Abstract
Objective
Two interferon-gamma release assays (IGRAs), the QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB (T-SPOT), are commercially available. The agreement between the two IGRAs in the screening of healthcare workers (HCWs) for latent tuberculosis is not well known.
Methods
The QFT-GIT and T-SPOT tests were performed for the baseline tuberculosis screening of 654 HCWs who worked at Mie University Hospital in Japan. The results of the two tests were directly compared.
Results
Nineteen (2.9%), 28 (4.3%) and 33 (5.0%) of the 654 HCWs were found to be positive by the QFT-GIT, T-SPOT, and the QFT-GIT and/or T-SPOT methods using cut-off values of 0.35 IU/mL (QFT-GIT) and 6 spots (T-SPOT). After excluding 4 cases with indeterminate results, there were 14 concordant positive (2.2%), 618 concordant negative (95.1%), and 18 discordant (2.8%) results using the cut-off values of 0.35 IU/mL (QFT-GIT) and 6 spots (T-SPOT). The agreement of the two IGRAs was 97.2% (κ=0.595). When cut-off values of 0.35 IU/mL (QFT-GIT) and 8 spots (T-SPOT) were applied, there were 11 concordant positive (1.7%), 626 concordant negative (96.3%), and 13 discordant (2.0%) results, with 98.0% agreement (κ=0.618). When the borderline criteria for the QFT-GIT (0.1 to <0.35 IU/mL) and T-SPOT (5-7 spots) were applied, there were 11 concordant positive (1.7%), 11 concordant borderline (1.7%), 586 concordant negative (90.2%), and 42 discordant (6.5%) results, with 93.5% agreement between the two methods (κ=0.538).
Conclusion
When standard cut-off values were used, the agreement between the two IGRAs in the tuberculosis screening of Japanese HCWs was moderate to high. Importantly, some HCWs showed discordant results, especially those whose results were in the borderline zones.
Keywords: infection control, healthcare worker, latent tuberculosis, interferon-gamma release assay, QuantiFERON-TB Gold In-Tube, T-SPOT.TB
Introduction
The diagnosis and treatment of latent tuberculosis infection (LTBI) are important components of tuberculosis (TB) control (1). Historically, the tuberculin skin test (TST) has been the only method for detecting LTBI; however, the TST is associated with several limitations, including a lack of specificity among bacille Calmette-Guérin (BCG)-vaccinated individuals, the need for two clinic visits to complete the test, and inaccuracy and bias in the reading of the TST results (2). Interferon-gamma release assays (IGRAs), which measure the interferon-gamma release in response to antigens representing TB, were developed to overcome the limitations of the TST.
Healthcare workers (HCWs) have an increased risk of being infected with TB through occupational exposure, and are an important group for targeted LTBI testing. The TB screening of HCWs may be beneficial for healthcare institutions, both in terms of nosocomial infection control and from the viewpoint of occupational health. There are numerous reports from several countries about the TB screening of HCWs using IGRAs (3-9).
In Japan, where BCG vaccination is routine, IGRAs have been used in place of the TST for applications such as contact investigations and the TB screening of HCWs (9-11). Two IGRAs, the QuantiFERON-TB Gold In-Tube (QFT-GIT; Cellestis, Carnegie, VIC, Australia) and the T-SPOT.TB (T-SPOT; Oxford Immunotec, Abingdon, UK), are currently commercially available (12). The QFT-GIT uses whole blood and an enzyme-linked immunosorbent assay method, whereas the T-SPOT uses purified peripheral blood mononuclear cells and an ELISpot method to detect interferon-gamma release. Although the methodologies of the two IGRAs are different, both methods show high sensitivity and specificity in the diagnosis of TB infection.
However, there are several issues that need to be addressed, including discordant results when the QFT-GIT and T-SPOT are simultaneously performed, and the interpretation of borderline results (2, 13-15). The T-SPOT results are calculated from the highest spot count difference(s) between Panel A and/or Panel B, and the Nil Control. In Japan and other countries, spot count differences of 5-7 are defined as borderline, and should be followed by retesting. For this secondary analysis, a cut-off of 6 spots was used to reclassify borderline results (positive, a spot count difference ≥6; negative, both spot count differences are ≤5) (14). In Japan, the criteria for the interpretation of the QFT-GIT differ from those in other countries in that a second cut-off is employed. A TB antigen level of 0.1-0.35 IU/mL above the Nil response is classified as borderline (intermediate), and is classified as a suspected positive result (16, 17).
The equivocality of the above-mentioned IGRAs makes it difficult to select an IGRA and to interpret the results when IGRAs are applied to the TB screening of HCWs. As there is no gold standard for the diagnosis of LTBI, a direct comparison is necessary to ascertain the differences between the two IGRAs. Accordingly, this study aimed to evaluate the agreement between the two IGRAs when they were applied to HCWs in a nation with a moderate prevalence of TB (the incidence of TB in Japan in 2014 was 15.4 per 100,000 people, with a total of 19,615 newly registered cases) (18-20).
Materials and Methods
Study setting
This study was conducted at Mie University Hospital, a 685-bed educational hospital, in Japan. Our hospital is not designated as a medical intuition for treating TB patients and does not have a TB ward. Over the past decade, there have been 9.2 outpatient cases and 3.6 inpatient cases of active TB infection per year (detected using a polymerase chain reaction-based assay and/or culture) in this hospital.
Study design and ethics approval
In 2014, a large-scale contact investigation (n=312) was performed at Mie University Hospital, and the Infection Control Committee decided to perform baseline TB screening, using IGRA tests, for all HCWs. During the contact investigation using QFT-GIT, some of the contacts with a borderline baseline results (QFT-GIT: 0.10 to <0.35 IU/mL) underwent follow-up testing with the QFT-GIT and T-SPOT at 2-3 months after the first test. Consequently, discordant results between two IGRAs were observed. We therefore decided to conduct both QFT-GIT and T-SPOT tests in the baseline TB screening of HCWs.
As HCWs who were newly hired within the past five years had already undergone IGRA testing, current HCWs who had worked at Mie University Hospital for more than five years were selected for baseline TB screening using the two IGRAs and a questionnaire from June to November in 2015.
The study was approved by the Institutional Ethics Committee of the Mie University Graduate School of Medicine (No.1467). All of the participants in the study provided their written informed consent.
Interferon-gamma release assay testing
The QFT-GIT and T-SPOT tests were performed in parallel. Twelve milliliters of blood were obtained at the hospital for use in the QFT-GIT and T-SPOT tests; the samples were then transported to an external laboratory (Bio Medical Laboratories (BML), Saitama, Japan), where they were analyzed according to the manufacturers' instructions. After the blood was drawn, the tubes were stored and transported in a designated box at 22℃ using a controlled thermal storage pack. The QFT-GIT test tubes were incubated at 37℃; incubation was started in the transit laboratory on the same day (within 13 hours after venipuncture). After 16 hours of incubation, the tubes were centrifuged, and transported to the main laboratory at 2-8℃ for the assay. The T-SPOT test tubes were directly transported to the main laboratory; the T-Cell Xtend reagent was added just after arrival (within 17 hours after venipuncture). The assay was started 6 hours after addition of the T-Cell Xtend reagent. The temperature was continuously measured using thermometers that passed quality checks. The QFT-GIT and T-SPOT assays were carried out according to the procedure of the package insert. The quality of both the QFT-GIT and the T-SPOT was evaluated by measuring the positive/negative controls for each of the test specimens.
BML, which performed the QFT-GIT and T-SPOT tests, is certified the College of American Pathologists for the QFT-GIT and the United Kingdom National External Quality Assessment Service (UK NEQAS) for the interferon gamma release assays that were used for the T-SPOT. The quality controls of the procedures, from transportation to the assay, were thoroughly consolidated.
A QFT-GIT result was considered to be positive if the interferon-gamma response in the TB antigen tube was ≥0.35 IU/mL after subtracting the response in the Nil tube. The T-SPOT result was considered positive if (antigen panel A minus Nil) and/or (antigen panel B minus Nil) yielded ≥6 spots. Negative and indeterminate results were also interpreted according to the manufacturer's instructions.
The cut-off values of the above-noted IGRAs (without borderline zones) were used for the management of IGRA-positive HCWs, who were assumed to have an LTBI. In addition, another T-SPOT cut-off value (≥8 spots) and borderline zone, which were based on the Japanese borderline criterion for the QFT-GIT (0.1 to <0.35 IU/mL), and the above-mentioned borderline zone for the T-SPOT (the highest of the antigen panel A or panel B minus the Nil spot count =5-7) were assessed (12, 13, 15).
Questionnaire
The demographic data, symptoms (fever, cough, sputum), previous chest X-ray and/or IGRA abnormalities, a history of TB treatment, the presence of any immunodeficiency-associated comorbidities (diabetes mellitus, immunodeficiency disorder, treatment with steroids or immunosuppressive agents, dialysis), and a history of TB exposure within two years were obtained from the subjects' questionnaire responses.
The management of the positive results
HCWs with positive QFT-GIT (≥0.35 IU/mL) and/or T-SPOT (≥6 spots) results were considered to be IGRA-positive, and referred for clinical to a pulmonologist for evaluation. When appropriate, LTBI treatment was recommended by the pulmonologist.
Statistical analysis
The statistical analyses were performed using the SPSS software program (version 22; SPSS Benelux, Gorinchem, The Netherlands). Continuous variables were presented as the mean±standard deviation. Categorical variables were compared using Pearson's χ-squared test. The concordance between the QFT-GIT and T-SPOT results was assessed using κ coefficients. p values of <0.05 were considered to indicate statistical significance.
Results
The characteristics of the study population
Among the 655 enrolled HCWs, 654 (210 physicians, 348 nurses, and 96 others) completed the IGRA tests and questionnaire; one HCW who declined to participate was excluded from the study. The mean age of the 654 participants was 42.7±9.7 years. Four hundred thirteen (63.1%) participants were women. The questionnaire responses indicated that 16 (2.4%) respondents had symptoms, 26 (4.0%) had previous chest X-ray and/or IGRA abnormalities, 16 (2.4%) had a history of TB treatment, 17 (2.6%) had an immunodeficiency-associated comorbidity, and 19 (2.9%) had a history of TB exposure (Table 1).
Table 1.
Description of the Study Population by Questionnaire.
| Group | All | IGRA positive | IGRA negative | *p value |
|---|---|---|---|---|
| (QFT-GIT≥0.35 and/or T-SPOT≥6 spots) | (other than positive) | |||
| n | 654 | 33 (5.0%) | 621 (95.0%) | |
| symptom | 16 (2.4%) | 1 (3.0%) | 15 (2.4%) | NS |
| previous | ||||
| abnormalities of chest | 26 (4.0%) | 11 (33.3%) | 15 (2.4%) | p<0.05 |
| X-ray and/or IGRAs | ||||
| history of TB treatment | 16 (2.4%) | 10 (30.3% | 6 (1.0%) | p<0.05 |
| co-morbidity | 17 (2.6%) | 1 (3.0%) | 16 (2.6%) | NS |
| history of TB | ||||
| exposure within 2 | 19 (2.9%) | 1 (3.0%) | 18 (2.9%) | NS |
| years |
Categorical variables were compared by the Pearson’s χ-squared test.
* p<0.05 was considered statistically significant. NS: not significant
The Rates of overall diagnosis with the QFT-GIT and T-SPOT results (with the exclusion of borderline criteria)
According to the QFT-GIT results, 19 of the 654 (2.9%) respondents were positive, 633 (96.8%) were negative, and two were indeterminate. According to the T-SPOT results, 28 of the 654 (4.3%) respondents were positive, 624 (95.4%) were negative, and two were indeterminate. The four cases with an indeterminate IGRA result were not immunocompromised, and no cases showed indeterminate results on both IGRA tests.
There were 33 cases (5.0%) that were positive according to the QFT-GIT test (≥0.35 IU/mL) and/or the T-SPOT test (≥6 spots). The IGRA-positive group (n=33) contained a significantly higher number of subjects with previous chest X-ray and/or IGRA abnormalities, and subjects with a history of TB treatment than the IGRA-negative group (Table 1). The proportion of IGRA-positive HCWs differed according to their age group and occupation. The IGRA-positive group included significantly higher numbers of older HCWs and physicians (Table 2).
Table 2.
Proportion of Latent TB Infection (IGRA Positive) among Healthcare Workers by Age, Gender, and Profession.
| Group | All | IGRA positive | IGRA negative | *p value | |
|---|---|---|---|---|---|
| (QFT-GIT≥0.35 and/or T-SPOT≥6 spots) | (other than positive) | ||||
| Total | 654 | 33 (5.0%) | 621 (95.0%) | ||
| Age | |||||
| 20-30 years | 58 | 3 (5.2%) | 55 (94.8%) | ||
| 30-40 years | 205 | 3 (1.5%) | 202 (98.5%) | ||
| 40-50 years | 231 | 12 (5.2%) | 219 (94.8%) | p<0.05 | |
| 50-60 years | 117 | 10 (8.5%) | 107 (91.5%) | ||
| 60-70 years | 43 | 5 (11.6%) | 38 (88.4%) | ||
| Gender | |||||
| Female | 413 | 18 (4.4%) | 395 (95.6%) | NS | |
| Male | 241 | 15 (6.2%) | 226 (93.8%) | ||
| Profession | |||||
| Physician | 210 | 17 (8.1%) | 193 (91.9%) | ||
| Nurse | 348 | 15 (4.3%) | 333 (95.7%) | p<0.05 | |
| Other | 96 | 1 (1.0%) | 95 (99.0%) | ||
Categorical variables were compared by the Pearson’s χ -squared test.
* p<0.05 was considered statistically significant. NS: not significant
Thirteen of the 33 IGRA-positive HCWs were considered to have had a previous TB infection after treatment. The other 20 IGRA-positive HCWs were referred for a clinical evaluation by a pulmonologist; 9 were started on LTBI treatment after further examination and counseling.
Comparison of the QFT-GIT and T-SPOT results (with the exclusion of borderline criteria)
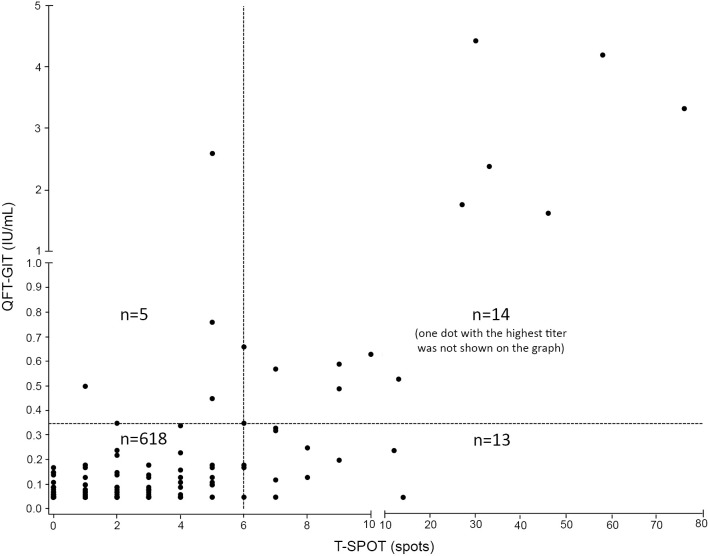
After excluding the four cases with indeterminate results (QFT-GIT, n=2; T-SPOT, n=2), the QFT-GIT and T-SPOT results (positive or negative) were directly compared. There were 14 concordant positive (2.2%), 618 concordant negative (95.1%), and 18 discordant results (2.8%) using cut-off values of 0.35 IU/mL (QFT-GIT) and 6 spots (T-SPOT). The agreement between the two IGRAs was 97.2%, with a κ value of 0.595 [95% confidence interval (CI), 0.41-0.78] (Figure and Table 3A).
Figure.
The QFT-GIT and T-SPOT results of 650 HCWs after excluding the indeterminate cases using cut-off values of 0.35 IU/mL (QFT-GIT) and 6 spots (T-SPOT) (with the exlusion of the borderline criteria).
Table 3A.
Comparison of QFT-GIT and T-SPOT Results for 650 HCWs after Excluding Indeterminate Cases Using Cut-offs of 0.35 IU/mL (QFT-GIT) and Six Spots (T-SPOT) Cut-off with the Exclusion of Borderline Zone.
| T-SPOT | Total | |||
|---|---|---|---|---|
| Positive (≥6 spot) | Negative (≤5 spot) | |||
| QFT-GIT | ||||
| Positive (≥0.35 IU/mL) | 14 | 5 | 19 | |
| Negative (<0.35 IU/mL) | 13 | 618 | 631 | |
| Total | 27 | 623 | 650 | |
The QFT-GIT result was considered positive if the interferon-gamma response in the TB antigen tube after subtracting the response in the Nil tube was ≥0.35 IU/mL, and the T-SPOT result was considered positive if (antigen panel A minus Nil) and/or (antigen panel B minus Nil) ≥6. The QFT-GIT result was considered negative if the interferon-gamma response in the TB antigen tube after subtracting the response in the Nil tube was <0.35 IU/mL, and the T-SPOT result was considered negative if (antigen panel A minus Nil) and/or (antigen panel B minus Nil) was ≤5.
When cut-off values of 0.35 IU/mL (QFT-GIT) and 8 spots (T-SPOT) were used, there were 11 concordant positive (1.7%), 626 concordant negative (96.3%), and 13 discordant (2.0%) with 98.0% agreement between the two tests (κ=0.618) (95% CI, 0.41 to 0.82) (Table 3B).
Table 3B.
Comparison of QFT-GIT and T-SPOT Results for 650 HCWs after Excluding Indeterminate Cases Using Cut-offs of 0.35 IU/mL (QFT-GIT) and Eight Spots (T-SPOT) with the Exclusion of Borderline Zone.
| T-SPOT | Total | |||
|---|---|---|---|---|
| Positive (≥8 spot) | Negative (≤7 spot) | |||
| QFT-GIT | ||||
| Positive (≥0.35 IU/mL) | 11 | 8 | 19 | |
| Negative (<0.35 IU/mL) | 5 | 626 | 631 | |
| Total | 16 | 634 | 650 | |
The QFT-GIT result was considered positive if the interferon-gamma response in the TB antigen tube after subtracting the response in the Nil tube was ≥0.35 IU/mL, and the T-SPOT result was considered positive if (antigen panel A minus Nil) and/or (antigen panel B minus Nil) ≥8. The QFT-GIT result was considered negative if the interferon-gamma response in the TB antigen tube after subtracting the response in the Nil tube was <0.35 IU/mL, and the T-SPOT result was considered negative if (antigen panel A minus Nil) and/or (antigen panel B minus Nil) was ≤7
Comparison of the QFT-GIT and T-SPOT results (with the inclusion of borderline criteria)
After excluding the 4 cases with indeterminate results (QFT-GIT, n=2; T-SPOT, n=2), the QFT-GIT and T-SPOT results (positive, borderline, negative) were directly compared. There were 11 concordant positive (1.7%), 11 concordant borderline (1.7%), 586 concordant negative (90.2%), and 42 discordant results (6.5%). The agreement between the two methods was 93.5%, with a κ value of 0.538 (95% CI, 0.40 to 0.67) (Table 4).
Table 4.
Comparison of QFT-GIT and T-SPOT Results for 650 HCWs after Excluding Indeterminate Cases Using Cut-off with the Inclusion of Borderline Zone.
| T-SPOT | ||||||
|---|---|---|---|---|---|---|
| Positive (≥8 spot) | Borderline (5,6,7 spot) | Negative (≤4 spot) | Total | |||
| QFT-GIT | ||||||
| Positive (≥0.35 IU/mL) | 11 | 6 | 2 | 19 | ||
| Borderline (0.1 to < 0.35 IU/mL) | 4 | 11 | 24 | 39 | ||
| Negative (<0.1 IU/mL) | 1 | 5 | 586 | 592 | ||
| Total | 16 | 22 | 612 | 650 | ||
The QFT-GIT result was considered positive if the interferon-gamma response in the TB antigen after subtracting the response in the Nil tube was ≥0.35 IU/mL, and the T-SPOT result was considered positive if (antigen panel A minus Nil) and/or (antigen panel B minus Nil) ≥8. The QFT-GIT result was considered borderline if the interferon-gamma response in the TB antigen tube after subtracting the response in the Nil tube was (0.1 to <0.35 IU/mL), and the T-SPOT result was considered borderline if (antigen pane l A minus Nil) and/or (antigen panel B minus Nil) was 5, 6, 7. The QFT-GIT result was considered negative if the interferon-gamma response in the TB antigen tube after subtracting the response in the Nil tube was <0.1 IU/mL, and the T-SPOT result was considered negative if (antigen panel A minus Nil) and/or (antigen panel B minus Nil) was ≤4.
Discussion
This study compared the two commercially available IGRAs (QFT-GIT and T-SPOT) in a cohort of HCWs in a nation with moderate TB prevalence. The results from this study showed that the baseline prevalence of positive IGRA results among the HCWs was 2.9% by the QFT-GIT, 4.1% by the T-SPOT, and 5.0% by either methods, and that older HCWs and physicians were thought to be at high risk. The agreement of the two IGRAs when they were used for TB screening was relatively high; however, discordant results were observed, especially in borderline cases.
The statistical data from The Tuberculosis Surveillance Center in Japan demonstrated that the age-specific incidence of TB per 100,000 individuals in 2014 was as follows: 9.2 (20-29 years), 7.7 (30-39 years), 7.8 (40-49 years), 9.8 (50-59 years), and 14.3 (60-69 years). As shown in Table 2, the cohort of the present study demonstrated a similar age-specific morbidity distribution. The rates of LTBI positivity (IGRA-positive) among physicians (8.1%) and nurses (4.3%) were much higher than those among other HCWs (1.0%, mainly pharmacists and radiation technologists) in the present study. It may be important to consider these risk factors for LTBI (age and profession) when planning TB screening programs among HCWs.
The absence of a true gold standard test for LTBI presents a major challenge for determining the accuracy of LTBI tests (21). Thus, it is not clear which of the IGRAs is better for detecting LTBI and the level of agreement between the two IGRAs has not been fully determined. Thus, the two IGRA tests were performed in parallel. The direct comparison of the two IGRAs using cut-off values of ≥0.35 IU/mL (QFT-GIT) and ≥6 spots (T-SPOT), showed moderate agreement (97.2% with a κ value of 0.595) in this cohort. When a cut-off value of 8 spots (T-SPOT) was used instead of 6 spots, the agreement between the two methods was higher (98.0% with a κ value of 0.618). A similar comparison performed in 111 healthy subjects (IGRA-positive rate, 1%) demonstrated 100% agreement between the two IGRAs (12); however, this report might have overestimated the concordance rate as there was only one positive case. The other direct comparison between the two IGRAs, which was performed in the contact investigation (n=812, QFT-GIT positive rate of 30.2%, and T-SPOT positive rate of 28.7%), demonstrated 93.9% agreement with a κ value of 0.852 using cut-off values of 0.35 IU/mL (QFT-GIT) and 6 spots (T-SPOT) and 93.6% agreement with a κ value of 0.843 using cut-off values of 0.35 IU/mL (QFT-GIT) and 8 spots (T-SPOT) (15). These results suggest that the agreement between the two IGRAs using the standard cut-off values is likely to be moderate to high under several circumstances.
In contrast, if the borderline cases were taken into consideration, the agreement between the two IGRAs decreased to 93.5% with a κ value of 0.538 in the present study. Only 10.3% (4 of 39) of the HCWs with borderline QFT-GIT (0.1 to <0.35 IU/mL) showed positive T-SPOT results in the present study. In a previous study comparing the two IGRAs among 111 healthy subjects, none of the 14 subjects with borderline QFT-GIT results were found to be positive by the T-SPOT (12). According to the Japanese guidelines for QFT-GIT, this intermediate range represents a buffer zone and assists with the comprehensive diagnosis. For example, a person with an intermediate test result who has been in close contact with a smear-positive TB patient is at a high risk for infection. This is therefore considered to be equivalent to a positive result, thus the patient is indicated for LTBI treatment (17). Accordingly, the QFT-GIT results in the borderline cases should be considered to be negative when they are applied to baseline TB screening.
Six of the 22 (27.3%) HCWs with borderline T-SPOT results (5-7 spots) were found to be positive by the QFT-GIT in this study. In Japan, similarly to the United States, borderline T-SPOT results should be followed by retesting, and reclassified with a cut-off value of 6 spots. T-SPOT retesting was not performed in this study, but an alternative cut-off of 6 spots for a positive T-SPOT result and the QFT-GIT result were used for the clinical evaluation. King et al. reported that 79.8% of the borderline T-SPOT results obtained from the routine TB screening of HCWs (n=465 pairs) became clearly positive or negative on retesting, with 23% progressing to a positive result (14). Thus, the T-SPOT results in the borderline zone should be followed by retesting, or alternatively, by the addition of a QFT-GIT test.
Four of the 654 (0.6%) tested cases had indeterminate results (QFT-GIT, n=2; T-SPOT, n=2) in the present study. Fortunately, none of the cases showed indeterminate results on both tests.
In conclusion, the present study demonstrated moderate to high agreement between the two IGRAs when they were applied to the baseline TB screening of HCWs, which suggests that the two IGRAs could be equivalent in the diagnosis of LTBI in this context. However, the borderline zones of the QFT-GIT and T-SPOT tended to yield inconsistent results; thus, caution is needed when interpreting the results, and the inclusion of the other IGRA test could be an option for improving the accuracy of the clinical evaluation of cases with borderline or indeterminate results.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank all of the participants for their time and commitment to this study, and Masahiro Shimojima, Bio Medical Laboratories Inc., for his support and critical comments on this project. Funding was received from the Mie University Hospital, Tsu, Japan (The Director Research Grant in 2014).
References
- 1. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 54(RR-17): 1-141, 2005. [PubMed] [Google Scholar]
- 2. Mazurek GH, Jereb J, Vernon A, LoBue P, Goldberg S, Castro K. Updated guidelines for using Interferon Gamma Release Assays to detect Mycobacterium tuberculosis infection - United States, 2010. MMWR Recomm Rep 59(RR-5): 1-25, 2010. [PubMed] [Google Scholar]
- 3. Joshi M, Monson TP, Woods GL. Use of interferon-gamma release assays in a health care worker screening program: experience from a tertiary care centre in the United States. Can Respir J 19: 84-88, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zwerling A, Cojocariu M, McIntosh F, et al. TB screening in Canadian health care workers using interferon-gamma release assays. PLoS One 7: e43014, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nienhaus A, Schablon A, Preisser AM, Ringshausen FC, Diel R. Tuberculosis in healthcare workers: a narrative review from a German perspective. J Occup Med Toxicol 9: 9, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lamberti M, Muoio M, Monaco MG, et al. Prevalence of latent tuberculosis infection and associated risk factors among 3,374 healthcare students in Italy. J Occup Med Toxicol 9: 34, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCarthy KM, Scott LE, Gous N, et al. High incidence of latent tuberculous infection among South African health workers: an urgent call for action. Int J Tuberc Lung Dis 19: 647-653, 2015. [DOI] [PubMed] [Google Scholar]
- 8. Chu H, Shih CJ, Lee YJ, et al. Risk of tuberculosis among healthcare workers in an intermediate-burden country: a nationwide population study. J Infect 69: 525-532, 2014. [DOI] [PubMed] [Google Scholar]
- 9. Hirama T, Hagiwara K, Kanazawa M. Tuberculosis screening programme using the QuantiFERON-TB Gold test and chest computed tomography for healthcare workers accidentally exposed to patients with tuberculosis. J Hosp Infect 77: 257-262, 2011. [DOI] [PubMed] [Google Scholar]
- 10. Ito Y, Nagao M, Iinuma Y, et al. Risk factors for nosocomial tuberculosis transmission among health care workers. Am J Infect Control 44: 596-598, 2016. [DOI] [PubMed] [Google Scholar]
- 11. Abe T, Hashimoto T, Kobayashi T, et al. Baseline screening using interferon-gamma release assay suggests an increased risk of mycobacterium tuberculosis infection among employees in a japanese general hospital. Kekkaku (Tuberculosis) 90: 625-630, 2015(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 12. Higuchi K, Sekiya Y, Igari H, Watanabe A, Harada N. Comparison of specificities between two interferon-gamma release assays in Japan. Int J Tuberc Lung Dis 16: 1190-1192, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Dorman SE, Belknap R, Graviss EA, et al. Interferon-γ release assays and tuberculin skin testing for diagnosis of latent tuberculosis infection in healthcare workers in the United States. Am J Respir Crit Care Med 189: 77-87, 2014. [DOI] [PubMed] [Google Scholar]
- 14. King TC, Upfal M, Gottlieb A, et al. T-SPOT.TB Interferon-γ release assay performance in healthcare worker screening at nineteen U.S. hospitals. Am J Respir Crit Care Med 192: 367-373, 2015. [DOI] [PubMed] [Google Scholar]
- 15. Diel R, Loddenkemper R, Meywald-Walter K, Gottschalk R, Nienhaus A. Comparative performance of tuberculin skin test, QuantiFERON-TB-Gold In Tube assay, and T-Spot. TB test in contact investigations for tuberculosis. Chest 135: 1010-1018, 2009. [DOI] [PubMed] [Google Scholar]
- 16. Harada N, Higuchi K, Yoshiyama T, et al. Comparison of the sensitivity and specificity of two whole blood interferon-gamma assays for M. tuberculosis infection. J Infect 56: 348-353, 2008. [DOI] [PubMed] [Google Scholar]
- 17. The Prevention Committee of the Japanese Society for Tuberculosis Guidelines for using QuantiFERON TB Gold in-tube. Kekkaku (Tuberculosis) 88: 33-37, 2013. [PubMed] [Google Scholar]
- 18. World Health Organization. Global tuberculosis report 2015. WHO/HTM/TB/201522 .
- 19. The International Exchanging Committee of the Japanese Society for Tuberculosis Report from the Committee of the Japanese Society for Tuberculosis: a study of tuberculosis among foreigners resident in Japan, 2008: with particular focus on those leaving Japan in the middle of treatment. Kekkaku (Tuberculosis) 89: 5-12, 2014. [PubMed] [Google Scholar]
- 20. Ministry of Health, Labour and Welfare, Japan. [cited 2016 July 8] Avaiable from: http://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou03/14.html (in Japanese) .
- 21. Ewer K, Deeks J, Alvarez L, et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361: 1168-1173, 2003. [DOI] [PubMed] [Google Scholar]