Abstract
The stoichiometric constraints of algal growth are well understood, whereas there is less knowledge for heterotrophic bacterioplankton. Growth of the marine bacterium Phaeobacter inhibens DSM 17395, belonging to the globally distributed Roseobacter group, was studied across a wide concentration range of NH4+ and PO43−. The unique dataset covers 415 different concentration pairs, corresponding to 207 different molar N:P ratios (from 10−2 to 105). Maximal growth (by growth rate and biomass yield) was observed within a restricted concentration range at N:P ratios (∼50−120) markedly above Redfield. Experimentally determined growth parameters deviated to a large part from model predictions based on Liebig's law of the minimum, thus implicating synergistic co-limitation due to biochemical dependence of resources. Internal elemental ratios of P. inhibens varied with external nutrient supply within physiological constraints, thus adding to the growing evidence that aquatic bacteria can be flexible in their internal elemental composition. Taken together, the findings reported here revealed that P. inhibens is well adapted to fluctuating availability of inorganic N and P, expected to occur in its natural habitat (e.g. colonized algae, coastal areas). Moreover, this study suggests that elemental variability in bacterioplankton needs to be considered in the ecological stoichiometry of the oceans.
Keywords: Phaeobacter inhibens DSM 17395, growth physiology, N:P ratio, Redfield, Liebig limitation, ecological stoichiometry
Phaeobacter inhibens DSM 17395, member of the marine Roseobacter group, grows optimally at N:P supply ratios >16, exhibits phytoplankton-like flexible internal elemental stoichiometry and different nutrients control growth synergistically.
INTRODUCTION
Activity and productivity of microorganisms in marine ecosystems are largely controlled by the availability of the macronutrients nitrogen (N) and phosphorous (P). Most marine organisms use N and P from inorganic (e.g. NO3−, NH4+, PO43−) as well as organic sources (e.g. proteins, amino acids, nucleic acids, nucleotides, phospholipids and other cell envelope components). Some marine microorganisms (in particular cyanobacteria) transitorily deposit N and P within intracellular storage compounds, e.g. the organic N-rich polymer cyanophycin or inorganic polyphosphates (e.g. Allen 1984; Brock et al.2012; Burnat, Herrero and Flores 2014). Across different marine systems, e.g. from nutrient-rich estuaries, coastal and upwelling regions to oligotrophic open ocean water bodies, the availability of both macronutrients varies strongly. Further variations emerge from spreading oxygen minimum zones and the associated N loss (Franz et al.2012; Tsementzi et al.2016). In the environment, concentrations of total N and P range approximately from 6 to 200 μM and 0.03 to 20 μM, respectively, and molar N:P ratios vary from ∼5 to 310 with an average of 37 (Downing 1997).
Despite this huge variability in nutrient ratios (Hecky and Kilham 1988), oceanographers noticed relatively constant N:P stoichiometry of the particulate matter (seston) in the open ocean (Redfield 1934, 1958). This has been related to the ability of phytoplankton to stabilize the oceanic N:P ratio through nitrogen fixation (Lenton and Klausmeier 2007). Following the same rationale, the canonical Redfield ratio (molar C:N:P of 106:16:1) was suggested to be a global attractor of elemental composition achieved by phytoplankton at growth rates close to μmax (Goldman, McCarthy and Peavey 1979) or through conserved homeostatic relationships between macromolecules (Loladze and Elser 2011). Empirical evidence suggests, however, that phytoplankton can be highly flexible in cellular N:P ratios, reflecting the strongly varying availability of both elements in nature (Guildford and Hecky 2000). This high flexibility is not only observed at the level of entire communities, but even within single species grown under different nutrient supply ratios (Hillebrand et al.2013). Resolving this discrepancy, Klausmeier et al. (2004) modeled optimal N:P ratios for phytoplankton during exponential growth and competitive equilibrium by differentially addressing the cellular machinery for uptake (N-rich proteins) and assembly (P-rich ribosomes). This and other studies indicated that optimal N:P ratios differ between species with the Redfield ratio representing only the median across environmental conditions and phylogenies (Quigg et al.2003; Klausmeier et al.2004; Klausmeier, Litchman and Levin 2004; Hillebrand et al.2013). At low nutrient concentrations, the external N:P ratio determines the elemental composition of phytoplankton (Sterner and Elsner 2002), whereas at high nutrient concentrations, optimal nutrient uptake results in optimal internal N:P stoichiometry independent of the external N:P supply ratio (Klausmeier et al.2004).
Generally, heterotrophic bacteria compete with phytoplankton for available sources of N and P (e.g. Kirchman 1994; Jørgensen, Kroer and Coffin 1994). Bacteria take up a large proportion of inorganic nutrients (Kirchman 1994) and can outcompete phytoplankton when nutrients are scarce (Currie 1990; Thingstad, Skjoldal and Bone 1993; Joint et al.2002). Moreover, the presence of bacteria can alter the relative availability of nutrients for autotrophs, and thus shift nutrient limitation (and internal N:P ratios) for phytoplankton (Danger et al.2007). The uptake and recycling of elements by bacteria is thus a cornerstone of aquatic biogeochemistry (Cotner and Biddanda 2002). Makino et al. (2003) paraphrased the central question by asking whether heterotrophic bacteria are more like plants or animals, i.e. whether they show flexibility in elemental composition (as most autotrophs) or not (as most metazoan animals). Heterotrophs are in general significantly more inflexible (homeostatic) than autotrophs (at least with respect to N:P ratios), but they also deviate from homeostasis to variable degrees (Persson et al.2010). Escherichia coli was reported to be rather inflexible (Makino et al.2003), whereas bacterial communities (Chrzanowski et al.1996; Vrede et al.2002; Godwin and Cotner 2014) and single strains (Chrzanowski and Grover 2008; Chan et al.2012; Godwin and Cotner 2015a,b) exhibited variable internal stoichiometry if nutrient supply varied. Given the central role of bacteria in elemental cycles, the question of how bacterial growth and nutrient incorporation responds to different supply rates and ratios of inorganic nutrients remains a central question for marine ecosystems ecology.
Roseobacters constitute a metabolically diverse group within the alphaproteobacterial Rhodobacterales and can account for ∼20% of coastal and ∼15% of mixed-layer ocean bacterioplankton communities. They inhabit coastal and open oceans, sea ice and the sea floor and occur in the planktonic state as well as associated to particles (Buchan, González and Moran 2005; Wagner-Döbler and Biebl 2006). Roseobacters contribute to the recycling of seasonal biomass peaks generated during phytoplankton blooms (Teeling et al.2012, 2016; Luo and Moran 2014; Buchan et al.2014) and possess a highly adaptive potential towards habitat changes (Luo et al.2014). Phaeobacter inhibens DSM 17395 is a nutritionally versatile representative of roseobacters (Thole et al.2012; Drüppel et al.2014; Wiegmann et al.2014), appears to preferentially interact with biotic (e.g. algae, higher eukaryotes) and abiotic surfaces (e.g. Seyedsayamdost et al.2011; Gram et al.2015).
Phaeobacter inhibens DSM 17395 (and roseobacters in general) dwells in marine systems with differing and/or fluctuating availabilities of nitrogen and phosphorous. This study combines comprehensive experiments on growth physiology with mathematical modeling (see Fig. 1 for conceptual framework) to investigate the nutritional plasticity of P. inhibens under widely varying concentrations of ammonium (NH4+) and phosphate (PO43−) against a constant background of glucose as sole source of carbon and energy. We assessed the impact of varying external N:P supply ratios on growth of P. inhibens by determining achieved biomass yields (reflected by optical density and cellular dry weight) and growth rates. We found that (i) maximal values for assessed growth parameters were achieved at external N:P ratios >16; (ii) growth was synergistically controlled by more than just one nutrient (C, N, P), suspending effectiveness of Liebig's law of the minimum; and that (iii) internal elemental ratios (N:P, C:N, C:P) were flexible. Together, the here reported findings broaden our current perception on the potential of marine bacterioplankton to influence the cycling of macronutrients.
Figure 1.
Conceptual design highlighting the interconnections of physiological and modeling approaches, as applied in this study.
MATERIALS AND METHODS
Strain, media, cultivation and harvesting of cells
Phaeobacter inhibens DSM 17395 was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ, Braunschweig, Germany) and since then maintained in our laboratory. Only chemicals of analytical grade and membrane-purified water were used. All cultures were incubated on a rotary shaker (100 rpm) at 28°C in the dark, and growth was monitored by measuring the optical density (OD) at 600 nm (UVmini-1240; Shimadzu, Duisburg, Germany). For purity control, each culture was inspected by light microscopy and spreading on agar plates containing marine broth (MB) medium.
Each individual growth experiment was started according to a defined procedure (Fig. S1, Supporting Information) from a glycerol stock (stored at −80°C) beforehand prepared from cultures of P. inhibens grown in MB medium (Zech et al.2009). Stock cultures were revived using mineral medium (Zech et al.2009) supplemented with 11 mM glucose, 2 mM ammonium (provided as NH4Cl), 0.03 mM phosphate (provided as KH2PO4) and trace elements. Each nutrient was added from sterile stock solutions to the autoclaved mineral medium. To eliminate residual glycerol and MB medium, a serial dilution (up to 10–5) was prepared with 13.5 mL mineral medium (in 50 mL Erlenmeyer flasks) and a 1.5 mL glycerol stock. This was followed by two successive passages (50 mL culture in 250 mL Erlenmeyer flasks) in the same mineral medium, but with NH4+ and PO43− adjusted to one of 415 different concentration pairs. Main experiments were conducted in 1 L Erlenmeyer flasks containing 250 mL medium and with at least three biological replicates. All cultures were consistently inoculated with 2% (v/v) of the respective preceding culture when it had reached approximately half-maximal OD (∼0.5 ODmax). To avoid carryover of any residual PO43− at very low PO43− concentrations (<50 μM), glassware was specifically cleansed by overnight incubation in HCl (1%, v/v), followed by thorough rinsing with membrane-purified water. Growth experiments with 415 different NH4+ and PO43− concentration pairs were conducted over a period of 5 years by seven different experimenters. Coherent experimental proceedings were ascertained for each experimenter by initial and repeated cultivation at the same NH4+ (2 mM) and PO43− (30 μM) concentration pair. The dataset compiled in Fig. S2 (Supporting Information) derives from ∼1300 individual cultures and ∼20 000 OD measurements.
Detailed cellular-physiological analyses were performed for five selected NH4+ and PO43− concentration pairs, corresponding to N:P supply ratios of 267:1, 67:1, 17:1, 4:1 and 1:1 (Fig. S3, Supporting Information). These are above, close to and below the Redfield ratio, respectively. Per supply ratio, high-resolution growth curves were obtained from four parallel cultures (250 mL in 1 L Erlenmeyer flasks) to monitor OD600 and absorbance at 398 nm (Abs398), as well as consumption of glucose, NH4+ and PO43−. Simultaneously, sufficient cell material was generated for biomass analyses from eight additional parallel cultures per N:P supply ratio, harvested by centrifugation (11 300 × g, 20 min, 4°C; Avanti J-25, Beckmann Coulter, Krefeld, Germany) at ∼0.5 ODmax and ODmax. In case of ODmax, 45 mL of the culture supernatant was immediately filtered (0.2 μm CA; Sartorius, Göttingen, Germany), shock frozen in liquid nitrogen and analyzed within 2 days to quantify the excreted antibiotic tropodithietic acid (TDA). Cell pellets were used to determine the cellular dry weight (CDW) and the elemental composition at ∼0.5 ODmax and ODmax (see below section ‘Chemical analyses’).
Standardized calculation of growth rates
Growth with these widely varying NH4+ and PO43− concentration pairs (415) was assessed by monitoring OD (Figs 2–5; Fig. S2, Supporting Information). A logistic dose–response (LDR) function (Beckon et al.2008)
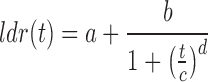 |
(a: OD directly after inoculation (ODstart), b: difference between ODmax and ODstart, c: time when the medium value a + 0.5b is reached, d: controls the shape of the curve) was fitted to experimental OD data, as recently described (Trautwein et al.2016). Based on this fit, the linear growth rate (μlin) was estimated as the slope of the LDR function at the inflection point (Trautwein et al.2016). Validity of applying the LDR function to this comprehensive dataset was verified based on high-resolution growth curves determined for five selected NH4+ and PO43− supply ratios (Table 1; Fig. S3, Supporting Information). For each of the 415 different NH4+ and PO43− concentration pairs, similarity of biological replicates was confirmed before fitting the LDR function to averaged OD measurements, minimizing the mean square error for all measurements before and at ODmax (Fig. S2, Supporting Information). Exponential growth was not included in the comprehensive dataset, as this growth phase was rather short and restricted to early growth, which could not be covered in sufficiently high resolution. Analytical calculations were performed with Maple 18.02 (Maplesoft, Waterloo, Canada); data fitting and numerical calculations were performed with MATLAB R2016a (The MathWorks, Natick, MA, USA).
Figure 2.
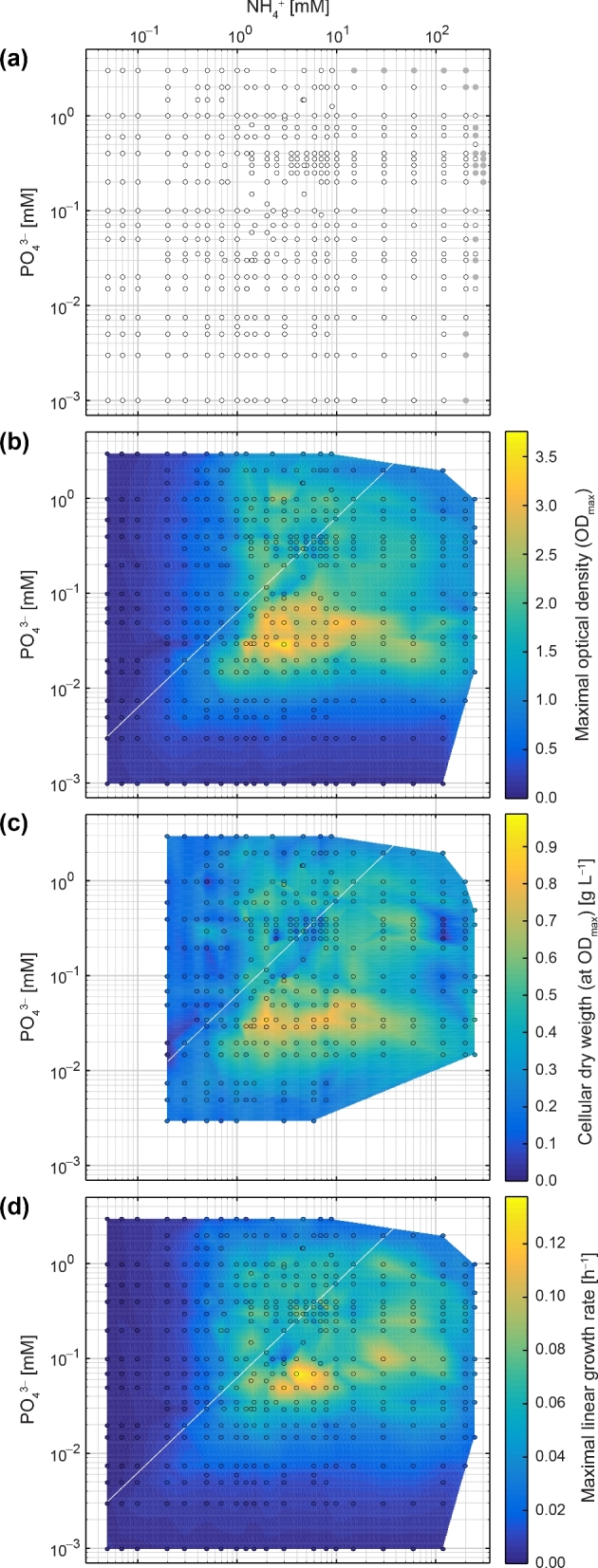
Growth of P. inhibens with varying concentrations of NH4+ (50 μM to 250 mM) and PO43− (1 μM to 3 mM). Glucose (sole source of carbon and energy) was in each case provided at the same initial concentration (11 mM). NH4+ and PO43− concentrations are plotted in logarithmic scale, growth parameters in colored linear scales. (a) Grid display of the analyzed 415 different concentration pairs of NH4+ and PO43− that correspond to 207 different N:P supply ratios ranging from 10–2 to 105. Filled gray circles indicate concentrations where growth was not observed. Colored data maps display the (b) maximal optical density (ODmax) reached upon entry into stationary growth phase, (c) the attained cellular dry weight at ODmax and (d) the calculated maximal growth rate (μlin) during linear growth (representing the major active growth phase) for each concentration pair of NH4+ and PO43−. The diagonal white line represents the canonical Redfield N:P ratio of 16:1. The circles in subfigures b–d represent experimentally determined values and colored areas between these data points were retrieved by linear interpolation. See Fig. 3 for analyzed growth parameters as a function of NH4+ or PO43− concentration. Corresponding growth curves with fitted logistic dose–response (LDR) function are compiled in Fig. S2 (Supporting Information).
Figure 5.
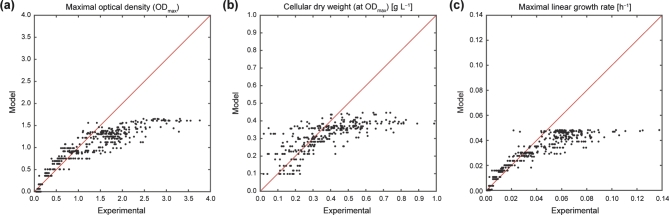
Comparison of growth parameters to Liebig's law of the minimum. Experimental values for P. inhibens were compared to modeled ones for (a) maximal optical density (ODmax), (b) cellular dry weight at ODmax and (c) maximal linear growth rate (μlin) across the studied concentration range of NH4+ and PO43−. The diagonal red line represents the exact match of measured values to model prediction for single nutrient-limited growth based on Liebig's law of the minimum. For data points above this line, predicted values were higher than measured, whereas measured values exceeded the model prediction below this line. A more detailed comparison of experimental values with those predicted by Liebig's law of the minimum is shown in Fig. S4 (Supporting Information).
Table 1.
Cellular and physiological characteristics of P. inhibens during growth with selected supply ratios of NH4+ and PO43− (for growth curves see Fig. S3, Supporting Information).
| Physiological parameters | External N:P supply ratioa | ||||
|---|---|---|---|---|---|
| 267 (P-limited) | 67 (P-limited) | 17 (NP-limited) | 4 (N-limited) | 1 (N-limited) | |
| ODmax (600 nm) | 3.24 ± 0.03 | 3.26 ± 0.04 | 0.92 ± 0.01 | 0.61 ± 0.02 | 0.58 ± 0.04 |
| μexp [h−1] | 0.163 ± 0.003 | 0.148 ± 0.003 | 0.225 ± 0.006 | 0.207 ± 0.003 | 0.250 ± 0.001 |
| μlin [h−1] | 0.090 ± 0.002 | 0.087 ± 0.002 | 0.040 ± 0.001 | 0.024 ± 0.001 | 0.025 ± 0.002 |
| YGlc [g dry cells (mol Glc)−1]b | 66.8 ± 1.2 | 63.3 ± 1.0 | 46.5 ± 1.2 | 63.0 ± 0.2 | 55.1 ± 0.2 |
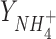 [g dry cells (mol NH4+)−1]b [g dry cells (mol NH4+)−1]b
|
494 ± 18 | 493 ± 2 | 360 ± 12 | 315 ± 6 | 296 ± 2 |
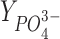 [g dry cells (mol PO43−)−1]b [g dry cells (mol PO43−)−1]b
|
23 883c | 21 185c | 5393c | 3178 ± 211 | 2,763 ± 46 |
| Internal N:P ratio (biomass)d | 36 | 43 | 16 | 12 | 13 |
| Internal N:P ratio (biomass)b | 46 | 57 | 19 | 11 | 12 |
| Internal C:N ratio (biomass)d | 18 | 19 | 14 | 11 | 10 |
| Internal C:N ratio (biomass)b | 30 | 26 | 22 | 16 | 17 |
| Internal C:P ratio (biomass)d | 661 | 821 | 233 | 129 | 129 |
| Internal C:P ratio (biomass)b | 1408 | 1460 | 420 | 183 | 197 |
| TDA (Abs398nm) b | 0.54 ± 0.01 | 0.58 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.06 ± 0.00 |
| TDA [nM] b | 0.19 ± 0.05 | 0.24 ± 0.09 | 0 | 0 | 0 |
aSelected molar N:P supply ratios with concentrations of NH4+ and PO43− (in brackets, respectively) in the medium: 267 (8.0 mM, 30 μM), 67 (2.0 mM, 30 μM), 17 (0.5 mM, 30 μM), 4 (0.5 mM, 125 μM) and 1 (0.5 mM, 0.5 mM).
bValues at ∼ODmax (i.e. at the transition into stationary growth phase). Molar growth yields were calculated according to the following formula:
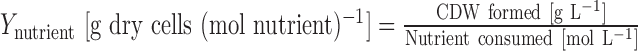 .
.
cComplete consumption of PO43− assumed (initial concentration below limit of quantitation [50 μM]; Ruppersberg et al.2016).
dValues at ∼0.5 ODmax during linear growth.
Data analysis with modeling
For each growth parameter, a 2D locally weighted regression (LOWESS) was computed to compensate for inhomogeneous data density across the studied range of 415 different NH4+ and PO43− concentration pairs (Cleveland 1979). The resulting surface has been used to calculate the quantiles (25%, 50% and 75%) for each growth parameter across the NH4+ and PO43− profiles, respectively. The median profile functions 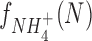 and
and 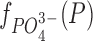 (black solid lines in Fig. 3) were combined according to Liebig's law of the minimum, which takes the smaller of the two values for any nutrient combination (N, P), resulting in a simple predictive model. Model predictions were computed and compared to actual measurements (Fig. 5; Fig. S4, Supporting Information). The LOWESS surface was further used to calculate mean values across the covered range of external N:P ratios (blue line in Fig. 4).
(black solid lines in Fig. 3) were combined according to Liebig's law of the minimum, which takes the smaller of the two values for any nutrient combination (N, P), resulting in a simple predictive model. Model predictions were computed and compared to actual measurements (Fig. 5; Fig. S4, Supporting Information). The LOWESS surface was further used to calculate mean values across the covered range of external N:P ratios (blue line in Fig. 4).
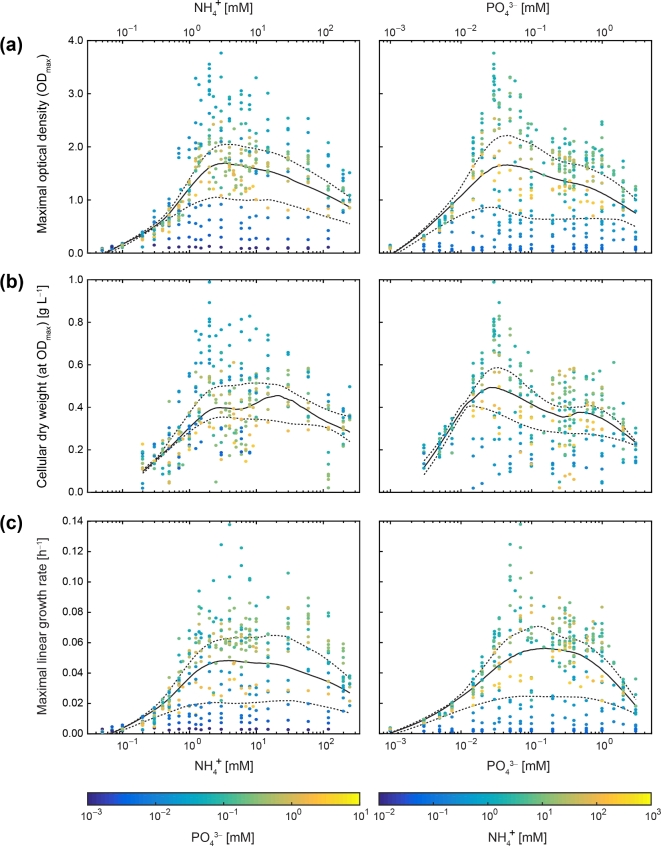
Figure 3.
Median of growth parameters across NH4+ and PO43− concentrations. Semilogarithmic profiles of (a) maximal optical density (ODmax), (b) cellular dry weight at ODmax and (c) maximal linear growth rate (μlin) for P. inhibens as a function of NH4+ or PO43− concentration (see Fig. 2b–d for joint display in color maps). Colors of data points indicate the corresponding NH4+ or PO43− concentration, respectively. The black solid line displays the calculated median and the dashed ones the 25% and 75% quantiles (based on 2D LOWESS fit to experimental data). A complementary fitting of a Monod function to the experimental data is shown in Fig. S5 (Supporting Information).
Figure 4.
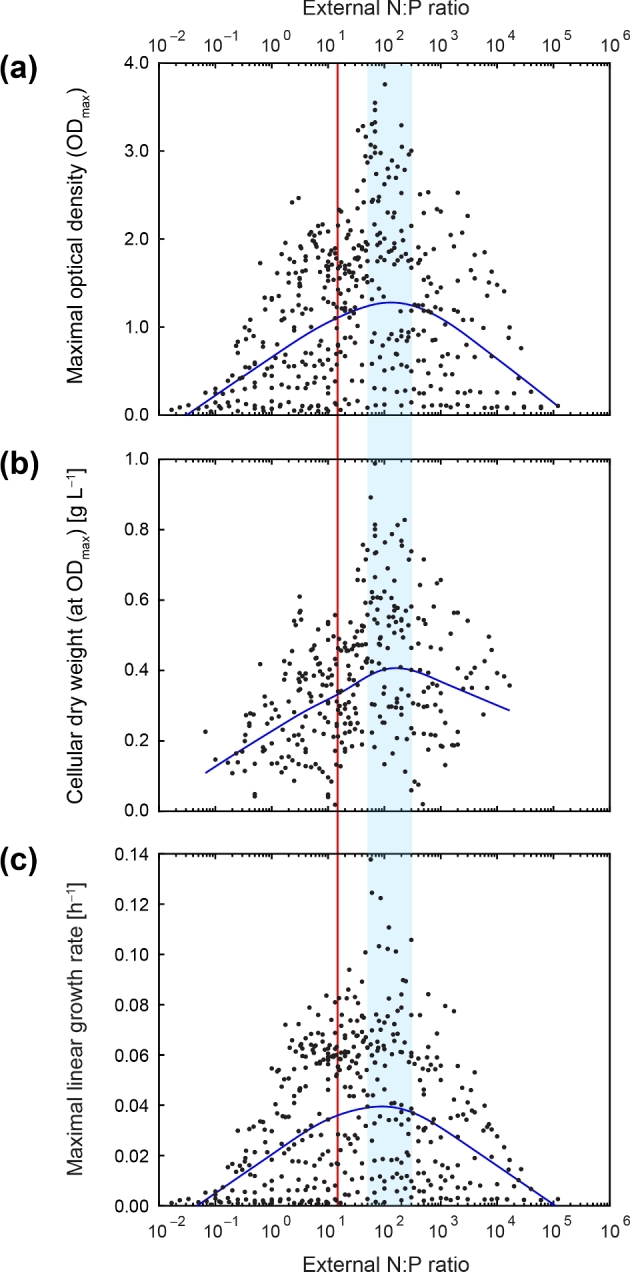
Mean of growth parameters across the N:P supply ratios. Semilogarithmic profiles of (a) maximal optical density (ODmax), (b) cellular dry weight at ODmax and (c) maximal linear growth rate (μlin) for P. inhibens as a function of the external N:P ratio. The blue line displays the calculated mean (based on 2D LOWESS fit) for the experimental data (black dots). The vertical red line marks the Redfield N:P ratio of 16:1. The blue shaded area delimits the approximate range of N:P supply ratios (∼50–120), at which the mean of the studied growth parameters was maximal.
Chemical analyses
The concentrations of NH4+ and PO43− in cell-free culture supernatants were determined with photometric assays, employing a microplate reader (MPR) as described in detail by Ruppersberg et al. (2016). The colorimetric determination of NH4+ was based on its reaction with sodium salicylate and sodium hypochlorite. The NH4+ assays were incubated for 15 min at 37°C in the MPR and then measured at 620 nm. The detection limit for NH4+ was 14 μM, and the linear range for quantitative determination was 36−200 μM. The colorimetric determination of PO43− was based on its complex formation with ammonium molybdate in the presence of ascorbate and zinc acetate at pH 5. The PO43− assays were incubated for 30 min at 30°C in the MPR and then measured at 620 nm. The detection limit for PO43− was 13 μM and the linear range for quantitative determination was 50 μM to 1 mM.
The concentration of glucose in cell-free culture supernatants was determined by HPLC analysis. The system consisted of an UltiMate 3000 Rapid Separation LC (ThermoFisher Scientific, Germering, Germany) equipped with a Eurokat separation column (8 × 300 mm, 5 μm bead size; Knauer, Berlin, Germany) temperature controlled at 75°C and a refractive index (RI) detector (RI-101; Shodex, Munich, Germany). The eluent was composed of 5 mM H2SO4 and administered at a flow rate of 1.2 mL min–1. The system was controlled by the Chromeleon (version 7.1) software (ThermoFisher Scientific). Calibration was performed with a glucose standard (retention time at 5.6 min) diluted in mineral medium. The linear range for quantitative glucose analysis was from 10 μM to 15 mM.
Cell pellets for analysis of the CDW were washed twice with 50 mM ammonium acetate, then resuspended in 300 μL 50 mM ammonium acetate and transferred into pre-dried and weighed 1.5 mL reaction tubes. The CDW was determined by gravimetric analysis after drying of tubes at 60°C to constant weight. Then, CDW samples were stored at room temperature until used for determination of the elemental composition of cells.
The cellular C, H, N and S content was determined by subjecting CDW samples (1–5 mg) to high temperature oxidation, sequential heat-dependent release from adsorption columns and thermal conductivity detection using a Vario EL cube (Elementar Analysensysteme GmbH, Hanau, Germany) essentially as described before (Zech et al.2013). The cellular P content was determined according to Schramel (1983) as follows. The samples were properly weighed into quartz vessels and 1 mL HNO3 (suprapure, sub-boiling distilled; Merck, Darmstadt, Germany) was added. The vessels were closed and introduced into a pressure digestion system (Seif Aufschlussapparatur; Seif, Unterschleissheim, Germany) for 10 h at 170°C. The resulting clear solution was filled up exactly to 5 mL with ultrapure and filtered H2O. An inductively coupled plasma atomic emission spectrometer (ICP-AES Optima 7300 system; Perkin Elmer, Rodgau-Jügesheim, Germany) was used for P and S determination. Samples were introduced into the system by a peristaltic pump (flow rate 0.8 mL min−1) connected to a Seaspray nebulizer with a cyclon spray chamber. The measured spectral element line was 177.495 nm for P and 182.034 nm for S. The radio frequency power was set to 1350 W, the plasma gas was supplied at 15 L Ar min−1, the auxiliary gas at 0.2 L Ar min−1 and the nebulizer gas at 0.6 L Ar min−1. Every 10 samples, three blanks and a certified standard (CPI, with Lots 08G043 and G9B079) were measured. Calculations were carried out with a computerized lab-data management system, relating sample measurements to calibration curves, blanks, CPIs and to the initial dry weight of digested samples. The S content of biomass was determined to compare and integrate the data from the two independent methods. Elemental ratios mentioned in this study describe exclusively the molar ratios of C, N or P.
Presence of TDA was estimated spectrophotometrically by the absorbance increase in cell-free culture supernatants at 398 nm (D’Alvise et al.2016). In addition, TDA was quantified from cell-free culture supernatant (45 mL) as follows: first, the pH of the supernatant was adjusted to 3.0 with 2 M HCl, followed by extraction with 20 mL ethyl acetate, which was repeated two times. Ethyl acetate was removed under vacuum and the precipitate was dissolved in 1 mL acetonitrile, from which 2 μL were injected into an HPLC system equipped with an LTQ XL mass detector operated in ESI-negative mode (both ThermoFisher Scientific). TDA was separated on a Hypersil GOLD C18 column (2.1 × 50 mm; ThermoFisher Scientific) using an acetonitrile-water gradient containing 0.25% (v/v) formic acid as the eluent. The gradient started with 5% (v/v) acetonitrile and increased linearly to 95% (v/v) within 4 min and was then held constant for 3 min. TDA concentrations were determined from peak areas following detection by MS/MS with Selected Reaction Monitoring of the m/z 166.9 fragment derived from m/z 211.1 (TDA). Calibration curves were prepared from purchased TDA (Sigma-Aldrich, St. Louis, USA) dissolved in acetonitrile:water (1:1). The retention time of TDA was 3.9 min, and the linear range for quantitative TDA analysis covered 1 to 25 μg mL–1.
RESULTS
Highest growth rates and biomass yields at N:P »16
The broad concentration range (NH4+: 50 μM to 250 mM; PO43–: 1 μM to 3 mM) tested in this study comprised 415 different NH4+ and PO43– concentration pairs (Fig. 2a; Fig. S2, Supporting Information), which represent 207 different N:P supply ratios that ranged from 0.02 to 120 000. For each concentration pair, we recorded OD values in regular intervals (to calculate growth rates) and analyzed biomass yields from determined CDW at ODmax (i.e. at the transition of cultures into stationary growth phase).
Across the complete dataset, maximal values for ODmax (Fig. 2b) and corresponding CDW (Fig. 2c), as well as for maximal linear growth rates (μlin; Fig. 2d), were observed at N:P supply ratios markedly above Redfield (N:P >16). Average ODmax values were 2.9 within a concentration range of ∼1.5−15 mM NH4+ and ∼30−70 μM PO43–, with the maximum (3.8) recorded at 3 mM NH4+ and 30 μM PO43–. Correspondingly, CDW values (average of 774 mg L–1) were highest at ∼30 μM PO43– within a similar NH4+ concentration range (∼1.5–10 mM), with the maximum (988 mg L–1) observed at 2.0 mM NH4+. Biomass yields were considerably lower (average OD of 1.8 and CDW of 452 mg L–1) for concentration pairs of 1−60 mM NH4+ and 15 μM to 1 mM PO43−, excluding the here embedded concentration pairs supporting maximal values. Strong growth limitation with an achieved average ODmax of 0.42 and CDW of 206 mg L–1 prevailed at concentrations <1 mM NH4+ or <10 μM PO43−. At concentrations of >120 mM NH4+ or >3 mM PO43−, growth was not observed. Exponential growth was short and always confined to early growth, not well covered by the experimental data. On average, the exponential growth phase contributed only ∼10% to the total time elapsed until ODmax was reached. Since growth was linear during the main active growth phase, μlin was determined by fitting an LDR function to experimental OD values (Fig. 2d). Values for μlin were highest (on average 0.100 h–1) within a similar concentration range of NH4+ (2.3−10 mM), but at slightly higher PO43– (50−70 μM) concentrations than observed for ODmax and CDW. The maximal value for μlin (0.138 h–1) was observed at 4.0 mM NH4+ and 70 μM PO43−, whereas lowest values (average of 0.012 h–1) were again tied to strong resource limitation (<1 mM NH4+ or <10 μM PO43–).
A 2D LOWESS fit was applied to the experimental data shown in Fig. 2, to correct for differences in data density. Within a concentration range of about one order of magnitude, the three growth parameters (Fig. 3) increased as a function of the external NH4+ or PO43– concentration (up to ∼2–3 mM or ∼20–40 μM, respectively). Above these concentrations, median values were quite stable (except for PO43– in Fig. 3a and b) until they declined with different slopes at the respective higher concentrations tested. The concentration–response profiles thus revealed zones, where growth rate and biomass yields (i) were apparently controlled and limited by the external NH4+ or PO43– concentration, (ii) became saturated and (iii) were inhibited at higher concentrations (especially in case of PO43–). Maximal mean values for growth rate and biomass yields were observed at external N:P supply ratios ranging from ∼50 to 120 (Fig. 4).
Detailed physiological growth experiments were conducted that covered N:P supply ratios from 1 to 267 (Table 1) to assess (i) substrate consumption, (ii) growth efficiency (by molar growth yields) and (iii) the internal elemental stoichiometry of P. inhibens. Physiological parameters varied strongly at external N:P ratios ranging from 4 to 67; beyond this range (i.e. at 1 and 267, respectively) they remained essentially unchanged. Molar growth yields were calculated from the formed biomass and associated resource consumption at ∼ODmax (Table 1). Obtained values were similar for glucose (YGlc), but varied ∼1.7-fold for NH4+ ( ) and ∼10-fold for PO43– (
) and ∼10-fold for PO43– (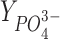 ) across the studied range of external N:P ratios. Notably, both,
) across the studied range of external N:P ratios. Notably, both, 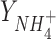 and
and 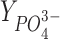 were maximal at N:P ratios >16 (PO43–-limited) and then declined with decreasing N:P ratios. The secondary metabolite TDA was produced in detectable amounts only at high NH4+ concentrations, i.e. at N:P supply ratios of 67 and 267.
were maximal at N:P ratios >16 (PO43–-limited) and then declined with decreasing N:P ratios. The secondary metabolite TDA was produced in detectable amounts only at high NH4+ concentrations, i.e. at N:P supply ratios of 67 and 267.
Synergistic interaction of nutrients
Experimental values were plotted against those predicted by Liebig's law of the minimum using the LOWESS fit-derived median profiles for NH4+ and PO43− (see Fig. 3). The experimental values for the three analyzed growth parameters (Fig. 5; Fig. S4, Supporting Information) diverted markedly above an ODmax of ∼1.0, a CDW of ∼0.354 g L–1 and a μlin of ∼0.04 h–1 from the diagonal line, which represents the exact match of experimental values with those predicted by Liebig's law of the minimum. Thus, the nutrient limitation model matched the experimental data only at low and strongly growth-limiting concentrations of NH4+ (<∼1 mM). At higher concentrations, realized growth rates and biomass yields were mostly larger than predicted.
Flexible internal elemental stoichiometry
Internal elemental ratios (N:P, C:N and C:P) of P. inhibens positively correlated with the consumption ratios of glucose, NH4+ and PO43– (at ODmax) for external N:P ratios from 4 to 67 (Fig. 6a). Within this range, internal elemental ratios varied ∼5-fold for the N:P, ∼1.6-fold for the C:N and ∼8-fold for the C:P ratio (for values at ODmax; Table 1), suggesting that the internal C:P stoichiometry is more flexible than C:N. For external N:P ratios of 4 and 67, μlin positively correlated with internal elemental ratios during linear growth (at ∼0.5 ODmax) (Fig. 6b). Within this range, values for μlin were ∼3.6-fold higher during P-limited as compared to N-limited growth (Table 1). In contrast, exponential growth rate (μexp; Table 1) was highest under conditions of P-excess, agreeing with the growth rate hypothesis, which suggests that low internal N:P ratios are characterized by P-rich rRNA and high μ (Elser et al.2000; Sterner et al.2008). Here, external N:P ratios apparently only positively affected the exponential growth rate (μexp; Table 1), which was short and confined to early growth. Below (i.e. 1) or above (i.e. 267), the before mentioned range of external N:P ratios, internal elemental composition and growth rates remained unchanged.
Figure 6.
Imprint of external NH4+ and PO43− supply ratios on internal elemental stoichiometry of P. inhibens. (a) Internal elemental ratios for C, N, and P as a function of the consumption ratio of glucose, NH4+ and PO43− (both at ODmax). (b) Relation of maximal linear growth rate (μlin) to internal elemental ratios (both at ∼0.5 ODmax). Linear growth represented the major active growth phase, whereas exponential growth was mostly very short and confined to early growth. Values for μlin were largest during P-limitation, whereas the exponential growth rate (μexp; Table 1) was higher under P-excess (as to be expected from the growth rate hypothesis; Elser et al.2000). (c) Internal N:P ratios of P. inhibens (blue) and phytoplankton (gray) as a function of external N:P ratios. Phytoplankton data (from marine and freshwater species) were compiled from a meta-analysis of phytoplankton stoichiometry and growth rate (Hillebrand et al.2013). The gray line displays the median across the phytoplankton data. LN represents the natural logarithm.
The range of supplied external N:P ratios (1 to 267) in this experiment matched the range of 55 phytoplankton studies (both freshwater and marine) compiled in the meta-analysis by Hillebrand et al. (2013) (Fig. 6c). Phytoplankton comprised diatoms, dinoflagellates, chlorophytes, prymnesiophytes and cyanobacteria (see Hillebrand et al.2013 for details on data retrieval). Comparing external to internal N:P ratios revealed a strong similarity between P. inhibens and phytoplankton: both are characterized by a flexible internal N:P stoichiometry, i.e. internal N:P ratios positively correlate with the supplied N:P ratio in the growth medium within physiologically determined constraints.
DISCUSSION
The Redfield ratio originally conceptualized that the internal elemental stoichiometry of marine phytoplankton (C:N:P of 106:16:1) in conjunction with its remineralization determines the oceanic contents of dissolved NO3− and PO43− (Redfield 1958). As noted later, this canonical concept of constant elemental ratios did not consider nutrient cycling (including availability of iron) and phytoplankton diversity (Falkowski 2000), nor differences between oceanic provinces and aquatic ecosystems, where nutrient concentrations and N:P ratios can vary over several orders of magnitude (Quan and Falkowski 2009; Weber and Deutsch 2010). Correspondingly, algae and cyanobacteria display largely varying internal N:P ratios from <5 under conditions of P excess to >100, when N was supplied in excess (Geider and La Roche 2002; Hillebrand et al.2013). In contrast to phytoplankton, however, the quantitative contribution of heterotrophic bacterioplankton to varying elemental ratios in the ocean is not well understood. The large range of external N:P ratios (at constant C) studied here with the marine bacterium Phaeobacter inhibens revealed that maximal growth occurred at external N:P ratios far above Redfield (Fig. 4), that synergistic nutrient interactions suspend Liebig's law of the minimum (Fig. 5) and that P. inhibens adapts its internal N:P stoichiometry to the external nutrient supply, as known from phytoplankton (Fig. 6c).
The observed growth maximum at N:P ratios >16 (Fig. 4) indicates that N concentration has a strong influence on growth performance of P. inhibens (Table 1). The strain was detected in harbors and coastal regions, where it appears to be a competitive colonizer of abiotic and biotic marine surfaces (e.g. Seyedsayamdost et al.2011; Gram et al.2015). Coastal and estuarine habitats are often limited primarily in N, corresponding to low N:P ratios (Vitousek and Howarth 1991; Downing 1997; Elser et al.2007). The assumption that P. inhibens is well adapted to changing availability of reduced inorganic nitrogen is supported by the recently observed rapid consumption of supplied NH4+ during early growth, accompanied by transitory intracellular storage of N in the form of proteins, RNA and DNA (Trautwein, Rabus et al., unpublished). Furthermore, high external NH4+ concentrations fueled the intracellular synthesis of community-shared secreted N-rich potential RTX toxins and of antibiotic TDA (this study; Trautwein, Rabus et al., unpublished). Considering that viral-induced cell lysis of algae results in liberation of vacuole-stored NH4+ (e.g. Dortch et al.1984), bacterial colonizers of dying algae should benefit from optimization of their cellular metabolism to a transitory high N availability. Alternatively, substantial loss of NH4+ (up to ∼50% of fixed N2) from the colony-forming cyanobacterium Aphanizomenon spp. (Adam et al.2016) may create microenvironments with very high N:P ratios. The growth maximum at external N:P ratios >16 (N excess) thus underpins the importance of N (as compared to P) for niche adaptation in P. inhibens.
Realized growth of P. inhibens in parts followed a Liebig-type limitation, but also exceeded the prediction over a wide range of NH4+ and PO43– concentrations (Fig. 5; Fig. S4, Supporting Information). The latter indicates synergistic co-limitation of interacting nutrients, apparently resulting in superadditive growth responses at elevated NH4+ and PO43– concentrations. If realized growth would have been lower than predicted, this would have pinpointed to a sequential Liebig-type limitation by other essential elements or to inhibitory effects at higher NH4+ and PO43– concentrations (see Harpole et al.2011 for a detailed discussion). The results obtained here for P. inhibens thus add further evidence that multiple resource limitation occurs regularly, as observed for autotrophs globally (Elser et al.2007; Harpole et al.2011). Considering that we used a pure culture, the mechanism underlying the observed non-Liebig-type limitation suggests a biochemical dependence (Saito, Goepfert and Ritt 2008), i.e. increasing the concentration of one resource enhances the efficiency to sequester and utilize a second one for growth (including, but not necessarily N or P).
Nutrient consumption ratios tightly coupled with internal elemental composition of P. inhibens, and affected exponential (μexp) and linear (μlin) growth rates diametrically at N:P supply ratios from 4 to 67 (Fig. 6a and b; Table 1). This external N:P ratio range apparently defines the physiological limits, outside of which homeostasis in P. inhibens attenuates and growth rate becomes independent of nutrient supply. Both are likely the result of constrained biochemical and stoichiometric variability in cellular components and their rates of biosynthesis. Thus, P. inhibens obviously represents a ‘conformer’ (Meunier, Malzahn and Boersma 2014) that has its internal elemental stoichiometry determined by the external nutrient supply ratio within the organism's specific range. In case of the markedly varying internal C:P ratio in P. inhibens (up to ∼11-fold), maximal values of 1460 obtained under P-limitation may reflect pronounced C storage in the form of poly-(3-hydroxyalkanoates) (Trautwein, Rabus et al., unpublished) at high (2 and 8 mM) external NH4+ concentrations. Highly flexible internal C:P ratios were also reported for other heterotrophic aquatic bacteria (Tezuka 1990; Godwin and Cotner 2015a,b) and for phytoplankton (Sterner et al.1998).
Overall, the marine bacterium P. inhibens closely resembles phytoplankton in its flexibility and physiological range to alter internal N:P stoichiometry (Fig. 6c; Klausmeier et al.2004; Franz et al.2012; Hillebrand et al.2013). Marine bacterioplankton isolates grown in batch cultures under N- or P-limitation also revealed variations in their internal N:P, C:N and C:P ratios (Vrede et al.2002). At a constant growth rate and under similar limiting conditions, the marine Roseobacter group member Ruegeria pomeroyi DSS-3 modulated its internal elemental stoichiometry (Chan et al.2012), as was also reported for aquatic heterotrophic bacteria from lakes (Godwin and Cotner 2015a,b). In contrast, the high growth rates achieved by Escherichia coli K-12 were suggested to determine its primarily homeostatic internal elemental composition (Makino et al.2003). Also soil microbial biomass appears to be constrained to rather constant elemental stoichiometries of C:N:P of 60:7:1, irrespective of external elemental ratios in the soil (Cleveland and Liptzin 2007). However, also here microbial internal N:P ratios ranged from 1 to >50 across different soils. Non-homeostasis in heterotrophic bacteria should rely on transitory storage of surplus nutrients, which appears advantageous especially in environments characterized by highly variable and fluctuating nutrient inputs (Persson et al.2010; Meunier, Malzahn and Boersma 2014). The here reported findings for P. inhibens (Figs 2–6) suggest that this bacterium is indeed well adapted to cope with such dynamic changes in N and P availability, expected to occur in its natural environment (e.g. colonized algae or higher eukaryotes, coastal areas).
Taken together, flexible internal stoichiometry is observed across organismic and trophic levels (Persson et al.2010), giving rise to complex controls of the elemental cycling and sequestration in marine and other aquatic systems alike. The flexibility of bacterial stoichiometry requires acknowledgement in the analysis of large-scale biogeochemical processes, given the central role of heterotrophic bacteria in matter and energy flows (Azam and Malfatti 2007). Bacterioplankton are not only central for carbon flux and remineralization of N and P (Cho and Azam 1988; Kirchman 1994), but also for iron (Tortell, Maldonado and Price 1996) and silicate (Bidle and Azam 1999). A flexible nutrient content in bacterioplankton potentially alters the ability of bacteria to sequester limiting nutrients and to compete with phytoplankton under inorganic nutrient limitation. At the same time, stoichiometric flexibility within strains, as shown here, is only one mechanism increasing the flexibility of bacterial effects on organic matter cycling and element fluxes. In complex natural communities, compositional shifts of strains within taxa and/or of different taxa should increase the overall stoichiometric flexibility of the bacterial component. This becomes even more striking given the fact that organic matter cycling and the relative importance of bacterioplankton respond interactively to nutrient supply and temperature (Wohlers-Zöllner et al.2011).
Supplementary Material
Supplementary data are available at FEMSEC online.
Acknowledgments
We are grateful to the undergraduate students M. Boelke, A. Bräuer, L. Lüdemann, M. Goebel, S. Kleinert and S. Bloem, as well as to M. Dörries (all Oldenburg) for help with the cultivations and measurements of ammonium, phosphate and glucose, and to U. Maschmann, C. Versteegen (both Oldenburg) and P. Grill (Munich) for technical assistance.
SUPPLEMENTARY DATA
Supplementary data are available at FEMSEC online.
FUNDING
This study was supported by the Deutsche Forschungsgemeinschaft (SFB TRR 51).
Conflict of interest. None declared.
REFERENCES
- Adam B, Klawonn I, Svedén JB et al. N2-fixation, ammonium release and N-transfer to the microbial and classical food web within a plankton community. ISME J 2016;10:450–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen MM. Cyanobacterial cell inclusions. Ann Rev Microbiol 1984;38:1–25. [DOI] [PubMed] [Google Scholar]
- Azam F, Malfatti F. Microbial structuring of marine ecosystems. Nat Rev Microbiol 2007;5:782–91. [DOI] [PubMed] [Google Scholar]
- Beckon WN, Parkins C, Maximovich A et al. A general approach to modeling biphasic relationships. Environ Sci Technol 2008;42:1308–14. [DOI] [PubMed] [Google Scholar]
- Bidle KD, Azam F. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 1999;397:508–12. [Google Scholar]
- Brock J, Rhiel E, Beutler M et al. Unusual polyphosphate inclusions observed in a marine Beggiatoa strain. Anton Leeuw 2012;101:347–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan A, González JM, Moran MA. Overview of the marine Roseobacter lineage. Appl Environ Microb 2005;71:5665–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan A, LeCleir GR, Gulvik CA et al. Master recyclers: features and functions of bacteria associated with phytoplankton blooms. Nat Rev Microbiol 2014;12:686–68. [DOI] [PubMed] [Google Scholar]
- Burnat M, Herrero A, Flores E. Compartmentalized cyanophycin metabolism in the diazotrophic filaments of a heterocyst-forming cyanobacterium. Proc Natl Acad Sci USA 2014;111:3823–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L.-K, Newton RJ, Sharma S et al. Transcriptional changes underlying elemental stoichiometry shifts in a marine heterotrophic bacterium. Front Microbiol 2012;3:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BC, Azam F. Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature 1988;332:441–3. [Google Scholar]
- Chrzanowski TH, Grover JP. Element content of Pseudomonas fluorescens varies with growth rate and temperature: a replicate chemostat study addressing ecological stoichiometry. Limnol Oceanogr 2008;53:1242–51. [Google Scholar]
- Chrzanowski TH, Kyle M, Elser JJ et al. Element ratios and growth dynamics of bacteria in an oligotrophic Canadian shield lake. Aquat Microb Ecol 1996;11:119–25. [Google Scholar]
- Cleveland CC, Liptzin D. C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007;85:235–52. [Google Scholar]
- Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 1979;74:829–36. [Google Scholar]
- Cotner JB, Biddanda BA. Small players, large role: microbial influence on biogeochemical processes in pelagic aquatic ecosystems. Ecosystems 2002;5:105–21. [Google Scholar]
- Currie DJ. Large-scale variability and interactions among phytoplankton, bacterioplankton, and phosphorus. Limnol Oceanogr 1990;35:1437–55. [Google Scholar]
- D’Alvise PW, Phippen CBW, Nielsen KF et al. Influence of iron on production of the antibacterial compound tropodithietic acid and its noninhibitory analog in Phaeobacter inhibens. Appl Environ Microbiol 2016;82:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danger M, Oumarou C, Benest D et al. Bacteria can control stoichiometry and nutrient limitation of phytoplankton. Funct Ecol 2007;21:202–10. [Google Scholar]
- Dortch Q, Clayton JR, Thoresen SS et al. Species differences in accumulation of nitrogen pools in phytoplankton. Mar Biol 1984;81:237–50. [Google Scholar]
- Downing JA. Marine nitrogen: phosphorus stoichiometry and the global N:P cycle. Biogeochemistry 1997;37:237–52. [Google Scholar]
- Drüppel K, Hensler M, Trautwein K et al. Pathways and substrate-specific regulation of amino acid degradation in Phaeobacter inhibens DSM 17395 (archetype of the marine Roseobacter clade). Environ Microbiol 2014;16:218–38. [DOI] [PubMed] [Google Scholar]
- Elser JJ, Bracken MES, Cleland EE et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 2007;10:1135–42. [DOI] [PubMed] [Google Scholar]
- Elser JJ, Sterner RW, Gorokhova E et al. Biological stoichiometry from genes to ecosystems. Ecol Lett 2000;3:540–50. [Google Scholar]
- Falkowski PG. Rationalizing elemental ratios in unicellular algae. J Phycol 2000;36:3–6. [Google Scholar]
- Franz JMS, Hauss H, Sommer U et al. Production, partitioning and stoichiometry of organic matter under variable nutrient supply during mesocosm experiments in the tropical Pacific and Atlantic Ocean. Biogeosciences 2012;9:4629–43. [Google Scholar]
- Geider RJ, La Roche J. Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur J Phycol 2002;37:1–17. [Google Scholar]
- Godwin CM, Cotner JB. Aquatic heterotrophic bacteria have highly flexible phosphorus content and biomass stoichiometry. ISME J 2015a;9:2324–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin CM, Cotner JB. Carbon:phosphorus homeostasis of aquatic bacterial assemblages is mediated by shifts in assemblage composition. Aquat Microb Ecol 2014;73:245–58. [Google Scholar]
- Godwin CM, Cotner JB. Stoichiometric flexibility in diverse aquatic heterotrophic bacteria is coupled to differences in cellular phosphorus quotas. Front Microbiol 2015b;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JC, McCarthy JJ, Peavey DG. Growth rate influence on the chemical composition of phytoplankton in oceanic waters. Nature 1979;279:210–5. [Google Scholar]
- Gram L, Rasmussen BB, Wemheuer B et al. Phaeobacter inhibens from the Roseobacter clade has an environmental niche as a surface colonizer in harbors. Syst Appl Microbiol 2015;38:483–93. [DOI] [PubMed] [Google Scholar]
- Guildford SJ, Hecky RE. Total nitrogen, total phosphorus, and nutrient limitation in lakes and oceans: Is there a common relationship? Limnol Oceanogr 2000;45:1213–23. [Google Scholar]
- Harpole WS, Ngai JT, Cleland EE et al. Nutrient co-limitation of primary producer communities. Ecol Lett 2011;14:852–62. [DOI] [PubMed] [Google Scholar]
- Hecky RE, Kilham P. Nutrient limitation of phytoplankton in freshwater and marine environments: A review of recent evidence on the effects of enrichment. Limnol Oceanogr 1988;33:796–822. [Google Scholar]
- Hillebrand H, Steinert G, Boersma M et al. Goldman revisited: Faster-growing phytoplankton has lower N:P and lower stoichiometric flexibility. Limnol Oceanogr 2013;58:2076–88. [Google Scholar]
- Joint I, Henriksen P, Fonnes GA et al. Competition for inorganic nutrients between phytoplankton and bacterioplankton in nutrient manipulated mesocosms. Aquat Microb Ecol 2002;29:145–59. [Google Scholar]
- Jørgensen NOG, Kroer N, Coffin RB. Utilization of dissolved nitrogen by heterotrophic bacterioplankton: effect of substrate C/N ratio. Appl Environ Microbiol 1994;60:4124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman DL. The uptake of inorganic nutrients by heterotrophic bacteria. Microb Ecol 1994;28:255–71. [DOI] [PubMed] [Google Scholar]
- Klausmeier CA, Litchman E, Daufresne T et al. Optimal nitrogen-to-phosphorus stoichiometry of phytoplankton. Nature 2004;429:171–4. [DOI] [PubMed] [Google Scholar]
- Klausmeier CA, Litchman E, Levin SA. Phytoplankton growth and stoichiometry under multiple nutrient limitation. Limnol Oceanogr 2004;49:1463–70. [Google Scholar]
- Lenton TM, Klausmeier CA. Biotic stoichiometric controls on the deep ocean N:P ratio. Biogeosciences 2007;4:353–67. [Google Scholar]
- Loladze I, Elser JJ. The origins of the Redfield nitrogen-to-phosphorus ratio are in a homeostatic protein-to-rRNA ratio. Ecol Lett 2011;14:244–50. [DOI] [PubMed] [Google Scholar]
- Luo H, Moran MA. Evolutionary ecology of the marine Roseobacter clade. Microbiol Mol Biol Rev 2014;78:573–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Swan BK, Stepanauskas R et al. Comparing effective population sizes of dominant marine alphaproteobacteria lineages. Environ Microbiol Rep 2014;6:167–72. [DOI] [PubMed] [Google Scholar]
- Makino W, Cotner JB, Sterner RW et al. Are bacteria more like plants or animals? Growth rate and resource dependence of bacterial C : N : P stoichiometry. Funct Ecol 2003;17:121–30. [Google Scholar]
- Meunier CL, Malzahn AM, Boersma M. A new approach to homeostatic regulation: towards a unified view of physiological and ecological concepts. PLoS One 2014;9:e107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J, Fink P, Goto A et al. To be or not to be what you eat: regulation of stoichiometric homeostasis among autotrophs and heterotrophs. Oikos 2010;119:741–51. [Google Scholar]
- Quan TM, Falkowski PG. Redox control of N:P ratios in aquatic ecosystems. Geobiology 2009;7:124–39. [DOI] [PubMed] [Google Scholar]
- Quigg A, Finkel ZV, Irwin AJ et al. The evolutionary inheritance of elemental stoichiometry in marine phytoplankton. Nature 2003;425:291–4. [DOI] [PubMed] [Google Scholar]
- Redfield AC. On the proportions of organic derivations in sea water and their relation to the composition of plankton. In: James Johnstone Memorial Volume. Daniel RJ. (ed). Liverpool: University Press of Liverpool; 1934, 176–192. [Google Scholar]
- Redfield AC. The biological control of chemical factors in the environment. Am Sci 1958;46:205–21. [PubMed] [Google Scholar]
- Ruppersberg HS, Goebel MR, Kleinert SI et al. Photometric determination of ammonium and phosphate in seawater using a microplate reader. J Mol Microbiol Biotechnol 2016;27:73–80. [DOI] [PubMed] [Google Scholar]
- Saito MA, Goepfert TJ, Ritt JT. Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnol Oceanogr 2008;53:276–90. [Google Scholar]
- Schramel P. Consideration of inductively coupled plasma spectroscopy for trace element analysis in the bio-medical and environmental fields. Spectrochim Acta B 1983;38:199–206. [Google Scholar]
- Seyedsayamdost MR, Case RJ, Kolter R et al. The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat Chem 2011;3:331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner RW, Andersen T, Elser JJ et al. Scale-dependent carbon:nitrogen:phosphorus seston stoichiometry in marine and freshwaters. Limnol Oceanogr 2008;53:1169–80. [Google Scholar]
- Sterner RW, Clasen J, Lampert W et al. Carbon:phosphorus stoichiometry and food chain production. Ecol Lett 1998;1:146–50. [Google Scholar]
- Sterner RW, Elsner JJ. Ecological Stoichiometry: The Biology of Elements From Molecules to the Biosphere. Princeton: Princeton University Press, 2002. [Google Scholar]
- Teeling H, Fuchs BM, Becher D et al. Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 2012;336:608–11. [DOI] [PubMed] [Google Scholar]
- Teeling H, Fuchs BM, Bennke CM et al. Recurring patterns in bacterioplankton dynamics during coastal spring algae blooms. eLife 2016;5:e11888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka Y. Bacterial regeneration of ammonium and phosphate as affected by the carbon : nitrogen : phosphorus ratio of organic substrates. Microb Ecol 1990;19:227–38. [DOI] [PubMed] [Google Scholar]
- Thingstad TF, Skjoldal EF, Bone RA. Phosphorus cycling and algal-bacterial competition in Sandsfjord, western Norway. Mar Ecol Prog Ser 1993;99:239–59. [Google Scholar]
- Thole S, Kalhoefer D, Voget S et al. Phaeobacter gallaeciensis genomes from globally opposite locations reveal high similarity of adaptation to surface life. ISME J 2012;6:2229–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortell PD, Maldonado MT, Price NM. The role of heterotrophic bacteria in iron-limited ocean ecosystems. Nature 1996;383:330–2. [Google Scholar]
- Trautwein K, Will SE, Hulsch R et al. Native plasmids restrict growth of Phaeobacter inhibens DSM 17395. Energetic costs of plasmids assessed by quantitative physiological analyses. Environ Microbiol 2016;18:4817–29. [DOI] [PubMed] [Google Scholar]
- Tsementzi D, Wu J, Deutsch S et al. SAR11 bacteria linked to ocean anoxia and nitrogen loss. Nature 2016;536:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Howarth RW. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991;13:87–115. [Google Scholar]
- Vrede K, Heldal M, Norland S et al. Elemental composition (C, N, P) and cell volume of exponentially growing and nutrient-limited bacterioplankton. Appl Environ Microbiol 2002;68:2965–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner-Döbler I, Biebl H. Environmental biology of the marine Roseobacter lineage. Annu Rev Microbiol 2006;60:255–80. [DOI] [PubMed] [Google Scholar]
- Weber TS, Deutsch C. Ocean nutrient ratios governed by plankton biogeography. Nature 2010;467:550–4. [DOI] [PubMed] [Google Scholar]
- Wiegmann K, Hensler M, Wöhlbrand L et al. Carbohydrate catabolism in Phaeobacter inhibens DSM 17395, member of the marine Roseobacter clade. Appl Environ Microb 2014;80:4725–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlers-Zöllner J, Breithaupt P, Walther K et al. Temperature and nutrient stoichiometry interactively modulate organic matter cycling in a pelagic algal-bacterial community. Limnol Oceanogr 2011;56:599–610. [Google Scholar]
- Zech H, Hensler M, Koßmehl S et al. Adaptation of Phaeobacter inhibens DSM 17395 to growth with complex nutrients. Proteomics 2013;13:2851–68. [DOI] [PubMed] [Google Scholar]
- Zech H, Thole S, Schreiber K et al. Growth phase-dependent global protein and metabolite profiles of Phaeobacter gallaeciensis strain DSM 17395, a member of the marine Roseobacter-clade. Proteomics 2009;9:3677–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data are available at FEMSEC online.