Abstract
Acute kidney injury (AKI) is now widely recognised as a serious health care issue, occurring in up to 25% of hospital in-patients, often with worsening of outcomes. There have been several reports of substandard care in AKI. This quality improvement (QI) programme aimed to improve AKI care and outcomes in a large teaching hospital.
Areas of documented poor AKI care were identified and specific improvement activities implemented through sequential Plan-Do-Study-Act (PDSA) cycles. An electronic alert system (e-alert) for AKI was developed, a Priority Care Checklist (PCC) was tested with the aid of specialist nurses whilst targeted education activities were carried out and data on care processes and outcomes monitored.
The e-alert had a sensitivity of 99% for the detection of new cases of AKI. Key aspects of the PCC saw significant improvements in their attainment: Detection of AKI within 24 hours from 53% to 100%, fluid assessment from 42% to 90%, drug review 48% to 95% and adherence to nine key aspects of care from 40% to 90%. There was a significant reduction in variability of delivered AKI care. AKI incidence reduced from 9% of all hospitalisations at baseline to 6.5% (28% reduction), AKI related length of stay reduced from 22.1 days to 17 days (23% reduction) and time to recovery (AKI days) 15.5 to 9.8 days (36% reduction). AKI related deaths also showed a trend towards reduction, from an average of 38 deaths to 34 (10.5%). The number of cases of hospital acquired AKI were reduced by 28% from 120 to 86 per month.
This study demonstrates significant improvements related to a QI programme combining e-alerts, a checklist implemented by a nurse and education in improving key processes of care. This resulted in sustained improvement in key patient outcomes.
Problem
Acute kidney injury (AKI) is a defined by a rapid reduction in kidney function resulting in reduced clearance of excess water, electrolytes and toxins. It is highly prevalent amongst hospitalised patients and is associated with poor outcomes such as increased length of stay (LoS) and increased mortality1.
The NCEPOD AKI audit to which our hospital contributed highlighted in 2009 significant deficiencies in AKI management. Subsequently, the study by Challiner et al. in 2013 found a high incidence of AKI in acute admissions, with significant consequences (2.5-fold increased LOS and 3.5-fold increased mortality)8. In 2014, an audit of the management of 50 cases of severe AKI (AKI Stage 3) found similar levels of management deficiencies as reported in the NCEPOD audit of 200915. It highlighted a wide variability in the treatment of AKI across the hospital, indicating a lack of reliable systems and processes for the detection and management of AKI. The detection of new AKI cases was missed in 47% of patients in this audit. Also, potentially “nephrotoxic” medications had not been discontinued in 52% of cases and appropriate fluid management had not been initiated in 60% of cases. This data underlined the need for a hospital-wide strategy to improve and standardise systems and processes to prevent, detect and manage AKI.
Intended Improvement
The AKI QI team was formed in 2014, with the aim of reducing the overall impact of AKI on patients admitted to our hospital. More specifically, it sought to achieve by the end of December 2015, a
i. 10% reduction in the total number of cases of AKI (incidence)
ii. 10% reduction in the length of stay of patients with AKI
iii. 20% reduction in AKI days or time to recovery
iv. 10% reduction in deaths of patients with AKI
v. 10% reduction in dialysis/haemofiltration requirement as a result of AKI
The areas of care which were considered to require improvement and their respective goals by the end of December 2015 (target attainment) were:
i. Recognition of AKI within 24 hours (95% attainment)
ii. Appropriate medications review (95% attainment)
iii. Appropriate fluid management (90% attainment)
iv. Adherence to all aspects of priority AKI care based on a 10 point checklist (80%)
An operational team consisting of professionals with a varied background (three nephrologists, an intensivist, an acute care physician, two renal clinical nurse specialists, a renal matron, a biochemist, a renal pharmacist and an IT business intelligence developer) was created. A steering committee provided supervision with direct reporting to the Trust Board.
Study Question
Will the introduction of an automated AKI detection system and the implementation of a Priority Care Checklist with the aid of specialist nurses and pharmacists lead to improvements in AKI care and outcomes?
Background
Acute kidney injury (AKI) is very common amongst hospitalised patients.1 Previous studies reported AKI to be present in 5-7% of hospitalised patients.2–4 More recently, Wang et al, in the US found AKI to occur in up to 22% of hospitalised patients.5 In the UK, using electronic alerts, Porter et al found the overall incidence of AKI in all hospitalised patients (emergency and elective admissions) in a large teaching hospital to be 10.7%.6 A survey of acute admission units of ten UK hospitals found an AKI incidence of 17.7%.7 In our hospital, a large study conducted by Challiner et al. in 2013 showed that AKI occurred in 25.3% of 745 unselected adult acute admissions.8 Of the new cases of AKI, at least 50% tend to be present at the time of admission (“community acquired AKI”), the remaining occurring during their inpatient stay (“hospital-acquired AKI”).
AKI has been consistently associated with increased morbidity and mortality. Chertow et al found a 6.5 fold increase in mortality and a 3.5 fold increase in hospital length of stay (LOS) in association with AKI..9A large Swedish cohort study of intensive care patients showed that those who developed AKI had a 48.4% mortality compared to 24.6% for those without AKI at one year.10 Interestingly, patients who develop AKI, even after clinical recovery tend to have a higher longer term mortality; 61.8% with AKI versus 39.1% without AKI at five years.10 Similarly, Hobson et al found that patients who developed AKI after cardiac surgery still had a 28% increased risk of death at 10 years, in spite of complete recovery of the AKI. AKI patients have also been shown to consistently have longer hospital length of stay (LOS) and a higher incidence of chronic kidney disease and end stage renal disease requiring dialysis.8 10 11 Challiner et al found the median LOS of AKI patients in our hospital was 10 days compared to four days in the non AKI group.8
In the UK, The National Clinical Enquiry into Patients Outcomes and Death (NCEPOD) in 2009 (“Adding Insult to Injury”) identified significant deficiencies in the identification and management of AKI.12 Only 50% of the patients in this study had been deemed to have received “good care”. Approximately one third of patients had received an inadequate basic clinical assessment, investigations and physiological monitoring. Hypovolaemia and sepsis, key causes of AKI were found to be poorly recognised. More recently, a study of ten acute medical units in the UK, found deficiencies in the care of patients with AKI; "nephrotoxic" medications were continued inappropriately in 28.6% of patients and fluid balance was not charted in 38.9% of patients when indicated.7 These findings of inconsistent and deficient management of AKI have been echoed by several other studies.11 13 These often simple clinical actions, if carried out appropriately could potentially prevent a significant proportion of cases of AKI or alter their course and hence potentially save lives.
In the UK, in recent years, AKI has been increasingly recognised as a national healthcare priority. It has been estimated that at least 1000 patients die unnecessarily every month in the UK from potentially avoidable AKI. This is compounded by the significant estimated costs of AKI to the UK National Health Service, estimated at one billion pounds a year.14
Baseline measurement
The chosen measures for AKI care processes and outcomes are shown in table 1. The care processes chosen were shown to have very poor attainment in both the NCEPOD study and a local audit carried out in 2014. Furthermore, studies in other hospitals, both in the UK and abroad, have demonstrated the potential for these processes and outcomes to be improved by targeted activities. A short pilot quality improvement (QI) project targeting the most severe AKI cases also confirmed the potential sensitivity of these measures to improvement. These key care processes are shown as part of the ten point Priority Care Checklist (see supplementary file).
Table 1.
Measures, their operational definitions and improvement goals
| Metric | Type | Operational Definition | Targets by 31/12/15 |
|---|---|---|---|
| PM1: AKI Detection | Process | Proportion of AKI cases appropriately diagnosed within 24 hours | 95% attainment |
| PM2: Fluid Assessment | Process | Proportion of AKI patients with documented fluid assessment and charts | 95% attainment |
| PM3: Drug Review | Process | Proportion of AKI patients with a documented appropriate drug review | 95% attainment |
| PM4: Adherence to AKI priority care | Process | Proportion of AKI patients in whom nine elements of the Priority Care Checklist (PCC) were adhered to | 80% attainment |
| OM1: AKI incidence | Outcome | Proportion of cases of AKI in all admissions | 10% Reduction |
| OM2: Incidence of Hospital Acquired AKI | Outcome | Count of number of new cases of AKI developed in hospital (AKI must not be present on admission/or first blood test) | 10% reduction |
| OM2: AKI LOS | Outcome | Average number of days AKI patients spend in hospital | 10% Reduction |
| OM3: AKI Days | Outcome | Average number of days a patient remains in AKI after diagnosis (time to recovery) | 20% Reduction |
| OM4: AKI Deaths | Outcome | Count of deaths with a diagnosis of AKI | 10% Reduction |
| OM5: Dialysis/Haemofiltration for AKI | Outcome | Count of AKI patients requiring dialysis/haemofiltration | 10% Reduction |
bmjqir.u219176.w7476supp.pdf (823.6KB, pdf)
Baseline data for the selected measures was continuously recorded from November 2013 to January 2015 prior to any QI interventions (15 months) and displayed in statistical process control charts (SPCs), with interventions starting from March 2015. Continuous measures have been reported up until June 2016 (16 months since the commencement of the QI programme)
Design
Ethical Considerations
After consultation with the National Health Service Research Ethics Committee and the Trust Research and Development department, the study was exempt from requiring a specific ethical approval as it was considered to be a quality improvement project. All patient information was anonymised for analysis and reporting.
Setting
Central Manchester University Hospitals is the leading provider of tertiary and specialist healthcare services in Manchester, treating more than a million patients every year. Manchester Royal Infirmary, the setting of this study, is one of the six specialist hospitals of the trust, focusing on adult general and specialist medical and surgical services. It has over 800 beds and admits over 20,000 patients a year from the metropolitan area of central Manchester but also further afield from the North West region for specialist services such as cardiac, renal, gastroenterology, vascular surgery, hepatobiliary surgery, kidney and pancreas transplantation and haematology. The patients are spread across 32 inpatient wards and 20 specialties served by over 1000 doctors, 5000 nurses and 100 pharmacists. It is also the main teaching hospital affiliated to the University of Manchester.
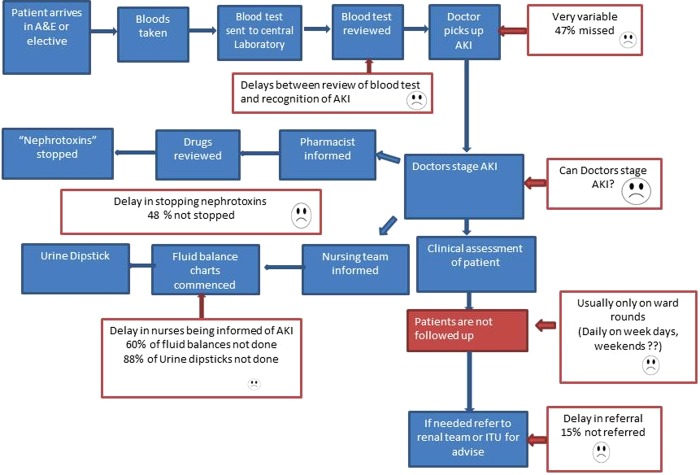
Prior to the study, AKI care in the hospital was found to be variable and inconsistent. It relied on the treating clinician's ability to recognise a significant rise in serum creatinine and to institute appropriate care according to their knowledge and experience. In spite of the presence of clear guidelines, audit data showed that this was very unreliable. Several High Level Clinical Incidents (HLIs) had been reported where severe cases of AKI had been missed or inadequately managed, sometimes with fatal consequences. Also, there was no evidence of integrated working between the nurses, doctors and pharmacists in the management of these cases. Figure 1 shows a process map for AKI diagnosis and management prior to any specific interventions in this QI programme.
Figure 1. Process map for AKI diagnosis and management prior to the formation of the AKI team.
Notes:This process map shows a convoluted and complicated process for AKI care prior to the QI programme, with several potential pitfall areas as highlighted in the red boxes.
Planning the intervention
Using available local audit data and other UK publications the project team identified key areas of intervention that would likely lead to improvement as in the driver diagram (see supplementary file). For each driver, the team performed a Factor Analysis and prioritised the sequence of testing based on feasibility and likely impact.
Driver A: Improved prevention of AKI.
To reduce the incidence of new cases of AKI, it is essential to address the prevention of new cases. Increased awareness of AKI risk factors, prompt blood tests in suspected cases and appropriate interventions (e.g. discontinuing potentially exacerbating ("nephrotoxic") medications are key to this). A multi-pronged education and awareness programme with risk assessment tools was created, tested and implemented.
Driver B: A reliable early detection system.
AKI electronic alert: AKI is mainly diagnosed based on a rise in serum creatinine. AKI is said to have occurred when there is a greater than 50% rise in serum creatinine from a baseline value. An electronic alert (e-alert) system that used a computer algorithm to automatically detect any such rise was developed and implemented. The output is linked to the patient identifiable data and a report is produced with a list of all patients with AKI within the hospital, including the AKI stage and each patient's location. The ability of this system to correctly detect all new cases of AKI (sensitivity and specificity) was tested by the specialist nurse in several cycles by manually cross-checking for accuracy. This process was repeated in several cycles with slight modification of the algorithm to maximise both sensitivity and specificity. The performance of this algorithm was also compared to a newly mandated algorithm for AKI e-alerts by the National AKI programme (Think Kidneys/NPSA Safety Alert NHS/PSA/RE/2016/007).
A reliable notification system for AKI: Once an AKI case was detected by the e-alert, in addition to the electronic flag on the electronic pathology reporting system, the AKI specialist nurse attended the ward and informed the treating doctor, nurse and pharmacist of the new case. Latterly, for a hospital-wide intervention, pharmacists were also used to perform the same function.
Driver C: Consistent prompt and appropriate treatment
Development of an AKI aide-mf#x00A9;moire checklist. Ten key aspects of priority AKI care were identified, some of which were the subject of significant inadequacies as indicated by an earlier audit and the NCEPOD study. A simple checklist list intended to help the treating teams recall what was expected of them when a patient was diagnosed with AKI was developed. The design and content of the checklist was tested using several Plan-Do-Study-Act (PDSA) cycles involving doctors, nurses and pharmacists with real cases of AKI and providing feedback through surveys on its key aspects, utility and user-friendliness.
Implementation of an AKI aide-mf#x00A9;moire checklist. Following the confirmation of AKI using the electronic alert, ward teams were encouraged to use the checklist to ensure that key aspects of care had been delivered. The overall goal of this checklist was to ensure consistency in AKI management regardless of location, time of day, staffing levels and the grades or knowledge of the treating/caring staff. The checklist was piloted in one ward, then four wards before hospital-wide.
Pharmacist led implementation of drug review. A prompt medications review with discontinuation of potentially nephrotoxic medications is a key component of AKI care. Audit data showed that such reviews were missed in half the cases. We tested the use of our network of pharmacists across the hospital, to instigate a prompt medication review in all cases of AKI following electronic alerts.
Improving AKI awareness and education. Improvement of the awareness of the importance and impact of AKI in the trust would likely improve compliance with targeted improvement activities. The awareness campaign used a multimedia strategy with tools such as posters, banners, videos, communiques and specific awareness events. In addition, targeted education sessions were carried out for various groups of clinicians and nurses to improve their knowledge on AKI. A particular innovation in the education was the use of a 4 slide micro teaching education package for small groups and individuals.
Evaluation
This was an interventional, quasi- experimental, longitudinal, before and after study aimed at testing several specific interventions in improving the management and outcomes of patients with AKI. To determine the impact of these interventions, clear systems were put in place to monitor baseline data on the key process and outcome measures for the 16 months prior to the full intervention (November 2014 to February 2015). Compliance to AKI key care processes was measured by a monthly review of randomly selected case notes by the AKI specialist nurses and recorded on a purpose designed database. Compliance with the ten elements of the checklist was considered individually and collectively. Data on patient outcomes including incidence, time to recovery, LoS and deaths were automatically uploaded by linking the Patient Administration System (PAS) through an SQL server to the AKI alerts system. Thus, such outcomes were automatically populated onto a purpose-designed database on a daily basis, but manually cross-checked by the AKI specialist nurse. Recovery was defined as a drop in serum creatinine to a level at which AKI would no longer be diagnosed (<50% higher than baseline or a new plateau attained). Deaths were analysed for all cases of AKI in which death resulted, with no attempt at cause attribution.
Factorial design and analysis: In order to determine which factor(s) led to improvement, nurse intervention, education, pharmacist intervention and PCC were tested in isolation and in various combinations in different clinical areas. Data was analysed using an Excel Add-in for QI charts with data expressed as time series statistical process control charts (SPC). A sustained shift in the desired direction (6 or more data points) of both the mean and control limits was considered to constitute an improvement. The impact of various factors of the intervention on the desired outcome (in this case reduction in AKI days) was evaluated using response plots. Where appropriate, Student's T tests were performed using GraphPad Prism® to determine “before” and “after” statistical differences with p<0.05 considered significant.
Strategy
To implement the changes, we used the Model for Improvement via sequential Plan-Do-Study-Act (PDSA) cycles. Before the start of implementation, the QI team underwent training in the model for improvement.
Key secondary drivers were identified for the beginning of study/implementation. We adopted a step-wise approach to implementation of tests of change by using a one ward-four wards-hospital wide strategy.
Results
The setting: The initial interventions were carried out at three levels; hospital-wide interventions which were limited to education and awareness events (mainly due to limited human resources) and an intensive intervention on one then four wards. Subsequently, the appointment of two AKI nurse specialists enabled an intensive hospital-wide intervention from March 2015.The interventions benefited from organisational support at the highest level and great enthusiasm and engagement from front-line clinical and nursing staff about the proposed improvements. There was however, significant initial scepticism about the intended improvement methods. This was probably related to a relative unawareness of quality improvement methodology across the organisation at the time. The limited number of full time team members at the beginning, coupled with illness and leave imposed a staggered, patchy implementation of the intervention. A four-week pilot in a single ward in October/November 2014 gave strong signals on the impact of the AKI checklist. This provided impetus for hospital-wide intervention from March 2015, following the appointment of two AKI nurse specialists.
Changes in processes of care
Small scale testing on one then four wards: The ability of the intervention to change the identified key processes of care was evaluated on one then four wards. Over a four-week period, for 27 new cases of AKI newly diagnosed on these wards, the adherence to the ten aspects of the PCC were grouped into three major categories and evaluated: “Fluid management” (fluid assessment, fluid balance charts and catheterisation) improved from a baseline of 40% to 95%. Evidence of an appropriate drug review improved from a baseline of 48% to 100%. However, when all aspects of the checklist were considered to be a bundle, the overall compliance was 40% at the end of the pilot. This low attainment was mainly due to a persistently poor compliance with the urine dipstick aspect of the checklist. Excluding the urine dipstick and considering only the other nine aspects showed an improvement from 67.4% to 92%.
Hospital-wide intervention: From March 2015, the interventions were implemented across all wards in the hospital and data for care processes and outcomes continuously monitored as shown on the SPCs.
Process Measures: Detection of AKI within 24 hours improved from a baseline of 47% to 84% by the ward team and to 100% with combination of AKI nurse and ward team (see supplementary file), fluid assessments were carried out in 90% of cases from a baseline of 40%, medication reviews took place in 95% of cases (previously 48%), whilst adherence to all 10 aspects of the checklist performed poorly (20%) due to the poor performance in carrying out urine dipsticks. If urine dipstick is excluded the adherence to the checklist would be 90%.
A new streamlined process map for AKI care has resulted from the QI programme (see supplementary file). This improves the more complicated and convoluted process previously, with several areas of potential failures (figure 1).
Outcome Measures
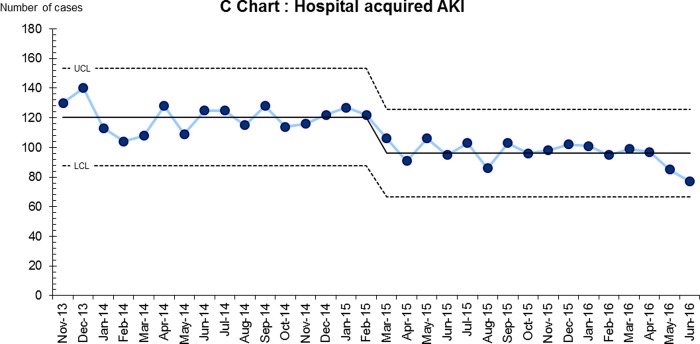
Incidence of AKI: There was an average of 313 new cases of AKI per month during the baseline period of November 2013 to February 2015; this improved progressively since the beginning of intervention with the most recent (April-June 2016) monthly average of 215 cases (31% reduction). Taking into account monthly hospital admissions, the AKI incidence has reduced from 9% to 6.5%. Hospital-acquired AKI consistently accounts for 40% of new AKI cases, this reduced from an average of 120 to 86 cases per month (28%), figure 2.
Figure 2. Number of cases of hospital-acquired or post-admission AKI.
Notes: Hospital acquired AKI (defined as AKI detected on a blood test taken 24 hours or more after admission) has fallen consistently from an average of 120 cases per month to 86 cases per month (28% reduction).
AKI Length of Stay: The average length of stay (LoS) during the baseline period November 2013 to February 2015 was 22.1 days; this was down to 17 days in the last three months of reporting (23% reduction) (see supplementary file).
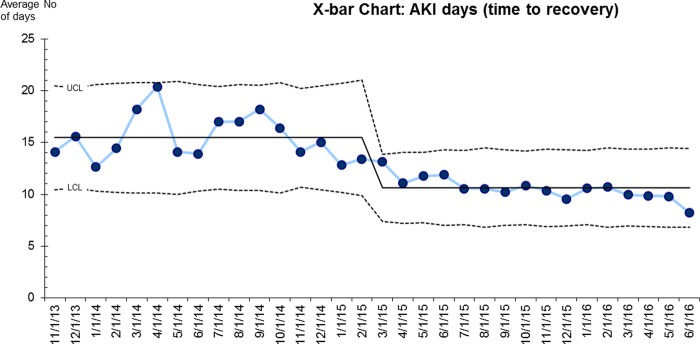
AKI Days or time to recovery: There was a marked sustained improvement in AKI days from 15.5 to 9.3 days (40% reduction, figure 3). This absolute number of AKI days reduction (6 days) is remarkably similar to that of LoS, indicating early recovery may be playing a key part in early discharge.
Figure 3. AKI days or time to recovery.
Notes: Prior to the QI programme, the average time taken to recover from an episode of AKI was 15.4 days; this is continuing to fall and is currently 9.3 days (40% reduction).
AKI Deaths: The monthly number of patients dying with a diagnosis of AKI reduced from 38 to 34 (10.5%) (see supplementary file)
Use of Dialysis and Haemofiltration: Throughout the study period, an average of 13.5 patients per month required dialysis and/or haemofiltration. This did not seem to change with the most recent monthly average being 11 patients. These other outcome measures are shown in the supplementary files.
Factors leading to improvement-outcome of factorial design experiment
A total of 135 patients were studied as part of the factorial design experiment that took place across the hospital during March and April 2015. The number of patients recruited in the various experimental units (wards) and the relevant factors studied are depicted in the supplementary file. All units had patients recruited apart from ward 3 (pharmacist intervention and education) and ward 36 (telephone alerting and pharmacist intervention). “AKI days” as the key outcome variable and AKI recognition, fluid and drug management as care process variables were used to determine the impact of each factor and their respective interactions. The analysis shows that the combination of nurse intervention (face-to face), PCC and education was associated with the shortest time to recovery of 5.4 days compared to 14.4 days for the units with different combinations of interventions.
Lessons and limitations
This improvement programme, has been very challenging in design, implementation and embedding.
It became clear from the beginning of the programme that all team members needed a good knowledge of quality improvement methodology to fully participate in project discussions. We enrolled in a quality improvement training programme but competing priorities in a busy health care environment meant that not all members could attend consistently. This resulted in significant delays in formulating objectives, and agreeing the final driver diagram.
We also learnt that the constitution of a dedicated, highly motivated, skilled team of individuals is essential for the success of such a programme. For instance, there were several delays in progressing to subsequent phases due to staff shortages. Only after a successful business case leading to the appointment of whole-time nurses could hospital-wide implementation be carried out, as all other project team members did not have sufficient allocated time to carry out improvement activities. We learnt that it is important from the outset to get the buy-in from the hospital senior management, to enable appropriate resourcing and help overcome barriers. For instance, we needed to develop and implement a new algorithm for AKI e-alerts, which needed significant input from the informatics team. Appropriate resourcing is also very important for sustainability as even with the improvement of systems and a culture change, some key activities need to be aligned to usual care. In our case, this has been achieved by creating the new permanent roles of AKI nurses.
We have learned that getting the commitment from front-line staff is key to achieving such improvement. By focusing on sensitisation activities, the problem of AKI has achieved a very high profile and visibility within the hospital, with has almost certainly resulted in a change in culture, with a missed case of AKI now becoming unacceptable.
Such a large QI project requires meticulous recording of data, especially of PDSA cycles as you go along. We have learnt that it can be very difficult to sustain this, and it is important to use aids such as audio recording.
Conclusion
This paper describes a series of interventions aimed at reducing the impact of AKI on in-patients at a large teaching hospital. To achieve this, it focused on interventions that were deemed likely to improve or strengthen key deficiencies previously identified in several areas of AKI care: early recognition, fluid management and medication management. These key aspects of care were encapsulated in a ten point AKI Priority Care Checklist designed and optimised with front-line staff using PDSA cycles, and implemented in a one-ward, then four ward pilot, before spreading across all areas.
The problem of early and accurate detection of AKI was addressed by the development of bespoke electronic alert algorithm that automatically generated a daily list of all new cases of AKI based on laboratory serum creatinine values. This algorithm when tested by manual cross-checking of identified AKI cases was found to have a sensitivity of 100% and a specificity of 97.3% (AUC 0.99). This compared favorably to a nationally recommended algorithm with a sensitivity of 72.4% (AUC 0.88). The high sensitivity was desirable so that no cases of AKI should be missed, but at the expense of over diagnosis of AKI in up 30% of cases. This is equivalent to about one false positive case per ward per week, usually of very early AKI (stage 1). These false positives were easily identified by the AKI team and did not represent a significant additional work load.
Following the design of the AKI PCC, its effectiveness on improving the identified processes of care was tested in an intensive four-week pilot across one then four wards. This showed a notable improvement in two broad areas of fluid management and medication management. The urine dipstick element saw no improvement, leading to an overall poor performance if considered as a bundle. When the urine dipstick was excluded, overall improvement in the other nine aspects was notable. The use of a bundle approach to improve care is well documented [18, 19]. Recently, Bhagwanani et al described a five aspect bundle that resulted in an improvement of compliance to all aspects of the bundle from 8% to 17 % [16]. This highlights difficulties in fully implementing a care bundle, when by definition the improvement is required in all the bundle constituents. In our case, "unbundling" these showed that all other areas of care other than urine dipstick showed quite significant improvement, from 40% to 90%. Further analysis identified several factors including the absence of a clear hospital-wide consistent documentation system for urine dipstick and competing priorities to be potential reasons for this poor attainment. This is currently being addressed by a urine dipstick work stream.
Encouraged by the results of the four ward pilot, we set about replicating the intervention across all hospital areas. Due to staffing difficulties, this could only be carried out three months later, from March 2015, when the two AKI specialist nurses were in post. Data was monitored for compliance to each of the ten key care processes for every new case of AKI, by the ward staff before or after the intervention(s) for the particular ward. This showed significant improvements in the compliance to nine of the ten care processes and even more importantly a significant improvement in the consistency of carrying out these actions across the hospital. This reduction of variability across ward areas was an additional benefit of the improvement programme.
The lack of consistency and the poor performance of UK hospitals in basic aspects of AKI care are well documented.7 12 Several initiatives to improve this have been published. Bhagwanani et al. saw a modest improvement in their five-point DONUT bundle [16]. Tsui et al used a specially designed care bundle and saw an improvement in at least eight aspects of what they considered key AKI care [20]. Our AKI PCC assesses very similar care components and shows significant improvements as detailed. Such interventional studies targeting improvement in specific actions related to AKI care are not common in the literature, although several papers have reported the potential utility of automated electronic alerts for detection [4, 21]. The utility of such electronic alerts could hardly be disputed. However, a recent randomised trial showed no difference in patient outcomes when the alerts alone were instituted [22], suggesting that targeted improvement actions are required to change overall care and potentially improve patient outcomes.
We monitored specific patient outcomes over the intervention period. AKI incidence, LoS, AKI days and perhaps mortality showed clear sustained reductions. The improvement in LoS and possibly AKI deaths is likely related to the improved management of newly diagnosed cases of AKI, with a probable alteration of disease course and consequent early recovery or stabilisation. Such an improvement will thus likely reduce the effective number of days the patients spend in AKI (“AKI days”).This new metric was our most sensitive outcome variable to AKI improvement. It has the advantage over measures such as LoS of excluding other factors that may come into play. We are not aware of previous reports of AKI days but propose this should be considered by organisations looking to measure improvement in AKI care.
Interestingly, new cases of hospital acquired AKI saw a reduction from 120 cases per month to 86 cases per month indicating that hospital wide activities to improve AKI care are also likely helping to prevent new cases.
Few interventional studies have demonstrated improvement in AKI care processes and patient outcomes . Our factorial design data is also unique, showing clearly the benefit of a nurse-led approach together with education and tools such as AKI alerting and a simple checklist. Due to the small numbers, the dialysis requirement data could not be interpreted; the trends will need to be monitored over a longer period or subgroup analysis performed. In spite of these cumulative shortcomings, this study is still unique and valuable in that it shows how a set of targeted interventions using a simple checklist administered by a nurse can result in an improvement in and an attainment of consistent care for AKI, with improvement in key patient outcomes. Furthermore, reduction in AKI length of stay and incidence will have resulted in the release of an estimated 2946 bed days, equivalent to ∼£1 million over one year.
Acknowledgments
We will like to offer sincere thanks to the Haelo team for supporting us through our development in QI methodology, particularly Maxine Power, Lloyd Provost, Brandon Bennett and Zoe Ashcroft for their patient supervision and guidance. None of this work would have been possible without the unwavering support of the CMFT Trust Board and the Improving Science for Academics Programme (IS4Ac) funded through the Manchester Academic health Sciences network (MAHSC); we are sincerely grateful. The Greater Manchester Kidneys Charity, Kidneys for Life has supported the production of promotional material and presentation of key findings. Finally we wish to thank all the staff from the various wards who have supported the implementation of improvement at the frontline, the AKI patients who have shared their stories and several other key staff members involved in various work streams of this programme that we have not named individually
Footnotes
Declaration of interests: Nothing to declare
Ethical approval: After consultation with the National Health Service Research Ethics Committee and the Trust Research department, the study was exempt from requiring a specific ethical approval as it was considered to be a service improvement project. All patient information was anonymised for analysis and reporting.
References
- 1.Kellum J, Lameire N, Group ftKAGW Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Critical Care 2013;17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman J, Dhakal M, Patel B et al. Community-acquired acute renal failure. Am J Kidney Dis 1991;17:191–8. [DOI] [PubMed] [Google Scholar]
- 3.Hoste EA, Schurgers M. Epidemiology of acute kidney injury: how big is the problem? Crit Care Med 2008;36:S146–51. [DOI] [PubMed] [Google Scholar]
- 4.Selby NM, Crowley L, Fluck RJ et al. Use of Electronic Results Reporting to Diagnose and Monitor AKI in Hospitalized Patients. Clinical Journal of the American Society of Nephrology 2012;7:533–540. [DOI] [PubMed] [Google Scholar]
- 5.Wang HE, Muntner P, Chertow GM et al. Acute Kidney Injury and Mortality in Hospitalized Patients. American Journal of Nephrology 2012;35:349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter CJ, Juurlink I, Bisset LH et al. A real-time electronic alert to improve detection of acute kidney injury in a large teaching hospital. Nephrology Dialysis Transplantation 2014. [DOI] [PubMed] [Google Scholar]
- 7.Finlay S, Bray B, Lewington AJ et al. Identification of risk factors associated with acute kidney injury in patients admitted to acute medical units. Clin Med 2013;13:233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Challiner R, Ritchie JP, Fullwood C et al. Incidence and consequence of acute kidney injury in unselected emergency admissions to a large acute UK hospital trust. BMC Nephrol 2014;15:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chertow GM, Burdick E, Honour M et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]
- 10.Rimes-Stigare C, Frumento P, Bottai M et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Crit Care 2015;19:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aitken E, Carruthers C, Gall L et al. Acute kidney injury: outcomes and quality of care. 2013. [DOI] [PubMed] [Google Scholar]
- 12.Outcome NCEiP, Death, Stewart J. Adding Insult to Injury: A Review of the Care of Patients who Died in Hospital with a Primary Diagnosis of Acute Kidney Injury (acute Renal Failure): a Report by the National Confidential Enquiry Into Patient Outcome and Death: NCEPOD; 2009. [Google Scholar]
- 13.Meran S, Wonnacott A, Amphlett B et al. How good are we at managing acute kidney injury in hospital? Clinical Kidney Journal 2014;7:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kerr M, Bedford M, Matthews B et al. The economic impact of acute kidney injury in England. Nephrology Dialysis Transplantation 2014;29:1362–1368. [DOI] [PubMed] [Google Scholar]
- 15.Subbegowda PH, Hutchison AJ, Challiner R et al. Acute Kidney Injury: Experience of Nurse led intervention by early identification and intervention for inpatients developing AKI using E-alert system. In. British Renal Society Annual Conference. Newcastle, UK; 2014. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjqir.u219176.w7476supp.pdf (823.6KB, pdf)