Abstract
Hemodialysis patients carry a large burden of cardiovascular disease; most onerous is the high risk of sudden cardiac death. Defining sudden cardiac death among hemodialysis patients as well as understanding its pathogenesis is challenging, but inferences from existing literature reveal differences between sudden cardiac death among hemodialysis patients and the general population. Vascular calcifications and left ventricular hypertrophy may play a role in the pathophysiology of sudden cardiac death whereas traditional cardiovascular risk factors seem to have a more muted effect. Arrhythmic triggers also differ in this group as compared to the general population, with some arising uniquely from the hemodialysis procedure. Combined, these factors may alter the types of terminal arrhythmias that lead to sudden cardiac death among hemodialysis patients, having important implications for prevention strategies. This review highlights current knowledge on the epidemiology, pathophysiology, and risk factors for sudden cardiac death among hemodialysis patients. We then examine strategies for prevention including use of specific cardiac medications and device-based therapies such as implantable defibrillators. We also discuss dialysis-specific prevention strategies including minimizing exposure to low potassium and calcium dialysate, extending dialysis treatment times or adding sessions to avoid rapid ultrafiltration, and lowering dialysate temperature.
Keywords: sudden cardiac death, sudden death, arrhythmia, risk factors, pathophysiology, pathogenesis, hemodialysis, dialysis, end-stage renal disease, end-stage kidney disease, prevention, prevention strategies
Case presentation
A 26-year-old African American man was brought to the hospital following a sudden cardiac arrest during hemodialysis at his outpatient dialysis center. He was being dialyzed on a 2 mEq/L potassium bath and 2 mEq/L calcium bath with a dialysate temperature of 37°C and ultrafiltration goal of 3.9 liters. He was 1.5 hours into his 4 hour and 45 minute hemodialysis treatment when he suddenly became unresponsive. Cardiopulmonary resuscitation (CPR) was started while an automated external defibrillator (AED) was attached and subsequently delivered a shock for ventricular fibrillation.
The patient had a history of obesity-related glomerulopathy and was on hemodialysis for ten months prior to his cardiac arrest. His other medical problems included paroxysmal atrial fibrillation, left and right sided systolic dysfunction with left sided diastolic dysfunction, and aortic valve infective endocarditis requiring replacement five months prior to his cardiac arrest. He frequently presented with large interdialytic weight gain leading to large ultrafiltration goals and intradialytic hypotension.
This case offers the opportunity to explore several aspects of sudden cardiac arrest and sudden cardiac death (SCD) among dialysis patients. What are the epidemiology and pathophysiology of SCD in dialysis patients? What are the modifiable dialysis-specific risk factors? What primary and secondary SCD prevention strategies are effective in this population? This article will review the epidemic of sudden cardiac death in the hemodialysis population with a focus on prevention.
Definition of SCD
SCD is the largest contributor to mortality among hemodialysis (HD) patients. Cardiac arrest accounts for a quarter of hemodialysis patient deaths1 (Figure 1). International data from the Dialysis Outcomes and Practice Patterns registry show that SCD among hemodialysis patients is more common in the United States (33% of all deaths) than in other countries including Japan (23%), Australia/New Zealand (19%), and Canada (18%). It is unknown whether these findings are from different reporting schemes, dialysis practices, or baseline patient characteristics.2
Figure 1.
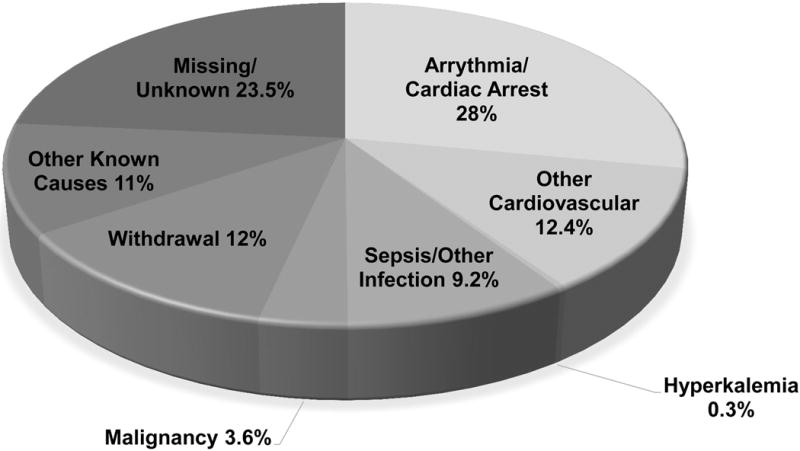
Cause of death among dialysis patients 2011–2013. Adapted from the United States Renal Data System, 2015 Annual Data Report, Figure 9.1b. Other cardiovascular causes include acute myocardial infarction, atherosclerotic heart disease, congestive heart failure, cerebrovascular accident, and other cardiac events.1
It is important to acknowledge that these SCD epidemiology data are derived from large population registries that lack a systemic adjudication process and may be prone to misclassification. There is no universally accepted and precise definition of SCD. First identified as a specific cause of death by Hinkle and Thaler,3 sudden cardiac death has been defined broadly as a natural, rapid, and unexpected cardiac death within an hour of symptom onset.4 Other definitions include death without an obvious non-cardiac cause in patients well within the last 24 hours.5 Paramount to the classification of death as sudden cardiac death is the ability to determine: 1) the clinical circumstances surrounding death, and 2) the timing of progression from symptoms to cardiac arrest. This poses several difficulties when applied to the hemodialysis population. First, many deaths are unwitnessed and limited information is available about the circumstances of death, making the exclusion of a non-cardiac cause challenging.6 Patients with end-stage renal disease (ESRD) may be susceptible to other etiologies of sudden, unexpected death such as cerebral hemorrhage, pulmonary or air embolism, or aortic dissection, which may be mistaken for SCD if there are no clinical or autopsy data available to confirm a primary cardiac etiology. The potential for misclassification of SCD was seen in an autopsy series of 93 Japanese dialysis patients who were apparent victims of SCD. Stroke was found as the most frequent etiology (25.8%), followed by cardiac disease (19.4%) and infections (17.2%).7 Second, the timing and unexpected nature of death can also be difficult to ascertain, since patients with ESRD are chronically ill with numerous co-morbidities and are frequently hospitalized. What qualifies a death as an “unexpected death” in this population can be subject to interpretation. Cardiac arrests in the setting of withdrawal from dialysis or after missing dialysis treatment have been included in SCD definitions in some studies, but consensus is that these circumstances should be excluded since cardiac arrest is not unexpected in these situations.2,8,9
Due to the difficulty in determining the circumstances and timing of sudden death, SCD definitions used in studies of the ESRD population have been variable, leading to wide variations in reported SCD rates. A recent systematic review evaluated 42 cohort studies and randomized controlled trials (RCTs) reporting on SCD rates in ESRD patients.10 Only 25 studies provided a specific definition for SCD, and among those, only 17 included any measure of time in their SCD definition. Reported SCD rates varied widely from 0.4 to 10.4% annually. Despite the troubling variability in SCD definitions across studies,11 clinical trials such as the Hemodialysis (HEMO) Study, Die Deutsche Diabetes Dialyse Studie (4D), and Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) Trial, where cause of death was carefully adjudicated, all reported a consistent proportion of 22–26% of all trial deaths attributed to SCD.12–14 This is similar to what has been consistently reported by large population registries such as the United States Renal Data System (USRDS), and two studies comparing causes of death reported by USRDS with adjudicated sources have shown reasonable sensitivity and specificity for cardiac causes of death.11,15 However, improved harmonization of SCD definitions across studies is needed in order to accurately track SCD rates and test interventions to reduce its incidence. Such a definition should exclude in-hospital and hospice patients as well as death following withdrawal of HD or after missing HD.
While ESRD patients receiving long-term dialysis are at the highest risk of SCD, it is important to note that patients with moderate kidney disease are also at elevated risk. The absolute number of individuals affected by SCD is much higher among CKD patients given the higher prevalence of CKD compared to ESRD in the general population (Figure 2). This highlights the opportunity for early SCD risk modification and the role of slowing CKD progression to reduce the impact of SCD overall.
Figure 2.
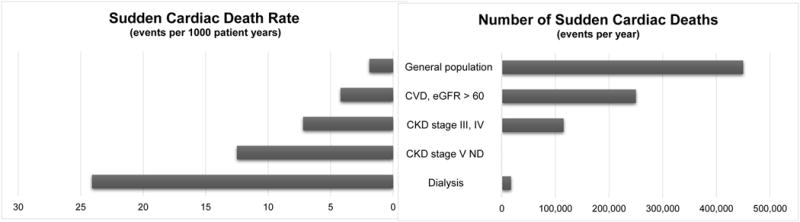
Rates of SCD in selected populations (left) and absolute numbers of affected individuals (right). CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; CKD, chronic kidney disease; ND, non-dialysis.16,23
Pathophysiology of SCD
The pathophysiology of SCD is thought to result from the combination of a vulnerable myocardium and an acute pro-arrhythmic trigger that leads to a terminal arrhythmia.16 In the general population, this manifests as ischemic cardiomyopathy with reduced left ventricular ejection fraction (LVEF) that is prone to disorganized cardiac conduction when subjected to acute ischemia from coronary plaque rupture. Ventricular fibrillation is the terminal arrhythmia in more than 80% of cases.16,17 It follows that therapies that prevent or slow the progression of coronary artery disease (CAD) as well as defibrillation have been proven the most beneficial in reducing SCD in the general population.18 The pathophysiology of SCD among hemodialysis patients, however, may be different in regards to coronary artery and heart structure pathology, arrhythmic triggers, and terminal arrhythmias.
Coronary Artery and Structural Heart Pathology
Differences in the underlying pathology of CAD in ESRD patients (characterized by arterial wall stiffening and calcification in the media layer, rather than lipid-laden intimal atheromas)19 may explain why traditional cardiovascular risk factors such as cholesterol levels,20 obesity,21 and blood pressure22 do not fully predict cardiovascular events and mortality in patients with kidney disease. An observational study of over 19,000 patients who underwent cardiac catheterization, 25% of whom had moderate to advanced CKD, found that severity of CAD lesions did not explain the heightened risk of SCD among CKD patients.23
In contrast to the general population, where ischemic cardiomyopathy with reduced LVEF is often seen in SCD, reduced ejection fraction is far less common among hemodialysis patients who suffer SCD.24 In a study of 80 hemodialysis patients who were victims of sudden death, only 46% had an LVEF of 50% or less and only 25% had an LVEF of 35% or less.9 Likewise, a cohort of over 1,200 consecutive incident hemodialysis patients who underwent echocardiography revealed that only 13% had a reduced LVEF.25 Another observational study also found that heart failure severity did not correlate with increased SCD risk in CKD patients.23 Thus, in contrast to the general population, low ejection fraction cannot fully account for the high rates of SCD in the hemodialysis population. Instead, diastolic dysfunction from left ventricular hypertrophy (LVH) is more often observed among hemodialysis patients who experience SCD. Two case series reported that LVH was seen in more than 70% of hemodialysis patients with sudden cardiac arrrest.9,26 In a 10-year observational study of HD patients, left ventricular mass index was the best predictor of SCD risk over time.27 Even in the absence of significant CAD, LVH with diffuse myocardial fibrosis has been demonstrated by MRI in patients on HD.28,29 Chronic hypertension, anemia, microvessel disease leading to capillary/myocyte mismatch,30 and repetitive myocardial injury brought on by hypoperfusion during dialysis may be to blame for this pattern of disease.31
Arrhythmic Triggers
It has been long observed that SCD occurs more frequently on hemodialysis days and especially on Mondays and Tuesdays following the long dialysis-free weekends for patients on a thrice-weekly hemodialysis schedule.9,24,32,33 In contrast, there was no ‘day-of the-week’ SCD pattern observed among peritoneal dialysis or HD patients undergoing treatment more than three times a week in an observational cohort of over 14,000 ESRD patients in Australia and New Zealand.’.34 Low calcium dialysate baths as well as both the excessive accumulation and aggressive removal of potassium and fluid may underlie this pattern of increased risk during the first dialysis day of the week.35–39 Finally, metabolic alkalosis from exposure to high bicarbonate dialysate has been associated with hypokalemia, hemodynamic instability, and QT prolongation.40–42
Genetic factors may play a role in hemodialysis patients’ susceptibility to arrhythmic triggers. A study examining SCD risk among related pairs of dialysis patients found a 70% increased risk of SCD if one relative suffered sudden cardiac death, compared to non-genetically related cohabitating dialysis pairs.43 Specific genetic loci underlying the increased SCD risk among genetically related hemodialysis patients are unknown, but common variants in loci encoding cardiac ion channels may be involved.44 Further investigations could identify novel SCD pathways and lead to better identification of those highest at risk for SCD.
Terminal Arrhythmias
Some data suggest that ventricular tachyarrhythmias are the most common terminal arrhythmia associated with SCD not only in the general population45 but also in the CKD population. A study of 111 Brazilian patients with moderate CKD who were monitored for 24 hours with a Holter monitor found that 35% of participants experienced a ventricular arrhythmia, predominantly ventricular extra-systoles. Left ventricular mass index was the strongest predictor of ventricular arrhythmias.46 Another group has shown that rats with CKD had lower induction thresholds for ventricular fibrillation with loss of repolarization reserve.47 In a retrospective study of 75 hemodialysis patients who were prescribed a wearable cardioverter defibrillator, 79% of the 84 sudden cardiac arrest events were recorded as ventricular tachycardia or ventricular fibrillation.48 Unlike most hemodialysis patients, however, most of these patients had significant systolic dysfunction.
Other data suggest that hemodialysis patients may experience a larger proportion of non-ventricular arrhythmias. A study of 400 patients who had a witnessed sudden cardiac arrest in outpatient dialysis clinics found that 15% of cardiac arrests with a documented arrhythmia were asystole.36 Another study found that non-ventricular arrhythmias including asystole and pulseless electrical activity were even more common at 33% of all sudden deaths in HD patients with a documented arrhythmia.49 Similarly, a retrospective study of 24 hemodialysis patients with cardiac arrest at an inpatient hemodialysis unit found that cardiac arrest from ventricular tachycardia and Ventricular fibrillation was only 31.6%. Bradycardia (26.3%), asystole (15.8%), and pulseless electrical activity (15.8%) accounted for the majority of arrhythmias (57.9%).50 In a study of 50 Australian HD patients with LVEF over 35% that were monitored with implantable loop recorders, bradycardia accounted for 65% of all significant arrhythmias detected. Of the eight sudden cardiac deaths, six had captured rhythm data that showed either bradycardia or asystole. Although not considered a significant arrhythmia in this study, paroxysmal atrial fibrillation was common and accounted for 62% of all documented arrhythmias. Only 0.8% of all arrhythmic events were related to ventricular tachycardia or ventricular fibrillation.33 Similar findings were observed in another cohort of HD patients that underwent brief Holter monitoring (86% of all events were supraventricular tachycardia while only 14% were ventricular arrhythmias).51
The lack of clear knowledge on the most common terminal arrhythmia among hemodialysis patients is concerning since non-ventricular arrhythmias will not respond to traditional resuscitative measures involving defibrillation.45 To help address this knowledge gap, the Monitoring in Dialysis study also used implantable loop recorders to provide information collected over six months on the type and frequency of arrhythmias.52 The final study reports are pending, but unpublished data on the first 66 patients enrolled in the study have suggested a large number of captured arrhythmic episodes predominantly due to atrial arrhythmias (57.4%) followed by bradycardia (15%), with most events occurring in the post-dialytic period. Ventricular arrhythmias comprised 9.1% of captured events.53 The WEarable cardioverter Defibrillator in HEmoDialysis (WED-HED) randomized trial will also provide arrhythmia data by assigning at least 650 hemodialysis patients to the wearable defibrillator arm and providing continuous monitoring data. Additionally, it will test device efficacy in preventing SCD (Clinicaltrials.gov, Registry number: NCT02481206).
Primary Prevention of SCD
The chances of surviving sudden cardiac arrest are poor, with studies of witnessed sudden cardiac arrest in dialysis clinics observing a long-term survival of only 8%.54,55 Thus, efforts towards primary prevention are likely to have the greatest public health impact. The first step in reducing SCD in the hemodialysis population is to identify risk factors that can be used for risk-stratification and targeted for risk-modification.
There are no large-scale studies specifically designed to develop and validate risk stratification scores for SCD. However, several inferences can be made from existing data. Since the first months following initiation of hemodialysis are a particularly high risk for SCD and risk accumulates with dialysis vintage, patients at both extremes should be considered a high risk population.8 Additionally, dialysis patients who are prone to large interdialytic weight gains, extremes of serum potassium, and those who fall out of the desired target ranges for mineral metabolism and nutrition also form the general profile of the high-risk patient.35,56 Victims of SCD are more likely to be diabetic and have a history of arrhythmias and pre-existing heart disease.8,57 Biomarker studies have established associations between SCD and inflammatory markers such as IL-6,58 C-reactive protein,58 and adiponectin59 as well as markers of nutrition including serum albumin58 and pre-dialysis serum creatinine.35
A secondary analysis of the HEMO study created a SCD prediction model that included age, serum creatinine, serum alkaline phosphatase, and a history of diabetes, peripheral vascular disease, and ischemic heart disease.60 While model discrimination (C-statistic 0.75) and calibration were good, this prediction model has not been validated in other populations and does not incorporate biomarkers or other risk factors identified by other studies to determine overall risk.61
Cardiovascular Medications
Certain classes of cardiovascular medications have been useful in reducing SCD risk in the general population. Beta-adrenergic blockers have been shown to reduce the risk of SCD after myocardial infarction.62 Among 114 hemodialysis patients with dilated cardiomyopathy, a randomized study of carvedilol showed a 24% reduction in mortality at two years and a trend towards reduction in SCD.63 More recently, a randomized study of 200 hemodialysis patients evaluated the efficacy of atenolol versus lisinopril to reduce LVH and found significantly fewer combined cardiovascular events and heart failure hospitalizations in the atenolol group.64 No difference in left ventricular mass was seen between the groups, but patients treated with atenolol had fewer arrhythmias and cardiac arrests, although the number of events was too small to establish statistical significance. In contrast, a secondary analysis of the HEMO study did not find an association between beta-blocker use and a decreased risk of SCD.65 Thus, based on the current state of evidence, it is premature to make recommendations regarding initiating beta blocker therapy in dialysis patients specifically to prevent SCD outside of the usual clinical indications such as systolic heart failure or coronary heart disease.
The evidence that cholesterol-lowering medications are beneficial in dialysis patients is not compelling. Both 4D (atorvastatin) and A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA) trials were adequately-powered studies that failed to establish a significant cardiovascular benefit from statins among hemodialysis patients.13,66 In the Study of Heart and Renal Protection (SHARP) Trial, the simvastatin/ezetimibe combination demonstrated an overall benefit in the primary outcome of major atherosclerotic events among patients with CKD (including two-thirds of patients with moderate CKD and one-third of patients on dialysis). However, this was based on a decreased need for coronary revascularization in the treated group, not all-cause mortality. As with prior trials, it also failed to detect a significant benefit of statin therapy among dialysis patients, although the study was not powered to detect a difference in this subgroup.67
The data regarding the benefit of renin-angiotensin-aldosterone system blockers on cardiovascular outcomes in dialysis patients is mixed. RCTs of fosinopril and olmesartan among hypertensive patients on hemodialysis both failed to demonstrate a reduction in cardiovascular events or all-cause mortality.68,69 Furthermore, a meta-analysis of RCTs of angiotensin converting enzyme inhibitors and angiotensin receptor blockers reinforced the paucity of evidence to support a cardiovascular or mortality benefit from these medications among hemodialysis patients.70 Yet, a significant reduction in left ventricular mass was observed, leaving open the possibility that these medications might reduce SCD in a larger, adequately powered trial.70 The Dialysis Outcomes Heart Failure Outcomes Study (DOHAS) examined the effect of spironolactone on cardiovascular and cerebrovascular events in 309 Japanese hemodialysis patients.71 Although the number of SCD events was too few to determine a treatment effect, patients randomized to spironolactone had a 60% reduced risk of the composite outcome of death or hospitalization from cardiovascular and cerebrovascular events.
A concluding observation on the use of cardiovascular medications to prevent SCD is that the medications used in the general population to reduce cardiovascular risk appear to have a muted effect in the dialysis population. Well-designed clinical trials are needed before such medications can be recommended for routine use to prevent SCD in the dialysis population.
Adjusting the Hemodialysis Prescription
The hemodialysis prescription offers several opportunities to reduce SCD risk. Avoidance of large electrolyte and volume shifts may be critical to reduce SCD risk among hemodialysis patients. Three large cohort observational studies have described associations between increased SCD risk and low potassium baths (< 2mEq/L) in outpatient dialysis clinics.2,36,72 It is important to note that in these studies, many patients prescribed low potassium dialysate already had pre-dialysis serum potassium levels in the normal range, suggesting that inattention to serum potassium levels and failure to adjust the dialysate potassium appropriately may have contributed to the risk of SCD. Further support for avoiding low potassium dialysate and large potassium shifts comes from studies comparing the direct arrhythmogenic effect of a constant dialysate potassium concentration versus dialysate potassium modeling (i.e. serum to dialysate potassium gradient is maintained at a constant level throughout treatment).73 Reduced incidence of premature ventricular contractions during and after dialysis was seen in 30 HD patients exposed to potassium modeling versus a fixed potassium dialysate, suggesting that more gradual potassium removal may be protective. Unfortunately, dialysis equipment capable of potassium modeling is not widely available, and precise individualization would also require the development of hemodialysis machines capable of measuring serum potassium in real time. The recent availability of new, well-tolerated potassium binding agents presents an intriguing opportunity to manage hyperkalemia without resorting to low potassium dialysate. They may even afford the opportunity to utilize higher potassium baths overall to reduce the arrythmogenicity of HD.74,75
Serum and dialysate calcium have also been associated with an increased risk of hemodialysis-related arrhythmias and SCD. Lowering of serum calcium during dialysis has been shown to promote QT interval prolongation and ventricular arrhythmias.41,76 In fact, exposure to low calcium dialysate (<2.5 mEq/L) has been associated with a 40% increase in the risk of SCD.77 A facility-level analysis of dialysis clinics that lowered the predominant calcium dialysate concentration used in the clinics from 2.5 mEq/L to <2.5 mEq/L found a higher incidence of intradialytic hypotension and hospitalization for heart failure exacerbation following the switch compared to facilities that maintained dialysate calcium levels at 2.5 mEq/L.78 Conversely, there is some observational evidence to suggest high corrected calcium may play a role in mortality by promoting vascular calcifications and a vulnerable myocardium.79 Consideration of the potential consequences of both too low and too high calcium dialysate highlights the need for further investigation and the role of other measures beyond calcium dialysate to optimize mineral metabolism including vitamin D analogues, phosphate binders, and calcimimetics. Further support for the role of these agents and optimal phosphate control comes from work demonstrating a link between hyperphosphatemia and mortality, possibly through myocardial calcifications and distorted microcirculatory hemodynamics.80
In addition to electrolyte shifts, large shifts in volume from high ultrafiltration rates have been consistently associated with cardiac events.39 Reviews from both the Dialysis Outcomes and Practice Patterns Study (DOPPS) I and II and the HEMO Study revealed that ultrafiltration rates over 10 ml/kg/hr were associated with increased mortality.81 A prospective observational study of 287 Italian HD patients also reported an association between high ultrafiltration rates and increased mortality risk.37 Efforts to reduce interdialytic weight gain and therefore the need for high ultrafiltration rates include patient dietary education, more frequent HD, extended dialysis treatment times, and smaller gradients between dialysate and serum sodium levels.82
Dialysate cooling can lead to less intradialytic hypotension and repetitive ischemic myocardial injury. A study in which the dialysate temperature was lowered to 2°C below body temperature reduced the occurrence of dialysis-induced myocardial wall motion abnormalities, which have been associated with an increased risk of cardiac death.83 Furthermore, a RCT evaluating 73 incident hemodialysis patients subject to either the control dialysate temperature of 37°C or lowering of dialysate temperatures to 0.5°C below body temperature found a reduction in left ventricular mass in the cooled dialysate group without major adverse effects or withdrawal.84 Frequent hemodialysis is another intervention that potentially reduces left ventricular mass. A prospective, parallel-group trial of 254 patients randomized to in-center hemodialysis six times per week or thrice weekly HD observed an association between more frequent HD and reduced left ventricular mass.85 The authors noted that ultrafiltration, interdialytic weight gain, and hypotension were less in the frequent HD group. However, the sample size was too small to determine the effects of frequent in-center hemodialysis on death.85
Use of Implantable Cardioverter Defibrillators (ICDs)
In the general population, ICDs are a proven but expensive therapy to reduce SCD in high-risk patients. However, multiple studies have demonstrated significantly reduced survival among dialysis ICD recipients compared to their non-dialysis counterparts.86–90 An observational study of 303 hemodialysis patients with heart failure found no significant survival benefit among patients who received primary prevention ICD compared to matched controls without ICDs (Figure 3).91 A meta-analysis of three trials of primary prevention ICDs found no significant benefit of ICD use compared to control in the 1,040 patients with eGFR < 60 ml/min per1.73 m2 analyzed.92 There is also an increased risk of ICD-related complications among dialysis patients including an increased risk of central venous stenosis from intravascular leads, which is particularly detrimental to the maintenance of hemodialysis vascular access.93 In fact, the rate of central venous stenosis has reported to be as high as 70% in a retrospective study of HD patients with an ipsilateral transvenous pacemaker.94 Treatment options for central venous stenosis are limited since percutaneous balloon angioplasty has a low primary patency rate and endovascular stenting is contraindicated with an indwelling device lead in place.95 Infectious-related complications are a major concern in hemodialysis patients; a study of over 9,500 hemodialysis patients with ICDs found high annual rates of bacteremia (52%) and device infections (4.2%).96 Lead-associated endocarditis requires removal of not only the ICD but also often removal of the vascular access.95
Figure 3.
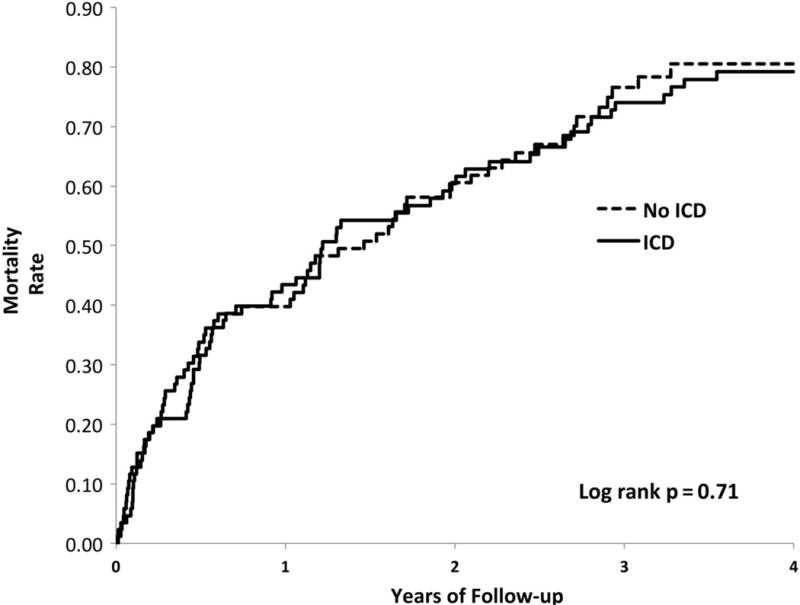
Mortality among hemodialysis patients with and without ICDs (matched cohorts). Log-rank P = 0.71; HR = 0.94 (95% CI: 0.67, 1.31).91 Abbreviations: CI, confidence interval; HR, hazard ratio; ICD, implantable cardioverter defibrillator. Reprinted from Pun et al91 with permission of Oxford University Press.
Newer leadless defibrillator devices including subcutaneous implantable defibrillators and wearable external defibrillators may be an advantageous alternative among hemodialysis patients to avoid vascular access complications and minimize infectious risk. While subcutaneous implantable defibrillators are not available for patients requiring transvenous pacing, two single-center cohorts reported favorable safety data — no subcutaneous implantable defibrillators device-related infections and no excess of inappropriate shocks in HD patients compared to the non-dialysis patients studied.97,98
Until the question of efficacy regarding ICD therapy in the HD population is definitively answered with well-designed randomized trials, increased communication between nephrologists and cardiologists is needed to counsel potential ICD recipients about the likelihood of increased risks and reduced benefits compared the general population. If ICD placement is deemed necessary, such discussions can also help optimize placement in order to limit vascular access compromise.
Improving Survival Following sudden cardiac arrest
As discussed earlier, the chances of survival following sudden cardiac arrest are poor,54,55 and this information should be included in advance directive discussions with patients. Notwithstanding, existing sudden cardiac arrest management strategies include CPR, defibrillation for ventricular arrhythmias, and subsequent evaluation for secondary prevention ICDs. Because of the high risk of sudden cardiac arrest in dialysis clinics, the National Kidney Foundation released guidelines in 2005 recommending that all outpatient dialysis clinics provide basic life support/CPR training for dialysis staff and on-site capabilities for defibrillation using AEDs.99 The effect of placing AEDs in hemodialysis clinics was evaluated in a retrospective study comparing survival post-sudden cardiac arrest among patients in hemodialysis clinics with and without AEDs on site.100 No significant benefit of AED-equipped clinics on sudden cardiac arrest survival was observed. However, this may have been due to significant deficiencies in CPR efforts in dialysis clinics including poor utilization of AEDs. In the study, deployment of the AED could be documented in only 27% of events in AED-equipped clinics. Similar evidence of poor AED utilization was seen in another study of sudden cardiac arrest events occurring in outpatient dialysis clinics where available AEDs were used by dialysis staff in only 53% of cardiac arrests.49,100 Thus, improving the capabilities and readiness of dialysis staff to perform adequate basic life support may be an opportunity to improve sudden cardiac arrest outcomes. Patients who are fortunate enough to survive a sudden cardiac arrest should be considered for a secondary prevention ICD. Although subject to selection bias, several observational studies have consistently shown a modest survival advantage of secondary prevention ICDs compared to propensity matched controls.96,101,102
Figure 4 and Table 1 contain a summary of known risk factors for SCD and suggestions to address them, based on the current state of evidence.
Figure 4.
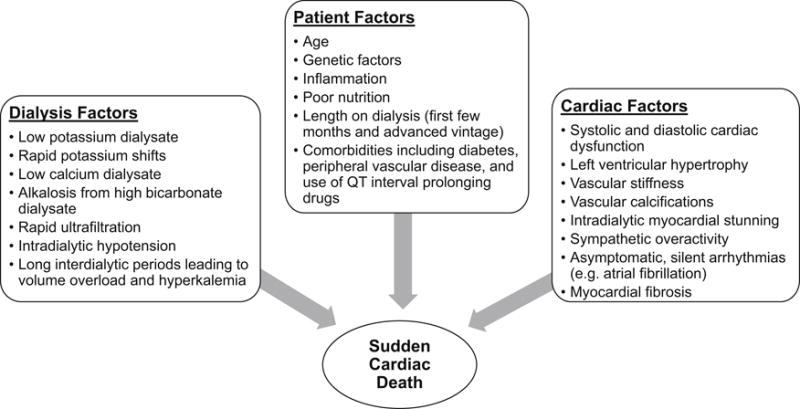
Major hypothesized risk factors for sudden cardiac death including postulated pathophysiology of sudden cardiac death, data source Di Lullo et al 80
Table 1.
Possible Strategies for SCD Prevention in HD Patients
| General Strategy | Specific Intervention |
|---|---|
| Manage cardiomyopathy | |
| Systolic Dysfunction | Use carvedilol in patients with dilated cardiomyopathy |
| Diastolic dysfunction/LVH | Consider more frequent HD to reduce left ventricular mass; consider use of spironolactone, ACE inhibitors, or ARBs |
| Minimize arrhythmic triggers | |
| Potassium shifts | Monitor pre-dialysis potassium frequently, especially after hospitalization and change dialysate bath accordingly; avoid low (< 2 mEq/L) potassium baths; consider potassium modeling and potassium binding agents to reduce interdialytic hyperkalemia |
| Calcium shifts | Avoid low (< 2.5 mEq/L) calcium baths, especially with concurrent use of QT interval prolonging medications |
| Metabolic alkalosis | Avoid high dialysate bicarbonate concentrations in alkalotic patients; account for all sources of base in dialysate, including acetate |
| Rapid ultrafiltration | Encourage patient adherence to salt and fluid restrictions; avoid sodium ramping and large dialysate/serum sodium gradients; extend dialysis time so that ultrafiltration rates do not exceed 10 ml/kg/hr |
| Dialysis-induced myocardial ischemia | Lower dialysate temperature to between 0.5°C and 2°C below patient temperature to reduce intradialytic hypotension |
| Medications | Avoid QT interval prolonging medications whenever possible and reconcile medication list regularly |
| Weigh risks and benefits of ICDs | Consider ICDs for secondary prevention; increase communication between nephrologists and cardiologists to consider risks and benefits of primary prevention ICDs; consider leadless defibrillators to reduce vascular and infectious risks |
| Improve response to cardiac arrest | Increase dialysis clinic staff awareness of cardiac arrest risk and readiness to provide basic life support; encourage awareness and CPR training among patients and families |
Adapted from Pun103 with permission of Elsevier.
Abbreviations: HD, hemodialysis; LVH, left ventricular hypertrophy; ACE inhibitors, angiotensin converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ICDs, implantable cardioverter defibrillators; CPR, cardiopulmonary resuscitation
Summary
SCD is a major problem in HD patients, and our understanding of this disease is underdeveloped. Further well-designed cohort studies are needed to understand disease pathophysiology and risk factors, and randomized intervention trials are needed before new and effective prevention strategies can be implemented. Based on current evidence, a common-sense approach to SCD prevention among HD patients would be to identify and treat those at highest risk with existing cardiovascular medications and reduce potential dialysis-related arrhythmic triggers. Strategies to reduce dialysis-related arrhythmic triggers include more frequent or extended dialysis sessions, monitoring pre-dialysis potassium more commonly, avoiding very low potassium and calcium dialysate baths when possible, and reducing dialysate temperatures. Other therapies such as ICDs should be used judiciously and on a case-by-case basis, with recognition of the associated hazards that these devices carry for the hemodialysis population.
Case Review
Returning to our case, our patient survived his cardiac arrest. Cardiac enzymes and electrocardiogram did not suggest acute myocardial infarction. Several prior electrocardiograms, however, did show a prolonged QT interval and he was referred to outpatient genetic testing as part of a thorough examination. It was presumed that structural heart disease including LVH and aortic valve replacement as well as the rapid potassium and fluid shifts during dialysis played a significant role in his cardiac arrest. These risk factors could not be entirely controlled. In fact, he was initially dialyzed on a 3 mEq/L potassium bath while hospitalized but due to subsequent hyperkalemia, he was returned to a 2 mEq/L potassium bath by discharge. To reduce his SCD risk as an outpatient, all QT interval prolonging medications were discontinued (including fluconazole and ondansetron) and the following changes were made to his dialysis prescription: raising his dialysate calcium to 2.5 mEq/L, lowering the dialysate temperature, and adding an extra dialysis session over the long weekend if interdialytic weight gain resulted in excessively high ultrafiltration goals. He also underwent dietary education regarding potassium restriction and he is being considered for treatment with novel potassium binding agents. Given the difficulty controlling his risk factors and history of cardiac arrest, the decision was made to implant a leadless subcutaneous ICD instead of a traditional ICD in hopes of preventing further events and reducing the risk of infection and central venous stenosis.
Acknowledgments
Support: Support provided by National Institutes of Health grants 5K23DK098281 and T32DK007731.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Peer Review: Evaluated by 3 peer reviewers, Deputy Editor Weiner, and Editor-in-Chief Levey.
References
- 1.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2015;66(1) doi: 10.1053/j.ajkd.2015.05.001. Supplement 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jadoul M, Thumma J, Fuller DS, et al. Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(5):765–774. doi: 10.2215/CJN.08850811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinkle LE, Jr, Thaler HT. Clinical classification of cardiac deaths. Circulation. 1982;65(3):457–464. doi: 10.1161/01.cir.65.3.457. [DOI] [PubMed] [Google Scholar]
- 4.Mann DL, Zipes DP, Libby P, Bonow RO, Braunwald E. Braunwald’s heart disease : a textbook of cardiovascular medicine. Tenth edition. Philadelphia, PA: Elsevier/Saunders; p. 2015. http://getitatduke.library.duke.edu/?sid=sersol&SS_jc=TC0001535525&title=Braunwald%27s%20heart%20disease%20%3A%20a%20textbook%20of%20cardiovascular%20medicine. [Google Scholar]
- 5.Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80(6):572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 6.Writing Group M. Mozaffarian D, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 7.Takeda K, Harada A, Okuda S, et al. Sudden death in chronic dialysis patients. Nephrol Dial Transplant. 1997;12(5):952–955. doi: 10.1093/ndt/12.5.952. [DOI] [PubMed] [Google Scholar]
- 8.Herzog CA. Cardiac arrest in dialysis patients: approaches to alter an abysmal outcome. Kidney Int Suppl. 2003;(84):S197–200. doi: 10.1046/j.1523-1755.63.s84.17.x. [DOI] [PubMed] [Google Scholar]
- 9.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69(12):2268–2273. doi: 10.1038/sj.ki.5000446. [DOI] [PubMed] [Google Scholar]
- 10.Ramesh S, Zalucky A, Hemmelgarn BR, et al. Incidence of sudden cardiac death in adults with end-stage renal disease: a systematic review and meta-analysis. BMC Nephrol. 2016;17(1):78. doi: 10.1186/s12882-016-0293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pun PH, Herzog CA, Middleton JP. Improving ascertainment of sudden cardiac death in patients with end stage renal disease. Clin J Am Soc Nephrol. 2012;7(1):116–122. doi: 10.2215/CJN.02820311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung AK, Sarnak MJ, Yan G, et al. Cardiac diseases in maintenance hemodialysis patients: results of the HEMO Study. Kidney Int. 2004;65(6):2380–2389. doi: 10.1111/j.1523-1755.2004.00657.x. [DOI] [PubMed] [Google Scholar]
- 13.Wanner C, Krane V, Marz W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler DC, London GM, Parfrey PS, et al. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: the EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc. 2014;3(6):e001363. doi: 10.1161/JAHA.114.001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocco MV, Yan G, Gassman J, et al. Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. The National Institutes of Health-funded Hemodialysis. Health Care Financing Administration. Am J Kidney Dis. 2002;39(1):146–153. doi: 10.1053/ajkd.2002.29905. [DOI] [PubMed] [Google Scholar]
- 16.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473–1482. doi: 10.1056/NEJMra000650. [DOI] [PubMed] [Google Scholar]
- 17.Bayes de Luna A, Coumel P, Leclercq JF. Ambulatory sudden cardiac death: mechanisms of production of fatal arrhythmia on the basis of data from 157 cases. Am Heart J. 1989;117(1):151–159. doi: 10.1016/0002-8703(89)90670-4. [DOI] [PubMed] [Google Scholar]
- 18.Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Eur Heart J. 2015;36(41):2793–2867. doi: 10.1093/eurheartj/ehv316. [DOI] [PubMed] [Google Scholar]
- 19.Ohtake T, Kobayashi S, Moriya H, et al. High prevalence of occult coronary artery stenosis in patients with chronic kidney disease at the initiation of renal replacement therapy: an angiographic examination. J Am Soc Nephrol. 2005;16(4):1141–1148. doi: 10.1681/ASN.2004090765. [DOI] [PubMed] [Google Scholar]
- 20.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15(5):458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 21.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63(3):793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 22.Zager PG, Nikolic J, Brown RH, et al. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int. 1998;54(2):561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 23.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76(6):652–658. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Genovesi S, Valsecchi MG, Rossi E, et al. Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant. 2009;24(8):2529–2536. doi: 10.1093/ndt/gfp104. [DOI] [PubMed] [Google Scholar]
- 25.Yamada S, Ishii H, Takahashi H, et al. Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(10):1793–1798. doi: 10.2215/CJN.00050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangrum AJ, Liu D, Dimarco JP, Bolton K, Mangrum M. Sudden Cardiac Death and Left Ventricular Function in Hemodialysis Patients. Heart Rhythm. 2005;2(5):S41. [Google Scholar]
- 27.Paoletti E, Specchia C, Di Maio G, et al. The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: a 10 year survey. Nephrol Dial Transplant. 2004;19(7):1829–1834. doi: 10.1093/ndt/gfh288. [DOI] [PubMed] [Google Scholar]
- 28.Mark PB, Johnston N, Groenning BA, et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int. 2006;69(10):1839–1845. doi: 10.1038/sj.ki.5000249. [DOI] [PubMed] [Google Scholar]
- 29.Schietinger BJ, Brammer GM, Wang H, et al. Patterns of late gadolinium enhancement in chronic hemodialysis patients. JACC Cardiovasc Imaging. 2008;1(4):450–456. doi: 10.1016/j.jcmg.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9(6):1018–1022. doi: 10.1681/ASN.V961018. [DOI] [PubMed] [Google Scholar]
- 31.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4(5):914–920. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365(12):1099–1107. doi: 10.1056/NEJMoa1103313. [DOI] [PubMed] [Google Scholar]
- 33.Wong MC, Kalman JM, Pedagogos E, et al. Temporal distribution of arrhythmic events in chronic kidney disease: Highest incidence in the long interdialytic period. Heart Rhythm. 2015;12(10):2047–2055. doi: 10.1016/j.hrthm.2015.06.033. [DOI] [PubMed] [Google Scholar]
- 34.Krishnasamy R, Badve SV, Hawley CM, et al. Daily variation in death in patients treated by long-term dialysis: comparison of in-center hemodialysis to peritoneal and home hemodialysis. Am J Kidney Dis. 2013;61(1):96–103. doi: 10.1053/j.ajkd.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 35.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79(2):218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 36.Karnik JA, Young BS, Lew NL, et al. Cardiac arrest and sudden death in dialysis units. Kidney Int. 2001;60(1):350–357. doi: 10.1046/j.1523-1755.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 37.Movilli E, Gaggia P, Zubani R, et al. Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant. 2007;22(12):3547–3552. doi: 10.1093/ndt/gfm466. [DOI] [PubMed] [Google Scholar]
- 38.Saran R, Bragg-Gresham JL, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69(7):1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 39.Flythe JE, Brunelli SM. The risks of high ultrafiltration rate in chronic hemodialysis: implications for patient care. Semin Dial. 2011;24(3):259–265. doi: 10.1111/j.1525-139X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 40.Gabutti L, Ferrari N, Giudici G, Mombelli G, Marone C. Unexpected haemodynamic instability associated with standard bicarbonate haemodialysis. Nephrol Dial Transplant. 2003;18(11):2369–2376. doi: 10.1093/ndt/gfg383. [DOI] [PubMed] [Google Scholar]
- 41.Di Iorio B, Torraca S, Piscopo C, et al. Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis: pilot study of single dialysis effects. J Nephrol. 2012;25(5):653–660. doi: 10.5301/jn.5000036. [DOI] [PubMed] [Google Scholar]
- 42.Pande S, Raja R, Bloom E, Chewaproug D, Dissanayake I. Effect of dialysate baths on serum bicarbonate levels in hemodialysis patients. Am J Kidney Dis. 2011;57(4):A75. [Google Scholar]
- 43.Chan KE, Newton-Cheh C, Gusella JF, Maddux FW. Heritability of Risk for Sudden Cardiac Arrest in ESRD. J Am Soc Nephrol. 2015;26(11):2815–2820. doi: 10.1681/ASN.2014090881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spooner PM. Sudden cardiac death: The larger problem… The larger genome. J Cardiovasc Electrophysiol. 2009;20(5):585–596. doi: 10.1111/j.1540-8167.2008.01419.x. [DOI] [PubMed] [Google Scholar]
- 45.Keller SP, Halperin HR. Cardiac arrest: the changing incidence of ventricular fibrillation. Curr Treat Options Cardiovasc Med. 2015;17(7):392. doi: 10.1007/s11936-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bonato FO, Lemos MM, Cassiolato JL, Canziani ME. Prevalence of ventricular arrhythmia and its associated factors in nondialyzed chronic kidney disease patients. PLoS One. 2013;8(6):e66036. doi: 10.1371/journal.pone.0066036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsueh C-HC, Neal X, Chen Peng-Sheng, Lin Shien F, Moe Sharon M. Increased Inducibility of Ventricular Arrhythmia in a Rat Model of Chronic Kidney Disease. J Am Soc Nephrol. 2013;24:139A. [Google Scholar]
- 48.Wan C, Herzog CA, Zareba W, Szymkiewicz SJ. Sudden Cardiac Arrest in Hemodialysis Patients with Wearable Cardioverter Defibrillator. Ann Noninvasive Electrocardiol. 2013 doi: 10.1111/anec.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis TR, Young BA, Eisenberg MS, Rea TD, Copass MK, Cobb LA. Outcome of cardiac arrests attended by emergency medical services staff at community outpatient dialysis centers. Kidney Int. 2008;73(8):933–939. doi: 10.1038/sj.ki.5002749. [DOI] [PubMed] [Google Scholar]
- 50.Lafrance JP, Nolin L, Senecal L, Leblanc M. Predictors and outcome of cardiopulmonary resuscitation (CPR) calls in a large haemodialysis unit over a seven-year period. Nephrol Dial Transplant. 2006;21(4):1006–1012. doi: 10.1093/ndt/gfk007. [DOI] [PubMed] [Google Scholar]
- 51.Verde E, Perez de Prado A, Lopez-Gomez JM, et al. Asymptomatic Intradialytic Supraventricular Arrhythmias and Adverse Outcomes in Patients on Hemodialysis. Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.04310416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charytan DM, Foley R, McCullough PA, et al. Arrhythmia and Sudden Death in Hemodialysis Patients: Protocol and Baseline Characteristics of the Monitoring in Dialysis Study. Clin J Am Soc Nephrol. 2016;11(4):721–734. doi: 10.2215/CJN.09350915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roy-Chaudhury PWD, Tumlin H, et al. American Society of Nephrology. San Diego: 2015. Monitoring in Dialysis (MiD) Study: Exploring the Timeline and Etiology of Increased Arrhythmias in Hemodialysis (HD) Patients. [Google Scholar]
- 54.Pun PH, Lehrich RW, Smith SR, Middleton JP. Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol. 2007;2(3):491–500. doi: 10.2215/CJN.02360706. [DOI] [PubMed] [Google Scholar]
- 55.Kudenchuk PJ, Stuart R, Husain S, Fahrenbruch C, Eisenberg M. Treatment and outcome of out-of-hospital cardiac arrest in outpatient health care facilities. Resuscitation. 2015;97:97–102. doi: 10.1016/j.resuscitation.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 56.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–2138. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 57.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76(6):652–658. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parekh RS, Plantinga LC, Kao WH, et al. The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int. 2008;74(10):1335–1342. doi: 10.1038/ki.2008.449. [DOI] [PubMed] [Google Scholar]
- 59.Drechsler C, Krane V, Winkler K, Dekker FW, Wanner C. Changes in adiponectin and the risk of sudden death, stroke, myocardial infarction, and mortality in hemodialysis patients. Kidney Int. 2009;76(5):567–575. doi: 10.1038/ki.2009.200. [DOI] [PubMed] [Google Scholar]
- 60.Shastri S, Tangri N, Tighiouart H, et al. Predictors of sudden cardiac death: a competing risk approach in the hemodialysis study. Clinical journal of the American Society of Nephrology : CJASN. 2012;7(1):123–130. doi: 10.2215/CJN.06320611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Passman R, Herzog CA. End-stage renal disease: sudden cardiac death: stratifying risk in dialysis patients. Nat Rev Nephrol. 2011;7(3):133–135. doi: 10.1038/nrneph.2010.166. [DOI] [PubMed] [Google Scholar]
- 62.Domanski MJ, Zipes DP, Schron E. Treatment of sudden cardiac death. Current understandings from randomized trials and future research directions. Circulation. 1997;95(12):2694–2699. doi: 10.1161/01.cir.95.12.2694. [DOI] [PubMed] [Google Scholar]
- 63.Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41(9):1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 64.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG. Hypertension in hemodialysis patients treated with atenolol or lisinopril: a randomized controlled trial. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gft515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tangri N, Shastri S, Tighiouart H, et al. beta-Blockers for prevention of sudden cardiac death in patients on hemodialysis: a propensity score analysis of the HEMO Study. Am J Kidney Dis. 2011;58(6):939–945. doi: 10.1053/j.ajkd.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 66.Fellstrom B, Zannad F, Schmieder R, et al. Effect of rosuvastatin on outcomes in chronic haemodialysis patients - design and rationale of the AURORA study. Curr Control Trials Cardiovasc Med. 2005;6(1):9. doi: 10.1186/1468-6708-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iseki K, Arima H, Kohagura K, et al. Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant. 2013;28(6):1579–1589. doi: 10.1093/ndt/gfs590. [DOI] [PubMed] [Google Scholar]
- 69.Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70(7):1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 70.Tai DJ, Lim TW, James MT, Manns BJ, Tonelli M, Hemmelgarn BR. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clinical journal of the American Society of Nephrology : CJASN. 2010;5(4):623–630. doi: 10.2215/CJN.07831109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsumoto Y, Mori Y, Kageyama S, et al. Spironolactone reduces cardiovascular and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63(6):528–536. doi: 10.1016/j.jacc.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 72.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP. Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int. 2011;79(2):218–227. doi: 10.1038/ki.2010.315. [DOI] [PubMed] [Google Scholar]
- 73.Santoro A, Mancini E, London G, et al. Patients with complex arrhythmias during and after haemodialysis suffer from different regimens of potassium removal. Nephrol Dial Transplant. 2008;23(4):1415–1421. doi: 10.1093/ndt/gfm730. [DOI] [PubMed] [Google Scholar]
- 74.Weir MR, Bakris GL, Bushinsky DA, et al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372(3):211–221. doi: 10.1056/NEJMoa1410853. [DOI] [PubMed] [Google Scholar]
- 75.Kosiborod M, Peacock WF, Packham DK. Sodium zirconium cyclosilicate for urgent therapy of severe hyperkalemia. N Engl J Med. 2015;372(16):1577–1578. doi: 10.1056/NEJMc1500353. [DOI] [PubMed] [Google Scholar]
- 76.Beaubien ER, Pylypchuk GB, Akhtar J, Biem HJ. Value of corrected QT interval dispersion in identifying patients initiating dialysis at increased risk of total and cardiovascular mortality. Am J Kidney Dis. 2002;39(4):834–842. doi: 10.1053/ajkd.2002.32005. [DOI] [PubMed] [Google Scholar]
- 77.Pun PH, Horton JR, Middleton JP. Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(5):797–803. doi: 10.2215/CJN.10000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brunelli SM, Sibbel S, Do TP, Cooper K, Bradbury BD. Facility Dialysate Calcium Practices and Clinical Outcomes Among Patients Receiving Hemodialysis: A Retrospective Observational Study. Am J Kidney Dis. 2015;66(4):655–665. doi: 10.1053/j.ajkd.2015.03.038. [DOI] [PubMed] [Google Scholar]
- 79.Kim ED, Parekh RS. Calcium and Sudden Cardiac Death in End-Stage Renal Disease. Semin Dial. 2015;28(6):624–635. doi: 10.1111/sdi.12419. [DOI] [PubMed] [Google Scholar]
- 80.Di Lullo L, Rivera R, Barbera V, et al. Sudden cardiac death and chronic kidney disease: From pathophysiology to treatment strategies. Int J Cardiol. 2016;217:16–27. doi: 10.1016/j.ijcard.2016.04.170. [DOI] [PubMed] [Google Scholar]
- 81.Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int. 2011;79(2):250–257. doi: 10.1038/ki.2010.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mendoza JM, Bayes LY, Sun S, Doss S, Schiller B. Effect of Lowering Dialysate Sodium Concentration on Interdialytic Weight Gain and Blood Pressure in Patients Undergoing Thrice-Weekly In-center Nocturnal Hemodialysis: A Quality Improvement Study. Am J Kidney Dis. 2011 doi: 10.1053/j.ajkd.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jefferies HJ, Burton JO, McIntyre CW. Individualised dialysate temperature improves intradialytic haemodynamics and abrogates haemodialysis-induced myocardial stunning, without compromising tolerability. Blood Purif. 2011;32(1):63–68. doi: 10.1159/000324199. [DOI] [PubMed] [Google Scholar]
- 84.Odudu A, Eldehni MT, McCann GP, McIntyre CW. Randomized Controlled Trial of Individualized Dialysate Cooling for Cardiac Protection in Hemodialysis Patients. Clin J Am Soc Nephrol. 2015;10(8):1408–1417. doi: 10.2215/CJN.00200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.In-Center Hemodialysis Six Times per Week versus Three Times per Week. New England Journal of Medicine. 2010;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eckart RE, Gula LJ, Reynolds MR, Shry EA, Maisel WH. Mortality following defibrillator implantation in patients with renal insufficiency. J Cardiovasc Electrophysiol. 2006;17(9):940–943. doi: 10.1111/j.1540-8167.2006.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wase A, Basit A, Nazir R, et al. Impact of chronic kidney disease upon survival among implantable cardioverter-defibrillator recipients. J Interv Card Electrophysiol. 2004;11(3):199–204. doi: 10.1023/B:JICE.0000048570.43706.34. [DOI] [PubMed] [Google Scholar]
- 88.Robin J, Weinberg K, Tiongson J, et al. Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm. 2006;3(10):1196–1201. doi: 10.1016/j.hrthm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 89.Sakhuja R, Keebler M, Lai TS, McLaughlin Gavin C, Thakur R, Bhatt DL. Meta-analysis of mortality in dialysis patients with an implantable cardioverter defibrillator. Am J Cardiol. 2009;103(5):735–741. doi: 10.1016/j.amjcard.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 90.Dasgupta A, Montalvo J, Medendorp S, et al. Increased complication rates of cardiac rhythm management devices in ESRD patients. Am J Kidney Dis. 2007;49(5):656–663. doi: 10.1053/j.ajkd.2007.02.272. [DOI] [PubMed] [Google Scholar]
- 91.Pun PH, Hellkamp AS, Sanders GD, et al. Primary prevention implantable cardioverter defibrillators in end-stage kidney disease patients on dialysis: a matched cohort study. Nephrol Dial Transplant. 2015;30(5):829–835. doi: 10.1093/ndt/gfu274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pun PH, Al-Khatib SM, Han JY, et al. Implantable Cardioverter-Defibrillators for Primary Prevention of Sudden Cardiac Death in CKD: A Meta-analysis of Patient-Level Data From 3 Randomized Trials. Am J Kidney Dis. 2014;64(1):32–39. doi: 10.1053/j.ajkd.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Drew DA, Meyer KB, Weiner DE. Transvenous cardiac device wires and vascular access in hemodialysis patients. Am J Kidney Dis. 2011;58(3):494–496. doi: 10.1053/j.ajkd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 94.Teruya TH, Abou-Zamzam AM, Jr, Limm W, Wong L, Wong L. Symptomatic subclavian vein stenosis and occlusion in hemodialysis patients with transvenous pacemakers. Ann Vasc Surg. 2003;17(5):526–529. doi: 10.1007/s10016-003-0048-4. [DOI] [PubMed] [Google Scholar]
- 95.Dhamija RK, Tan H, Philbin E, et al. Subcutaneous implantable cardioverter defibrillator for dialysis patients: a strategy to reduce central vein stenoses and infections. Am J Kidney Dis. 2015;66(1):154–158. doi: 10.1053/j.ajkd.2015.01.028. [DOI] [PubMed] [Google Scholar]
- 96.Charytan DM, Patrick AR, Liu J, et al. Trends in the use and outcomes of implantable cardioverter-defibrillators in patients undergoing dialysis in the United States. Am J Kidney Dis. 2011;58(3):409–417. doi: 10.1053/j.ajkd.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 97.Koman E, Gupta A, Subzposh F, Saltzman H, Kutalek SP. Outcomes of subcutaneous implantable cardioverter-defibrillator implantation in patients on hemodialysis. J Interv Card Electrophysiol. 2016;45(2):219–223. doi: 10.1007/s10840-015-0093-2. [DOI] [PubMed] [Google Scholar]
- 98.El-Chami MF, Levy M, Kelli HM, et al. Outcome of Subcutaneous Implantable Cardioverter Defibrillator Implantation in Patients with End-Stage Renal Disease on Dialysis. J Cardiovasc Electrophysiol. 2015;26(8):900–904. doi: 10.1111/jce.12705. [DOI] [PubMed] [Google Scholar]
- 99.National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) Guideline 8: External Defibrillation. 2005 http://www2.kidney.org/professionals/KDOQI/guidelines_cvd/guide8.htm.
- 100.Lehrich RW, Pun PH, Tanenbaum ND, Smith SR, Middleton JP. Automated external defibrillators and survival from cardiac arrest in the outpatient hemodialysis clinic. J Am Soc Nephrol. 2007;18(1):312–320. doi: 10.1681/ASN.2006040392. [DOI] [PubMed] [Google Scholar]
- 101.Herzog CA, Li S, Weinhandl ED, Strief JW, Collins AJ, Gilbertson DT. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int. 2005;68(2):818–825. doi: 10.1111/j.1523-1755.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 102.Hiremath S, Punnam SR, Brar SS, et al. Implantable defibrillators improve survival in end-stage renal disease: results from a multi-center registry. Am J Nephrol. 2010;32(4):305–310. doi: 10.1159/000319461. [DOI] [PubMed] [Google Scholar]
- 103.Pun PH. The interplay between CKD, sudden cardiac death, and ventricular arrhythmias. Adv Chronic Kidney Dis. 2014;21(6):480–488. doi: 10.1053/j.ackd.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


