Abstract
Dysbiosis of the gut microbiome via antibiotics, changes in diet and infection can select for bacterial groups that more frequently harbor antimicrobial resistance genes (ARGs) and mobile genetic elements (MGEs). However, the impact of environmental toxicants on the reservoir of ARGs in the gut microbiome has received less attention. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a potent aryl hydrocarbon receptor (AhR) agonist with multiple toxic health effects including immune dysfunction. The selective pressure of TCDD on the abundance of ARG and MGE-harboring gut populations was examined using C57BL/6 mice exposed to 0–30 μg/kg TCDD for 28 and 92 days with the latter having a 30-day recovery period. DNA extracted from temporally collected fecal pellets was characterized using a qPCR array with 384 assays targeting ARGs and MGEs. Fourteen genes, typically observed in Enterobacteriaceae, increased significantly within 8 days of initial dosing, persisted throughout the treatment period, and remained induced 30 days post dosing. A qPCR primer set targeting Enterobacteriaceae also showed 10- to 100-fold higher abundance in TCDD-treated groups, which was further verified using metagenomics. Results show a bloom of ARG-harboring bacterial groups in the gut due to a xenobiotic compound that is not a metal, biocide or antimicrobial.
Keywords: TCDD; antibiotic resistance genes (ARGs); gut microbiome; persistent organic pollutant, immune-suppression, mobile genetic elements, dysbiosis, Enterobacteriaceae
An environmental toxicant that is not a metal, biocide or antimicrobial indirectly (via a non-inflammatory host response) influences the reservoir of antibiotic resistance genes in the murine gut.
INTRODUCTION
Dysregulation of the gut microbiome has been implicated in a number of diseases (Moore and Moore 1995; Ley et al.2005; Turnbaugh et al.2006; Bollyky et al.2009; Damman et al.2012; Serino et al.2014), and is associated with environmental stimuli such as diet (Penders et al.2005; Turnbaugh et al.2009), stress (Bailey, Lubach and Coe 2004), antibiotics (Tanaka et al.2009), infection (Barman et al.2008) and environmental contaminants such as subtherapeutic antimicrobials (Cho et al.2012) and xenobiotics (Zhang et al.2015). Typically, bacterial dysbiosis is either a direct response to therapeutics (e.g. antibiotics) or an indirect response due to changes in host that influence availability of metabolites or immune defenses such as cytokines, lymphocytes, antimicrobial peptide (Stelter et al.2011) or reactive nitrogen and oxygen species (Winter et al.2013). For example, commensal facultative Enterobacteriaceae (e.g. Escherichia coli) bloomed over fermenting obligate anaerobic bacteria (e.g. Bacteriodetes and Firmicutes) due to host-mediated inflammation (Winter et al.2013). Increased levels of Enterobacteriaceae were also reported due to gastrointestinal infection (Lupp et al.2007), and antibiotic therapies including streptomycin, vancomycin (Sekirov et al.2008), amoxicillin, metronidazole and bismuth (Antonopoulos et al.2009).
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a persistent organic pollutant, causes several aryl hydrocarbon receptor (AhR)-mediated effects (Whitlock 1990) including immune dysfunction, hepatic damage and steatosis, and gastric epithelial hyperplasia. TCDD and other dioxins are formed as a side product of manufacturing herbicides or burning organic materials such as waste incarnation, fossil fuel and wood combustion (DHHS 2016). In general, exposure can occur from food such as fish or dairy, and is partly the result of persistence and accumulation in river sediment and floodplains (Yamashita et al.2000). Following oral exposure, TCDD elicits an immunosuppressive response in the gut (Vorderstrasse et al.2001; Marshall et al.2008) that depletes immune cell populations and expression (Fader et al.2015; Stedtfeld et al.2016), and increases host susceptibility to infectious diseases including Salmonella, Trichinella spirali and Listeria (Thigpen et al.1975; Luebke et al.1994; Sugita-Konishi et al.2003). Since TCDD influences immune response and microbial dysbiosis, this study focused on the elicited impact on the reservoir of antibiotic resistance genes (ARGs) and mobile genetic elements (MGEs) in the gut.
Based on previous studies, we hypothesized that TCDD-induced response may also select for ARG-harboring bacterial groups in the gut resistome. Levels of xenobiotic exposure required to shift gut bacterial populations are also untested. As such, this study examined C57BL/6 mouse models orally gavaged with 0–30 μg/kg TCDD every 4 days for 92 days (long-term study, LS group) followed by a 30-day recovery period (response study, RS group), and a short-term high dose of 30 μg/kg every 4 days for 28 days (short-term study, SS group). Fecal pellets were collected temporally and extracted DNA was tested using a 384-assay qPCR array targeting ARGs and MGEs and metagenomics.
MATERIALS AND METHODS
Animal treatment
Animal handling procedures were carried out with the approval of the Michigan State University All-University Committee on Animal Use and Care as previously described (Fader et al.2015). Briefly, experiments (Fig. S1, Supporting Information) included a long-term 92-day dose–response study (LS group) followed by a 30-day recovery study (RS group), and a short-term 28-day study (SS group). Female C57BL/6 mice were obtained from Charles Rivers Laboratories (Portage, MI for LS and RS studies, Kingston, NY for SS study) postnatal day 25, and acclimatized for 3–4 days. Mice in the LS and RS groups were housed in polycarbonate cages with cellulose fiber chips (Aspen Chip Laboratory Bedding, Northeastern Products, Warrensburg, NY, USA). Mice in the SS group were housed in Innocages (Innovive, San Diego, CA, USA) with ALPHA-dri® bedding (Shepherd Specialty Papers, Chicago, IL, USA). Mice in all three groups were housed in a 23°C environment with 30%–40% humidity and a 12-h light/dark cycle and fed ad libitum with Harlan Teklad 22/5 Rodent Diet 8940 (Madison, WI, uSA) and had free access to deionized water (LS and RS) or Aquavive water (SS; Innovive).
Mice in the LS experiments were dosed with 0, 0.01, 0.03, 0.1, 0.3, 1, 3, 10 or 30 μg/kg of TCDD every 4 days for 92 days, for a total of 23 exposures. Mice in the RS group were dosed with 0 or 30 μg/kg of TCDD every 4 days for 92 days, followed by a recovery for 30 days with no treatment. Mice in the SS group were dosed with 0 or 30 μg/kg TCDD every 4 days for 28 days, for a total of seven exposures. For the LS and RS groups, mice were housed five per cage, with two cages for each dose. Mice in the SS group were housed individually with eight mice per dose group. Groups with 0 μg/kg TCDD were orally gavaged with vehicle (0.1 ml sesame oil), which was also used to administer TCDD via oral gavage.
Fecal pellets were collected every 8 days for the RS and LS groups, and pellets were collected daily before and after initiation of dosing for the SS group. In all groups, fecal pellets were <24 h old at the time of collection and freezing. All pellets were stored at –80°C until analyzed.
DNA extraction
For the SS group, pellets collected from five separate time points were selected for analysis including 1 day prior to initial treatment and 4, 8, 18 and 28 days following initial treatment. Pellets collected 75 days following initial treatment were selected for analysis of the LS group. Pellets collected 30 days following cessation of treatment for the RS group were used for analysis. DNA was extracted from fecal pellets (100 mg) using the PowerMax Soil DNA Isolation Kit (MOBIO Laboratories). DNA samples were quantified on a Nanodrop ND-1000 UV-Vis spectrophotometer (Nanodrop Products, Wilmington, DE, USA). DNA integrity was determined by gel electrophoresis. Extracted DNA was stored at –20°C.
qPCR ARG array
The qPCR array contained 384 assays targeting ARGs and MGEs (Table S1, Supporting Information). All assays were described in previous studies (Wang et al.2016). qPCR reactions were performed on a Wafergen SmartChip Real-time PCR system as reported (Wang et al.2016). Sample and primers were dispensed into the SmartChip using a Multi-sample Nanodispenser. Extracted DNA (30 μg per μl) was used for each sample, DNA extracted from individual groups was pooled and all qPCR reactions were run in triplicate. Cycling conditions and data processing were performed as previously described (Wang et al.2016). The 16S rRNA gene assay targeting Enterobacteriaceae (Barman et al.2008) was run separately on the Chromo4 BioRad using reagents and cycling parameters recommended with the Power SYBR Green PCR Master Mix. For calculating relative abundance of Enterobacteriaceae, estimated copies of total 16S rRNA gene were quantified using the universal bacteria primer set targeting the 16S rRNA gene (Lane 1991), which was run in parallel on the Chromo4 BioRad.
qPCR data and statistical analysis
Estimated genomic copies, relative abundance and fold difference were calculated as previously described (Looft et al.2012; Zhu et al.2013; Wang et al.2014). Briefly, relative abundance of ARGs in the total bacterial community was determined by qPCR using 16S rRNA gene designed to be universal to bacteria, and fold difference was calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001). The Shapiro-Wilk test was used to evaluate normality of data. If necessary, variance between compared groups was corrected with the Geisser-Greenhouse method. One-way ANOVA followed by a multiple comparison Sidak test was performed to compare fold difference of enriched ARGs among days of SS group and doses among LS and RS group. When normal distribution was satisfied, a Student's t-test was used for comparing differences between two groups. Statistical analysis and some plots were generated using Prism (version 7 for Windows, GraphPad Software, San Diego, CA, USA), additional plots were made using Excel.
Shotgun metagenomics analysis
To verify qPCR results and examine structural shifts in the whole bacterial community, samples were pooled and analyzed using Illumina HiSeq. In detail, an equal mass of gDNA extracted from individual samples was pooled to yield 400 ng. Samples included SS study treated and vehicle on day 28, LS treated and vehicle with 30 μg/kg TCDD on day 75 of treatment, and RS treated and vehicle 30 days following cessation of dosing. The six pooled samples were submitted for library preparation and sequencing to the Research Technology Support Facility at Michigan State University. Libraries were prepared using the Illumina TruSeq Nano DNA Library Preparation Kit. After QC and quantitation, all six libraries were combined into a single pool. The pool was loaded on an Illumina HiSeq 2500 Rapid Run flow cell (v1) and sequenced using Rapid SBS reagents in a 2 × 150 bp paired-end format.
Base calling was done by Illumina Real Time Analysis (RTA) v1.18.64, and output of RTA was de-multiplexed and converted to FastQ format with Illumina Bcl2fastq v1.8.4. With the combined output of three flow cells (three HiSeq lanes), all six samples were represented at >22 Gbp, yielding a total of 137 Gbp.
The MSU High Performance Computing Center was used for all sequence analyses. In detail, pair-end reads were assembled using PandaSeq (Masella et al.2012), and reads from individual lanes were concatenated. Xander was used to assemble and retrieve the rplB gene, which was used to analyze taxonomic structure (Wang et al.2015). Xander parameters included a k-size of 45. Following Xander, chimeras were removed using UCHIME in de novo mode (Edgar et al.2011), and FrameBot (Wang et al.2013) was used to correct sequencing errors and output closest matches to the reference database. The Search Engine for Antimicrobial Resistance was used to detect ARGs from sequence data (Rowe et al.2015) using Illumina BaseSpace® (https://basespace.illumina.com).
RESULTS AND DISCUSSION
Previous studies have examined dysbiosis due to infection, antibiotic treatment or heavy metal selection (Baker-Austin et al.2006); however, this study investigated the influence of an environmental toxicant with no known antimicrobial characteristics on ARG-harboring populations in the gut. The abundance of ARGs and MGEs in fecal pellets was measured temporally (SS group) for mice orally gavaged with TCDD, with varying doses of TCDD long term (LS group), and 30 days following cessation of dosing (RS group). Host hepatic weight, TCDD levels in the host, histology, host gene expression, intestinal and hepatic immune cell populations, and abundance of obligate anaerobes from these experiments are described elsewhere (Bhaduri 2015; Fader et al.2015).
Higher abundance of ARGs in TCDD-dosed mice
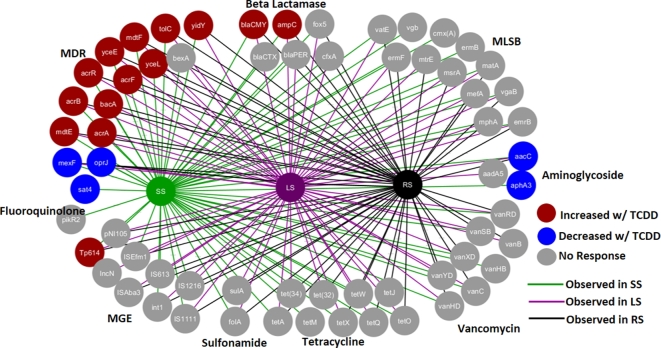
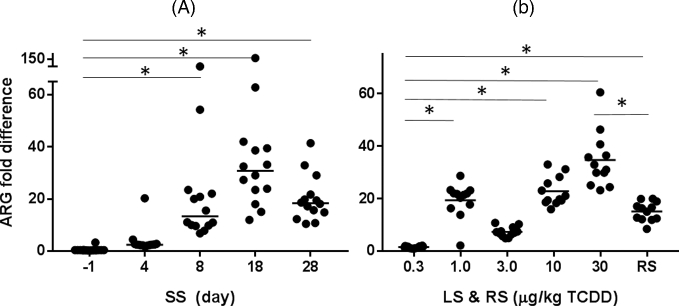
In total, 14 of 65 genes (ARGs and MGEs) in the murine gut microbiome had >2-fold increase in response to TCDD (Fig. 1). Thirteen out of 14 genes were enriched in all three groups (SS, LS and RS), with the Tp614 gene only enriched in the SS group (Tables S2 and S3, Supporting Information). For the LS group, the same 13 enriched ARGs were observed in all doses >0.3 μg/kg TCDD (Fig. 2B). For the SS experiment, ARGs were enriched 4 to 8 days after the initial dose and increased with time (Fig. 2A). The fold difference of enriched ARGs was highest on day 18 of the experiment. Only ARGs and MGEs that were originally present in all three groups of mice showed higher abundance with exposure to TCDD, suggesting that unintentional colonization with non-commensal transient bacteria did not occur.
Figure 1.
qPCR ARG analysis of TCDD-induced response measured in DNA from fecal pellets for SS, LS and RS groups (fold change ≥ |2.0| and P value < 0.05). Node color indicates genes with higher (red), lower (blue) or no change (gray) in ARG abundance in response to TCDD. Edge or line color indicates genes were detected in SS group (green), LS group (purple), RS group (black). MLSB: macrolide-lincosamide-streptogramin, MGE: mobile genetic elements, MDR: multiple drug resistance.
Figure 2.
Fold difference of 14 enriched genes (13 ARGs and one MGE) in response to TCDD (A) over time for SS group days since start of dosing (x-axis), and (B) dose response with LS group, and 30 days following cessation of dosing (RS group). Individual bars represent median fold change for all enriched ARGs with (fold change ≥ |2.0| and P value < 0.05). Stars indicate significant difference (P < 0.05) between before and after start of dosing for SS group and difference among LS groups compared to 0.3 μg/kg TCDD, and vehicle and TCDD-dosed RS group.
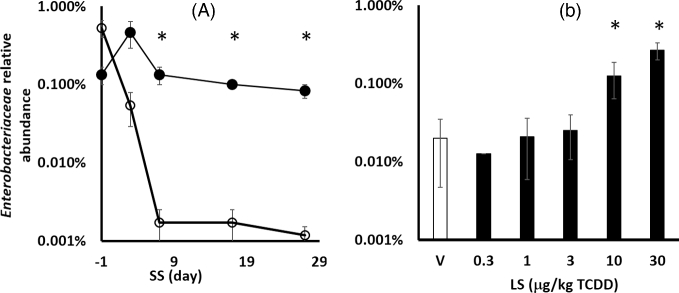
Based on in silico BLAST analysis of primer sets, 13 out of 14 enriched genes are found in Enterobacteriaceae family including Citrobacter, Enterobacter, Escherichia, Klebsiella, Salmonella and Shigella. Only the Tp614 gene does not appear to be present in Enterobacteriaceae. Measured using 16S rRNA gene primer set targeting Enterobacteriaceae (Fig. 3), the higher abundance of this group was confirmed to be 10- to 100-fold in response to TCDD in SS and LS groups. Shotgun metagenomics analysis also verified an increased abundance of Enterobacteriaceae in response to TCDD (Fig. S2A, Supporting Information) in SS, LS and RS groups based on the rplB gene. The bacA gene, in which expression confers bacitracin resistance, has been observed in multiple non Enterobacteriaceae species; however, the primer set only targets alleles observed in Escherichia coli.
Figure 3.
Relative abundance of Enterobacteriaceae (A) in SS groups before (-1 day) and throughout dosing. Open and closed circles indicate vehicle and 30 μg/kg TCDD, respectively. (B) Dose response (LS group). Stars indicates significant difference (P < 0.05) between TCDD and vehicle-dosed mice for the same time point. Error bars represent standard deviation of technical replicates.
The observed response of ARGs with 1 μg/kg TCDD corresponds with doses of TCDD that have previously been shown to elicit an immune response. For example, the effect of TCDD on immune function in mice, evaluated with anti-sheep red blood cell immunoglobulin M antibody forming cells, induced significant suppression of humoral activity at doses down to 1 μg/kg TCDD and 0.5 μg/kg TCDD (Kaplan et al.2011; Boyd et al.2016). In addition, TCDD was shown to deplete immune cell expression and populations of macrophage and dendritic cells in the intestinal lamina propria of these same mice (Fader et al.2015). Interestingly, host gene expression and hepatic phenotypes were more often observed at higher doses (>1 μg/kg TCDD) in these mice (Fader et al.2015).
Accordingly, TCDD-elicited influence on immune cells in the gut may provide an environment that is more favorable to specific populations (Van Kaer, Parekh and Wu 2015). For example, increased levels of flagellin-producing Proteobacteria were observed in mice lacking the flagellin receptor Toll-like receptor 5 gene TLR5 (Cullender et al.2013). Several members of gut commensal Firmicutes and Enterobacteriaceae (excluding Bacteroidetes) produce flagella (Lozupone et al.2012). In agreement, shotgun metagenomics analysis of these mice also showed a higher abundance of Firmicutes in fecal pellets in response to TCDD (Fig. S2B, Supporting Information) based on the rplB gene. Furthermore, TCDD tended (p = 0.06) to reduce the expression of TLR5 gene 2.0-fold in ileum of mice devoid of microbiota (Stedtfeld et al.2016).
Commensal dysbiosis may also be due to other TCDD-induced changes in host such as availability of luminal metabolites. Additional jejunal epithelial genes that were differentially expressed in response to TCDD in these mice included lipid, fatty acid and cholesterol uptake and metabolism (Fader et al.2015). Alternatively, observed TCDD-elicited changes to host may partially be the result of bacterial dysbiosis that is initially caused by an immune response. These questions warrant additional studies.
Previous studies have also observed dysbiosis with AhR ligands. Similar to our study, TCDD dramatically shifted taxonomic composition with increased relative abundance of Firmicutes at the expense of Bacteroidetes (Lefever et al.2016). On the contrary, a study with wild-type mice orally dosed with 2,3,7,8-tetrachlorodibenzofuran (TCDF), a less potent AhR activator, heightened host inflammation, and increased abundance of Bacteroides over Firmicutes (Zhang et al.2015). Both studies surmised that dysregulation may be the results of TCDD-induced immune response. Dissimilar responses in immune response and subsequent bacterial shifts may be due to differences in AhR ligand potency, which has recently been reported in a study comparing TCDD and 6-Formylindolo[3,2-b] carbazole (Farmahin et al.2016). However, neither of these previous studies reported shifts in Enterobacteriaceae or associated ARGs. This may be due the methods used for community analysis.
The TCDD-elicited commensal response does not appear to be direct. Our own previous studies showed that 30-nM TCDD-dosed batch reactors did not influence gut commensals (including Enterobacteriaceae) in vitro (Stedtfeld et al.2016). No effects were observed with 1–1000 nM TCDD on bacteria grown on agar plates (Lefever et al.2016). In addition, TCDF did not influence gut commensals in AhR knockout mice (Zhang et al.2015). Taken together, results suggest that the response of ARG-harboring bacteria is due to TCDD-induced influences on the host.
Enriched ARGs included blaCMY2 and ampC genes that are associated to beta-lactam resistance (e.g. carbapenem, cefoxitin, ceftazidime, ceftriaxone, cephalosporin and cephamycin). Enriched multiple drug resistance (MDR) genes included mdtE, F, mdtG, H and acrA, B which are resistance-nodulation-cell division (RND) multidrug resistance flux pumps. These MDR genes require accompaniment of the tolC gene to confer resistance, which was also enriched with TCDD. The enriched group of MDR genes is associated with resistance to beta-lactam (Nishino et al.2003), acriflavin, aminoglycoside, glycylcycline, macrolide, doxorubicin, erythromycin, deoxycholate and fosfomycin (Liu and Pop 2009). It should be noted that the abundance of five ARGs decreased in some groups (Fig. 1, Table S2, Supporting Information). However, the measured decreases was not as prominent as ARGs that were enriched in response to TCDD.
A commensal shift towards ARG-harboring organisms could potentially increase horizontal gene transfer and antibiotic resistance infections. For example, a study with E. coli and Salmonella reported that inflammation-induced dysbiosis appeared to drive horizontal gene transfer and assortment of plasmids between pathogens and commensals in the gut (Stecher et al.2012), potentially promoting the spread of AR and virulence determinants. Higher levels of Enterobacteriaceae in the gut are typically associated with inflammation (Lupp et al.2007; Winter et al.2013). In another study, antibiotic perturbation of the microbiota caused blooms of multidrug-resistant E. coli in the intestine, which subsequently caused bacteremia and sepsis (Ayres, Trinidad and Vance 2012). In addition, the genomic structure of Proteobacteria contains the highest number of MGEs (Hu et al.2016). Another study reported that E. coli was the most highly represented carrier of antibiotic resistance in all tested groups (Osterblad et al.2000). Higher levels of Enterobacteriaceae type subfamilies such as E. coli have also been associated with diseases such as irritable bowel disease (Fava and Danese 2011).
Temporal decrease of Enterobacteriaceae in vehicle groups
Temporally measured pellets from the SS group showed that the relative (Fig. 3A) abundance of ARG-harboring Enterobacteriaceae decreased in the vehicle-dosed group. Initially, the relative abundance of Enterobacteriaceae was ∼ 0.1% in SS group and decreased in the vehicle-dosed group, while higher levels were observed in all LS groups. This level corresponds with previous studies showing that Enterobacteriaceae typically constitutes 0.1% of the commensal population (Eckburg et al.2005).
The temporal decrease in Enterobacteriaceae may be due to maturation of mice, sesame oil vehicle or other factors. Studies have reported that Enterobacteriaceae are early colonizers among the gut microbiota, which decreases with host maturation as the gut is depleted of oxygen and becomes increasingly anaerobic (Adlerberth and Wold 2009). Complementary analysis with these mice also observed a temporal increase in levels of Bacteroides fragilis and Clostridia in both vehicle and TCDD-dosed groups of mice (Bhaduri 2015), which would subsequently decrease relative abundance of Enterobacteriaceae. What's more, sesame oil is a traditional health food in Asian countries, and has been reported to attenuate fibrosis induced by 2,4,6-trinitrobenzenesulfonic acid (Periasamy et al.2013), and reduce inflammatory cytokines (Narasimhulu et al.2015; Selvarajan et al.2015). Similarly, these effects may be indirectly influencing the levels of ARG-harboring Enterobacteriaceae in vehicle-dosed mice.
Total ARGs and MGEs in mice
In total, 56 ARGs and 9 MGEs (Fig. 1, Table S3, Supporting Information) were present in mouse fecal pellets. Amplified genes were associated with resistance to aminoglycosides, beta-lactams, amphenicols, Macrolide-Lincosamide-Streptogramin B (MLSB), sulfonamides, MDR, tetracycline, vancomycin and MGEs. The initial presence of ARGs in fecal pellets can be contributed to many factors. In humans, ARGs and associated microbial communities are passed between healthy mother–infant pairs (de Vries et al.2011), with ARGs detected in the mouth of newborns 15 min after birth (Alicea-Serrano et al.2013). The presence of multidrug-resistance efflux pumps can be attributed to mechanisms that are not associated with resistance (Piddock 2006).
ARGs that did not respond to TCDD may be from bacterial groups that do not have a casual response to TCDD. In some instances, ARGs primer sets (e.g. tetO gene) target a taxonomically diverse group of bacteria and thus a measured increase or decrease may be masked by responses in varying directions.
Shotgun metagenomics was also performed to verify presence and TCDD-induced shift of ARGs. However, the half-lane of Hi-Seq 2500 used per sample did not provide sequence depth required to observe shifts in ARGs with lower abundance. For example, 53 ARGs were detected using the qPCR array for the SS group treated with TCDD, and only 8 ARGs were detected with metagenomics (Table S4, Supporting Informatiob). The eight ARGs detected with metagenomics also had the highest relative abundance observed with the ARG qPCR array. Based on qPCR measurements, the detection limit of the qPCR array was ∼0.0004% relative abundance and ∼0.05% relative abundance for metagenomics. For another example, analyses performed using qPCR and metagenomics observed detectable levels (0.08% and 0.13%, relative abundance, respectively) of Enterobacteriaceae for the SS group dosed with TCDD. However, Enterobacteriaceae was not detected in metagenomics-derived sequences from vehicle-dosed SS group while it was detected with qPCR at 0.001% relative abundance. Others have also observed higher sensitivity of PCR compared to metagenomics (Pärnänen et al.2016).
Abundance of ARGs 30 days post cessation of dosing
Dysbiosis has been observed for long periods following antibiotic treatment (Larsen et al.2010). As such, we examined the level of Enterobacteriaceae and associated ARGs following cessation of dosing (RS group). Results showed that the fold difference of ARGs was lower compared to the LS study, but remained high compared to the vehicle-dosed group (Fig. 2B). The level of Enterobacteriaceae was also higher in the RS group dosed with TCDD (Fig. S2, Supporting Information) compared to the vehicle. This suggests that TCDD-induced dysbiosis may have similar temporal effects as antibiotic therapy (Antonopoulos et al.2009). Sjölund et al. (2003) reported that resistant enterococci harboring the ermB gene were isolated immediately after and up to 3 years following antibiotic treatment. Reports have also observed that older individuals have higher levels of ARGs (Lu et al.2014), which may be due to increased exposure to antimicrobials. Our observation suggests that such accumulation can also be due to exposure to an environmental toxicant such as TCDD.
Overall, TCDD enriched ARG and MGE-harboring members of Enterobacteriaceae in the gut microbiome by 10- to 100-fold. The higher abundance of Enterobacteriaceae may also promote transfer of ARGs among bacteria in the gut. Strains of bacteria associated with human/animal microbiome are 25 times more likely to transfer genetic material compared to organisms from environmental samples (Smillie et al.2011). Moreover, 50% of horizontal gene transfers included ARGs. The immunosuppressive host response to TCDD with increased susceptibility to infections (Thigpen et al.1975; Luebke et al.1994) combined with increased levels of ARG-harboring gut commensals may synergistically influence the likelihood for transfer of resistance to bacterial pathogens. Studies have also suggested that the gut microbiota is a potential reservoir for environmental spread of ARGs (Sommer, Dantas and Church 2009). As such, increased exposure to TCDD and perhaps other non-expected environmental xenobiotics may further contribute to enrichment of ARGs in gut microbiomes.
Supplementary Material
Supplementary data are available at FEMSEC online.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSEC online.
FUNDING
This work was supported by the National Institute of Environmental Health Sciences Superfund Basic Research Program (NIEHS SBRP P42ES04911) with contributions from Project 3, 4, 5 and Core B.
Conflict of interest. None declared.
REFERENCES
- Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr 2009;98:229–38. [DOI] [PubMed] [Google Scholar]
- Alicea-Serrano A, Contreras M, Magris M et al. Tetracycline resistance genes acquired at birth. Arch Microbiol 2013;195:447–51. [DOI] [PubMed] [Google Scholar]
- Antonopoulos DA, Huse SM, Morrison HG et al. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun 2009;77:2367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres J, Trinidad N, Vance RE. Lethal inflammasome activation by a multidrug-resistant pathobiont upon antibiotic disruption of the microbiota. Nat Med 2012;18:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastr Nutr 2004;38:414–21. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C, Wright MS, Stepanauskas R et al. Co-selection of antibiotic and metal resistance. Trends Microbiol 2006;14:176–82. [DOI] [PubMed] [Google Scholar]
- Barman M, Unold D, Shifley K et al. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun 2008;76:907–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaduri P. Exposure to 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin Differentially Impacts Key Members of Mice Gut Microbiome. Michigan State University, Environmental Engineering. Phd Dissertation2015. [Google Scholar]
- Bollyky PL, Bice JB, Sweet IR et al. The Toll-like receptor signaling molecule myd88 contributes to pancreatic beta-cell homeostasis in response to injury. PLoS One 2009;4:2–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd SA, Sallach JB, Zhang Y et al. Sequestration of TCDD by activated carbon eliminates bio availability and the suppression of immune function in mice. Environ Toxicol Chem 2017. doi: 10.1002/etc.3815. [DOI] [PMC free article] [PubMed]
- Cho I, Yamanishi S, Cox L et al. Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature 2012;488:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullender TC, Chassaing B, Janzon A et al. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe 2013;14:571–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damman CJ, Miller SI, Surawicz CM et al. The microbiome and inflammatory bowel disease: is there a therapeutic role for fecal microbiota transplantation? Am J Gastroenterol 2012;107:1452–9. [DOI] [PubMed] [Google Scholar]
- de Vries LE, Valle Y, Agersø Y et al. The gut as reservoir of antibiotic resistance: microbial diversity of tetracycline resistance in mother and infant. PLoS One 2011;6:e21644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DHHS: Report on Carcinogens, 14th edn2016, https://ntp.niehs.nih.gov/ntp/roc/content/introduction_508.pdf (29 May 2017, date last accessed). [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN et al. Diversity of the human intestinal microbial flora. Science (80-) 2005;308:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC et al. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011;27:2194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fader KA, Nault R, Ammendolia DA et al. 2, 3, 7, 8-Tetrachlorodibenzo-p-Dioxin (TCDD) alters lipid metabolism and depletes immune cell populations in the jejunum of C57BL/6 mice. Toxicol Sci 2015;148:567–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmahin R, Crump D, O’Brien JM et al. Time-dependent transcriptomic and biochemical responses of 6-Formylindolo [3, 2-b] carbazole (FICZ) and 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) are explained by AHR activation time. Biochem Pharmacol 2016;115:134–43. [DOI] [PubMed] [Google Scholar]
- Fava F, Danese S. Intestinal microbiota in inflammatory bowel disease: Friend of foe? World J Gastroenterol 2011;17:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Yang X, Li J et al. The transfer network of bacterial mobile resistome connecting animal and human microbiome. Appl Environ Microb 2016, DOI: 10.1128/AEM.01802-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan BLF, Crawford RB, Kovalova N et al. TCDD adsorbed on silica as a model for TCDD contaminated soils: evidence for suppression of humoral immunity in mice. Toxicology 2011;282:82–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane DJ. 16S/23S rRNA sequencing in nucleic acid techniques in bacterial systematics. In: Stackebrandt E, Goodfellow M, (eds). Chichester, UK: John Wiley & Sons; 1991, 115–75. [Google Scholar]
- Larsen N, Vogensen FK, van den Berg FWJ et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 2010;5:e9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever DE, Xu J, Chen Y et al. TCDD modulation of gut microbiome correlated with liver and immune toxicity in streptozotocin (STZ)-induced hyperglycemic mice. Toxicol Appl Pharmacol 2016;304:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P et al. Obesity alters gut microbial ecology. P Natl Acad Sci USA 2005;102:11070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Pop M. ARDB–Antibiotic Resistance Genes Database. Nucleic Acids Res 2009;37:D443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K, Schmittgen T. Analysis of relative gene expression data using realtime quantitative PCR and the 2∧-Delta Delta Ct Method. Methods 2001;25:402–8. [DOI] [PubMed] [Google Scholar]
- Looft T, Johnson TA, Allen HK et al. In-feed antibiotic effects on the swine intestinal microbiome. P Natl Acad Sci USA 2012;109:1691–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozupone C, Faust K, Raes J et al. Identifying genomic and metabolic features that can underlie early successional and opportunistic lifestyles of human gut symbionts. Genome Res 2012;22:1974–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu N, Hu Y, Zhu L et al. DNA microarray analysis reveals that antibiotic resistance-gene diversity in human gut microbiota is age related. Sci Rep 2014;12:4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebke R, Copeland C, Diliberto J et al. Assessment of host resistance to Trichinella spiralis in mice following preinfection exposure to 2,3,7,8-TCDD. Toxicol Appl Pharmacol 1994;125:7–26. [DOI] [PubMed] [Google Scholar]
- Lupp C, Robertson ML, Wickham ME et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of enterobacteriaceae. Cell Host Microbe 2007;2:119–29. [DOI] [PubMed] [Google Scholar]
- Marshall NB, Vorachek WR, Steppan LB et al. Functional characterization and gene expression analysis of CD4+ CD25+ regulatory T cells generated in mice treated with 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin. J Immunol 2008;181:2382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella AP, Bartram AK, Truszkowski JM et al. PANDAseq: PAired-eND assembler for Illumina sequences. BMC Bioinformatics 2012;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WEC, Moore LH. Intestinal floras of populations that have a high risk of colon cancer. Appl Environ Microb 1995;61:3202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhulu CA, Selvarajan K, Litvinov D et al. Anti-atherosclerotic and anti-inflammatory actions of sesame oil. J Med Food 2015;18:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino K, Yamada J, Hirakawa H et al. Roles of tolC-dependent multidrug transporters of Escherichia coli in resistance to Beta-Lactams. Antimicrob Agents Ch 2003;47:3030–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterblad M, Hakanen A, Manninen R et al. A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora. Antimicrob Agents Ch 2000;44:1479–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pärnänen K, Karkman A, Tamminen M et al. Evaluating the mobility potential of antibiotic resistance genes in environmental resistomes without metagenomics. Sci Rep 2016;6:35790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penders J, Vink C, Driessen C et al. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett 2005;243:141–7. [DOI] [PubMed] [Google Scholar]
- Periasamy S, Hsu D, Chandrasekaran V et al. Sesame oil accelerates healing of 2,4,6-trinitrobenzenesulfonic acid-induced acute colitis by attenuating inflammation and fibrosis. JPEN- Parenter Enter 2013;37:674–82. [DOI] [PubMed] [Google Scholar]
- Piddock L. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol 2006;4:629–36. [DOI] [PubMed] [Google Scholar]
- Rowe W, Baker KS, Verner-Jeffreys D et al. Search engine for antimicrobial resistance: a cloud compatible pipeline and web interface for rapidly detecting antimicrobial resistance genes directly from sequence data. PLoS One 2015;10:e0133492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekirov I, Tam NM, Jogova M et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun 2008;76:4726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan K, Narasimhulu C, Bapputty R et al. Anti-inflammatory and antioxidant activities of the nonlipid (aqueous) components of sesame oil: potential use in atherosclerosis. J Med Food 2015;18:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino M, Blasco-baque V, Nicolas S et al. Far from the eyes, close to the heart: dysbiosis of gut microbiota and cardiovascular consequences. Curr Cardiol Rep 2014;16:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjölund M, Wreiber K, Andersson D et al. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori. Ann Intern Med 2003;139:483–7. [DOI] [PubMed] [Google Scholar]
- Smillie C, Smith M, Friedman J et al. Ecology drives a global network of gene exchange connecting the human microbiome. Nature 2011;480:241–4. [DOI] [PubMed] [Google Scholar]
- Sommer M, Dantas G, Church G. Functional characterization of the antibiotic resistance reservoir in the human microflora. Science (80-) 2009;325:1128–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stecher B, Denzler R, Maier L et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. P Natl Acad Sci USA 2012;109:1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedtfeld RD, Chai B, Crawford RB et al. Modulatory influence of segmented filamentous bacteria on transcriptomic response of gnotobiotic mice exposed to TCDD. Environ Health Persp 2016. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelter C, Kappeli R, Konig C et al. Salmonella-induced mucosal lectin RegIIIβ kills competing gut microbiota. PLoS One 2011;6:e20749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita-Konishi Y, Kobayashi K, Naito H et al. Effect of lactational exposure to 2, 3, 7, 8-tetrachlorodibenzo- p -dioxin on the susceptibility to Listeria infection. Biosci Biotech Bioch 2003;67:89–93. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kobayashi T, Songjinda P et al. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Micr 2009;56:80–7. [DOI] [PubMed] [Google Scholar]
- Thigpen J, Faith R, McConnell E et al. Increased susceptibility to bacterial infection as a sequela of exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Infect Immun 1975;12:1319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006;444:1027–31. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ridaura VK, Faith JJ et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med 2009;1:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L, Parekh VV, Wu L. The response of CD1d-restricted invariant NKT vells to microbial pathogens and their products. Front Immunol 2015;6:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorderstrasse BA, Steppan LB, Silverstone AE et al. Aryl hydrocarbon receptor-deficient mice generate normal immune responses to model antigens and are resistant to TCDD-induced immune suppression. Toxicol Appl Pharm 2001;171:157–64. [DOI] [PubMed] [Google Scholar]
- Wang F, Stedtfeld RD, Kim O-S et al. Influence of soil characteristics and proximity to antarctic research stations on abundance of antibiotic resistance genes in soils. Environ Sci Technol 2016;50:12621–9. [DOI] [PubMed] [Google Scholar]
- Wang F-H, Qiao M, Su J-Q et al. High throughput profiling of antibiotic resistance genes in urban park soils with reclaimed water irrigation. Environ Sci Technol 2014;48:9079–85. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fish JA, Gilman M et al. Xander: employing a novel method for efficient gene-targeted metagenomic assembly. Microbiome 2015;3:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Quensen J, Fish J et al. Ecological patterns of nifH genes in four terrestrial climatic zones explored with targeted metagenomics using FrameBot, a new informatics tool. MBio 2013;4:e00592–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock JP. Genetic and molecular aspects of 2,3,7,8-tetra chlorodibenzo-p-dioxin action. Annu Rev Pharmacal 1990;30:251–77. [DOI] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN et al. Host-derived nitrate boosts growth. Science (80-) 2013;339:708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Kannan K, Imagawa T et al. Vertical profile of polychlorinated dibenzo-p-dioxins, dibenzofurans,naphthalenes, biphenyls, polycyclic aromatic hydrocarbons, and alkylphenols in a sediment core from Tokyo Bay, Japan. Environ Sci Technol 2000;34:3560–7. [Google Scholar]
- Zhang L, Nichols RG, Correll J et al. Persistent organic pollutants modify gut microbiota-host metabolic homeostasis in mice through aryl hydrocarbon receptor activation. Environ Health Persp 2015;123:679–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y-G, Johnson TA, Su J-Q et al. Diverse and abundant antibiotic resistance genes in Chinese swine farms. P Natl Acad Sci USA 2013;110:3435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at FEMSEC online.