Abstract
Background
Pulmonary histoplasmosis is a fungal infection caused by histoplasma capsulatum, rarely diagnosed in non endemic areas and/or immunocompromised patients. Complication of pulmonary histoplasmosis with bronchocentric granulomatosis is extremely rare.
Case Report
A 48-year-old man with prolonged fever and nausea was admitted to our hospital. Clinical examination revealed pathological auscultatory sounds to the left lung. Computed tomography was performed and revealed a large solid mass of the left upper lobe, limited pleural and pericardial effusion and calcified lymphadenopathy of mediastinum. A computed tomography guided core biopsy of the lung lesion was performed and three samples were obtained. Culture and polymerase chain reaction (PCR) revealed Histoplasma capsulatum. Histological findings were compatible with bronchocentric granulomatosis. Extended laboratory investigation excluded immunosuppresion. Our patient although immunocompetent was diagnosed with chronic pulmonary histoplasmosis complicated with bronchocentric granulomatosis and treatment with antifungal medication and methylprednisoline started.
Conclusion
Description of a rare case of chronic pulmonary histoplasmosis in a non endemic area like Greece, with atypical radiological findings, complicated with bronchocentric granulomatosis.
Keywords: Pulmonary histoplasmosis, Bronchocentric granulomatosis, Non endemic area
1. Introduction
Histoplasmosis is caused by a dimorphic fungus which grows in soil fertilized by bird and bat excreta, Histoplasma capsulatum. It can be found in temperate climates worldwide, but it is endemic in the United States, primarily seen in the Ohio and Mississippi River Valleys. While the central United States seemed to have the greatest prevalence of Histoplasmosis capsulatum Brazil, Argentina, India, West Africa and South Africa have all reported small case series. Additionally, sporadic cases have been reported from Central and South America, Oceania, northern sub-Saharan Africa, and Europe (Italy, Po River valley) [1]. To our knowledge, none case has ever been reported in Greece since now. Clinical presentations include asymptomatic pulmonary histoplasmosis, acute diffuse pulmonary histoplasmosis, chronic pulmonary histoplasmosis, disseminated histoplasmosis, endobronchial histoplasmosis, fibrosing mediastinitis, mediastinal granulomas and lung nodules.
Bronchocentric granulomatosis (BG) was first described by Liebow in 1973. This entity represents a common pathological result of a heterogeneous group of diseases and does not represent a single clearly defined clinical syndrome [2], [3]. Exclusive peribronchial localization of granulomas can occur in infections and may cause confusion with bronchocentric granulomatosis. Diagnosis is only established with biopsy.
2. Case report
A 48-year-old man was admitted to our hospital due to prolonged fever and nausea. Physical examination indicated the following: Body temperature, 38.5 °C; heart rate, 109 beats/min; blood pressure, 140/80 mmHg and lack of ausculatory sounds in the upper lobe of the left lung. Laboratory examination performed revealed: normal white blood cell count, 8.5 × 109/l, an elevated C reactive protein, 7mg/dl (0.01–0.8mg/dl) and negative tuberculosis antibody test. Computed tomography (CT) showed a large solid mass of the left upper lobe, limited pleural and pericardial effusion and calcified lymphadenopathy of mediastinum, Fig. 1. The patient underwent a CT-guided core biopsy of the lung lesion and three samples were obtained. Specimens were sent for culture, histological and polymerase chain reaction (PCR) assay. The possibility of malignancy was excluded, at the same time culture and PCR were positive for Histoplasma capsulatum and histological findings were compatible with BG, Fig. 2. Due to the positive culture we investigated patient's traveling history and he referred traveling in United States many years ago just for a few days.
Fig. 1.
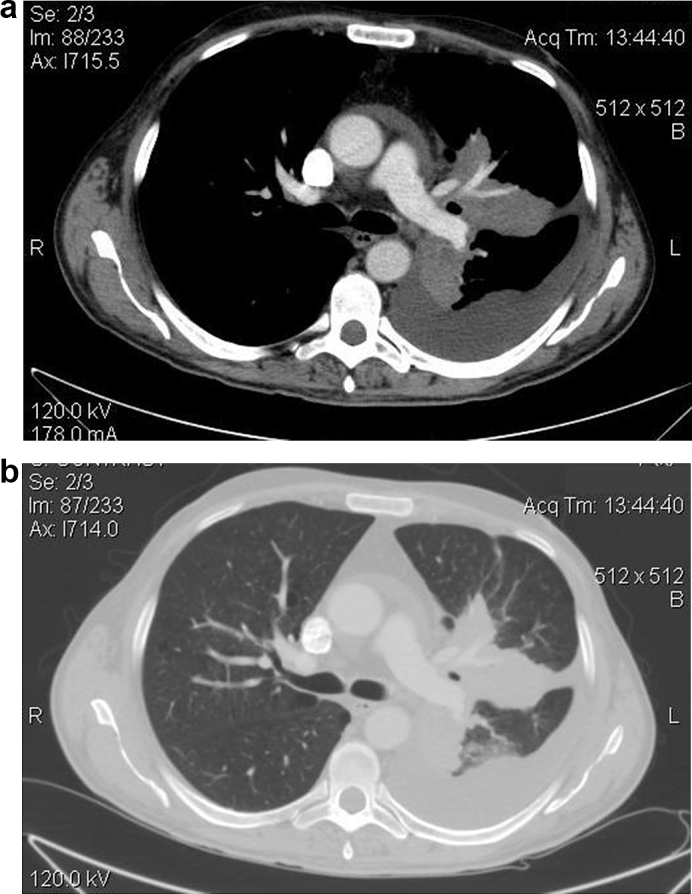
a-b. CT image revealing large solid mass of the left upper lobe, limited pleural and pericardial effusion and calcified lymphadenopathy of mediastium.
Fig. 2.
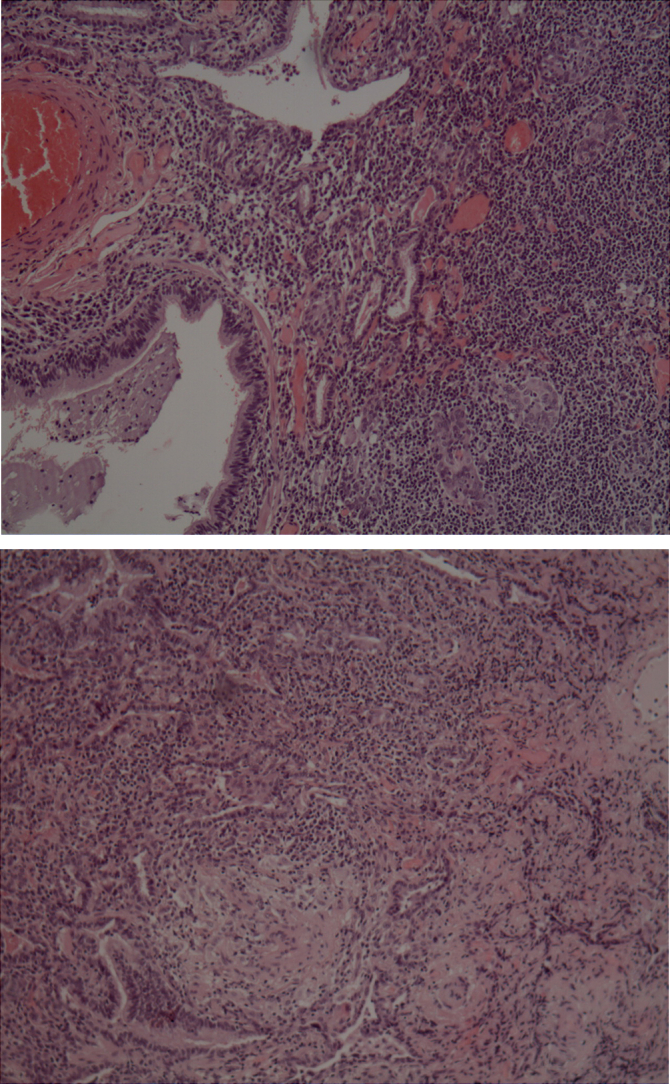
Lung biopsy histopathological findings compatible with Bronchocentric granulomatosis. Necrotizing granulomas with bronchocentric localization.
Complete laboratory investigation excluded any kind of immunosuppresion in our patient.
The patient was diagnosed as a chronic pulmonary histoplasmosis case with bronchogenic granulomatosis. Initially he was treated with amphotericin B and itraconazole. One month later he revealed complete clinical recovery, improvement of the inflammatory index and decrease of solid mass size, nevertheless, pleural and pericardial effusions increased. Having in mind the histological diagnosis of bronchocentric granulomatosis and the aggravation of effusions, corticosteroids were added to the medication. Our patient was discharged two weeks later, in a good clinical status with itraconazole and methylprednisolone.
CT imaging follow up one month later demonstrated a great decrease of the left lobe mass size. No pleural and pericardial effusion was reported Fig. 3.
Fig. 3.
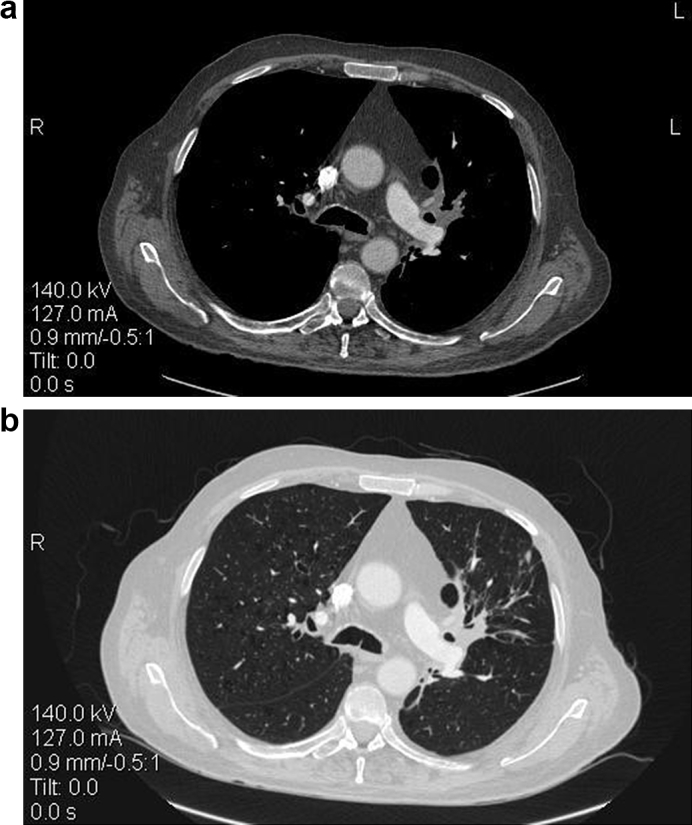
a-b. Follow up with CT two months after treatment reveals great decrease of the lung lesion. None pleural and pericardial effusion is reported.
3. Discussion
Histoplasmosis is not an endemic illness in Greece. The most common cause for histoplasmosis in non-endemic areas is prior exposure to endemic areas, through travel or residence. Indeed, our patient had visited America many years ago, but he never referred any clinical signs or symptoms of histoplasmosis. By 2015, only 76 cases had been reported in the literature. Among these patients, 52 cases were found to present increased risk factors or primary diseases. The majority of healthy persons infected with H. capsulatum develop subclinical infection or a self-limiting respiratory disease. Severe or disseminated forms of histoplasmosis usually occur in immunocompromised individuals, particularly in HIV patients [4], [5]. In our patient immunosuppresion was excluded after extended laboratory investigation however he suffered from severe chronic pulmonary form of the disease. Imaging manifestations of pulmonary histoplasmosis vary depending on the disease chronicity. At the acute stage it may be diagnosed as airspace shadowing with consolidation involving multiple lung segments or lobes similar to bacterial pneumonia, well-defined nodules with central calcifications while pleural effusions are possible but uncommon, [6]. Chronic pulmonary histoplasmosis is characterized in 90% by cavitations, predominantly in the upper lobes while hilar lymphadenopathy is rare. Fibrosing mediastinitis follows acute pulmonary histoplasmosis in a small number of patients and is not directly related to mediastinal lymphadenopathy. Progressive superior vena cava obstruction, narrowing of pulmonary arteries or veins and narrowing of the major airways is common on detailed assessment [4]. Underlying emphysematous changes are common.
Unlikely with the literature our patient was diagnosed with large solid mass of the left upper lobe, pleural and pericardial effusion and calcified lymphadenopathy of mediastinum, atypical and rare imaging findings for chronic pulmonary histoplasmosis.
Lung tissue biopsy and fungal culture have been widely recognized as the gold standards for diagnosing pulmonary histoplasmosis. CT-guided percutaneous lung biopsy is recommended to obtain histopathologic evidence and enhance the diagnostic accuracy when the diagnosis is ambiguous and undetermined [7]. In our patient culture for fungi was performed on two tubes of Sabouraud's dextrose agar, one incubated at room temperature and the other at 37 °C. As fungal culture endured for at least 3 weeks and the positive rate is not high; its clinical application is limited. Although culture was positive diagnosis in our case was confirmed in only 3 days with a positive PCR of the tissue sample.
Tissue biopsy results may reveal the presence of yeast forms in tissue through hematoxylin and eosin staining, Best visualization of H. capsulatum is obtained with methenamine silver or periodic acid-Schiff stains. Using the Grocott-Gomori methenamine-silver procedure, yeast may be detected in areas of caseation necrosis from histoplasmomas and calcified lymph nodes. Yeast forms in circulating neutrophils and monocytes are rarely detected using Wright-Giemsa staining. Yeasts are typically found within macrophages, most biopsies do not reveal organisms. In our patient hematoxylin staining was performed and none yeast form was observed.
Bronchocentric granulomatosis represents a common pathological result of a heterogeneous group of diseases. It is characterized by necrotizing granulomatous inflammation of bronchial and bronchiolar epithelium with chronic inflammatory changes in the surrounding lung parenchyma. Patients with BG are divided in two groups, the first including the majority of patients, are individuals with asthma and allergic bronchopulmonary aspergillosis and the second the non-asthmatic patients which are usually older and tissue eosinophilia is not a predominant finding. It is though that these cases are idiopathic, although associations with mycobacterial and fungal infection, rheumatologic disease, angiitis, chronic granulomatosis disease and other conditions have been reported [3]. Such a case is the patient we describe as the histological findings were compatible with BG and no medical history of asthma was reported [8]. BG in our case was considered to be an immunologic reaction against endobronchial fungal antigens. According to the literature he was treated with short-term corticosteroids and had a favourable overall prognosis.
We described a rare case of histoplasmosis as it is reported in a non endemic area, with a severe clinical presentation of chronic pulmonary histoplasmosis although our patient had no immunosuppresion. Furthermore imaging manifestations were atypical for the diagnosis and histopathological findings revealed complication with bronchocentric granulomatosis, entity rare in non asthmatic patients.
Conflict-of-interest statement
All authors declare any conflict interest.
References
- 1.Joseph Wheat L. Histoplasmosis: a review for clinicians from non-endemic areas. Mycoses. 2006;49(4):274–282. doi: 10.1111/j.1439-0507.2006.01253.x. [DOI] [PubMed] [Google Scholar]
- 2.Myers Jeffrey L. Bronchocentric granulomatosis. Chest. 1989;96(1):3–4. doi: 10.1378/chest.96.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Clee M.D., Lamb D., Clark R.A. Bronchocentric granulomatosis: a review and thoughts on pathogenesis. Br. J. Dis. Chest. 1983;77:227–234. [PubMed] [Google Scholar]
- 4.Kauffman C.A. Histoplasmosis: a clinical and laboratory update. Clin. Microbiol. Rev. 2007;20(1):115–132. doi: 10.1128/CMR.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu Cui, Wang Ge, Chen Qiong, He Bixiu, Wang Lijing. Pulmonary histoplasmosis in a immunocompetent patient: a case report and literature review. Exp. Ther. Med. 2016 Nov;12(5):3256–3260. doi: 10.3892/etm.2016.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L.F., Guo L., Zhu M. CT features of primary pulmonary histoplasmosis. Lin. Chuang Fang. She Xue Za Zhi. 2012;31:1727–1729. [Google Scholar]
- 7.Cai R.Z., Li G., Cai Y.Q. Misdiagnosis of pulmonary histoplasmosis: one case report and literature review. Lin. Chuang Hui Cui. 2012;27:1912–1913. [Google Scholar]
- 8.Wheat L. Joseph, Freifeld Alison G., Kleiman Martin B., Baddley John W., McKinsey David S., Loyd James E., Kauffman Carol A. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the infectious diseases society of America. Clin. Infect. Dis. 2007;45(7):807–825. doi: 10.1086/521259. [DOI] [PubMed] [Google Scholar]


