Abstract
Introduction
Brain surgery in the language dominant hemisphere remains challenging due to unintended post-surgical language deficits, despite using pre-surgical functional magnetic resonance (fMRI) and intraoperative cortical stimulation. Moreover, patients are often recommended not to undergo surgery if the accompanying risk to language appears to be too high. While standard fMRI language mapping protocols may have relatively good predictive value at the group level, they remain sub-optimal on an individual level. The standard tests used typically assess lexico-semantic aspects of language, and they do not accurately reflect the complexity of language either in comprehension or production at the sentence level. Among patients who had left hemisphere language dominance we assessed which tests are best at activating language areas in the brain.
Method
We compared grammar tests (items testing word order in actives and passives, wh-subject and object questions, relativized subject and object clauses and past tense marking) with standard tests (object naming, auditory and visual responsive naming), using pre-operative fMRI. Twenty-five surgical candidates (13 females) participated in this study. Sixteen patients presented with a brain tumor, and nine with epilepsy. All participants underwent two pre-operative fMRI protocols: one including CYCLE-N grammar tests (items testing word order in actives and passives, wh-subject and object questions, relativized subject and object clauses and past tense marking); and a second one with standard fMRI tests (object naming, auditory and visual responsive naming). fMRI activations during performance in both protocols were compared at the group level, as well as in individual candidates.
Results
The grammar tests generated more volume of activation in the left hemisphere (left/right angular gyrus, right anterior/posterior superior temporal gyrus) and identified additional language regions not shown by the standard tests (e.g., left anterior/posterior supramarginal gyrus). The standard tests produced more activation in left BA 47. Ten participants had more robust activations in the left hemisphere in the grammar tests and two in the standard tests. The grammar tests also elicited substantial activations in the right hemisphere and thus turned out to be superior at identifying both right and left hemisphere contribution to language processing.
Conclusion
The grammar tests may be an important addition to the standard pre-operative fMRI testing.
Abbreviations: fMRI, functional magnetic resonance imaging; CYCLE-N, Curtiss-Yamada Comprehensive Language Evaluation: Neurological Measures; LH, left hemisphere; RH, right hemisphere
Keywords: Language, Grammar, fMRI, Brain mapping, Surgery, Tumor, Epilepsy
Highlights
-
•
We added comprehensive grammar tests to standard presurgical fMRI of language.
-
•
The grammar tests generated more volume of activation bilaterally.
-
•
The tests identified additional language regions not shown by the standard tests.
-
•
The grammar tests may be an important addition to standard pre-operative fMRI.
1. Introduction
1.1. Challenges of clinical language mapping
While most agree that the ability to communicate is critical to patient outcome after surgery, little attention is given to the complexity of language structures in clinical mapping procedures (Połczyńska, 2009, Połczyńska et al., 2014, Rofes and Miceli, 2014). The goal of this study is to evaluate whether including an assessment of grammar comprehension and production in clinical language functional magnetic resonance imaging (fMRI) can provide us with additional areas of activation in the language network and to compare these results with a standard fMRI testing protocol.
An increasing number of centers use functional MRI because it is a particularly valuable and non-invasive method assessing language organization in the brain (e.g., Sabsevitz et al., 2003, Połczyńska et al., 2015, Połczyńska et al., 2016). Frequently used language tests involve a wide range of lexical-semantic tasks, e.g., object naming, auditory responsive naming and word generation (Bookheimer, 2007, Fernández Coello et al., 2013, Wang et al., 2012). However, there is no single established protocol for pre-surgical language fMRI.
Presurgical language mapping remains sub-optimal. In our clinical practice patients can be denied surgery if a lesion is in close proximity to eloquent language sites because the procedure could result in new, pronounced language deficits. Brain surgeries carry a risk of new postoperative language deficits (Sabsevitz et al., 2003, Wilson et al., 2015). In a recently-completed survey we found approximately 25% of responding epilepsy programs reported one or more instances where a patient experienced a persisting (> 3 months) postoperative language deficit in spite of preserving all areas that were positive with pre-operative language fMRI (Benjamin et al., 2015). Neurosurgical language evaluations typically do not account for particular aspects of grammar (Połczyńska, 2009, Połczyńska et al., 2014). Without mapping grammar, patients may suffer post-operative language deficits (Rofes and Miceli, 2014). This is because grammar and lexico-semantic aspects of language have a partially segregated representation at the neural and behavioral level in adults (Ardila, 2011, Friederici, 2011, Jackendoff, 2007, Rodd et al., 2015, Skeide et al., 2014). The ability to name objects can be spared in the face of impaired action naming or grammatical processing (e.g., Miceli et al., 1984, Hillis et al., 2002, Mätzig et al., 2009, Rofes et al., 2015a). Moreover, severely impaired production of verbal morphology may be accompanied by an intact ability to produce nominal morphology (e.g., Shapiro and Caramazza, 2003, Tsapkini et al., 2002).
Ojemann and Mateer (1979) were the first to use direct cortical electrical stimulation to identify areas of the brain that were exclusively devoted to more complex aspects of language involving syntax. Since then there have been only a few studies that investigated aspects of grammar in the clinical language mapping context (Ojemann and Mateer, 1979, Hamberger et al., 2003, Roux et al., 2003, Bello et al., 2007, Papagno et al., 2011, De Witte et al., 2015, Lubrano et al., 2014; see also a review by Rofes and Miceli, 2014, Rofes et al., 2015b). Those studies have examined and even mapped specific tasks to specific brain regions. Below are examples of grammar tests used in those studies. In some cases tasks were labeled as “syntactic” or “grammar” but in fact were lexical tasks:
-
(1)
Object naming – a naming to picture test included in standard protocols, not a syntactic test,
-
(2)
Auditory responsive naming – naming object to oral description. If the task contains a verb (e.g., “it tells time” for “a watch”), it taps on verb processing. Yet, this is not a syntactic task.
-
(3)
Action naming – evaluates single word verb production, with only third person singular verb forms required. Since no other forms were used, subject-verb agreement was not really being tested, except in this very limited sense,
-
(4)
Verb generation – assesses only the ability to produce a single word, one that is semantically associated with a singular noun. This is not a syntactic test,
-
(5)
Syntactic fluency – a lexical task, not one that tests knowledge of syntax structure. The only syntactic aspect of the test is in requiring knowledge of the lexical category (noun, verb) of a word. Moreover, accessing verbs is very different from using verbs in sentences.
Examples of tasks tackling grammatical aspects of language applied in those studies are:
-
(1)
Naming finite verbs – a sentence frame is provided. The subject has to complete the sentence with the correctly inflected verb,
-
(2)
Sentence-completion – requires the ability to process the sentence frame given and complete it with a syntactically correct form of the word,
-
(3)
Syntactic sentence judgment – requires the participant to assess whether a sentence containing a given syntactic structure is correct or not,
-
(4)
Sentence comprehension – requires indicating which picture corresponds to the sentence heard or read (e.g., “a man poking a woman versus a woman poking a man”). The task typically assesses comprehension of reversible active versus passive voice word order.
1.2. Assessment of grammar
Grammar refers to the implicit knowledge of what can be a well-formed word, phrase or sentence that then allows one to produce, comprehend and judge the grammaticality of words and their combinations. Grammar goes beyond simple word meaning and more accurately reflects and comprises the complexity of human language. Grammar is subserved in part by procedural (implicit) memory in contrast to lexical knowledge that is subserved by declarative memory (Ullman, 2001; see also a review by Perani and Abutalebi, 2005). Those two systems can be selectively impaired, as evidenced, e.g., by studies on dementia that report lexical disturbances but few morpho-syntactic impairments (Kempler et al., 1987, Léger and Jonhnson, 2007; but see Wilson et al., 2012). Testing grammar thus not only offers a fuller picture of language function, but an essential component of that picture. Grammar includes (1) syntax—the rules and constraints that govern word order in phrases and sentences, and (2) morphology—processes that, in part, govern affixation: inflections added to word stems, e.g., adding tense to verbs, such as, sign-signed where sign is the stem and –ed is the inflection. Under the most current version of minimalist theory, morphology is completely subsumed under syntax, and thus, inflection is syntax (e.g., Sportiche et al., 2013).
Assessing grammar in people with brain tumors is relevant because inflections can be selectively disturbed, while the ability to generate word stems is preserved (Miozzo et al., 2010). In the left hemisphere (LH), syntax engages a wide range of areas. Based on lesion and neuroimaging studies areas implicated in frontal cortex include the operculum, inferior frontal gyrus (BA 47, 45, 44) and mid-frontal (BA 46) cortex; temporal regions implicated include the anterior and posterior superior temporal gyrus as well as posterior middle temporal gyrus, and the superior temporal sulcus; parietal regions include the angular and supramarginal gyri as well as superior parietal cortex and precuneus (den Ouden et al., 2012, Dronkers et al., 2004, Grodzinsky and Friederici, 2006, Hickok and Rogalsky, 2011, Newman et al., 2010, Turken and Dronkers, 2011, Tyler et al., 2013, Wright et al., 2012). Inflectional morphology recruits left inferior frontal areas (Justus et al., 2011, Ullman, 2001), though the non-dominant right hemisphere (RH) may also play an important role (Grodzinsky and Friederici, 2006, Pulvermüller, 2010).
In Połczyńska et al. (2014) we added grammar tests to standard lexico-semantic tasks during the recovery phase of the Wada test. The results showed that the grammar tests (syntax and morphology) were superior at lateralizing language function to the dominant LH (p = 0.01), compared to the standard tests (p = 0.2). Because grammar tests elucidate the complexity of language rather than concentrating on word knowledge, they may be more sensitive in identifying core aspects of communication that are not normally detected by current testing, e.g., inability to form and/or understand sentences, such as in The girl who the boy is pushing is wearing yellow. This sentence requires understanding who the subject and the object of the main clause are and which of these the relative clause modifies, as well as knowing that the object in the relative clause has been moved.
1.3. Anterior versus posterior language areas
In our clinical practice we found that different tasks differentially activate more anterior (i.e., Broca's) versus more posterior (i.e., Wernicke's) areas, such as tasks requiring production versus language comprehension, respectively. Task differences in Broca's versus Wernicke's region have also been shown in the literature. For example, lexico-semantic tasks, such as auditory responsive naming have been shown to activate the frontal language areas (orbital frontal areas; Gaillard et al., 2004). We also found using Wada testing that some patients have mixed language dominance, where expressive and receptive language is located in different hemispheres. Furthermore, we found that the standard lexico-semantic tests generate higher fMRI activations in anterior as compared to posterior language sites. In particular, the standard lexico-semantic tasks activate the frontal language areas, e.g., an auditory responsive naming task has been shown to activate the orbital frontal regions. We typically do not see much neural activity outside Wernicke's area in the left posterior language regions, such as, e.g., the angular gyrus, supramarginal gyrus, or posterior middle temporal gyrus (e.g., Bookheimer, 2007, Połczyńska et al., 2016). A recent study by Ivanova et al. (2016) demonstrated that the integrity of the more posterior segments of the major language tracts in the dominant left hemisphere (e.g., the inferior fronto-occipital fasciculus) was strongly related to performance in grammar. Further, the majority of surgical candidates undergoing language fMRI have a lesion neighboring either Broca's or Wernicke's area. Therefore, it should be useful, even necessary, to analyze those regions separately in order to verify which language tests (lexico-semantic or grammar) best engage anterior and posterior language areas. Hence, we chose to divide language areas into anterior and posterior ones.
1.4. Hypothesis
In this study we used a comprehensive grammar protocol in pre-surgical language fMRI in epilepsy and tumor patients. We investigated aspects of grammar that are particularly vulnerable to brain pathology: syntactic movement (in relative clauses and questions) and inflectional morphology, particularly Tense (Linebarger et al., 1983, Grodzinsky and Finkel, 1998, van der Lely, 1998, Friedmann, 2001, Bastiaanse and Thompson, 2003, Edwards and Varlokosta, 2007, Friedmann et al., 2010, Shetreet and Friedmann, 2014). Since this work is hypothesis-driven, we focused on regions that were damaged in those studies. We thus selected nine language regions of interest (ROI) in each hemisphere: four anterior (BA 44, BA 45, BA 47 and the anterior superior temporal gyrus) and five posterior (the posterior middle temporal gyrus, posterior superior temporal gyrus, anterior and posterior supramarginal gyrus and angular gyrus). The regions were also indicated in studies using a full-brain analysis (e.g., Gaillard et al., 2004, Friederici et al., 2000, Bornkessel et al., 2005). We chose the ROI approach because we did not want to correct for the whole brain in our analysis. We know that other language regions (e.g., the visual cortex) are irrelevant for the language processes we tested, and power is a problem. We hypothesized that grammar tests would produce more volume of activation in the LH, both in anterior and posterior language areas. Since the grammar process is strongly left-lateralized (e.g., Połczyńska et al., 2014), we are expecting to see far less activation in the RH. Studies on split-brain individuals have shown that the RH performed at chance level even on semantically reversible subject-verb-object (active declarative) sentences (Gazzaniga and Hillyard, 1971). If, however, there is more substantial activity in the RH, it might be caused by functional compensation (Deng et al., 2015, Thiel et al., 2006). In that case we should see differences between LH and RH lesion patients with the former group showing more volume of activation in the RH. To our knowledge the current study is the first to investigate the neuroarchitecture of specific aspects of morpho-syntax via research and theoretically motivated grammar comprehension and production items with fMRI in surgical candidates.
2. Materials and methods
2.1. Subjects
Twenty-five patients (13 females; 16 epilepsy, 9 brain tumors) participated in the study (see Table 1). A total of 47 patients with brain tumor or epilepsy participated. Twenty-two patients were excluded due to excessive movement in the scanner (N = 17) or RH dominance on the standard language tasks and/or Wada testing (N = 5). Mean age was 38.8 years (± 11.7). Eighteen patients had LH lesions and seven had RH lesions. Twenty subjects were right-handed; four left-handed; and one was ambidextrous. Six patients had previously undergone resections to treat their epilepsy/brain tumor. Fourteen participants had mild or moderate aphasia on standard presurgical neurocognitive testing and/or on a pre-fMRI interview. Due to the treatment urgency of most of our tumor patients (the needs of particular patients were sometimes inconsistent with getting formal testing), we were only able to obtain results from formal neurocognitive assessments for 4 of 25 patients. Assessment included assessment of language, verbal executive ability, working memory and attention (Boston Naming Test-II; Boston Diagnostic Aphasia Exam (BDAE), BDAE Complex Ideational Material; Controlled Oral Word Association test: letters (F, A, S), category (animals); Wechsler Adult Intelligence Scale IV Digit span and Vocabulary; Woodcock Johnson-III Word Attack). The assessment was conducted at the UCLA neuropsychology clinic.
Table 1.
Patient demographics. E = epilepsy, T = tumor, L = left, R = right, Y = yes, N = no.
| Patient # | Etiology | Lesion | Lobe | Sex | Age | Years of education | Handedness | Previous surgery | Language deficits |
|---|---|---|---|---|---|---|---|---|---|
| 1 | E | L | Temporal | M | 37 | 12 | L | Y | Y |
| 2 | E | L | Temporal | M | 38 | 12 | R | Y | Y |
| 3 | E | L | Temporal | M | 23 | 12 | L | Y | Y |
| 4 | E | L | Temporal | F | 31 | 12 | L | N | N |
| 5 | E | R | Temporal | F | 49 | 12 | R | N | N |
| 6 | E | L | Temporal | F | 48 | 14 | R | N | N |
| 7 | E | L | Temporal | M | 56 | 12 | R | N | Y |
| 8 | E | L | Temporal | F | 21 | 16 | R | N | N |
| 9 | E | L | Temporal | F | 40 | 13 | R | Y | N |
| 10 | T | L | Fronto-temporal | F | 44 | 18 | R | Y | Y |
| 11 | T | L | Frontal | F | 26 | 18 | R | N | N |
| 12 | T | R | Temporal | M | 36 | 12 | R | N | N |
| 13 | T | R | Temporal | F | 58 | 12 | R | N | Y |
| 14 | T | L | Temporo-parietal | M | 26 | 16 | R | N | Y |
| 15 | T | L | Temporal | M | 35 | 16 | R | N | Y |
| 16 | T | R | Fronto-parietal | M | 31 | 12 | R | N | N |
| 17 | T | L | Temporal | F | 27 | 14 | R | Y | N |
| 18 | T | L | Temporal | F | 22 | 12 | R | N | Y |
| 19 | T | L | Frontal | M | 27 | 12 | R | N | N |
| 20 | T | R | Fronto-temporal | F | 48 | 16 | R | N | N |
| 21 | T | L | Frontal | M | 36 | 14 | R | N | Y |
| 22 | T | R | Fronto-temporal | M | 51 | 12 | R | N | Y |
| 23 | T | R | Parietal | M | 49 | 16 | L | N | Y |
| 24 | T | L | Temporo-parietal | F | 39 | 18 | R | N | Y |
| 25 | T | L | Temporal | F | 60 | 20 | A | N | Y |
Average age of seizure onset in the epilepsy subjects was 24.7 years (± 12.2). All participants had an adult seizure onset, with the exception of one patient who had his first seizure at age seven. The participants received direct instruction and task practice prior to beginning the fMRI session. Only participants who were able to complete the practice run were included in the study.
2.2. fMRI
2.2.1. fMRI tasks
2.2.1.1. The standard tests
The participants performed three standard language tests:
-
(1)
Object naming (the patient looked at a black and white drawing of a concrete object and thought of its name, e.g., a watch, a sock),
-
(2)
Auditory responsive naming (the patient heard a phrase, e.g., “wear them on feet” and thought of the word being described,
-
(3)
Visual responsive naming (reading: the patient read a phrase, e.g., “color of the sky” and thought of the word being defined) (e.g., Gaillard et al., 2004).
2.2.1.2. The CYCLE-N
Next, the participants performed seven grammar tasks from the CYCLE-N (an adaptation of a well-validated clinical instrument for grammar evaluation, the CYCLE; Curtiss and Yamada, 2004). The CYCLE-N evaluates aspects of grammar that are known to be particularly vulnerable to brain damage (Bastiaanse and Thompson, 2003, Edwards and Varlokosta, 2007, Friedmann, 2001, Friedmann et al., 2010, Linebarger et al., 1983, Shetreet and Friedmann, 2014, van der Lely, 1998). The test uses pictures that can be interpreted by very young children (even those suffering from substantial cognitive deficits) and adults with progressive dementia (Curtiss and Yamada, 2004; CYCLE manual). The vocabulary used in the CYCLE-N fMRI tasks consists of highly frequent nouns and verbs. The same vocabulary items are used throughout the test, so that one can rule out knowing the vocabulary involved as a reason for poor performance on a specific set of items. The CYCLE-N includes both “simple” structures, such as marking plural or tense to more complex grammatical structures (e.g., relative clauses and movement), such as those that involve movement of parts of a sentence (“constituents”) from their original position to another position in their clause (e.g., moving the direct object of the verb in a clause, such as “the girl” in the clause “the girl who the boy is pulling” where “the girl” is the direct object of the verb “pull” and would follow the verb in the original form of the clause, which would be “the boy is pulling the girl”). The test uses minimal pair sentences that differ only in morphosyntax, e.g., “Which girl is pulling the boy? versus Which girl is the boy pulling?”. We tested both comprehension and production because the two language modalities have been shown to engage, in part, distinct language networks (Neuhaus and Penke, 2008).
A subset of CYCLE-N items was selected for this study to balance assessment with time constraints. The participants first underwent a preliminary assessment that involved all the grammar aspects tested in the scanner (N = 7). We used three test items per each grammatical structure (total N of test items = 21). Pre-testing used stimuli not applied during MR imaging. After that, participants underwent fMRI imaging with comprehension and production tests. In the grammar production tests the participants were asked to silently finish a sentence that described pictures presented on a screen (Fig. 1a and d). We administered three production tests with 16 sentences each. In the grammar comprehension tests the subjects were asked to (a) look at two pictures and silently choose the one that matched a sentence they heard (three tests, 16 sentences each) (Fig. 1b), or (b) silently answer a question about a picture they were looking at (one test, 16 sentences) (see Fig. 1c; see Table 2 for the distribution of production and comprehension tests). The grammar tasks evaluated:
-
(1)syntax:
-
(a)reversible active and passive sentences,
-
(b)single clause “which-X” subject and object questions,
-
(c)relativized subject and object clauses,
-
(a)
-
(2)
morphology: irregular and regular past tense marking on frequent and infrequent verbs (see Table 2 for examples of specific grammar structures).
Fig. 1.
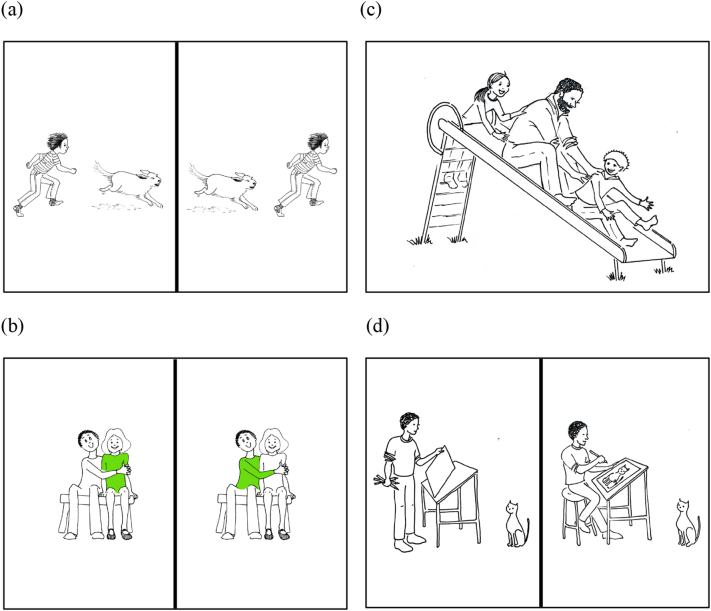
Sample fMRI stimuli from the CYCLE-N: (a) Production test for passive voice. The subject is instructed to look at two pictures and finish a sentence they hear: Here the boy is chasing the dog but here the boy…, (b) Comprehension test for relativized object clauses. The subject is looking at two pictures and chooses one that matches a sentence they hear: The girl who the boy is hugging is wearing green, (c) Comprehension test for “wh”-subject questions. The subject is looking at the picture and silently answers a question: Which person is pushing the man?, and (d) Production test for regular and irregular past tense. The subject is instructed to look at the pictures and finish a sentence they hear: Here the boy is about to paint a picture but here he already….
Table 2.
Design of the CYCLE-N.
| Grammar aspect | Syntax or morphology | Comprehension A: button press Instruction: Look at two pictures and silently choose the one that matched a sentence you hear. |
Comprehension B: Instruction: Silently answer a question about a picture you are looking at |
Production Instruction: Finish my sentence. |
|---|---|---|---|---|
| Active voice | Syntax | The girl is kicking a boy. | – | Here the clown is pulling the dog but here… |
| Passive voice | Syntax | Here the girl is pushing the boy. | – | Here the boy is chasing the dog but here the boy… (Fig. 1a) |
| Relativized subjects | Syntax | The boy who is kicking the clown is wearing brown. | – | One of these boys is carrying some boxes, one of these boys is making a cake. This is the boy… |
| Relativized objects | Syntax | The girl who the boy is hugging is wearing green. (Fig. 1b) | – | The boy is making one cake. The father is making another cake. This is the cake… |
| Subject questions | Syntax | – | Which person is pushing the man? | – |
| Object questions | Syntax | – | Which person is the cat chasing? | – |
| Regular past tense marking | Morphology | The mother dressed the baby. | – | Here the boy is about to pour the juice but here he already… |
| Irregular past tense marking | Morphology | The boy washed his face. | – | Here the boy is about to draw a picture but here he already… (Fig. 1d) |
All tasks were presented in a blocked design and each grammar test began with instructions, followed by alternating blocks of rest and task (test items) (5 × 20 s and 4 × 20 s, respectively), with four trials per task block. After acquiring initial sequences, including T2 (up to 5 min), the patients performed two runs of the standard tests (30 min), followed by the grammar tests (25 min). Thus, the total time the participants spent in the scanner was about 1 h.
2.2.2. MRI acquisition
Scanning was performed on a Siemens Allegra head-only 3 Tesla scanner. Functional blood oxygenation level dependent (BOLD) echo-planar images (EPI) were collected using: repetition time (TR) 2.5 s; echo time (TE) 35 ms; flip angle, 90°; voxel dimensions, 3.1 × 3.1 × 3.1 mm; 0.75 mm gap; field-of-view, 200 mm; matrix, 64 × 64; 96 measurements; 28 slices. Data collected during the first three TRs were discarded for T1 equilibration. A high-resolution T1-weighted image (MPRAGE) was obtained to provide detailed brain anatomy with: TR 2.3 s, TE 2.93 ms, and voxel dimensions 1.3 × 1.3x1mm. An additional T2 structural scan, co-planar to the EPIs, was acquired to improve alignment to a standard coordinate system: TR of 5 s; TE, 33 ms; flip angle, 90°; 32 slices; voxel dimensions, 1.55 × 1.55 × 3 mm, field-of-view, 200 mm; and matrix, 128 × 128.
Visual stimuli were presented using a set of MRI-compatible stereoscopic goggles (Resonance Technology, Northridge, California). Participants were also provided a button box to make their responses for three of the grammar comprehension tasks (relativized subject and object clauses, active and passive voice, and irregular and regular past).
2.2.3. fMRI data processing
Functional MRI data were processed using tools from the FMRIB Software Library (FSL), Version 6.0. Preprocessing steps included motion correction, skull-stripping, spatial smoothing, normalization, and temporal filtering. Functional images were first registered to the co-planar structural image, then to the high-resolution T1 image (MPRAGE), and finally to standard space (Montreal Neurological Institute (MNI). Registration was visually inspected, motion was evaluated using relative and absolute motion estimates. We conducted first-level within-subject FEAT analyses using a general linear model (GLM) including six motion parameters and regressors for motion outlier volumes as determined by differential frame-to-frame variance (dVARS) calculations. The number of images omitted due to motion did not differ between groups (all p > 0.1).
First-level contrast Z-statistic images were entered into between group analyses using each subject as a random factor. All Z-statistic images were cluster thresholded by Z > 2.3, with a cluster-corrected significance threshold of p = 0.05 (Worsley, 2001).
2.2.4. Statistical analysis of ROI
Based on the literature showing certain areas of brain damage being linked to impairments in the structures we tested (Bastiaanse and Thompson, 2003, Dronkers et al., 2004, Edwards and Varlokosta, 2007, Friedmann, 2001, Friedmann et al., 2010, Linebarger et al., 1983, Shetreet and Friedmann, 2014) we selected nine ROI in each hemisphere (total ROI N = 18). There were four anterior ROI (BA 44, BA 45, BA 47 and the anterior superior temporal gyrus) and five posterior ROI (the posterior middle temporal gyrus, posterior superior temporal gyrus, anterior and posterior supramarginal gyrus and angular gyrus). Mean percent signal change was extracted for each ROI to compare (1) epilepsy versus tumor patients, and (2) LH- versus RH-lesioned patients. Spheres with a 5 mm radius were created at the gravitational center for a series of language ROI taken from a Brodmann's area atlas and from FSL's Harvard-Oxford Cortical Atlas (Drury et al., 1999; see Table 3). Percent signal change was extracted across each participant's time course using fslmeants. Analyses of variance (ANOVA) were also conducted using MATLAB R2014a to compare: standard (all tasks combined) vs. CYCLE-N (all tasks combined) activation in each individual ROI. We used a composite measure for the standard and the CYCLE-N tests because using a panel of language tasks (versus a single task) has been shown to improve sensitivity and specificity of fMRI signal in clinical language mapping (e.g., Gaillard et al., 2004, de Guibert et al., 2010). Paired-sample t-tests compared mean percent signal change in response to either standard vs. CYCLE-N in ROI averaged into anterior and posterior clusters. All statistical tests were conducted using MATLAB R2014a and were corrected for multiple comparisons using Bonferroni correction.
Table 3.
MNI Coordinates for ROI used in percent signal change comparisons of language regions. BA: Brodmann's area. MTG: middle temporal gyrus. SMG: supramarginal gyrus. STG: superior temporal gyrus.
| Left hemisphere | Right hemisphere | |||||
|---|---|---|---|---|---|---|
| ROI | X | Y | Z | X | Y | Z |
| Angular gyrus | 70 | 34 | 50 | 19 | 36 | 52 |
| BA 44 | 69 | 70 | 51 | 21 | 70 | 51 |
| BA 45 | 69 | 79 | 42 | 21 | 79 | 43 |
| BA 46 | 63 | 85 | 46 | 27 | 85 | 46 |
| BA 47 | 62 | 80 | 33 | 28 | 80 | 33 |
| MTG posterior | 75 | 49 | 29 | 14 | 51 | 29 |
| SMG anterior | 73 | 46 | 54 | 15 | 49 | 55 |
| SMG posterior | 72 | 39 | 52 | 17 | 42 | 52 |
| STG anterior | 73 | 61 | 31 | 16 | 62 | 30 |
| STG posterior | 75 | 49 | 36 | 14 | 51 | 36 |
2.2.5. Individual analysis
We ran two-way ANOVAs to test for significant interaction effects between task types and ROI on ROI percent signal change for each patient. ANOVA tests were corrected for multiple comparisons using Bonferroni Correction. Follow-up two-sample t-tests (uncorrected) were run for significant ANOVA tests to determine whether patients displayed greater activation across standard or grammar tasks for each ROI.
3. Results
3.1. Group results
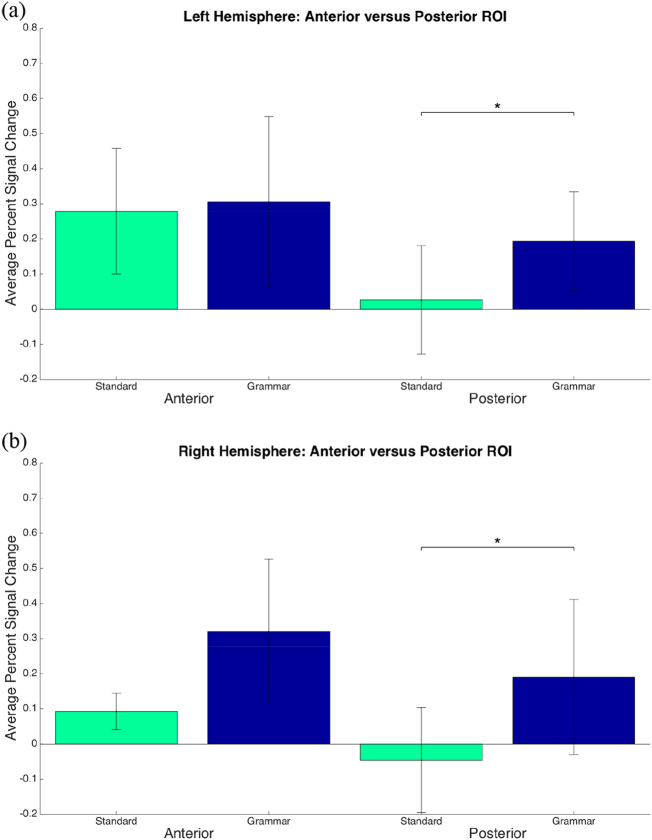
Overall, patients displayed increased bilateral ROI activation during the CYCLE-N when compared with the standard tests. Greater mean percent signal change was produced by the CYCLE-N (all tasks combined) than the standard tests (all tasks combined) in the posterior ROI of the left hemisphere (t(4) = − 4.066, p = 0.015) and the posterior ROI of the right hemisphere (t(4) = − 5.947, p = 0.004). There were no significant differences in mean percent signal change produced by the standard and CYCLE-N tests in the anterior ROI in the left or right hemisphere (Fig. 2).
Fig. 2.
ROI analysis for anterior and posterior fMRI activations in the standard, and the grammar tests.
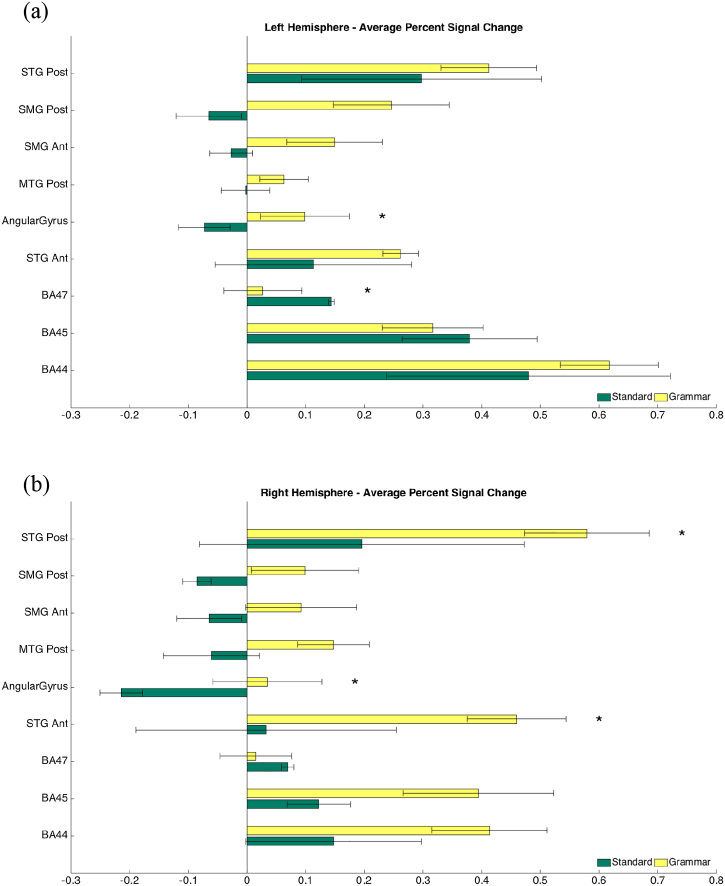
Left hemisphere ROI comparisons showed that of nine ROI, four were identified exclusively with the CYCLE-N (see Fig. 3a). The CYCLE-N generated higher activation in the left angular gyrus (p = 0.0006), while the standard tests produced higher activation in BA 47 (p = 0.0005). The standard language tests only produced negative percent signal changes within the former region.
Fig. 3.
Functional MRI activations in language ROI in the left and right hemisphere.
Analysis of the right hemisphere ROI also revealed that of nine ROI, four were identified exclusively with the CYCLE-N: the anterior and posterior supramarginal gyrus, posterior middle temporal gyrus and angular gyrus (see Fig. 3b). The CYCLE-N generated a higher volume of activation in three regions: the anterior STG (p = 0.0002), posterior STG (p = 0.0008) and angular gyrus (p = 0.00017).
3.2. Individual results
Individual subject analysis showed that within the LH, ten patients had significantly increased activation in the CYCLE-N, while three patients (T8, T12 and T27) had significantly increased activation in the standard tests (see Table 4). Within the RH, twelve patients had significantly more volume of activation in the CYCLE-N and two patients had more volume of activation in the standard tests. Detailed brain images of each patient can be seen in Fig. 1 in the Supplementary materials; signal percent change in specific LH and RH ROI of individual subjects can be seen in Table 1 in the Supplementary materials.
Table 4.
Two-way ANOVA testing for significant interaction effects between task type (grammar and standard tasks) and ROI. ANOVAs corrected for multiple comparisons using Bonferroni Correction (p < 0.05/50).
| LH p-values |
RH p-values |
||||
|---|---|---|---|---|---|
| Patient | Standard Skewed | Grammar Skewed | Patient | Standard Skewed | Grammar Skewed |
| T_11 | 5.6E − 07⁎⁎⁎ | E_3 | 7.5E − 13⁎⁎⁎ | ||
| T_27 | 1.6E − 06⁎⁎⁎ | E_12 | 4.1E − 05⁎⁎⁎ | ||
| E_5 | 1.9E − 04⁎⁎⁎ | T_21 | 5.2E − 05⁎⁎⁎ | ||
| T_12 | 5.0E − 04⁎⁎⁎ | T_3 | 1.1E − 04⁎⁎⁎ | ||
| T_3 | 0.002⁎ | T_25 | 1.4E − 04⁎⁎⁎ | ||
| E_8 | 0.002⁎ | E_5 | 2.6E − 04⁎⁎⁎ | ||
| T_25 | 0.003⁎ | T_27 | 3.2E − 04⁎⁎⁎ | ||
| T_10 | 0.003⁎ | E_7 | 3.2E − 04⁎⁎⁎ | ||
| T_16 | 0.02⁎ | T_11 | 7.5E − 04⁎⁎⁎ | ||
| E_3 | 0.02⁎ | T_2 | 0.002⁎ | ||
| T_22 | 0.02⁎ | T_10 | 0.004⁎ | ||
| T_7 | 0.03⁎ | T_7 | 0.005⁎ | ||
| T_8 | 0.03⁎ | E_2 | 0.02⁎ | ||
| T_13 | 0.06 | E_8 | 0.02⁎ | ||
| T_26 | 0.07 | E_13 | 0.05 | ||
| E_12 | 0.07 | T_16 | 0.09 | ||
| E_2 | 0.1 | T_8 | 0.09 | ||
| T_21 | 0.1 | E_4 | 0.1 | ||
| E_6 | 0.2 | T_12 | 0.2 | ||
| E_4 | 0.3 | E_6 | 0.2 | ||
| T_2 | 0.4 | T_22 | 0.4 | ||
| E_13 | 0.4 | T_6 | 0.4 | ||
| T_4 | 0.4 | T_4 | 0.5 | ||
| E_7 | 0.5 | T_13 | 0.5 | ||
| T_6 | 0.9 | T_26 | 0.6 | ||
| Total significant | 3 | 10 | 2 | 12 | |
p < 0.001.
p < 0.05.
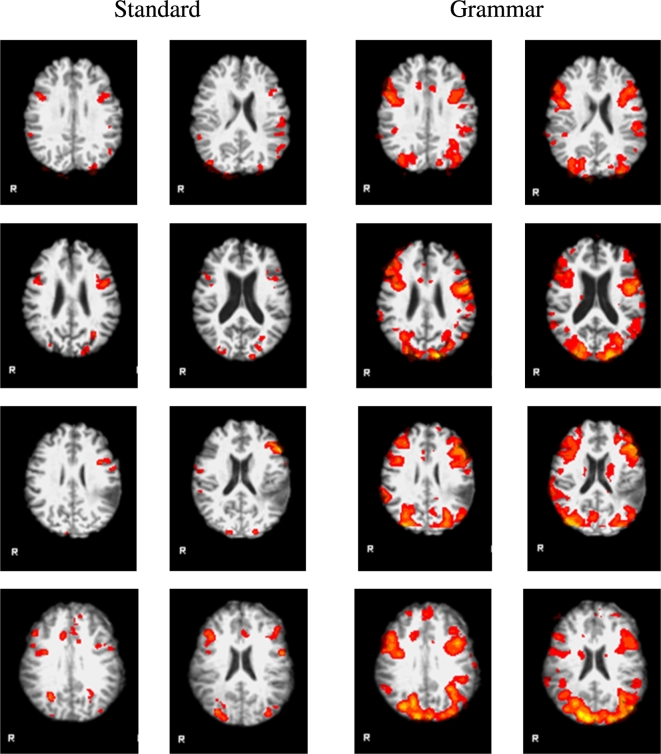
Fig. 4 presents functional language maps for standard versus CYCLE-N in four patients. The CYCLE-N elicited more volume of activation in bilateral BA 44, BA 45, posterior superior temporal gyri angular and supramarginal gyri. Detailed images showing activation the CYCLE-N and the standard tests in each patient can be seen in Fig. 1 in the Supplementary materials.
Fig. 4.
Comparison between functional activations in all standard versus all grammar tests in four patients: two with epilepsy – E2 (row 1) and E5 (row 2) and two with brain tumor – T 8 (row 3) and T11 (row 4). The grammar tests generated more volume of activation bilaterally in BA 44, BA 45, posterior superior temporal gyrus angular and supramarginal gyrus.
Lesion location (LH versus RH) had very little effect on the volume of activation either in the CYCLE-N or the standard tests. Similarly, volume of activation between the epilepsy and tumor group did not reveal significant differences.
4. Discussion
The goal of this study was to evaluate whether including an assessment of grammar comprehension and production in clinical language fMRI can provide us with additional areas of activation in the language network, thus enriching and advancing our knowledge of the neuroarchitecture of language. The CYCLE-N grammar test, at least in our sample (25 patients with tumor and epilepsy), was an excellent testing measure for localizing functional language areas within the posterior ROI (the angular gyrus) of the LH.
4.1. Group results
Surprisingly, the CYCLE-N also produced more volume of activation in the posterior RH. Our results within the posterior ROI of the RH also generated more volume of activation. Since the CYCLE-N seem to be less lateralizing than the standard tests (due to more volume of activation bilaterally), they may be an important addition to pre-operative fMRI in people with brain tumors and people with epilepsy in cases in which language laterality is known because they will help identify additional and more specific language areas.
Compared to studies on language lateralization (e.g., Janecek et al., 2013, Bauer et al., 2014, Nadkarni et al., 2014, DeSalvo et al., 2016, Morrison et al., 2016), clinical fMRI research has not been sufficiently focused on language localization within a hemisphere. This is the first foray into developing a protocol that is optimal for revealing areas of activity within either hemisphere. Including tests accounting for more complex linguistic aspects is an important step towards delineating a more accurate neuroanatomy of specific language structures in surgical candidates. Through a comprehensive assessment of grammar, we are more likely to adequately determine the functional anatomy of language in individual patients (Połczyńska et al., 2014, Rofes and Miceli, 2014, Rofes et al., 2015b).
We saw substantially greater volume of activation within the left posterior language ROI with the CYCLE-N (specifically the angular gyrus). This result is in line with previous studies in which we saw involvement of the posterior language regions (including the underlying white matter) in grammatical processing (Dronkers et al., 2004, Turken and Dronkers, 2011, Ivanova et al., 2016). There was no activation in the anterior and posterior supramarginal gyrus, posterior middle temporal gyrus or angular gyrus with the standard tasks. This result is consistent with our earlier reports using lexico-semantic tasks in clinical fMRI in which we saw insignificant activity in the left posterior language areas, including the angular gyrus, supramarginal gyrus (e.g., Bookheimer, 2007, Połczyńska et al., 2016). Furthermore, we observed activations in those areas that were absent in the standard tasks. Considerable neuroimaging and lesion studies have shown that grammar, and syntax in particular, is strongly lateralized to the LH in most individuals (e.g., Antonenko et al., 2013, Batterink and Neville, 2013, Charles et al., 2014, Grodzinsky and Friederici, 2006, Miozzo et al., 2010, Newman et al., 2010, Hickok and Rogalsky, 2011, Turken and Dronkers, 2011, den Ouden et al., 2012, Friederici et al., 2012, Griffiths et al., 2013, Makuuchi et al., 2013, Magnusdottir et al., 2013, Papoutsi et al., 2011, Tyler et al., 2010, Tyler et al., 2013, Wilson et al., 2011, Wilson et al., 2012, Wright et al., 2012). Lesion studies have uniformly indicated that damage to the LH results in grammar deficits. For example, Dronkers et al. (2004) investigated comprehension of syntactic structures including simple declaratives, possession, active and passive (agentless and agentive) word order, double embedding, subject and object relative clauses, negative passive, object clefting and object relatives with relativized objects and found that all these structures were impaired to a various degree in patients having lesions in the LH. Further, the right hemisphere of split-brain individuals performed at chance level even on semantically reversible subject-verb-object; active declarative sentences, e.g., The boy is pushing the girl versus The girl is pushing the boy. This is a very simple syntactic structure, but one for which world knowledge alone cannot yield good comprehension, but rather requires syntactic knowledge (Gazzaniga and Hillyard, 1971). Moreover, Foki et al. (2008) pointed out that sentential level tasks are superior at identifying activation in Broca's and Wernicke's areas (> 95%) than word level tasks, e.g., object naming – 85% in Wernicke's area and 75% on Broca's area (Gaillard et al., 2004, word generation – 81% in Wernicke's area and 81% and 92% in Broca's area (Stippich et al., 2003). Those findings are in line with our results because the CYCLE-N comprised stimuli at the sentence level, whereas the standard tests included only word level tasks.
Compared to the standard tests, the CYCLE-N produced significantly higher activity in the left angular gyrus. This area was not identified using the standard tests. Damage to the left angular gyrus has been associated with impaired performance on reversible passive sentences, object-cleft sentences, conceptual combination (where single basic concepts are synthesized to form a mentally composite/complex concept), short-term memory and verbal working memory (Dronkers et al., 2004, Newman et al., 2010, Newhart et al., 2012, Price et al., 2015, Thothathiri et al., 2012). In a meta-analysis of 120 studies Binder et al. (2009) found a network of seven regions in the LH, including the angular gyrus, that were consistently reported for semantic processing. The authors postulated that semantic knowledge is stored and retrieved through widespread neural systems located in the cortex (Binder et al., 2009). While we found the lexical process to be subserved by several (mainly anterior) ROI, we found no activity in the angular gyrus.
However, our results are in line with a recent study by Humphreys et al. (2015). These authors investigated the left angular gyrus, which is part of the default network (it shows deactivation in many cognitive tasks), and found that it was consistently deactivated in various cognitive semantic and non-semantic tasks (e.g., synonym and number judgment, category judgment of words, pictures and sounds).
Among all the language ROI, the CYCLE-N and the standard tests produced the highest activation in the left hemisphere BA 44. The region has been identified as the primary processor of syntax in the brain (Dapretto and Bookheimer, 1999, Friederici, 2011, Haller et al., 2005, Skeide et al., 2014, Tyler et al., 2013). BA44 participates in building of syntactic structures (Friederici, 2011). It is activated by long-distance dependencies (structures whose grammaticality depends on rules or operations being applied to non-adjacent parts of a sentence) (e.g., Opitz and Friederici, 2007). In addition, BA 44 has been shown to be particularly vulnerable to syntactically complex (non-canonical) sentences (i.e., sentences involving movement operations) in primary progressive aphasia (Wilson et al., 2012). Concurrently, a recent meta-analysis of 54 fMRI and PET studies (Rodd et al., 2015) showed that this area is involved both in syntactic and semantic processing (language stimuli were single words, pairs and triples of words, fragments of sentences or narratives). Our results are consistent with this study in that both the standard and the CYCLE-N generated the highest amount of activity in BA 44. Rodd et al.'s study, as well as others, also demonstrated that the anterior inferior frontal gyrus (BA 47) was primarily associated with semantic processing (Friederici, 2011, Hagoort, 2005, Rodd et al., 2015). There was no significant difference is activity in BA 45 between the CYCLE-N and the standard tasks. At the same time, the inferior frontal gyrus centered on BA 44/45 has been shown to be involved in thematic role assignment (Friederici, 2011) (which may be construed to be part of syntax, e.g., theta-role assignment). The area has been indicated to participate in artificial grammar learning. BA 44/45 is thought to unify syntactic information from various sources in an incremental (sequentially processed) and recursive manner (Petersson et al., 2012) (syntactic structures which embody what is referred to as “recursivity” – the property by which syntactic rules generate an unbounded number of sentences and by which sentences are unbounded in length).
Three ROI in the RH were activated more by the CYCLE-N than the standard tests. This considerable involvement of the RH was unexpected because the LH seems to be the neural substrate for syntactic processing even in very young children with typical language development. The LH has been shown to specialize for processing syntax in two to three-year-olds (Oberecker et al., 2005), and it is the LH that is recruited when discriminating verbs from nouns in children as young as two years who are still at the one-word stage (Bernal and Ardila, 2014). However, there is little evidence to believe that our results were due to functional reorganization of language areas in our patient sample. Reorganization is known to occur in younger onset individuals. All of our patients with epilepsy had an older onset with the exception of one individual. As noted in Section 2.1, we analyzed only patients with LH language dominance. Yet, the results of our study were not significantly altered by the location of lesion (LH versus RH) or etiology (tumor versus epilepsy). Thus, our results were specific to tasks we used in this study and not due to atypical language organization. However, Sammler et al. (2013) also found bilateral activity in a grammar test in epileptic individuals. The authors performed intracranial EEG over the temporal lobe while study participants were exposed to syntactic violations of a sentence structure. We believe that there may be increased support of the RH in processing grammar in both epilepsy and tumor patients and that this support is not fully due to functional compensation (Deng et al., 2015, Thiel et al., 2006). Moreover, since damage only to the right hemisphere very rarely leads to aphasia, right hemisphere ROI activated by fMRI may reveal a broader neural network involved in language processing, only a part of which may, in fact, be critical or necessary for language processing. According to Hickok and Poeppel (2007) language comprehension (subserved by the ventral system processing speech signals) is bilaterally organized. At the same, time the authors pointed out that there are substantial computational differences between the RH and LH systems. Studies using pre- and post-operative test performance alone, not fMRI performance, may produce key data bearing on this important issue of differentiating clinical vs. experimental findings regarding mapping language in the brain. Nonetheless, our results fit into a growing body of work that shows that RH areas are recruited in language tasks, though an understanding of what these RH regions contribute to language processing in not yet understood and requires more research specifically devoted to understanding just that (Hartwigsen et al., 2010, Vigneau et al., 2011, Wlotko and Federmeier, 2013, Passeri et al., 2015).
4.2. Individual results
Individual subject results matched our group results in that we observed significantly more patients had more robust activity in the language ROI bilaterally (Table 4; Supplementary Fig. 1). The three patients (T8, T12 and T27) who had more volume of activation in the standard tasks than the grammar tasks had extensive lesions: tumor with widespread edematous tissue; T12 additionally had a prior resection. The lesions directly affected several posterior language ROI and were masked in the three patients. We thus recorded no activity in those regions. As shown in our group results, the grammar tasks produced more volume of activation in the posterior language ROI compared to the standard lexico-semantic tasks. After extracting much of the left posterior activity associated with the grammar tasks we may have seen more activity associated with the standard tasks in the frontal language ROI. There were three more individuals with tumors within/neighboring the posterior language ROI: T6, T7 and T26. In patients T6 and T26 the results did not significantly differ between the grammar and the standard tasks, while T7 had more volume of activation in the standard tasks. Patient T7 had a large yet well confined tumor that seemed to have pushed the left superior temporal gyrus more posteriorly preserving the functional cortex. Patient T6 had a lesion extending from the middle posterior to inferior temporal gyrus, thus affecting only one posterior language ROI the left posterior middle temporal gyrus. Finally, patient T26 had a small lesion affecting only the left angular gyrus. In sum, after excluding individual tumor cases with extensive lesions in the posterior ROI, there were no patients who had significantly more volume of activation in the standard tests versus the grammar tests.
4.3. Importance of grammar assessment
Grammar assessment may be an important addition to pre-operative fMRI because it may help identify additional and more specific areas in the brain dedicated to language. The fMRI literature has suggested that the neurosubstrate of the language system is much more complex than the standard Broca's and Wernicke's area. For example, substantial attention has been paid recently to the role of the anterior temporal lobe (Binder et al., 2011, Brennan and Pylkkänen, 2016). A historically known but under-discussed region is the basal temporal language area. Stimulation of this area has been shown to cause anomia (Lüders et al., 1986). It is difficult to assess how relevant any of these areas are for grammar tasks because grammar is not tested perioperatively. According to Cervenka et al. (2013) more efficient, comprehensive language mapping protocols (including the syntactic level) are required to avoid language deficits after brain surgery. With no proper assessment of grammar, neurosurgical decisions may be made based on incomplete language maps that do not account for brain areas engaged in grammatical processing, including complex linguistic processes. Consequently, despite language testing, patients may have their language compromised after brain surgery (Połczyńska, 2009, Połczyńska et al., 2014). Disrupted grammar processes may be less apparent in the standard language evaluations, but because grammatical knowledge is central to normal communication, grammatical deficits will substantially affect quality of life. In many cases such impairments may require years of expensive language intervention (Basso et al., 2003). The magnitude of impact of resection of brain areas engaged with grammar processing on long-term outcome is yet to be studied. Further, the impact may vary according to what the patient's needs are (e.g., what their profession is). Those issues are not completely understood, nonetheless, we think it is important to begin a discussion that includes assessment of grammar. Hopefully this will be a basis on which future studies will start to examine grammar function perioperatively.
4.4. Study limitations
This study has limitations. Cerebral lesions may impair reliability of fMRI images in the pre-surgical language mapping context (Hou et al., 2006, Zacà et al., 2012). Larger lesions, such as mass defects and severe atrophy can decrease the laterality index measure (Wellmer et al., 2009). Moreover, brain tumors have been associated with edema and altered oxygenation in the brain. These changes may hamper the accuracy of fMRI and reduce the BOLD signal (Giussani et al., 2010). However, a comparison between our lesional and non-lesional patients did not show significant differences in the laterality index measure. At the same time, we admit that fMRI as it is currently used should not be an alternative method to language mapping with intraoperative cortical stimulation (Giussani et al., 2010) or direct, nonexperimental testing.
We lacked behavioral monitoring for our fMRI tasks, which may have impacted task involvement and accuracy. However, after several years of study we believe we have established that tasks that require an internal generation of a response generate as much activity as tasks involving a verbal response (see also Partovi et al., 2012). We assessed accuracy and involvement of our participants in three ways: (1) the subjects received direct instruction and task practice prior to beginning the fMRI session, (2) right after each fMRI task we asked the subjects whether they had any problems with it, and (3) we analyzed the primary visual and auditory cortices to assure that the subjects actively participated in the task.
Another caveat in this study is using rest as the contrast task for our language fMRI tasks. Contrast tasks are still controversial. We chose to use a baseline that was equally relevant to tasks with different modalities. To remove perceptual activation we used a conjunction model.
Choosing an ROI approach we designed our study on a priori knowledge. However, it is difficult to run a full brain analysis when there is a space occupying lesion and likely reorganization. Therefore, we decided to use ROI that have been shown to be associated with impaired grammar processing after brain damage (van der Lely, 1998, Linebarger et al., 1983, Friedmann, 2001, Bastiaanse and Thompson, 2003, Edwards and Varlokosta, 2007, Friedmann et al., 2010, Shetreet and Friedmann, 2014;) and were also indicated using a whole-brain analysis (e.g., (e.g., Friederici et al., 2000, Dronkers et al., 2004, Gaillard et al., 2004, Bornkessel et al., 2005, Turken and Dronkers, 2011).
Finally, in this study we combined all the grammar tests and were not able to test specific grammar structures and link them to particular brain areas. We think it is an important future goal that should further advance our understanding of the neural architecture of language.
5. Conclusions
In this study we introduced a comprehensive grammar test (the CYCLE-N) to pre-operative fMRI. The test assessed language comprehension and production of a variety of linguistic structures at a sentence level. The CYCLE-N generated more volume of activation in the LH and identified additional language regions not shown by the standard tests. Contrary to what was expected, the CYCLE-N also evoked substantial activations in the RH and thus turned out to be superior at identifying RH contributions to language processing. Thus, the CYCLE-N appears to be an important addition to the standard pre-operative fMRI.
The following are the supplementary data related to this article.
Activation in grammar and standard tests in epilepsy (E) and tumor (T) patients. Lesion images are included for all tumor patients. Images of three epilepsy patients (E3, E4, E13) show a prior resection cavity. Overall, the grammar tests generate more robust activations in language areas than the standard test.
Two-sample t-tests (uncorrected) run for patients with significant ANOVA tests (Table 4) to determine task type skew directionality (greater percent signal change in grammar or standard tests) within each ROI. Region stats with NaN denote regions which have been ablated by tumor masses or edema.
Acknowledgment
This work was supported by the Polish Ministry of Science and Higher Education, grant no. 608/MOB/2011/0 (Investigator MP). We would like to thank Jason Yamada-Hanff, Ph.D. for his thoughtful comments and suggestions on our work.
Contributor Information
Monika Połczyńska, Email: plmonik@wa.amu.edu.pl.
Susan Curtiss, Email: scurtiss@ucla.edu.
Teena Moody, Email: tmoody@g.ucla.edu.
Christopher Benjamin, Email: christopher.benjamin@yale.edu.
Taylor Kuhn, Email: tkuhn@mednet.ucla.edu.
Susan Bookheimer, Email: sbook@ucla.edu.
References
- Antonenko D., Brauer J., Meinzer M., Fengler A., Kerti L., Friederici A.D., Flöel A. Functional and structural syntax networks in aging. NeuroImage. 2013;83:513–523. doi: 10.1016/j.neuroimage.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Ardila A. There are two different language systems in the brain. J. Behav. Brain Sci. 2011;01:23–36. [Google Scholar]
- Basso U., Tosoni A., Vastola F., Brandes A.A. Guidelines for the treatment of malignant gliomas in elderly patients. Forum (Genova) 2003;13(1):46–60. [PubMed] [Google Scholar]
- Bastiaanse R., Thompson C.K. Verb and auxiliary movement in agrammatic Broca's aphasia. Brain Lang. 2003;84:286–305. doi: 10.1016/s0093-934x(02)00553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L., Neville H.J. The human brain processes syntax in the absence of conscious awareness. J. Neurosci. 2013;33:8528–8533. doi: 10.1523/JNEUROSCI.0618-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.R., Reitsma J.B., Houweling B.M., Ferrier C.H., Ramsey N.F. Can fMRI safely replace the Wada test for preoperative assessment of language lateralisation? A meta-analysis and systematic review. J. Neurol. Neurosurg. Psychiatry. 2014;85(5):581–588. doi: 10.1136/jnnp-2013-305659. [DOI] [PubMed] [Google Scholar]
- Bello L., Gallucci M., Fava M., Carrabba G., Giussani C., Acerbi F., Baratta P., Songa V., Conte V., Branca V., Stocchetti N., Papagno C., Gaini S.M. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving speech areas. Neurosurgery. 2007;60:67–82. doi: 10.1227/01.NEU.0000249206.58601.DE. [DOI] [PubMed] [Google Scholar]
- Benjamin C.F., Walshaw P., Polczynska M., Hale L., Alkawadri R., Bookheimer S. National Academy of Neuropsychology; Austin, Texas: 2015. A Clinical Model of Language for Presurgical Language Localization Using fMRI. (Oral, Nov 2015) [Google Scholar]
- Bernal B., Ardila A. Bilateral representation of language: a critical review and analysis of some unusual cases. J. Neurolinguistics. 2014;28:63–80. [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb. Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Gross W.L., Allendorfer J.B., Bonilha L., Chapin J., Edwards J.C., Grabowski T.J., Langfitt J.T., Loring D.W., Lowe M.J., Koenig K., Morgan P.S., Ojemann J.G., Rorden C., Szaflarski J.P., Tivarus M.E., Weaver K.E. Mapping anterior temporal lobe language areas with fMRI: a multicenter normative study. NeuroImage. 2011;54(2):1465–1475. doi: 10.1016/j.neuroimage.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Pre-surgical language mapping with functional magnetic resonance imaging. Neuropsychol. Rev. 2007;17:145–155. doi: 10.1007/s11065-007-9026-x. [DOI] [PubMed] [Google Scholar]
- Bornkessel I., Zysset S., Friederici A.D., von Cramon D.Y., Schlesewsky M. Who did what to whom? The neural basis of argument hierarchies during language comprehension. NeuroImage. 2005;26(1):221–233. doi: 10.1016/j.neuroimage.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Brennan J.R., Pylkkänen L. MEG evidence for incremental sentence composition in the anterior temporal lobe. Cogn. Sci. 2016 doi: 10.1111/cogs.12445. [DOI] [PubMed] [Google Scholar]
- Cervenka M.C., Corines J., Boatman-Reich D.F., Eloyan A., Sheng X., Franaszczuk P.J., Crone N.E. Electrocorticographic functional mapping identifies human cortex critical for auditory and visual naming. NeuroImage. 2013;69:267–276. doi: 10.1016/j.neuroimage.2012.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles D., Olm C., Powers J., Ash S., Irwin D.J., McMillan C.T., Rascovsky K., Grossman M. Grammatical comprehension deficits in non-fluent/agrammatic primary progressive aphasia. J. Neurol. Neurosurg. Psychiatry. 2014;85:249–256. doi: 10.1136/jnnp-2013-305749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss S., Yamada J. A Fine Line; Baltimore: 2004. The Curtiss-Yamada Comprehensive Language Evaluation: The CYCLE. [Google Scholar]
- Dapretto M., Bookheimer S.Y. Form and content: dissociating syntax and semantics in sentence comprehension. Neuron. 1999;24:427–432. doi: 10.1016/s0896-6273(00)80855-7. [DOI] [PubMed] [Google Scholar]
- De Witte E., Satoer D., Robert E., Colle H., Verheyen S., Visch-Brink E., Mariën P. The Dutch linguistic intraoperative protocol: a valid linguistic approach to awake brain surgery. Brain Lang. 2015;140:35–48. doi: 10.1016/j.bandl.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Deng X., Zhang Y., Xu L., Wang B., Wang S. Comparison of language cortex reorganization patterns between cerebral arteriovenous malformations and gliomas: a functional MRI study. J. Neurosurg. 2015;122:996–1003. doi: 10.3171/2014.12.JNS14629. [DOI] [PubMed] [Google Scholar]
- DeSalvo M.N., Tanaka N., Douw L., Leveroni C.L., Buchbinder, B.R.1 Greve, D.N., Stufflebeam, S.M. Resting-state functional MR imaging for determining language laterality in intractable epilepsy. Radiology. 2016;281(1):264–269. doi: 10.1148/radiol.2016141010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dronkers N.F., Wilkins D.P., Van Valin R.D., Redfern B.B., Jaeger J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Drury H.A., Van Essen D.C., Corbetta M., Snyder A.Z. Surface-based analyses of the human cerebral cortex. In: Toga A.W., editor. Brain Warping. Academic Press; San Diego: 1999. pp. 337–363. [Google Scholar]
- Edwards S., Varlokosta S. Pronominal and anaphoric reference in agrammatism. J. Neurolinguistics. 2007;20:423–444. [Google Scholar]
- Fernández Coello A., Moritz-Gasser S., Martino J., Martinoni M., Matsuda R., Duffau H. Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J. Neurosurg. 2013;119:1380–1394. doi: 10.3171/2013.6.JNS122470. [DOI] [PubMed] [Google Scholar]
- Foki T., Gartus A., Geissler A., Beisteiner R. Probing overtly spoken language at sentential level—a comprehensive high-field BOLD–fMRI protocol reflecting everyday language demands. NeuroImage. 2008;39:1613–1624. doi: 10.1016/j.neuroimage.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Meyer M., von Cramon D.Y. Auditory language comprehension: an event-related fMRI study on the processing of syntactic and lexical information. Brain Lang. 2000;74(2):289–300. doi: 10.1006/brln.2000.2313. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Oberecker R., Brauer J. Neurophysiological preconditions of syntax acquisition. Psychol. Res. 2012;76:204–211. doi: 10.1007/s00426-011-0357-0. [DOI] [PubMed] [Google Scholar]
- Friedmann N. Agrammatism and the psychological reality of the syntactic tree. J. Psycholinguist. Res. 2001;30:71–90. doi: 10.1023/a:1005256224207. [DOI] [PubMed] [Google Scholar]
- Friedmann N., Reznick J., Dolinski-Nuger D., Soboleva K. Comprehension and production of movement-derived sentences by Russian speakers with agrammatic aphasia. J. Neurolinguistics. 2010;23:44–65. [Google Scholar]
- Gaillard W.D., Balsamo L., Xu B., McKinney C., Papero P.H., Weinstein S., Conry J., Pearl P.L., Sachs B., Sato S., Vezina L.G., Frattali C., Theodore W.H. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M.S., Hillyard S.A. Language and speech capacity of the right hemisphere. Neuropsychologia. 1971;9:273–280. doi: 10.1016/0028-3932(71)90022-4. [DOI] [PubMed] [Google Scholar]
- Giussani C., Roux F.-E., Ojemann J., Sganzerla E. Pietro, Pirillo D., Papagno C. Is preoperative functional magnetic resonance imaging reliable for language areas mapping in brain tumor surgery? Review of language functional magnetic resonance imaging and direct cortical stimulation correlation studies. Neurosurgery. 2010;66:113–120. doi: 10.1227/01.NEU.0000360392.15450.C9. [DOI] [PubMed] [Google Scholar]
- Griffiths J.D., Marslen-Wilson W.D., Stamatakis E.A., Tyler L.K. Functional organization of the neural language system: dorsal and ventral pathways are critical for syntax. Cereb. Cortex. 2013;23:139–147. doi: 10.1093/cercor/bhr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky Y., Finkel L. The neurology of empty categories aphasics' failure to detect ungrammaticality. J. Cogn. Neurosci. 1998;10:281–292. doi: 10.1162/089892998562708. [DOI] [PubMed] [Google Scholar]
- Grodzinsky Y., Friederici A.D. Neuroimaging of syntax and syntactic processing. Curr. Opin. Neurobiol. 2006;16:240–246. doi: 10.1016/j.conb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- de Guibert C., Maumet C., Ferré J.C., Jannin P., Biraben A., Allaire C., Barillot C., Le Rumeur E. FMRI language mapping in children: a panel of language tasks using visual and auditory stimulation without reading or metalinguistic requirements. NeuroImage. 2010;51(2):897–909. doi: 10.1016/j.neuroimage.2010.02.054. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends Cogn. Sci. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Haller S., Radue E.W., Erb M., Grodd W., Kircher T. Overt sentence production in event-related fMRI. Neuropsychologia. 2005;43:807–814. doi: 10.1016/j.neuropsychologia.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Hamberger M., Seidel W., Goodman R.R., Perrine K., McKhann G.M. Temporal lobe stimulation reveals anatomic distinction between auditory naming processes. Neurology. 2003;60(9):1478–1483. doi: 10.1212/01.wnl.0000061489.25675.3e. [DOI] [PubMed] [Google Scholar]
- Hartwigsen G., Price C.J., Baumgaertner A., Geiss G., Koehnke M., Ulmer S., Siebner H.R. The right posterior inferior frontal gyrus contributes to phonological word decisions in the healthy brain: evidence from dual-site TMS. Neuropsychologia. 2010;48(10):3155–3163. doi: 10.1016/j.neuropsychologia.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. The cortical organization of speech processing. Nat. Rev. Neurosci. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hickok G., Rogalsky C. What does Broca's area activation to sentences reflect? J. Cogn. Neurosci. 2011;23:2629–2635. doi: 10.1162/jocn_a_00044. [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Tuffiash E., Caramazza A. Modality-specific deterioration on naming verbs in nonfluent, primary progressive aphasia. J. Cogn. Neurosci. 2002;14:1099–1108. doi: 10.1162/089892902320474544. [DOI] [PubMed] [Google Scholar]
- Hou B.L., Bradbury M., Peck K.K., Petrovich N.M., Gutin P.H., Holodny A.I. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. NeuroImage. 2006;32:489–497. doi: 10.1016/j.neuroimage.2006.04.188. [DOI] [PubMed] [Google Scholar]
- Humphreys G.F., Hoffman P., Visser M., Binney R.J., Lambon Ralph M.A. Establishing task- and modality-dependent dissociations between the semantic and default mode networks. Proc. Natl. Acad. Sci. U. S. A. 2015;112:7857–7862. doi: 10.1073/pnas.1422760112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova M.V., Isaev D.Y., Dragoy O.V., Akinina Y.S., Petrushevskiy A.G., Fedina O.N., Shklovsky V.M., Dronkers N.F. Diffusion-tensor imaging of major white matter tracts and their role in language processing in aphasia. Cortex. 2016;85:165–181. doi: 10.1016/j.cortex.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Jackendoff R. A parallel architecture perspective on language processing. Brain Res. 2007;1146:2–22. doi: 10.1016/j.brainres.2006.08.111. [DOI] [PubMed] [Google Scholar]
- Janecek J.K., Swanson S.J., Sabsevitz D.S., Hammeke T.A., Raghavan M., E. Rozman. M., Binder, J.R. Language lateralization by fMRI and Wada testing in 229 patients with epilepsy: rates and predictors of discordance. Epilepsia. 2013;54(2):314–322. doi: 10.1111/epi.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justus T., Larsen J., Yang J., Davies P. de M., Dronkers N., Swick D. The role of Broca's area in regular past-tense morphology: an event-related potential study. Neuropsychologia. 2011;49:1–18. doi: 10.1016/j.neuropsychologia.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler D., Curtiss S., Jackson C. Syntactic preservation in Alzheimer's disease. J. Speech Hear. Res. 1987;30:343350. doi: 10.1044/jshr.3003.343. [DOI] [PubMed] [Google Scholar]
- Léger G.C., Jonhnson N. A review on primary progressive aphasia. Neuropsychiatr. Dis. Treat. 2007;3(6):745–752. doi: 10.2147/ndt.s1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lely H.K.J. SLI in children: movement, economy, and deficits in the computational-syntactic system. Lang. Acquis. 1998;7:161–192. [Google Scholar]
- Linebarger M.C., Schwartz M.F., Saffran E.M. Sensitivity to grammatical structure in so-called agrammatic aphasics. Cognition. 1983;13:361–392. doi: 10.1016/0010-0277(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Lubrano V., Filleron T., Démonet J.-F., Roux F.-E. Anatomical correlates for category-specific naming of objects and actions: a brain stimulation mapping study. Hum. Brain Mapp. 2014;35:429–443. doi: 10.1002/hbm.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders H., Lesser R.P., Hahn J., Dinner D.S., Morris H., Resor S., Harrison M. Basal temporal language area demonstrated by electrical stimulation. Neurology. 1986;36:505–510. doi: 10.1212/wnl.36.4.505. (http://www.ncbi.nlm.nih.gov/pubmed/3960324) [DOI] [PubMed] [Google Scholar]
- Magnusdottir S., Fillmore P., den Ouden D.B., Hjaltason H., Rorden C., Kjartansson O., Bonilha L., Fridriksson J. Damage to left anterior temporal cortex predicts impairment of complex syntactic processing: a lesion-symptom mapping study. Hum. Brain Mapp. 2013;34:2715–2723. doi: 10.1002/hbm.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuuchi M., Grodzinsky Y., Amunts K., Santi A., Friederici A.D. Processing non-canonical sentences in Broca's region: reflections of movement distance and type. Cereb. Cortex. 2013;23:694–702. doi: 10.1093/cercor/bhs058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mätzig S., Druks J., Masterson J., Vigliocco G. Noun and verb differences in picture naming: past studies and new evidence. Cortex. 2009;45(6):738–758. doi: 10.1016/j.cortex.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Miceli G., Silvieri C., Villa G., Caramazza A. On the basis for the agrammatic's difficulty in producing main verbs. Cortex. 1984;20(2):207–220. doi: 10.1016/s0010-9452(84)80038-6. [DOI] [PubMed] [Google Scholar]
- Miozzo M., Fischer-Baum S., Postman J. A selective deficit for inflection production. Neuropsychologia. 2010;48:2427–2436. doi: 10.1016/j.neuropsychologia.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Morrison M.A., Churchill N.W., Cusimano M.D., Schweizer T.A., Das S., Graham S.J. Reliability of task-based fMRI for preoperative planning: a test-retest study in brain tumor patients and healthy controls. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni T.N., Andreoli M.J., Nair V.A., Yin P., Young B.M., Kundu B., Pankratz J., Radtke A., Holdsworth R., Kuo J.S., Field A.S., Baskaya M.K., Moritz C.H., Meyerand M.E., Prabhakaran V. Usage of fMRI for pre-surgical planning in brain tumor and vascular lesion patients: task and statistical threshold effects on language lateralization. Neuroimage Clin. 2014;7:415–423. doi: 10.1016/j.nicl.2014.12.014. (eCollection 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E., Penke M. Production and comprehension of wh-questions in German Broca's aphasia. J. Neurolinguistics. 2008;21:150–176. [Google Scholar]
- Newhart M., Trupe L.A., Gomez Y., Cloutman L., Molitoris J.J., Davis C., Leigh R., Gottesman R.F., Race D., Hillis A.E. Asyntactic comprehension, working memory, and acute ischemia in Broca's area versus angular gyrus. Cortex. 2012;48:1288–1297. doi: 10.1016/j.cortex.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A.J., Supalla T., Hauser P., Newport E.L., Bavelier D. Dissociating neural subsystems for grammar by contrasting word order and inflection. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7539–7544. doi: 10.1073/pnas.1003174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberecker R., Friedrich M., Friederici A.D. Neural correlates of syntactic processing in two-year-olds. J. Cogn. Neurosci. 2005;17:1667–1678. doi: 10.1162/089892905774597236. [DOI] [PubMed] [Google Scholar]
- Ojemann G., Mateer C. Human language cortex: localization of memory, syntax, and sequential motor-phoneme identification systems. Science. 1979;205:1401–1403. doi: 10.1126/science.472757. [DOI] [PubMed] [Google Scholar]
- Opitz B., Friederici A.D. Neural basis of processing sequential and hierarchical syntactic structures. Hum. Brain Mapp. 2007;28:585–592. doi: 10.1002/hbm.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Ouden D.-B., Saur D., Mader W., Schelter B., Lukic S., Wali E., Timmer J., Thompson C.K. Network modulation during complex syntactic processing. NeuroImage. 2012;59:815–823. doi: 10.1016/j.neuroimage.2011.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno C., Galluci M., Casarotti A., Castellano A., Falini A., Fava E., Giussani C., Carrabba G., Bello L., Caramazza A. Connectivity constraints on cortical reorganization of neural circuits involved in object naming. NeuroImage. 2011;55(3):1306–1313. doi: 10.1016/j.neuroimage.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Papoutsi M., Stamatakis E.A., Griffiths J., Marslen-Wilson W.D., Tyler L.K. Is left fronto-temporal connectivity essential for syntax? Effective connectivity, tractography and performance in left-hemisphere damaged patients. NeuroImage. 2011;58:656–664. doi: 10.1016/j.neuroimage.2011.06.036. [DOI] [PubMed] [Google Scholar]
- Partovi S., Konrad F., Karimi S., Rengier F., Lyo J.K., Zipp L., Nennig E., Stippich C. Effects of covert and overt paradigms in clinical language fMRI. Acad. Radiol. 2012;19(5):518–525. doi: 10.1016/j.acra.2011.12.017. [DOI] [PubMed] [Google Scholar]
- Passeri A., Capotosto P., Di Matteo R. The right hemisphere contribution to semantic categorization: a TMS study. Cortex. 2015;64:318–326. doi: 10.1016/j.cortex.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Perani D., Abutalebi J. The neural basis of first and second language processing. Curr. Opin. Neurobiol. 2005;15(2):202–206. doi: 10.1016/j.conb.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Petersson K.-M., Folia V., Hagoort P. What artificial grammar learning reveals about the neurobiology of syntax. Brain Lang. 2012;120:83–95. doi: 10.1016/j.bandl.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Połczyńska M. New tests for language mapping with intraoperative electrical stimulation of the brain to preserve language in individuals with tumors and epilepsy: a preliminary follow-up study. Poznań Stud. Contemp. Linguist. 2009;45:261–279. [Google Scholar]
- Połczyńska M., Curtiss S., Walshaw P., Siddarth P., Benjamin C., Moseley B.D., Vigil C., Jones M., Eliashiv D., Bookheimer S. Grammar tests increase the ability to lateralize language function in the Wada test. Epilepsy Res. 2014;108:1864–1873. doi: 10.1016/j.eplepsyres.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Połczyńska M., Benjamin C., Moseley B., Walshaw P., Eliashiv D., Vigil C., Jones,_M., Bookheimer, S. Role of the Wada test and functional magnetic resonance imaging in preoperative mapping of language and memory: two atypical cases. Neurocase: Neural Basis Cogn. 2015;21(6):707–720. doi: 10.1080/13554794.2014.977300. [DOI] [PubMed] [Google Scholar]
- Połczyńska M.M., Benjamin C.F.A., Japardi K., Frew A., Bookheimer S.Y. Language system organization in a quadrilingual with a brain tumor: implications for understanding of the language network. Neuropsychologia. 2016;86:167–175. doi: 10.1016/j.neuropsychologia.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Price A.R., Bonner M.F., Peelle J.E., Grossman M. Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. J. Neurosci. 2015;35:3276–3284. doi: 10.1523/JNEUROSCI.3446-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F. Brain embodiment of syntax and grammar: discrete combinatorial mechanisms spelt out in neuronal circuits. Brain Lang. 2010;112:167–179. doi: 10.1016/j.bandl.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Rodd J.M., Vitello S., Woollams A.M., Adank P. Localising semantic and syntactic processing in spoken and written language comprehension: an activation likelihood estimation meta-analysis. Brain Lang. 2015;141:89–102. doi: 10.1016/j.bandl.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Rofes A., Miceli G. Language mapping with verbs and sentences in awake surgery: a review. Neuropsychol. Rev. 2014;24:185–199. doi: 10.1007/s11065-014-9258-5. [DOI] [PubMed] [Google Scholar]
- Rofes A., Capasso R., Miceli G. Verb production tasks in the measurement of communicative abilities in aphasia. J. Clin. Exp. Neuropsychol. 2015;37(5):483–502. doi: 10.1080/13803395.2015.1025709. [DOI] [PubMed] [Google Scholar]
- Rofes A., Spena G., Miozzo A., Fontanella M.M., Miceli G. Advantages and disadvantages of intraoperative language tasks in awake surgery: a three-task approach for prefrontal tumors. J. Neurosurg. Sci. 2015;59:337–349. [PubMed] [Google Scholar]
- Roux F.E., Boulanouar K., Lotterie J.A., Mejdoubi M., LeSage J.P., Berry I. Language functional magnetic resonance imaging in preoperative assessment of language areas: correlation with direct cortical stimulation. Neurosurgery. 2003;52(6):1335–1347. doi: 10.1227/01.neu.0000064803.05077.40. [DOI] [PubMed] [Google Scholar]
- Sabsevitz D.S., Swanson S.J., Hammeke T.A., Spanaki M.V., Possing E.T., Morris G.L., Mueller W.M., Binder J.R. Use of preoperative functional neuroimaging to predict language deficits from epilepsy surgery. Neurology. 2003;60:1788–1792. doi: 10.1212/01.wnl.0000068022.05644.01. [DOI] [PubMed] [Google Scholar]
- Sammler D., Koelsch S., Ball T., Brandt A., Grigutsch M., Huppertz H.-J., Knösche T.R., Wellmer J., Widman G., Elger C.E., Friederici A.D., Schulze-Bonhage A. Co-localizing linguistic and musical syntax with intracranial EEG. NeuroImage. 2013;64:134–146. doi: 10.1016/j.neuroimage.2012.09.035. [DOI] [PubMed] [Google Scholar]
- Shapiro K., Caramazza A. Grammatical processing of nouns and verbs in left frontal cortex? Neuropsychologia. 2003;41(9):1189–1198. doi: 10.1016/s0028-3932(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Shetreet E., Friedmann N. The processing of different syntactic structures: fMRI investigation of the linguistic distinction between wh-movement and verb movement. J. Neurolinguistics. 2014;27:1–17. [Google Scholar]
- Skeide M.A., Brauer J., Friederici A.D. Syntax gradually segregates from semantics in the developing brain. NeuroImage. 2014;100:106–111. doi: 10.1016/j.neuroimage.2014.05.080. [DOI] [PubMed] [Google Scholar]
- Sportiche D., Koopman H.J., Stabler E.P. John Wiley & Sons Inc.; Hoboken: 2013. An Introduction to Syntactic Analysis and Theory. [Google Scholar]
- Stippich C., Mohammed J., Kress B., Hähnel S., Günther J., Konrad F., Sartor K. Robust localization and lateralization of human language function: an optimized clinical functional magnetic resonance imaging protocol. Neurosci. Lett. 2003;346:109–113. doi: 10.1016/s0304-3940(03)00561-5. [DOI] [PubMed] [Google Scholar]
- Thiel A., Habedank B., Herholz K., Kessler J., Winhuisen L., Haupt W.F., Heiss W.-D. From the left to the right: how the brain compensates progressive loss of language function. Brain Lang. 2006;98:57–65. doi: 10.1016/j.bandl.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Thothathiri M., Kimberg D.Y., Schwartz M.F. The neural basis of reversible sentence comprehension: evidence from voxel-based lesion symptom mapping in aphasia. J. Cogn. Neurosci. 2012;24:212–222. doi: 10.1162/jocn_a_00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsapkini K., Jarema G., Kehayia E. A morphological processing deficit in verbs but not in nouns: a case study in a highly inflected language. J. Neurolinguistics. 2002;15:265–288. [Google Scholar]
- Turken A.U., Dronkers N.F. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Front. Syst. Neurosci. 2011;5:1–20. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L.K., Wright P., Randall B., Marslen-Wilson W.D., Stamatakis E.A. Reorganization of syntactic processing following left-hemisphere brain damage: does right-hemisphere activity preserve function? Brain. 2010;133:3396–3408. doi: 10.1093/brain/awq262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L.K., Cheung T.P.L., Devereux B.J., Clarke A. Syntactic computations in the language network: characterizing dynamic network properties using representational similarity analysis. Front. Psychol. 2013;4:1–19. doi: 10.3389/fpsyg.2013.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman M.T. A neurocognitive perspective on language: the declarative/procedural model. Nat. Rev. Neurosci. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Hervé P.Y., Jobard G., Petit L., Crivello F., Mellet E., Zago L., Mazoyer B., Tzourio-Mazoyer N. What is right-hemisphere contribution to phonological, lexico-semantic, and sentence processing? Insights from a meta-analysis. NeuroImage. 2011;54(1):577–593. doi: 10.1016/j.neuroimage.2010.07.036. [DOI] [PubMed] [Google Scholar]
- Wang A., Peters T.M., de Ribaupierre S., Mirsattari S.M. Functional magnetic resonance imaging for language mapping in temporal lobe epilepsy. Epilepsy Res. Treat. 2012;2012:1–8. doi: 10.1155/2012/198183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J., Weber C., Mende M., von der Groeben F., Urbach H., Clusmann H., Elger C.E., Helmstaedter C. Multitask electrical stimulation for cortical language mapping: hints for necessity and economic mode of application. Epilepsia. 2009;50:2267–2275. doi: 10.1111/j.1528-1167.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Galantucci S., Tartaglia M.C., Rising K., Patterson D.K., Henry M.L., Ogar J.M., DeLeon J., Miller B.L., Gorno-Tempini M.L. Syntactic processing depends on dorsal language tracts. Neuron. 2011;72:397–403. doi: 10.1016/j.neuron.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.M., Galantucci S., Tartaglia M.C., Gorno-Tempini M.L. The neural basis of syntactic deficits in primary progressive aphasia. Brain Lang. 2012;122:190–198. doi: 10.1016/j.bandl.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S.M., Lam D., Babiak M.C., Perry D.W., Shih T., Hess C.P., Berger M.S., Chang E.F. Transient aphasias after left hemisphere resective surgery. J. Neurosurg. 2015;123:581–593. doi: 10.3171/2015.4.JNS141962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlotko E.W., Federmeier K.D. Two sides of meaning: the scalp-recorded n400 reflects distinct contributions from the cerebral hemispheres. Front. Psychol. 2013;4:181. doi: 10.3389/fpsyg.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley K.J. Statistical analysis of activation images. In: Jezzard P., Matthews P.M., Smith S.M., editors. Functional Magnetic Resonance Imaging: An Introduction to Methods. Oxford University Press; New York: 2001. pp. 251–270. [Google Scholar]
- Wright P., Stamatakis E.A., Tyler L.K. Differentiating hemispheric contributions to syntax and semantics in patients with left-hemisphere lesions. J. Neurosci. 2012;32:8149–8157. doi: 10.1523/JNEUROSCI.0485-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacà D., Nickerson J.P., Deib G., Pillai J.J. Effectiveness of four different clinical fMRI paradigms for preoperative regional determination of language lateralization in patients with brain tumors. Neuroradiology. 2012;54:1015–1025. doi: 10.1007/s00234-012-1056-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Activation in grammar and standard tests in epilepsy (E) and tumor (T) patients. Lesion images are included for all tumor patients. Images of three epilepsy patients (E3, E4, E13) show a prior resection cavity. Overall, the grammar tests generate more robust activations in language areas than the standard test.
Two-sample t-tests (uncorrected) run for patients with significant ANOVA tests (Table 4) to determine task type skew directionality (greater percent signal change in grammar or standard tests) within each ROI. Region stats with NaN denote regions which have been ablated by tumor masses or edema.