Key Clinical Message
Trisomy 9 can be suspected and confirmed in the prenatal period since the 11–13.6 weeks of screening. In cases of partial trisomy 9, the diagnosis is important especially to counseling the couple due to the increased likelihood of recurrence in subsequent pregnancies.
Keywords: Partial trisomy 9 first‐trimester diagnosis
What is already known about this topic?
Prenatal diagnosis of complete and partial trisomy 9 is rare and is usually made after second trimester of pregnancy due to severe malformations, especially in the central nervous system.
What does this study add?
Trisomy 9 can be suspected and confirmed since the 11–13.6 weeks of pregnancy through the first‐trimester scan screening, providing the parents with complete information, and an early genetic counseling.
Introduction
Trisomy 9 (T9) is a rare chromosomic anomaly with multiple malformations 1. Sonographic suspicion during the first and second trimesters is uncommon due to high lethality rate of the disease.
Clinical reports mention multiple and severe malformations primarily occurring in the central nervous system, heart, kidneys, and limbs (Table 1). Prenatal growth restriction, postnatal mental retardation, and early mortality are also reported 1, 2, 3, 4, 5, 6 .
Table 1.
Sonographic findings in trisomy 9
| Organ/system | Alteration |
|---|---|
| Skull/head | Abnormal morphology (“strawberry shape”), brachycephaly, dolichocephaly. |
| Central nervous system | Ventriculomegaly |
| Vermis hypoplasia | |
| Megacisterna magna | |
| Face | Dismorphic |
| Hypotelorism | |
| Microphthalmia | |
| Micrognathia | |
| Neck | Edema |
| Thorax | Thoracic narrowing |
| Pleural effusion | |
| Pericardial effusion | |
| Diaphragmatic hernia | |
| Heart | Septal defects |
| Hypoplasia | |
| Thickened muscle wall | |
| Abdomen | Ascites |
| Echogenic bowel | |
| Kidneys | Policystic kidneys |
| Dysplasia | |
| Hydronephrosis | |
| Limbs | Bilateral clubfoot |
| Clenched hands | |
| Overlaying fingers | |
| Other findings | Single umbilical artery |
| Reversed ductus venous | |
| Short femur | |
| Oligohydramnios |
Cytogenetically, all trisomies can be classified either as full (complete) or partial 7, 8. A full trisomy is when an extra chromosome in all cells is found. A partial trisomy is the presence of an additional chromosomic fragment (usually as the result of gamete segregation patterns from a balanced carrier but could also be de novo) 8. If there are two or more cellular lines in one individual (usually one with normal number of chromosomes), somatic mosaicism is present and the expression of the phenotype relies on the different chromosomic complement and the number of cellular lines that are affected 9, 10.
The majority of full and partial T9 end in spontaneous abortion. Clinical reports of partial T9 that show second‐ and third‐trimester survival and postnatal outcomes have usually a variable amount of extra genetic material that correlates with the severity of the malformations or the phenotype 11, 12, 13, 14, 15, 16, 17. We present the familiar case of a couple with two subsequent pregnancies affected by partial T9.
Case Report
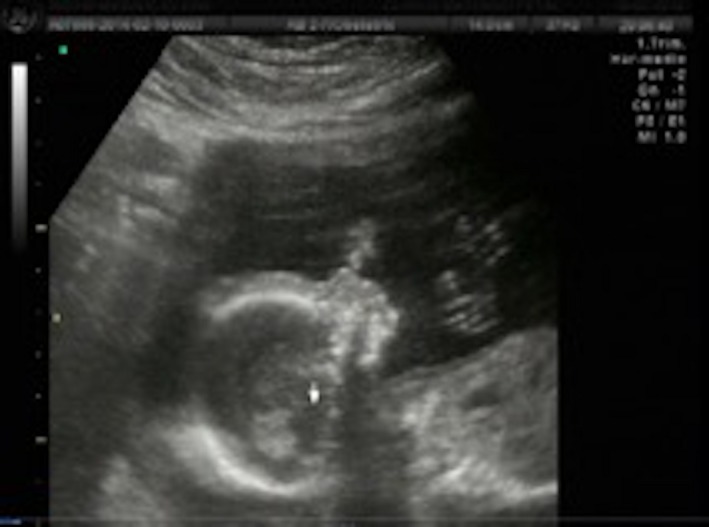
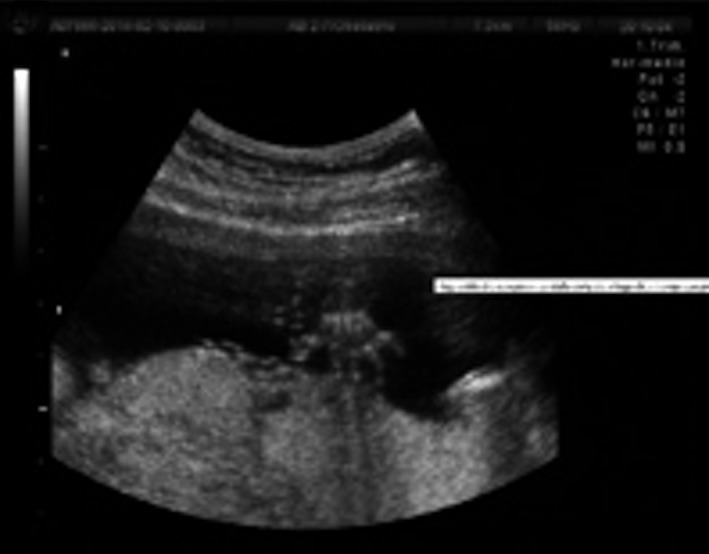
A 28‐year‐old woman arrived to the maternal fetal clinic in order to perform a first‐trimester combined screening at 12.3 weeks. Sonographic findings were as follows: crown‐rump length (CRL) of 53 mm according with gestational age (Fig. 1), nuchal translucency of 2.35 mm (Fig. 2), normal ductus venosus, absent nasal bone (Fig. 3), and left pleural effusion (Fig. 4). The biochemical markers showed PAPP‐A 2.97 mIU/mL (1.07 MoM) and free β hCG 15.3 ng/mL (0.36 MoM). The first‐trimester risk calculation program Fetal Test® (Dr. Domingo J. Ramos‐Corpas, Spain) showed a high risk for trisomy 18 with a 1 in 234 risk (established cutoff is 1/250).
Figure 1.
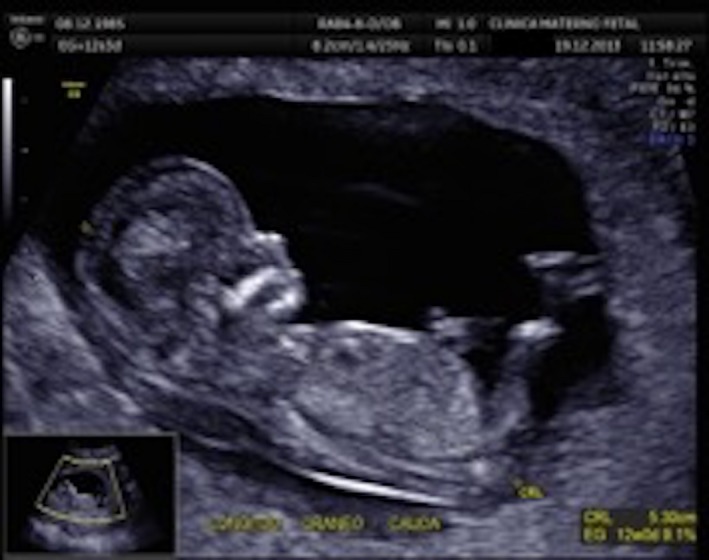
Crown‐rump length (CRL).
Figure 2.
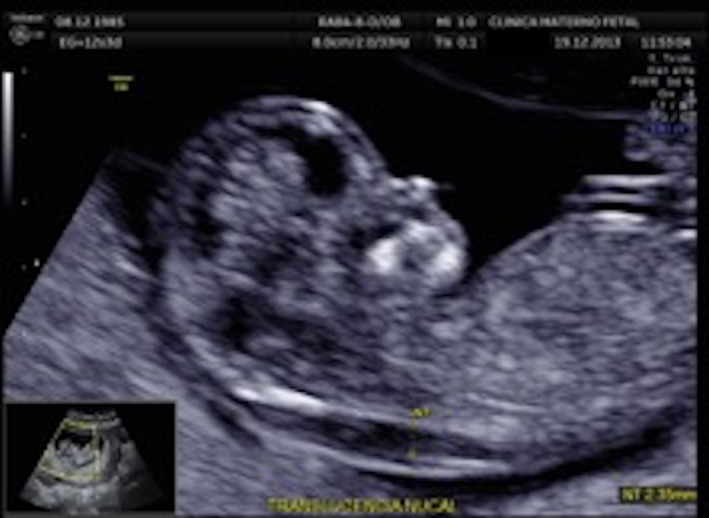
Nuchal translucency.
Figure 3.
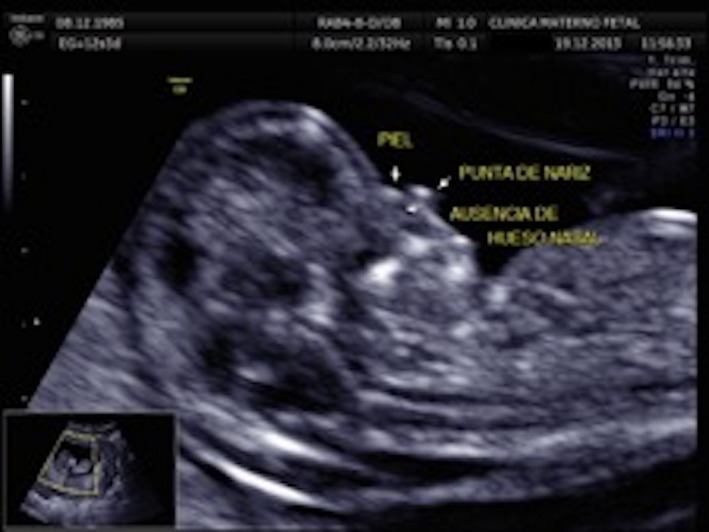
Absence of nasal bone.
Figure 4.
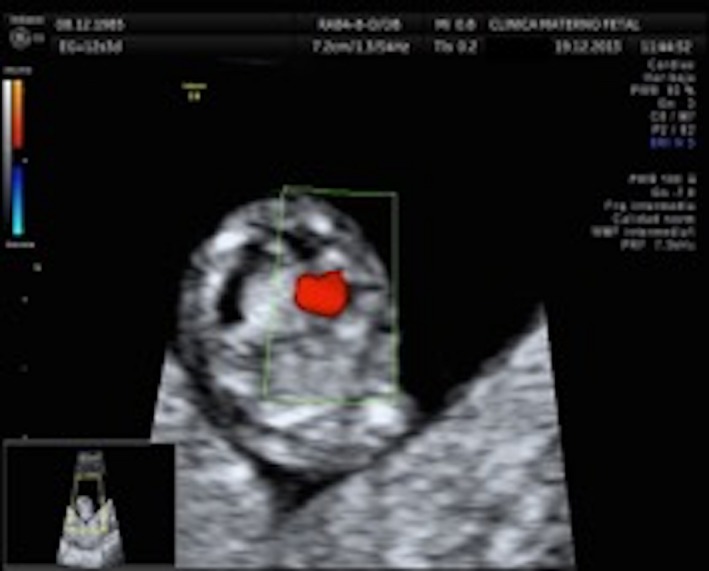
Axial thoracic section with pleural effusion.
A second ultrasound was performed at 15.1 weeks of gestation with sonographic findings of CRL of 77.6 mm, (which accorded to 13.6 gestational weeks) and no pleural effusion. Amniocentesis was performed at 17.4 weeks by last menstrual period (LMP) with a GTG banding conventional karyotype result of 47,XX,+der(9)(pter→?q32) in 25 cells from four primary cultures (Fig. 5). A third ultrasound at 20 gestational weeks by LPM showed a fetus of 18 weeks with absent nasal bone (Fig. 6), thickness and dilatation of right cardiac walls (Fig. 7), echogenic focus in left ventricle, and “claw‐like” hands (Fig. 8). After the karyotype results, pregnancy termination was decided by the couple and they refused pathological anatomy analysis.
Figure 5.
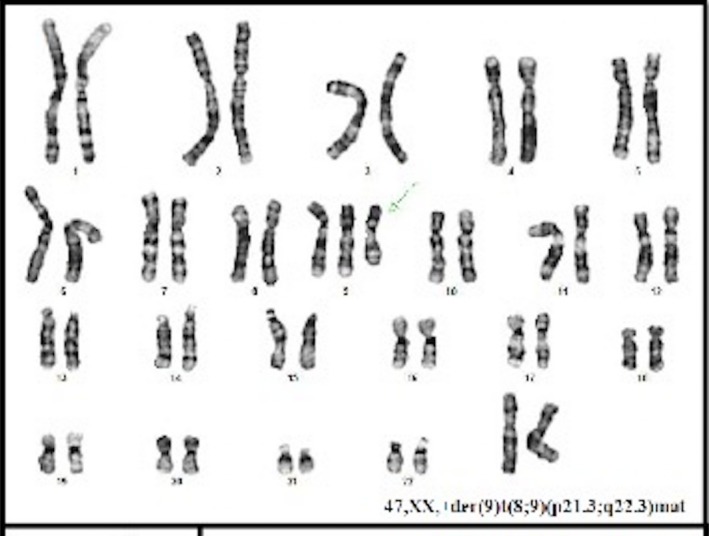
GTG banding conventional karyotype result of 47,XX,+der(9)(pter→?q32) in 25 cells from four primary cultures.
Figure 6.
Fetal profile at 20 weeks with absent nasal bone.
Figure 7.
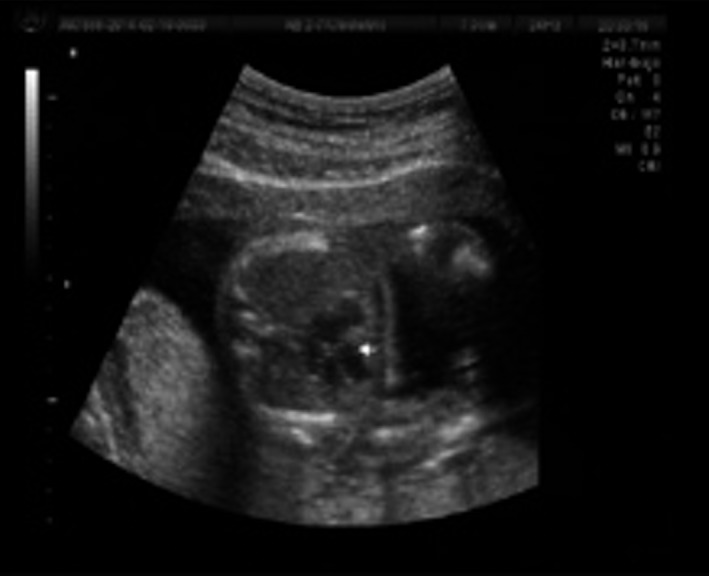
Thoracic axial view with right ventriculum wall thickening and dilation.
Figure 8.
Hand “claw‐like”.
The family's past medical history only revealed childhood seizures of the mother and maternal uncle with successful oral treatment. Parents received genetic counseling during the follow‐up period. A karyotype analysis in the blood sample from both parents was performed: father's karyotype resulted 46,XY, and the mother's karyotype revealed a reciprocal balanced translocation 46,XX, t(8;9) (p21.3;q22.3) (Fig. 9A and B).
Figure 9.
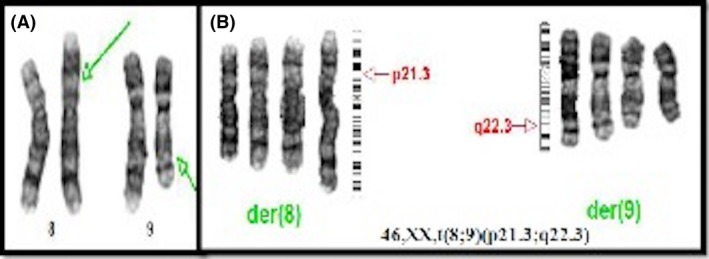
(A and B) Mother karyotype with balanced translocation between chromosomes 8 and 9.
During their second pregnancy, chromosomal microarray analysis (CMA) was performed in chorionic villus sample (CVS) at the eleventh week of pregnancy (Fig. 10). The result revealed multiple copy number gains including approximately 25.837 Mb of the distal short arm of chromosome 8 (8p), and approximately 88.745 Mb of chromosome 9, from 9pter to 9q21.33. No increased blocks of region of heterozygosity (ROH) suggestive of uniparental disomy (UPD) were detected. Termination of pregnancy was decided.
Figure 10.
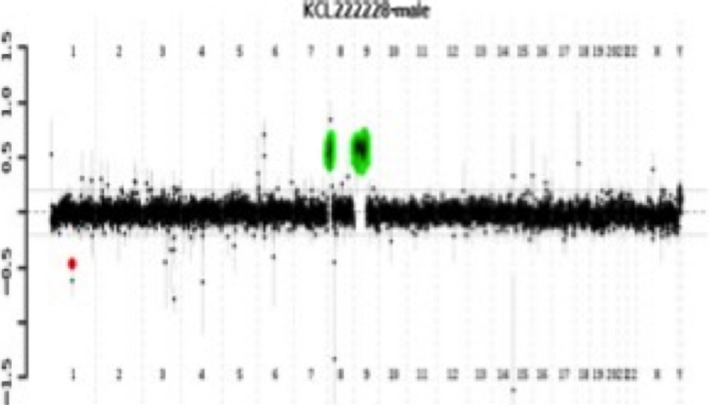
Whole genome summary. Green dots represent copy number gains in chromosomes 8 and 9. Red dot represents copy number losses that in this specific case was a polymorphism.
Case Review
The literature search in major databases was performed looking for articles reporting on prenatal diagnosis of trisomy 9 (either complete or partial). Few case reports as well as a recompilation of nine cases within a 12‐year period were found 7.
According to these reports, partial trisomy 9 was only found in five cases 15, 18, 19, 20, 21, all of them during second and third trimesters (Table 2).
Table 2.
Prenatal diagnosis of partial trisomy 9 in the literature research
| Author/Year | Karyotype | Fetal scan (weeks) | Prenatal findings | Postnatal findings |
|---|---|---|---|---|
| Sherer (1993) 18 | 47,XX+i(9p) | 23 | Twin pregnancy: One fetus with abnormal cerebellum, echogenic kidneys, bilateral clubfoot, cleft palate. |
Corpus callosum and cerebellar vermis agenesis. Died 1 week after. |
| Chen (1999) 19 | Trisomy 9p with Trisomy 21p (Amniocentesis at 17 weeks by amniocentesis for familial chromosomal translocation) | 24 | Bilateral ventriculomegaly, wide cisterna magna, intrauterine growth restriction. | Pregnancy termination. Microcephaly, short stature, hypertelorism and low‐set ears. |
| Von Kaisenberg (2000) 15 |
47,XX,+der(9)t(7;9) (q35;q22.2) |
23 | Cerebellar vermis hypoplasia, wide cisterna magna, bilateral ventriculomegaly |
Pregnancy termination Mother Translocation (7;9)(q35;q22.2). |
| Hengstschläger (2002) 20 | Trisomy 9p with trisomy 10p | 18 | Facial cleft, clubfoot, abnormal cerebellum, kidney cysts. |
Pregnancy termination at 18th week of gestation Postmortem examination: nose anomalies (snout‐like), bilateral cleft lip palate, low set ears, club feet, lung anomalies, cystic kidney and aplasia of the uterus |
| Chen (2002) 21 | Trisomy 9p with distal deletion of 12p (Amniocentesis at 17 weeks for a 5‐year‐old daughter with trisomy 9p) | 20 | Bilateral ventriculomegaly, brachycephaly, Dandy Walker malformation with enlarged cisterna magna and absence of the cerebellar vermis. |
Pregnancy termination Mother balanced translocation |
| López (Present case) | 47,XX+der(9)t(8;9)(p21.3;q22.3)mat (Amniocentesis at 17 weeks by ultrasound findings) | 12.3 | Nuchal translucency 2.35 mm, absent nasal bone, pleural effusion, early growth restriction, “claw‐like” hands, heart malformations |
Pregnancy interruption. Mother balanced translocation 46,XXt(8;9)(p21.3;q22.3) |
Discussion
Partial or full trisomy 9 (T9) is a rare chromosomal alteration. Its high lethality and very low prevalence make prenatal and postnatal diagnosis difficult. Few medical reports can be found in the literature and its suspicion usually raises during second and third trimesters when the severe malformations (detected by ultrasound) precede diagnostic procedures. Technology advances in fetal ultrasound allow an earlier diagnosis of severe structural malformations and suspicion of chromosomal aberrations during the first trimester with combined screening. Findings in previous reports of full trisomy 9 during the first trimester are only nuchal translucency over 3 mm and abnormal reverse ductus venosus wave, which are not really specific of trisomy 9 7, 22. Second‐ and third‐trimester findings are malformations in different organs with particular attention to central nervous system, heart, and limbs 23.
Our case was detected at the 12th week of gestational age, with altered sonographic findings as absent nasal bone, abnormal reverse ductus venosus wave, pleural effusion, and nuchal translucency of 2.35 mm (above 95 percentile for CRL). Three weeks after initial appearance, pleural effusion was not visualized, but early growth restriction could be documented. Combined first‐trimester screening aims for physical and biochemical search for the most common aneuploidies (T21, T18, and T13). Rare chromosomal alterations such as trisomy 9 are not usually part of the screening program. There are some clinical reports where biochemical abnormal values result positive for trisomy 18 (T18) due to very low quantity of PAPP‐A and free β‐hCG, suggesting the suspicion not only for trisomy 18, but also for trisomy 9 when these values are decreased 24, 25, 26. In our case, even though the values of PAPP‐A were normal, free β‐hCG was decreased and the global calculation result was high risk for trisomy 18, as reported in the literature.
There are few case reports in medical literature of partial trisomy 9. And only five reports were made in prenatal period (Table 1). All of them are reported in the second and third trimesters of pregnancy, and the earlier gestational age reported was 17 weeks. Our case had similar malformations as those reported in the literature, but they were detected in the first trimester (12th week) by sonographic and biochemical findings in the first pregnancy, and the second pregnancy was affected by the same chromosomic alteration due to the same 3:1 segregation pattern.
The breaking point in 9q21.3 represents a big amount of genes/material in chromosome 9 (64.12%), and sonographic findings showed a similar phenotype as complete trisomy 9. Only two other articles with similar breaking points were found in newborn babies. In both reports, maternal translocations between chromosomes 1 and 9 with 3:1 segregation patterns were found. The karyotypes were t(1,9)(p36;q22)mat and t(1;9)(q41;q21.32)mat. These phenotypes were similar to partial trisomy 9 with few different characteristics due to the presence of chromosome 1 material. Five additional cases of carrier mothers of chromosomal rearrangements in 9q21‐22 with an estimate of 23% risk of unbalanced products by a 3:1 segregation pattern exist. This risk and segregation pattern can be applied to our case because the second pregnancy was also affected by partial trisomy 9 27, 28, 29.
Conclusions
Complete and partial trisomy 9 are a rare chromosomal alteration with high lethality rates. Uncommon pathologies can be now detected in earlier stages of pregnancy due to technologic advances in prenatal care allowing us to understand natural history of rare and lethal diseases.
There are no specific sonographic findings for trisomy 9 in the first trimester, but the suspicion of a chromosomic alteration by abnormal nuchal translucency, absent nasal bone, altered reverse ductus venosus wave, and pleural effusion, along with low values of PAPP‐A and β‐hCG, can be considered suspects not only for trisomy 18, but also for trisomy 9. Trisomy 9 can also be suspected in the second trimester, primarily when sonographic findings consist of malformations of the central nervous system and abnormal limbs' postures. Parents' karyotype is mandatory for a precise genetic counseling.
Conflict of Interest
All authors declare that they have no conflict of interest in relation to this work.
Authorship
JLF: took part in maternal–fetal medicine, bibliographic research, and first‐trimester ultrasound scan detecting anomaly. LFG: participated in bibliographic research and first‐trimester ultrasound scan detecting anomaly. LGZ: performed cytogenetic analysis of the couple and both pregnancies. LJG: involved in second ultrasound scan and maternal–fetal medicine. RMH: performed cytogenetic analysis of the couple and both pregnancies. ECM: took part in Ob‐gyn management in both pregnancies. DMM: Head of Genetic laboratory and Genetic Conuseling of the couple.
References
- 1. Feingold, M. , and Atkins L.. 1973. A case of trisomy 9. J. Med. Genet. 10:184–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurnick, J. , Atkins L., Feingold M., et al. 1974. Trisomy 9: predominance of cardiovascular, liver, brain, and skeletal anomalies in the first diagnosed case. Hum. Pathol. 5:223–232. [DOI] [PubMed] [Google Scholar]
- 3. Jones, K. L. , Jones, M. C. , & Del Campo, M. 2013. Trisomy 9 mosaic syndrome Pp.28–29 in: Smith's recognizable patterns of human malformation, 7th ed. Elsevier Health Sciences. [Google Scholar]
- 4. Arnold, G. L. , Kirby R. S., Stern T. P., and Sawyer J. R.. 1995. Trisomy 9: review and report of two new cases. Am. J. Med. Genet. 56:252–257. [DOI] [PubMed] [Google Scholar]
- 5. Cantú, E. S. , Eicher D. J., Pai G. S., Donahue C. J., and Harley R. A.. 1996. Mosaic vs non mosaic trisomy 9: report of a liveborn infant evaluated by fluorescence in situ hybridization and review of the literature. Am. J. Med. Genet. 62:330–335. [DOI] [PubMed] [Google Scholar]
- 6. Smart, R. D. , Viljoen D. L., and Fraser B.. 1988. Partial trisomy 9 – further delineation of the phenotype. Am. J. Med. Genet. 31:947–951. [DOI] [PubMed] [Google Scholar]
- 7. Sepulveda, W. , Wimalasundera R. C., Taylor M. J., Blunt S., Be C., De La Fuente S., et al. 2003. Prenatal ultrasound findings in complete trisomy 9. Ultrasound Obstet. Gynecol. 22:479–483. [DOI] [PubMed] [Google Scholar]
- 8. Chitayat, D. , Hodgkinson K., Luke A., Winsor E., Rose T., Kalousek D., et al. 1995. Prenatal diagnosis and fetopathological findings in five fetuses with trisomy 9. Am. J. Med. Genet. 56:247–251. [DOI] [PubMed] [Google Scholar]
- 9. Saura, R. , Traore W., Taine L., Wen Z. Q., Roux D., Maugey‐Laulom B., et al. 1995. Prenatal diagnosis of trisomy 9. Six cases and a review of the literature. Prenat. Diagn. 15:609–614. [DOI] [PubMed] [Google Scholar]
- 10. Zen, P. R. , Rosa R. F., Rosa R. C., Graziadio C., and Paskulin G. A.. 2011. New report of two patients with mosaic trisomy 9 presenting unusual features and longer survival. Sao Paulo Med. J. 129:428–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pruksanusak, N. , Rujirabanjerd S., Kanjanapradit K., Kor‐anantakul O., Suntharasaj T., Suwanrath C., et al. 2014. Prenatal diagnosis of complete trisomy 9 with a novel sonographic finding of heart calcification. J. Ultrasound Med. 33:1871–1873. [DOI] [PubMed] [Google Scholar]
- 12. Schwendemann, W. D. , Contag S. A., and Wax J. R.. 2009. Sonographic Findings in Trisomy 9. J. Ultrasound Med. 28:39–42. [DOI] [PubMed] [Google Scholar]
- 13. Wilson, G. N. , Raj A., and Baker D.. 1985. The phenotypic and cytogenetic spectrum of partial trisomy 9. Am. J. Med. Genet. 20:277–282. [DOI] [PubMed] [Google Scholar]
- 14. Vaglio, A. , Búrix B., Quadrelli A., and Quadrelli R.. 2007. Síndrome de trisomía 9p: características clínico‐evolutivas y citogenéticas. Seguimiento de doce años. Arch. Pediatr. Urug. 78:151–156. [Google Scholar]
- 15. von Kaisenberg, C. S. , Caliebe A., Krams M., Hackelöer B. J., and Jonat W.. 2000. Absence of 9q22‐9qter in trisomy 9 does not prevent a Dandy‐Walker phenotype. Am. J. Med. Genet. 95:425–428. [PubMed] [Google Scholar]
- 16. Littooij, A. S. , Hochstenbach R., Sinke R. J., van Tintelen P., and Giltay J. C.. 2002. Two cases with partial trisomy 9p: molecular cytogenetic characterization and clinical follow‐up. Am. J. Med. Genet. 109:125–132. [DOI] [PubMed] [Google Scholar]
- 17. San Román Muñoz, M. , Herranz Fernández J. L., Tejerina Puente A., et al. 2004. Trisomy 9p. An. Pediatr. (Barc) 61:336–339. [DOI] [PubMed] [Google Scholar]
- 18. Sherer, D. M. , Abramowicz J. S., Jaffe R., and Woods J. R. Jr. 1993. Cleft palate: confirmation of prenatal diagnosis by color Doppler Ultrasound. Prenat. Diagn. 13:953–956. [DOI] [PubMed] [Google Scholar]
- 19. Chen, C. P. , and Shih J. C.. 1999. Prenatal diagnosis of bilateral ventriculomegaly and an enlarged cisterna magna in a fetus with partial trisomy 9 and partial trisomy 21. Prenat. Diagn. 19:1175–. [DOI] [PubMed] [Google Scholar]
- 20. Hengstschläger, M. , Bettelheim D., Repa C., Lang S., Deutinger J., Bernaschek G., et al. 2002. A fetus with trisomy 9p and trisomy 10p originating from unbalanced segregation of a maternal complex chromosome rearrangement t(4;10;9). Fetal Diagn. Ther. 17:243–246. [DOI] [PubMed] [Google Scholar]
- 21. Chen, C. P. , Chang T. Y., Shih J. C., Lin S. P., Lin C. J., Wang W., et al. 2002. Prenatal diagnosis of the Dandy‐Walker malformation and ventriculomegaly associated with partial trisomy 9p and distal 12p deletion. Prenat. Diagn. 22:1063–1066. [DOI] [PubMed] [Google Scholar]
- 22. Murta, C. , Moron A., Avila M., França L., and Vargas P.. 2000. Reverse flow in the umbilical vein in a case of trisomy 9. Ultrasound Obstet. Gynecol. 16:575–577. [DOI] [PubMed] [Google Scholar]
- 23. Chen, C. P. , Chern S. R., Cheng S. J., Chang T. Y., Yeh L. F., Lee C. C., et al. 2004. Second‐ trimester diagnosis of complete trisomy 9 associated with abnormal serum screen results, open sacral spina bifida, and congenital diaphragmatic hernia, and review of the literature. Prenat. Diagn. 24:455–462. [DOI] [PubMed] [Google Scholar]
- 24. Lam, Y. H. , Lee C. P., and Tang M. H.. 1998. Low second‐trimester maternal serum human chorionic gonadotropin in a trisomy 9 pregnancy. Prenat. Diagn. 18:1212. [PubMed] [Google Scholar]
- 25. Khoury‐Collado, F. , Anderson V. M., Haas B. R., Fisher A. J., Bombard A. T., Weiner Z., et al. 2004. Trisomy 9 screened positive for trisomy 18 by maternal serum screening. Prenat. Diagn. 24:836–838. [DOI] [PubMed] [Google Scholar]
- 26. Tørring, N , Petersen O. B., Becher N., Vogel I., Uldbjerg N, Danish Fetal Medicine Study Group, Danish Clinical Genetics Study Group . 2015. First trimester screening for other trisomies than trisomy 21, 18, and 13. Prenat. Diagn. 35:612–619. [DOI] [PubMed] [Google Scholar]
- 27. Akalin, I. , Bozdag S., Spielmann M., Basaran S. Y., Nanda I., Klopocki E., et al. 2014. Partial trisomy 1q41‐qter and partial trisomy 9pter‐9q21.32 in a newborn infant: an array CGH analysis and review. Am. J. Med. Genet. A 164A:490–494. [DOI] [PubMed] [Google Scholar]
- 28. Neu, R. L. , Dennis N. R., Lanman J. T. Jr, and Bannerman R. M.. 1979. An infant with trisomy 9pter yields 9q22 resulting from 3:1 segregation in a 46, XX t(1;9) (p36;q22) mother. Ann. Genet. 22:151–154. [PubMed] [Google Scholar]
- 29. Fisch, G. S. , Davis R., Youngblom J., and Gregg J.. 2011. Genotype‐phenotype association studies of chromosome 8p inverted duplication deletion syndrome. Behav. Genet. 41:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]


