Abstract
Pulmonary artery sarcoma is highly malignant and easily metastasizes to the systemic organs. Both the introduction of novel diagnostic procedures and the development of new treatment modalities are required to achieve long-term survival. Several studies have shown that platelet-derived growth factor receptor α (PDGFRα) gene amplification is frequently observed in pulmonary artery sarcoma. PDGFRα is known to be involved in cell proliferation in certain malignancies. PDGFRα may become a potential biological marker in pulmonary artery sarcoma. We report a case in which a diagnosis of pulmonary artery sarcoma overexpressing PDGFRα was made using endovascular catheter biopsy following positron emission tomography with integrated computed tomography (PET/CT) scans.
Keywords: pulmonary artery sarcoma, PDGFRα, PET/CT scans, endovascular catheter biopsy
Introduction
Pulmonary artery sarcoma is a rare and highly malignant neoplasm arising from the multipotential mesenchymal cells of the intimal vessel layer. It presents as vascular obstruction due to intraluminal growth and associated thrombosis. The clinical findings often mimic chronic pulmonary thromboembolism, as does the progression to pulmonary artery hypertension (1, 2). The diagnosis is difficult and therefore frequently delayed. Previously, the definitive diagnosis was made by surgery or autopsy. Additionally, pulmonary artery sarcoma easily metastasizes to organs such as the lung and brain, because the tumor is located within the circulation to the lung and the systemic organs. The results of surgical treatment are not favorable, even when the tumor is completely resected, with a median overall survival of 17.0-26.8 months after surgery (2-4). Both the introduction of novel diagnostic procedures and the development of new treatment modalities are required to achieve long-term survival.
Platelet-derived growth factor receptor α (PDGFRα) is involved in cell proliferation, mainly through the mitogen-activated protein kinase (MAPK) and protein kinase B (AKT) signaling pathways in certain malignancies (5). Several studies have shown that PDGFRα gene amplification is frequently observed and overexpression of PDGFRα activates the MAPK and AKT signaling pathways in pulmonary artery sarcoma (6, 7). These findings suggested that PDGFRα may become a potential biological marker in pulmonary artery sarcoma. We describe a case in which a diagnosis of pulmonary artery sarcoma overexpressing PDGFRα was made using an endovascular catheter biopsy following positron emission tomography with integrated computed tomography (PET/CT) scans.
Case Report
A 52-year-old woman who had never smoked visited our hospital because of fatigue and dyspnea for 3 months. The patient had undergone complete resection of thyroid papillary adenocarcinoma at the age of 51 years. There was no medical history of factors predisposing her to embolism or episodes of venous thromboembolism. On a physical examination, the respiratory rate was 20 breaths/min, blood pressure was 132/70 mmHg, pulse rate was 88 beats/min, and the temperature was 36.6℃. Cardiac and pulmonary auscultation revealed no abnormal sounds. No leg edema was present. Laboratory blood tests and plasma coagulation function parameters were in the normal range. An analysis of arterial blood gases revealed a PaO2 of 77.8 Torr, PaCO2 of 41.6 Torr, and O2 saturation of 94.1%.
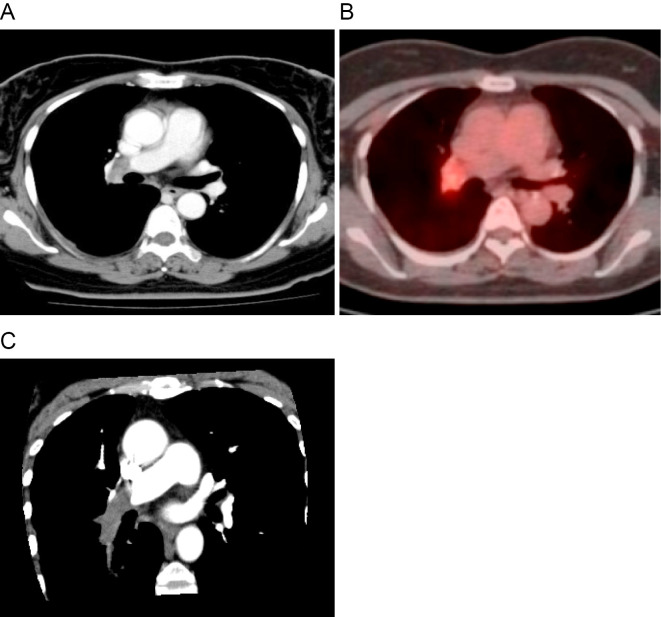
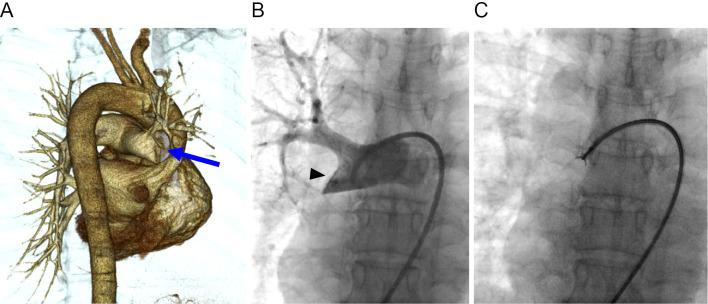
A chest roentgenogram showed a post-inflammatory change in the subpleural region of the right middle lung field. Contrast-enhanced chest CT (Fig. 1A) and 18F-fluorodeoxyglucose PET/CT (Fig. 1B) revealed a 2.7-cm, soft-tissue density mass with high uptake of 18F-fluorodeoxyglucose in the right distal main pulmonary artery. A multiplanar reconstruction image (oblique plane, Fig. 1C) showed the mass within the right distal main pulmonary artery that extends to the descending interlobar artery and the segmental branches. Three-dimensional CT angiography (posterior oblique view, Fig. 2A) and pulmonary angiography (Fig. 2B) showed a large filling defect in the right distal main pulmonary artery adjacent to the right upper lobe artery, and the descending interlobar artery was completely occluded by the lesion. An endovascular catheter biopsy using forceps for myocardial biopsy was then performed (Fig. 2C).
Figure 1.
Radiological examinations. (A) Contrast-enhanced computed tomography (CT) shows a soft-tissue density mass in the right distal main pulmonary artery. (B) 18F-fluorodeoxyglucose positron emission tomography with integrated CT shows the mass with a high uptake of 18F-fluorodeoxyglucose in the right distal main pulmonary artery. (C) A multiplanar reconstruction image (oblique plane) shows an intravascular mass within the right distal main pulmonary artery that extends to the descending interlobar artery and the segmental branches.
Figure 2.
Angiographic examinations. (A) Three-dimensional computed tomographic angiography (posterior oblique view) shows complete occlusion of the right distal main pulmonary artery (arrow). (B) pulmonary angiography shows a filling defect occupying the entire lumen of the right distal main pulmonary artery (arrowhead). (C) An endovascular catheter biopsy using forceps is performed following pulmonary angiography.
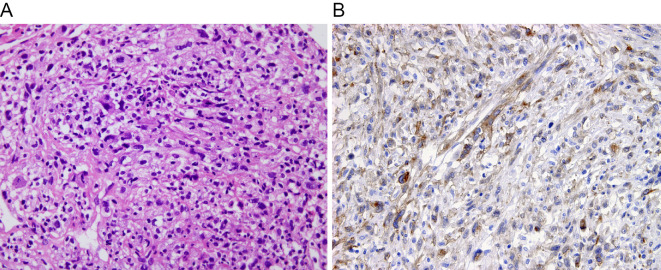
The biopsy specimens showed fascicular proliferation of spindle cells with pleomorphic nuclei in a background of fibrin and necrosis (Fig. 3A). The tumor cells were immunohistochemically positive for vimentin and weakly positive for CD31, but negative for CD34, factor VIII, desmin, α-smooth muscle actin, and c-KIT. The tumor cells overexpressed Ki-67 (labeling index of 50%), a cell proliferation marker. Thus, the tumor was diagnosed as a high-grade sarcoma of the pulmonary artery. Further immunohistochemical examination using the PDGFRα antibody (catalogue number SC338, Santa Cruz) showed that more than 50% of the tumor cells were positive for PDGFRα (Fig. 3B).
Figure 3.
Histological examinations. (A) The tumor is composed of fascicular and poorly-arranged proliferation of atypical spindle cells. The tumor cells are anaplastic, with atypical nuclei and some multinucleated cells (Hematoxylin and Eosin staining, original magnification 200×). (B) The tumor cells are immunohistochemically positive for platelet-derived growth factor receptor α (original magnification 200×).
A Doppler echocardiogram showed a tricuspid regurgitant pressure gradient (TRPG) of 55.4 mmHg, reflecting pulmonary artery hypertension. Pulmonary function tests showed a vital capacity (VC) of 2.03 L, 79% of the predicted value; forced expiratory volume in one second (FEV1) of 1.63 L, 71% of the predicted value; carbon monoxide diffusing capacity (DLco) of 9.33 mL/min/mmHg, 48% of the predicted value; and DLco adjusted for volume (DLco/VA) of 3.60 mL/min/mmHg/L, 73% of the predicted value. Underlying pulmonary diseases such as chronic obstructive pulmonary disease and pulmonary fibrosis were not detected. The reduced pulmonary function may have been due to both the obstruction of the pulmonary artery and the unknown lesions of the pulmonary parenchyma and airway.
As a treatment, surgical resection including right pneumonectomy was considered quite difficult. The coexistence of pulmonary artery hypertension with a reduced pulmonary function increased the risk of operative complications, including respiratory failure, right cardiac failure, and mortality. Treatment with chemotherapy was selected for the patient. Although 2 cycles of doxorubicin 60 mg/m2 were administered every 4 weeks, the tumor grew. Anticoagulant treatment with warfarin and beraprost, however, decreased the pulmonary artery hypertension from a TRPG of 55.4 mmHg to 30.0 mmHg. Since no metastasis was found at that time, surgical resection was re-considered. Resection of the right lung and pulmonary artery was performed six months after the first visit. The tumor, measuring 4.0 cm, extended into the pulmonary artery and invaded the surrounding lung parenchyma and hilar lymph nodes. No tumor cells were seen in the mediastinal lymph nodes or the stump of resected tissues. Histologically, the center part of the tumor was replaced with hyalinizing tissue, and the peripheral evolving part consisted of fascicular proliferation of spindle tumor cells. Thereafter, the disease metastasized to the brain, lung, and adrenal glands despite treatment. The patient died from respiratory failure 12 months after the first visit.
Discussion
Since pulmonary artery sarcoma originates from the pluripotent mesenchymal stem cells of the intimal vessel layer, the histological features are undifferentiated or show heterogeneous components. The immunohistochemical profiles also vary with differentiation towards the components (1). In general, undifferentiated sarcoma is diffusely positive for vimentin and focally positive for α-smooth muscle actin. Angiosarcoma expresses endothelial markers such as CD31, CD34, and factor VIII. Leiomyosarcoma exhibits a different extent of α-smooth muscle actin and desmin. In the present case, the tumor was weakly positive for CD31 but negative for CD34, factor VIII, α-smooth muscle actin, and desmin. Although the tumor may differentiate, at least in part, towards the characteristics of endothelial cells, a diagnosis of angiosarcoma was not made because of the absence of CD34 and factor VIII expression. Thus, the patient was diagnosed with sarcoma of the pulmonary artery without showing distinct differentiation.
The diagnosis of pulmonary artery sarcoma is usually delayed, since symptoms are insidious and nonspecific. In particular, pulmonary artery sarcoma is frequently misdiagnosed as pulmonary thromboembolism (1, 2). Most pulmonary artery sarcomas arise from the pulmonary artery trunk or the main pulmonary artery. When a filling defect in the central pulmonary artery is seen on contrast-enhanced chest CT in patients without predisposing factors for embolism or who have no symptom relief after anticoagulant therapy, the alternative diagnosis of pulmonary artery sarcoma should be considered. Several investigators have recently reported the diagnostic usefulness of PET/CT scans for distinguishing pulmonary artery sarcoma from pulmonary thromboembolism (2, 8). Although a pathological examination is required to establish the correct diagnosis of pulmonary artery sarcoma, the diagnosis is usually made at surgery or autopsy. There are a few reports of a pathological diagnosis obtained using an endovascular catheter biopsy (2, 9). When the lesion within the pulmonary artery shows a high uptake of 18F-fluorodeoxyglucose, an endovascular catheter biopsy should be performed to make an early diagnosis.
Pulmonary artery sarcoma is highly lethal and easily metastasizes to the lung, brain, kidney, and adrenal glands. The median overall survival is approximately 17 months, although this includes patients receiving surgical treatment (2-4). Most patients die from metastatic disease, probably due to the early spread of tumor cells into the circulation from the pulmonary artery. Several investigators have reported that pulmonary artery sarcoma has amplification of the PDGFRα gene and constitutive activation of the PDGFRα protein (6, 7). Additionally, overexpression of PDGFRα activates MAPK and AKT signaling pathways in cultured tumor cells obtained from a patient with pulmonary artery sarcoma (7). Further studies are required in order to explore whether or not PDGFRα has utility as a biological marker or therapeutic target in pulmonary artery sarcoma.
To our knowledge, there have been no reports of cases in which PDGFRα positive pulmonary artery sarcoma was diagnosed with endovascular catheter biopsy following PET/CT scans. An early diagnosis with PET/CT scans and an endovascular catheter biopsy and the evaluation of biological markers may encourage the introduction of novel treatment, leading to long-term survival in patients with pulmonary artery sarcoma.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Chen D, Zhu G, Wang D, Zhang Z, Fang W, Qu Z. Clinicopathological and immunohistochemical features of pulmonary artery sarcoma: a report of three cases and review of the literature. Oncol Lett 11: 2820-2826, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mussot S, Ghigna MR, Mercier O, et al. . Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg 43: 787-793, 2013. [DOI] [PubMed] [Google Scholar]
- 3. Wong HH, Gounaris I, McCormack A, et al. . Presentation and management of pulmonary artery sarcoma. Clin Sarcoma Res 5: 3, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Grazioli V, Vistarini N, Morsolini M, et al. . Surgical treatment of primary pulmonary artery sarcoma. J Thorac Cardiovasc Surg 148: 113-118, 2014. [DOI] [PubMed] [Google Scholar]
- 5. Heldin CH. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun Signal 11: 97, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao J, Roth J, Bode-Lesniewska B, Pfaltz M, Heitz PU, Komminoth P. Combined comparative genomic hybridization and genomic microarray for detection of gene amplifications in pulmonary artery intimal sarcomas and adrenocortical tumors. Genes Chromosomes Cancer 34: 48-57, 2002. [DOI] [PubMed] [Google Scholar]
- 7. Dewaele B, Floris G, Finalet-Ferreiro J, et al. . Coactivated platelet-derived growth factor receptor α and epidermal growth factor receptor are potential therapeutic targets in intimal sarcoma. Cancer Res 70: 7304-7314, 2010. [DOI] [PubMed] [Google Scholar]
- 8. Chong S, Kim TS, Kim BT, Cho EY, Kim J. Pulmonary artery sarcoma mimicking pulmonary thromboembolism: integrated FDG PET/CT. Am J Roentgenol 188: 1691-1693, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Yamada N, Kamei S, Yasuda F, Isaka N, Yada I, Nakano T. Primary leiomyosarcoma of the pulmonary artery confirmed by catheter suction biopsy. Chest 113: 555-556, 1998. [DOI] [PubMed] [Google Scholar]