Highlights
-
•
Quantitative Perfusion CT parameters are not affected substantially by increasing ASIR levels.
-
•
ASIR improves image noise and CNR at a reduced peak kilovoltage of 100 kV.
-
•
Peak tumour CNR is greater than 1.5 with 60% ASIR and above.
Keywords: Perfusion imaging, Multidetector computed tomography, Colorectal neoplasms, Computer-assisted image processing, Radiation dosage
Abstract
Objectives
To determine the effect of Adaptive Statistical Iterative Reconstruction (ASIR) on perfusion CT (pCT) parameter quantitation and image quality in primary colorectal cancer.
Methods
Prospective observational study. Following institutional review board approval and informed consent, 32 patients with colorectal adenocarcinoma underwent pCT (100 kV, 150 mA, 120 s acquisition, axial mode). Tumour regional blood flow (BF), blood volume (BV), mean transit time (MTT) and permeability surface area product (PS) were determined using identical regions-of-interests for ASIR percentages of 0%, 20%, 40%, 60%, 80% and 100%. Image noise, contrast-to-noise ratio (CNR) and pCT parameters were assessed across ASIR percentages. Coefficients of variation (CV), repeated measures analysis of variance (rANOVA) and Spearman’ rank order correlation were performed with statistical significance at 5%.
Results
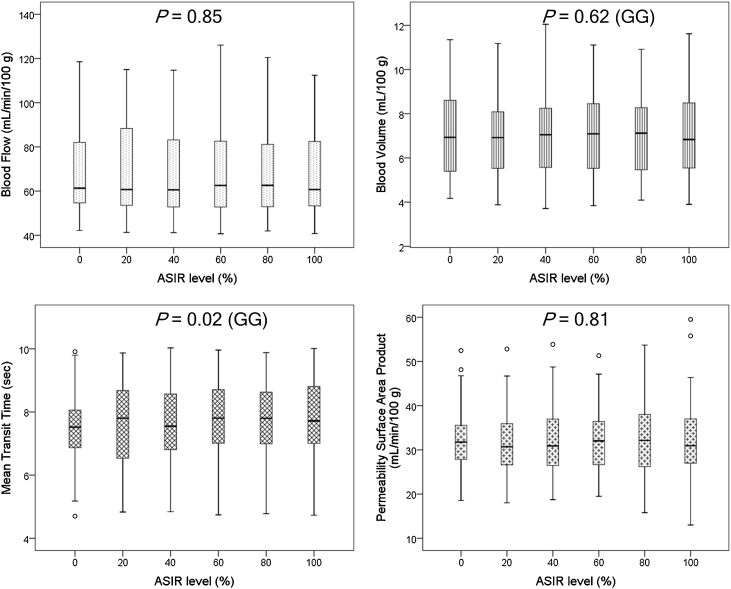
With increasing ASIR percentages, image noise decreased by 33% while CNR increased by 61%; peak tumour CNR was greater than 1.5 with 60% ASIR and above. Mean BF, BV, MTT and PS differed by less than 1.8%, 2.9%, 2.5% and 2.6% across ASIR percentages. CV were 4.9%, 4.2%, 3.3% and 7.9%; rANOVA P values: 0.85, 0.62, 0.02 and 0.81 respectively.
Conclusions
ASIR improves image noise and CNR without altering pCT parameters substantially.
1. Introduction
Perfusion computed tomography (pCT) techniques enable in-vivo tumour vascularisation to be evaluated in clinical practice [1]. Regional blood flow (BF), blood volume (BV) and permeability surface area product (PS), derived from the temporal changes in vascular and tissue attenuation following an intravenous bolus injection of standard CT iodinated contrast agent, act as surrogate measures of tumour angiogenesis and perfusion-related hypoxia across different tumour types [2], [3], [4], [5], [6]. Perfusion CT is clinically relevant in colorectal cancer. Staging blood flow is lower in patients with nodal metastases [7], those who subsequently present with metastases despite curative surgery [8], and those who have a poorer overall survival [7]. This is thought to reflect perfusion-related hypoxia, supporting the use of pCT in oncologic clinical trials as a prognostic and response biomarker [1].
In recent years, the clinical emphasis on dose-reduction has led all major CT manufacturers to implement hybrid iterative image reconstruction techniques instead of traditional analytical filtered back projection (FBP) reconstruction, in order to compensate for the increase in image noise with low-dose CT acquisitions. These hybrid algorithms combine analytical and iterative methods in a number of ways. Adaptive statistical iterative reconstruction (ASIR) uses the image information obtained from FBP as an initial building block for further iterative reconstruction to improve image characteristics, including noise. This has enabled dose reductions between 32% and 65% in phantom studies without substantially affecting image quality [9], [10] subsequently confirmed by clinical studies, both paediatric [11], [12], [13] and adult [14], [15], [16], [17], [18], [19], [20], [21]. The impact of iterative reconstruction techniques on quantitative pCT analysis has not been investigated and is clinically relevant, given their widespread use. The aim of this prospective study was to assess the effect of ASIR on image quality and pCT quantitation in primary colorectal cancer.
2. Materials and methods
2.1. Patients
Institutional review board approval and written informed consent were obtained prospectively for this single-centre observational study, as part of a multicentre NIHR HTA funded study aimed at evaluating a prognostic model of conventional predictive variables and novel variables derived from pCT to improve the prediction of metastatic disease in primary colorectal cancer. Inclusion criteria were adult patients with a diagnosis of colorectal cancer. Exclusion criteria were standard contraindications to iodinated contrast agent (including previous iodinated contrast agent reaction, impaired renal function, lack of intravenous access) and no visible tumour on the planning CT acquisition. Thirty-four patients agreed to participate from January 2012 to July 2014. In two patients technical errors precluded analysis, leaving 32 patients (27 men, 5 women) in this study group. Mean (SD) patient age was 71.3 years ± 10.5 (men: 71.1 years ± 10.6; range, 38–91 years; women: 72.2 ± 11.5; range, 54–84 years). Mean (SD) patient size, measured in the axial plane immediately cranially to the iliac crest, was 24.28 ± 3.23 cm (latero-lateral; range, 17.89-31.06 cm) × 35.67 ± 2.73 cm (antero-posterior; range, 30.45–40.99). Tumours had a mean diameter of 4.5 cm ± 2.1 (range, 2.0–10.1 cm) and were located as follows: cecum [5], ascending colon [4], descending colon [1], sigmoid colon [7], rectum [15]. Radiological tumour (T) and nodal (N) staging, evaluated on CT images only and based on the UICC TNM Classification of Malignant Tumours, Seventh Edition, was as follows: T2 [12], T3 [17], T4 [3], N0 [16], N1 [11], N2 [5].
2.2. CT perfusion technique
To minimise bowel peristaltic movement, 20 mg of the spasmolytic agent hyoscine butylbromide (Buscopan; Boehringer Ingelheim, Ingelheim am Rhein, Germany) were administered intravenously prior to data acquisition unless contraindicated. Perfusion CT was performed on a Discovery 750 HD multi-detector CT(GE Healthcare, Waukesha, USA). An initial low dose abdominal or pelvic planning sequence was performed, without intravenous contrast material, to locate the colorectal tumour (100 kV, 50 mA with smart mA, 5-mm slice collimation, 50-cm scan field of view, 512 × 512 mm matrix). The tumour was identified by the supervising radiologist and the acquisition coordinates were used to plan the subsequent dynamic pCT acquisition, which was centred at mid-tumour level. 50 mL of 370 mg/mL iodinated contrast agent (Niopam, Bracco S.p.A, Milan, Italy) were injected intravenously through a pump injector (Medrad Stellant dual syringe, Bayer Healthcare, Leverkusen, Germany) at a rate of 5 mL/sec, followed by a 50 mL saline chaser injected at the same rate. The pCT acquisition consisted of 8 contiguous 5 mm slices (4 cm z-axis coverage), obtained at 1.5 s intervals for 45 s (perfusion phase) and at 15 s intervals for a further 75 s (interstitial phase), giving a total number of 35 acquisition time points with the following acquisition parameters: 100 kV; 150 mA without tube current modulation; axial mode; 0.5 s rotation time; 50 cm scan field of view; 512 × 512 mm matrix; B30 soft reconstruction kernel; 10 s delay after IV contrast agent injection; breath intermittently held in expiration during the perfusion and interstitial phase to minimise respiratory excursion. Mean CTDIVol and DLP were 137.8 ± 15.3 mGy and 551.0 ± 61.2 mGy cm respectively.
2.3. Image analysis
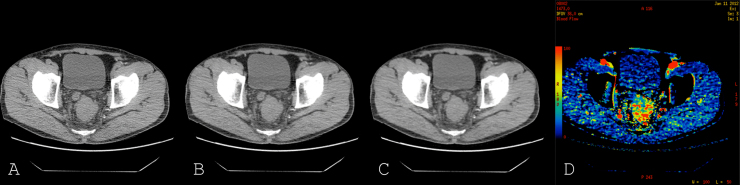
All pCT acquisitions were reconstructed at 6 different ASIR percentages: 0% ASIR (equivalent to FBP) and with increasing percentages of 20%, 40%, 60%, 80% and 100% ASIR, resulting in 6 separate pCT datasets per patient (Fig. 1). With the aim of eliminating interobserver variability, image data were analysed by two readers in consensus [with 1 year (SV) and >15 years’ (VG) CT perfusion experience respectively] on a workstation (Advantage Windows 4.6; GE Healthcare, Chicago, USA) using commercial perfusion software (Perfusion 4; GE Healthcare, Chicago, USA). A processing threshold of −50 to 150 HU was chosen to optimise soft tissue visualisation. No motion correction algorithm was applied. An arterial input was defined by placing a 1 cm2 circular region-of-interest (ROI) within the best visualised artery (aorta, iliac, or femoral) to derive the arterial time-enhancement curve. Parametric maps (BF, BV, MTT and PS) were generated, with each pixel representing a parameter value, based on the maximum slope method algorithm and a modified Johnson and Wilson distributed parameter model (GE Healthcare, Chicago, USA). A tumour ROI was outlined on each axial image where tumour was visualised, ensuring that the entire tumour was encompassed within the ROIs; luminal gas was excluded where present.
Fig. 1.
Axial perfusion CT image reconstructed with 0% (A), 60% (B) and 100% ASIR (C). Parametric blood flow colour map (D).
The analysis of 0% ASIR images was conducted first; the delineated ROIs were saved by the software to allow these to be propagated onto subsequent datasets with different ASIR percentages (20–100%), thus avoiding any measurement variation related to ROI placement. The mean values for each of the four tumour pCT parameters – BF, BV, MTT and PS – were recorded. The entire process was repeated in the same manner for each patient and for all the subsequent ASIR datasets- 20%, 40%, 60%, 80% and 100% – using the exact same ROI.
2.4. Assessment of image quality
Image noise and contrast to noise ratio (CNR) were calculated from the grey-scale images. For each patient and ASIR percentage, tumour baseline density and peak enhancement were recorded from the tissue time-density curve on a single gray-scale image; a fixed circular 1 cm2 ROI was placed on skeletal muscle and the Hounsfield unit (HU) standard deviation recorded to provide a measure of image noise. CNR was calculated by dividing tumour enhancement (tumour peak enhancement – baseline density) by the muscle ROI standard deviation.
2.5. Statistical analysis
The mean and standard deviation for each pCT parameter, image noise and CNR were calculated for the different ASIR percentages (0–100%). Within-patient coefficients of variation (CV) were calculated for each parameter across the different ASIR percentages (standard deviation divided by mean), and expressed as a percentage value. Repeated measures analysis of variance (rANOVA) of pCT parameters was performed, using Greenhouse-Geisser (GG) correction when appropriate based on Mauchly’s test of sphericity, and followed by post hoc testing using the Bonferroni method. Spearman rank order correlation was performed to assess the associations of image noise and CNR with ASIR percentage. Statistical significance was assigned at the 5% level. Statistical analysis was performed using IBM SPSS Statistics, Version 22 (IBM Corporation, New York, USA).
2.6. Sample size justification
Retrospective power analysis under the guidance of a statistician (GPower 3.1) showed that, with a 5% significance level and 90% power, 13 subjects would be required to be able to detect a difference of 7 units between two sets of parameter measurements (based on BF values), equivalent to a clinically significant 10% variation. Our actual sample size of 32 meets these criteria with ample margins.
3. Results
3.1. Image quality
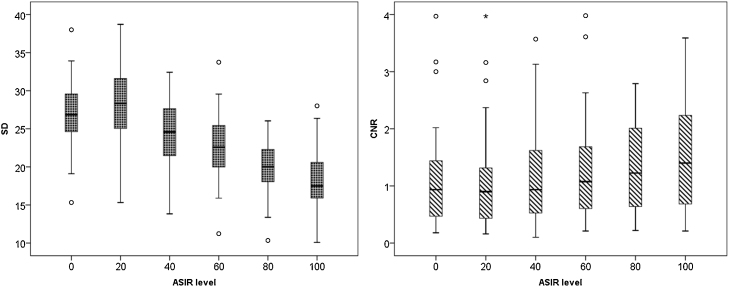
Mean image noise (±standard deviation) decreased with increasing ASIR percentages, changing from 27.06 ± 4.57 (range, 15.32-38.00) at 0% ASIR to 18.16 ± 4.41 (range, 10.08-28.01) at 100% ASIR, a relative decrease of 32.8%. Mean tumour enhancement remained relatively stable across ASIR levels, ranging on average between 33.01 ± 4.94 HU and 35.59 ± 8.47 HU (a relative change of 7.8%). Consequently, mean CNR increased from 1.22 ± 1.08 (range, 0.18–4.59) to 1.96 ± 1.92 (range, 0.21–9.23), a relative increase of 60.7% (Table 1, Fig. 2). As expected, there was a strong negative rank correlation between image noise and ASIR level (r = −0.639; P < 0.001); a weak positive rank correlation was found between CNR and ASIR level(r = 0.206; P = 0.004).
Table 1.
Mean ± SD for image noise and contrast to noise ratios for each ASIR percentage and the relative change between 0% and 100% ASIR are shown. Data are means ± standard deviations, with ranges in parentheses.
| Image quality | ASIR percentage |
Relative change (%) | |||||
|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 60% | 80% | 100% | ||
| Noise | 27.06 ± 4.57 (15.32–38.00) |
28.24 ± 4.75 (15.32–38.71) |
24.37 ± 4.32 (13.83–32.42) |
22.40 ± 4.45 (11.23–33.74) |
19.64 ± 3.72 (10.34–26.04) |
18.16 ± 4.41 (10.08–28.01) |
−32.8% |
| Contrast to noise | 1.22 ± 1.08 (0.18–4.59) |
1.20 ± 1.12 (0.16–4.92) |
1.36 ± 1.28 (0.10–5.73) |
1.53 ± 1.48 (0.21–6.73) |
1.73 ± 1.69 (0.22–7.90) |
1.96 ± 1.92 (0.21–9.23) |
+60.7% |
Fig. 2.
Box and whisker plots of perfusion CT parameters across increasing ASIR levels. Solid line in box represents median value, upper and lower bars represent first and third quartiles, whiskers represent 95% confidence intervals. P values of repeated measures ANOVA (with or without GG correction) are reported.
3.2. Perfusion CT parameters
Mean BF, BV, MTT, PS and coefficients of variation across increasing ASIR percentages are summarised in Table 2 and Fig. 3. Mean parameter values differed by less than 1.8%, 2.9%, 2.5% and 2.6% for BF, BV, MTT and PS respectively across the different ASIR percentages. Coefficients of variation ranged between 3.3% and 7.9% for each of the parameters. Repeated measures ANOVA showed that mean BF, BV and PS measurements did not differ substantially, with P values of 0.85, 0.62 and 0.81 respectively. Mean MTT measurements yielded a P value of 0.02; post hoc tests using the Bonferroni correction, however, revealed no significant difference between pairs of ASIR levels (P values = 0.23 to 1.00).
Table 2.
Tumour perfusion CT measurements for each ASIR percentage, P values for repeated measures ANOVA and coefficients of variation (CV) are shown. Data are means ± standard deviations, with ranges in parentheses.
| Perfusion CT parameter | ASIR percentage |
P- value | CV (%) |
|||||
|---|---|---|---|---|---|---|---|---|
| 0% | 20% | 40% | 60% | 80% | 100% | |||
| Blood flow (mL/min/100 g) | 68.08 ± 20.31 (42.17–118.55) |
67.75 ± 20.38 (41.31–114.99) |
67.51 ± 20.62 (41.18–114.69) |
68.13 ± 21.11 (40.68–126.09) |
67.18 ± 20.10 (41.95–120.48) |
66.93 ± 19.39 (40.80–112.45) |
0.85 | 4.9 ± 3.4 |
| Blood volume (mL/100 g) | 7.09 ± 1.99 (4.17–11.35) |
7.01 ± 1.99 (3.88–11.18) |
7.03 ± 1.97 (3.71–12.05) |
7.15 ± 1.96 (3.84–11.11) |
7.10 ± 1.95 (4.09–10.92) |
7.12 ± 2.03 (3.90–11.62) |
0.62 | 4.2 ± 3.0 |
| Mean transit time (sec) | 7.54 ± 1.15 (4.70–9.91) |
7.60 ± 1.29 (4.83–9.87) |
7.62 ± 1.20 (4.84–10.03) |
7.74 ± 1.24 (4.74–9.96) |
7.74 ± 1.21 (4.78–9.88) |
7.78 ± 1.29 (4.73–10.01) |
0.02 | 3.3 ± 2.3 |
| Permeability surface area product (mL/min/100 g) | 32.51 ± 7.65 (18.54–52.47) |
32.20 ± 8.90 (18.00–52.82) |
32.03 ± 8.84 (18.73–53.87) |
32.08 ± 8.21 (19.49–51.32) |
32.82 ± 9.17 (15.78–53.70) |
32.76 ± 9.95 (12.99–59.52) |
0.81 | 7.9 ± 3.6 |
Fig. 3.
Box and whisker plots of image noise and CNR across increasing ASIR levels. Solid line in box represents median value, upper and lower bars represent first and third quartiles, whiskers represent 95% confidence intervals.
4. Discussion
In recent years there has been a step change in CT practice. The emphasis on dose reduction has led to the widespread implementation of hybrid iterative reconstruction techniques by all the major CT manufacturers, in order to improve or maintain image quality with low dose acquisitions. These hybrid algorithms combine filtered back projection data with iterative reconstruction methods. The potential impact of iterative reconstruction on quantitative perfusion CT techniques has not been explored for body tumours but is clearly relevant to oncologic imaging.
In this study we showed that ASIR does not alter the tumour pCT parameters BF, BV and PS substantially, with repeated measurements ANOVA P values greater than 0.6; MTT differed significantly between ASIR levels on ANOVA, though not so on post hoc pairwise comparisons; within-subject coefficients of variation for all parameters were smaller than 10%.
Image noise and CNR improved with increasing levels of ASIR at a reduced peak kilovoltage of 100 kV, strengthening the case for the implementation of low dose CT perfusion protocols within the constraints of an adequate CNR for quantitation.
Contrast enhancement within tumours, typically higher and more heterogeneous than in normal solid organs, altered by less than 8% across the different ASIR percentages.
In general terms, the first stage of iterative reconstruction produces artificial raw data from the forward projection of a volumetric estimate taken from either an empty image or a FBP reconstruction. Modelling of the volumetric estimate is then undertaken and the many possible path lengths, including the different angles and orientations, at which the x-ray photons could pass through the object are calculated; this way the estimate mimics a more realistic pathway in which the x-ray photons travel through the object to the detector; furthermore, instead of placing the initial location of the x-ray photons over a single point, like in FBP, it is placed over a small area. This artificial projection is then compared to the actual measurement to construct an updated image. Modification of this updated image is repeated a number of times until very little differences are present between the artificial projection and real measurement. In the last stage, the updated image is back projected onto the volumetric estimate. A phantom study comparing two first generation hybrid iterative reconstruction methods, ASIR and iDose, has shown they provide similar benefits, mainly acting on image noise reduction [22].
Our results are in line with previous studies that have assessed the effect of commercially implemented iterative reconstruction techniques within normal organs. Studies have shown that iDose and AIDR did not substantially affect quantitative perfusion CT parameters in normal brain tissue [23], [24], normal liver [25] and normal pancreas [26], allowing dose reductions ranging between 20% and 56%. These hybrid algorithms are different to ASIR but have a similar aim of reducing noise. iDose implemented by Philips applies an optimal anatomical model in the image domain to iteratively eliminate the quantum image noise and attempts to maintain the appearance of a ‘full dose FBP image [27]; in adaptive iterative dose reduction, AIDR implemented by Toshiba, statistical and scanner based models combined with projection noise estimation are applied to FBP reconstructions and followed by an iterative technique for optimisation [28].
The reported coefficients of variation for CT Perfusion parameters (3.3%–7.9%) are well within those previously reported for intra- and inter-observer variation, supporting their clinical acceptability; for example, a previous quantitative perfusion CT study in primary colorectal cancer reported mean intra-observer CV, based on same-study repeated measurements, ranging between 6.2% (PS) and 27.9% (BV) [28].
ASIR levels of 30–50% have been shown to provide acceptable image noise and diagnostic confidence in abdominal CT without a substantial change in image appearance [18]: these levels have been typically adopted in clinical practice for viewing purposes. Published phantom- and patient-based CT perfusion image quality assessments, for a reconstructed slice thickness of 5 mm in the pelvis, have shown typical peak CNR values greater than 1.5 at 100 kV [29]. In our study, a CNR greater than 1.5 was achieved with ASIR levels of 60% and above: with the aim of striking a balance between achieving adequate CNR and avoiding major changes in image appearance, typical of pure ASIR algorithms, 60% will be our favored and recommended ASIR level for tumour CT perfusion analysis below 100 kV in future studies, particularly for tumours located in the pelvis, representing 27 out of 32 tumours in the current cohort (including rectum, sigmoid and cecum).
A reduced peak kilovoltage of 100 kV was adopted instead of the “standard” 120 kV previously used for quantitative perfusion analysis of colorectal tumours [30], [31], [32], aiming to strike a balance between dose reduction and clinically acceptable image quality in a typical patient cohort from the United Kingdom.
The current study has a few limitations. We only assessed the effect of ASIR on image quality and pCT quantitation at fixed kV and mA levels, having to adhere to a fixed imaging protocol as part of a prospective multicentric study; ethical issues would have to be considered with repeated acquisitions at multiple kV or mA levels in the same patient. The patient cohort size (n = 32), albeit relatively small, has been shown to have >99.9% power, with a 5% significance level, to show a difference of 7 units or more between two sets of parameter measurements, equivalent to the clinically acceptable 10% parameter variation (based on BF values).
In summary we found that ASIR improves image noise and contrast-to-noise ratio and does not alter pCT tumour parameters substantially at a reduced peak kilovoltage of 100 kV. These supportive data strengthen the case for implementing lower dose pCT protocols in future clinical practice, within the constraints of an adequate CNR for quantification.
Declaration of interest
-
•
D Prezzi, FRCR (no conflict of interest and/or commercial involvement to disclose)
-
•
V Goh, FRCR (no conflict of interest; research agreement with Siemens Healthcare)
-
•
S Virdi, MBBS (no conflict of interest and/or commercial involvement to disclose)
-
•
S Mallett, DPhil (no conflict of interest and/or commercial involvement to disclose)
-
•
C Grierson, FRCR (no conflict of interest and/or commercial involvement to disclose)
-
•
DJ Breen, FRCR (no conflict of interest and/or commercial involvement to disclose)
Acknowledgements
The authors would like to acknowledge the following for their contribution to the NIHR HTA funded PROSPeCT study. Site principal investigators: M Betts, I Britton, D Breen, J Brush, P Correa, N Dodds, C Grierson, N Griffin, S Gourtsoyianni, J Hampton, A Higginson, A Lowe, R Mannion, C Oliver, A Slater, M Strugnell, D Tolan, R Vinayagam, I Zealley. Site radiographers & research staff: D Barrie, G Cattini, P Clewer, N Cowan, M Dodds, S Dundas, N Gibbons, A Green, M Harris, G Haley, L Houston, K Jones, L Jones, M Lewis, C Marsh, E Ngandwe, B Pharoah, V Ritchie, C Roe, R Smith, D Tew, K Wallace, A Williams, P Woodburn, N Wragg. Trial management group, steering committee, data monitoring committee and ad hoc advisors: D Altman, C Bartram, V Boulter, R Glynne-Jones, A Groves, A Hackshaw, S Halligan, P Hoskin, S Mallett, D Miles, K Miles, A Rockall, M Rodriguez-Justo, SA Taylor.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR HTA Programme, NIHR, NHS or the Department of Health.
References
- 1.Miles K.A., Lee T.Y., Goh V. Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur. Radiol. 2012;22(7):1430–1441. doi: 10.1007/s00330-012-2379-4. [DOI] [PubMed] [Google Scholar]
- 2.Yi C.A., Lee K.S., Kim E.A. Solitary pulmonary nodules: dynamic enhanced multi-detector row CT study and comparison with vascular endothelial growth factor and microvessel density. Radiology. 2004;233(1):191–199. doi: 10.1148/radiol.2331031535. [DOI] [PubMed] [Google Scholar]
- 3.Li Y., Yang Z.G., Chen T.W., Chen H.J., Sun J.Y., Lu Y.R. Peripheral lung carcinoma: correlation of angiogenesis and first-pass perfusion parameters of 64-detector row CT. Lung Cancer. 2008;61(1):44–53. doi: 10.1016/j.lungcan.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Ma S.H., Le H.B., Jia B.H. Peripheral pulmonary nodules: relationship between multi-slice spiral CT perfusion imaging and tumor angiogenesis and VEGF expression. BMC Cancer. 2008;8:186. doi: 10.1186/1471-2407-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sauter A.W., Winterstein S., Spira D. Multifunctional profiling of non-small cell lung cancer using 18 F-FDG PET/CT and volume perfusion CT. J. Nucl Med. 2012;53(4):521–529. doi: 10.2967/jnumed.111.097865. [DOI] [PubMed] [Google Scholar]
- 6.Spira D., Neumeister H., Spira S.M. Assessment of tumor vascularity in lung cancer using volume perfusion CT (VPCT) with histopathologic comparison: a further step toward an individualized tumor characterization. J. Comput. Assist. Tomogr. 2013;37(1):15–21. doi: 10.1097/RCT.0b013e318277c84f. [DOI] [PubMed] [Google Scholar]
- 7.Hayano K., Shuto K., Koda K., Yanagawa N., Okazumi S., Matsubara H. Quantitative measurement of blood flow using perfusion CT for assessing clinicopathologic features and prognosis in patients with rectal cancer. Dis. Colon. Rectum. 2009;52(9):1624–1629. doi: 10.1007/DCR.0b013e3181afbd79. [DOI] [PubMed] [Google Scholar]
- 8.Goh V., Halligan S., Wellsted D.M., Bartram C.I. Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur. Radiol. 2009;19(1):79–89. doi: 10.1007/s00330-008-1128-1. [DOI] [PubMed] [Google Scholar]
- 9.Hara A.K., Paden R.G., Silva A.C., Kujak J.L., Lawder H.J., Pavlicek W. Iterative reconstruction technique for reducing body radiation dose at CT: feasibility study. AJR Am. J. Roentgenol. 2009;193(3):764–771. doi: 10.2214/AJR.09.2397. [DOI] [PubMed] [Google Scholar]
- 10.Silva A.C., Lawder H.J., Hara A., Kujak J., Pavlicek W. Innovations in CT dose reduction strategy: application of the adaptive statistical iterative reconstruction algorithm. AJR Am. J. Roentgenol. 2010;194(1):191–199. doi: 10.2214/AJR.09.2953. [DOI] [PubMed] [Google Scholar]
- 11.Brady S.L., Moore B.M., Yee B.S., Kaufman R.A. Pediatric CT: implementation of ASIR for substantial radiation dose reduction while maintaining pre-ASIR image noise. Radiology. 2014;270(1):223–231. doi: 10.1148/radiol.13122578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S., Kalra M.K., Shenoy-Bhangle A.S. Radiation dose reduction with hybrid iterative reconstruction for pediatric CT. Radiology. 2012;263(2):537–546. doi: 10.1148/radiol.12110268. [DOI] [PubMed] [Google Scholar]
- 13.Vorona G.A., Ceschin R.C., Clayton B.L., Sutcavage T., Tadros S.S., Panigrahy A. Reducing abdominal CT radiation dose with the adaptive statistical iterative reconstruction technique in children: a feasibility study. Pediatr. Radiol. 2011;41(9):1174–1182. doi: 10.1007/s00247-011-2063-x. [DOI] [PubMed] [Google Scholar]
- 14.Desai G.S., Uppot R.N., Yu E.W., Kambadakone A.R., Sahani D.V. Impact of iterative reconstruction on image quality and radiation dose in multidetector CT of large body size adults. Eur. Radiol. 2012;22(8):1631–1640. doi: 10.1007/s00330-012-2424-3. [DOI] [PubMed] [Google Scholar]
- 15.Kaza R.K., Platt J.F., Al-Hawary M.M., Wasnik A., Liu P.S., Pandya A.C.T. enterography at 80 kVp with adaptive statistical iterative reconstruction versus at 120 kVp with standard reconstruction: image quality, diagnostic adequacy, and dose reduction. AJR Am. J. Roentgenol. 2012;198(5):1084–1092. doi: 10.2214/AJR.11.6597. [DOI] [PubMed] [Google Scholar]
- 16.Kambadakone A.R., Chaudhary N.A., Desai G.S., Nguyen D.D., Kulkarni N.M., Sahani D.V. Low-dose MDCT and CT. enterography of patients with Crohn disease: feasibility of adaptive statistical iterative reconstruction. AJR Am. J. Roentgenol. 2011;196(6):W743–W752. doi: 10.2214/AJR.10.5303. [DOI] [PubMed] [Google Scholar]
- 17.Singh S., Kalra M.K., Gilman M.D. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology. 2011;259(2):565–573. doi: 10.1148/radiol.11101450. [DOI] [PubMed] [Google Scholar]
- 18.Singh S., Kalra M.K., Hsieh J. Abdominal CT: comparison of adaptive statistical iterative and filtered back projection reconstruction techniques. Radiology. 2010;257(2):373–383. doi: 10.1148/radiol.10092212. [DOI] [PubMed] [Google Scholar]
- 19.Flicek K.T., Hara A.K., Silva A.C., Wu Q., Peter M.B., Johnson C.D. Reducing the radiation dose for CT colonography using adaptive statistical iterative reconstruction: a pilot study. AJR Am. J. Roentgenol. 2010;195(1):126–131. doi: 10.2214/AJR.09.3855. [DOI] [PubMed] [Google Scholar]
- 20.Marin D., Nelson R.C., Schindera S.T. Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm–initial clinical experience. Radiology. 2010;254(1):145–153. doi: 10.1148/radiol.09090094. [DOI] [PubMed] [Google Scholar]
- 21.Sagara Y., Hara A.K., Pavlicek W., Silva A.C., Paden R.G., Wu Q. Abdominal CT: comparison of low-dose CT with adaptive statistical iterative reconstruction and routine-dose CT with filtered back projection in 53 patients. AJR Am. J. Roentgenol. 2010;195(3):713–719. doi: 10.2214/AJR.09.2989. [DOI] [PubMed] [Google Scholar]
- 22.Miéville F.A., Gudinchet F., Brunelle F., Bochud F.O., Verdun F.R. Iterative reconstruction methods in two different MDCT scanners: physical metrics and 4-alternative forced-choice detectability experiments–a phantom approach. Phys. Med. 2013;29(1):99–110. doi: 10.1016/j.ejmp.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Lin C.J., Wu T.H., Lin C.H. Can iterative reconstruction improve imaging quality for lower radiation CT perfusion? Initial experience. AJNR Am. J. Neuroradiol. 2013;34(8):1516–1521. doi: 10.3174/ajnr.A3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niesten J.M., van der Schaaf I.C., Riordan A.J. Radiation dose reduction in cerebral CT perfusion imaging using iterative reconstruction. Eur. Radiol. 2014;24(2):484–493. doi: 10.1007/s00330-013-3042-4. [DOI] [PubMed] [Google Scholar]
- 25.Negi N., Yoshikawa T., Ohno Y. Hepatic CT perfusion measurements: a feasibility study for radiation dose reduction using new image reconstruction method. Eur. J. Radiol. 2012;81(11):3048–3054. doi: 10.1016/j.ejrad.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Xie Q., Wu J., Tang Y. Whole-organ CT perfusion of the pancreas: impact of iterative reconstruction on image quality, perfusion parameters and radiation dose in 256-slice CT-preliminary findings. PLoS One. 2013;8(11):e80468. doi: 10.1371/journal.pone.0080468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Funama Y., Taguchi K., Utsunomiya D. Combination of a low-tube-voltage technique with hybrid iterative reconstruction (iDose) algorithm at coronary computed tomographic angiography. J. Comput. Assist. Tomogr. 2011;35(4):480–485. doi: 10.1097/RCT.0b013e31821fee94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schindera S.T., Odedra D., Raza S.A. Iterative reconstruction algorithm for CT: can radiation dose be decreased while low-contrast detectability is preserved? Radiology. 2013;269(2):511–518. doi: 10.1148/radiol.13122349. [DOI] [PubMed] [Google Scholar]
- 29.Goh V., Dattani M., Farwell J. Radiation dose from volumetric helical perfusion CT of the thorax, abdomen or pelvis. Eur. Radiol. 2011;21(5):974–981. doi: 10.1007/s00330-010-1997-y. [DOI] [PubMed] [Google Scholar]
- 30.Bellomi M., Petralia G., Sonzogni A., Zampino M.G., Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244(2):486–493. doi: 10.1148/radiol.2442061189. [DOI] [PubMed] [Google Scholar]
- 31.Goh V., Halligan S., Gharpuray A., Wellsted D., Sundin J., Bartram C.I. Quantitative assessment of colorectal cancer tumor vascular parameters by using perfusion CT: influence of tumor region of interest. Radiology. 2008;247(3):726–732. doi: 10.1148/radiol.2473070414. [DOI] [PubMed] [Google Scholar]
- 32.Dighe S., Blake H., Jeyadevan N. Perfusion CT vascular parameters do not correlate with immunohistochemically derived microvessel density count in colorectal tumors. Radiology. 2013;268(2):400–410. doi: 10.1148/radiol.13112460. [DOI] [PubMed] [Google Scholar]