Abstract
Liver failure induced by systemic inflammatory response (SIRS) is often associated with mitochondrial dysfunction but the mechanism linking SIRS and mitochondria-mediated liver failure is still a matter of discussion. Current hypotheses suggest that causative events could be a drop in ATP synthesis, opening of mitochondrial permeability transition pore, specific changes in mitochondrial morphology, impaired Ca2+ uptake, generation of mitochondrial reactive oxygen species (mtROS), turnover of mitochondria and imbalance in electron supply to the respiratory chain. The aim of this review is to critically analyze existing hypotheses, in order to highlight the most promising research lines helping to prevent liver failure induced by SIRS. Evaluation of the literature shows that there is no consistent support that impaired Ca++ metabolism, electron transport chain function and ultrastructure of mitochondria substantially contribute to liver failure. Moreover, our analysis suggests that the drop in ATP levels has protective rather than a deleterious character. Recent data suggest that the most critical mitochondrial event occurring upon SIRS is the release of mtROS in cytoplasm, which can activate two specific intracellular signaling cascades. The first is the mtROS-mediated activation of NADPH-oxidase in liver macrophages and endothelial cells; the second is the acceleration of the expression of inflammatory genes in hepatocytes. The signaling action of mtROS is strictly controlled in mitochondria at three points, (i) at the site of ROS generation at complex I, (ii) the site of mtROS release in cytoplasm via permeability transition pore, and (iii) interaction with specific kinases in cytoplasm. The systems controlling mtROS-signaling include pro- and anti-inflammatory mediators, nitric oxide, Ca2+ and NADPH-oxidase. Analysis of the literature suggests that further research should be focused on the impact of mtROS on organ failure induced by inflammation and simultaneously providing a new theoretical basis for a targeted therapy of overwhelmed inflammatory response.
Abbreviations: ADP, adenosine diphosphate; ANT, adenine nucleotide translocator; ATP, adenosine triphosphate; Bax, BCL2-associated X protein; Bcl-xL, B-cell lymphoma-extra large; CLP, cecal ligation and puncture; CyP, cyclophilin; DAMP, damage-associated molecular pattern; ER, endoplasmic reticulum; ERK, extracellular-signal regulated; GRIM19, retinoic-interferon-induced mortality; H2O2, hydrogen peroxide; iNOS, inducible nitric oxide synthase; IL, interleukin; i.v., intravenous; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; LD50, median lethal dose; LPS, lipopolysaccharide; MAP, mitogen-activated protein; MKK4, MAP kinase kinase; MODS, multiple organ dysfunction syndrome; MOF, multiple organ failure; mPTP, mitochondrial permeability transition pore; mtROS, mitochondrial reactive oxygen species; NO, nitric oxide; O2•−, superoxide radical; ONOO−, peroxynitrite; PAMP, pathogen-associated molecular pattern; PCLS, precision cut liver slices; PiC, phosphate carrier; PK, protein kinase; ONOO−, peroxynitrite; RIP, receptor-interacting protein; ROS, reactive oxygen species; SHP1, src homology 1 domain containing protein tyrosine phosphatase; SIR, systemic inflammatory response; SIRS, systemic inflammatory response syndrome; STAT3, signal transducer and activator of transcription 3; TLR, Toll-like receptor; TNF alpha, tumor necrosis factor alpha; TNFR1, TNF receptor type 1
Keywords: Liver failure, Mitochondria, Reactive oxygen species, Signaling, Inflammation
Highlights
-
•
Relationship between mitochondrial dysfunction and high lethality upon sepsis.
-
•
Criteria to define critical for lethality mitochondrial dysfunction.
-
•
ATP, calcium, mitochondrial ultrastructure and apoptosis, upon inflammation.
-
•
Regulation of inflammatory processes by mitochondrial ROS.
Graphical abstract
1. Introduction
Mitochondrial dysfunction is often associated with multiple organ failure (MOF), also referred to as multiple organ dysfunction syndrome (MODS), induced by dysregulated systemic inflammatory response (SIR). This pathological process, known as systemic inflammatory response syndrome (SIRS) [1], [2], is a common cascade accompanying sepsis, trauma, burns, acute pancreatitis, ischemia, anaphylaxis and a number of other diseases. Despite huge efforts in the preclinical and clinical research field, pathogenesis of MOF is still not clearly understood. Mitochondria are good candidates for playing a key role in this process (reviewed in [3], [4], [5]), because they are essential components of almost all eukaryotic cells, controlling several important cellular functions, such as adenosine triphosphate (ATP) synthesis [6], regulation of Ca++ homeostasis (reviewed in [7], [8]), generation of reactive oxygen species (ROS) (reviewed in [9], [10], [11], [12], [13], [14]), activation of apoptosis (reviewed in [15]) and maintenance of intracellular redox potential.
The liver is one of the most susceptible organs to SIR [16], and manifestation of liver dysfunction nearly always accompanies SIRS [17]. Liver dysfunction, in turn, has dramatic consequences for the whole body, inducing encephalopathy and cerebral edema, coagulopathy, cardiovascular instability, respiratory and renal failure. Thus, liver failure itself may induce MOF contributing to the lethal fate of SIR. Consequently, prevention of liver dysfunction may ameliorate MOF/MODS and improve clinical outcome and survival of patients with SIRS.
Liver failure has already been associated with mitochondrial dysfunction [18], [19] and inflammation (reviewed in [20]), both contributing to a wide range of liver diseases. It has been shown that liver inflammation, accompanied by the activation of immune cells in liver tissue, is highly associated with hepatocellular carcinoma (reviewed in [21]) and both alcoholic and non-alcoholic fatty liver diseases (reviewed in [22]). This suggests that mitochondrial dysfunction links inflammation and liver failure.
Single studies usually address only selected mitochondrial functions instead of providing a complete view in the context of inflammation. This may be a reason for controversial data in the literature on the pathologic impact of mitochondrial dysfunction, ranging from critical to unimportant contribution to MOF/MODS. Another body of literature suggests that an increase in mitochondrial turnover can be a sign of mitochondrial dysfunction. These reports address the changes in mitochondrial biogenesis [23], [24], [25] or/and autophagy [26]; (reviewed in [27], [28]), assuming that these changes are due to an increased number of damaged/dysfunctional mitochondria, although the majority of mitochondria appeared normal [29].
The aim of this review was to summarize data which confirm or contradict contribution of specific mitochondrial functions to liver inflammation-induced organ failure. We analyzed the existing literature hypothesizing that there are two prerequisites for the critical role of mitochondrial dysfunction: (i) in all species which are susceptible to SIR, either mitochondrial structure or function(s) will be impaired in a similar manner, and (ii) if such impairment of mitochondrial function(s) occurs, then a similar damage should cause liver failure also in other pathological settings. Consequently, we consider that mechanisms of liver dysfunction have similar pathways in humans and in animal models.
1.1. Factors inducing acute SIR
The activation of the inflammatory response aims at combating pathogens. This reaction is induced by so-called damage-associated molecular pattern molecules (DAMPs) ([30], reviewed in [31]). A sub-group of DAMPs are microbial pathogen-associated molecular pattern molecules (PAMPs), such as structural components and nucleic acids of viruses (reviewed in [32]), lipopolysaccharide (LPS) of Gram-negative bacteria and peptidoglycan of Gram-positive bacteria (reviewed in [33]). Both DAMPs and PAMPs activate innate and adaptive immune responses predominantly via Toll-like receptors [34]. If the regulatory mechanisms of the inflammation fail, the response may become excessive, resulting in an overwhelming SIR. Although intended as a self-protection program, this undesirable side effect of the activation of the immune system can cause damage to host cells and induce MODS/MOF [35], [36], [37]. The mechanisms and pathways of MODS/MOF-inducing systemic inflammatory response are subject of many investigations and have already been extensively reviewed [38], [39], [40], [41], [42], [43], [44]. However, the exact mechanisms underlying this deleterious effect have still not clearly been uncovered. As a matter of fact, many mammals develop MOF in response to SIR, often leading to death. The mortality resulting from SIR in the clinics is still very high [45], [46], [47], even in modern intensive care units (reviewed in [4]).
The most common and reproducible way to induce SIR in experimental animal models is administration of the pathogen-associated molecular pattern molecule LPS, a Gram-negative bacterial toxin. To date, the majority of mechanistic data on SIR are based on LPS models. LPS induces MOF/MODS in different species in a similar way as can be characterized by elevated levels of circulating tissue damage markers [48], [49]. However, the concentrations of LPS needed to induce similar damage as well as LD50 differ from species to species (Table 1). A prominent feature of LPS-induced SIR is a dose-dependent increase in TNF-α levels. Xenobiotics, like D-Galactosamine sensitize hepatocytes to apoptosis triggered by TNF-α, at least partly by a transcriptional block. In such models, a small amount of LPS, as little as a few micrograms/kg is able to induce severe hepatic damage [50]. Moreover, it was demonstrated that desensitization of the mitochondrial permeability transition pore leads to a protection against liver injury despite the maintained early TNF-α response [18]. Accordingly, on the one hand, the above mentioned evidences demonstrate crucial role of mitochondria in the process. On the other hand, inter-species differences in the sensitivity to LPS might be connected to regulatory characteristics of pore opening as well. If we consider mitochondrial dysfunction as the critical issue for organ failure upon SIR, we expect that different doses of LPS in different species should induce similar changes in mitochondria. This consideration assumes that intracellular signaling pathways mediating mitochondrial dysfunction, predominantly define the sensitivity to LPS and/or other DAMPs/PAMPs in various species. The question whether or not different species in fact manifest similar impairment of liver mitochondria upon SIR will be discussed in the following sections.
Table 1.
Susceptibility of different species to LPS.
Registry of toxic effects of chemical substances [51].
Maximum dose reported.
1.2. Effect of SIR on mitochondrial structure
Ultra-structural alterations of liver mitochondria have been reported from various animal species and as well as from septic patients. Crouser et al. observed mild to moderate mitochondrial swelling, and occasionally high-amplitude swelling with concomitant loss of mitochondrial membrane integrity in experiments with LPS-treated feline [52]. They also reported that cyclosporine A pretreatment attenuated LPS-induced mitochondrial ultra-structural abnormalities, suggesting the involvement of permeability transition pore opening in mitochondrial swelling [53]. Hypertrophic mitochondria with reduced matrix electron density and irregular cristae were also found in post-mortem samples of critically ill patients [54]. In contrast, in another study, most mitochondria of liver samples taken from septic patients were normal in appearance, with intact organelle membrane [29]. These results were confirmed in a parallel study performed in mice. Livers of animals subjected to cecal ligation and puncture (CLP) did not manifest consistent abnormalities in mitochondria or nuclei [29]. Similarly, in another CLP mice study, the vast majority of liver mitochondria in the CLP group appeared normal, but increased number of intracellular vacuoles were observed in hepatocytes and identified as autophagosomes [55]. Furthermore, electron microscopic examination of isolated liver mitochondria and liver tissue taken from rats subjected to LPS did not evoke changes in the morphology of mitochondria neither in isolated mitochondria nor in liver tissue [56]. Also an increased number of intracellular vacuoles were observed in hepatocytes in close vicinity to mitochondria in liver samples from LPS-treated rats, which were identified as dilated endoplasmic reticulum (ER) [57].
Taken together, the majority of papers reported normal appearance of mitochondria. However, intracellular vesicles have often been found which were either interpreted as autophagosomes or dilated ER. Both of these structures occurred being linked to mitochondria either functionally (autophagosomes) or spatially (dilated), suggesting that mitochondria could be indirectly involved. The findings discussed above show that there is no obvious coincidence between SIR and mitochondrial ultra-structural changes, although the data indirectly imply involvement of mitochondria. This suggests that not morphological but functional changes in mitochondria may be the key for understanding their pathological impact.
1.3. Respiratory function of mitochondria
Oxidative phosphorylation is the major function of mitochondria providing energy in form of ATP for eukaryotic cells. The conversion rate of adenosine diphosphate (ADP) to ATP linked to oxygen consumption by the electron transport chain of mitochondria can easily be evaluated by measuring the rate of mitochondrial respiration. State 3 respiration upon addition of ADP is directly linked to ATP synthesis and this respiration state is determined in the majority of reports on mitochondrial respiration. State 3 respiration can be determined only in freshly isolated mitochondria [58] or in freshly prepared homogenates [59]. In frozen samples, only enzyme activities of single respiratory chain complexes can be determined. This, however, does not necessarily reflect the capacity to synthesize ATP. Tissue homogenate is probably the best way to estimate mitochondrial function, because the isolation of mitochondria may theoretically lead to the loss of a fraction of mitochondria, and additional damage (e.g. uncoupling).
Impaired respiratory function of mitochondria caused by LPS treatment was predominantly determined in the pig [60], cat [52] and baboon [61]. A recent review by Jeger et al. analyzed the existing publications on rodents in experimental models of CLP, fecal peritonitis and LPS challenge [62]. The authors summarized publications on mitochondrial function in skeletal muscles, heart and liver. The majority of publications showed that there were no changes in mitochondrial function in any organ. However, in a few cases, mitochondrial function was either improved or impaired. The majority of published reports suggest that rodents are less susceptible to mitochondrial dysfunction than porcine or feline although all these species manifest MOF. Thus, there is no consistent coincidence between SIR and respiratory dysfunction of mitochondria. The fact that respiratory function shows such strong variations from species to species and between different experimental models suggests that impairment may not be due to primary but secondary processes occurring in SIR models. In fact, SIR often leads to circulatory disturbances, resulting in impaired tissue perfusion and secondary hypoxia. Consequently, not inflammatory mediators directly, but circulatory failure accompanied by tissue hypoxia may be the reason for SIR-induced mitochondrial impairment.
1.4. The impact of hypoxia and inflammatory mediators on mitochondria upon SIRS
SIRS and sepsis is characterized by profound alterations of hemodynamics. This is observed, on one hand, at the level of systemic circulation, and on the other hand, in deteriorating tissue perfusion and microcirculation disturbances [63], [64], which are associated with a poor outcome [65]. It is important to note that the survival in porcine and sheep models of severe SIR is defined not by direct effect of DAMPs/PAMPs on target cells, but by secondary circulatory effects such as increase in pulmonary arterial pressure probably via release of endothelin-1 [66]. Acute pulmonary hypertension may cause respiratory failure, hypoxemia and hypoxia including liver tissue. SIR also causes alteration in hepatic circulation at the level of both macro- and microcirculation. As a specific feature of liver dysfunction, it has been reported that severe SIR impairs portal venous blood inflow (reviewed in [67]). Nitric oxide (NO), endothelin-1, and carbon monoxide are considered to be the major factors disturbing liver circulation [68]. NO can be the mediator which predominantly defines the vascular reaction upon systemic inflammation. In contrast to pigs and humans, white blood cells of rodents strongly express inducible nitric oxide synthase (iNOS) causing excessive NO production. For example, there were no increased NO levels upon SIRS in pigs [60], while a drastic increase in NO levels was observed in a rat model [69]. By its well-known vasorelaxant effect, NO is able to lower vascular resistance. This particular difference in the regulation of circulation upon inflammation may be the reason for different susceptibility of circulation to SIR in different species. Thus, circulatory failure can be the reason for cellular damage and mitochondrial dysfunction upon SIR, even explaining the differences between species. Beside circulatory failure, it was shown that cellular damage can be induced by pro-inflammatory cytokines such as tumor necrosis factor (TNF) alpha via induction of oxidative stress (reviewed in [70]) or via intracellular signaling pathways of acute-phase genes controlling cell damage [71]. Therefore, it is difficult to define exactly the reason for cellular damage in in vivo models. Additionally, since hypoxia and inflammatory mediators occur and interact simultaneously, it is very difficult to separate their biological effects in vivo.
In a recent study, this issue was addressed in a model with precisely cut liver slices (PCLS) [72]. PCLS are an ex vivo model of liver tissue, maintaining cell-cell and cell-extracellular matrix interactions without the influence of systemic processes. In this study, hypoxic incubation of PCLS resulted in accumulation of liver cellular damage markers and mitochondrial dysfunction, whereas incubation with inflammatory mediators caused only release of liver damage markers. With regard to the effects of NO and hypoxia during inflammation, the rapid diffusion of NO and its potent action as an inhibitor of mitochondrial respiration has been proposed to perform a salutatory function, extending the tissue oxygen gradient away from vessels to distal hypoxic regions [73] There is evidence disturbances in this effect may adversely affect tissue perfusion under pathologic conditions [74], [75].
This data confirm the aforementioned assumption that respiratory function of mitochondria in in vivo SIR-models is impaired by secondary circulatory failure inducing hypoxia rather than by direct interaction of inflammatory mediators with liver cells. This can explain the species- and model-specific differences in mitochondrial respiration upon SIR.
Above, we described reports describing mitochondrial morphology/function determined ex vivo. However, the absence of mitochondrial dysfunction in ex vivo tests does not necessarily mean that mitochondrial function is also not impaired in vivo because inhibition of mitochondria can be reversible, for instance, by inducing de-phosphorylation of cytochrome c oxidase [76], [77] or reversible binding of NO to cytochrome c oxidase [78]. The performance of mitochondrial oxidative phosphorylation in liver tissue in vivo can be estimated by measurement of ATP levels.
1.5. ATP levels in tissues upon SIRS
In contrast to reports on mitochondrial morphology and function, data on ATP levels upon SIR are rather homogeneous, showing a consistent decrease in ATP levels in different tissues. The study on septic patients carried out by the group of Singer demonstrated that skeletal muscle ATP concentrations were approximately two times lower in patients with sepsis who subsequently died compared to septic patients who survived and to control group; ADP levels were also increased in non-survivors [3]. Similar difference in ATP levels was determined in lymphocytes of septic patients. Survivors had approximately two times higher ATP levels compared to non-survivors [79]. Also studies performed in various animal models reported a similar decrease in ATP content in rodents and other species, as well as a decline in energy charge (defined as [ATP] + 0.5 [ADP]/[AMP]) which is a more precise measure of bioenergetic status [80]; see also corresponding references [81], [82], [83], [84] in Table 2. Importantly, in contrast to mitochondrial respiratory function, there are no clear species specific differences in ATP levels.
Table 2.
Papers reporting decreased ATP levels and energy charge upon SIR in animal models.
In animal models, Hart et al. [83] point out that the results on ATP levels in sepsis are conflicting and appear to be important in more severe models and may depend on the loss of compensatory mechanisms. Duvigneau et al. demonstrated that the changes in ATP levels do not correlate with the data on respiratory activity of mitochondria determined ex vivo [91]. It has been shown that the respiratory activity drops and recovers after LPS injection, but ATP levels drop and do not recover [91]. This set of data again suggests that mitochondria are reversibly inhibited in the liver tissue in situ, and that this inhibition is abolished after their isolation.
There are two methodological explanations for the reversible inhibition of mitochondrial function in vivo. First, from methodological considerations, mitochondrial function is tested with saturating amounts of substrates (e.g. tricarboxylic acid cycle substrates), which is not the case in vivo. Second, SIR is accompanied by the release of a number of gas messengers such as NO and carbon monoxide which can inhibit mitochondria in vivo, but will be substituted by non-physiologically high oxygen concentrations ex vivo. Irrespective of which process decreases ATP levels, the question is whether the drop in ATP levels can cause liver dysfunction.
1.6. ATP levels in other disorders
Summarizing the results discussed to this point, the drop in ATP levels by a factor of two was the most reproducible change related to mitochondrial function. The next question is whether or not such a 2-fold decrease in ATP levels alone is sufficient to cause organ/liver failure observed in SIRS. An approximately 2-fold (40%) drop in ATP level in liver was observed after partial hepatectomy and was shown to give a stimulus for regeneration [92]. Of particular interest is the paper by Latta et al. The authors reduced the levels of ATP to 20% compared to control levels by an infusion of phosphate-trapping fructose and tested how ATP depletion influences the rate of TNF-induced hepatocyte death [93]. Unexpectedly, decreased levels of ATP prevented mitochondrial dysfunction, activation of type II caspases, DNA fragmentation, and the levels of apoptotic markers after exposure to TNF alpha [93], suggesting that partial ATP depletion can be even beneficial.
Intracellular ATP levels play an important role in the crosstalk between necrosis and apoptosis (reviewed in [94]). However, elevation of apoptotic markers is not always accompanied by increased occurrence of apoptotic cells. In a rat model, severe SIR has been shown to initiate apoptotic signaling (Bax/Bcl-xL ratio) [56]. Interestingly, the apoptosis inducing factor was not translocated from mitochondria to the nucleus suggesting that apoptosis was not executed [56]. In the same model, ATP levels dropped approximately 2-fold [91], suggesting that partial ATP depletion might prevent apoptosis.
Probably, partial depletion of ATP, on one hand, can already inhibit development of apoptosis, while, on the other hand, ATP levels are still high enough to also prevent necrosis. Induction of apoptosis has been shown to require ATP levels above 40–50% compared to control levels [93]. This suggests that a moderate decrease in levels of ATP can be an adaptive reaction preventing the execution of programmed cell death. In contrast, nearly full ATP depletion occurs during total liver ischemia, or incubation of hepatocytes with oligomycin, which increases the death rate of hepatocytes [95] and induces liver necrosis [96]. Additionally, an approximate 2-fold drop in ATP levels was shown in erythrocytes of elderly individuals compared to young ones [97], muscles of patients with Friedrich ataxia [98] and trauma patients [99]. In our opinion, these results suggest that a mild drop in ATP levels is likely an unspecific response to diverse pathological settings which can be protective rather than deleterious by inhibiting the activation of apoptosis, in particular, those pathways mediated by mitochondrial dysfunction.
1.7. Mitochondrial calcium
There is a number of reports suggesting that altered Ca++ metabolism has an impact in SIR [100], [101], [102], although impaired Ca++ metabolism is predominantly associated directly with hypoxia and ischemia, occurring for instance during transplantation [103]. LPS treatment has been reported to produce a slight, but significant increase in total hepatic Ca++ content while CLP did not affect this parameter neither in liver nor in heart, demonstrating a slight Ca++ overload in endotoxicosis but not in abdominal inflammation [104]. It has been suggested that hepatic intracellular regulation of Ca++ may play a role in acute inflammation [105]. Furthermore, it has been shown that Ca-ethylenediaminetetraacetic acid (Ca-EDTA) reduces the bacterial burden in liver, suggesting that Ca++ interferes with immune response, likely stimulating killing of bacteria [106]. In addition, it has been shown that, upon inflammatory stimuli, iNOS is up-regulated in hepatocytes in a Ca++ dependent manner [107]. On the other hand, up-regulation of iNOS is driven by ROS too, implying that Ca++ pathway may operate via/ or in a synergistic way with ROS release. The link between Ca++ and mitochondrial ROS was also suggested by Rowlands et al. [108]. They provided evidence that controlled TNF alpha infusion leads to sustained mitochondrial Ca++ elevation and mitochondrial ROS generation in the pulmonary endothelium. The data on Ca++ metabolism suggest that Ca++ is not a key player involved in acute SIR and its involvement is likely mediated by mitochondrial reactive oxygen species (mtROS).
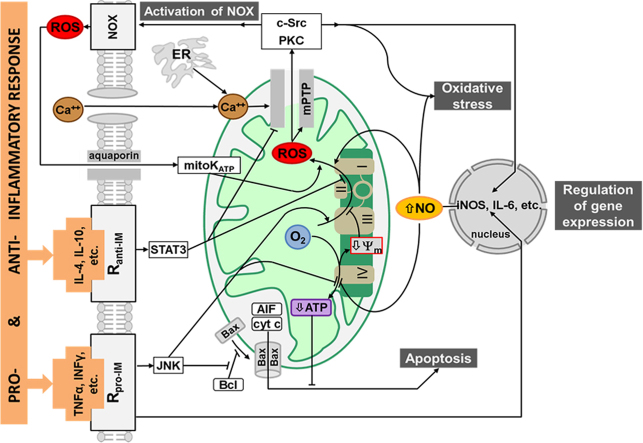
1.8. Mitochondrial ROS (Fig. 1)
Fig. 1.
Mitochondrial ROS-signaling in liver cells upon acute inflammation. Pro-inflammatory mediators (e.g. TNFα) decrease ATP levels and activate mtROS production, but do not control directly the release of mtROS in cytoplasm. Anti-inflammatory mediators (e.g. IL-4) decrease mtROS generation. The release of mtROS via mPTP is controlled by Ca2+, and ROS originated from both mitochondria and NOX. In addition, pro-inflammatory mediators shift the Bcl/Bax balance in the direction of apoptosis, but the execution of apoptosis is inhibited by low levels of ATP in hepatocytes. Simultaneously, pro-inflammatory mediators up-regulate iNOS thereby increasing NO levels, which activates feed forward loop including mtROS and iNOS. This loop may also induce oxidative stress if NO and ROS are generated in a great excess. Another feed forward loop includes mtROS and NOX, the latter further accelerates both mtROS production and NOX activity. Abbreviations: AIF–apoptosis inducing factor, ATP–adenosine triphosphate, Bax–B-cell lymphoma-associated X protein, c-Src–cellular sarcoma, cyt c–cytochrome c, ER–endoplasmic reticulum; IL–interleukin; INF–interferon; iNOS–inducible nitric oxide synthase, JNK–c-Jun N-terminal kinase, mitoKATP–adenosine triphosphate sensitive potassium channel, mPTP–mitochondrial permeability transition pore, NO–nitric oxide, NOX–NADPH-oxidase, Ranti-IM–receptors for anti-inflammatory mediators, O2–molecular oxygen, PKC–protein kinase C, ROS–reactive oxygen species, Rpro-IM–receptors for pro-inflammatory mediators, STAT-signal transducer and activator of transcription, TNF–tumor necrosis factor, Δψ–membrane potential, I-IV–complexes I-IV of electron transport chain.
Depending on cell type and state, mitochondria are the major source of ROS in cells. Primary species generated by mitochondria are superoxide radicals (O2•−) and hydrogen peroxide (H2O2), the latter resulting from dismutation of two O2•−. Superoxide and H2O2 are not especially dangerous ROS, but toxic peroxynitrite (ONOO−) can be formed upon reaction of O2•− with NO, and H2O2 can give rise to toxic hydroxyl radicals upon reaction with transition metals. In the past, generation of mitochondrial ROS (mtROS) was only associated with oxidative stress. In the liver, for instance, excessive generation of mtROS is associated with the development of non-alcoholic steatohepatitis (reviewed in [109]). Recently, ROS were recognized as signaling molecules in liver and other organs (reviewed in [9], [11], [12], [13], [14], [110]). Critical role of mitochondrial ROS was suggested for signal transducer and activator of transcription (STAT) signaling and development of hepatocellular carcinoma [111], [112].
An increasing number of studies demonstrate the essential role of mtROS in the regulation of the activity of the immune system. It has been shown that mtROS can modulate activity of NADPH oxidase in immune cells and in endothelium [113], [114], [115], thereby influencing bactericidal activity [116]. MtROS are also involved in the production of inflammatory cytokines [117] and in the regulation of T cell activation [118], [119] and antigen presentation by Kupffer cells [120]. In the latter study, Kupffer cells were either incubated with antioxidants or inhibitors of NADPH oxidase/xanthine oxidase, or with inhibitors of the electron transport chain. Each treatment significantly decreased T-cell proliferation in response to antigen presentation by Kupffer cells [120]. These data suggest that also in Kupffer cells the activity of NADPH oxidase is regulated by mtROS.
More recently, it has been shown that mtROS regulate inflammatory response not only in immune cells, but also in hepatocytes. Using direct detection of mitochondrial ROS and mitochondria targeted antioxidants, Weidinger et al. have demonstrated that mtROS and NO generate a feed forward loop, so-called NOS-ROS cycle, which is activated by inflammatory mediators and accelerated by mtROS in hepatocytes [69]. NOS providing NO for this cycle is located in cytosol rather than in mitochondria [121]. Since both O2•− and NO are involved in this cycle, it is likely that they react to yield ONOO−, which damages hepatocytes as shown by release of hepatocyte damage markers [69]. Recent reports revealed that an increase in mtROS production may be due to sustained activation of c-Jun N-terminal kinase (JNK) pathway [122]. The activation of the JNK pathway, occurring in liver upon inflammation [123], resulted in inactivation of Src tyrosine kinase on the inner mitochondrial. This, in turn, inhibits electron transport and increases ROS release [122]. The STAT3 pathway, another signaling cascade, occurring upon inflammation, inhibits mtROS production [124], and has been shown to inhibit the opening of the mitochondrial permeability transition pore [125]. Of note, participation of mtROS in intracellular signaling can be regulated not only by increase in ROS production but also by the release of ROS into the cytoplasm. It has been reported that permeability transition pore may regulate the release of ROS for cellular signaling (reviewed in [126]). Furthermore, treatment with LPS facilitates the release of ROS from liver mitochondria [127]. These data indicate that mtROS generation is tightly controlled at different levels and, upon release to cytoplasm, accelerate inflammatory reactions.
Thus, the contribution of mtROS to intracellular signaling is controlled at least three sites, (i) the generation of ROS at the respiratory chain, (ii) the release of ROS into cytoplasm, and (iii) the reaction of ROS with targets mediating signal transduction in the cytoplasm. Below, we consider in details how mtROS generation is regulated at each of these sites (Fig. 1).
2. Control of ROS production at the mitochondrial respiratory chain (Fig. 2)
Fig. 2.
Interaction of regulatory factors with ATP and ROS production of the mitochondrial electron transport chain. At least three major signaling pathways (STAT3, JNK and NO) regulate mtROS production under acute inflammation. Signal transducer and activator of transcription protein 3 is activated by phosphorylation by Janus Kinase (JAK) proteins. After phosphorylation, STAT3 binds directly to GRIM-19, a subunit of mitochondrial ETC complex I, and is then transported into mitochondria, where it enhances activity of complex I, thereby enhancing ATP synthesis, and reduces ROS production. Phosphorylated JNK1/2 forms a complex in the outer mitochondrial membrane with MKK4, Bax and Sab. Then, this signal will be transmitted to the inner mitochondrial membrane by SHP1, which decreases complex IV protein phosphorylation causing a decrease in ATP synthesis and an increase in ROS production. As a third pathway, nitric oxide and peroxynitrite are able to directly inhibit complex I and IV causing a drop in ATP levels, and increase ROS formation. Abbreviations: ATP–adenosine triphosphate, Bax– B-cell lymphoma-associated X protein, cyt c–cytochrome c, GRIM-19–gene associated with retinoid interferon induced cell mortality 19, H2O2–hydrogen peroxide, IMM–inner mitochondrial membrane, IMS–intermembrane space, JNK–cJun N-terminal kinase, MKK–mitogen-activated protein kinase kinase, NO–nitric oxide, ONOO–peroxynitrite, O2•-–superoxide radical, O2–molecular oxygen, OMM–outer mitochondrial membrane, Q–Q-cycle, Sab–SH3BP5, STAT–signal transducer and activator of transcription, CI-CV–mitochondrial electron transport chain complex I–V.
The primary ROS, the superoxide radical, is produced upon inflammation predominantly at complex I of mitochondrial respiratory chain. MtROS production is linked to mitochondrial membrane potential and ATP production. There are four major mechanisms regulating ROS at respiratory chain upon acute inflammation, namely STAT3, JNK, NO signaling and p66Shc, exerting direct control of mitochondrial ROS production, interacting with complex I, IV and cytochrome c, respectively.
2.1. STAT 3
STAT 3 will first be phosphorylated by receptor-interacting protein (RIP) 1 on serine 727 as a result of various activation signals through extracellular-signal regulated (ERK) 1, ERK2, p38, JNK and mitogen-activated protein (MAP) kinases [128] (in addition to tyrosine 705 phosphorylation, mediated by Janus kinase (JAK) [129]). This increases its affinity to retinoic-interferon-induced mortality (GRIM)19 and formation of STAT3-GRIM19 complexes. Formed complexes will be transported to mitochondria and subsequently accumulate at complex I. Since GRIM19 is a functional element of complex I, this association will accelerate ATP synthesis and simultaneously decrease ROS formation (Fig. 2). It has been assumed that not only GRIM19, but also STAT3 is required for optimal functioning of complex I [130].
2.2. JNK
JNK 1–3 are members of the MAP kinase family and are activated by a wide range of pro-inflammatory cytokines, such as TNF-alpha and interleukin (IL)-1 (reviewed in [131]). The best characterized pathway of JNK activation is the TNF-TNF receptor type (TNFR)1 cascade. The formation of TNF-TNFR1 (Fig. 1) rapidly activates JNK. Activated (phosphorylated) JNK forms a protein complex with MAP kinase kinase (MKK)4, Sab and Bax in the outer mitochondrial membrane [132] (Fig. 2).
Phosphorylation of Sab by p-JNK leads to dephosphorylation of Src in the inner mitochondrial membrane, presumably by a src homology 1 domain containing protein tyrosine phosphatase (SHP1)-dependent mechanism [122]. Consequently, mitochondrial respiratory capacity declines and ROS production rises, leading to sustained activation of JNK-signaling by a self-sustaining circle, ultimately resulting in high ROS levels and subsequent mitochondrial permeability transition pore opening.
An additional regulatory pathway is exerted by JNK through the activation of p66Shc, an alternatively spliced transcript from gene SHC1. It was first published in 2005 that p66Shc can produce ROS acting as an oxidoreductase and transferring electrons from mitochondrial cytochrome c to molecular oxygen, producing ROS [133]. The role of p66Shc was shown so far in hypoxia-reoxygenation and pathological states associated with elevated ROS levels. The role of mitochondrial p66Shc was postulated in the ROS-induced ROS release [134], [135], leading ultimately to induction of mitochondrial permeability transition.
2.3. NO
Nitric oxide interacts with complex I [136] and complex IV [137] of the mitochondrial respiratory chain. The inhibition of electron flow through the respiratory chain from either NADH or succinate to molecular oxygen causes electron accumulation in the electron transport chain. This increases the probability of electron leak from respiratory chain and one-electron reduction of oxygen yielding O2•− [138]. Simultaneously with increased O2•− generation, the inhibition of electron transfer also decreases membrane potential and consequently ATP synthesis in mitochondria [139].
3. Control of ROS release to cytoplasm (Fig. 3)
Fig. 3.
Mechanism regulating reactive oxygen species release into cytoplasm. Under normal conditions, mPTP is impermeable (A). Under inflammatory and other pathologic conditions, two pathways are repeatedly described in the literature. The first one is ROS-dependent ROS release (B). Elevated ROS levels facilitate oxidation of cysteines at different sites of mitochondrial F-ATP synthase. The first, called S-site, is associated with the voltage sensor of the mPTP. The second, termed as “P-site”, can be affected by oxidation-reduction state of pyridine nucleotides. An additional site is located on the outer surface of the inner mitochondrial membrane (here indicated as “O”) which can modulate the mPTP opening in a two-step reaction. The second mechanism is Ca++-dependent (C). Increased concentrations of Ca++ and cyclophilin D convert the F-ATP synthase to a non-specific pore which facilitates the release of ROS from mitochondria to the cytosol. Phosphorylated STAT3 can inhibit the opening of the mitochondrial permeability transition pore by binding to Cyp D. Abbreviations: ATP—adenosine triphosphate, Cyp D—cyclophilin D, ROS—reactive oxygen species.
The regulation of ROS release into cytoplasm is controlled by two distinct mechanisms, namely the Ca++- and ROS- mediated ROS release into cytoplasm. Both mechanisms are realized by ROS diffusion via opened mPTP (Fig. 3). The exact structure of the mPTP is not yet clarified, although there are different models. In the first model, the mPTP is formed at the adenine nucleotide translocator (ANT). The ANT is the most abundant protein in the inner mitochondrial membrane, transporting ATP out of mitochondria and ADP back into the matrix. Different reports suggest that this channel consist of two ANT units or of one ANT and one phosphate carrier (PiC) unit (reviewed in [140]). More recently, it has been suggested that not the ANT but the F-ATP synthase (Fig. 3a) forms the mPTP channel [141]. This assumption is supported by the fact that, in the absence of ANT, the formation of the mPTP can be detected [142].
3.1. ROS induced ROS release
It has been shown that, upon inflammation, complex I generates substantially more ROS than at normal conditions [4] and induces the formation of mPTP. As a result, mtROS are released to the cytoplasm via mPTP. This phenomenon is called ROS-induced ROS release. There are, at least, three targets of oxidative agents contributing to increased probability to mPTP opening (Fig. 3b). The first class of cysteines, termed as ‘S-site’ is associated with the voltage sensor of the mPTP, where vicinal SH groups on cysteine residues can be oxidized or reduced (probably through glutathione on the matrix side of IMM) [143]. The second target, called ‘P-site’, is modulated by the oxidation-reduction state of pyridine nucleotides [144]. Finally, an additional site of action was described by Costantini et al. [145], as part of a two-step reaction. In this reaction, SH groups, located most probably on the outer surface of IMM, react with oxidants, which subsequently expose a thiol group for a second oxidation step, modulating the mPTP opening directly.
3.2. Ca++ mediated ROS release
The pore-forming mitochondrial F-ATP synthase could also be activated by Ca++ [146]. The critical component of this mechanism is mitochondrial cyclophilin (CyP)-D exhibiting peptidyl-prolyl cis–trans isomerase activity. Pi facilitates the binding of CyP-D to the F-ATP synthase, thereby converting it to a non-specific pore, which enables mtROS to be released into the cytoplasm. [146] (Fig. 3c). It has been suggested that a decrease in the membrane potential, induced by NO or other factors (Fig. 1) can also open the mPTP [147].
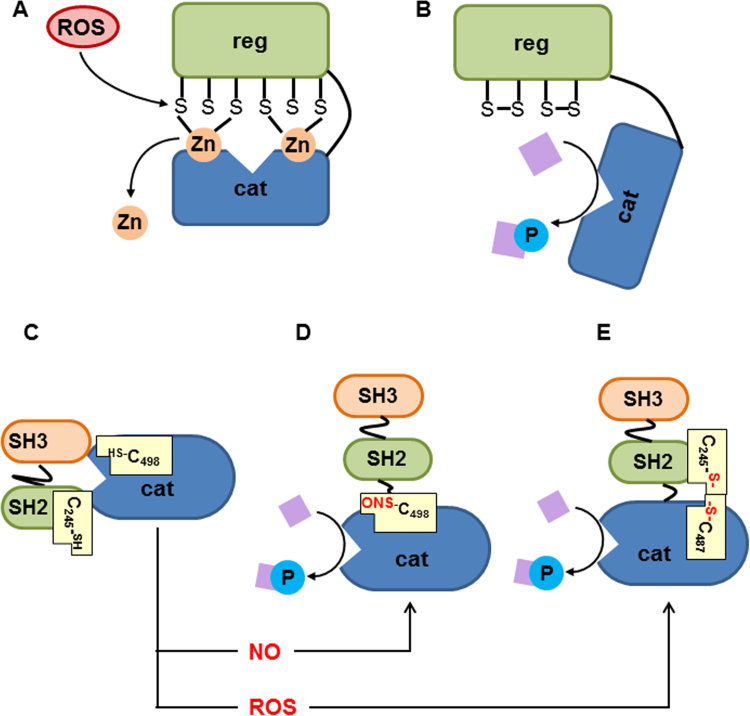
4. Activation of signaling pathways (Fig. 4)
Fig. 4.
Mechanism of ROS-mediated signaling pathways in cytoplasm. Upon inflammation, ROS predominantly activate two kinases, PKC and c-Src. Under normal conditions, PKC is kept in an inactive “closed” configuration. The zinc finger at the regulatory unit, a complex between SH groups and zinc atoms, defines this configuration, which does not allow phosphorylation. ROS can interact with SH groups, thereby disrupting this binding. This results in the release of zinc (A) and activation (B) of PKC. Similar to PKC, c-Src is inactive under normal conditions. In this catalytically inactive conformation, the kinase domain of c-Src interacts with the SH2-domain (C). The activation of c-Src may occur through S-nitrosylation of cysteine 498 of kinase domain by NO (D) or intermolecular disulfide bond formation between cysteine 245 and cysteine 487 by ROS (E). ROS oxidize these SH groups linking catalytic domain and SH2 by means of S-S bridge (E). Abbreviations: cat—catalytic subunit, cys—cysteine, H2O2—hydrogen peroxide, NO—nitric oxide, reg—regulatory subunit, SH—Src homolog.
As mentioned above, the two major pathways regulated by mtROS in liver are the activation of NADPH oxidase and up-regulation of several inflammatory genes. These signaling pathways are turned on by two kinases, PKC and c-Src tyrosine kinase (c-Src). Both kinases are located in the cytoplasm and can be activated by ROS. Thus, the interaction between ROS and kinases is the third and the last phase involving mtROS mediated activation of signaling cascades. Logically, this phase is dependent on the concentration and availability of kinases in single types of cells and can also be influenced by endogenous antioxidant systems. The activation of kinases by ROS is based on the reaction with specific SH groups of PKC and c-Src. This reaction can be catalyzed by superoxide, hydrogen peroxide and other ROS. It has been shown that thiyl radicals are intermediates of this interaction. Thiyl radicals further generate ROS, suggesting that the reaction between protein thiols and ROS is a specific signaling interaction, which does not influence antioxidant capacity of the cells [148] (Fig. 4).
4.1. ROS induced activation of PKC
PKC has a unique structure containing two domains, regulatory and catalytic, and has two types of cysteine-rich regions present in both domains. Critical for activation are the cysteine-rich domain-binding zinc atoms, keeping PKC in an inactive configuration. Oxidative modification of these groups by ROS causes the so-called cofactor independent activation of PKC, which becomes able to phosphorylate target proteins [149]. However, further increase in ROS levels causing modification of SH residues in catalytic center can inactivate PKC (reviewed in [150]).
4.2. ROS induced activation of c-Src
It has been shown that c-Src can be activated by two mechanisms linked to the formation of ROS, namely those mediated by NO and ROS. c-Src tyrosine kinase contains three major domains, the catalytic domain, and SH2 and SH3, domains critical for the activation of enzyme. The inactive enzyme occurs in a “closed form” which does neither allow interacting with downstream proteins at SH2 and SH3 domains nor executing kinase activity at the catalytic domain. This “closed configuration” can be turned in the active form by NO [151]. NO nitrosylates Cys498, which forms a link between SH2 and catalytic domain. In this configuration, the SH2 and SH3 domains are enabled to bind downstream signaling proteins and the catalytic domain phosphorylates them [151]. Another ROS-mediated mechanism linking SH2 and catalytic domain includes ROS-mediated oxidation of SH groups of Cys245 located in SH2 and Cys487 located in catalytic domain. Cys245 and Cys487 form an S-S bridge activating the enzyme [152].
5. Conclusion
The available literature does not uniformly report changes in mitochondrial structure and major functions upon acute inflammation. Inflammation does only slightly and reversibly inhibit mitochondrial respiratory function, causing an approximate 2-fold decrease in tissue ATP levels. This decrease seems to be sufficient to inhibit apoptosis, but not sufficient to induce acute necrotic cell death, although in the long run, upon chronic inflammation, this may lead to pathological changes in liver metabolism. We assume that necrotic cell death arises from profound impairment of mitochondrial function as a result of secondary to inflammation circulation failure followed by hypoxia. MtROS rather than low ATP levels contribute to acute liver failure induced by inflammation. MtROS are acting either via intracellular signaling cascades, or via induction of oxidative stress, damaging intracellular structures. The available literature suggests that signaling cascades, such as feed forward activation of NADPH-oxidase and up-regulation of inflammatory genes (ROS-NOS cycle) play a predominant role in inflammation-related action of mtROS. ONOO−, which escapes from the ROS-NOS cycle, and free iron, released from intracellular depots, seem to be the major pro-oxidants operating upon inflammation and hypoxia, respectively. The signaling action of mtROS is strictly controlled at, at least, three points, (i) at the site of ROS generation at complex I, (ii) the site of mtROS release in cytoplasm via permeability transition pore, and (iii) interaction with specific kinases in cytoplasm. The systems controlling mtROS-signaling include pro- and anti-inflammatory mediators, nitric oxide, Ca++ and NADPH-oxidase. This review highlights mitochondria-dependent mechanisms that may be associated with liver failure upon acute inflammation. However, further liver cell-specific studies on mtROS signaling are necessary to translate these data into clinical practice, in order to develop mitochondria-targeted diagnostic and therapeutic approaches.
Acknowledgment
We thank Prof. Paolo Bernardi for stimulating and helpful discussion and critical review of the manuscript.
References
- 1.Bone R.C. Toward an epidemiology and natural history of SIRS (systemic inflammatory response syndrome) J. Am. Med. Assoc. 1992;268:3452–3455. [PubMed] [Google Scholar]
- 2.Levy M., Fink M., Marshall J., Abraham E., Angus D., Cook D. SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit. Care Med. 2001;31(2003):1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 3.Brealey D., Brand M., Hargreaves I., Heales S., Land J., Smolenski R. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 4.Kozlov A.V., Bahrami S., Calzia E., Dungel P., Gille L., Kuznetsov A.V. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann. Intensive Care. 2011;1:41. doi: 10.1186/2110-5820-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arulkumaran N., Deutschman C.S., Pinsky M.R., Zuckerbraun B., Schumacker P.T., Gomez H. Mitochondrial function in sepsis. Shock. 2016;45:271–281. doi: 10.1097/SHK.0000000000000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls D.G. Mitochondria and calcium signaling. Cell Calcium. 2005;38:311–317. doi: 10.1016/j.ceca.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D.X., Gutterman D.D. Mitochondrial reactive oxygen species-mediated signaling in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2023–H2031. doi: 10.1152/ajpheart.01283.2006. [DOI] [PubMed] [Google Scholar]
- 10.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic. Biol. Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finkel T. Signal transduction by mitochondrial oxidants. J. Biol. Chem. 2012;287:4434–4440. doi: 10.1074/jbc.R111.271999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy M.P., Siegel R.M. Mitochondrial ROS fire up T cell activation. Immunity. 2013;38:201–202. doi: 10.1016/j.immuni.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Weidinger A., Kozlov A.V. Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules. 2015;5:472–484. doi: 10.3390/biom5020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tait S.W.G., Green D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- 16.Deitch E.A., Goodman E.R. Prevention of multiple organ failure. Surg. Clin. North Am. 1999;79:1471–1488. doi: 10.1016/s0039-6109(05)70088-8. [DOI] [PubMed] [Google Scholar]
- 17.Nesseler N., Launey Y., Aninat C., Morel F., Mallédant Y., Seguin P. Clinical review: the liver in sepsis. Crit. Care. 2012;16:235. doi: 10.1186/cc11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soriano M.E., Nicolosi L., Bernardi P. Desensitization of the permeability transition pore by cyclosporin A prevents activation of the mitochondrial apoptotic pathway and liver damage by tumor necrosis factor-alpha. J. Biol. Chem. 2004;279:36803–36808. doi: 10.1074/jbc.M405297200. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y., Yang Y., Miller M.L., Shen D., Shertzer H.G., Stringer K.F. Hepatocyte-specific Gclc deletion leads to rapid onset of steatosis with mitochondrial injury and liver failure. Hepatology. 2007;45:1118–1128. doi: 10.1002/hep.21635. [DOI] [PubMed] [Google Scholar]
- 20.Seki E., Schwabe R.F. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wan S., Kuo N., Kryczek I., Zou W., Welling T.H. Myeloid cells in hepatocellular carcinoma. Hepatology. 2015;62:1304–1312. doi: 10.1002/hep.27867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tilg H., Moschen A.R., Szabo G. Interleukin-1 and inflammasomes in alcoholic liver disease/acute alcoholic hepatitis and nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatology. 2016;00:1–39. doi: 10.1002/hep.28456. [DOI] [PubMed] [Google Scholar]
- 23.Carré J.E., Orban J.-C., Re L., Felsmann K., Iffert W., Bauer M. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am. J. Respir. Crit. Care Med. 2010;182:745–751. doi: 10.1164/rccm.201003-0326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacGarvey N.C., Suliman H.B., Bartz R.R., Fu P., Withers C.M., Welty-Wolf K.E. Activation of mitochondrial biogenesis by heme oxygenase-1-mediated NF-E2-related factor-2 induction rescues mice from lethal Staphylococcus aureus sepsis. Am. J. Respir. Crit. Care Med. 2012;185:851–861. doi: 10.1164/rccm.201106-1152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2013;5:1–7. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunst J., Derese I., Aertgeerts A., Ververs E.-J., Wauters A., Van den Berghe G. Insufficient autophagy contributes to mitochondrial dysfunction, organ failure, and adverse outcome in an animal model of critical illness. Crit. Care Med. 2013;41:182–194. doi: 10.1097/CCM.0b013e3182676657. [DOI] [PubMed] [Google Scholar]
- 27.Yin X.M., Ding W.X., Gao W. Autophagy in the liver. Hepatology. 2008;47:1773–1785. doi: 10.1002/hep.22146. [DOI] [PubMed] [Google Scholar]
- 28.Tang D., Kang R., Coyne C.B., Zeh H.J., Lotze M.T. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol. Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watanabe E., Muenzer J.T., Hawkins W.G., Davis C.G., Dixon D.J., McDunn J.E. Sepsis induces extensive autophagic vacuolization in hepatocytes: a clinical and laboratory-based study. Lab. Investig. 2009;89:549–561. doi: 10.1038/labinvest.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matzinger P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi M.E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 32.Thompson A.J.V., Locarnini S.A. Toll-like receptors, RIG-I-like RNA helicases and the antiviral innate immune response. Immunol. Cell Biol. 2007;85:435–445. doi: 10.1038/sj.icb.7100100. [DOI] [PubMed] [Google Scholar]
- 33.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nüsslein-Volhard C., Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- 35.Davies M.G., Hagen P.O. Systemic inflammatory response syndrome. Br. J. Surg. 1997;84:920–935. doi: 10.1002/bjs.1800840707. [DOI] [PubMed] [Google Scholar]
- 36.Yao Y.M., Redl H., Bahrami S., Schlag G. The inflammatory basis of trauma/shock-associated multiple organ failure. Inflamm. Res. 1998;47:201–210. doi: 10.1007/s000110050318. [DOI] [PubMed] [Google Scholar]
- 37.Baue A.E. MOF, MODS, and SIRS: what is in a name or an acronym? Shock. 2006;26:438–449. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- 38.Cobb J.P., Buchman T.G., Karl I.E., Hotchkiss R.S. Molecular biology of multiple organ dysfunction syndrome: injury, adaptation, and apoptosis. Surg. Infect. 2000;1:207–215. doi: 10.1089/109629600750018132. [DOI] [PubMed] [Google Scholar]
- 39.Abraham E., Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit. Care Med. 2007;35:2408–2416. doi: 10.1097/01.ccm.0000282072.56245.91. [DOI] [PubMed] [Google Scholar]
- 40.Castellheim A., Brekke O.L., Espevik T., Harboe M., Mollnes T.E. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand. J. Immunol. 2009;69:479–491. doi: 10.1111/j.1365-3083.2009.02255.x. [DOI] [PubMed] [Google Scholar]
- 41.Barie P.S., Hydo L.J., Pieracci F.M., Shou J., Eachempati S.R. Multiple organ dysfunction syndrome in critical surgical illness. Surg. Infect. 2009;10:369–377. doi: 10.1089/sur.2009.9935. [DOI] [PubMed] [Google Scholar]
- 42.De Jong H.K., Van Der Poll T., Wiersinga W.J. The systemic pro-inflammatory response in sepsis. J. Innate Immun. 2010;2:422–430. doi: 10.1159/000316286. [DOI] [PubMed] [Google Scholar]
- 43.Iskander K.N., Osuchowski M.F., Stearns-Kurosawa D.J., Kurosawa S., Stepien D., Valentine C. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol. Rev. 2013;93:1247–1288. doi: 10.1152/physrev.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee I., Hüttemann M. Energy crisis: the role of oxidative phosphorylation in acute inflammation and sepsis. Biochim. Biophys. Acta. 1842;2014:1579–1586. doi: 10.1016/j.bbadis.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deitch E.A. Multiple organ failure. Pathophysiology and potential future therapy. Ann. Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayr V.D., Dünser M.W., Greil V., Jochberger S., Luckner G., Ulmer H. Causes of death and determinants of outcome in critically ill patients. Crit. Care. 2006;10:R154. doi: 10.1186/cc5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vincent J.-L., Nelson D.R., Williams M.D. Is worsening multiple organ failure the cause of death in patients with severe sepsis? Crit. Care Med. 2011;39:1050–1055. doi: 10.1097/CCM.0b013e31820eda29. [DOI] [PubMed] [Google Scholar]
- 48.Yu D.-H., Kim B., Park J. Pathophysiologic and immunologic changes in a canine endotoxemia over a period of 24 h. J. Vet. Med. Sci. 2012;74:537–544. doi: 10.1292/jvms.11-0321. [DOI] [PubMed] [Google Scholar]
- 49.Hao E., Lang F., Chen Y., Zhang H., Cong X., Shen X. Resveratrol alleviates endotoxin-induced myocardial toxicity via the Nrf2 transcription factor. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leist M., Gantner F., Kunstle G., W. A Cytokine-mediated hepatic apoptosis. Rev. Physiol. Biochem. Pharmacol. 1998;133:109–155. doi: 10.1007/BFb0000614. [DOI] [PubMed] [Google Scholar]
- 51.Sweet D.V. Cincinnati; Ohio: 1997. Registry of Toxic Effects of Chemical Substances (RTECS ®) Comprehensive Guide To the RTECS®. [Google Scholar]
- 52.Crouser E., Julian M., Blaho D., Pfeiffer D. Endotoxin-induced mitochondrial damage correlates with impaired respiratory activity. Crit. Care Med. 2002;30:276–284. doi: 10.1097/00003246-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Crouser E.D., Julian M.W., Huff J.E., Joshi M.S., Bauer J.A., Gadd M.E. Abnormal permeability of inner and outer mitochondrial membranes contributes independently to mitochondrial dysfunction in the liver during acute endotoxemia. Crit. Care Med. 2004;32:478–488. doi: 10.1097/01.CCM.0000109449.99160.81. [DOI] [PubMed] [Google Scholar]
- 54.Vanhorebeek I., De Vos R., Mesotten D., Wouters P.J., De Wolf-Peeters C., Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi W., Hatano H., Hirasawa H., Oda S. Protective role of autophagy in mouse cecal ligation and puncture-induced sepsis model. Crit. Care. 2013;17:P1. doi: 10.1186/cc12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kozlov A.V., Duvigneau J.C., Miller I., Nürnberger S., Gesslbauer B., Kungl A. Endotoxin causes functional endoplasmic reticulum failure, possibly mediated by mitochondria. Biochim. Biophys. Acta. 1792;2009:521–530. doi: 10.1016/j.bbadis.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Nürnberger S., Miller I., Duvigneau J.C., Kavanagh E.T., Gupta S., Hartl R.T. Impairment of endoplasmic reticulum in liver as an early consequence of the systemic inflammatory response in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;303:G1373–G1383. doi: 10.1152/ajpgi.00056.2012. [DOI] [PubMed] [Google Scholar]
- 58.Kozlov A.V., Staniek K., Haindl S., Piskernik C., Ohlinger W., Gille L. Different effects of endotoxic shock on the respiratory function of liver and heart mitochondria in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290:G543–G549. doi: 10.1152/ajpgi.00331.2005. [DOI] [PubMed] [Google Scholar]
- 59.Kozlov A.V., Gille L., Miller I., Piskernik C., Haindl S., Staniek K. Opposite effects of endotoxin on mitochondrial and endoplasmic reticulum functions. Biochem. Biophys. Res. Commun. 2007;352:91–96. doi: 10.1016/j.bbrc.2006.10.180. [DOI] [PubMed] [Google Scholar]
- 60.Kozlov A.V., van Griensven M., Haindl S., Kehrer I., Duvigneau J.C., Hartl R.T. Peritoneal inflammation in pigs is associated with early mitochondrial dysfunction in liver and kidney. Inflammation. 2010;33:295–305. doi: 10.1007/s10753-010-9185-4. [DOI] [PubMed] [Google Scholar]
- 61.Gellerich F.N., Trumbeckaite S., Hertel K., Zierz S., Müller-Werdan U., Werdan K. Impaired energy metabolism in hearts of septic baboons: diminished activities of Complex I and Complex II of the mitochondrial respiratory chain. Shock. 1999;11:336–341. [PubMed] [Google Scholar]
- 62.Jeger V., Djafarzadeh S., Jakob S.M., Takala J. Mitochondrial function in sepsis. Eur. J. Clin. Invest. 2013;43:532–542. doi: 10.1111/eci.12069. [DOI] [PubMed] [Google Scholar]
- 63.Backer D. De, Creteur J., Preiser J.C., Dubois M.J., Vincent J.L. Microvascular blood flow is altered in patients with sepsis. Am. J. Respir. Crit. Care Med. 2002;166:98–104. doi: 10.1164/rccm.200109-016oc. [DOI] [PubMed] [Google Scholar]
- 64.Backer D. De, Cortes D. Orbegozo, Donadello K., Vincent J.-L. Pathophysiology of microcirculatory dysfunction and the pathogenesis of septic shock. Virulence. 2014;5:73–79. doi: 10.4161/viru.26482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sakr Y., Dubois M.-J., Backer D. De, Creteur J., Vincent J.-L. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit. Care Med. 2004;32:1825–1831. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 66.Forni M., Mazzola S., Ribeiro L.A., Pirrone F., Zannoni A., Bernardini C. Expression of endothelin-1 system in a pig model of endotoxic shock. Regul. Pept. 2005;131:89–96. doi: 10.1016/j.regpep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 67.Marzano C., Cazals-Hatem D., Rautou P.E., Valla D.C. The significance of nonobstructive sinusoidal dilatation of the liver: impaired portal perfusion or inflammatory reaction syndrome. Hepatology. 2015;62:956–963. doi: 10.1002/hep.27747. [DOI] [PubMed] [Google Scholar]
- 68.Spapen H. Liver perfusion in sepsis, septic shock, and multiorgan failure. Anat. Rec. 2008;291:714–720. doi: 10.1002/ar.20646. [DOI] [PubMed] [Google Scholar]
- 69.Weidinger A., Müllebner A., Paier-Pourani J., Banerjee A., Miller I., Lauterböck L. Vicious inducible nitric oxide synthase-mitochondrial reactive oxygen species cycle accelerates inflammatory response and causes liver injury in rats. Antioxid. Redox Signal. 2015;22:572–586. doi: 10.1089/ars.2014.5996. [DOI] [PubMed] [Google Scholar]
- 70.Sakaguchi S., Furusawa S. Oxidative stress and septic shock: metabolic aspects of oxygen-derived free radicals generated in the liver during endotoxemia. FEMS Immunol. Med. Microbiol. 2006;47:167–177. doi: 10.1111/j.1574-695X.2006.00072.x. [DOI] [PubMed] [Google Scholar]
- 71.Bauer M., Press A.T., Trauner M. The liver in sepsis: patterns of response and injury. Curr. Opin. Crit. Care. 2013;19:123–127. doi: 10.1097/MCC.0b013e32835eba6d. [DOI] [PubMed] [Google Scholar]
- 72.Weidinger A., Dungel P., Perlinger M., Singer K., Ghebes C., Duvigneau J.C. Experimental data suggesting that inflammation mediated rat liver mitochondrial dysfunction results from secondary hypoxia rather than from direct effects of inflammatory mediators. Front. Physiol. 2013;4:138. doi: 10.3389/fphys.2013.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thomas D.D., Liu X., Kantrow S.P., Lancaster J.R. The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mantena S.K., Vaughn D.P., Andringa K.K., Eccleston H.B., King A.L., Abrams G.A. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem. J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hill B.G., Dranka B.P., Bailey S.M., Lancaster J.R., Darley-Usmar V.M. What part of NO don’t you understand? Some answers to the cardinal questions in nitric oxide biology. J. Biol. Chem. 2010;285:19699–19704. doi: 10.1074/jbc.R110.101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bender E., Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 77.Lee I., Bender E., Kadenbach B. Control of mitochondrial membrane potential and ROS formation by reversible phosphorylation of cytochrome c oxidase. Mol. Cell. Biochem. 2002;234–235:63–70. [PubMed] [Google Scholar]
- 78.Brown G.C. Nitric oxide and mitochondria. Front. Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 79.Lawrence K.L., White P.H., Morris G.P., Jennemann J., Phelan D.L., Hotchkiss R.S. CD4+ lymphocyte adenosine triphosphate determination in sepsis: a cohort study. Crit. Care. 2010;14:R110. doi: 10.1186/cc9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hill B.G., Benavides G.A., Lancaster J.J.R., Ballinger S., Dell’Italia L., Zhang J. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kang H., Mao Z., Zhao Y., Yin T., Song Q., Pan L. Ethyl pyruvate protects against sepsis by regulating energy metabolism. Ther. Clin. Risk Manag. 2016;12:287–294. doi: 10.2147/TCRM.S97989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ozkok E., Yorulmaz H., Ates G., Aksu A., Balkis N., Şahİn Ö. Amelioration of energy metabolism by melatonin in skeletal muscle of rats With LPS induced endotoxemia. Physiol. Res. 2016;65:833–842. doi: 10.33549/physiolres.933282. [DOI] [PubMed] [Google Scholar]
- 83.Hart D.W., Gore D.C., Rinehart A.J., Asimakis G.K., Chinkes D.L. Sepsis-induced failure of hepatic energy metabolism. J. Surg. Res. 2003;115:139–147. doi: 10.1016/s0022-4804(03)00284-1. [DOI] [PubMed] [Google Scholar]
- 84.Hatano E., Tanaka A., Iwata S., Satoh S., Kitai T., Tsunekawa S. Induction of endotoxin tolerance in transgenic mouse liver expressing creatine kinase. Hepatology. 1996;24:663–669. doi: 10.1053/jhep.1996.v24.pm0008781340. [DOI] [PubMed] [Google Scholar]
- 85.Chaudry I.H., Wichterman K.A., Baue A.E. Effect of sepsis on tissue adenine nucleotide levels. Surgery. 1979;85:205–211. [PubMed] [Google Scholar]
- 86.Aslami H., Pulskens W.P., Kuipers M.T., Bos A.P., van Kuilenburg A.B.P., Wanders R.J.A. Hydrogen sulfide donor NaHS reduces organ injury in a rat model of pneumococcal pneumosepsis, associated with improved bio-energetic status. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carchman E.H., Rao J., Loughran P.A., Rosengart M.R., Zuckerbraun B.S. Heme oxygenase-1-mediated autophagy protects against hepatocyte cell death and hepatic injury from infection/sepsis in mice. Hepatology. 2011;53:2053–2062. doi: 10.1002/hep.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirai F., Aoyama H., Ohtoshi M., Kawashima S., Ozawa K., Tobe T. Significance of mitochondrial enhancement in hepatic energy metabolism in relation to alterations in hemodynamics in septic pigs with severe peritonitis. Eur. Surg. Res. 1984;16:148–155. doi: 10.1159/000128402. [DOI] [PubMed] [Google Scholar]
- 89.Corrêa T.D., Vuda M., Blaser A.R., Takala J., Djafarzadeh S., Dünser M.W. Effect of treatment delay on disease severity and need for resuscitation in porcine fecal peritonitis. Crit. Care Med. 2012;40:2841–2849. doi: 10.1097/CCM.0b013e31825b916b. [DOI] [PubMed] [Google Scholar]
- 90.Regueira T., Djafarzadeh S., Brandt S., Gorrasi J., Borotto E., Porta F. Oxygen transport and mitochondrial function in porcine septic shock, cardiogenic shock, and hypoxaemia. Acta Anaesthesiol. Scand. 2012;56:846–859. doi: 10.1111/j.1399-6576.2012.02706.x. [DOI] [PubMed] [Google Scholar]
- 91.Duvigneau J.C., Piskernik C., Haindl S., Kloesch B., Hartl R.T., Hüttemann M. A novel endotoxin-induced pathway: upregulation of heme oxygenase 1, accumulation of free iron, and free iron-mediated mitochondrial dysfunction. Lab. Investig. 2008;88:70–77. doi: 10.1038/labinvest.3700691. [DOI] [PubMed] [Google Scholar]
- 92.Crumm S., Cofan M., Juskeviciute E., Hoek J.B. Adenine nucleotide changes in the remnant liver: an early signal fro regeneration after partial hepatectomy. Hepatology. 2008;48:898–908. doi: 10.1002/hep.22421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Latta M., Künstle G., Leist M., Wendel A. Metabolic depletion of ATP by fructose inversely controls CD95- and tumor necrosis factor receptor 1-mediated hepatic apoptosis. J. Exp. Med. 2000;191:1975–1985. doi: 10.1084/jem.191.11.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nikoletopoulou V., Markaki M., Palikaras K., Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochim. Biophys. Acta – Mol. Cell Res. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 95.Kehrer J.P., Jones D.P., Lemasters J.J., Farber J.L., Jaeschke H. Mechanisms of hypoxic cell injury. Toxicol. Appl. Pharmacol. 1990;106:165–178. doi: 10.1016/0041-008x(90)90238-p. [DOI] [PubMed] [Google Scholar]
- 96.Leist M., Single B., Castoldi A.F., Kühnle S., Nicotera P. Intracellular Adenosine Triphosphate (ATP), a switch in the decision between apoptosis and necrosis. J. Exp. Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rabini R., Petruzzi E., Staffolani R., Tesei M., Fumelli P., Pazzagli M. Diabetes mellitus and subjects' ageing: a study on the ATP content and ATP-related enzyme activities in human erythrocytes. Eur. J. Clin. Invest. 1997;27:327–332. doi: 10.1046/j.1365-2362.1997.1130652.x. [DOI] [PubMed] [Google Scholar]
- 98.Lodi R., Cooper J.M., Bradley J.L., Manners D., Styles P., Taylor D.J. Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia [see comments] Proc. Natl. Acad. Sci. USA. 1999;96:11492–11495. doi: 10.1073/pnas.96.20.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liaw K.Y., Askanazi J., Michelson C., Kantrowitz L.R., Fürst P., Kinney J.M. Effect of injury and sepsis on high-energy phosphates in muscle and red cells. J. Trauma. 1980;20:755–759. doi: 10.1097/00005373-198009000-00008. [DOI] [PubMed] [Google Scholar]
- 100.Bhattacharyya J., Thompson K., Sayeed M.M. Calcium-dependent and calcium-independent protease activities in skeletal muscle during sepsis. Circ. Shock. 1991;35:117–122. [PubMed] [Google Scholar]
- 101.Todd J.C., Mollitt D.L. Effect of sepsis on erythrocyte intracellular calcium homeostasis. Crit. Care Med. 1995;23:459–465. doi: 10.1097/00003246-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 102.Hotchkiss R.S., Karl I.E. Calcium: a regulator of the inflammatory response in endotoxemia and sepsis. New Horiz. 1996;4:58–71. [PubMed] [Google Scholar]
- 103.Trocha M., Szelag A. The role of calcium and calcium channel blocking drugs in damage to the liver preserved for transplantation. Ann. Transplant. 2004;9:5–11. [PubMed] [Google Scholar]
- 104.Deaciuc I.V., Spitzer J.A. Calcium content in liver and heart and its intracellular distribution in liver during endotoxicosis and sepsis in rats. Cell Calcium. 1987;8:365–376. doi: 10.1016/0143-4160(87)90011-x. [DOI] [PubMed] [Google Scholar]
- 105.Rose S., Baumann H., Jahreis G.P., Sayeed M.M. Diltiazem and superoxide dismutase modulate hepatic acute phase response in gram-negative sepsis. Shock. 1994;1:87–93. doi: 10.1097/00024382-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 106.Yoshizumi A., Ishii Y., Livermore D.M., Woodford N., Kimura S., Saga T. Efficacies of calcium-EDTA in combination with imipenem in a murine model of sepsis caused by Escherichia coli with NDM-1 β-lactamase. J. Infect. Chemother. 2013;19:992–995. doi: 10.1007/s10156-012-0528-y. [DOI] [PubMed] [Google Scholar]
- 107.Zhang B., Crankshaw W., Nesemeier R., Patel J., Nweze I., Lakshmanan J. Calcium-mediated signaling and calmodulin-dependent kinase regulate hepatocyte inducible nitric oxide synthase expression. J. Surg. Res. 2015;193:795–801. doi: 10.1016/j.jss.2014.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rowlands D.J., Islam M.N., Das S.R., Huertas A., Quadri S.K., Horiuchi K. Activation of TNFR1 ectodomain shedding by mitochondrial Ca2+ determines the severity of inflammation in mouse lung microvessels. J. Clin. Invest. 2011;121:1986–1999. doi: 10.1172/JCI43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Begriche K., Massart J., Robin M.A., Bonnet F., Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 110.Schulz E., Wenzel P., Münzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Friedbichler K., Themanns M., Mueller K.M., Schlederer M., Kornfeld J.-W., Terracciano L.M. Growth-hormone-induced signal transducer and activator of transcription 5 signaling causes gigantism, inflammation, and premature death but protects mice from aggressive liver cancer. Hepatology. 2012;55:941–952. doi: 10.1002/hep.24765. [DOI] [PubMed] [Google Scholar]
- 112.Mueller K.M., Kornfeld J.W., Friedbichler K., Blaas L., Egger G., Esterbauer H. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011;54:1398–1409. doi: 10.1002/hep.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dikalov S.I., Li W., Doughan A.K., Blanco R.R., Zafari A.M. Mitochondrial reactive oxygen species and calcium uptake regulate activation of phagocytic NADPH oxidase. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;302:R1134–R1142. doi: 10.1152/ajpregu.00842.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kröller-Schön S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bulua A.C., Simon A., Maddipati R., Pelletier M., Park H., Kim K.-Y. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J. Exp. Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sena L.A., Li S., Jairaman A., Prakriya M., Ezponda T., Hildeman D.A. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaminski M.M., Sauer S.W., Kaminski M., Opp S., Ruppert T., Grigaravicius P. T cell activation is driven by an ADP-Dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep. 2012;2:1300–1315. doi: 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 120.Maemura K., Zheng Q., Wada T., Ozaki M., Takao S., Aikou T. Reactive oxygen species are essential mediators in antigen presentation by Kupffer cells. Immunol. Cell Biol. 2005;83:336–343. doi: 10.1111/j.1440-1711.2005.01323.x. [DOI] [PubMed] [Google Scholar]
- 121.Lacza Z., Kozlov A.V., Pankotai E., Csordas A., Wolf G., Redl H. Mitochondria produce reactive nitrogen species via an arginine-independent pathway. Free Radic. Res. 2006;40:369–378. doi: 10.1080/10715760500539139. [DOI] [PubMed] [Google Scholar]
- 122.Win S., Than T.A., Min R.W.M., Aghajan M., Kaplowitz N. JNK mediates mouse liver injury through a novel Sab (SH3BP5) dependent pathway leading to inactivation of intramitochondrial Src. Hepatology. 2016 doi: 10.1002/hep.28486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chai J., He Y., Cai S.Y., Jiang Z., Wang H., Li Q. Elevated hepatic multidrug resistance-associated protein 3/ATP-binding cassette subfamily C 3 expression in human obstructive cholestasis is mediated through tumor necrosis factor alpha and C-Jun NH2-terminal kinase/stress-activated protein kinase-signali. Hepatology. 2012;55:1485–1494. doi: 10.1002/hep.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mantel C., Messina-Graham S., Moh A., Cooper S., Hangoc G., Fu X.-Y. Defects, mitochondrial dysfunction, ROS overproduction, and a rapid mouse hematopoietic cell – targeted STAT3 deletion: stem/progenitor cell defects, mitochondrial dysfunction, ROS overproduction, and a rapid aging – like phenotype. Blood. 2012;120:2589–2599. doi: 10.1182/blood-2012-01-404004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boengler K., Hilfiker-Kleiner D., Heusch G., Schulz R. Inhibition of permeability transition pore opening by mitochondrial STAT3 and its role in myocardial ischemia/reperfusion. Basic Res. Cardiol. 2010;105:771–785. doi: 10.1007/s00395-010-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Piskernik C., Haindl S., Behling T., Gerald Z., Kehrer I., Redl H. Antimycin A and lipopolysaccharide cause the leakage of superoxide radicals from rat liver mitochondria. Biochim. Biophys. Acta. 1782;2008:280–285. doi: 10.1016/j.bbadis.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 128.Decker T., Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 129.Guschin D., Rogers N., Briscoe J., Witthuhn B., Watling D., Horn F. A major role for the protein tyrosine kinase JAK1 in the JAK/STAT signal transduction pathway in response to interleukin-6. EMBO J. 1995;14:1421–1429. doi: 10.1002/j.1460-2075.1995.tb07128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wegrzyn J., Potla R., Chwae Y.-J., Sepuri N.B.V., Zhang Q., Koeck T. Function of mitochondrial Stat3 in cellular respiration. Science. 2009;323:793–798. doi: 10.1126/science.1164551. (80-) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seki E., Brenner D.A., Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schwabe R.F., Uchinami H., Qian T., Bennett B.L., Lemasters J.J., Brenner D.A. Differential requirement for c-Jun NH 2 -terminal kinase in TNF-α- and Fas-mediated apoptosis in hepatocytes. FASEB J. 2004 doi: 10.1096/fj.03-0771fje. [DOI] [PubMed] [Google Scholar]
- 133.Giorgio M., Migliaccio E., Orsini F., Paolucci D., Moroni M., Contursi C. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 134.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Galimov E.R., Chernyak B.V., Sidorenko A.S., Tereshkova A.V., Chumakov P.M. Prooxidant properties of p66shc are mediated by mitochondria in human cells. PLoS One. 2014;9:e86521. doi: 10.1371/journal.pone.0086521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clementi E., Brown G.C., Feelisch M., Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Brown G.C., Cooper C.E. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 138.Chen J., Chen C.L., Alevriadou B.R., Zweier J.L., Chen Y.R. Excess no predisposes mitochondrial succinate-cytochrome c reductase to produce hydroxyl radical. Biochim. Biophys. Acta – Bioenerg. 2011;1807:491–502. doi: 10.1016/j.bbabio.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Salzman A.L., Menconi M.J., Unno N., Ezzell R.M., Casey D.M., Gonzalez P.K. Nitric oxide dilates tight junctions and depletes ATP in cultured Caco-2BBe intestinal epithelial monolayers. Am. J. Physiol. 1995;268:G361–G373. doi: 10.1152/ajpgi.1995.268.2.G361. [DOI] [PubMed] [Google Scholar]
- 140.Kung G., Konstantinidis K., Kitsis R.N. Programmed necrosis, not apoptosis, in the heart. Circ. Res. 2011;108:1017–1036. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 141.Bernardi P., Giorgio V. EBEC 2012 – an energetic time in Freiburg. EMBO Rep. 2013;14:7–9. doi: 10.1038/embor.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kokoszka J.E., Waymire K.G., Levy S.E., Sligh J.E., Cai J., Jones D.P. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petronilli V., Costantini P., Scorrano L., Colonna R., Passamontil S. The voltage sensor of the mitochondrial permeability transition pore is tuned by the oxidation-reduction state of vicinal thiols. J. Biol. Chem. 1994;269:16638–16642. [PubMed] [Google Scholar]
- 144.Costantini P., Chernyak B.V., Petronilli V., Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J. Biol. Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- 145.Costantini P., Colonna R., Bernardi P. Induction of the mitochondrial permeability transition by N-ethylmaleimide depends on secondary oxidation of critical thiol groups. Potentiation by copper-ortho-phenanthroline without dimerization of the adenine nucleotide translocase. Biochim. Biophys. Acta – Bioenerg. 1998;1365:385–392. doi: 10.1016/s0005-2728(98)00090-5. [DOI] [PubMed] [Google Scholar]
- 146.Bernardi P., Rasola A., Forte M., Lippe G. The mitochondrial permeability transition pore: channel formation by F-ATP synthase, integration in signal transduction, and role in pathophysiology. Physiol. Rev. 2015;95:1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scorrano L., Petronilli V., Bernardi P. On the voltage dependence of the mitochondrial permeability transition pore. J. Biol. Chem. 1997;272:12295–12299. doi: 10.1074/jbc.272.19.12295. [DOI] [PubMed] [Google Scholar]
- 148.Winterbourn C.C. Revisiting the reactions of superoxide with glutathione and other thiols. Arch. Biochem. Biophys. 2016;595:68–71. doi: 10.1016/j.abb.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 149.Truong T.H., Carroll K.S. Redox regulation of protein kinases. Crit. Rev. Biochem. Mol. Biol. 2013:1–25. doi: 10.3109/10409238.2013.790873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gopalakrishna R., Gundimeda U., Zhou S., Zung K., Forell K., Holmgren A. Imbalance in protein thiol redox regulation and cancer-preventive efficacy of selenium. React. Oxyg. Species. 2016;2:272–289. doi: 10.20455/ros.2016.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rahman M.A., Senga T., Ito S., Hyodo T., Hasegawa H., Hamaguchi M. S-nitrosylation at cysteine 498 of c-Src tyrosine kinase regulates nitric oxide-mediated cell invasion. J. Biol. Chem. 2010;285:3806–3814. doi: 10.1074/jbc.M109.059782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Giannoni E., Buricchi F., Raugei G., Ramponi G., Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol. Cell. Biol. 2005;25:6391–6403. doi: 10.1128/MCB.25.15.6391-6403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]