Abstract
This study aimed to investigate the relationship between carotid atherosclerosis and left ventricular hypertrophy on electrocardiogram (ECG-LVH) on adults living in the community. A total of 9,266 adults who participated in the Namwon Study were included in this analysis. Carotid atherosclerosis, including intima-media thickness (IMT) and plaques, were assessed using high-resolution B-mode ultrasound. ECG-LVH was determined using the Sokolow-Lyon voltage (SokV) and Cornell voltage (CorV) criteria. The prevalence of ECG-LVH was 12.7% using the SokV criteria and 9.7% using the CorV criteria. After full adjustment, compared to the lowest quartile of common carotid artery IMT (CCA-IMT), the odds ratios and 95% confidence intervals for ECG-LVH of the carotid IMT quartiles 2, 3, and 4 increased linearly as follows: 1.54 (1.24-1.90), 1.62 (1.31-2.02), and 1.91 (1.54-2.38), respectively, for the SokV criteria (p<0.001); and 1.33 (1.05-1.68), 1.41 (1.11-1.78), and 1.48 (1.16-1.88), respectively, for the CorV criteria (p=0.003). Positive associations between the carotid bulb IMT (CB-IMT) quartiles and the ECG-LVH were also observed, although the magnitudes of association between CB-IMT and ECG-LVH were slightly lower than those of CCA-IMT. However, no significant association between carotid plaques and ECG-LVH as defined by the SokV or CorV criteria was found. The present study demonstrated that increased carotid IMT, but not carotid plaques, is significantly associated with LVH defined by various ECG criteria in a large population.
Keywords: Hypertrophy, Left Ventricular; Carotid Intima-Media Thickness; Plaque, Atherosclerotic; Adult
INTRODUCTION
Cardiovascular disease (CVD) accounts for nearly one-third of all deaths worldwide, and coronary artery disease (CAD) is the main contributor to CVD.1,2 Although the total CVD mortality rate has decreased over a 30-year period in South Korea, the CAD mortality rate has continuously increased until recently.3 Left ventricular hypertrophy (LVH) is a strong predictor of CVD morbidity and mortality, including CAD, stroke, and congestive heart failure.4,5,6 Epidemiological studies have shown that LVH on the electrocardiogram (ECG-LVH) is a potent risk factor for future CVD events and death.7,8 Moreover, randomized clinical trials have shown that regression of ECG-LVH during medical treatment is associated with a decrease in the prevalence and incidence of CVD.9,10,11
Evaluation of carotid arteries with B-mode ultrasound is a reliable, cost-effective, and widely used method for the assessment of carotid atherosclerosis. Epidemiological studies have determined that carotid atherosclerotic findings, such as carotid intima-media thickness (IMT) and plaque, are a surrogate of subclinical atherosclerosis and a predictor of CVD.12,13,14 Moreover, it has recently been reported that carotid ultrasonographic indices are potent predictors of death and cardiovascular events in patients with CAD, suggesting that carotid plaque might have greater prognostic significance than IMT.15,16
Although carotid atherosclerosis and LVH are principal risk factors for CVD morbidity and mortality, little is known about the relationship between carotid atherosclerosis and ECG-LVH in the general population.17 Therefore, to date, the relationship between carotid atherosclerosis and ECG-LVH remains undetermined. To gain additional insights into the potential role of carotid atherosclerosis in the development of LVH, it is important to examine the effect of carotid atherosclerotic phenotypes on ECG-LVH. Thus, in this large cross-sectional population-based study, we evaluated the association between carotid atherosclerosis and LVH (defined by commonly used electrocardiographic voltage criteria) in Korean adults.
MATERIALS AND METHODS
1. Study population
A total of 10,667 subjects aged 45-74 years were enrolled in the baseline survey of the Namwon Study between 2004 and 2007. The Namwon Study, whose cohort profile was published previously,18 is an ongoing population-based prospective study designed to examine the prevalence, incidence, and risk factors for chronic disease in rural areas of Korea. Of the total subjects, 444 patients with missing or poor carotid ultrasonography images, and 791 subjects with missing information on ECG, were excluded. Finally, a total of 9,266 participants were included in the analyses after further excluding 166 subjects whose records lacked information regarding anthropometric or laboratory measures. This study was conducted in accordance with the Declaration of Helsinki guidelines. All subjects were well-informed of the study contents and provided informed consent. The study protocol was independently approved by the Institutional Review Board of Chonnam National University Hospital (I-2007-07-062).
2. Carotid ultrasonography
A high-resolution B-mode ultrasound system (SONOACE 9900; Medison, Seoul, Korea) equipped with a 7.5-MHz linear array transducer was used to measure carotid IMT and to determine the presence of carotid plaque. IMT was defined as the distance from the intima-lumen interface to the media-adventitia interface on the far wall in a region free of plaque. Carotid plaque was defined as focal structures that encroached into the lumen by an area at least 100% thicker than the surrounding IMT value of the common carotid artery (CCA), carotid bulb (CB), or internal carotid artery (ICA). IMT of the CCA (CCA-IMT) was measured between the origin of the CB and a point 10 mm proximal to the CCA. IMT of the CB (CB-IMT) was measured in the CB region. In the present study, the CCA-IMT and CB-IMT values were determined as the average of the maximum IMT values of the right and left CCA and CB, respectively. Two sonographers performed carotid ultrasounds. The correlation coefficients for inter-sonographer and intra-sonographer variability of the carotid IMT were 0.86 and 0.90, respectively. The kappa coefficients of the carotid plaques were 0.76 for inter-sonographer and 0.85 for intra-sonographer agreement. One trained reader analyzed still images obtained from carotid ultrasound. The correlation coefficient for intra-reader variability of carotid IMT was 0.94. The kappa coefficient of carotid plaques was 0.91 for intra-reader agreement.
3. Electrocardiographic measurement
Trained research technicians performed a 12-lead ECG examination using a 12-channel ECG device (PageWriter-200; Hewlett-Packard, MA, USA). Patients were able to freely breathe and did not talk during the procedure, and ECG recordings were performed in a comfortable supine position at a speed of 25 mm/sec and a voltage of 0.1 mV/mm. The heart rate (30 sec), pulse rate interval, QRS duration, QT interval, and corrected QT interval were obtained from ECG records. Research staff trained by cardiologists measured the R-wave amplitudes in leads aVL, V5, and V6, and the S-wave amplitudes in leads V1 and V3. LVH was determined as the voltage sum of SV 1+RV 5 or V 6≥35 mm using the Sokolow-Lyon voltage (SokV) criteria19 and as RaVL+SV 3≥28 mm for males and ≥20 mm for females using the Cornell voltage (CorV) criteria.20
4. Measurements and assessments of covariates
The participants' heights were measured to the nearest 0.1 cm; their weights were measured to the nearest 0.1 kg in light clothing without shoes. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). After a 5-min rest in the sitting position, blood pressure was measured to the nearest 2 mmHg on the right upper arm using a standard mercury sphygmomanometer (Baumanometer; WA Baum Co., Inc., Copiague, NY, USA). Three consecutive measurements of systolic blood pressure (SBP) and diastolic blood pressure (DBP) were performed at 1-min intervals, and the average values were used in the analysis. Venous blood samples and urinary specimens were collected after a 12-h overnight fast. Total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, triglycerides, and fasting blood glucose (FBG) were analyzed using an automatic analyzer with enzymatic methods (Model 7600 Chemical Analyzer; Hitachi Ltd., Tokyo, Japan). High-sensitivity C-reactive protein (hsCRP) was measured by latex-enhanced nephelometry using a high-sensitivity assay analyzer (Behring Nephelometer II; Dade-Behring Diagnostics, Marburg, Germany).
Information concerning cigarette smoking, alcohol consumption, and medication (hypertension, diabetes, and dyslipidemia) was obtained with a standardized questionnaire by trained research staff. Smoking status was classified as never, former, or current smoker. Alcohol consumption was calculated using the usual quantity of alcohol consumed and drinking frequency, and grouped into the following categories: none, 0.1-1.9 drinks/day, 2.0-3.9 drinks/day, and ≥4.0 drinks/day.
5. Statistical analysis
Data was presented as means ± standard deviation for continuous variables and frequencies (percentages) for categorical variables. Characteristics were compared between subjects with and without LVH using the chi-squared test and the independent t-test. Carotid IMT values were classified into quartiles for CCA-IMT (quartile 1: median value 0.594, interquartile range 0.549-0.609, range [min-max] 0.387-0.641 mm; quartile 2: median value 0.688, interquartile range 0.670-0.698, range [min-max], 0.642-0.732 mm, quartile 3: median value 0.775, interquartile range 0.733-0.779, range [min-max] 0.733-0.822 mm; quartile 4: median value 0.876, interquartile range 0.859-0.959, range [min-max] 0.823-1.839 mm) and for CB-IMT (quartile 1, median value 0.688, interquartile range 0.640-0.707, range [min-max] 0.420-0.733 mm; quartile 2: median value 0.785, interquartile range 0.777-0.823, range [min-max] 0.734-0.830 mm, quartile 3: median value 0.875, interquartile range 0.869-0.914, range [min-max] 0.831-0.932 mm; quartile 4: median value 1.01, interquartile range 0.966-1.10, range [min-max] 0.933-2.070 mm). The differences and linear trends in the proportions of ECG-LVH by carotid IMT quartile, and the presence of carotid plaque, were compared using the independent t-test. Furthermore, independent associations between carotid atherosclerosis and ECG-LVH were analyzed sequentially using multiple logistic regression. Model 1 was adjusted for age and gender and model 2 was further adjusted for smoking status, alcohol consumption, BMI, heart rate, SBP, FBG, TC, HDL cholesterol, hsCRP, and anti-hypertensive, anti-diabetic, and anti-dyslipidemic medications. The adjusted odds ratios (ORs) for ECG-LVH across the carotid IMT quartiles were presented with 95% confidence intervals (CIs) using the first quartile as the reference category. Linear trends were evaluated after viewing carotid IMT categories as continuous variables. Additionally, the ORs and 95% CIs for ECG-LVH, associated with the presence versus absence of carotid plaque, were compared. All statistical analyses were performed using SPSS software (ver. 22.0; SPSS Inc., Chicago, IL, USA). A value of p<0.05 was considered to indicate statistical significance.
RESULTS
1. Characteristics of the study population
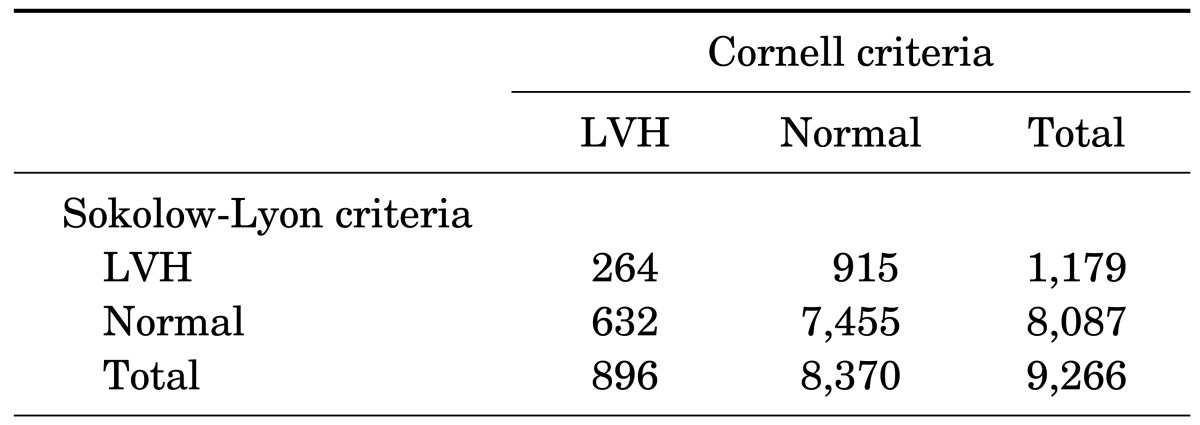
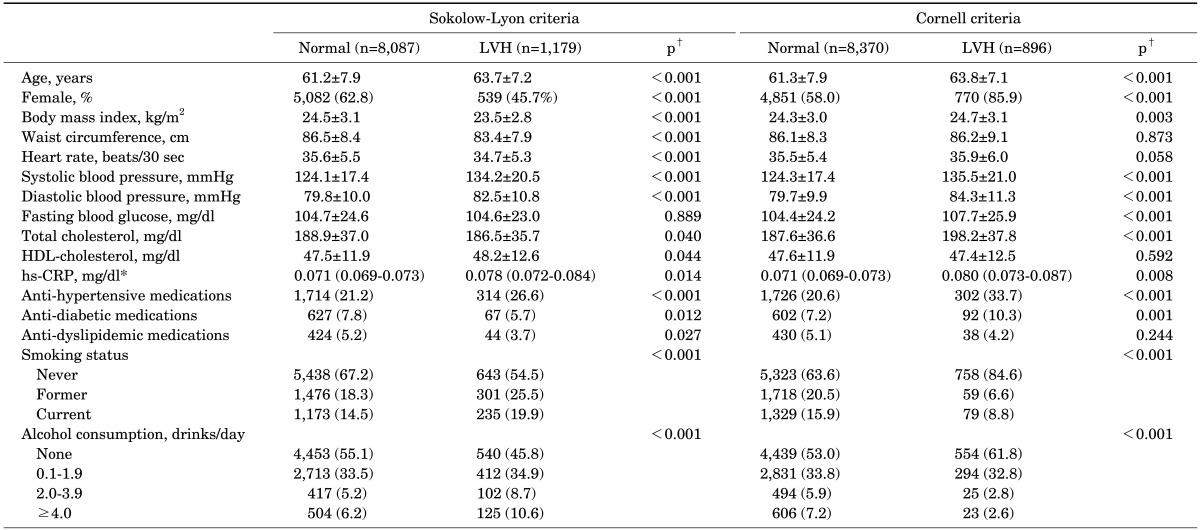
The prevalence of ECG-LVH was 12.7% using the SokV criteria and 9.7% using the CorV criteria. The percent agreement was high (83.3%), but the Kappa statistics value was poor (0.16) (Table 1). The study population characteristics according to ECG-LVH are shown in Table 2. For both the SokV and CorV criteria, subjects with LVH were older than those without LVH (p<0.001). The proportion of females was lower in the LVH group compared with the control group when using the SokV criteria, while it was greater in the LVH group compared with the control group when using the CorV criteria. Significant differences in smoking status and alcohol consumption between the control and LVH groups were observed using both the SokV and CorV criteria.
TABLE 1. Agreement of LVH using Kappa statistics.
LVH: left ventricular hypertrophy.
TABLE 2. Baseline characteristics according to the presence of LVH.
Values are expressed as means ± standard deviation or geometric means (95% confidence intervals)* for continuous variables, and frequency (percentage) for categorical variables.
LVH, left ventricular hypertrophy; HDL, high-density lipoprotein; hs-CRP, high sensitivity C-reactive protein.
†p-values were obtained using analysis of variance for continuous variables and the chi-square test for categorical variables.
Using the SokV criteria, SBP and DBP, HDL cholesterol, and hsCRP were significantly higher in subjects with LVH than in the control group, while BMI, waist circumference, heart rate, and TC were lower. The proportion of patients taking anti-hypertensive medication was higher in the LVH group defined by the SokV criteria, while the proportion of patients taking anti-diabetic and anti-dyslipidemic medications was lower than in the control group. Using the CorV criteria, BMI, SBP and DBP, FBG, TC, and hsCRP were significantly higher in subjects with LVH compared with control subjects. The proportion of patients taking anti-hypertensive and anti-diabetic medications was higher in subjects with LVH defined by the CorV criteria than in the control group.
2. LVH prevalence by carotid IMT and plaque
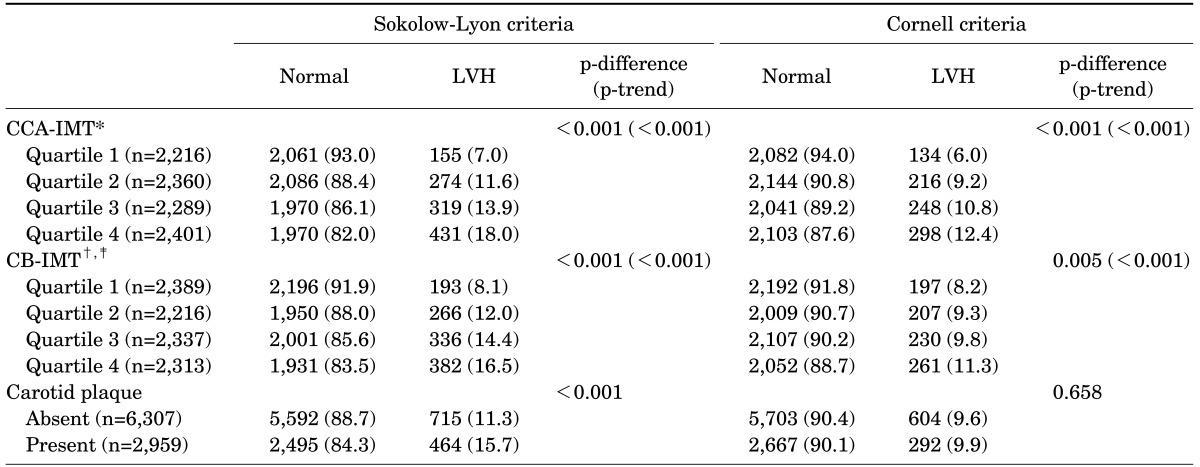
The prevalence of LVH defined by the SokV criteria increased with increasing CCA-IMT quartiles (7.0%, 11.6%, 13.9%, and 18.0%, respectively) (p<0.001) and that of LVH defined by the CorV criteria showed a similar trend according to increasing quartiles (6.0%, 9.2%, 10.8%, and 12.4%, respectively) (p<0.001). The prevalence of LVH defined by the SokV criteria increased by 8.1%, 12.0%, 14.4%, and 16.5% (p-trend<0.001) for the first, second, third, and fourth CB-IMT quartiles, respectively, and that defined by the CorV criteria increased by 8.2%, 9.3%, 9.8%, and 11.3% (p<0.001), respectively. According to the SokV criteria, the prevalence of LVH was 15.7% in subjects with carotid plaque and 11.3% in those without carotid plaque (p<0.001). However, according to the CorV criteria, no significant difference was observed between subjects with carotid plaque and those without (p=0.658; Table 3).
TABLE 3. Prevalence of LVH assessed with carotid IMT and plaque.
Values are expressed as number (percentile).
LVH, left ventricular hypertrophy; CCA, common carotid artery; IMT, intima-media thickness; CB, carotid bulb.
*Quartile 1: median value 0.594, interquartile range 0.549-0.609, range (min-max) 0.387-0.641 mm; quartile 2: median value 0.688, interquartile range 0.670-0.698, range (min-max), 0.642-0.732 mm, quartile 3: median value 0.775, interquartile range 0.733-0.779, range (min-max) 0.733-0.822 mm; quartile 4: median value 0.876, interquartile range 0.859-0.959, range (min-max) 0.823-1.839 mm.
†Quartile 1, median value 0.688, interquartile range 0.640-0.707, range (min-max) 0.420-0.733 mm; quartile 2: median value 0.785, interquartile range 0.777-0.823, range (min-max) 0.734-0.830 mm, quartile 3: median value 0.875, interquartile range 0.869-0.914, range (min-max) 0.831-0.932 mm; quartile 4: median value 1.01, interquartile range 0.966-1.10, range (min-max) 0.933-2.070 mm.
‡Eleven subjects with missing CB-IMT information were excluded.
3. Association between carotid IMT, plaque, and LVH
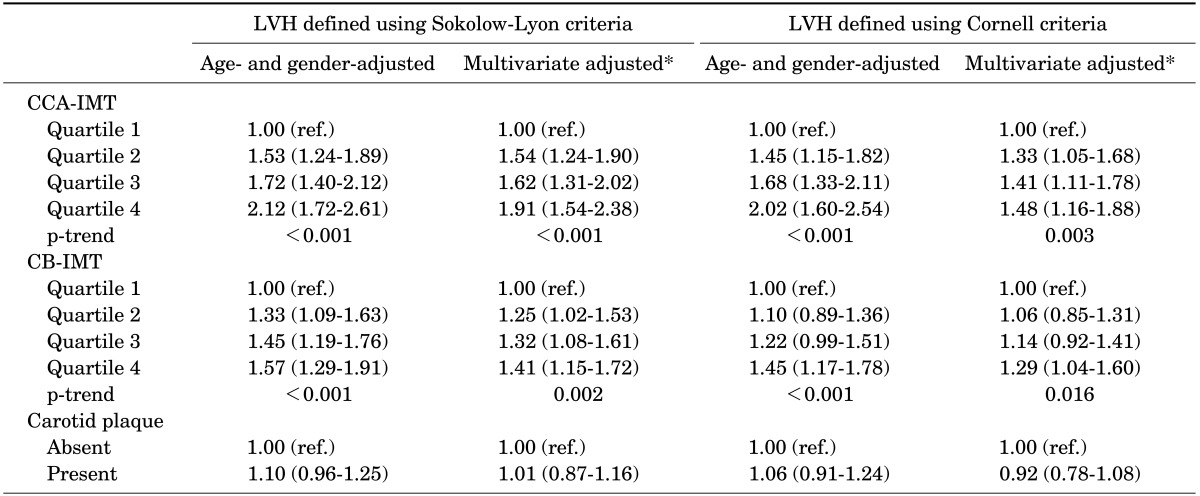
The associations between carotid atherosclerosis and ECG-LVH using logistic regression analysis are presented in Table 4. Using both the SokV and CorV criteria, the age- and gender-adjusted ORs for LVH increased as the CCA-IMT quartiles increased (p<0.001). After multivariate adjustment, compared to the lowest CCA-IMT quartile, the ORs and 95% CIs for ECG-LVH carotid IMT quartiles increased linearly and were 1.54 (1.24-1.90) for the second quartile, 1.62 (1.31-2.02) for the third quartile, and 1.91 (1.54-2.38) for the fourth quartile using the SokV criteria (p<0.001) and 1.33 (1.05-1.68) for the second quartile, 1.41 (1.11-1.78) for the third quartile, and 1.48 (1.16-1.88) for the fourth quartile using the CorV criteria (p=0.003). In addition, the age- and gender-adjusted ORs for LVH increased as the CB-IMT quartiles increased (p<0.001) using both the SokV and CorV criteria. After full adjustment, compared to the lowest CB-IMT quartile, the ORs and 95% CI for LVH increased for both the SokV (1.25 (1.02-1.53), 1.32 (1.08-1.61), and 1.41 (1.15-1.72) for the second, third, and fourth quartiles, respectively; p<0.001) and the CorV (1.06 (0.85-1.31), 1.14 (0.92-1.41), and 1.29 (1.04-1.60) for the second, third, and fourth quartiles, respectively; p=0.017) criteria. However, no significant association between carotid plaque and LVH (defined by the SokV or CorV criteria) was found in a logistic regression analysis.
TABLE 4. Associations between carotid atherosclerosis and LVH using multiple logistic regression.
Values are expressed as odds ratios (95% confidence intervals).
LVH, left ventricular hypertrophy; CCA, common carotidartery; IMT, intima-media thickness; CB, carotid bulb.
*Adjusted for age, gender, smoking status, alcohol consumption, body mass index, heart rate, systolic blood pressure, fasting blood glucose, total cholesterol, high-density lipoprotein cholesterol, high-sensitivity C-reactive protein (log), anti-hypertensive medication, anti-diabetic medication, and anti-dyslipidemic medication.
DISCUSSION
We explored the cross-sectional associations between carotid atherosclerosis and ECG-LVH in a community-based Korean population aged 45-74 years. Carotid IMT, including CCA-IMT and CB-IMT, was independently and linearly associated with ECG-LVH, while this association was more strongly present in ECG-LVH defined by the SokV criteria than by the CorV criteria. However, no significant association between carotid plaque and ECG-LVH was determined.
Although echocardiography is the preferred method used to diagnose LVH in clinical practice, ECG is commonly used as the first-line instrument for detecting LVH due to its convenience, good reproducibility, cost-effectiveness, and availability in large cohort settings.21 However, ECG-LVH may be a better predictor of cardiovascular complications than LVH detected by echocardiography (Echo-LVH).22 Both ECG and echocardiography should be performed as they predict total and cardiovascular mortality independently of each other; ECG-LVH provides independent prognostic information on cardiovascular risk even after adjusting for left ventricular mass.23,24 Recent studies have shown that both ECG-LVH and Echo-LVH are independent risk factors for various CVDs.25,26,27 ECG-LVH and Echo-LVH can be used alternately for the prediction of stroke or congestive heart failure because, both have been shown to be similarly associated with increased risk.25,26 Therefore, ECG-LVH, independent of Echo-LVH, is regarded as an electrophysiological marker for future CVD.27
The present study showed that among carotid atherosclerotic indices, IMT, but not plaque, is significantly associated with ECG-LVH. It is not clear why this is so. One possible explanation is that increased IMT and atherosclerotic plaque formation reflect different stages, and distinct pathobiological aspects, of atherosclerosis,28 although they have been used as a surrogate of CVD.12,14 Carotid IMT thickening, which is associated with hypertrophy of the intima and media as a compensatory non-atherosclerotic alteration, represents an early stage of atherosclerosis.29,30 Plaque formation, which is more prevalent in CB or ICA than CCA, is a pathology of the intima and reflects a more advanced phase of atherosclerotic disease, caused by oxidation, inflammation, endothelial dysfunction, and smooth muscle cell proliferation.31 Epidemiological studies revealed that carotid IMT thickening is more closely associated with hypertension and ischemic stroke, while carotid plaque is more strongly associated with hyperlipidemia and CAD.12,32,33
Variability in subjects and devices, as well as differences in IMT measurements, plaque definition, and methods of determining LVH (ECG or Echo) between previous studies and our study should be considered. Epidemiological studies have shown that carotid atherosclerosis was significantly associated with Echo-LVH.34,35,36 The study by Cuspidi et al. in untreated and uncomplicated essential hypertension found a significant association between carotid thickening and Echo-LVH defined as an increase in left ventricular mass.34 Cardiac hypertrophy defined as Echo-LVH was significantly associated with the presence of carotid plaque.35,36 In addition to these studies, carotid plaque was significantly associated with ECG-LVH in 349 asymptomatic subjects.17 However, the abovementioned studies included only small sample sizes and hypertensive subjects, used plaque as a marker and not IMT, or used Echo-LVH. Our results revealed that carotid IMT thickening was positively associated with ECG-LVH in a large population, while carotid plaque was not.
In our study investigating LVH determined by both the SokV and CorV criteria, the strength of the association between carotid IMT and ECG-LVH was greater when using the CCA region for the IMT measurement compared with the CB region. A possible explanation for this is as follows. When plaque was present in the IMT measurement region of CCA and CB in our study, we measured IMT in a region free of plaque. Plaque was more prevalent in the CB and the origin of the ICA than in the CCA. Although ultrasound identifies later stages of atherosclerosis such as plaque, which may be identified either in the absence of, or in conjunction with, increased IMT, it cannot clearly identify the intermediate stages between increasing IMT and plaque formation.30 Thus, particularly in the CB region, IMT and plaque may not have been definitively separated in the present study. Compared with the other regions used for IMT measurements, the segment that is most useful for assessment, and which produces the most reproducible results for IMT measurement, is the CCA.37 Although there are no superior carotid artery segments clearly associated with CVD, CCA-IMT is associated with a higher relative risk for stroke, whereas ICA-IMT is associated with a higher relative risk for CHD.38
The present study had certain limitations. First, our cross-sectional study design did not allow us to infer a causal relationship between carotid atherosclerosis and ECG-LVH. In addition, this study used the presence of carotid plaque as an outcome variable. However, more precise analyses using Doppler flow quantification to assess the degree of plaque stenosis are needed because carotid Doppler measurements, in addition to IMT and plaque evaluation, are independently associated with future CVD.39 Third, because this study measured only ECG-LVH instead of Echo-LVH, there may be a distortion in the results of this study that suggested a relationship between carotid atherosclerosis and LVH. Despite these limitations, our work is valuable as it provides insight into the potential role of carotid atherosclerosis in the development of LVH, as determined by ECG in large populations.
In conclusion, our study demonstrated that high carotid IMT, but not carotid plaque, was significantly associated with LVH as assessed by ECG in the general Korean population. These associations were more powerful in the CCA than the CB. Moreover, carotid IMT measured at both the CCA and CB was more strongly associated with ECG-LVH defined using the SokV criteria than using the CorV criteria. Further longitudinal, population-based studies are warranted to explore the relationship between carotid atherosclerosis and ECG-LVH. In addition, further research designs are needed to evaluate the clinical impact of carotid atherosclerosis on ECG-LVH.
ACKNOWLEDGMENTS
This study was supported by Wonkwang University in 2016.
Footnotes
CONFLICT OF INTEREST STATEMENT: None declared.
References
- 1.Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11:276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- 2.Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. World Health Organization; 2011. [Google Scholar]
- 3.Lee SW, Kim HC, Lee HS, Suh I. Thirty-year trends in mortality from cardiovascular diseases in Korea. Korean Circ J. 2015;45:202–209. doi: 10.4070/kcj.2015.45.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin EJ, Levy D. Why is left ventricular hypertrophy so predictive of morbidity and mortality? Am J Med Sci. 1999;317:168–175. doi: 10.1097/00000441-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J. 2000;140:848–856. doi: 10.1067/mhj.2000.111112. [DOI] [PubMed] [Google Scholar]
- 6.Antikainen RL, Grodzicki T, Palmer AJ, Beevers DG, Webster J, Bulpitt CJ, et al. Left ventricular hypertrophy determined by Sokolow-Lyon criteria: a different predictor in women than in men? J Hum Hypertens. 2006;20:451–459. doi: 10.1038/sj.jhh.1002006. [DOI] [PubMed] [Google Scholar]
- 7.Cuspidi C, Facchetti R, Bombelli M, Sala C, Grassi G, Mancia G. Accuracy and prognostic significance of electrocardiographic markers of left ventricular hypertrophy in a general population: findings from the Pressioni Arteriose Monitorate E Loro Associazioni population. J Hypertens. 2014;32:921–928. doi: 10.1097/HJH.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 8.Porthan K, Niiranen TJ, Varis J, Kantola I, Karanko H, Kähönen M, et al. ECG left ventricular hypertrophy is a stronger risk factor for incident cardiovascular events in women than in men in the general population. J Hypertens. 2015;33:1284–1290. doi: 10.1097/HJH.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 9.Okin PM, Devereux RB, Jern S, Kjeldsen SE, Julius S, Nieminen MS, et al. Regression of electrocardiographic left ventricular hypertrophy during antihypertensive treatment and the prediction of major cardiovascular events. JAMA. 2004;292:2343–2349. doi: 10.1001/jama.292.19.2343. [DOI] [PubMed] [Google Scholar]
- 10.Larstorp AC, Okin PM, Devereux RB, Olsen MH, Ibsen H, Dahlöf B, et al. Changes in electrocardiographic left ventricular hypertrophy and risk of major cardiovascular events in isolated systolic hypertension: the LIFE study. J Hum Hypertens. 2011;25:178–185. doi: 10.1038/jhh.2010.52. [DOI] [PubMed] [Google Scholar]
- 11.Bang CN, Devereux RB, Okin PM. Regression of electrocardiographic left ventricular hypertrophy or strain is associated with lower incidence of cardiovascular morbidity and mortality in hypertensive patients independent of blood pressure reduction - A LIFE review. J Electrocardiol. 2014;47:630–635. doi: 10.1016/j.jelectrocard.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 13.Prati P, Tosetto A, Vanuzzo D, Bader G, Casaroli M, Canciani L, et al. Carotid intima media thickness and plaques can predict the occurrence of ischemic cerebrovascular events. Stroke. 2008;39:2470–2476. doi: 10.1161/STROKEAHA.107.511584. [DOI] [PubMed] [Google Scholar]
- 14.Baldassarre D, Veglia F, Hamsten A, Humphries SE, Rauramaa R, de Faire U, et al. Progression of carotid intima-media thickness as predictor of vascular events: results from the IMPROVE study. Arterioscler Thromb Vasc Biol. 2013;33:2273–2279. doi: 10.1161/ATVBAHA.113.301844. [DOI] [PubMed] [Google Scholar]
- 15.Park HW, Kim WH, Kim KH, Yang DJ, Kim JH, Song IG, et al. Carotid plaque is associated with increased cardiac mortality in patients with coronary artery disease. Int J Cardiol. 2013;166:658–663. doi: 10.1016/j.ijcard.2011.11.084. [DOI] [PubMed] [Google Scholar]
- 16.Yuk HB, Park HW, Jung IJ, Kim WH, Kim KH, Yang DJ, et al. Analysis of carotid ultrasound findings on cardiovascular events in patients with coronary artery disease during seven-year follow-up. Korean Circ J. 2015;45:28–37. doi: 10.4070/kcj.2015.45.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okin PM, Roman MJ, Devereux RB, Kligfield P. Association of carotid atherosclerosis with electrocardiographic myocardial ischemia and left ventricular hypertrophy. Hypertension. 1996;28:3–7. doi: 10.1161/01.hyp.28.1.3. [DOI] [PubMed] [Google Scholar]
- 18.Kweon SS, Shin MH, Jeong SK, Nam HS, Lee YH, Park KS, et al. Cohort profile: The Namwon Study and the Dong-gu Study. Int J Epidemiol. 2014;43:558–567. doi: 10.1093/ije/dys244. [DOI] [PubMed] [Google Scholar]
- 19.Sokolow M, Lyon TP. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am Heart J. 1949;37:161–186. doi: 10.1016/0002-8703(49)90562-1. [DOI] [PubMed] [Google Scholar]
- 20.Casale PN, Devereux RB, Kligfield P, Eisenberg RR, Miller DH, Chaudhary BS, et al. Electrocardiographic detection of left ventricular hypertrophy: development and prospective validation of improved criteria. J Am Coll Cardiol. 1985;6:572–580. doi: 10.1016/s0735-1097(85)80115-7. [DOI] [PubMed] [Google Scholar]
- 21.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2013;31:1281–1357. doi: 10.1097/01.hjh.0000431740.32696.cc. [DOI] [PubMed] [Google Scholar]
- 22.Dijkstra RF, van Schayck CP, Bakx JC, Thien T, Verheugt FW, Mokkink HG. Left ventricular hypertrophy; differences in the diagnostic and prognostic value of electrocardiography and echocardiography. Ned Tijdschr Geneeskd. 1997;141:1969–1972. [PubMed] [Google Scholar]
- 23.Sundström J, Lind L, Arnlöv J, Zethelius B, Andrén B, Lithell HO. Echocardiographic and electrocardiographic diagnoses of left ventricular hypertrophy predict mortality independently of each other in a population of elderly men. Circulation. 2001;103:2346–2351. doi: 10.1161/01.cir.103.19.2346. [DOI] [PubMed] [Google Scholar]
- 24.Bacharova L, Schocken D, Estes EH, Strauss D. The role of ECG in the diagnosis of left ventricular hypertrophy. Curr Cardiol Rev. 2014;10:257–261. doi: 10.2174/1573403X10666140514103220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Neal WT, Almahmoud MF, Qureshi WT, Soliman EZ. Electrocardiographic and echocardiographic left ventricular hypertrophy in the prediction of stroke in the elderly. J Stroke Cerebrovasc Dis. 2015;24:1991–1997. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almahmoud MF, O'Neal WT, Qureshi W, Soliman EZ. Electrocardiographic versus echocardiographic left ventricular hypertrophy in prediction of congestive heart failure in the elderly. Clin Cardiol. 2015;38:365–370. doi: 10.1002/clc.22402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leigh JA, O'Neal WT, Soliman EZ. Electrocardiographic left ventricular hypertrophy as a predictor of cardiovascular disease independent of left ventricular anatomy in subjects aged ≥65 years. Am J Cardiol. 2016;117:1831–1835. doi: 10.1016/j.amjcard.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Sarnowski B, Lüdemann J, Völzke H, Dorr M, Kessler C, Schminke U. Common carotid intima-media thickness and framingham risk score predict incident carotid atherosclerotic plaque formation: longitudinal results from the study of health in Pomerania. Stroke. 2010;41:2375–2377. doi: 10.1161/STROKEAHA.110.593244. [DOI] [PubMed] [Google Scholar]
- 29.Spence JD. Measurement of intima-media thickness vs. carotid plaque: uses in patient care, genetic research and evaluation of new therapies. Int J Stroke. 2006;1:216–221. doi: 10.1111/j.1747-4949.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 30.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 2012;34:290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnsen SH, Mathiesen EB. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr Cardiol Rep. 2009;11:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 32.Johnsen SH, Mathiesen EB, Joakimsen O, Stensland E, Wilsgaard T, Løchen ML, et al. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromsø Study. Stroke. 2007;38:2873–2880. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 33.Inaba Y, Chen JA, Bergmann SR. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Cuspidi C, Mancia G, Ambrosioni E, Pessina A, Trimarco B, Zanchetti A, et al. Left ventricular and carotid structure in untreated, uncomplicated essential hypertension: results from the Assessment Prognostic Risk Observational Survey (APROS) J Hum Hypertens. 2004;18:891–896. doi: 10.1038/sj.jhh.1001759. [DOI] [PubMed] [Google Scholar]
- 35.Roman MJ, Pickering TG, Schwartz JE, Pini R, Devereux RB. Association of carotid atherosclerosis and left ventricular hypertrophy. J Am Coll Cardiol. 1995;25:83–90. doi: 10.1016/0735-1097(94)00316-i. [DOI] [PubMed] [Google Scholar]
- 36.Guarini P, De Michele M, Tedeschi C, Accadia M, Giordano G, Corigliano FG, et al. Presence and severity of carotid atherosclerosis in asymptomatic hypertension patients with left ventricular hypertrophy. G Ital Cardiol. 1999;29:910–914. [PubMed] [Google Scholar]
- 37.Nambi V, Chambless L, He M, Folsom AR, Mosley T, Boerwinkle E, et al. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur Heart J. 2012;33:183–190. doi: 10.1093/eurheartj/ehr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 39.Chung H, Jung YH, Kim KH, Kim JY, Min PK, Yoon YW, et al. Carotid artery end-diastolic velocity and future cerebro-cardiovascular events in asymptomatic high risk patients. Korean Circ J. 2016;46:72–78. doi: 10.4070/kcj.2016.46.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]