Supplemental Digital Content is available in the text
Keywords: Chinese herbal injections, network meta-analysis, pancreatic cancer, systematic review
Abstract
Background:
This study sought to use a network meta-analysis to assess the effectiveness and safety of Chinese herbal injections (CHIs) combined with the chemotherapy for the treatment of pancreatic cancer.
Methods:
Randomized controlled trials (RCTs) regarding CHIs to treat pancreatic cancer were searched in PubMed, the Cochrane library, Embase, the China National Knowledge Infrastructure Database (CNKI), the Wan-Fang Database, the Chinese Scientific Journals Full-text Database (VIP), and the Chinese Biomedical Literature Database (SinoMed) up to November 2016. The quality assessment was conducted by the Cochrane risk of bias tool and network meta-analysis was performed to compare the effectiveness and safety of different CHIs combined with the chemotherapy. Data were analyzed using STATA 12.0 and Win-BUGS 1.4 software.
Results:
A total of 278 records were searched, and 22 eligible RCTs involving 1329 patients and 9 CHIs were included. The results of the network meta-analysis demonstrated that compared with the chemotherapy alone, Compound Kushen, Kangai or Kanglaite injection combined with chemotherapy yielded significantly higher probability of improving performance status. Aidi injection combined with chemotherapy was more effective in relieving leucopenia than using chemotherapy single. And these between-group differences were statistically significant. However, CHIs combined with chemotherapy could not achieve a better effect in the total clinical effect, nausea and vomiting. As for the cluster analysis for the adverse reactions (ADRs), the chemotherapy alone and Huachansu injection combined with the chemotherapy were inferior to relieve ADRs than the other CHIs plus chemotherapy for patients with pancreatic cancer.
Conclusions:
The current evidence showed that using CHIs on the basis of the chemotherapy could be beneficial for patients with pancreatic cancer in improving performance status and reducing the ADRs.
1. Introduction
Pancreatic cancer is one of the common malignancies in the digestive system.[1] According to the World Health Organization (WHO) statistics, the global morbidity and mortality of pancreatic cancer respectively account for the first 13, 7 in 2008.[2] And in China, the morbidity and mortality of pancreatic cancer ranks 7 and 6 of all malignant tumors.[3] About 60% patients with pancreatic cancer may occur distant metastasis of tumor when they are diagnosed, and the median survival time is only 6 to 15 months, 5-year survival rate is close to 5%.[4,5] The results of the present studies clarify that the incidence of pancreatic cancer is related to smoking, alcohol, diabetes, dietary habits, and other factors.[6–11] In traditional Chinese medicine (TCM) theory, pancreatic cancer belongs to “lump in the abdomen causing distension and pain” or “mass located in the upper or lower abdomen,” the lump in the abdomen, jaundice, and pain appears as its main manifestation in clinic.[12,13] TCM serve as an important part of the comprehensive treatment for pancreatic cancer, it not only can enhance immune function and anti-tumor ability, reduce the toxicity of radiotherapy and chemotherapy; but also can improve the clinical symptoms and performance status of patients with pancreatic cancer, furthermore, it is possible to prolong the survival time.[3] Apart from the using TCM decoction of treatment based on syndrome differentiation, CHIs have the advantages of rapid absorption and high-bioavailability, and widely used in clinics. Especially, CHIs play an important role of anti-cancer in the treatment of tumor diseases by reducing toxicity, enhancing efficiency when it combined with radiotherapy and chemotherapy.[14,15] Such as Kanglaite, Kangai, and compound Kushen injection can improve performance status, alleviating symptoms, control the development and metastasis of tumors.[16–19]
Currently, the majority of clinical trials are 2-arm trials which focus on the comparisons between CHIs plus chemotherapy and chemotherapy; however, the head-to-head comparisons of different CHIs about their effectiveness and safety are relatively lack.[20] Network meta-analysis allows for the simultaneous evaluation of multiple interventions, and provides valuable information for clinical decision-making through indirect comparisons in the absence of direct comparisons.[21,22] In addition, network meta-analysis can sort the different interventions based on their therapeutic effect and the probability of optimal interventions.[23] CHIs plus the chemotherapy has been in the clinical application of patients with pancreatic cancer, while there is a lack of high-level evidence-based medical researches about it recently. Given above, this study sought to use a network meta-analysis to assess the effectiveness and safety of CHIs combined with the chemotherapy for pancreatic cancer.
2. Methods
This study, including its inclusion and exclusion criteria, literature search, data extraction, quality assessment, and statistical analysis components, was conducted in accordance with Cochrane criteria and PRISMA guidelines.
2.1. Inclusion and exclusion criteria
Only RCTs meeting the following criteria were included in this network meta-analysis: (1) Study type: RCTs regarding CHIs were combined with the chemotherapy for the treatment of pancreatic cancer, with irrespective of blinding methods the publishing language. (2) Study objects: the included participants met the pathological or cytological diagnostic criteria of pancreatic cancer, and no gender, race, or nationality limitations were imposed. And patients were without contraindications to chemotherapy and obvious abnormalities in their electrocardiograms and liver and kidney functions. (3) Interventions: The CHIs group was treated by CHIs plus the chemotherapy, while the chemotherapy group solely used the chemotherapy. The chemotherapeutic drugs included gemcitabine (GEM), docetaxel (DOC), tegafur (S1), cisplatin (DDP), oxaliplatin (L-OHP), 5-fluorouracil (5-Fu), leucovorin (LV). (4) Outcomes: The primary outcomes of the research were the clinical effectiveness rate and the performance status. The criterion of the rapeutical effect met the WHO for solid tumors released in 1979.[24] The clinical effectiveness rate was calculated by the following formula: the clinical effectiveness rate = [number of complete response (CR) patients + partial response (PR)]/ total number of patients × 100%. The clinical effectiveness rate was defined as a complete response (CR) or a partial response (PR). Performance status was assessed by the Karnofsky performance score (KPS), which was calculated as follows: KPSs that increased by ≥10 points after treatment were considered to improve performance status; KPSs that decreased by ≥10 points after treatment were considered to lower performance status, and KPSs that increased or decreased by <10 points were considered stable. The secondary outcomes were the ADRs involving leucopenia, nausea and vomiting. And the criterion of the ADRs met the WHO for common toxicity criteria of chemotherapy drugs released in 1981.[25] The incidence of ADRs was calculated by the following formula: the incidence of ADRs = (number of patients occurred ADRs)/ total number of patients × 100%.
RCTs meeting the following criteria were excluded in this network meta-analysis: (1) study type: as for the repeatedly published studies, only retaining the latest or more comprehensive ones. (2) Study objects: the patients suffered from other primary tumors, obstructive jaundice, or severe infections. (3) Interventions: the joints interventions were not chemotherapy (radiotherapy, hyperthermia, interventional therapy, etc.). CHIs’ route of administration was not intravenous infusion. The information of chemotherapeutic drugs, dose and duration of treatment was incomplete or incorrect. (4) Outcomes: the rapeutical effect or ADRs of RCTs was not in accordance with the criterion of WHO, and RCTs did not report the clinical effectiveness rate, the improvement of performance status and ADRs.
2.2. Literature search
RCTs involving regarding CHIs to treat pancreatic cancer were retrieved by searching the following databases from January 1979 to November 2016: PubMed, the Cochrane library, Embase, CNKI, VIP, CBM and the Wan-Fang Database. In English databases, the searching words in the pancreatic cancer category were “Pancreatic Neoplasms, Insulinoma, Gastrinoma, Glucagonoma, Somatostatinoma, Vipoma, Pancreatic Neoplasm, Pancreas Neoplasm∗, Pancreas Cancer∗, Pancreatic Cancer∗.” The specific Chinese and English search terms for each CHIs and specific retrieval strategies were shown in Attachment 1.
2.3. Data extraction and quality assessment
Two researchers (DZ and JW) independently read the titles and abstracts of the identified RCTs, excluding not relevant ones, reviews, and pharmacological experiments. Full texts of the possibly relevant RCTs were checked to further determine whether they met the inclusion criteria. The reference sections of the retrieved articles and meeting abstracts were also screened. Case reports, animal experiments, editorials, letters, and review articles were excluded. In the case that 1 publication overlapped with another publication of the same trial, only the article with more detail or the most recent article was included. The two researchers (DZ and JW) conducted their quality evaluations independently. If there was disagreement occurred, discussion or further inquiry to a third researcher (SL) was chosen as a way to decide. The main components of the extracted data were as follows: (1) general information: title, authors’ names, publication date, and literature sources; (2) patient information: the number of patients, patient ages, patient genders, patients’ KPSs before treatment, tumor types, and tumor stages; (3) intervention: the names, dosages, and treatment cycles of CHIs; (4) outcomes: the measured data about clinical effectiveness rate, performance status and ADRs.
The quality assessment was conducted by the Cochrane risk of bias tool, which included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias.[26] Each RCT was rated “high,” “unclear,” or “low.” “High” meant incorrect random methods, no allocation concealment or no blinding. “Unclear” meant no description in the text with which to assess bias. “Low” meant that a detailed description of correct random methods, the appropriate blinding without being violated through implementation.
2.4. Statistical analysis
First, the chi-squared test was used to evaluate heterogeneity among studies, and I2 was used to show the magnitude of this heterogeneity. Results of P ≥ .1 and I2 ≤ 50% suggested a lack of significant heterogeneity; in such cases, the Mantel-Haenszel fixed-effects model was used for meta-analysis. For cases with P < .1 and I2 > 50%, we explored sources of heterogeneity via subgroup analysis and meta-regression. When no clinical heterogeneity was indicated, the Mantel-Haenszel random-effects model was used to perform the meta-analysis.[27] Moreover, the evaluation of the inconsistency between direct and indirect comparisons was unnecessary because a loop connecting the 3 arms did not exist in our study. Second, all calculations and graphs were made using STATA 12.0 software (Stata Corporation, College Station, TX), and Markov Chain Monte Carlo (MCMC) methods were performed using Win-BUGS 1.4.3 software (MRC Biostatistics Unit, Cambridge, UK). The network graph presented indirect comparative relationship between different interventions by STATA software.[28] The data were analyzed by the Win-BUGS software, the posterior probability was deduced from the prior probabilities, and conducted the Bayesian inference under the assumption that the MCMC had reached a stable convergence state.[29] Setting the number of iterations was 200,000, the first 10,000 for the annealing algorithm in order to eliminate the influence of initial value when running the Win-BUGS program. Third, dichotomous data was presented as the odds ratios (OR) and 95% confidence intervals (CI), and OR value used the median. Finally, we applied surface under the cumulative ranking probabilities (SUCRA) values to rank the examined treatments, with SUCRA values of 100% and 0% assigned to the best and worst treatments, respectively.[30,31] Besides, a comparison-adjusted funnel plot was used to test for the publication bias. Furthermore, we used clustering methods and 2-dimensional plots to produce clusters of treatments to account for the incidence of ADRs.
3. Results
3.1. Literature search and the characteristics of included RCTs
A total of 278 articles were retrieved via a primary search of the aforementioned literature databases. After reading titles and abstracts to remove the irrelevant articles and reading the full texts to remove those that did not meet the inclusion criteria, a total of 22 RCTs that evaluated CHIs combined with the chemotherapy for the treatment of pancreatic cancer were identified. In total, 9 types of CHIs were identified, including Compound kushen, Kanglaite, Kangai, Shenqifuzheng, Huanchansu, Aidi, Javanica oil emulsion, Disodium cantharidinate and vitamin B6, Astragalus polysaccharide injections. 1 RCT was published in English, and the other trials were published in Chinese (Fig. 1).
Figure 1.
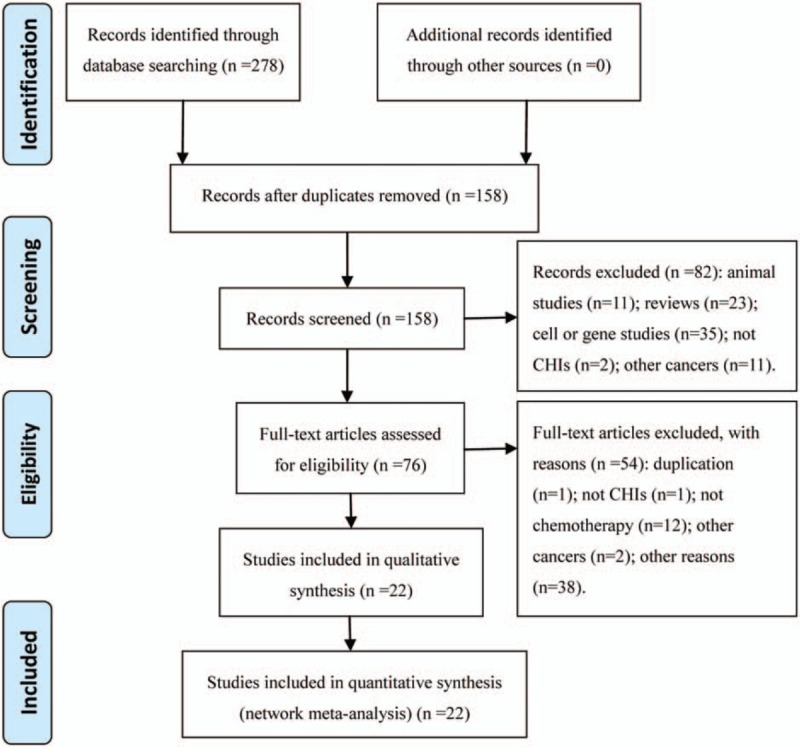
Flow chart of the search for eligible studies.
The 22 RCTs included 9 types of CHIs and 1329 patients, among which 675 patients were in the CHIs groups and 654 were in the chemotherapy groups.[32–53] All of the included RCTs reported patient numbers and ages, whereas 21 (95.45%), 10 (45.45%), 9 (40.91%) and 11 (50.00%) trials separately described the patients’ gender, tumor staging of pancreatic cancer, expected survival time and KPSs before treatment, respectively. More details regarding the individual trials are presented in Table 1. And Fig. 2 depicted a network graph of leucopenia for with 7 types of CHIs combined with chemotherapy and chemotherapy alone for pancreatic cancer.
Table 1.
The basic characteristics of the included studies.
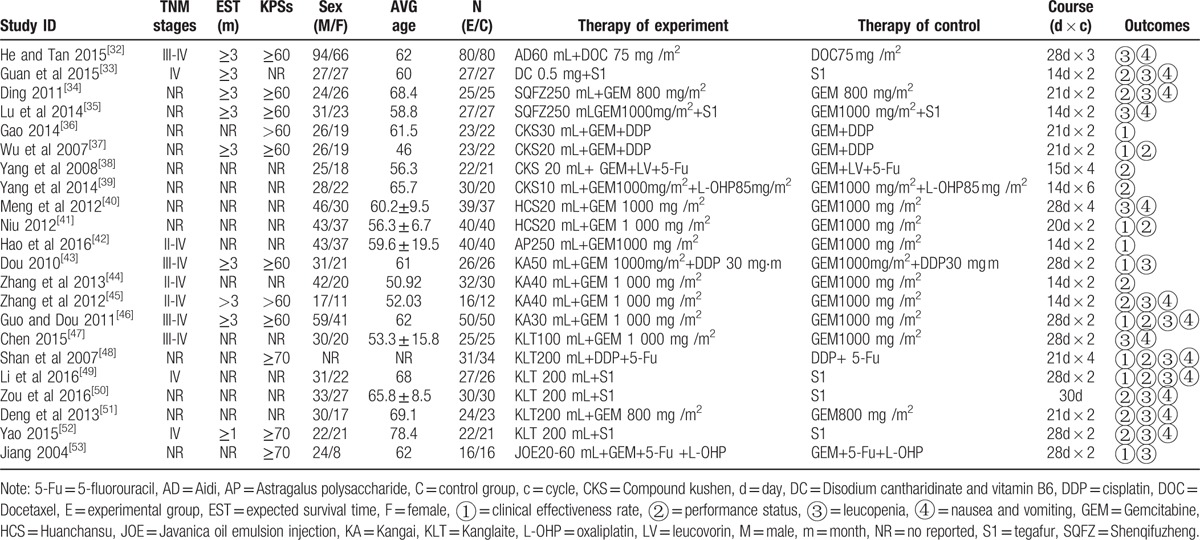
Figure 2.
Network graph for leucopenia. Note: Node sizes indicate total sample sizes for treatments. Line thicknesses correspond to the number of trials used for comparisons. AD = Aidi, C = chemotherapy, DC = disodium cantharidinate and vitamin B6, HCS = Huanchansu, JOE = Javanica oil emulsion injection, KA = Kangai, KLT = Kanglaite, SQFZ = Shenqifuzheng.
3.2. Quality assessment
Although all trials mentioned randomization, only 4 RCTs (18.18%) used a random number table, 1 RCT (4.55%) used an envelope method for randomization, 1 RCT (4.55%) used the lot drawing method, and 1 RCT (4.55%) adopted the method of hospitalized time difference. Only 1 RCT (4.55%) referred to the method of blinding. Regarding allocation concealment, the included RCTs did not mention it. Except for 1 RCT had selective outcome reporting, the other 21 RCTs (95.45%) did not select outcome reporting or have incomplete outcome data. And the included RCTs did not provide information about other bias (Fig. 3). In addition, the included RCTs described the inclusion and exclusion criteria but did not mention the sample size estimation and funding. Follow-up information for 4 RCTs (18.18%) was available. As for ADRs, 15 RCTs (68.18%) evaluated the ADRs which caused by chemotherapeutic drugs. And 6 RCTs (27.27%) described the details about medical ethics.
Figure 3.
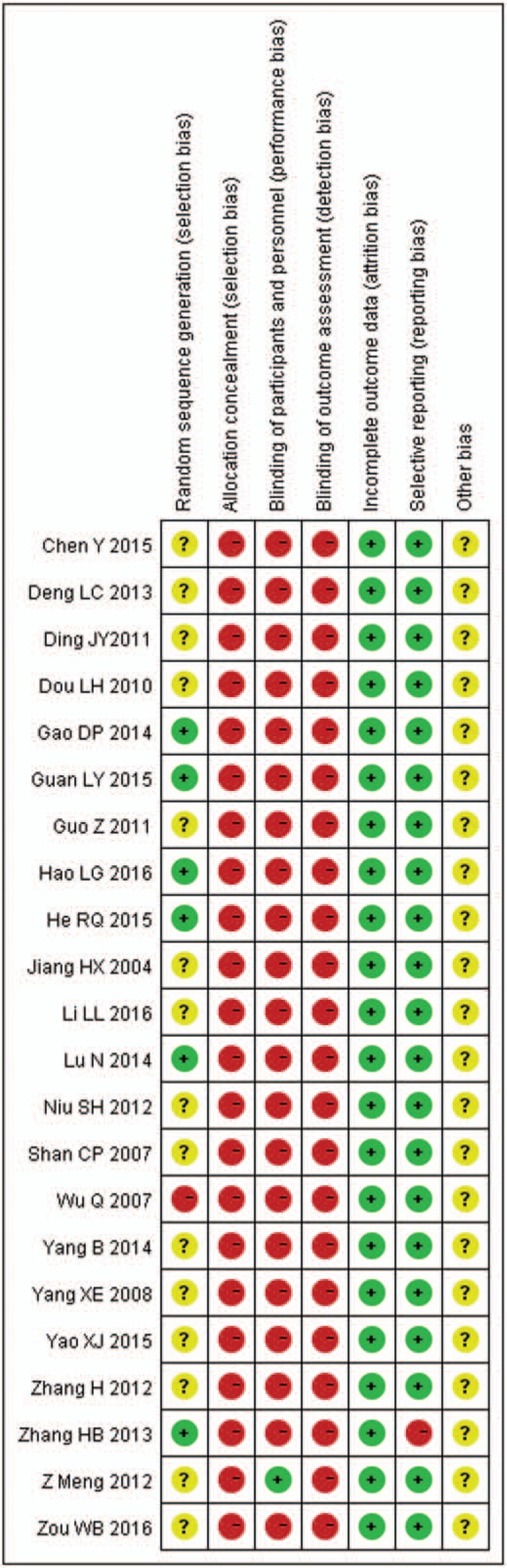
Risk of bias graph.
3.3. Outcomes
3.3.1. The clinical effectiveness rate
A total of 9 RCTs with 6 types CHIs reported the clinical effectiveness rate. Indirect comparisons demonstrated that the experimental groups that on the basis of chemotherapy also received Compound kushen, Huanchansu, Astragalus polysaccharide, Kangai, Kanglaite, Javanica oil emulsion injections could not experience the better clinical effectiveness rates than the control group that received the chemotherapy alone; and there were no statistically significant between-group differences. And among the CHIs groups, there was no statistically significant between-group difference. Based on the calculated probabilities for clinical effectiveness rate, the examined CHIs were ranked as follows: Javanica oil emulsion > Astragalus polysaccharide > Huachansu > Kangai > Compound kushen > Kanglaite. The results of indirect comparisons of the clinical effectiveness rate of each CHIs were shown in Table 2, and their calculated probabilities were shown in Fig. 4.
Table 2.
Results of the network meta-analysis of the clinical effectiveness rate (upper right quarter) and performance status (lower left quarter).
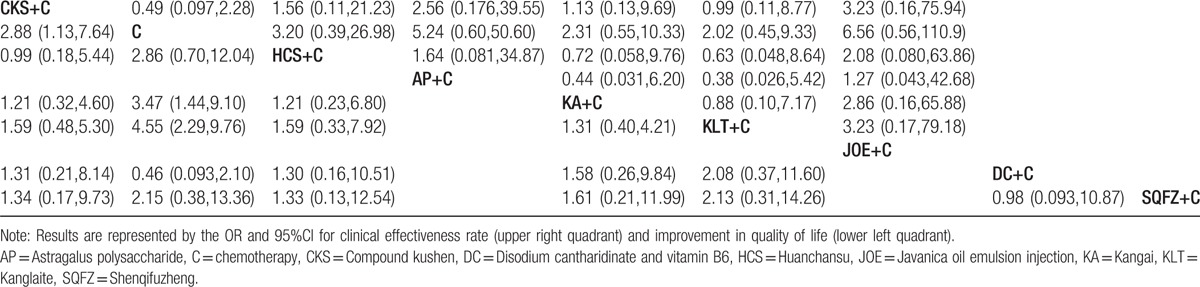
Figure 4.
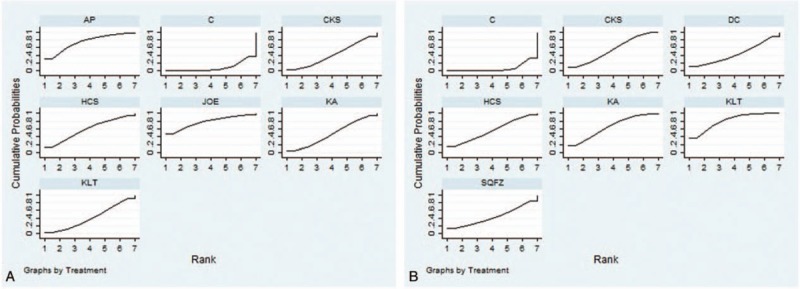
Rank of the cumulative probabilities of the clinical effectiveness rate and performance status. Note: A = the clinical effectiveness rate, AP = Astragalus polysaccharide, B = performance status, C = chemotherapy, CKS = Compound kushen, DC = disodium cantharidinate and vitamin B6, HCS = Huanchansu, JOE = Javanica oil emulsion, KA = Kangai, KLT = Kanglaite, SQFZ = Shenqifuzheng injection.
3.3.2. Performance status
A total of 14 RCTs with 6 types of CHIs reported the improvement in performance status. Indirect comparisons demonstrated that the experimental groups that received Compound kushen + chemotherapy, Kangai + chemotherapy, and Kanglaite + chemotherapy experienced superior improvement in performance status relative to that of the control group which only received the chemotherapy; these between-group differences were statistically significant, with ORs and 95% CIs of 62.88 (1.13,7.64), 3.47 (1.44,9.1), and 4.55 (2.29,9.76), respectively (Table 2). However, among the CHIs groups, there were no statistically significant between-group differences. Based on the calculated probabilities for improvement in performance status, the CHIs were ranked as follows: Kanglaite > Kangai > Compound kushen > Huachansu > Disodium cantharidinate and vitamin B6 > Shenqifuzheng. The results of indirect comparisons of the improvement in performance status were shown in Table 2, and their calculated probabilities were shown in Fig. 4.
3.3.3. ADRs
3.3.3.1. Leukopenia
A total of 14 RCTs with 7 types of CHIs reported the leukopenia. Indirect comparisons demonstrated that the experimental groups that received Aidi + chemotherapy could relieve leukopenia than the control group which only received the chemotherapy; these between-group differences were statistically significant, with ORs and 95% CIs of 5.34 (1.7,17.05) (Table 3). And among the CHIs groups, there was no statistically significant between-group difference. Based on the calculated probabilities for leukopenia, the CHIs were ranked as follows: Aidi > Javanica oil emulsion > Disodium cantharidinate and vitamin B6 > Kangai > Shenqifuzheng > Kanglaite > Huachansu. The results of indirect comparisons of leukopenia were shown in Table 3.
Table 3.
Results of the network meta-analysis of the leukopenia (upper right quarter), nausea, and vomiting (lower left quarter).

3.3.3.2. Nausea and vomiting
13 RCTs with 6 types of CHIs reported the nausea and vomiting. Indirect comparisons indicated that the experimental groups that on the basis of chemotherapy also received Aidi, Disodium cantharidinate and vitamin B6, Shenqifuzheng, Huachansu, Kanglaite, and Kangai could not relieve the nausea and vomiting than the control group that received the chemotherapy alone; these between-group differences were not statistically significant (Table 3). And among the CHIs groups, there was no statistically significant between-group difference. Based on the calculated probabilities for nausea and vomiting, the CHIs were ranked as follows: Shenqifuzheng > Aidi > Kangai > Disodium cantharidinate and vitamin B6 > Kanglaite > Huachansu. The results of indirect comparisons of nausea and vomiting were shown in Table 3.
3.3.4. Publication bias
A funnel plot was used to measure the publication bias. The funnel plot of the improvement in performance status analysis showed potential publication bias of the included RCTs (Fig. 5).
Figure 5.
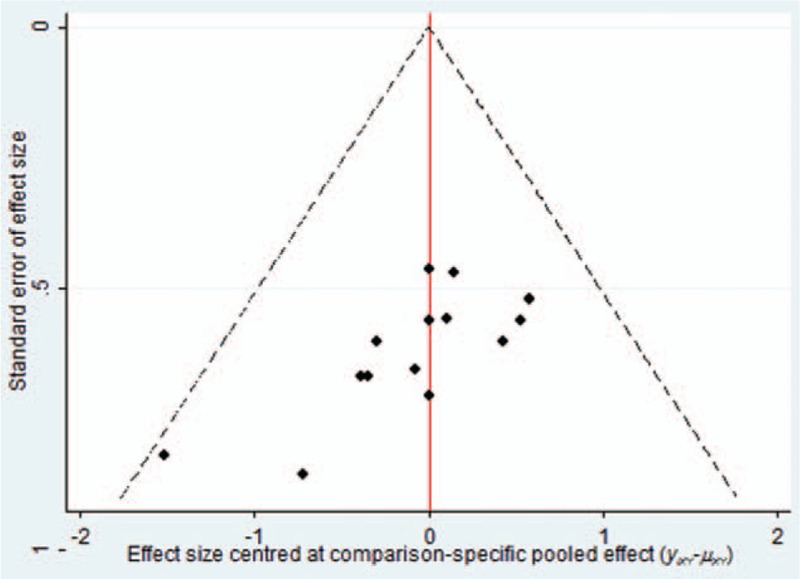
Funnel plot of performance status.
3.3.5. Cluster analysis
A cluster analysis was conducted for 6 types of CHIs that reported both nausea and vomiting, and leukopenia. The plot was based on cluster analysis of SUCRA values, and each plot shows SUCRA values for ADRs. Each color represents a group of treatments that belong to the same cluster. Treatments located in the upper right corner were superior to other CHIs for relieve ADRs. The results of the cluster analysis demonstrated that the chemotherapy alone and Huachansu injection combined with the chemotherapy were inferior to relieve ADRs than the other CHIs plus chemotherapy for patients with pancreatic cancer. Aidi, Disodium cantharidinate and vitamin B6 were the most beneficial CHIs for alleviating leukopenia in combination with the chemotherapy. Shenqifuzheng was associated with having a good effect of relieving nausea and vomiting (Fig. 6).
Figure 6.
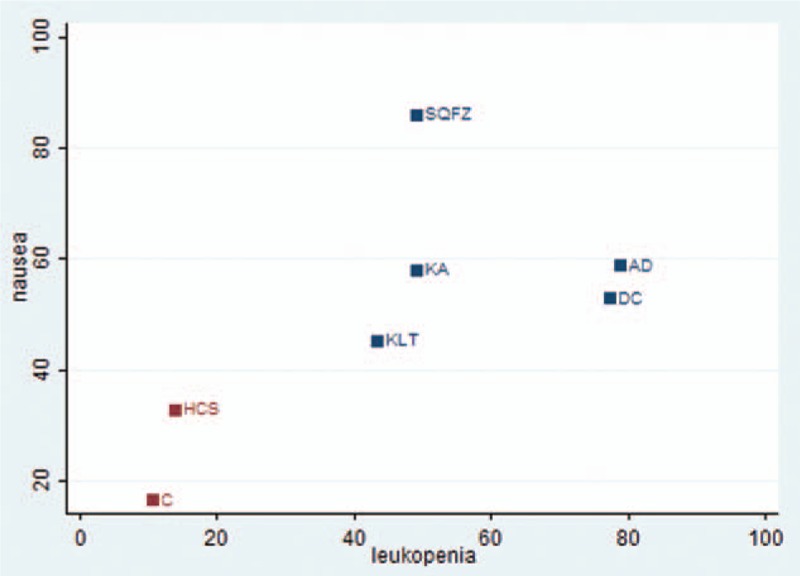
Cluster analysis plot of ADRs. Note: AD = Aidi, C = chemotherapy, DC = disodium cantharidinate and vitamin B6, HCS = Huanchansu, KA = Kangai, KLT = Kanglaite injection, SQFZ = Shenqifuzheng, X axis = leukopenia, Y-axis = nausea and vomiting.
4. Discussion
Our study evaluated the clinical effect, improvement in performance status, and ADRs after the application of CHIs combined with the chemotherapy for the treatment of pancreatic cancer by conducting a network meta-analysis. After analysis of the included RCTs, our study indicated that compared with the chemotherapy alone, Compound Kushen, Kangai or Kanglaite injection plus chemotherapy yielded significantly higher probability of improving the performance status. Aidi injection combined with chemotherapy was more effective in relieve leucopenia than using chemotherapy single. And these between-group differences were statistically significant. However, CHIs combined with chemotherapy could not achieve a better effect in the total clinical effect, nausea and vomiting. The cluster analysis for ADRs indicated that the chemotherapy alone and Huachansu injection combined with the chemotherapy were inferior to relieve ADRs than the other CHIs plus chemotherapy for patients with pancreatic cancer.
As one kind of malignant tumors in the digestive tract, pancreatic cancer has the features of occult clinical manifestation, rapid development, and poor prognosis. And at present, chemotherapy is still one of the effective treatments for pancreatic cancer.[54] GEM has been in the first-line treatment for pancreatic cancer over 10 years,[55] recently, the chemotherapy regime of GEM combined with other chemotherapeutic drugs such as 5-FU, L-OHP has been applied in the clinical studies.[56,57] However, some studies found that there are 2 major obstacles in the chemotherapy treatment. One is that the chemotherapeutic drugs could lose their potency over time due to the development of multidrug resistance. The other is that chemotherapy would produce serious side effects during medical practice, and the side effects might cause systemic multi-organ damages including hematological system, circulatory system, and even nervous system.[54,58] TCM may provide solutions to the above problems because of its own unique advantages: TCM has characterized by overall regulation, syndrome differentiation treatment, specimen and centralizer. And to achieve therapeutic effects to cancer, it can balance of yin and yang, promoting the body resistance and eliminate pathogenic factors by multiaspect, multilink, multitarget.[59] The antitumor effectiveness of TCM injections, such as Javanica oil emulsion, Huachansu, and Aidi mainly involved shrinking the tumor along with amelioration of symptoms, thereby improving the performance status.[60,61] Relevant studies has proved that Kanglaite injection can inhibits proliferation and induces apoptotic the xenografts of human pancreatic cancer, the pharmacological mechanism of its main components, coixan were anti-angiogenesis of tumors, promoting the apoptosis of cancer cells, and decreasing the enzyme activity.[62] Aidi injection was extracted from ginseng, Astragalus, Radix Acanthopanacis Semticosi, Chinese blister beetle; it can be used to treat cancer due to its heat-clearing and detoxifying effects.[63,64] Correlative studies have reported that Kangai injection was made from ginseng, Astragalus, Sophora flavescens, and its functions were replenishing qi and strengthening the body resistance owing to its active components, namely Astragalussaponins, ginsenoside, and matrine.[65,66] Astragalus polysaccharide and ginsenoside Rg3 can inhibit tumor cell proliferation, regulate immune function and induce tumor cell apoptosis,[67–69] and matrine can improve the treatment effects of advanced primary tumors and reduce the ADRs of interventional chemotherapy.[70,71] Compound Kushen injection was composed of Rhizoma Heterosmilacis Japonicae and Sophora flavescens, and it had the effects of heat-clearing and damp-inhibiting, blood-cooling and toxin-relieving, stagnation-eliminating and pain-relieving.[72] Moreover, validated modern pharmacological findings have shown that the anti-cancer components, matrine and oxymatrine can effectively reduce, stabilize, or even inhibit the growth of tumor, and improve the clinical symptoms of pain, fever, and fatigue.[73–74]
The advantages of this study were shown in the following aspects: first, this is the first network meta-analysis to compare the effectiveness and safety of CHIs in patients with pancreatic cancer. Literature searches were conducted about 22 types of CHIs which have been used for cancer treatment at the present, and the inclusion and exclusion criteria were established strictly. Second, the retrieval of this study was relatively comprehensive. On the one hand, apart from searching the database of domestic and foreign, we also search RCTs at related academic organization websites. On the other hand, the search words were divided into 3 parts: pancreatic cancer, CHIs and RCTs, the search strategy used a combination of subject words and random words. Third, the common interventions were chemotherapy of included RCTs, the criterion of the rapeutical effect met the WHO for solid tumors. Finally, this study not only analyzed the clinical effectiveness rate and the improvement of performance status, but also focused on the ADRs.
5. Limitation
Our study had several limitations. First, survival time was an important outcome for evaluating the curative effect of cancer, whereas in the included RCTs, only 4 trails reported the information of survival time or follow-up. Second, this study was limited by the quantity and quality of the included RCTs, the majority of RCTs included in the analysis exhibited a high risk of bias, largely due to inadequate allocation concealment and blinding. No direct head-to-head comparison was conducted between different CHIs. And the quantity of RCTs regarding CHIs for pancreatic cancer was not large. Third, all of the included RCTs were performed in patients of Asian descent; therefore, it is unclear whether the conclusions of our study apply to other populations. Despite the above limitations, our network meta-analysis provides a complete evaluation of the clinical effect, performance status, and ADRs of different CHIs for pancreatic cancer patients. However, large-sample and multicenter, head-to-head RCTs are needed to confirm these conclusions.
6. Conclusion
The current evidence shows that using CHIs on the basis of the chemotherapy could be beneficial for patients with pancreatic cancer in improving performance status and reducing the ADRs.
Addditional Information: S1 Text. Search Strategy.(DOC)
Supplementary Material
Footnotes
Abbreviations: 5-Fu = 5-fluorouracil, ADRs = adverse reactions, CHIs = Chinese herbal injections, CI = confidence interval, CNKI = the China National Knowledge Infrastructure Database, CR = complete response, DDP = cisplatin, DOC = docetaxel, GEM = gemcitabine, KPS = Karnofsky performance score, L-OHP = oxaliplatin, LV = leucovorin, OR = odds ratios, PR = partial response, RCTs = randomized controlled trials, S1 = tegafur, SinoMed = the Chinese Biomedical Literature Database, SUCRA = surface under the cumulative ranking probabilities, TCM = traditional Chinese medicine, VIP = the Chinese Scientific Journals Full-text Database, WHO = World Health Organization.
Authorship: Conception and design of the experiments: DZ, JW. Performance of the experiments: DZ, SL. Analysis of study data: DZ, JW. Contribution of reagents/materials/analysis tools: DZ, SL. Writing of the paper: DZ, JW. Quality control for the study: JW, BZ.
Funding: The National Natural Science Foundation of China (No. 81473547; 81673829).
Ethics approval: The ethical approval was not necessary in current meta-analysis because our meta-analysis just gathered the RCTs from literature search, this procedure was without deal with any patients’ personal data and harm to any patient.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Chen WQ, Wang QS, Zhang SW, et al. An analysis of incidence and mortality of pancreas cancer in China, 2003–2007. China Cancer 2012;21:248–53. [Google Scholar]
- [2].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burdenof cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [3].Wang LW, Chen DH, Li Q, et al. Pancreatic cancer diagnosis and treatment of Chinese experts consensus (2014 edition). Chin Clin Oncol 2014;19:358–70. [Google Scholar]
- [4].Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69–90. [DOI] [PubMed] [Google Scholar]
- [5].Witkowski ER, Smith JK, Tseng JF. Outcomes following resection of pancreatic cancer. J Surg Oncol 2013;107:97–103. [DOI] [PubMed] [Google Scholar]
- [6].Shi J, Wu C, Liu S, et al. Meta analyses of risk factors for pancreatic cancer in China. Chin J Pancreatol 2004;4:154–8. [Google Scholar]
- [7].Genkinger JM, Spiegelman D, Anderson KE, et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev 2009;18:765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Perrin MC, Terry MB, Kleinhaus K, et al. Gestational diabetes as a risk factor for pancreatic cancer: a prospective cohort study. BMC Med 2007;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Li GL, Chen WQ, Wang QS, et al. Analysis of the association between pancreas cancer and diabetes. J Cancer Comtral Treat 2010;23:132–6. [Google Scholar]
- [10].Larsson SC, Giovannucci E, Wolk A. Methionine and vitamin B6 intake and risk of pancreatic cancer: a prospective study of Swedish women and men. Gastroenterology 2007;132:113–8. [DOI] [PubMed] [Google Scholar]
- [11].Zhang J, Dhakal IB, Gross MD, et al. Physical activity, diet, and pancreatic cancer: a population-based, case-control study in Minnesota. Nutr Cancer 2009;61:457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fan ZZ, Liang F, Li Q, et al. Analysis of traditional Chinese medicine diagnosis and treatment status for treating advanced pancreatic cancer. Liaoning J Tradit Chin Med 2008;35:679–81. [Google Scholar]
- [13].Cao HT, Li FJ, He YM, et al. Current status of TCM treatment of pancreatic carcinoma. Tradit Chin Med Res 2005;18:49–53. [Google Scholar]
- [14].Huo SJ, Wang YC, Cao GZ, et al. The anticancer effect of traditional Chinese medicine injection in the chemotherapy of malignant tumor. Occup Health 2008;24:91. [Google Scholar]
- [15].Jiang S, Zhang M, Li P. Discussion on the application of traditional Chinese medicine injection in tumor. Chin Med Mod Distance Educ China 2014;12:17–9. [Google Scholar]
- [16].Wang W, You RL, Qin WJ, et al. Anti-tumor activities of active ingredients in Compound Kushen Injection Acta Pharmacol Sin 2015;6:676–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li YS. New progress of Kanglaite injection in the treatment of advanced pancreatic cancer. Chin J Clin Oncol 2012;39:1158–60. [Google Scholar]
- [18].Zhang YR, Dong Q. Study of Kangai injection in the treatment of malignant tumors of digestive system. Chin J Surg Integrated Tradit Western Med 2014;20:97–100. [Google Scholar]
- [19].Zhen J, Liang Z. Application in anti-head and neck carcinoma of matrine compound injections. Fudan Univ J Med Sci 2016;43:236–40. [Google Scholar]
- [20].Bhatnagar N, Lakshmi PV, Jeyashree K. Multiple treatment and indirect treatment comparisons: an overview of network meta-analysis. Perspect Clin Res 2014;5:154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med 2013;11:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med 2002;21:2313–24. [DOI] [PubMed] [Google Scholar]
- [23].Migliore A, Broccoli S, Massafra U, et al. Mixed-treatment comparison of anabolic (teriparatide and PTH 1-84) therapies in women with severe osteoporosis. Curr Med Res Opin 2012;28:467–73. [DOI] [PubMed] [Google Scholar]
- [24].Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer 1981;1:207–14. [DOI] [PubMed] [Google Scholar]
- [25].People's Republic of China Department of Health Management. Guidelines for the Diagnosis and Treatment of Cancer. 1991;Beijing: Pecking Union Medical College Press, 11–15. [Google Scholar]
- [26].Higgins JPT, Green S, editors: Assessment of study quality. Cochrane Handbook for Systematic Reviews of Interventions 5, 1.0 [updated March 2011]; Section 6. In The Cochrane Library Issue 4 Chichester, UK: John Wiley & Sons, Ltd; 2011. [Google Scholar]
- [27].Salanti G, Higgins JPT, Ades AE, et al. Evaluation of networks of randomized trials. Slat Methods Med Res 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- [28].Tian JH, Li L, Yang KH. The realization of network meta analysis of frequency statistics method in STATA software. Chin J Evid Based Pediatr 2014;9:472–4. [Google Scholar]
- [29].Lunn DJ, Thomas A, Best N, et al. WinBUGS—a Bayesian modeling framework: concepts, structure, and extensibility. Statist Comput 2000;10:325–37. [Google Scholar]
- [30].Cope S, Jansen JP. Quantitative summaries of treatment effect estimates obtained with network meta-analysis of survival curves to inform decision-making. BMC Med Res Methodol 2013;13:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Salanti G, Dias S, Welton NJ, et al. Evaluating novel agent effects in multiple-treatments meta-regression. Stat Med 2010;29:2369–83. [DOI] [PubMed] [Google Scholar]
- [32].He RQ, Tan XM. Curative effect of ai’di injection combination with docetaxel in the treatment of middle-advanced pancreatic carcinoma. Chin J Clin Oncol Rehab 2015;22:1054–6. [Google Scholar]
- [33].Guan LY, Ma J, Yuan SF, et al. Disodium cantharidinate and vitamin B6 combined with tegafur, gimeracil and oteracil potassium for treating stage IV pancreatic cancer in 27 cases. China Pharm 2015;24:124–5. [Google Scholar]
- [34].Ding JY. Clinical observation of gemcitabine combined with Shenqi injection in the treatment of elderly patients with pancreatic cancer. Chin Primary Health Care 2011;25:106–7. [Google Scholar]
- [35].Lu N, Hang S, Jing D, et al. Clinical observation on 27 cases of advanced pancreatic cancer patients with Qi deficiency syndrome treated by Shenqifuzheng injection combined with chemotherapy. J Tradit Chin Med 2014;55:1038–40. [Google Scholar]
- [36].Gao DP. Clinical benefit and short term efficacy of compound matrine injection combined with GP regimen in the treatment of advanced pancreatic cancer. Shanxi J Tradit Chin Med 2014;30:20–1. [Google Scholar]
- [37].Wu Q, Xia ZQ, Yan JH, et al. Curative effect observation of compound matrine injection in the treatment of 45 cases of pancreatic cancer. Chin Remedies Clin 2007;7:784–5. [Google Scholar]
- [38].Yang XE, Liu T, Dong YH, et al. Clinical observation of the effect of Yanshu injection on quality of life of patients with advanced pancreatic cancer. Shanxi J Tradit Chin Med 2008;24:18–9. [Google Scholar]
- [39].Yang B, Liang CW, Pan Y, et al. Clinical observation of Yanshu injection combined with chemotherapy of advanced pancreatic carcinoma. Liaoning J Tradit Chin Med 2014;41:1926–7. [Google Scholar]
- [40].Meng Z, Garrett CR, Shen Y, et al. Prospective randomised evaluation of traditional Chinese medicine combined with chemotherapy: a randomised phase II study of wild toad extract plus gemcitabine in patients with advanced pancreatic adenocarcinomas. Brit J Cancer 2012;107:411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Niu SH. Observation of Huachansu injection combined with gemcitabine in treatment of advanced pancreatic cancer. Chin J Clin Rational Drug Use 2014;7:61–2. [Google Scholar]
- [42].Hao LG, Cui HS, Sui XF, et al. Application effect of gemcitabine combined with Astragalus polysaccharide in the treatment of pancreatic cancer. China Health Care Nutr 2016;26:36. [Google Scholar]
- [43].Dou LH. Kang’ai injection combined with GP regimen in the treatment of locally advanced pancreatic cancer. Chin J Exp Tradit Med Formulae 2010;16:207–9. [Google Scholar]
- [44].Zhang HB, Shan LZ, Zhang J, et al. Influence of Kangai injection combined with chemotherapy on immune function in patients with pancreatic cancer. Shanxi J Tradit Chin Med 2013;29:23–4. [Google Scholar]
- [45].Zhang H, Shan LZ, Zhang J, et al. Clinical observation on pancreatic cancer's cancer-related fatigue with Kangai injection combined chemotherapy. Chinese J Surg Integrated Tradit Western Med 2012;5:438–40. [Google Scholar]
- [46].Guo Z, Dou LH. Clinical observation of Kang’ ai injection combined with gemcitabine in the treatment of advanced pancreatic carcinoma. China Pharmacy 2011;22:699–701. [Google Scholar]
- [47].Chen Y. Safety and effectiveness of Kanglaite injection combined with gemcitabine on advanced pancreatic cancer. Clin J Chin Med 2015;16:24–5, 28. [Google Scholar]
- [48].Shan CP, Yang XQ, Cui W, et al. Clinical observation of Kanglaite injection combined with small dose chemotherapy in the treatment of advanced pancreatic cancer. Tradit Chin Drug Res Clin Pharmacol 2007;18:72–3. [Google Scholar]
- [49].Li LL, Chen J, Zhang DR, et al. Efficacy of tegafur gimeracil oteracil potassium capsule combined with Kanglaite injection for advanced pancreatic carcinoma. China Mod Doctor 2016;54:67–9. [Google Scholar]
- [50].Zou WB, Dong YY, Yan L, et al. Clinical observation on tegafur gimeracil oteracil potassium capsules combined with Kanglaite injection in palliative treatment of advanced pancreatic carcinoma. China Health Standard Management 2016;7:136–8. [Google Scholar]
- [51].Deng LC, Shen WS, Zhang Y, et al. Clinical observation on coicis oil injection combined with gemcitabine in old patients with advanced pancreatic cancer. Mod J Integrated Tradit Chin Western Med 2013;22:2281–3. [Google Scholar]
- [52].Yao XJ. Clinical observation of S-1 combined with Kanglaite injection in the treatment of elderly patients with advanced pancreatic cancer. J Basic Clin Oncol 2015;28:350–2. [Google Scholar]
- [53].Jiang HX. Curative effect observation of javanica oil emulsion combined with chemotherapy in the treatment of advanced pancreatic cancer. Chin J Hemorheol 2004;14:596–7. [Google Scholar]
- [54].Pan B, Guo JC, Liao Q, et al. Mechanism of gemcitabine resistance in pancreatic cancer chemotherapy. Chin J Bases Clin General Surg 2005;12:530–2. [Google Scholar]
- [55].Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients withadvanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13. [DOI] [PubMed] [Google Scholar]
- [56].Berlin JD, Catalano P, Thomas JP, et al. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol 2002;20:3270–5. [DOI] [PubMed] [Google Scholar]
- [57].Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol 2005;23:3509–16. [DOI] [PubMed] [Google Scholar]
- [58].Gong JF, Zhang XD, Li J, et al. Efficacy of gemcitabine-based chemotherapy on advanced pancreatic cancer. Chin J Cancer 2007;17:890–4. [PubMed] [Google Scholar]
- [59].Sun JP. Research status of traditional Chinese medicine in the treatment of pancreatic cancer. Chin J Clin Oncol Rehab 2011;18:571–3. [Google Scholar]
- [60].Bai XJ. Analysis of anti-tumor varieties of traditional Chinese medicine injections. Pharmacol Clin Chin Materia Medica 2009;25:72–3. [Google Scholar]
- [61].Liu Y, Zhang W, Wang X-J, et al. Antitumor effect of Kanglaite® injection in human pancreatic cancer xenografts. BMC Complementary Alternative Med 2014;14:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wu Y, Yuan YF. Progress of Coix studies on chemical constituents and pharmacological activity. West China J Pharm Sci 2010;25:111–3. [Google Scholar]
- [63].Liu XL, Chen JX, Liu Y, et al. Study on norcantharidin induced apoptosis of HL60 cells. J Beijing Univ Tradit Chin Med 2002;23:35–7. [Google Scholar]
- [64].Zhang H, Zhou QM, Lu YY, et al. Aidi injection alters the expression profiles of microRNAs in human breast cancer cells. J Tradit Chin Med 2011;31:10–6. [DOI] [PubMed] [Google Scholar]
- [65].Song WX, Chen M, Cheng MJ, et al. Progress on clinical application of Kangai injection. Mod Hospital 2014;14:47–52. [Google Scholar]
- [66].Sun CH, Li YX, Zhang Y. Progress in clinical research of Kangai injection and adverse reactions. Clin Misdiagnosis Misther 2013;26:100–2. [Google Scholar]
- [67].Huang HF, Qian JY, Xie SR, et al. Effects of astragalus polysaccharide on apoptosis and cell cycle of human gastric carcinoma cell line MKN45. J Clin Med Pract 2010;10:17–20. [Google Scholar]
- [68].Zhang LS, Zhang XH, Li WF. Research progress of ginsenoside Rg3. Herald Med 2006;25:687–9. [Google Scholar]
- [69].Gao WQ, Jia L, Zhao YQ. Advances in the study on the anticancer effect and mechanism of Panax ginseng. Drug Eval Res 2011;34:53–8. [Google Scholar]
- [70].Liu QR, Li YH. Effect of Marin solution combined with transcatheter arterial embolization on 75 cases of primary hepatocelluar carcinoma. China J Mod Med 2004;14:33–5. [Google Scholar]
- [71].Ma JQ. To observe the curative effect of 24 cases of Kangai injection in the treatment of radiation-induced lung injury. N J Tradit Chin Med 2006;8:40–1. [Google Scholar]
- [72].Ma Y, Zhang QZ, Wang ZM. Research progress of compound matrine injection. Chinese J Exp Tradit Med Formulae 2012;18:342–4. [Google Scholar]
- [73].Li CM. Clinical application of compound matrine injection. J Dis Monit Control 2010;14:330. [Google Scholar]
- [74].Xu JX. Research progress of Yanshu injection in the treatment of tumor. Chin J Inform Tradit Chin Med 2006;13:94. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


