Key Clinical Message
Measles infection, postliver transplant, may lead to a fatal graft loss. Individuals who have been previously exposed to the measles antigen may have a modified disease presentation. Although vaccination may not provide solid immunity, it ameliorates the severity of the disease.
Keywords: Liver transplant, measles, rejection
Background
Transplant recipients have an increased susceptibility to a variety of infections owing to immunosuppression in the post‐transplant period. They are prone to serious consequences of any infection due to impaired clinical signs and rapid progression of disease. Pretransplant immunization is part of routine prophylaxis against many viral infections but does not confirm long‐lasting protection against these infections in the post‐transplant period due to waning immunity 1, 2. Post‐transplant vaccination with live vaccines has been suggested by many reports but is not a part of guidelines due to risk of clinical disease from the live vaccine strain 3, 4. Measles infection or immunization against measles virus is thought to confer long‐lasting immunity. We report an unusual and rare presentation of measles virus infection in a previously vaccinated, liver transplant recipient manifesting as rash and hepatitis and pneumonia.
Case
A 43‐year‐old woman, with postliver transplant (2008) secondary to primary sclerosing cholangitis‐related cirrhosis, maintained on tacrolimus, presented to our hospital with 4‐day history of sore throat and fever and 2‐day history of skin rash. On examination, patient was found to be febrile with temperature of 38.2 celsius, B.P 100/80 mmHg, respiratory rate (R.R) 24/min, and heart rate (H.R.) 130/min. Oxygen saturation on room air was 85%. Systemic examination revealed congested eyes, pharyngeal erythema, maculopapular skin rash involving face, upper limbs, and trunk. Chest examination revealed decreased breath sounds on the right basal area and crackles on the left side posteriorly till the mid‐zone. Basic laboratory work‐up was as follows: WBC: 9.8 × 103/μL, neutrophil: 7.7 × 103/UL, lymphocyte: 0.9 × 103/UL, BUN:2.80 mmol/L, creatinine: 88 μmol/L, Na: 129 mmol/L, K: 4 mmol/L, Cl: 99 mmol/L, HCO3: 18 mmol/L mmol/L, bilirubin T: 7.6 μmol/L, albumin: 28 gm/L, ALT: 50 U/L, AST: 84 U/L, ALP: 268 U/L, INR: O.9, CRP: 53, ESR: 29 and tacrolimus level: 3.4. Hepatic transaminases showed progressive increase over 2 days. Ultrasound of abdomen and liver was normal. A CT pulmonary angiogram was ordered due to the presence of tachypnoea, tachycardia and drop in oxygen saturation. It did not show any evidence of PE but showed bilateral basal lung infiltrates more on the right side with associated two large size basal lung cysts (Fig. 1) .
Figure 1.

CT chest.
Patient was admitted as a case of viral exanthemata fever and bronchopneumonia with airborne precautions and was treated with intravenous antibiotics and fluids. The respiratory viral panel came negative. Viral serology results were positive for CMV Ab IgG, EBV nuclear antigen IgG, EBV capsid antigen IgG, rubella IgG Ab, measles IgG Ab, and measles IgM Ab. Autoimmune serologies and tuberculosis work‐up were negative. On day 2 of admission, patient's skin rash started to fade from the face and patient showed clinical improvement with regard to fever and constitutional symptoms. Bronchoscopy with bronchoalveolar lavage to evaluate lung infiltrates and cystic lung disease showed negative work‐up for acid‐fast bacilli, Pneumocystis jiroveci pneumonia, and fungi. Measles virus PCR in serum was reported positive. She was managed with fluids and supportive measures. The patient improved clinically with the fading of skin rash gradually and normalization of liver enzymes over the next 5 days and was discharged home.
Discussion
Immunosuppression in solid‐organ transplant recipients is an absolute necessity to prevent organ rejection, but carries a risk for a variety of infections. Viral infections are common 1–6 months post‐transplant. Although to a lesser degree, they continue to pose a problem after 6 months owing to continued but less intensive immunosuppression.
Measles is a highly contagious viral illness with a 90% attack rate in susceptible exposed individuals 5. It is characterized by fever, malaise, rash, cough, coryza, and conjunctivitis 6. Immunity after measles infection is thought to be lifelong, although rare cases of reactivation have been reported 7, 8. Modified measles is an attenuated infection that occurs in patients with preexisting measles immunity (either via wild‐type disease or vaccination) 9. It may result from antibody titer lower than that considered protective. The diagnosis of measles virus infection is usually made based on at least one of the following: positive serologic test for serum measles IgM antibody, significant rise in measles IgG antibody between acute and convalescent titers, isolation of measles virus in culture, or detection of measles virus RNA by reverse transcription polymerase chain reaction (RT‐PCR), but the gold standard test for serologic evidence of recent measles infection is a fourfold rise in titer as measured in a measles virus plaque reduction neutralization test (PRN or PRNT) 9.
Measles infection in adults is known to cause some degree of hepatitis, which is mainly hepatocellular 10. Cholestatic pattern of hepatitis, although rare, is not unknown 11. Satoh et al. 12 reported that the rise in transaminases due to measles returned back to normal 3 weeks after onset of rash, which is consistent with our case, where the LFTs in our patient returned to baseline in about 2 weeks after onset of rash (Fig. 2). The hepatitis in the course of measles is attributed to direct viral invasion of the hepatocytes, as detected by RT‐PCR in the hepatocytes 12.
Figure 2.
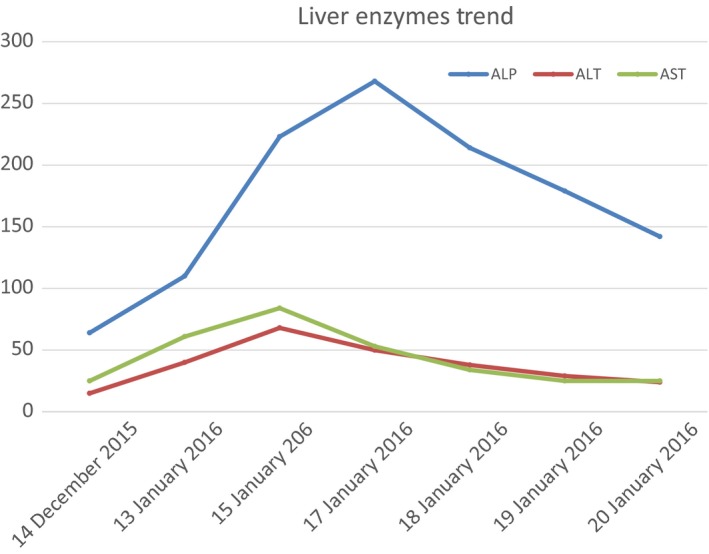
Liver enzyme trend.
Infection with measles virus postliver transplant manifesting as clinical illness is rare among adults. Graft rejection was reported in one case of measles infection in a cadaveric liver transplant recipient 13. On the contrary, our patient presented with resolving hepatitis. Although measles infection was confirmed by IgM positivity and PCR, but interestingly IgG was positive in our case, who reported two doses of measles vaccines, raising the suggestion, that the patient had some degree of immunity against the virus. This may be the cause of ameliorating the course of measles, resulted in self‐limiting transaminitis. Possible mechanisms for infection in our case include either infection by a wild strain of measles virus or antibody levels not enough to confer adequate immunity due to immunosuppressive state.
Liu et al. reported five cases of measles postliver transplant with serious consequences of pneumonia and respiratory failure requiring mechanical ventilation in two of them. Furthermore, one of those cases ended in death due to respiratory failure 14. Lung cysts are a characteristic of measles in the presence of cellular immune deficiency 15. The respiratory symptoms in our patient were limited to pneumonitis and lung cysts and did not progress to full blown respiratory failure.
In our center and others, pretransplant vaccination is routinely given to candidates awaiting any solid‐organ transplant as a protective measure (Fig. 3).
Figure 3.

A time line graph for the events.
A review conducted by Isabella et al. identified 72 papers addressing the issue of serological response rate against a host of viral vaccines in solid‐organ transplants (SOT) 16. They concluded that SOT recipients mount a lower immune response than healthy controls. The degree of this impaired response depended on the type of vaccine, age, and the organ transplanted. A study by Dehghani et al. in pediatric liver transplant recipients reported a low incidence of adequate immunization against HBV, DTwP, and MMR vaccines given pretransplant 4. Similar findings were concluded by other studies 5, 6. We think the degree of immunity in our patient was adequate to protect the graft from serious injury and possible rejection, but still not adequate enough to cause a clinical illness and transient hepatitis (a partial immune state).
Individuals who have been previously exposed to the measles antigen may have a modified disease presentation. These cases are usually detected during an outbreak, exposure to a confirmed measles case, and rarely, without a known exposure. In our case, no related cases were diagnosed nor outbreak was reported simultaneously 10, 17.
Immunization post‐transplant may be necessary, but is complicated by the fact that response to vaccines can be impaired by immunosuppression. As a general rule, live vaccines are not licensed for use in this population because of the increased risk of clinical disease from the vaccine strain of the virus. In 1999, Kano et al. 3 reported that pretransplant vaccination confers immunity for up to 6 months post‐transplant and that post‐transplant live vaccine under immunosuppressive conditions was safe and effective. Recently, Masayoshi et al. 4 studied revaccination for primary vaccine failure in postliver transplant patients and concluded that live attenuated vaccine was safe and effective postliver transplant in patients who were not severely immunosuppressed.
In conclusion, we report a liver transplant recipient acquiring measles infection manifesting as transient hepatitis and pneumonia. It resolved over 10 days owing to a partial immune state and did not progress to a full blown graft rejection as previously reported. It is clear from the literature reviewed that pretransplant vaccination did not confer adequate immunity in spite of IgG positivity in our patient. Good quality evidence is needed on the subject of post‐transplant vaccination to formulate guidelines.
Conclusions
Immunity against pretransplant vaccination wanes over time.
Clinicians need to be aware of the waning immunity and keep a broad perspective when faced with diagnostic challenges in transplant recipients. Although vaccination may not provide solid immunity, it ameliorates the severity of the disease.
More prospective trials are needed to address the issue of vaccination post‐transplant.
Authorship
All authors have contributed to and agreed on the content of the manuscript. They shared substantial contributions to the conception of the work and interpretation of data for the work, drafting the work, and final approval of the version to be published.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1. Eckerle, I. , Rosenberger K., Zwahlen M., and Junghanss T.. 2013. Serologic vaccination response after solid organ transplantation: a systematic review. PLoS ONE 8:e56974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dehghani, S. M. , Shakiba M. A., Ziaeyan M., Imanieh M. H., Haghighat M., Sedaghat M., et al. 2014. Evaluation of immunity status to routine vaccination in pediatric liver transplant candidates. Turk. J. Gastroenterol. 25(Suppl. 1):26–31. [DOI] [PubMed] [Google Scholar]
- 3. Kano, H. , Mizuta K., Sakakihara Y., Kato H., Miki Y., Shibuya N., et al. 2002. Efficacy and safety of immunization for pre‐ and post‐ liver transplant children. Transplantation 74:543–550. [DOI] [PubMed] [Google Scholar]
- 4. Shinjoh, M. , Hoshino K., Takahashi T., and Nakayama T.. 2015. Updated data on effective and safe immunizations with live‐attenuated vaccines for children after living donor liver transplantation. Vaccine 33:701–707. [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention . Epidemiology and Prevention of Vaccine‐Preventable Diseases. Hamborsky J., Kroger A., Wolfe S., eds. 13th ed. Washington, D.C. Public Health Foundation, 2015Chapter 13; Page 214. [Google Scholar]
- 6. Moss, W. J. , and Griffin D. E.. 2012. Measles. Lancet 379:153. [DOI] [PubMed] [Google Scholar]
- 7. Welliver, R. C. , Cherry J. D., and Holtzman A. E.. 1977. Typical, Modified, and Atypical MeaslesAn Emerging Problem in the Adolescent and Adult. Arch Intern Med. 137(1):39–41. [PubMed] [Google Scholar]
- 8. Schaffner, W. , Schluederberg A. E., and Byrne E. B.. 1968. Clinical epidemiology of sporadic measles in a highly immunized population. N. Engl. J. Med. 279:783. [DOI] [PubMed] [Google Scholar]
- 9. Siennicka, J. , Częścik A., and Trzcińska A.. 2014. The significance for epidemiological studies anti‐measles antibody detection examined by enzyme immunoassay (EIA) and plaque reduction neutralization test (PRNT). Przegl. Epidemiol. 68:417–420. 527‐9. [PubMed] [Google Scholar]
- 10. Merino, E. , Ramos J. M., Reus S., Boix V., Zurita A., Alzate E., et al. 2014. Measles in adults during an outbreak in Spain: hospitalization associated with gastrointestinal and liver involvement. Infection 42:763–765. [DOI] [PubMed] [Google Scholar]
- 11. Gavish, D. , Kleinman Y., Morag A., and Chajek‐Shaul T.. 1983. Hepatitis and jaundice associated with measles in young adults. An analysis of 65 cases. Arch. Intern. Med. 143:674–677. [PubMed] [Google Scholar]
- 12. Satoh, A. , Kobayashi H., Yoshida T., Tanaka A., Kawajiri T., Oki Y., et al. 1999. Clinicopathological study on liver dysfunction in measles. Intern. Med. 38:454–457. [DOI] [PubMed] [Google Scholar]
- 13. Sternfeld, T. , Spo ¨ ri‐Byrtus V., Riediger C., Langer R., Friess H., Schmid R. M., et al. 2010. Acute measles infection triggering an episode of liver transplant rejection Schulte‐Frohlinde. Int. J. Infect. Dis. 14:e528–e530. [DOI] [PubMed] [Google Scholar]
- 14. Liu, Y. , Sun L. Y., Zhu Z. J., Lin W., Qu W., and Zeng Z. G.. 2015. Measles virus infection in pediatric liver transplantation recipients. Transplant. Proc. 47:2715–2718. [DOI] [PubMed] [Google Scholar]
- 15. Becroft, D. M. , and Osborne D. R.. 1980. The lungs in fatal measles infection in childhood: pathological, radiological and immunological correlations. Histopathology 4:401–412. [DOI] [PubMed] [Google Scholar]
- 16. Danerseau, A. M. , and Robinson J. L.. 2008. Efficacy and safety of measles, mumps, rubella and varicella live viral vaccines in transplant recipients receiving immunosuppressive drugs. World J. Pediatr. 4:254–258. [DOI] [PubMed] [Google Scholar]
- 17. Rota, J. S. , Hickman C. J., Sowers S. B., et al. 2011. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: high risk of infection but low risk of transmission. J. Infect. Dis. 204(Suppl. 1):S559–S563. [DOI] [PubMed] [Google Scholar]


