Abstract
We experienced two patients with serious arrhythmias associated with the administration of ledipasvir (LDV) and sofosbuvir (SOF). Neither patient took amiodarone, an agent for which concomitant use is prohibited. One patient was 82 years old and hypertensive; the other was 72 years old and had no cardiovascular risk factors such as hypertension or diabetes mellitus. The arrhythmias were both serious ventricular tachycardia (VT) that converted spontaneously to sinus rhythm, without treatment, and both patients had good outcomes. These cases suggest that LDV/SOF may be associated with an increased risk of heart-related events.
Keywords: chronic hepatitis C, ledipasvir and sofosbuvir, ventricular tachycardia
Introduction
Hepatitis C virus (HCV) infects an estimated 2.3% to 2.8% of the human population worldwide. A total of 25% of patients with cirrhosis develop hepatocellular carcinoma and/or decompensated cirrhosis. In Japan, HCV infection is the main underlying factor in the progression of liver disease to hepatic cancer (1).
Since 2015, reports have shown that the use of directly acting antiviral agents (DAAs), such as sofosbuvir (SOF), to treat HCV is associated with high sustained virological response (SVR) rates, and that nearly 95% of DAA-treated patients appear to have fewer adverse reactions than those given interferon-based regimens (2-4).
In spring 2015, the Food and Drug Administration and the European Medicines Agency warned that bradyarrhythmias may occur when SOF is used with amiodarone. However, to date, no serious arrhythmias have been reported except in patients co-administered DAAs with amiodarone. We herein report our experience with two patients who developed ventricular tachycardia (VT) likely caused by treatment with ledipasvir (LDV) plus SOF (the LDV/SOF combination regimen).
Case Reports
Case 1
The patient was an 82-year-old woman with a medical history of primary aldosteronism who received treatments for hypertension, gastritis and hyperactive bladder. She had not undergone surgery for aldosteronism. She had no history of smoking or alcohol consumption. Her height was 155 cm, weight 68 kg, and body mass index (BMI) 28.3 kg/m2. Her HCV genotype was 1b. The Fibroscan testing result was 7.9 kPa, her Child-Pugh score was class A5, and the model for end-stage liver disease (MELD) score was 6, consistent with a diagnosis of compensated cirrhosis. She had no history of prior antiviral therapies including interferon. Her laboratory test results were as follows: albumin 4.3 g/dL, total bilirubin 0.52 mg/dL, aspartate aminotransferase (AST) 26 IU/L, alanine aminotransferase (ALT) 34 IU/L, alkaline phosphatase (ALP) 251 IU/L, lactate dehydrogenase (LDH) 231 IU/L, potassium (K) 4.3 mmoL/L, total cholesterol 171 mg/dL, triglyceride 79 mg/dL, high-density lipoprotein (HDL) cholesterol 50 mg/dL, low-density lipoprotein (LDL) cholesterol 96 mg/dL, blood glucose 107 mg/dL, and hemoglobin A1c 5.9%, indicating good glycemic control (Table 1). The electrocardiogram (ECG) obtained prior to admission showed sinus rhythm. The QTc ratio was 438 mm (Fig. 1).
Table 1.
Blood Test Findings on Admission.
| case 1 | case 2 | |
|---|---|---|
| White blood cells (/μL) | 4,900 | 7,600 |
| Hemoglobin (g/dL) | 12.5 | 12.7 |
| Platelets (/μL) | 10.1×104 | 17.2×104 |
| Prothrombin INR | 0.98 | 0.96 |
| Albumin (g/dL) | 4.3 | 3.7 |
| Total bilirubin (mg/dL) | 0.52 | 0.37 |
| Asparate aminotranferase (IU/L) | 34 | 60 |
| Alanine aminotranferase (IU/L) | 26 | 44 |
| Alkaline phosphatase (IU/L) | 251 | 578 |
| Lactate dehydrogenase (IU/L) | 231 | 260 |
| Blood urea nitrogen (mg/dL) | 10.3 | 17.8 |
| Creatinine (mg/dL) | 0.77 | 0.67 |
| Na (mmoL/L) | 144 | 137 |
| K (mmoL/L) | 4.3 | 4.5 |
| Cl (mmoL/L) | 107 | 107 |
| Total cholesterol (mg/dL) | 171 | 132 |
| Triglyceride (mg/dL) | 79 | 68 |
| HDL-cholesterol (mg/dL) | 50 | Not tested |
| LDL -cholesterol (mg/dL) | 96 | Not tested |
| Blood glucose (mg/dL) | 107 | 101 |
| Hemoglobin A1c (%) | 5.90 | 5.80 |
| Hepatitis B surface antigen | (-) | (-) |
| Antinuclear antibody | (-) | (-) |
| Antimitochondrial membrane (M2) | (-) | (-) |
Figure 1.
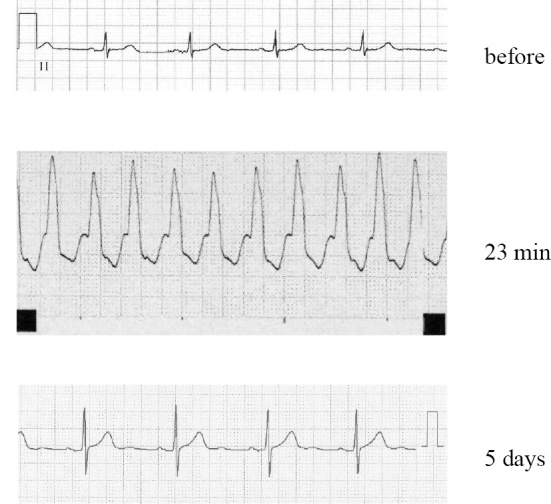
The electrocardiograms (ECGs) of patient 1. The ECG obtained prior to admission showed a sinus rhythm. There were no premature ventricular contractions (VPcs). At 23 minutes after the administration of LDV/SOF, the patient experienced dizziness, and ECG monitoring demonstrated VT for approximately 20 seconds. Holter ECG monitoring results then remained normal for 5 days after treatment.
On the first day of treatment with SOF (400 mg) and LDV (90 mg), the patient took her usual medications orally: atenolol 50 mg, amlodipine 2.5 mg, etizolam 0.5 mg, famotidine 20 mg, teprenone 50 mg and oxybutynin 73.5 mg. Twenty-three minutes after taking the LDV/SOF, the patient experienced dizziness and ECG monitoring demonstrated VT for approximately 20 seconds (Fig. 1). The VT resolved spontaneously.
She was then followed-up without the reintroduction of LDV/SOF. Her cardiac function was assessed on the seventh day after hospital admission. The findings of an echocardiogram, Holter monitoring, and heart rate monitoring 5 days after treatment were all normal (Fig. 1). The patient was discharged from the hospital 13 days after admission. VT did not recur after the initial episode.
Case 2
The patient was a 72-year-old woman with a history of surgery for pulmonary tuberculosis at 19 years of age. She had no history of allergies, smoking, or drinking consumption. Her height was 154 cm, weight 44 kg, and BMI 18.5 kg/m2. Her HCV genotype was 1b, and she had not previously received treatment for HCV infection. She was found to have compensated cirrhosis: 23.3 kPa on Fibroscan testing, a Child-Pugh score of class A5, and a HCV RNA level of 6.8 log IU/mL. She had no comorbidities and had been taking only an oral ursodeoxycholic acid agent. Her laboratory test results were as follows: albumin 3.7 g/dL, total bilirubin 0.37 mg/dL, AST 60 IU/L, ALT 44 IU/L, ALP 578 IU/L, LDH 260 IU/L, total cholesterol 132 mg/dL, triglyceride 68 mg/dL, and blood glucose 101 mg/dL (Table 1). The hepatitis B virus test result was negative. The ECG QTc ratio was 430 mm, and there was no evidence of premature ventricular contractions (VPcs).
SOF (400 mg) and LDV (90 mg) were administered. Two days later, ECG monitoring showed VT, although the patient remained asymptomatic. The VT resolved spontaneously. However, 5 days later, ECG monitoring detected a similar episode of VT.
LDV/SOF was discontinued after consultation with a cardiologist. The patient had no specific symptoms during the VT episodes. Her cardiac function was assessed using a standard ECG, echocardiogram, Holter monitoring, and heart rate monitoring; the results were unremarkable. She was discharged from the hospital 20 days after admission (Fig. 2).
Figure 2.
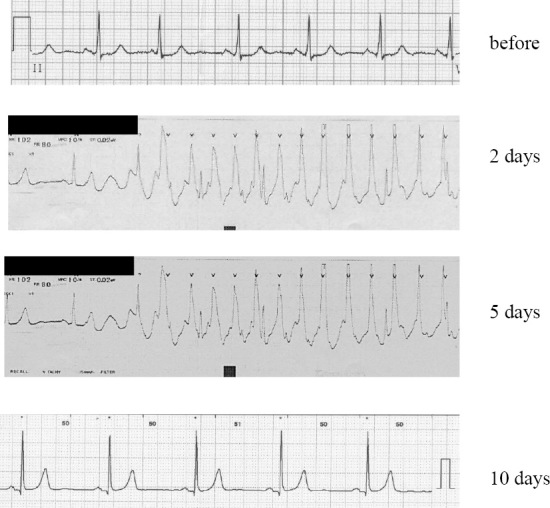
The electrocardiograms (ECGs) of patient 2. An ECG obtained upon admission showed sinus rhythm. ECGs recorded 2, 5, and 10 days after treatment are shown. VT developed 2 days after the treatment. LDV/SOF administration was continued, and 5 days later, VT developed again. After discontinuation of LDV/SOF for 10 days, no arrhythmias were detected.
Discussion
There are no reports, to our knowledge, systematically reviewing the LDV/SOF side effects with respect to cardiotoxicity. However, previous case reports and articles have warned that serious slowing of the heart rate can occur when HCV treatments, including SOF plus another antiviral drug, are administered with amiodarone (5-9). Renet et al. reported two patients experiencing extreme bradycardia when administered LDV/SOF in combination with amiodarone (5).
Renet et al.'s first patient was a 61-year-old woman with cardiovascular risk factors (hypertension and dyslipidemia) whose medical history included basilar artery aneurysm rupture, pulmonary embolism, acute coronary syndrome, persistent atrial fibrillation, and ischemic stroke. Her Child-Pugh score was class A6. She had been administered atenolol 50 mg, atorvastatin 20 mg, fluindione, and amiodarone 200 mg. Their second patient was a 50-year-old man who had diabetes mellitus and paroxysmal atrial fibrillation; his Child-Pugh score was class B9. Renet et al. described serious bradycardia as having developed when he concurrently received propranolol 40 mg and amiodarone 200 mg.
In both of Renet et al.'s patients, as described above, serious bradycardia also developed on the first day of treatment with LDV/SOF. The occurrence of the arrhythmia on the first day suggests that this adverse reaction develops relatively early after the initiation of treatment with DAAs. However, neither of our patients was given amiodarone. No bradycardia developed in our patients. The VT episodes occurred suddenly and resolved spontaneously, without resulting in cardiac arrest.
In the ION-1 trial, serious adverse events occurring in more than one patient included cellulitis, chest pain, gastroenteritis, hand fracture, non-cardiac chest pain, and pneumonia; the most common adverse events were fatigue, headache, insomnia, and nausea (2). In the ION-2 trial, no patient receiving the 12-week treatment with LDV/SOF experienced a serious adverse event (3). In subsequent trials based on the ION-3 trial, the common adverse events were fatigue, headache, nausea, and insomnia. Most of the adverse events were more common in the LDV/SOF plus ribavirin-containing arms. No serious adverse reactions were reported in the LDV/SOF-containing arms (4). One study reported that when SOF either with or without ribavirin was administered to patients with advanced cirrhosis, including those with Child-Pugh scores of class B and C, serious adverse reactions occurred in 43% and lactic acidosis in 14%. These results suggested that patients with advanced cirrhosis and those with renal failure are potentially at risk for the development of adverse reactions (10). Case 1 in the present study had cirrhosis: 7.9 kPa on Fibroscan testing, Child-Pugh score of class A5, and MELD score of 6. Renal function tests showed a creatinine level of 0.77 mg/dL, and the estimated glomerular filtration rate (eGFR) was 53.9 mL/min/1.73 m2. The same was true for Case 2, who also had cirrhosis: 23.3 kPa on Fibroscan testing, Child-Pugh score of class A5, and MELD score of 6. Her creatinine level was 0.67 mg/dL, and her eGFR was 65.1 mL/min/1.73 m2. She therefore had neither advanced cirrhosis nor an impaired renal function.
A prior case report described LDV/SOF as exacerbating glucose intolerance (11). Hyperglycemia did not occur in Case 1; her blood glucose level was 107 mg/dL on admission and 122 mg/dL on the second day of treatment (after the development of VT). The blood glucose levels of Case 2 were within the normal range of 97 to 101 mg/dL. Thus, hyperglycemia was unlikely to have induced VT in either of our patients.
Long QT syndrome (LQTS) is also associated with VT and sudden death (12, 13). We considered the possibility that LDV/SOF treatment might have affected LQTS. Before treatment, the patients' QTc values were 438 and 430 mm in Cases 1 and 2, respectively. After treatment, these values were unchanged. Secondary LQTS is known to be associated with other drugs and with the K channel structure. The K levels of our patients were within the normal range. Case 1 was being treated with 20 mg of famotidine. However, this was stopped after LDV/SOF administration, after which VT did not recur.
VT is reportedly associated with VPc (14, 15). VPc was not detected before treatment in Case 2, but Case 1 showed sporadic VPc both before and after treatment with LDV/SOF. However, her VPc was Lown grade 1, and after discontinuation of LDV/SOF, no VPc of Lown grade 2 was detected.
In Case 1, arrhythmia manifested just 23 minutes after LDV/SOF administration, a remarkably short time. LDV median peak concentrations were observed 4 to 4.5 hours after administration. However, SOF was absorbed quickly, and the peak median plasma concentration was observed 0.8 to 1 hours after administration (16). We thus cannot rule out the possibility that administering LDV/SOF triggered this arrhythmia.
In our hospital, a total of 82 (37 men and 45 women) patients had been administered LDV/SOF during the period reviewed herein. The mean age ± standard deviation and eGFR of these patients were 65.9±10.9 years and 74.6±15.6 mL/min/1.73 m2, respectively (Table 2). Seventy-nine patients had a Child-Pugh score of 5 points, and 3 had a score of 6 points. Both of our patients had a Child-Pugh score class of A and eGFR exceeding 30 mL/min/1.73 m2 (Table 2). Compared with prior reports, our patients were slightly older, but their eGFR and Child-Pugh score classes did not differ markedly from those of previously reported patients (2-4).
Table 2.
Backgrounds of Our Patients.
| Gender (M/F) | 37/45 |
| Age (years) | 65.9 ± 10.9 |
| eGFR (mL/min/1.73m2) | 74.6 ± 15.6 |
| Child-Pugh score (class A) | |
| 5 points | 79 patients |
| 6 points | 3 patients |
Given the observations made in our present cases, LDV/SOF may be associated with an increased risk of heart-related events. We report these patients' experiences to raise awareness that LDV/SOF should be administered cautiously.
Author's disclosure of potential Conflicts of Interest (COI).
Kazushige Nirei: Honoraria, Gilead Sciences. Shunichi Matsuoka: Honoraria, Gilead Sciences.
References
- 1.Katayama K, Tanaka J, Komiya Y, Kumagai J, Yoshizawa H. Significance of medical examination for HCV infection as a national project for prevention of hepatocellular carcinoma in Japan. Nihon Rinsho 62: 248-252, 2004. [PubMed] [Google Scholar]
- 2.Afdhal N, Zeuzem S, Kwo P, et al. ; ION-1 Investigators Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 15: 1889-1898, 2014(in Japanese). [DOI] [PubMed] [Google Scholar]
- 3.Afdhal N, Reddy KR, Nelson DR, et al. ; ION-2 Investigators Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 17: 1483-1493, 2014. [DOI] [PubMed] [Google Scholar]
- 4.Kowdley KV, Gordon SC, Reddy KR, et al. ; ION-3 Investigators Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 15: 1879-1888, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Renet S, Chaumais MC, Antonini T, et al. . Extreme bradycardia after first doses of sofosbuvir and daclatasvir in patients receiving amiodarone: 2 cases including a rechallenge. Gastroenterology 149: 1378-1380.e1, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Sofosbuvir + amiodarone: bradycardia and conduction disturbances. Prescrire Int 24: 294-295, 2015. [PubMed] [Google Scholar]
- 7.Brainard DM, McHutchison JG. Bradyarrhythmias associated with sofosbuvir treatment. N Engl J Med 373: 1888, 2015. [DOI] [PubMed] [Google Scholar]
- 8.Back DJ, Burger DM. Interaction between amiodarone and sofosbuvir-based treatment for hepatitis C virus infection: potential mechanisms and lessons to be learned. Gastroenterology 149: 1315-1317, 2015. [DOI] [PubMed] [Google Scholar]
- 9.Wilder JM, Jeffers LJ, Ravendhran N, et al. . Safety and efficacy of ledipasvir-sofosbuvir in black patients with hepatitis C virus infection: a retrospective analysis of phase 3 data. Hepatology 63: 437-444, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welker MW, Luhne S, Lange CM, et al. . Lactic acidosis in patients with hepatitis C virus cirrhosis and combined ribavirin/sofosbuvir treatment. J Hepatol 64: 790-799, 2016. [DOI] [PubMed] [Google Scholar]
- 11.Premji R, Roopnarinesingh N, Qazi N, Nylen ES. New-onset diabetes mellitus with exposure to ledipasvir and sofosbuvir. J Investig Med High Impact Case Rep 3: 2324709615623300, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Ponti F, Poluzzi E, Montanaro N. Organising evidence on QT prolongation and occurrence of Torsades de Pointes with non-antiarrhythmic drugs: a call for consensus. Eur J Clin Pharmacol 57: 185-209, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Clancy CE, Kurokawa J, Tateyama M, Wehrens XH, Kass RS. K+ channel structure-activity relationships and mechanisms of drug-induced QT prolongation. Annu Rev Pharmacol Toxicol 43: 441-461, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Lown B, Wolf M. Approaches to sudden death from coronary heart disease. Circulation 44: 130-142, 1971. [DOI] [PubMed] [Google Scholar]
- 15.Lown B, Graboys TB. Management of patients with malignant ventricular arrhythmias. Am J Cardiol 26: 910-918, 1977. [DOI] [PubMed] [Google Scholar]
- 16. Giliead HARVONIⓇ (ledipasvir and sofosbuvir) tablets, for oral use Initial U.S. Giliead; 15: 2016. [cited 2017 Feb 3] Available from: https://www.gilead.com/~/media/Files/pdfs/medicines/liver-disease/harvoni/harvoni_pi.pdf . [Google Scholar]


