Abstract
Identifying molecular targets and an appropriate targeting vehicle, i.e., monoclonal antibodies (mAb) and their various forms, for radioimmunotherapy (RIT) remains an active area of research. Panitumumab, a fully human and less immunogenic mAb that binds to the epidermal growth factor receptor (Erb1; HER1), was evaluated for targeted α-particle radiation therapy using 212Pb, an in vivo α generator. A single dose of 212Pb-panitumumab administered to athymic mice bearing LS-174T intraperitoneal (i.p.) tumor xenografts was found to have greater therapeutic efficacy when directly compared with 212Pb-trastuzumab, which binds to HER2. A dose escalation study determined a maximum effective working dose of 212Pb-panitumumab to be 20 μCi with a median survival of 35 days versus 25 days for the untreated controls. Pretreatment of tumor-bearing mice with paclitaxel and gemcitabine 24 hours prior to injection of 212Pb-pantiumumab at 10 or 20 μCi resulted in the greatest enhanced therapeutic response at the higher dose with median survivals of 106 versus 192 days, respectively. The greatest therapeutic impact, however, was observed in the animals that were treated with topotecan 24 hours prior to RIT and then again 24 hours after RIT; the best response from this combination was also obtained with the lower 10-μCi dose of 212Pb-panitumumab (median survival >280 days). In summary, 212Pb-panitumumab is an excellent candidate for the treatment of HER1-positive disseminated i.p. disease. Furthermore, the potentiation of the therapeutic impact of 212Pb-pantiumumab by chemotherapeutics confirms and validates the importance of developing a multimodal therapy regimen.
Introduction
Successful preclinical investigations to treat disseminated peritoneal disease with 212Pb, an α-radiation source, have resulted in the first-ever 212Pb-radioimmunotherapy (RIT) clinical study at the University of Alabama at Birmingham. The phase 1 trial, designed to assess the safety of 212Pb-1, 4, 7, 10-tetraaza-1, 4, 7,10-tetra-(2-carbamoyl methyl)-cyclododecane-trastuzumab (212Pb-TCMC-trastuzumab) RIT recruited patients with HER2-positive peritoneal neoplasms of ovarian, pancreatic, and gastric origin (NCT01384253). No adverse reactions or toxicities were reported for patients receiving 212Pb-trastuzumab by intraperitoneal (i.p.) injection [1], [2]. The progress of the phase 1 trial has ignited great interest in 212Pb radiopharmaceuticals for targeted α-therapy applications.
HER2 expression ranges from 25% to 30% (e.g., breast cancer) to 90% to 100% (colorectal cancer and ovarian cancer), with pancreatic cancer ranging from 35% to 45% [3], [4], [5], [6]. These percentages are a reflection of the heterogenic nature of tumors, which in turn presents difficulties in providing patients with appropriate traditional or conventional treatments [7], [8]. Targeting other molecules expressed by tumor cells presents a further opportunity to overcome tumor heterogeneity. Towards this end, studies in this laboratory have included identifying additional molecules that could serve as targets for α-particle RIT for the treatment of disseminated peritoneal disease [9], [10], [11], [12].
One molecule of great interest for the development of targeted therapies is the epidermal growth factor receptor (EGFR; HER1). Several investigators have demonstrated the potential of radiolabeled cetuximab (Erbitux) for imaging and RIT applications targeting this receptor [12], [13], [14], [15], [16], [17], [18], [19], [20]. Most recently, the potential of cetuximab as a vehicle for the delivery of α-particle radiation, via 212Pb, was demonstrated in a disseminated i.p. tumor mouse model [10]. Furthermore, 212Pb-cetuximab therapy was found to be augmented by chemotherapeutics. Despite the evidence that cetuximab is an effective vehicle for α-radiation therapy of HER1-positive tumors, there are underlying concerns with the high percent of injected dose per gram (%ID/g) consistently observed in the liver with this mAb [11], [12], [17]. Until dosimetric calculations are performed, the consequence of this hepatic sequestration of radioactivity remains unknown.
Clinical trials with cetuximab have also highlighted concerns about infusion reactions (IRs) noted in patients as well as its immunogenicity, both of which could limit the number of treatments that could be administered to a patient [21]. Mild-to-moderate IRs have occurred in 12% to 19% of the patients treated with cetuximab; severe IRs have occurred in 3% of the patients. Cetuximab is a chimeric mAb of which 34% of the mAb structure is murine. Anti-cetuximab antibodies have been detected in 5% of the evaluable patients with a median onset of the response occurring at 44 days [22]. Panitumumab (Vectibix), a fully human mAb, the first of its type to gain Food and Drug Administration approval, appears to be less immunogenic than cetuximab with an incidence of 0.4% or 3.2% depending on the detection format [23]. Furthermore, only 4% of the patients treated with panitumumab have developed IR, with 1% being severe. Imaging and tumor targeting studies with radiolabeled panitumumab (111In, 86Y, and 89Y) in mouse tumor models have indicated that the hepatic uptake of radioactivity is less than what has been observed with cetuximab [24]. Studies from this laboratory have demonstrated excellent tumor targeting of s.c., i.p. or i.t. tumor xenografts by radiolabeled (111In, 89Zr, or 86Y) panitumumab when injected i.v., i.p., or i.t. [12], [17], [24], [25], [26]. These studies suggest that panitumumab would be an excellent candidate to explore for targeted α therapy.
The present report details studies assessing the therapeutic efficacy of panitumumab when labeled with 212Pb. The work includes a pilot study directly comparing the effectiveness of targeting HER1 versus HER2 using panitumumab and trastuzumab, respectively, as the targeting vehicles. A reassessment of the effective therapeutic dose was performed followed by studies combining 212Pb-panitumumab with each of the chemotherapeutics gemcitabine, paclitaxel, or topotecan.
Materials and Methods
Cells
Media and supplements were purchased from Lonza unless otherwise indicated. Therapy studies were conducted using the LS-174T, a human colon carcinoma cell line, grown in Dulbecco's minimum essential medium (12-614Q). The medium was supplemented with 1 mM glutamine (17-605E), 10% FetalPlex (Gemini Bioproducts, Inc.; 100-602), and 1 mM nonessential amino acids 13-114E as previously described [27], [28].
Chelate Synthesis and mAb Conjugation
Panitumumab (Vectibix; Amgen, Inc., Thousand Oaks, CA) was purchased through the National Institutes of Health (NIH), Division of Veterinary Resources Pharmacy. Conjugation of panitumumab with the bifunctional ligands, 1, 4, 7, 10-tetraaza-1, 4, 7,10-tetra-(2-carbamoyl methyl)-cyclododecane (TCMC) or trans-cyclohexyl-diethylenetriamine-pentaacidic acid (CHX-A″), was performed at a 10-fold molar excess of ligand to panitumumab according to established methods previously described in detail [24], [29], [30], [31]. The final concentration of panitumumab was determined by the Lowry method using a BSA standard [32]. The number of TCMC and CHX-A″ molecules bound to panitumumab was quantitated using spectrophotometric assays based on the titration of lead-Arsenazo(III) and yttrium-Arsenazo(III) complex, respectively [33], [34]. Polyclonal human immunoglobulin (HuIgG; MP Biochemicals, 64145) served as a negative control in these studies and was similarly conjugated with TCMC or CHX-A″ in parallel and evaluated as described above. The HuIgG is purified from human serum, and to date, no known antigen has been described with which it reacts. Trastuzumab and cetuximab conjugated with TCMC, as previously described, were utilized in one study to allow a direct comparison of the therapeutic efficacy of panitumumab to that of trastuzumab and cetuximab when radiolabeled with 212Pb [10], [16], [30], [35], [36], [37].
Radiolabeling
Radioiodination of panitumumab (50 μg) with Na125I (0.5-1 mCi; PerkinElmer, Shelton, CT) was performed using Iodo-Gen (Pierce Chemical, Rockford, IL) [16], [38]. Radiolabeling of CHX-A″-panitumumab (50 μg) with 111In (1-2 mCi) was performed as previously described [39]. The radiolabeled products were purified with a PD-10 desalting column (GE Healthcare, Piscataway, NJ) using PBS as the eluent.
The 212Pb was obtained from a 224Ra/212Pb generator (Oak Ridge National Laboratories, U Batelle, Oak Ridge, TN). Elution of the 212Pb for radiolabeling of panitumumab-, cetuximab-, trastuzumab-, and HuIgG-TCMC and subsequent purification were performed as detailed elsewhere [30], [40].
Radioimmunoassays
The immunoreactivity of the TCMC-panitumumab conjugate was evaluated in a competition radioimmunoassay (RIA) as outlined in an earlier publication using purified human epidermal growth factor receptor (EGFR; Sigma-Aldrich, E3641-500UN) [24]. Briefly, EGFR was allowed to adsorb onto the wells of a 96-well plate, excess EGFR was removed, and 1% bovine serum albumin in phosphate-buffered saline (BSA/PBS; 100 μl) was added to each well. Following a 0.5- to 1-hour incubation at room temperature, the solution was removed, and serial dilutions of the immunoconjugate (1000 to 0.017 ng in 25 μl) in BSA/PBS were added to the wells in triplicate. Following the addition of 125I-panitumumab (50,000 cpm/25 μl) to each of the wells, the plates were incubated for 4 hours at 37°C. The wells were washed, and the radioactivity was dissociated from the wells with 0.1 M NaOH (100 μl), adsorbed to cotton filters, and counted in a γ-scintillation counter. The immunoconjugate was compared with unmodified panitumumab. The percent inhibition was calculated using the buffer control and plotted. HuM195, a mAb that reacts with human CD33 (provided by Dr. M. McDevitt, Memorial Sloan Kettering Cancer Center), served as a negative control.
The immunoreactivity of the 212Pb-panitumumab was assessed in an RIA using purified EGFR (100 ng per well). After adsorption of EGFR to the wells of a 96-well plate, the nonadsorbed EGFR was removed and the wells treated as described above. Serial dilutions of radiolabeled panitumumab (~200,000 cpm to 12,500 cpm in 50 μl of BSA/PBS) were added to the wells and incubated for 4 hours at 37°C. The wells were washed, and the radioactivity was harvested and counted in a γ-scintillation counter again as just described. The percentage binding was calculated for each dilution and averaged. The specificity of the radiolabeled panitumumab was confirmed by incubating one set of wells with radiolabeled panitumumab and 10 μg of unlabeled panitumumab.
In Vivo Studies
All in vivo studies were performed using 8- to 12-week-old female athymic (NCr-nu/nu) mice (NCI-Frederick, Cat#01B70). The studies were conducted per protocols approved by the National Cancer Institute Animal Care and Use Committee.
Tumor Localization
Mice (5 per time point) were injected i.p. with 1 × 108 LS-174T cells in 1 ml of medium and utilized in tumor targeting studies 5 days later. Following i.p. injection with 111In-CHX-A″-panitumumab (~7.5 μCi on 0.6 μg), the mice were euthanized by CO2 inhalation at 24, 48, 72, 96, and 168 hours. The blood, tumor, and major organs were collected, wet-weighed, and counted in a γ-scintillation counter. The %ID/g and the average cpm along with the standard deviations were calculated and plotted.
Therapy
RIT studies detailed below were initiated at 2 to 3 days following i.p. injection of the mice with LS-174T as described above. At this time point, the tumor burden presents as cell pellets either free floating in the peritoneum or showing evidence of adhering to organs and may have developing vasculature. 212Pb-labeled mAb was administered i.p. to mice in 0.5 ml of PBS; the activity is indicated in each study description that follows. 212Pb-HuIgG served as a nonspecific control in these studies. The mice were monitored at a minimum of 2 times weekly, and the body weight was measured and recorded 1 to 2 times per week for 4 to 6 weeks as a measure of toxicity due to therapy. Progression of disease was observed as an extension of the abdomen; development of ascites or noticeable, palpable nodules in the abdomen; or, conversely, as weight loss. Mice were euthanized if found to be in distress, moribund, or cachectic or when disease progression was evident as cited above. Euthanasia was also performed when a ~20% weight loss occurs or when disease progression was evident as cited above.
Study 1 was a pilot study exploring the potential of 212Pb-panitumumumab as a therapeutic agent. 212Pb-panitumumab was compared directly to 212Pb-trastuzumab and 212Pb-cetuximab. Tumor-bearing mice (n = 10) were injected (i.p.) with 10 μCi of 212Pb-labeled panitumumab, trastuzumab, cetuximab, or HuIgG. A fourth group was left untreated.
Study 2 was conducted to assess the maximum effective working dose of 212Pb-panitumumab. Tumor-bearing mice (groups of n = 10) were given increasing doses of 212Pb-panitumumab (10, 20, 30, or 40 μCi) by i.p. injection, 212Pb-HuIgG (20 or 40 μCi), or no RIT.
Lastly, in study 3, a series of investigations with 212Pb-panitumumab at the maximum effective working dose was conducted to assess potential enhancement of therapeutic efficacy by the inclusion of chemotherapeutics in the treatment regimen. The chemotherapeutics GEM (GEMZAR; Eli Lilly and Company, Indianapolis, IN), paclitaxel (Hospira, Inc., Lake Forest, IL), and topotecan (Fresenius Kabi USA, LLC, Lake Zurich, IL) were purchased through the NIH, Division of Veterinary Resources Pharmacy. Established from previous studies, mice (groups of n = 10) bearing i.p. LS-174T tumors were injected i.p. with 1 mg of GEM or 0.6 mg of paclitaxel 24 hours prior to i.p. administration of 10 or 20 μCi of 212Pb-panitumumab. These treatment groups were compared with mice pretreated with GEM or paclitaxel followed by 212Pb-HuIgG. Control groups included mice receiving no treatment, 212Pb-panitumumab, 212Pb-HuIgG, paclitaxel, or GEM only. Based on a preliminary investigation of combining topotecan with 212Pb-trastuzumab (Table S1), topotecan was included in the third study. The mice were given two injections (i.p.) of topotecan hydrochloride (0.25 mg): the day before and the day after administration of 10 or 20 μCi of 212Pb-panitumumab. As with the other chemotherapeutic studies, the controls here included sets of mice treated with topotecan only and topotecan with 10 or 20 μCi of 212Pb-HuIgG on this same schedule.
Quantitation of HER1 and HER2
Total protein isolates were prepared from LS-174T i.p. xenografts (n = 5) that were harvested 3 days after tumor cell inoculation. Briefly, tumors were removed, wet-weighed, washed in cold PBS, and homogenized in 1 ml of 50 mM Tris (pH 7.4) containing 150 mM NaCl, 5 mM EDTA, and 0.5% NP-40. The homogenates were placed on ice for 30 minutes and then subjected to centrifugation for 30 minutes at 10,000×g. The supernatant fraction was collected, the protein determined by the Lowry method, aliquoted, and stored at −80°C until assayed.
The HER1 and HER2 contents of the tumor lysates were determined using ELISA kits (Invitrogen, Camarillo, CA) for the quantitation of human HER1 (Cat# KHR9061) and HER2 (Cat. # KHO0701).
Statistical Analyses
Kaplan-Meier survival (time to sacrifice or natural death) analysis was conducted using GraphPad Prizm 7; groups were compared using a log-rank test. A pairwise comparison was performed to test for differences between treatment groups (Holm-Sidak method). All reported P values correspond to two-sided tests.
Results
Characterization of TCMC-Panitumumab
In vitro analysis
Conjugation of panitumumab with the TCMC ligand resulted in a chelate:protein ratio of 2.5 ± 1.6. A competition RIA was performed to determine if the modification with the TCMC chelate impacted the immunoreactivity of the panitumumab. As illustrated in Figure 1, the TCMC-panitumumab conjugate was found to have retained immunoreactivity with 4.7 ng required to achieve 50% inhibition of the 125I-labeled panitumumab versus 4.3 ng for the unmodified panitumumab.
Figure 1.
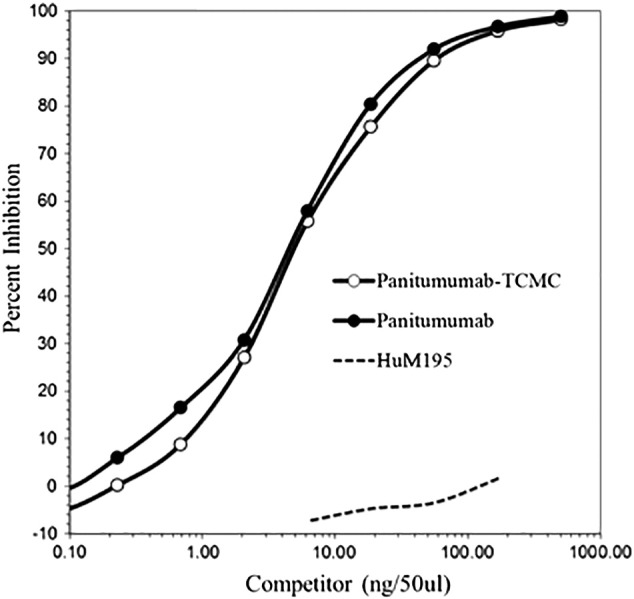
The retention of the panitumumab-TCMC conjugate immunoreactivity was demonstrated by a competition radioimmunoassay.
Radiolabeling of the TCMC-panitumumab with 212Pb was facile, resulting in a specific activity of 13.0 ± 5.1 mCi/mg, consistent with previous radiolabeling results with trastuzumab and cetuximab [10], [11], [21], [30], [36], [37]. The radioimmunoconjugate maintained its ability to recognize its cognate epitope in an RIA with a percent bound of 75.4 following a 4-hour incubation with EGFR coated in the wells of a 96-well plate. Specificity of this reaction was confirmed by the addition of 10 μg of unlabeled panitumumab to a set of wells to compete with the 212Pb-panitumumab. The excess unlabeled panitumumab reduced the percent bound to 0.3.
In Vivo Studies
Tumor localization
Panitumumab proved as effective in targeting i.p. tumor xenografts as it was in targeting subcutaneous tumors [24]. Twenty-four hours after the 111In-CHX-A″-panitumumab was administered i.p. to the tumor-bearing mice, a %ID/g of 47.03 ± 33.62 was obtained in the tumors (Figure 2A). The peak %ID/g was observed at 48 hours with a value of 48.3 ± 29.12. The %ID/g then steadily decreased to a final value of 7.20 ± 4.57 at the 168-hour time point. As shown in Figure 2B, when the average decay-corrected cpm was plotted, a different perspective is gained. From 48 to 168 hours, the amount of radioactivity in the tumor is constant, whereas the normal organs show a decrease in radioactivity, consistent with clearance of the radiolabeled mAb from the blood and retention of the radioactivity in the tumor. The LS-174T tumor is an aggressive tumor, and the rapid growth of the tumor skews the %ID/g values at the later time points. At 24 hours, the amount of tumor tissue harvested was 110 ± 112 mg, and by 168 hours, the amount collected was 1228 ± 826 mg. Of the normal tissues, meanwhile, the highest %ID/g was observed in the spleen (10.94 ± 2.68) at 48 hours. In general, the level of radioactivity in the normal organs was similar to what was reported when 111In-panitumumab was administered i.v. for targeting s.c. tumor xenografts [24].
Figure 2.
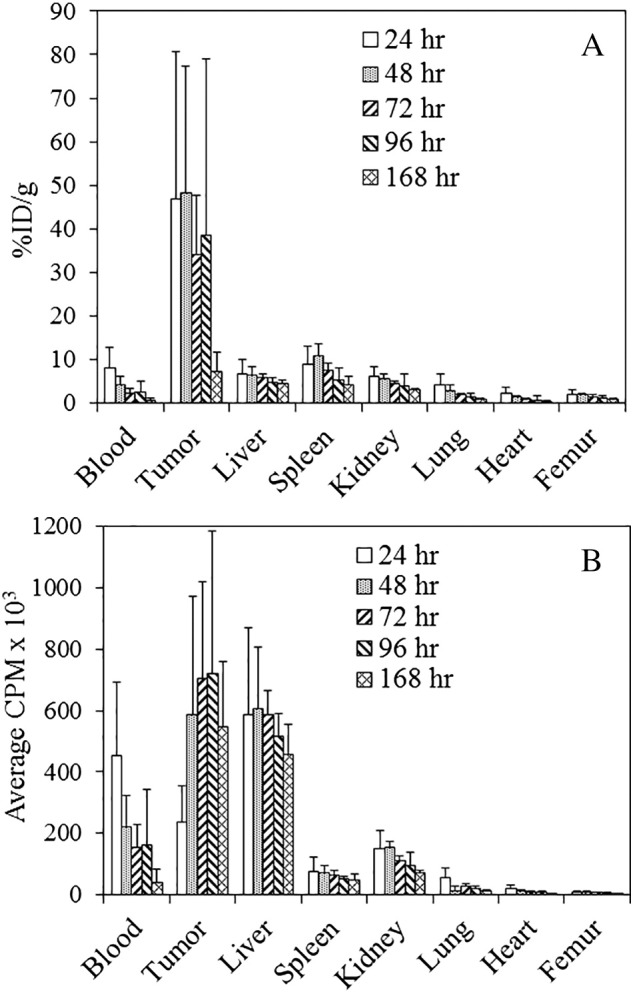
Tumor and normal tissue distribution of 111In-panitumumab.
(A) Athymic mice bearing 5-day LS-174T i.p. tumor xenografts were injected i.p. with 111In-panitumumab (~7.5 μCi) and euthanized (n = 5) at 24, 48, 72, 96, and 168 hours after the injection. The tumor and tissues were harvested and wet-weighed, and the radioactivity was measured in a γ-counter. The %ID/g and standard deviation were calculated. (B) The average cpm of the tumor and tissues was also plotted along with the standard deviation.
Pilot RIT targeting HER1 with 212Pb-panitumumab
A pilot study was conducted to obtain an initial evaluation of the therapeutic efficacy of 212Pb-panitumumab before proceeding with the larger, more complex studies. In this study, the 212Pb-panitumumab was directly compared with 212Pb-labeled trastuzumab and cetuximab. 212Pb-HuIgG served as a nonspecific control. Groups of mice (n = 10) bearing i.p. LS-174T tumor xenografts were injected with 10 μCi of each 212Pb-labeled mAb. As illustrated in Figure 3 and provided in Table 1, the median survival (MS) for the group receiving 212Pb-panitumumab was >293 (5 of 10 were still alive at 293 days), whereas for the groups treated with 212Pb-trastuzumab and 212Pb-cetuxmab, the MS was 182 and 147 days, respectively. Weight loss was observed at 8 days for the 212Pb-labeled trastuzumab, panitumumab, and HuIgG (3.2%, 3.1%, and 3.4%, respectively). By 12 days, all the groups had returned to their pretherapy weights (Supplemental Material, Table S2).
Figure 3.
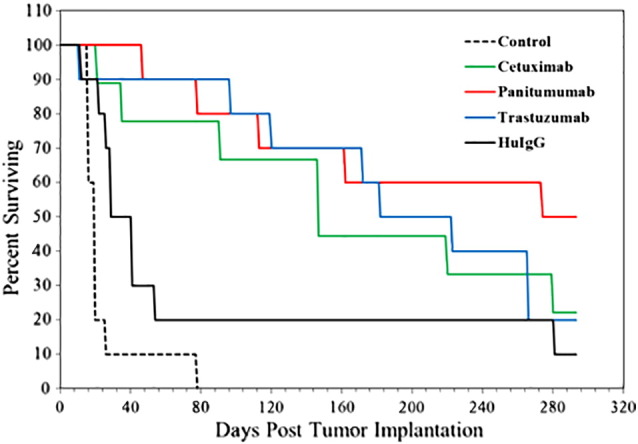
The therapeutic potential of 212Pb-panitumumab was directly compared with 212Pb-trastuzumab and 212Pb-cetuximab. The 212Pb-labeled mAbs (10 μCi per mouse) were administered i.p. to athymic mice bearing LS-174T i.p. tumor xenografts. Two other groups of mice were included in the experiment. One group received 10 μCi of 212Pb-HuIgG, a nonspecific control, and the other was left untreated.
Table 1.
Comparison of 212Pb-RIT Targeting HER1- and HER2-Positive Tumor Xenografts
| Vehicle | Target | Median Survival (Days) |
|---|---|---|
| None | None | 20 |
| Trastuzumab | HER2 | 182 |
| Cetuximab | HER1 | 147 |
| Panitumumab | HER1 | >293 |
| HuIgG | None | 29 |
A pilot study was performed to compare the therapeutic efficacy of 212Pb-panitumumab to 212Pb-labeled trastuzumab and cetuximab. Athymic mice (n = 10) bearing 3-day i.p. LS-174T tumor xenografts were injected with 10 μCi of each of the 212Pb-labeled mAb. Additional groups included those that received no treatment and those that were injected with 10 μCi of the nonspecific control, 212Pb-HuIgG.
A dose escalation study was then performed to determine an effective working dose for 212Pb-panitumumab. Previous experience had demonstrated the necessity of determining treatment conditions for each radioimmunoconjugate. Cohorts of mice (n = 10) bearing i.p. tumors were treated (i.p.) with 10, 20, 30, or 40 μCi of 212Pb-panitumumab. The MS for these groups was 27, 35, 32, and 12 days, respectively (Figure 4). The MS for the untreated group was 25 days; 20 or 40 μCi of the nonspecific control, 212Pb-HuIgG, resulted in an MS of 19 and 10 days, respectively. Clearly, the higher nonspecific injected dose contributed to toxicity.
Figure 4.
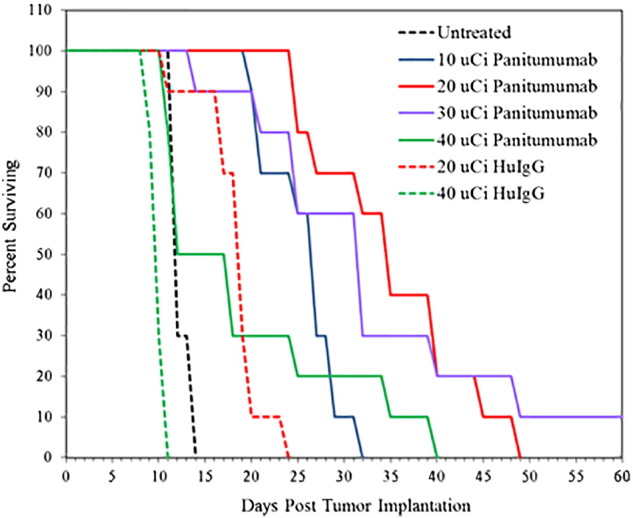
A dose escalation study was performed with 212Pb-panitumumab to determine an effective therapeutic dose. Groups of athymic mice (n = 10) bearing 3d LS-174T i.p. tumor xenografts were injected with 10, 20, 30, or 40 μCi of 212Pb-panitumumab. Additional groups of mice were administered 20 or 40 μCi of 212Pb-HuIgG which served as a nonspecific control.
In addition to the MS of the groups, the weights of the mice were monitored for ~5 weeks following administration of the 212Pb-panitumumab (Table S3). The mice experienced their greatest weight loss at 15 days with a 6.2%, 6.5%, 8.0%, and 15.1% loss for the 10, 20, 30, and 40 μCi, respectively. Whereas weight was regained for the first three dosages, those mice treated with 40 μCi of 212Pb-panitumumab failed to show evidence of recovery. A similar pattern of weight loss was observed with the 20- and 40-μCi doses of 212Pb-HuIgG. Based on the MS and the weight data, a dose of 20 μCi of 212Pb-panitumumab was selected as the effective therapeutic dose for subsequent studies.
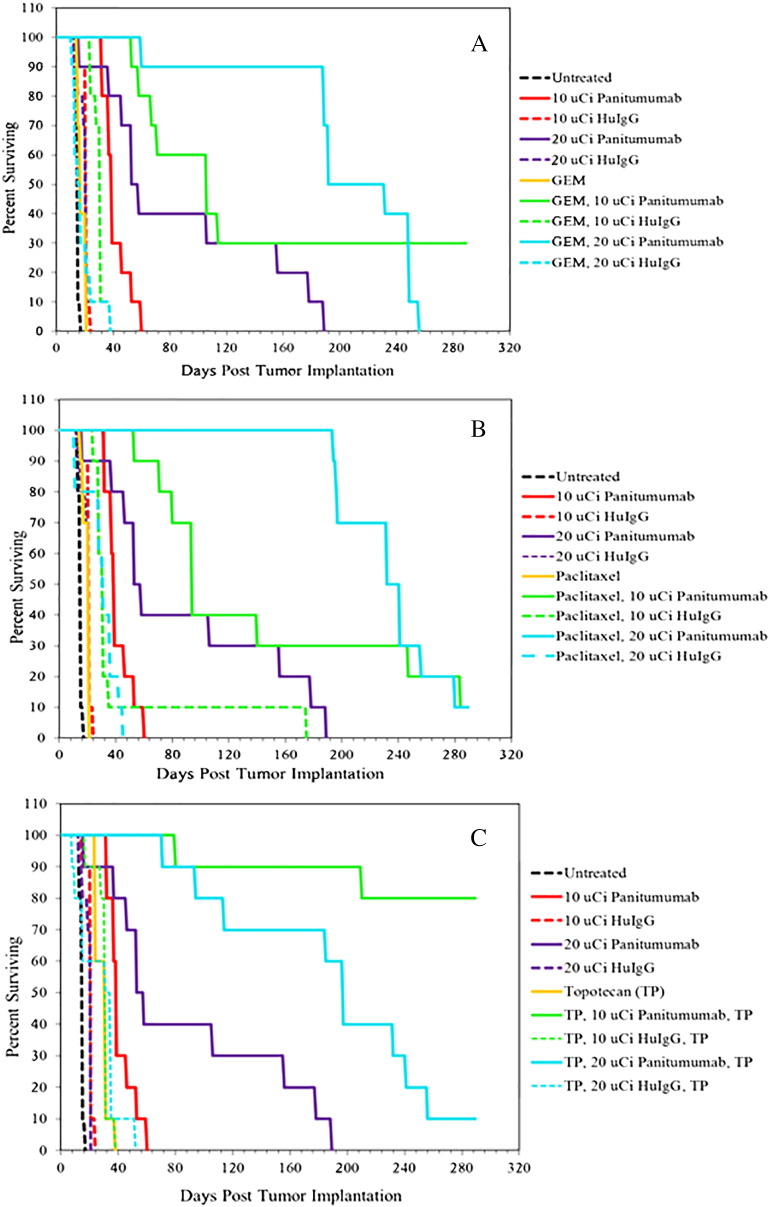
Having established the effective therapeutic dose, the investigation moved forward to culminate in that aspect of combining chemotherapeutics with 212Pb-RIT at two doses, 10 and 20 μCi. The choice of two doses of 212Pb-panitumumab permitted 1) a direct comparison with the previous studies conducted in this laboratory with 212Pb-labeled trastuzumab and cetuximab at 10 μCi [10], [16], [30], [37], [41] and 2) an assessment as to whether or not greater therapeutic efficacy would actually be realized with 212Pb-panitumumab at the 20-μCi dose in combination with chemotherapeutics or whether this dose would result in some measure of immediate toxicity. Based on previously published data, GEM (1 mg; Figure 5A) or paclitaxel (0.6 mg; Figure 5B) was administered to tumor-bearing mice (n = 10) 24 hours before the administration of 212Pb-panitumumab [16], [37]. Also included in the study were groups of mice that received two injections of topotecan (0.25 mg each): one 24 hours prior to 212Pb-RIT and a second dose 24 hours after the 212Pb-RIT (Figure 5C). A pilot study combining single doses of topotecan prior to or post-RIT with 212Pb-trastuzumab provided sufficient data to suggest that this chemotherapeutic was worth further investigation (Table S1). Controls for the study included groups of mice treated with 212Pb-labeled panitumumab or 212Pb-labeled HuIgG, each of the chemotherapeutics alone, as well as a group that was left untreated.
Figure 5.
Effect of chemotherapeutics in combination with 212Pb-panitumumab on the survival of athymic mice bearing LS-174T i.p. tumor xenografts. Mice were pretreated with gemcitabine (A) or paclitaxel (B) 24 hours prior to administration of 212Pb-panitumumab or 212Pb-HuIgG. Another set of mice received topotecan (C) 24 hours before and 24 hours after injection of the 212Pb-RIT. The combined modalities were assessed at two doses of 212Pb-RIT, 10 and 20 μCi.
Consistent with the previous study, treatment of tumor-bearing mice with 10 or 20 μCi of 212Pb-panitumumab resulted in an MS of 39 and 58 days, respectively, compared with 15 days for the untreated group (P < .002; Table 2 and Figure 5). Correspondingly, this translates to a therapeutic index (TI = MS of the treatment group divided by the MS of the untreated group) of 2.6 and 3.9. The MS of mice injected with 10 or 20 μCi of 212Pb-HuIgG was 21 days with a calculated TI of 1.4. Mice pretreated with 1 mg GEM the day before the RIT (Figure 5A) realized an increase in the MS: 106 days for those administered 10 μCi 212Pb-panitumumab (7.1 TI) and 192 days (12.8 TI) for those receiving 20 μCi. There was not a significant difference, however, between these two groups (P = 1), although 3 of 10 mice in the group treated with GEM and 10 μCi 212Pb-panitumumab survived to 289 days. GEM alone had a negligible influence on survival with an MS of 17 days, whereas treatment with GEM in combination with the control antibody, 212Pb-HuIgG, resulted in an MS of 31 and 15 days for the 10- and 20-μCi doses, respectively.
Table 2.
Enhancement of the Therapeutic Efficacy of 212Pb-RIT by Chemotherapeutics
| Chemotherapeutic |
|||||
|---|---|---|---|---|---|
| mAb | Activity (μCi) | None | Gemcitabine | Paclitaxel | Topotecan |
| None | None | 15a | 17 | 21 | 31 |
| Panitumumab | 10 | 39 | 106 | 94 | >289b |
| 20 | 58 | 192 | 241 | 197 | |
| HuIgG | 10 | 21 | 31 | 31 | 31 |
| 20 | 21 | 15 | 31 | 35 | |
Median survival (days) of athymic mice bearing LS-174T i.p. tumor xenografts following pretreatment with chemotherapeutics and a single injection of 212Pb-panitumumab. Paclitaxel (0.6 mg) and gemcitabine (1 mg) were administered i.p. to tumor-bearing mice 24 hours before RIT. Two doses of topotecan (0.25 mg each) were injected: 24 hours before and again 24 hours after the administration of the RIT. Additional groups of mice included those that were treated with each of the chemotherapeutics alone, 212Pb-HuIgG alone, 212Pb-HuIgG in combination with each of the chemotherapeutics, as well as a group of mice that were left untreated.
Eight of 10 mice remained alive at 289 days.
Paclitaxel also potentiated the therapeutic efficacy of 212Pb-panitumumab RIT dramatically by increasing survival of tumor-bearing mice with an MS of 94 days for paclitaxel combined with 10 μCi of 212Pb-panitumumab, and 241 days when combined with the 20-μCi dose of 212Pb-panitumumab (Figure 5B). Interestingly, the difference between these two treatment groups was not significant with a P value of .468. The corresponding doses with 212Pb-HuIgG resulted in an MS of 31 days following the pretreatment with paclitaxel, whereas paclitaxel alone resulted in an MS of 21 days.
The most dramatic enhancement of the therapeutic efficacy of 212Pb-panitumumab was observed in those groups treated with two doses of topotecan (0.25 mg each): one dose given the day before RIT and the second dose given the day after the α-therapy (Figure 5C). At 289 days, the MS of the group of tumor-bearing mice that had been treated with two doses of topotecan and a single dose of 10 μCi 212Pb-panitumumab could not be determined because 8 of the 10 mice remained alive. The MS of the group that received topotecan and 20 μCi 212Pb-panitumumab was also respectable with an MS of 197 days. Meanwhile, topotecan alone, topotecan with 10 μCi 212Pb-HuIgG, and topotecan with 20 μCi 212Pb-HuIgG therapies resulted in MSs of 31, 31, and 35 days, respectively.
Among the treatment groups, the greatest weight loss was observed in the group of mice that had been treated with two doses of topotecan and the 212Pb-RIT. Four days after receiving the 212Pb-panitumumb, the mice exhibited a 23.9% and 21.8% weight loss in the 10- and 20-μCi groups, respectively (Table S4). The mice then recovered from this weight loss, and by 19 days, both groups had returned to their pretherapy weights. A similar degree of weight loss was also observed at both dose levels of 212Pb-HuIgG (19.2% and 22.4%) in combination with the topotecan. These mice, however, never returned to their pretherapy weights. In fact, both the 10-and 20-μCi groups remained 10.7% below their original weights at 28 days.
Weight loss was also observed in the rest of the treatment groups. Except for the group treated with just 10 μCi of 212Pb-panitumumab, the mice given 20 μCi of 212Pb-RIT (panitumumab or HuIgG) demonstrated weight loss. Weight recovery was evident in all of the groups receiving the 212Pb-panitumumab beginning 6 to 13 days after RIT; at 4 weeks, the mice had recovered at least 91% of their body weight (91.1% to 100%). In contrast, as with the groups that were treated with topotecan and 212Pb-HuIgG, the recovery appears slower in the groups injected with either GEM or paclitaxel combined with 212Pb-HuIgG. Furthermore, those animals that were still alive at 4 weeks were at 83% to 92.7% of their original weights.
Flow cytometric analysis of the LS-174T cell line had indicated that ~77% of the cells were positive for HER2 expression with a mean fluorescence intensity (MFI) of 30.3, whereas 99% (MFI = 62) of the cells express HER1 [24], [30]. More cells do express HER1; however, the level of HER1 is not much greater than HER2 when the MFI is compared. Considering the therapeutic efficacy that is observed with both 212Pb-labeled trastuzumab and panitumumab and that 212Pb-panitumumab provided greater therapeutic benefit, quantitation of HER1 and HER2 in LS-174T tumor xenografts was warranted. Tumors were harvested from untreated mice (n = 5) 3 days post tumor cell implantation, and whole tumor lysates were prepared. HER1 and HER2 levels were then quantitated using an ELISA kit specific for the detection of each molecule. As outlined in Table 3, the amount of HER1 per mg of tumor was 1.00 ± 0.37 ng, ranging from 0.56 to 1.60 ng. Meanwhile, HER2 levels were found to be 12.5-fold lower with an average of 0.08 ± 0.04 ng per mg of tumor (0.03-0.11 ng HER2/mg tumor).
Table 3.
Quantitation of HER1 and HER2 Content in LS-174T Tumors
| Tumor # | Tumor Weight (mg) | HER1 (ng/mg Tumor) | HER2 (ng/mg Tumor) |
|---|---|---|---|
| 1 | 21.0 | 1.07 ± 0.01 | 0.11 ± 0.04 |
| 2 | 34.1 | 0.56 ± 0.08 | 0.03 ± 0.02 |
| 3 | 28.0 | 0.78 ± 0.10 | 0.05 ± 0.03 |
| 4 | 29.0 | 1.00 ± 0.16 | 0.09 ± 0.02 |
| 5 | 20.4 | 1.60 ± 0.13 | 0.10 ± 0.03 |
| Average ± S.D. | 26.5 ± 5.8 | 1.00 ± 0.37 | 0.08 ± 0.04 |
Lysates were prepared from LS-174T i.p. tumor xenografts (n = 5) harvested 3 days post–tumor cell implantation.
The amount of HER1 and HER2 in each of the tumor lysates was then quantitated using commercially available.
ELISA kits for the detection of each molecule. The assays were performed twice, each sample in duplicate. The values represent the average of the results from both assays.
Discussion
All indications are that locoregional administration of 212Pb-RIT can be performed safely. Eighteen patients with HER2-positive peritoneal malignancies that had failed standard therapies received 212Pb-trastuzumab (0.2 to 0.74 mCi/m2; 2-3 patients per cohort) by i.p. infusion. The single i.p. injection was reported as being well tolerated with grade 1 toxicity, mostly asymptomatic [42].
Prior to the approval of the evaluation of 212Pb-trastuzumab in a clinical trial at the University of Alabama at Birmingham, the Food and Drug Administration required two toxicological studies. One study was an evaluation of the effects of free 212Pb which was conducted with normal Balb/c mice to respond to concerns regarding what effects free 212Pb would have if prematurely released from the mAb-chelate conjugate, i.e., a worst case injection scenario [43]. The other study was a biodistribution and toxicological study of 212Pb-trastuzumab in nonhuman primates [44]. In the mouse study, changes in blood counts, chemistries, or tissues were observed at 7 and 90 days. These changes were interpreted to be not severe enough to impact organ function. The data from the cynomolgus monkey study were even more encouraging in that there was a lack of toxicity following i.p. injection of the 212Pb-trastuzumab reported [2]. Perhaps more importantly, the results from the clinical trial have advocated the safety of 212Pb-RIT. No late renal, hepatic, or cardiac toxicity was observed in patients up to 1 year after receiving a single infusion of 212Pb-trastuzumab in the clinical trial conducted at the University of Alabama at Birmingham [42].
Renal toxicity may be even less of a concern when other factors are considered. The TCMC chelate was developed specifically for Pb(II) radioisotopes. The Pb[TCMC]2+ complex is less likely to release Pb(II) than Pb[DOTA]2− at pH ≤ 3.5 [29]. When radioimmunoconjugates are catabolized, excretion of the radiometal occurs following lysosomal degradation, and it is in the form of a metabolite, not free metal [45], [46]. In addition, when administered by i.p. injection, 212Pb-trastuzumab demonstrates minimal redistribution out of the peritoneal cavity and no significant uptake in major organs in humans. The toxicology study of i.p. injection of 212Pb-trastuzumab in cynomolgus monkeys showed that ~90% to 100% of the 212Pb and 83% to 92% of the 212Bi daughter remained in the peritoneum [2].
The heterogeneous nature of tumors presents challenges to those devising treatment regimens for patients. Fortunately for RIT, expression of the target molecule by every tumor cell is not a requisite for success, nor does the target need to be expressed at high levels. The omnidirectional nature of radioactive decay and cross-fire effects can result in the delivery of a cytotoxic dose to neighboring cells, both malignant and normal. The bystander effect that occurs during cell death/damage due to the targeted radioactivity also exerts deleterious effects on cells in proximity [47], [48], [49]. If the target molecule is a receptor, the success of RIT is also not dependent on eliciting a biological effect upon interacting with that receptor; the injected protein doses when used as a targeting vector are generally well below therapeutic amounts. Regardless of the above-stated advantages of RIT, accruing additional target molecules and hence targeting vehicles such as mAbs broaden the options available for the treatment of cancer patients.
Earlier studies from this laboratory have demonstrated that exploiting HER1 for α-particle RIT might have the same potential as that of targeting HER2 and would expand the repertoire of available treatment options as well as address aspects of target heterogeneity by having the ability to direct therapy to multiple sites within the malignancy. Those studies were performed with cetuximab as the delivery vehicle of 212Pb [10]. As outlined earlier, however, cetuximab is a chimeric mAb, and its success in RIT applications may be restricted by the development of significant adverse responses by patients including anticetuximab responses. The information in the product label states that 5% of the patients treated with cetuximab develop an antibody response to cetuximab. However, there are reports of incidence rates as high as 22% [21], [50], [51], [52]. Having shown HER1 as a viable target for RIT, the development of a targeting vehicle devoid of some of the problems associated with cetuximab was deemed a worthwhile pursuit [10], [15], [20], [53].
Panitumumab has clear advantages over cetuximab. It is a fully human monoclonal antibody, and as such, immune responses in patients have been minimal: 0.4% or 3.2% depending on the assay format utilized [23]. When radiolabeled with 111In, panitumumab exhibited four-, three-, and two-fold lower levels of radioactivity in the liver, spleen, and kidneys, respectively, than what was reported for equivalent cetuximab analogs in tumor localization studies [10], [24]. Higher liver uptake with 177Lu-labeled cetuximab was also noted in a recent study in which 177Lu-labeled panitumumab and cetuximab were directly compared in a tumor targeting study as well as by single photon emission computed tomography/computed tomography imaging [15]. In that same report, 177Lu-panitumumab also demonstrated greater therapeutic efficacy. The superior tumor targeting of radiolabeled panitumumab versus cetuximab has also been demonstrated via PET imaging using 86Y [17]. Considering that these two mAbs both target EGFR, albeit different epitopes, the difference in the normal organ distribution along with the superior quality of the images obtained with panitumumab may be an indication that cetuximab is undergoing catabolism. Another possibility may be due to the differences in their affinity constants with panitumumab (Kd ~ 0.05 nM) having a greater affinity than cetuximab (Kd = 0.39 nM) for HER1 [54]. Given the patient experience and the demonstrated advantages of radiolabeled panitumumab over the equivalent cetuximab analogs in preclinical in vivo models, 212Pb-panitumumab has the potential to obviate the difficulties anticipated in the use of 212Pb-cetuximab. The studies presented within this report validate 212Pb-panitumumab for targeting HER1 and show its potential of providing yet another treatment option for cancer patients.
The RIT studies described herein began with a pilot study directly comparing the therapeutic efficacy of panitumumab to trastuzumab and to cetuximab radiolabeled with 212Pb at 10 μCi each. At 293 days, when the experiment was terminated, 50% of the mice that had received 212Pb-panitumumab were still alive versus 20% of the mice that received either 212Pb-trastuzumab or 212Pb-cetuximab. A dose escalation of the 212Pb-panitumumab dose (μCi) in a subsequent experiment led to the decision that 20 μCi would be the highest dose to evaluate in conjunction with the chemotherapeutics. Admittedly, the median survival of mice treated with 212Pb-panitumumab only was not as dramatic in subsequent experiments as what was observed in the pilot study. This is not the first time that such a phenomenon has been observed. Looking over the many therapy studies that this laboratory has conducted and published investigating targeted α-particle radiation therapy, there can be differences of 20 to 30 days in the median survival of mice treated with 212Pb-trastuzumab. In one set of studies, there was a difference of ~100 days in the median survival, and yet the untreated mice or the mice receiving the radiolabeled nonspecific antibody only varied 10 to 15 days in their median survival [16]. Another example may be found in the therapy studies with 212Pb-cetuximab [10]. Inclusion of the appropriate controls in the studies (i.e., treatment with a nonspecific antibody along with groups that are not treated) ensures that the model itself exhibits the expected characteristics and is not compromised. The controls also demonstrate that the therapeutic response is specific and is due to the targeting vehicle.
Great care is taken to ensure that the animal model is prepared the same for each experiment. The LS-174T cells are harvested during log-phase growth and are >95% viable when injected into the mice. There is also no more than 5% variance in the number of cells inoculated. One explanation for the differences in median survival between studies is differences in tumor burden when the RIT is administered. For example, at the initiation of the first time point of the biodistribution studies described in this report, albeit 6 days, the average tumor burden was 110 mg with a standard deviation of 112 mg. Another possibility is the accessibility of the target antigen/tumor to the 212Pb conjugate. Differences in the expression of the target molecule or accessibility of the target on the tumor would also influence the success of the 212Pb-RIT. One study that has yet to be performed is to assess the level of EGFR (and HER2) on the surface of LS-174T cells freshly isolated from i.p. tumor xenografts using a method such as flow cytometry. In addition, the 212Pb-RIT is administered by i.p. injection of a 0.5-ml solution. The distribution of that volume in the peritoneum could also greatly influence the outcome of the therapy.
The combination of chemotherapeutics (i.e., gemcitabine, paclitaxel) with α-particle radiation therapy for the treatment of HER2-positive peritoneal xenografts potentiates therapeutic efficacy using trastuzumab as the delivery vehicle [10], [16], [35], [37]. Demonstrating the success of these treatment regimens exploiting another mAb and target, albeit of the same family of growth factor molecules, would validate the strategy. The efficacy of 212Pb-panitumumab in combination with the chemotherapeutics was assessed at both 20 μCi, the dose chosen from the dose escalation study, and at 10 μCi, as it was deemed possible that the higher dose combined with a chemotherapeutic might lead to toxicity. The latter dose was also added to allow a direct comparison with the preexisting body of data generated with 212Pb-trastuzumab [10], [16], [35], [37].
The 20-μCi dose of 212Pb-panitumumab combined with either GEM or paclitaxel provided greater benefit than 10 μCi of 212Pb-panitumumab combined with either of these two drugs. Comparing the paclitaxel plus 212Pb-panitumumab versus the paclitaxel plus 212Pb-trastuzumab (10 μCi) treatment regimens, the calculated TIs were 6.2 and 10.7, respectively. At the higher dose of 20 μCi of 212Pb-panitumumab, however, the TI increased to 16.1, surpassing both results at 10 μCi. The combination of GEM with 212Pb-RIT (10 μCi) resulted in a TI of 7.1 for 212Pb-panitumumab and 4.4 for 212Pb-trastuzumab. The TI for mice pretreated with GEM 24 hours before administration of 20 μCi of 212Pb-panitumumab was 12.8. Thus, at the higher dose, 20 μCi of 212Pb-panitumumab in combination with either paclitaxel or GEM provided improved therapeutic efficacy.
Interestingly, it was the combination of topotecan, the topoisomerase I inhibitor, with 212Pb-panitumumab that yielded the best results. A pilot study combining topotecan with 212Pb-trastuzumab had provided sufficient data to suggest that this chemotherapeutic was worth further investigation (Table S1). That study also indicated that a single dose of topotecan 24 hours before or 24 hours after the 212Pb-RIT might be more effective than a concurrent administration of the two modalities. With that possibility in mind and since the single dose of topotecan was not impressive as what was observed with paclitaxel or GEM when combined with 212Pb-trastuzumab, the decision was made to give the mice two injections of topotecan: one the day before and a second the day after injection of the 212Pb-panitumumab. The selected dose, 0.25 mg per mouse, was derived from reports demonstrating the feasibility of combining topotecan with RIT that showed that a single dose of topotecan at 12.5 mg/kg would result in minimal weight loss and no deaths due to toxicity [55], [56].
In contrast to the therapeutic potentiation of 212Pb-panitumumab observed with paclitaxel or GEM, the two doses of topotecan on this schedule proved more effective in concert with 10 μCi than the 20-μCi dose. The MS of the topotecan/10-μCi 212Pb-panitumumab group could not be determined due to their lengthy survival (80% at 330+ days), and the TI was > 19.2 versus 13.1 for the group of mice that were treated with topotecan and 20 μCi of 212Pb-panitumumab. The topotecan combined with 212Pb-RIT regimen did result in greater weight loss in the mice in the first week following the therapy; however, the weights rebounded to pretreatment levels by ~3 weeks. It is worthy of note that the results for the RIT plus topotecan combination study provide a basis for investigating the other chemotherapeutics in a similar fashion by giving GEM or paclitaxel 24 hours before and after RIT treatment. This will provide a direct comparison of the three chemotherapeutics in the same treatment schedule with RIT.
In a series of papers from this laboratory, initial investigations into the molecular mechanisms of cell killing by α-particle radiation using trastuzumab to target HER2 were methodically described. These studies also provided insight into the pathways invoked by GEM and paclitaxel to potentiate the sensitivity of tumor cells to 212Pb-RIT with each chemotherapeutic affecting different pathways [57], [58], [59], [60], [61], [62], [63]. RIT with 212Pb-trastuzumab was found to induce double-stranded DNA breaks, impaired DNA damage repair, arrested the cell cycle at the G2-M phase, and induced chromatin remodeling [57]. Subsequently, gene profiling demonstrated that the expression of genes involved in apoptosis and cell cycle arrest was affected by the 212Pb-RIT [63]. Pretreatment with GEM was found to abrogate the G2-M checkpoint and inhibit DNA damage repair [59], [61]. Meanwhile, paclitaxel sensitization of tumor cells to α-particle radiation perturbed the mitotic spindle checkpoint which results in mitotic catastrophe [58]. It will be interesting to investigate the mechanism that leads to cell death when topotecan is included in the treatment regimen with 212Pb-RIT. Such studies have yet to be initiated.
The studies described herein indicate that α-particle radiation therapy of HER1 may provide greater therapeutic benefit than the targeting of HER2. Flow cytometric analysis of LS-174T cells has shown that the expression of either of these molecules on the cell surface is not high (i.e., mean fluorescence intensity). However, localization of radiolabeled panitumumab to tumors in biodistribution studies would argue that expression of this target might be higher in LS-174T tumor xenografts. Differences in antigen expression in cell culture and in vivo have been previously reported [64]. Quantitation of HER1 and HER2 levels in the LS-174T i.p. tumor xenografts was the first step towards gaining a better understanding of the differences between the therapeutic efficacies of targeting these two molecules with 212Pb-labeled mAbs. The data revealed that the tumors contained 12.5 times more HER1 than HER2 per mg of tumor. This differences in the two targeting molecules provide a significant explanation for the greater therapeutic benefit provided by 212Pb-panitumumab over that of 212Pb-trastuzumab. Admittedly, the quantitation was performed with whole lysates of the tumor. At this juncture, the amount of HER1 and HER2 available on the cell surface of these tumors is not known and will be investigated further. Included among those studies to be conducted will be dual targeting of HER1 and HER2 with 212Pb-labeled panitumumab and trastuzumab in combination with the above scheduled administration of topotecan [10].
Despite the fact that panitumumab is a human mAb, there are few reports investigating its potential as an RIT agent [15], [24], [53], [65], [66]. Consistent with the objectives of this laboratory to systematically develop multimodal treatment regimens for cancer patients, the data described within this report presents an evaluation of panitumumab for the therapy of HER1-positive disease with α-particle radiation. A single dose of 212Pb-panitumumab was found to have therapeutic efficacy that surpassed either 212Pb-trastuzumab or 212Pb-cetuximab. Clearly, incorporation of chemotherapeutics into the treatment design increases the therapeutic impact of the α-particle radiation therapy. Equally clear however is the need to understand and develop optimal dosing and scheduling of the components of such therapies. Having methodically assessed three mAbs in this approach, it is perhaps safe to state that the strategy possesses great potential for the treatment and management of cancer patients.
Disclosure of Potential Conflicts of Interest
There are no potential conflicts of interest to disclose.
Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.tranon.2017.04.004.
Appendix A. Supplementary data
Supplementary tables.
References
- 1.Meredith R, Torgue J, Shen S, Fisher DR, Banaga E, Bunch P, Morgan D, Fan J, Straughn JM., Jr. Dose escalation and dosimetry of first-in-human alpha radioimmunotherapy with 212Pb-TCMC-trastuzumab. J Nucl Med. 2014;55:1636–1642. doi: 10.2967/jnumed.114.143842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meredith RF, Torgue J, Azure MT, Shen S, Saddekni S, Banaga E, Carlise R, Bunch P, Yoder D, Alvarez R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm. 2014;29:12–17. doi: 10.1089/cbr.2013.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agus DB, Bunn PA, Jr., Franklin W, Garcia M, Ozols RF. Her-2/Neu as a therapeutic target in non–small cell lung cancer, prostate cancer, and ovarian cancer. Semin Oncol. 2000;27:53–63. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11236029] [PubMed] [Google Scholar]
- 4.Lanitis E, Dangaj D, Hagemann IS, Song DG, Best A, Sandaltzopoulos R, Coukos G, Powell DJ., Jr. Primary human ovarian epithelial cancer cells broadly express Her2 at immunologically-detectable levels. PLoS One. 2012;7:e49829. doi: 10.1371/journal.pone.0049829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Natali PG, Nicotra MR, Bigotti A, Venturo I, Slamon DJ, Fendly BM, Ullrich A. Expression of the P185 encoded by Her2 oncogene in normal and transformed human tissues. Int J Cancer. 1990;45:457–461. doi: 10.1002/ijc.2910450314. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1968437] [DOI] [PubMed] [Google Scholar]
- 6.Ross JS, McKenna BJ. The Her-2/Neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554–568. doi: 10.1081/cnv-100103852. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11458821] [DOI] [PubMed] [Google Scholar]
- 7.Hand PH, Nuti M, Colcher D, Schlom J. Definition of antigenic heterogeneity and modulation among human mammary carcinoma cell populations using monoclonal antibodies to tumor-associated antigens. Cancer Res. 1983;43:728–735. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6848188] [PubMed] [Google Scholar]
- 8.Natali PG, Giacomini P, Bigotti A, Imai K, Nicotra MR, Ng AK, Ferrone S. Heterogeneity in the expression of HLA and tumor-associated antigens by surgically removed and cultured breast carcinoma cells. Cancer Res. 1983;43:660–668. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6336658] [PubMed] [Google Scholar]
- 9.Milenic D, Garmestani K, Dadachova E, Chappell L, Albert P, Hill D, Schlom J, Brechbiel M. Radioimmunotherapy of human colon carcinoma xenografts using a 213Bi-labeled domain-deleted humanized monoclonal antibody. Cancer Biother Radiopharm. 2004;19:135–147. doi: 10.1089/108497804323071904. [DOI] [PubMed] [Google Scholar]
- 10.Milenic DE, Baidoo KE, Kim YS, Brechbiel MW. Evaluation of cetuximab as a candidate for targeted α-particle radiation therapy of HER1-positive disseminated intraperitoneal disease. MAbs. 2015;7:255–264. doi: 10.4161/19420862.2014.985160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milenic DE, Wong KJ, Baidoo KE, Ray GL, Garmestani K, Williams M, Brechbiel MW. Cetuximab: preclinical evaluation of a monoclonal antibody targeting EGFR for radioimmunodiagnostic and radioimmunotherapeutic applications. Cancer Biother Radiopharm. 2008;23:619–631. doi: 10.1089/cbr.2008.0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nayak TK, Garmestani K, Baidoo KE, Milenic DE, Brechbiel MW. Preparation, biological evaluation, and pharmacokinetics of the human anti-HER1 monoclonal antibody panitumumab labeled with 86Y for quantitative PET of carcinoma. J Nucl Med. 2010;51:942–950. doi: 10.2967/jnumed.109.071290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Parry JJ, Laforest R, Rogers BE, Anderson CJ. The role of P53 in combination radioimmunotherapy with 64Cu-DOTA-cetuximab and cisplatin in a mouse model of colorectal cancer. J Nucl Med. 2013;54:1621–1629. doi: 10.2967/jnumed.112.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koi L, Bergmann R, Bruchner K, Pietzsch J, Pietzsch HJ, Krause M, Steinbach J, Zips D, Baumann M. Radiolabeled anti–EGFR-antibody improves local tumor control after external beam radiotherapy and offers theragnostic potential. Radiother Oncol. 2014;110:362–369. doi: 10.1016/j.radonc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Ma T, Liu H, Jin Z, Sun X, Zhao H, Shi J, Jia B, Li F, Wang F. 177Lu-labeled antibodies for EGFR-targeted SPECT/CT imaging and radioimmunotherapy in a preclinical head and neck carcinoma model. Mol Pharm. 2014;11:800–807. doi: 10.1021/mp4005047. [DOI] [PubMed] [Google Scholar]
- 16.Milenic DE, Garmestani K, Brady ED, Baidoo KE, Albert PS, Wong KJ, Flynn J, Brechbiel MW. Multimodality therapy: potentiation of high linear energy transfer radiation with paclitaxel for the treatment of disseminated peritoneal disease. Clin Cancer Res. 2008;14:5108–5115. doi: 10.1158/1078-0432.CCR-08-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nayak TK, Garmestani K, Milenic DE, Baidoo KE, Brechbiel MW. HER1-targeted 86Y-panitumumab possesses superior targeting characteristics than 86Y-cetuximab for PET imaging of human malignant mesothelioma tumors xenografts. PLoS One. 2011;6:e18198. doi: 10.1371/journal.pone.0018198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Niu G, Sun X, Cao Q, Courter D, Koong A, Le QT, Gambhir SS, Chen X. Cetuximab-based immunotherapy and radioimmunotherapy of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16:2095–2105. doi: 10.1158/1078-0432.CCR-09-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perk LR, Visser GW, Vosjan MJ, Stigter-van Walsum M, Tijink BM, Leemans CR, van Dongen GA. (89)Zr as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals (90)Y and (177)Lu in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J Nucl Med. 2005;46:1898–1906. [ http://www.ncbi.nlm.nih.gov/pubmed/16269605] [PubMed] [Google Scholar]
- 20.Song H, Hedayati M, Hobbs RF, Shao C, Bruchertseifer F, Morgenstern A, Deweese TL, Sgouros G. Targeting aberrant DNA double-strand break repair in triple-negative breast cancer with alpha-particle emitter radiolabeled anti-EGFR antibody. Mol Cancer Ther. 2013;12:2043–2054. doi: 10.1158/1535-7163.MCT-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist. 2008;13:725–732. doi: 10.1634/theoncologist.2008-0012. [DOI] [PubMed] [Google Scholar]
- 22.ImClone Systems Inc.; Branchburg, NJ: 2016. Erbitux (cetuximab) [package insert] [Google Scholar]
- 23.Amgen; Thousand Oaks, CA: 2015. Vectibix (panitumumab) [package insert] [Google Scholar]
- 24.Ray GL, Baidoo KE, Wong KJ, Williams M, Garmestani K, Brechbiel MW, Milenic DE. Preclinical evaluation of a monoclonal antibody targeting the epidermal growth factor receptor as a radioimmunodiagnostic and radioimmunotherapeutic agent. Br J Pharmacol. 2009;157:1541–1548. doi: 10.1111/j.1476-5381.2009.00327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nayak TK, Bernardo M, Milenic DE, Choyke PL, Brechbiel MW. Orthotopic pleural mesothelioma in mice: SPECT/CT and MR imaging with HER1- and HER2-targeted radiolabeled antibodies. Radiology. 2013;267:173–182. doi: 10.1148/radiol.12121021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nayak TK, Garmestani K, Milenic DE, Brechbiel MW. PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J Nucl Med. 2012;53:113–120. doi: 10.2967/jnumed.111.094169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Milenic DE, Wong KJ, Baidoo KE, Nayak TK, Regino CA, Garmestani K, Brechbiel MW. Targeting HER2: a report on the in vitro and in vivo pre-clinical data supporting trastuzumab as a radioimmunoconjugate for clinical trials. MAbs. 2010;2:550–564. doi: 10.4161/mabs.2.5.13054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tom BH, Rutzky LH, Jakstys MH. Human colonic adenocarcinoma cells. establishment and description of a new cell line. In Vitro. 1976;12:180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- 29.Chappell LL, Dadachova E, Milenic DE, Garmestani K, Wu C, Brechbiel MW. Synthesis, characterization, and evaluation of a novel bifunctional chelating agent for the lead isotopes 203Pb and 212Pb. Nucl Med Biol. 2000;27:93–100. doi: 10.1016/s0969-8051(99)00086-4. [ http://www.ncbi.nlm.nih.gov/pubmed/10755652] [DOI] [PubMed] [Google Scholar]
- 30.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. α-Particle radioimmunotherapy of disseminated peritoneal disease using a 212Pb-labeled radioimmunoconjugate targeting HER2. Cancer Biother Radiopharm. 2005;20:557–568. doi: 10.1089/cbr.2005.20.557. [DOI] [PubMed] [Google Scholar]
- 31.Wu C, Kobayashi H, Sun B, Yoo TM, Paik CH, Gansow OA, Carrasquillo JA, Pastan I, Brechbiel MW. Stereochemical influence on the stability of radio-metal complexes in vivo. synthesis and evaluation of the four stereoisomers of 2-(P-nitrobenzyl)-trans-Cydtpa. Bioorg Med Chem. 1997;5:1925–1934. doi: 10.1016/s0968-0896(97)00130-2. [ http://www.ncbi.nlm.nih.gov/pubmed/9370037] [DOI] [PubMed] [Google Scholar]
- 32.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 33.Dadachova E, Chappell LL, Brechbiel MW. Spectrophotometric method for determination of bifunctional macrocyclic ligands in macrocyclic ligand-protein conjugates. Nucl Med Biol. 1999;26:977–982. doi: 10.1016/s0969-8051(99)00054-2. [ http://www.ncbi.nlm.nih.gov/pubmed/10708314] [DOI] [PubMed] [Google Scholar]
- 34.Pippin CG, Parker TA, McMurry TJ, Brechbiel MW. Spectrophotometric method for the determination of a bifunctional DTPA ligand in DTPA-monoclonal antibody conjugates. Bioconjug Chem. 1992;3:342–345. doi: 10.1021/bc00016a014. [ http://www.ncbi.nlm.nih.gov/pubmed/1390990] [DOI] [PubMed] [Google Scholar]
- 35.Milenic DE, Baidoo KE, Shih JH, Wong KJ, Brechbiel MW. Evaluation of platinum chemotherapy in combination with HER2-targeted α-particle radiation. Cancer Biother Radiopharm. 2013;28:441–449. doi: 10.1089/cbr.2012.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Milenic DE, Brady ED, Garmestani K, Albert PS, Abdulla A, Brechbiel MW. Improved efficacy of α-particle–targeted radiation therapy: dual targeting of human epidermal growth factor receptor-2 and tumor-associated glycoprotein 72. Cancer. 2010;116:1059–1066. doi: 10.1002/cncr.24793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milenic DE, Garmestani K, Brady ED, Albert PS, Abdulla A, Flynn J, Brechbiel MW. Potentiation of high-Let radiation by gemcitabine: targeting HER2 with trastuzumab to treat disseminated peritoneal disease. Clin Cancer Res. 2007;13:1926–1935. doi: 10.1158/1078-0432.CCR-06-2300. [DOI] [PubMed] [Google Scholar]
- 38.Fraker PJ, Speck JC., Jr. Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=637870] [DOI] [PubMed] [Google Scholar]
- 39.Garmestani K, Milenic DE, Plascjak PS, Brechbiel MW. A new and convenient method for purification of 86Y using a Sr(Ii) selective resin and comparison of biodistribution of 86Y and 111In labeled herceptin. Nucl Med Biol. 2002;29:599–606. doi: 10.1016/s0969-8051(02)00322-0. [ http://www.ncbi.nlm.nih.gov/pubmed/12088731] [DOI] [PubMed] [Google Scholar]
- 40.Baidoo KE, Milenic DE, Brechbiel MW. Methodology for labeling proteins and peptides with lead-212 (212Pb) Nucl Med Biol. 2013;40:592–599. doi: 10.1016/j.nucmedbio.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milenic DE, Garmestani K, Brady ED, Albert PS, Ma D, Abdulla A, Brechbiel MW. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin Cancer Res. 2004;10:7834–7841. doi: 10.1158/1078-0432.CCR-04-1226. [DOI] [PubMed] [Google Scholar]
- 42.Meredith RF, Torgue JJ, Rozgaja TA, Banaga EP, Bunch PW, Alvarez RD, Straughn JM, Jr.;Dobelbower, M. C.Lowy, A. M. Safety and outcome measures of first-in-human intraperitoneal α radioimmunotherapy with 212Pb-TCMC-trastuzumab. Am J Clin Oncol. 2016 doi: 10.1097/COC.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milenic DE, Molinolo AA, Solivella MS, Banaga E, Torgue J, Besnainou S, Brechbiel MW, Baidoo KE. Toxicological studies of 212Pb intravenously or intraperitoneally injected into mice for a phase 1 trial. Pharmaceuticals (Basel) 2015;8:416–434. doi: 10.3390/ph8030416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kasten BB, Azure MT, Schoeb TR, Fisher DR, Zinn KR. Imaging, biodistribution, and toxicology evaluation of 212Pb-TCMC-trastuzumab in nonhuman primates. Nucl Med Biol. 2016;43:391–396. doi: 10.1016/j.nucmedbio.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Yao Z, Garmestani K, Wong KJ, Park LS, Dadachova E, Yordanov A, Waldmann TA, Eckelman WC, Paik CH, Carrasquillo JA. Comparative cellular catabolism and retention of astatine-, bismuth-, and lead-radiolabeled internalizing monoclonal antibody. J Nucl Med. 2001;42:1538–1544. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11585870] [PubMed] [Google Scholar]
- 46.Rogers BE, Franano FN, Duncan JR, Edwards WB, Anderson CJ, Connett JM, Welch MJ. Identification of metabolites of 111In-diethylenetriaminepentaacetic acid-monoclonal antibodies and antibody fragments in vivo. Cancer Res. 1995;55:5714s–5720s. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7493333] [PubMed] [Google Scholar]
- 47.Hall EJ. The Bystander Effect. Health Phys. 2003;85:31–35. doi: 10.1097/00004032-200307000-00008. [ https://www.ncbi.nlm.nih.gov/pubmed/12852468] [DOI] [PubMed] [Google Scholar]
- 48.Hall EJ, Hei TK. Genomic instability and bystander effects induced by high-let radiation. Oncogene. 2003;22:7034–7042. doi: 10.1038/sj.onc.1206900. [DOI] [PubMed] [Google Scholar]
- 49.Nagar S, Smith LE, Morgan WF. Characterization of a novel epigenetic effect of ionizing radiation: the death-inducing effect. Cancer Res. 2003;63:324–328. [ https://www.ncbi.nlm.nih.gov/pubmed/12543783] [PubMed] [Google Scholar]
- 50.ImClone LLC; Bridgewater, NJ: 2016. Erbitux (cetuximab) [package insert] [Google Scholar]
- 51.Messersmith WA, Hidalgo M. Panitumumab, a monoclonal anti epidermal growth factor receptor antibody in colorectal cancer: another one or the one? Clin Cancer Res. 2007;13:4664–4666. doi: 10.1158/1078-0432.CCR-07-0065. [DOI] [PubMed] [Google Scholar]
- 52.O'Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, Goldberg RM. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 53.Liu Z, Liu Y, Jia B, Zhao H, Jin X, Li F, Chen X, Wang F. Epidermal growth factor receptor-targeted radioimmunotherapy of human head and neck cancer xenografts using 90Y-labeled fully human antibody panitumumab. Mol Cancer Ther. 2010;9:2297–2308. doi: 10.1158/1535-7163.MCT-10-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakobovits A, Amado RG, Yang X, Roskos L, Schwab G. From xenomouse technology to panitumumab, the first fully human antibody product from transgenic mice. Nat Biotechnol. 2007;25:1134–1143. doi: 10.1038/nbt1337. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17921999] [DOI] [PubMed] [Google Scholar]
- 55.Chastagner P, Merlin JL, Marchal C, Hoffstetter S, Barberi-Heyob M, Vassal G, Duprez A. In vivo potentiation of radiation response by topotecan in human rhabdomyosarcoma xenografted into nude mice. Clin Cancer Res. 2000;6:3327–3333. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10955820] [PubMed] [Google Scholar]
- 56.Guichard S, Montazeri A, Chatelut E, Hennebelle I, Bugat R, Canal P. Schedule-dependent activity of topotecan in OVCAR-3 ovarian carcinoma xenograft: pharmacokinetic and pharmacodynamic evaluation. Clin Cancer Res. 2001;7:3222–3228. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=11595718] [PubMed] [Google Scholar]
- 57.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. 212Pb-radioimmunotherapy induces G(2) cell-cycle arrest and delays DNA damage repair in tumor xenografts in a model for disseminated intraperitoneal disease. Mol Cancer Ther. 2012;11:639–648. doi: 10.1158/1535-7163.MCT-11-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. 212Pb-radioimmunotherapy potentiates paclitaxel-induced cell killing efficacy by perturbing the mitotic spindle checkpoint. Br J Cancer. 2013;108:2013–2020. doi: 10.1038/bjc.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. Sensitization of tumor to 212Pb-radioimmunotherapy by gemcitabine involves initial abrogation of G2 arrest and blocked DNA damage repair by interference with Rad51. Int J Radiat Oncol Biol Phys. 2013;85:1119–1126. doi: 10.1016/j.ijrobp.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. Impact of α-targeted radiation therapy on gene expression in a pre-clinical model for disseminated peritoneal disease when combined with paclitaxel. PLoS One. 2014;9:e108511. doi: 10.1371/journal.pone.0108511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. Cell killing mechanisms and impact on gene expression by gemcitabine and 212Pb-trastuzumab treatment in a disseminated i.p. tumor model. PLoS One. 2016;11:e0159904. doi: 10.1371/journal.pone.0159904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yong KJ, Milenic DE, Baidoo KE, Brechbiel MW. Mechanisms of cell killing response from low linear energy transfer (LET) radiation originating from 177Lu-radioimmunotherapy targeting disseminated intraperitoneal tumor xenografts. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17050736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yong KJ, Milenic DE, Baidoo KE, Kim YS, Brechbiel MW. Gene expression profiling upon 212Pb-TCMC-Trastuzumab treatment in the LS-174T i.p. xenograft model. Cancer Med. 2013;2:646–653. doi: 10.1002/cam4.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horan Hand P, Colcher D, Salomon D, Ridge J, Noguchi P, Schlom J. Influence of spatial configuration of carcinoma cell populations on the expression of a tumor-associated glycoprotein. Cancer Res. 1985;45:833–840. [ http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=3881173] [PubMed] [Google Scholar]
- 65.Wong KJ, Baidoo KE, Nayak TK, Garmestani K, Brechbiel MW, Milenic DE. In vitro and in vivo pre-clinical analysis of a F(ab')2 fragment of panitumumab for molecular imaging and therapy of HER1 positive cancers. EJNMMI Res. 2011;1 doi: 10.1186/2191-219X-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yook S, Cai Z, Lu Y, Winnik MA, Pignol JP, Reilly RM. Radiation nanomedicine for EGFR-positive breast cancer: panitumumab-modified gold nanoparticles complexed to the beta-particle-emitter, 177Lu. Mol Pharm. 2015;12:3963–3972. doi: 10.1021/acs.molpharmaceut.5b00425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.