scholar]
- [9].Appel J, Potter E, Shen Q, et al. A comparative analysis of structural brain MRI in the diagnosis of Alzheimer's disease. Behav Neurol 2009;21:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke 2004;35:1857–61. [DOI] [PubMed] [Google Scholar]
- [11].Contrafatto D, Mostile G, Nicoletti A, et al. [(123) I]FP-CIT-SPECT asymmetry index to differentiate Parkinson's disease from vascular parkinsonism. Acta Neurol Scand 2012;126:12–6. [DOI] [PubMed] [Google Scholar]
- [12].Kagi G, Bhatia KP, Tolosa E. The role of DAT-SPECT in movement disorders. J Neurol Neurosurg Psychiatry 2010;81:5–12. [DOI] [PubMed] [Google Scholar]
- [13].Zijlmans JC. The role of imaging in the diagnosis of vascular parkinsonism. Neuroimaging Clin N Am 2010;20:69–76. [DOI] [PubMed] [Google Scholar]
- [14].Tolosa E, Borght TV, Moreno E. Accuracy of DaTSCAN (123I-Ioflupane) SPECT in diagnosis of patients with clinically uncertain parkinsonism: 2-year follow-up of an open-label study. Mov Disord 2007;22:2346–51. [DOI] [PubMed] [Google Scholar]
- [15].Suwijn SR, van Boheemen CJM, de Haan RJ, et al. The diagnostic accuracy of dopamine transporter SPECT imaging to detect nigrostriatal cell loss in patients with Parkinson's disease or clinically uncertain parkinsonism: a systematic review. EJNMMI Res 2015;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gupta D, Kuruvilla A. Vascular parkinsonism: what makes it different? Postgrad Med J 2011;87:829–36. [DOI] [PubMed] [Google Scholar]
- [17].Vallabhajosula S, Nikolopoulou A. Radioiodinated metaiodobenzylguanidine (MIBG): radiochemistry, biology, and pharmacology. Semin Nucl Med 2011;41:324–33. [DOI] [PubMed] [Google Scholar]
- [18].Yamashina S, Yamazaki J. Neuronal imaging using SPECT. Eur J Nucl Med Mol Imaging 2007;34:939–50. [DOI] [PubMed] [Google Scholar]
- [19].Flotats A, Carrió I, Agostini D, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. Eur J Nucl Med Mol Imaging 2010;37:1802–12. [DOI] [PubMed] [Google Scholar]
- [20].Martins da Silva MI, Vidigal Ferreira MJ, Morão Moreirac AP. Iodine-123-metaiodobenzylguanidine scintigraphy in risk stratification of sudden death in heart failure. Rev Port Cardiol 2013;32:509–16. [DOI] [PubMed] [Google Scholar]
- [21].Verberne HJ, Brewster LM, Somsen GA, et al. Prognostic value of myocardial 123Imetaiodobenzylguanidine (MIBG) parameters in patients with heart failure: a systematic review. Eur Heart J 2008;29:1147–59. [DOI] [PubMed] [Google Scholar]
- [22].Orimo S, Suzuki M, Inaba A, et al. 123I-MIBG myocardial scintigraphy for differentiating Parkinson's disease from other neurodegenerative parkinsonism: a systematic review and meta-analysis. Parkinsonism Relat Disord 2012;18:494–500. [DOI] [PubMed] [Google Scholar]
- [23].Pace L, Bettocchi S, Losi MA, et al. Sympathetic nervous function in patients with hypertrophic cardiomyopathy assessed by 123I MIBG: relationship with ventricular perfusion and function. Q J Nucl Med Mol Imaging 2004;48:20–5. [PubMed] [Google Scholar]
- [24].Oka H, Toyoda C, Yogo M, et al. Reduced cardiac 123I-MIBG uptake reflects cardiac sympathetic dysfunction in de novo Parkinson's disease. J Neural Transm 2011;118:1323–7. [DOI] [PubMed] [Google Scholar]
- [25].Shin DH, Lee PH, Bang OY, et al. Clinical implications of cardiac-MIBG SPECT in the differentiation of parkinsonian syndromes. J Clin Neurol 2006;2:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim JS, Lee PH, Lee KS, et al. Cardiac [123I] metaiodobenzylguanidine scintigraphy for vascular parkinsonism. Mov Disord 2006;21:1990–4. [DOI] [PubMed] [Google Scholar]
- [27].Kalra S, Grosset DG, Benamer HTS. Differentiating vascular parkinsonism from idiopathic Parkinson's disease: a systematic review. Mov Disord 2010;25:149–56. [DOI] [PubMed] [Google Scholar]
- [28].Darcourt J, Booij J, Tatsch K, et al. EANM procedure guidelines for brain neurotransmission SPECT using 123I-labelled dopamine transporter ligands. Version 2. Eur J Nucl Med Mol Imaging 2010;37:443–50. [DOI] [PubMed] [Google Scholar]
- [29].Nuvoli S, Palumbo B, Fravolini M, et al. 123I MIBG cardiac scintigraphy in differentiating movement disorders by a Random Forest classifier. J Nucl Med 2014;55:1845. [Google Scholar]
- [30].Nuvoli S, Palumbo B, Fravolini M, et al. The Classification tree (CIT) classifier applied to 123I MIBG cardiac scintigraphy in differentiating Parkinson's disease (PD) from parkinsonism (P). J Nucl Med 2015;56:1582. [Google Scholar]
- [31].Holtbernd F, Eidelberg D. The utility of neuroimaging in the differential diagnosis of parkinsonian syndromes. Semin Neurol 2014;34:202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marshall VL, Reininger CB, Marquardt M, et al. Parkinson's disease is overdiagnosed clinically at baseline in diagnostically uncertain cases: a 3-year European Multicenter Study with Repeat [123I]FP-CIT SPECT. Mov Disord 2009;24:500–8. [DOI] [PubMed] [Google Scholar]
- [33].Gerschlager W, Bencsits G, Pirker W, et al. [123I]beta-CIT SPECT distinguishes vascular parkinsonism from Parkinson's disease. Mov Disord 2002;17:518–23. [DOI] [PubMed] [Google Scholar]
- [34].Scherfler C, Schwarz J, Antonini A, et al. Role of DAT-SPECT in the diagnostic work up of parkinsonism. Mov Disord 2007;22:1229–38. [DOI] [PubMed] [Google Scholar]
- [35].Antonini A, Vitale C, Barone P, et al. The relationship between cerebral vascular disease and parkinsonism: the VADO study. Parkinsonism Relat Disord 2012;18:775–80. [DOI] [PubMed] [Google Scholar]
- [36].Umemura A, Oeda T, Hayashi R, et al. Diagnostic accuracy of apparent diffusion coefficient and 123I-metaiodobenzylguanidine for differentiation of multiple system atrophy and Parkinson's disease. PLoS One 2013;8:e61066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yoshita M, Arai H, Arai H, et al. Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PLoS One 2015;10:e0120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Spiegel J, Möllers MO, Jost WH, et al. FP-CIT and MIBG scintigraphy in early Parkinson's disease. Mov Disord 2005;20:552–61. [DOI] [PubMed] [Google Scholar]
- [39].Südmeyer M, Antke C, Zizek T, et al. Diagnostic accuracy of combined FP-CIT, IBZM, and MIBG scintigraphy in the differential diagnosis of degenerative parkinsonism: a multidimensional statistical approach. J Nucl Med 2011;52:733–40. [DOI] [PubMed] [Google Scholar]
- [40].Novellino F, Arabia G, Bagnato A, et al. Combined use of DAT-SPECT and cardiac MIBG scintigraphy in mixed tremors. Mov Disord 2009;24:2242–8. [DOI] [PubMed] [Google Scholar]
- [41].Treglia G, Cason E, Cortelli P, et al. Iodine-123 metaiodobenzylguanidine scintigraphy and iodine-123 ioflupane single photon emission computed tomography in Lewy body diseases: complementary or alternative techniques? J Neuroimaging 2014;24:149–54. [DOI] [PubMed] [Google Scholar]
- [42].Shimizu S, Hirao K, Kanetaka H, et al. Utility of the combination of DAT SPECT and MIBG myocardial scintigraphy in differentiating dementia with Lewy bodies from Alzheimer's disease. Eur J Nucl Med Mol Imaging 2016;43:184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Navarro-Otano J, Gaig C, Muxi A, et al. 123I-MIBG cardiac uptake, smell identification and 123I-FP-CIT SPECT in the differential diagnosis between vascular parkinsonism and Parkinson's disease. Parkinsonism Relat Disord 2014;20:192–7. [DOI] [PubMed] [Google Scholar]
- [44].Koga S, Aoki N, Uitti RJ, et al. When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology 2015;85:404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Joutsa J, Gardberg M, Röyttä M, et al. Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord 2014;20:840–4. [DOI] [PubMed] [Google Scholar]
- [46].Goldstein DS, Sharabi Y, Karp BI, et al. Cardiac sympathetic denervation preceding motor signs in Parkinson disease. Cleve Clin J Med 2009;76(suppl 2):S47–50. [DOI] [PubMed] [Google Scholar]
- [47].Goldstein DS, Holmes C, Sewell L, et al. Sympathetic noradrenergic before striatal dopaminergic denervation: relevance to Braak staging of synucleinopathy. Clin Auton Res 2012;22:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sakamoto F, Shiraishi S, Tsuda N, et al. (123)I-MIBG myocardial scintigraphy for the evaluation of Lewy body disease: are delayed images essential? Is visual assessment useful? Br J Radiol 2016;10:20160144. [DOI] [PMC free article] [PubMed] [Google Scholar]


