Abstract
Background
Micronutrient deficiencies are common among adults living with HIV disease, particularly in low‐income settings where the diet may be low in essential vitamins and minerals. Some micronutrients play critical roles in maintenance of the immune system, and routine supplementation could therefore be beneficial. This is an update of a Cochrane Review previously published in 2010.
Objectives
To assess whether micronutrient supplements are effective and safe in reducing mortality and HIV‐related morbidity of HIV‐positive adults (excluding pregnant women).
Search methods
We performed literature searches from January 2010 to 18 November 2016 for new randomized controlled trials (RCTs) of micronutrient supplements since the previous review included all trials identified from searches prior to 2010. We searched the CENTRAL (the Cochrane Library), Embase, and PubMed databases. Also we checked the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and the ClinicalTrials.gov trials registers. We also checked the reference lists of all new included trials.
Selection criteria
We included RCTs that compared supplements that contained either single, dual, or multiple micronutrients with placebo, no treatment, or other supplements. We excluded studies that were primarily designed to investigate the role of micronutrients for the treatment of HIV‐positive participants with metabolic morbidity related to highly active antiretroviral therapy (HAART). Primary outcomes included all‐cause mortality, morbidity, and disease progression.
Data collection and analysis
Two review authors independently selected trials for inclusion, and appraised trial quality for risk of bias. Where possible, we presented results as risk ratios (RR) for dichotomous variables, as hazard ratios (HRs) for time‐to‐event data, and as mean differences (MD) for continuous variables, each with 95% confidence intervals (CIs). Since we were often unable to pool the outcome data, we tabulated it for each comparison. We assessed the certainty of the evidence using the GRADE approach.
Main results
We included 33 trials with 10,325 participants, of which 17 trials were new trials. Ten trials compared a daily multiple micronutrient supplement to placebo in doses up to 20 times the dietary reference intake, and one trial compared a daily standard dose with a high daily dose of multivitamins. Nineteen trials compared supplementation with single or dual micronutrients (such as vitamins A and D, zinc, and selenium) to placebo, and three trials compared different dosages or combinations of micronutrients.
Multiple micronutrients
We conducted analyses across antiretroviral therapy (ART)‐naive adults (3 trials, 1448 participants), adults on antiretroviral therapy (ART) (1 trial, 400 participants), and ART‐naive adults with concurrent active tuberculosis (3 trials, 1429 participants). Routine multiple micronutrient supplementation may have little or no effect on mortality in adults living with HIV (RR 0.91, 95% CI 0.72 to 1.15; 7 trials, 2897 participants, low certainty evidence).
Routine supplementation for up to two years may have little or no effect on the average of mean CD4+ cell count (MD 26.40 cells/mm³, 95% CI −22.91 to 75.70; 6 trials, 1581 participants, low certainty evidence), or the average of mean viral load (MD −0.1 log10viral copies, 95% CI −0.26 to 0.06; 4 trials, 840 participants, moderate certainty evidence). One additional trial in ART‐naïve adults did report an increase in the time to reach a CD4+ cell count < 250 cells/mm³ after two years of high dose supplementation in Botswana (HR 0.48, 95% CI 0.26 to 0.88; 1 trial, 439 participants). However, the trial authors reported this effect only in the trial arm that received multiple micronutrients plus selenium (not either supplementation alone), which is inconsistent with the findings of other trials that used similar combinations of micronutrients and selenium.
In one additional trial that compared high‐dose multiple micronutrient supplementation with standard doses in people on ART, peripheral neuropathy was lower with high dose supplements compared to standard dose (incidence rate ratio (IRR) 0.81, 95% CI 0.7 to 0.94; 1 trial, 3418 participants), but the trial was stopped early due to increased adverse events (elevated alanine transaminase (ALT) levels) in the high dose group.
Single or dual micronutrients
None of the trials of single or dual micronutrient supplements were adequately powered to assess for effects on mortality or morbidity outcomes. No clinically significant changes in CD4 cell count (data not pooled, 14 trials, 2370 participants, very low or low certainty evidence) or viral load (data not pooled, seven studies, 1334 participants, very low or low certainty evidence), were reported. Supplementation probably does increase blood concentrations of vitamin D and zinc (data not pooled, vitamin D: 4 trials, 299 participants, zinc: 4 trials, 484 participants, moderate certainty evidence) and may also increase blood concentrations of vitamin A (data not pooled, 3 trials, 495 participants, low certainty evidence), especially in those who are deficient.
Authors' conclusions
The analyses of the available trials have not revealed consistent clinically important benefits with routine multiple micronutrient supplementation in people living with HIV. Larger trials might reveal small but important effects.
These findings should not be interpreted as a reason to deny micronutrient supplements for people living with HIV where specific deficiencies are found or where the person's diet is insufficient to meet the recommended daily allowance of vitamins and minerals.
12 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (18 Nov, 2016) were included and six ongoing studies have been identified (see 'Characteristics of ongoing studies' section)
Keywords: Adult; Child; Female; Humans; Pregnancy; Dietary Supplements; HIV Infections; HIV Infections/complications; HIV Infections/mortality; CD4 Lymphocyte Count; Cause of Death; HIV‐1; HIV‐2; Hospitalization; Hospitalization/statistics & numerical data; Micronutrients; Micronutrients/administration & dosage; Micronutrients/deficiency; Pregnancy Complications, Infectious; Pregnancy Complications, Infectious/mortality; Randomized Controlled Trials as Topic; Selenium; Selenium/administration & dosage; Viral Load; Vitamin A; Vitamin A/administration & dosage; Vitamin D; Vitamin D/administration & dosage; Vitamins; Vitamins/administration & dosage; Zinc; Zinc/administration & dosage; beta Carotene; beta Carotene/administration & dosage
Plain language summary
Micronutrient supplements for non‐pregnant adults with HIV infection
Cochrane researchers conducted a review of the effects of micronutrient supplements for people living with HIV. This is an update of a Cochrane Review previously published in 2010. After searching for relevant trials up to 18 November 2016, the review authors included 33 trials. Thirteen of these trials included people not on HIV treatment and were conducted in Thailand, Peru, and eight African countries. Nineteen trials included people on HIV treatment and were conducted in North America, Europe, Brazil, Singapore, Thailand, Botswana, and Uganda. One trial from China did not state whether people living with HIV were on treatment or not. Some trials looked at the effects of taking supplements with multiple micronutrients whereas others looked at supplementation with single vitamins or minerals.
What are micronutrient supplements and how might they help people living with HIV?
Micronutrient supplements contain vitamins or minerals, or both, that are essential to good health. Many of these vitamins play important roles in maintaining the human immune system, which helps to fight off infections.
Infection with HIV causes a progressive destruction of the immune system, which leaves people vulnerable to frequent infections. Many people living with HIV, especially in low‐income countries, are also undernourished and many consume diets deficient that these essential micronutrients. Supplementation could therefore help people living with HIV to stay healthy for longer by strengthening their immune system or assisting recovery from infections.
What the research says
Multiple micronutrients
Providing a daily supplement that contains multiple vitamins and minerals may have little or no effect on reducing deaths in people living with HIV, whether they are taking antiretroviral drugs or not (low certainty evidence). Daily supplements may have little or no effect on HIV disease progression as measured by CD4 cell count (low certainty evidence) or HIV viral load (low or moderate certainty evidence).
Single or dual micronutrients
We do not know whether supplements that contain single vitamins or minerals reduce deaths (very low certainty evidence) or slow disease progression (very low/low certainty evidence) in people living with HIV. Supplementation with vitamin A, D, zinc, or selenium may improve the level of each vitamin in a person's blood, especially those with low levels before supplementation (low/moderate certainty evidence).
These findings do not mean that an adequate dietary intake for people living with HIV is not important. It is also not a reason to deny micronutrient supplements for those in whom a deficiency has been clinically demonstrated, or who are unlikely to meet the recommended daily allowance of vitamins and minerals.
Summary of findings
Summary of findings for the main comparison. Multiple micronutrients compared to placebo for adults with HIV infection.
| Multiple micronutrients compared to placebo for adults with HIV infection | ||||||
| Participant or population: adults with HIV infection (with and without concurrent tuberculosis, with and without ART) Settings: all settings Intervention: multiple micronutrient supplementation (standard or high dose daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Micronutrients | |||||
|
Mortality Follow‐up: 8 to 24 months |
100 per 1000 | 91 per 1000 (72 to 115) |
RR 0.91 (0.72 to 1.15) |
2897 (7 trials) | ⊕⊕⊝⊝
low1,2,3,4 due to indirectness and imprecision |
Multiple micronutrients may have little or no effect on mortality |
|
Hospital admissions Follow‐up: 11 to 18 months |
139 per 1000 |
120 per 1000 (85 to 170) |
RR 0.86 (0.61 to 1.22) |
881 (2 trials) |
⊕⊝⊝⊝
very low1,4,5 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on hospital admissions |
|
CD4 cell count Follow‐up: 6 weeks to 2 years |
The mean in the placebo groups ranged from 147 to 483 cells/mm³ |
The mean in the multiple micronutrient group was 26.40 cells/mm³ higher (22.91 lower to 75.70 higher) |
— | 1581 (6 trials) | ⊕⊕⊝⊝ low1,3,6 due to indirectness and inconsistency |
Multiple micronutrients may have little or no effect on CD4 cell count |
|
Viral load Follow‐up: 6 weeks to 2 years |
The mean in the placebo groups ranged from 4.1 to 5.4 log10copies/mL |
The mean in the multiple micronutrient groups was 0.10 log10copies/mL lower (0.26 lower to 0.06 higher) |
— | 840 (4 trials) |
⊕⊕⊕⊝
moderate1,7 due to indirectness |
Multiple micronutrients probably have little or no effect on viral load |
|
Nutritional status Follow‐up: 4 weeks to 1.9 years |
— | — | Not pooled | 1007 (3 trials) | ⊕⊝⊝⊝
very low1,8,9 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on nutritional status parameters |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ART: antiretroviral therapy; BMI: body mass index; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: most trials were at low risk of selection bias and used placebos to prevent performance or detection bias. 2No serious heterogeneity: none of the trials found statistically significant effects overall (although one small subgroup from one trial in Tanzania did find a statistically significant difference this is probably a chance finding). 3Downgraded by 1 for serious indirectness: although most trials reported this outcome, only one of these (from Uganda using standard dose micronutrients) included a substantial number of adults on ART in line with current recommendations. The other trials used standard or high dose micronutrients and were conducted in ART‐naive adults (in Botswana, Zambia, and Thailand), and adults with concurrent tuberculosis (in Tanzania and Malawi). 4Downgraded by 1 for serious imprecision: the 95% CI is wide and includes both clinically important effects and no effect. The overall meta‐analysis remains underpowered to confidently exclude effects. 5Downgraded by 2 for very serious indirectness: these two trials were conducted in Thailand (high dose micronutrients in ART‐naive adults) and Uganda (standard dose micronutrients in adults on ART). The finding of no effect may not apply to all populations and settings. 6Downgraded by 1 for serious inconsistency: in total eight trials reported a measure of CD4+ cell count although we could only include six trials in this meta‐analysis. Of note, one recent trial in Botswana among ART‐naive adults (not included in the meta‐analysis) reported a reduced risk of reaching a CD4+ cell count of less than 250 cells/mm³ after two years of high dose supplementation. This finding is inconsistent with other trials that used similar combinations of micronutrients and selenium. 7Downgraded by 1 for serious indirectness: in total four trials in ART‐naive adults, with concurrent TB (in Tanzania and Malawi) or without TB (in Kenia and Thailand), reported viral load. The finding of no effect may not apply to people on ART or other populations and settings. 8Downgraded by 2 for serious indirectness: only three trials (from Uganda, Zambia, and Tanzania) reported measures of nutritional status (BMI, weight, mid‐upper arm circumference (MUAC), lean body mass). The finding of no effect may not apply to all populations and settings. 9Downgraded by 1 for serious imprecision: we were unable to pool data but the 95% CIs of the individual trials were wide and included clinically important effects and no effect.
Summary of findings 2. Vitamin A compared to placebo.
| Vitamin A compared to placebo for adults with HIV infection currently taking ART or not | ||||||
| Participant or population: adults with HIV infection Settings: any Intervention: vitamin A (single dose or daily dose) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin A | |||||
| Mortality | — | — | — | (0 trials) | — | — |
| Morbidity | — | — | — | (0 trials) | — | — |
| CD4 cell count (cells/mm³) Follow‐up: 6 to 8 weeks | — | — | Not pooled | 464 (2 trials) | ⊕⊕⊝⊝
low1,2,3,4 due to risk of bias and indirectness |
Vitamin A may have little or no short‐term effect on CD4 cell count |
| Viral load (log10copies/mL) Follow‐up: 6 to 8 weeks | — | — | Not pooled | 495 (3 trials) | ⊕⊕⊝⊝
low1,2,3,4 due to risk of bias and indirectness |
Vitamin A may have little or no short‐term effect on viral load |
| Change in vitamin A concentrations (µmol/L) Follow‐up: 6 to 8 weeks | — | — | Not pooled | 495 (3 trials) |
⊕⊕⊝⊝
low1,3,4,5 due to risk of bias and indirectness |
Vitamin A may increase blood concentrations of persons with HIV with low baseline concentrations |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ART: antiretroviral therapy; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1Downgraded by 1 for serious risk of bias: one trial in Kenya with 400 participants reported high attrition overall (11.5%) and the trial authors stated that participants who were lost to follow‐up had more advanced HIV disease and were more likely to be vitamin A deficient (Baeten 2002 KEN). 2No serious heterogeneity: none of the trials found statistically significant effects. 3Downgraded by 1 for serious indirectness: trials were conducted in the USA and Kenya, and most participants were not on antiretroviral therapy (ART). This may not completely exclude the possibility of effects in some settings or populations. 4No serious imprecision: no statistically significant differences were seen. Although two trials were underpowered, one trial in Kenya with 400 participants was adequately powered to reliably detect a clinically beneficial effect on CD4 cell count, viral load, and blood vitamin A concentrations (Baeten 2002 KEN). 5No serious heterogeneity: a statistical significant increase in blood vitamin concentrations was reported in one trial from Kenya with 400 participants. Baseline blood vitamin concentrations of these participants were much lower than the 95 participants in the other two trials in the USA.
Summary of findings 3. Vitamin D compared to placebo.
| Vitamin D compared to placebo for adults with HIV infection | ||||||
| Participant or population: adults with HIV infection Settings: any Intervention: vitamin D (repeated single doses or daily dose with or without additional calcium) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Vitamin D | |||||
|
Mortality Follow‐up: 12 months |
254 per 1000 |
292 per 1000 (165 to 513) |
RR 1.15 (0.65 to 2.02) |
131 (1 trial) | ⊕⊝⊝⊝
very low1,2,3 due to indirectness and imprecision |
We don't know if vitamin D supplements have any effect on mortality |
| Morbidity | — | — | — | (0 trials) | — | — |
| CD4 cell count (cells/mm³) Follow‐up: 16 weeks to 12 months | — | — | Not pooled | 288 (4 trials) | ⊕⊕⊝⊝
low1,4 due to indirectness |
Vitamin D supplements may have little or no effect on CD4 cell count |
|
Viral load (log10copies/mL) Follow‐up: 12 months |
The mean in the placebo group was 3.78 |
The mean in the multiple micronutrient groups was 0.66 lower (1.37 lower to 0.05 higher) |
— | 28 participants (1 trial) |
⊕⊝⊝⊝
very low1,5,6 due to indirectness and imprecision |
We don't know if vitamin D supplements have an effect on viral load |
| Change in 25(OH) vitamin D concentrations (ng/mL) Follow‐up: 16 weeks to 12 months | — | — | Not pooled | 299 (4 trials) | ⊕⊕⊕⊝
moderate1,7,8 due to indirectness |
Vitamin D supplements probably increase blood 25(OH) vitamin D levels |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: the included trials were generally at low risk of bias. 2Downgraded by 2 for serious indirectness: only a single trial from Guinea‐Bissau reports the number of deaths after 12 months follow‐up in HIV‐positive participants on treatment for active tuberculosis (Wejse 2009 GNB). 3Downgraded by 1 for serious imprecision: the 95% CI is wide and includes both a relative risk reduction and relative risk increase of greater than 25%. 4Downgraded by 2 for serious indirectness: no changes in mean or median CD4 cell counts were reported from these four small trials from Italy (Giacomet 2013 ITA), the USA (Overton 2015 USA), Guinea‐Bissau (Wejse 2009 GNB), or Denmark (Bang 2012 DEN). This doesn't exclude the possibility of effects in some populations. 5Downgraded by 2 for very serious indirectness: this is a single very small trial from the USA. 6Downgraded by 1 for serious imprecision: the trial is very small, and the 95% CI is wide and includes no effect. 7No serious heterogeneity: all four studies report a statistical significant increase in blood 25(OH) vitamin D concentrations (ng/mL). 8Downgrade by 1 for serious risk of indirectness: all studies were conducted in high income countries (Italy, Canada, Denmark, and the USA).
Summary of findings 4. Zinc compared to placebo.
| Zinc compared to placebo for adults with HIV infection | ||||||
| Participant or population: adults with HIV infection Settings: any Intervention: zinc (daily or weekly dose) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Zinc | |||||
| Mortality Follow‐up: 6 to 18 months | 110 per 1000 |
135 per 1000 (58 to 315) |
RR 1.24 (0.53 to 2.86) | 433 (3 trials) | ⊕⊝⊝⊝
very low1,2,3 due to indirectness and imprecision |
We don't know if zinc supplements have any effect on mortality |
| Rate of diarrhoea Follow‐up: 18 months | — | — | OR 0.40 (0.18 to 0.87) | 231 (1 trial) |
⊕⊝⊝⊝
very low1,4,5 due to indirectness and imprecision |
We don't know if zinc supplements have any effect on the frequency of diarrhoea |
|
Change in CD4 cell count (cells/mm³) Follow‐up: 1 to 18 months |
— | — | Not pooled | 431 (4 trials) | ⊕⊕⊝⊝
low1,2,6 due to indirectness and inconsistency |
Zinc supplements may have little or no effect on CD4 cell count |
|
Change in viral load (log10copies/mL) Follow‐up: 1 to 18 months |
— | — | Not pooled | 400 (3 trials) | ⊕⊕⊝⊝
low1,2,7 due to indirectness and imprecision |
Zinc supplements may have little or no effect on viral load |
|
Change in blood zinc concentrations Follow‐up: 1 to 18 months |
— | — | Not pooled | 484 (4 trials) |
⊕⊕⊕⊝
moderate1,2,8 due to indirectness |
Zinc supplements probably increase blood zinc concentrations of persons with HIV with low baseline concentrations |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio; OR: odds ratio; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: the included studies were generally at low risk of bias. 2Downgraded by 1 for serious indirectness: the available data is from limited settings and populations. The findings are not easily generalized to other populations. 3Downgraded by 2 for serious imprecision: the 95% CI around the absolute effect is very wide and crosses 1. The overall meta‐analysis is underpowered to confidently exclude effects. 4Downgraded by 2 for very serious indirectness: this finding is from a single study in the USA and may not be applicable to other settings. 5Downgraded by 1 for serious imprecision: although the 95% CI does not cross the line of no effect this trial is underpowered to detect or exclude clinically important differences. 6Downgrade by 1 for serious inconsistency: one very small trial from Singapore reports a marginal improvement in median CD4 count after 6 months of standard dose supplements (Asdamongkol 2013 THA), and one study reports a significant reduction in the risk of decline of CD4+ to < 200 in those taking standard supplements (Baum 2010 USA). Two other small studies using high dose supplements report no statistically significant difference (Green 2005 SGP; Range 2006 TZA). 7Downgraded by 1 for serious imprecision: all three trials were underpowered to include or exclude clinically important effects (Baum 2010 USA; Green 2005 SGP; Range 2006 TZA). 8No serious inconsistency: three trials report an increase in blood zinc concentrations over time. The participants in one trial that did not report an increase in blood concentrations after supplementation, were not deficient in zinc at baseline (Green 2005 SGP).
Summary of findings 5. Selenium compared to placebo.
| Selenium compared to placebo for adults with HIV infection | ||||||
| Participant or population: adults with HIV infection Settings: all settings Intervention: selenium (daily dose) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Selenium | |||||
| Mortality | — | — | — | (0 trials) | — | — |
| Hospital admissions Follow‐up: 12 months | 309 per 1000 | 124 per 1000 (65 to 232) | RR 0.4 (0.21 to 0.75) | 186 (1 trial) | ⊕⊝⊝⊝
very low1,2,3 due to risk of bias, indirectness, and imprecision |
We don't know if selenium supplements reduce hospital admissions |
|
Change in CD4 cell count (cells/mm³) Follow‐up: 9 to 24 months |
— | — | Not pooled | 1187 participants (4 trials) | ⊕⊕⊝⊝
low4,5 due to risk of bias and imprecision |
Selenium supplements may have little or no effect on CD4 cell count |
|
Change in viral load (log10copies/mL) Follow‐up: 24 months |
— | — | Not estimable | 439 participants (1 trial) | ⊕⊕⊝⊝ low6,7 | Selenium supplements may have little or no effect on viral load |
| Change in selenium concentrations (µg/L) Follow‐up: 6 to 12 months | — | — | Not pooled | 527 (3 trials) | ⊕⊕⊝⊝ low4,8,9 | Selenium supplements may increase blood selenium concentrations |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ART: antiretroviral therapy; CI: confidence interval; RR: risk ratio; HR: hazard ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1Downgraded by 1 for serious risk of bias: high attrition due to participants with incomplete medical records. In addition, fewer participants in the placebo group compared to the selenium group were on antiretroviral therapy (ART) at baseline (Burbano 2002 USA). 2Downgraded by 1 for serious indirectness: only a single trial is available from the USA in HIV‐positive intravenous drugs users. This is not easily generalized to other HIV‐positive populations. 3Downgraded by 1 for serious imprecision: this trial is underpowered to detect clinically important differences. 4Downgraded by 1 for serious risk of bias: two of the three trials reported high attrition rates (Burbano 2002 USA; Hurwitz 2007 USA). In one trial fewer participants in the placebo group compared to the selenium group were on ART at baseline (Burbano 2002 USA). 5Downgraded by 1 for serious imprecision: three of the four trials were underpowered to include or exclude clinically important effects (Burbano 2002 USA; Hurwitz 2007 USA; Kamwesiga 2015 RWA). One trial from Botswana was adequately powered and reported no effect on the decline in CD4 cell counts of ART‐naive participants (Baum 2013 BWA). 6No serious risk of bias: the included trial was at low risk of selection and performance bias (Baum 2013 BWA). The trial authors performed multiple imputation of viral load data. The trial authors did not provide details. 7Downgraded by 2 for serious indirectness: only a single trial is available from Botswana in participants not on ART (Baum 2013 BWA). 8No serious heterogeneity: all three trials reported either an increase in the mean blood selenium concentration of participants or the proportion of participants with selenium concentrations above a certain threshold level. 9Downgraded by 1 for indirectness: participants in two of the three included trials were not deficient in selenium at baseline (Burbano 2002 USA; Hurwitz 2007 USA). The third trial reported data on participants who were selenium deficient at baseline; however it was a small subsample of the main trial from Botswana (Sales 2010).
Background
Description of the condition
Despite a substantial decrease in the number of new HIV infections during the past decade, recent estimates from the United Nations Joint Programme on HIV/AIDS (UNAIDS) indicate that 35 million people were still living with HIV worldwide in 2013 (UNAIDS 2014). The HIV/AIDS pandemic has severely affected sub‐Saharan Africa, more than any other part of the world. With about a tenth of the world's population, the region is home to more than two‐thirds of all people living with HIV worldwide, an estimated 24.7 million adults and children (UNAIDS 2014). Globally, more than one‐third of HIV‐positive adults receive antiretroviral therapy (ART) (UNAIDS 2014). Earlier initiation of ART, in line with recent recommendations, is a challenge to implement in many countries, especially those in resource‐limited settings (WHO 2015).
Adults living with HIV may also have micronutrient deficiencies, particularly those from communities at high risk of food insecurity since diets are frequently inadequate to meet the recommended daily requirements (Gebrehiwot 2014). A recent review reported that people living with HIV who experience food insecurity tended to have lower CD4 counts than their counterparts (Aibibula 2016). Deficiencies of micronutrients are more pronounced in individuals with advanced disease, as a consequence of reduced nutrient intake due to AIDS and opportunistic infections, and excessive losses due to diarrhoea, malabsorption, and parasitic infections. Furthermore, in sub‐Saharan Africa, a region severely affected by the HIV/AIDS pandemic, protein energy malnutrition (PEM) is common. PEM refers to inadequate protein and energy intake and is usually associated with multiple micronutrient insufficiency (Irlam 2007).
Description of the intervention
Micronutrient supplements are either single or multiple formulations of vitamins and trace elements.
How the intervention might work
Micronutrients play a critical role in the maintenance of a functional immune system. The interactions between micronutrients and the components of the immune response are multifaceted and complex Chandra 1997; Raiten 2015). Several observational studies have suggested that micronutrient deficiencies may hasten clinical disease progression in HIV‐positive adults. Low blood levels of vitamin A, B12, zinc, and selenium have been related to increased HIV progression (Graham 1991; Kupka 2004; Tang 1997) or death in this population (Baum 1997; Baum 2003; Semba 1993). Most participants in these earlier studies were not receiving ART at the time. More recently, vitamin D deficiency, which is assessed by low 25‐hydroxy vitamin D levels, has been associated with increased disease progression of untreated (Mehta 2010) or treated HIV disease (Sudfeld 2012; Viard 2011), and impaired CD4 cell count recovery of HIV‐positive men and women on antiretroviral therapy (ART) (Aziz 2013; Ross 2011).
Widespread micronutrient supplementation may lessen the effects of concurrent micronutrient deficiency and help to reduce the morbidity and mortality due to HIV (Semba 1999). It has also been suggested that micronutrient supplementation may enhance the CD4 cell responses of individuals on ART who demonstrate adequate viral suppression (Tang 2005).
Assessing the effectiveness of micronutrient supplementation in participants with inflammation requires special consideration. Acute inflammation results in the redistribution of micronutrients due to changes in plasma proteins and may therefore impact on the validity of nutrient biomarkers, such as serum micronutrient concentrations (Raiten 2015).
Why it is important to do this review
A previous version of this Cochrane review included 30 trials: 20 trials of single micronutrient supplements (vitamin A, vitamin D, zinc, and selenium) and 10 of multiple micronutrient supplements. Eight trials were undertaken in child populations and four trials were conducted among pregnant and lactating women (Irlam 2010). The review found no conclusive evidence that micronutrient supplementation effectively reduces or increases morbidity or mortality in HIV‐positive adults.
The HIV/AIDS pandemic has had a major impact on global health, nutrition, and overall socioeconomic development. An update of the review based on recent, valid research is therefore important. Micronutrient supplements have potential benefit for people living with HIV infection. However, in order to understand the magnitude of this benefit and how supplements should be positioned alongside the proven advantages of antiretroviral drugs, a robust evidence‐base to guide policy and practice is required.
At the request of the World Health Organization (WHO), two separate Cochrane Reviews on the role of micronutrient supplementation were published for HIV‐positive pregnant women (Siegfried 2012) and children (Irlam 2013). The primary focus of this Cochrane review is therefore on the role of micronutrient supplementation in HIV‐positive non‐pregnant adults.
Objectives
To assess whether micronutrient supplements are effective and safe in reducing mortality and HIV‐related morbidity in HIV‐positive adults (excluding pregnant women).
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) of micronutrient supplements compared with placebo, no treatment, or other supplements (including variations in quantity or formulation).
Types of participants
HIV‐positive adults, defined as people ≥ 15 years of age who were HIV‐positive (WHO 2007). Two other Cochrane reviews have addressed micronutrient supplementation for HIV‐positive children and pregnant women (Irlam 2013; Siegfried 2012). We included trials that recruited both HIV‐positive adults and children if 80% or more of the participants were HIV‐positive adults.
We included trials that recruited antiretroviral therapy (ART)‐naive participants, as well as those that recruited participants on ART. Since the objective of this Cochrane Review is on the adjunctive role of micronutrients on mortality and HIV‐related morbidity, we excluded studies that were primarily designed to investigate the role of micronutrients for the treatment of HIV‐positive participants with metabolic morbidity related to ART. A Cochrane review on treatment for dyslipidaemia in HIV infection is in progress (Martí‐Carvajal 2010).
We included trials conducted in populations with and without HIV infection if outcome data were available for HIV‐positive participants.
We included trials that involved participants with tuberculosis with and without HIV infection if outcome data were available for participants with HIV, regardless of whether the trial authors stratified the randomization of trial participants according to HIV infection status. We excluded studies that did not report outcome data for HIV‐positive participants.
Types of interventions
We included trials of micronutrient supplementation that included vitamins (A, D, E, C, B1, B2, niacin, B6, B12, K, folate, beta‐carotene), trace elements (zinc, selenium, magnesium, iron, iodine, copper, manganese, chromium, cobalt, molybdenum), or combinations of the above only. We described a supplement as a standard dose supplement if the trial provided a single micronutrient, or a combination of micronutrients, at the level of the Recommended Daily Intake (RDA). We described any supplement containing a single micronutrient, or a combination of micronutrients in multiples of the RDA, as a high‐dose supplement. We excluded studies that assessed the effect of adding micronutrients to foods (food fortification).
Types of outcome measures
Primary outcomes
All‐cause mortality
Morbidity (frequency, types, and duration of episodes of opportunistic infections; incidence of AIDS as defined by each trial; hospital admissions; and other types of illnesses related to HIV infection as reported in each study)
Disease progression according to either the World Health Organization (WHO 2007), or the Centers for Disease Control and Prevention (CDC) clinical staging system (Schneider 2008), as reported in each included trial
Secondary outcomes
Virological response: proportion of participants who maintained an undetectable viral load and change in HIV‐RNA levels (mean relative change (percent) or mean absolute change, compared with baseline, and standard deviation (SD))
Virological failure: proportion of participants who discontinued or switched ART due to virological failure, as defined by each included trial
Markers of immune response, such as change in absolute CD4+ T lymphocyte count (mean relative change (percent) or mean absolute change, compared with baseline, and SD)
Nutritional status, including measurements such as bodyweight, Body Mass Index (BMI), and body composition
Biochemical markers, such as serum micronutrient concentrations
We excluded studies that only reported data that related to biochemical markers from this review.
Adverse events
We extracted data on all adverse events that we judged to be associated with micronutrient supplementation, as reported by each included trial. If the trial authors had classified these events according to the Adverse Event Toxicity Scale, we extracted the data accordingly: grade 1 or 2 (mild to moderate symptoms), grade 3 (serious symptoms), or grade 4 symptoms (denotes life‐threatening events that require a significant clinical intervention) (DAIDS 2014).
Search methods for identification of studies
Electronic searches
We searched the CENTRAL (Appendix 1), PubMed (Appendix 2), and Embase (Appendix 3) databases from January 2010 up to 18 November 2016. We limited the search date from January 2010, since Irlam 2010 included all trials identified from searches prior to and including January 2010. In addition we checked the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (Appendix 4) and the ClinicalTrials.gov trial register (Appendix 5). We also searched the reference lists of the included trials.
Searching other resources
For this update, we searched the reference lists of all the included trials. We also contacted investigators of ongoing studies that have been completed by email to enquire about any new or imminent publications.
Data collection and analysis
Selection of studies
Two review authors (MV and SD) independently screened the titles and abstracts identified through the electronic searches for potentially eligible citations for full‐text screening. In the case of uncertainty regarding eligibility, we screened the full‐text article(s). Two review authors (MV and SD) screened full‐text articles using a standardized eligibility form based on the inclusion criteria of the review. In the case of disagreement or uncertainty, a third review author (NS) provided their opinion. We listed all studies that we excluded after full‐text assessment and their reasons for exclusion in a 'Characteristics of excluded studies' table. We constructed a PRISMA diagram to illustrate the study selection process.
Data extraction and management
Two review authors (MV and SD) independently extracted data from the included trials for the review update using an updated standardized electronic data extraction form. We extracted the following information from each trial.
Administrative details: trial identification number; author(s); published or unpublished; year of publication
Details of the trial: country and location of trial, trial design, duration and completeness of follow‐up; informed consent and ethics approval, source of funding
Details of participants: age, gender, disease progression according to clinical staging, relevant baseline characteristics including CD4 count and viral load
Details of intervention and control group: type, dosage, and frequency of micronutrient(s); additional co‐interventions (such as ART, tuberculosis treatment, or other management of opportunistic infections)
Details of outcomes: all prespecified outcomes and any additional outcomes reported in the study; adverse events and toxicity
Details of data analysis: numbers and reported statistics for each reported outcome. Where trial outcomes were reported in more than one reference, we used all the trial reports to extract data as comprehensively as possible
We entered data into the Review Manager 5 (RevMan 5) software (Review Manager 5). The trial ID for each included trial consisted of the name of the first trial author followed by the date of publication and the country code where the study was conducted (see Appendix 6).
Assessment of risk of bias in included studies
Two review authors (MV and SD) independently assessed the risk of bias of each new included trial using the Cochrane 'Risk of bias' assessment tool (Higgins 2011). Please see Appendix 7 for the additional assessment of risk of bias in included cluster‐randomized trials.For each trial we assessed the following domains as either at high, low, or unclear risk of bias: sequence generation, allocation concealment, blinding (participants, personnel, and outcome assessor), incomplete outcome data, selective outcome reporting, and other sources of bias.
Measures of treatment effect
For the measures of treatment effect, we used the risk ratio (RR) for dichotomous data, the weighted mean difference (WMD) for continuous data measured on the same scale, and the standardized mean difference (SMD) for continuous data measured on different scales, presented with 95% confidence intervals (CIs). For time‐to‐event data we extracted the hazard ratio (HR). We used RevMan 5 for data analysis (Review Manager 5).
Unit of analysis issues
We included two trials with factorial designs (Baum 2013 BWA; Range 2006 TZA). For Range 2006 TZA, we halved the number of events and participants in the placebo group for dichotomous outcomes and the number of participants for continuous outcomes in our meta‐analysis in order to avoid double counting. Since Baum 2013 BWA reported time‐to‐event data we were unable to incorporate the data into a meta‐analysis.
We described the outcome data narratively for the cross‐over trial by Coodley 1993 USA, since the study authors did not report outcome data before trial cross‐over. For Kelly 2008 ZMB we did not include the data after trial cross‐over as the trial authors did not clearly describe the wash‐out period.
Dealing with missing data
We contacted the authors of three published conference abstracts in order to obtain further information regarding the trial protocol and study outcomes (Baum 2010 USA; Sales 2010; Scrimgeour 2010). We also contacted other trial authors in order to clarify data or statistical analysis where needed. Where possible, we conducted a complete‐case analysis. For trial outcomes where this was unclear, we used the number of participants randomized to each trial arm. We documented the attrition rate for each included trial in the 'Risk of bias' table.
Assessment of heterogeneity
First we assessed trials for clinical heterogeneity by examining variability in the participants, interventions, and outcomes. We assessed statistical heterogeneity visually and by means of the Chi² test for heterogeneity and the I² statistic. We classified heterogeneity according to the I² statistic values as follows (Higgins 2002).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
Assessment of reporting biases
To prevent reporting biases we searched multiple sources and searched for unpublished studies in trials registers. We did not examine funnel plots to assess the likelihood of publication bias as there were an insufficient number of trials per outcome.
Data synthesis
In view of the anticipated heterogeneity between trial populations and interventions, we used a random‐effects model. When we were unable to pool data due to differences in the statistical methods and measures the study authors used, we presented the data in tables with a narrative summary.
Subgroup analysis and investigation of heterogeneity
We conducted stratified analyses according to whether participants were taking ART or not, and whether they were on concurrent treatment for tuberculosis or not. We stratified outcome data, such as CD4+ cell count and viral load, by time points (baseline and at longest time follow‐up) in order to demonstrate changes over time.
Certainty of the evidence
Two review authors (MV and SD) independently assessed the certainty of the evidence for the outcomes under each comparison (type of micronutrient intervention) using the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach. According to this approach the certainty rating of evidence for each outcome is determined by an assessment of the available study data in terms of its risk of bias, inconsistency, indirectness, imprecision, and publication bias. We used GRADEpro Guideline Development Tool (GDT) software to create 'Summary of findings' tables for each comparison (GRADEpro 2014).
Sensitivity analysis
We could not perform a sensitivity analysis to assess the robustness of the results against the 'Risk of bias' domains as there were too few studies for each comparison.
Results
Description of studies
Results of the search
The previous version of this review, which included pregnant women and children, included 30 trials (Irlam 2010). Only 16 of these were eligible for inclusion in this Cochrane Review.
The PRISMA flow diagram summarizes the results of the searches for this update (Figure 1). Electronic database searches identified 1835 records, of which there were 1572 records after we removed duplicates. After we screened these records by title/abstract, we identified 68 articles for full‐text assessment. Handsearching identified three new trials. Seventeen new trials met the inclusion criteria of this review, which gave a total of 33 included trials. Of the new included trials, four reported outcome data in two articles (Asdamongkol 2013 THA; Bang 2012 DEN; Baum 2010 USA; Baum 2013 BWA), and two letters of correspondence were related to one included trial (Isanaka 2012 TZA). Two included trials published their trial protocols (Guwatudde 2015 UG; Kamwesiga 2015 RWA). We identified one additional trial from our most recent search (18 November 2016), which we included in the 'Characteristics of studies awaiting classification' section. We identified six ongoing trials from searching trial registries.
1.
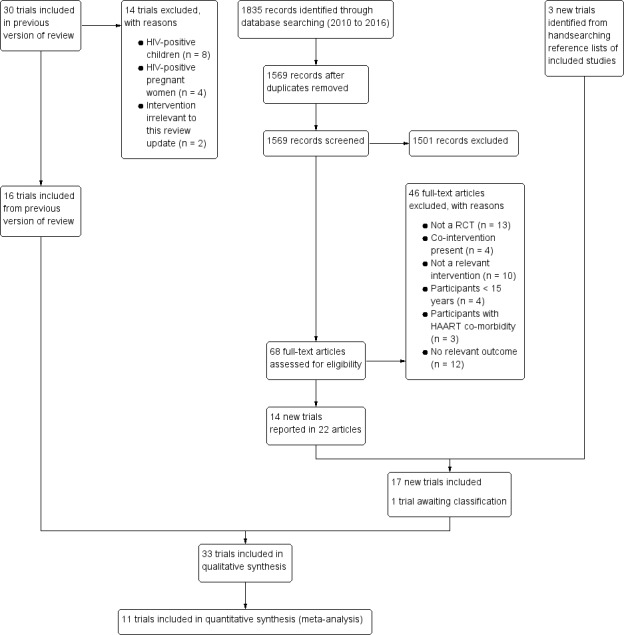
Study flow diagram
Included studies
Participants
Thirteen trials, which included 4493 participants, were conducted in antiretroviral therapy (ART)‐naive participants (Baeten 2002 KEN; Baum 2013 BWA; Cárcamo 2006 PER; Jiamton 2003 THA; Kamwesiga 2015 RWA; Kelly 1999 ZMB; Kelly 2008 ZMB; Lawson 2010 NIG; McClelland 2004 KEN; Range 2006 TZA; Semba 2007 MWI; Villamor 2008 TZA; Wejse 2009 GNB). One small trial did not state whether participants received ART or not (Zhao 2010 CHN).
The remaining 19 trials included 5730 participants, with most receiving either mono‐ or combination ART (Allard 1998 CAN; Coodley 1993 USA; Coodley 1996 USA; Humphrey 1999 USA; Semba 1998 USA), or highly active antiretroviral therapy (HAART) (Asdamongkol 2013 THA; Bang 2012 DEN; Baum 2010 USA; Burbano 2002 USA; Dougherty 2015 USA; Giacomet 2013 ITA; Guwatudde 2015 UG; Green 2005 SGP; Grigoletti 2013 BRA; Hurwitz 2007 USA; Isanaka 2012 TZA; Overton 2015 USA; Semba 2007 USA; Stallings 2014 USA). Many of these trials were small with fewer than 100 participants, with the exception of Isanaka 2012 TZA which had more than 3000 participants.
In five trials participants were on concurrent treatment for active tuberculosis (Lawson 2010 NIG; Range 2006 TZA; Semba 2007 MWI; Villamor 2008 TZA; Wejse 2009 GNB).
Trial site
Trials were undertaken in the following places.
Africa: Botswana (Baum 2013 BWA), Kenya (Baeten 2002 KEN; McClelland 2004 KEN), Guinea‐Bissau (Wejse 2009 GNB), Malawi (Semba 2007 MWI), Nigeria (Lawson 2010 NIG), Rwanda (Kamwesiga 2015 RWA), Tanzania (Range 2006 TZA; Isanaka 2012 TZA; Villamor 2008 TZA), Uganda (Guwatudde 2015 UG), and Zambia (Kelly 1999 ZMB; Kelly 2008 ZMB)
Asia: China (Zhao 2010 CHN), Singapore (Green 2005 SGP), and Thailand (Asdamongkol 2013 THA; Jiamton 2003 THA)
North America: Canada (Allard 1998 CAN) and USA (Baum 2010 USA; Burbano 2002 USA; Coodley 1993 USA; Coodley 1996 USA; Dougherty 2015 USA; Humphrey 1999 USA; Hurwitz 2007 USA; Overton 2015 USA; Semba 1998 USA; Semba 2007 USA; Stallings 2014 USA)
South America: Brazil (Grigoletti 2013 BRA) and Peru (Cárcamo 2006 PER)
Europe: Denmark (Bang 2012 DEN) and Italy (Giacomet 2013 ITA)
Interventions
Twenty‐nine placebo‐controlled studies met the inclusion criteria. Of these, two had factorial designs (Baum 2013 BWA; Range 2006 TZA). For both of these trials we extracted data for two treatment comparisons. Trials assessed the effectiveness of the supplementation of the following.
Multiple micronutrients (10 trials; 3533 participants: Baum 2013 BWA; Guwatudde 2015 UG; Jiamton 2003 THA; Kelly 1999 ZMB; Kelly 2008 ZMB; McClelland 2004 KEN; Range 2006 TZA; Semba 2007 MWI; Villamor 2008 TZA; Zhao 2010 CHN)
Vitamin A (4 trials; 581 participants: Baeten 2002 KEN; Coodley 1993 USA; Humphrey 1999 USA; Semba 1998 USA)
Vitamin D (5 trials; 447 participants: Bang 2012 DEN; Giacomet 2013 ITA; Overton 2015 USA; Stallings 2014 USA; Wejse 2009 GNB)
Vitamin E combined with vitamin C (1 trial, 49 participants: Allard 1998 CAN)
Folinic acid (1 trial, 30 participants: Grigoletti 2013 BRA)
Zinc (6 trials, 826 participants: Asdamongkol 2013 THA; Baum 2010 USA; Cárcamo 2006 PER; Green 2005 SGP; Lawson 2010 NIG, Range 2006 TZA)
Selenium (4 trials, 1308 participants: Baum 2013 BWA; Burbano 2002 USA; Hurwitz 2007 USA; Kamwesiga 2015 RWA)
In addition, we identified four trials that assessed the effectiveness of the supplementation of the following.
High dose versus standard dose multiple micronutrients (Isanaka 2012 TZA)
4000 IU vitamin D versus 7000 IU vitamin D (Dougherty 2015 USA)
Multiple micronutrients with iron versus multiple micronutrients (Semba 2007 USA)
β‐carotene with multivitamins versus multivitamins (Coodley 1996 USA)
The follow‐up periods of these trials ranged from two weeks to 24 months.
Sample size
Trials were generally underpowered to demonstrate effects on mortality. For example, to demonstrate a 25% reduction in deaths of HIV‐positive participants not on ART 2412 trial participants would be required, and to identify the same reduction for those on ART 7314 trial participants would be required (Table 6). This far exceeds the number of participants in the three included trials that reported on this outcome (Isanaka 2012 TZA; Jiamton 2003 THA; Villamor 2008 TZA). Isanaka 2012 TZA based their sample calculation of 3000 participants on the basis of a 25% reduction in the composite outcome of death and disease progression (any new or recurrent AIDS‐defining illness). We have provided the optimal information sizes for nutritional outcomes in Table 7.
1. Optimal information size calculations (dichotomous outcomes).
| Outcome | Power | Two‐sided significance level | Risk in control group | Relative risk reduction | Risk in intervention group | Sample size (total) |
| Death | 80% | 95% | 15.5%1 | 25% | 11.6% | 2412 |
| Death | 80% | 95% | 8.3%2 | 25% | 6.2% | 4782 |
| Death | 80% | 95% | 5.5%3 | 25% | 4.1% | 7314 |
| CD4 cell count ≤ 350 cells/mm3 4 | 80% | 95% | 10% | 60% | 55% | 2312 |
| CD4 cell count ≤ 350 cells/mm3 4 | 80% | 95% | 25% | 60% | 44% | 314 |
| CD4 cell count ≤ 350 cells/mm3 4 | 80% | 95% | 50% | 60% | 29% | 76 |
1Estimated annual risk of death of antiretroviral naive HIV‐infected persons (≥10 years after seroconversion) (Collaborative Group on AIDS Incubation 2000). 2Estimated annual risk of death of antiretroviral naive HIV‐infected persons (5 to 9 years after seroconversion) (Collaborative Group on AIDS Incubation 2000). 3Estimated risk of death of HIV‐infected persons after receiving first‐line antiretroviral therapy regimens for 12 months (Mbuagbaw 2010). 4Antiretroviral naive HIV‐infected participants who experience a decline in CD4 count (Kamwesiga 2011a, which is under Kamwesiga 2015 RWA).
2. Optimal information size calculations (continuous outcomes).
| Outcome | Power | Two‐sided significance level | Ratio of group 1: group 2 | Mean in control group | SD | Mean in supplement group | SD | Mean difference | Sample size (total) |
| Mean blood 25(OH) vitamin D level at 12 months1 | 80% | 95% | 1 | 17 ng/ml | 9 | 28 ng/ml | 9 | 11.5 | 22 |
| Mean blood 25(OH) vitamin D level at 12 months1 | 80% | 95% | 1 | 17 ng/ml | 20 | 28 ng/ml | 20 | 11.5 | 104 |
| Mean BMI at 24 months3 | 80% | 95% | 1 | 21 kg/m2 | 3 | 22 kg/m2 | 3 | 1 kg/m2 | 284 |
| Mean BMI at 24 months 4 | 80% | 95% | 1 | 21 kg/m2 | 3 | 23 kg/m2 | 3 | 2 kg/m2 | 72 |
Abbreviations: BMI: body mass index; SD: standard deviation.
1This example is based on data from Stallings 2014 USA. This example uses the SD from the control group. 2This example is based on data from Stallings 2014 USA. This example uses the SD from the supplemented group. 3This example uses the SD from Villamor 2008 TZA, but uses a 1 kg/m2 mean difference for illustrative purposes. 4This example uses the SD from Villamor 2008 TZA, but uses a 2 kg/m2 mean difference for illustrative purposes.
For full details of the included studies refer to the 'Characteristics of included studies' section.
Excluded studies
We excluded 14 trials that were included in the previous version of this review, Irlam 2010, from the current version. Eight trials were in HIV‐positive children; four trials were in HIV‐positive pregnant women; and in two trials the study participants received micronutrient supplements that did not contain micronutrients exclusively (Austin 2006; Kaiser 2006).
We excluded 46 records after full‐text assessment, 13 of which were not RCTs. We excluded the remaining studies because they addressed interventions that were not exclusively micronutrients (n = 10), had a co‐intervention (n = 4), involved trial participants aged less than 15 years (n = 4) or those with HAART co‐morbidity (n = 3), or reported study outcomes not relevant to this review (n = 12) (Figure 1).
See the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We evaluated the risk of bias of included studies for each of the six domains in the Methods section above (see the 'Characteristics of included studies' table). Figure 2 and Figure 3 present a graphical summary of the 'Risk of bias' assessments.
2.
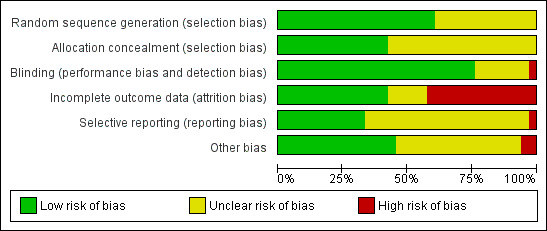
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
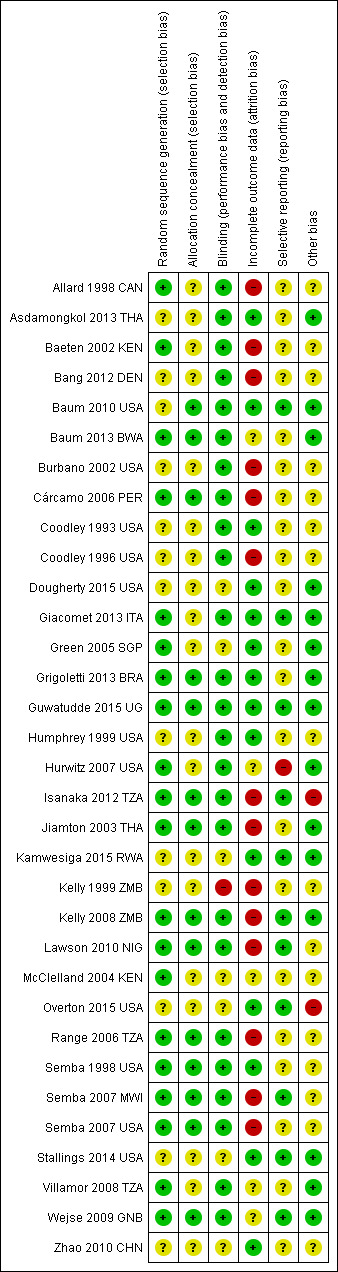
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Overall 20 trials adequately described a low risk method of random sequence generation. In 13 trials the methods were unclear. Fourteen trials adequately described a method of allocation concealment, and in 19 the methods were unclear.
Blinding
Blinding of participants, treatment providers and outcome assessors was well described in 25 trials, which we judged to be at low risk of detection and performance bias. The remaining trials were at unclear risk except Kelly 1999 ZMB, which we considered to be high risk due to the use of a non‐identical placebo. The main reason for trials being at unclear risk was that no information was provided about the blinding of the investigators or outcome assessors.
Incomplete outcome data
We judged 14 trials to be at high risk of attrition bias due to incomplete outcome data. In two trials this risk only applied to the measures of viral load which were only reported on a subset of trial participants (Isanaka 2012 TZA; Jiamton 2003 THA). Eleven trials had high attrition overall or differential attrition, or both (Allard 1998 CAN; Bang 2012 DEN; Burbano 2002 USA; Cárcamo 2006 PER; Coodley 1996 USA; Kelly 1999 ZMB; Kelly 2008 ZMB; Lawson 2010 NIG; Range 2006 TZA; Semba 2007 MWI; Semba 2007 USA), and in Baeten 2002 KEN participants lost to follow‐up had more advanced HIV disease and vitamin A deficiency. Thus we considered these trials to be at high risk for attrition bias.
Selective reporting
Insufficient information was available to permit judgment about the extent of bias due to selective outcome reporting in all but 11 included studies. We judged 10 of these as at low risk (Baum 2010 USA; Giacomet 2013 ITA; Guwatudde 2015 UG; Isanaka 2012 TZA; Kamwesiga 2015 RWA; Lawson 2010 NIG; Overton 2015 USA; Semba 2007 MWI; Stallings 2014 USA; Wejse 2009 GNB), and one as at high risk of reporting bias (Hurwitz 2007 USA).
Other potential sources of bias
One trial was stopped early due to evidence of increased alanine transaminase (ALT) levels with the intervention (Isanaka 2012 TZA).
Fourteen trials did not declare potential conflicts of interest (Allard 1998 CAN; Asdamongkol 2013 THA; Baeten 2002 KEN; Bang 2012 DEN; Burbano 2002 USA; Cárcamo 2006 PER; Coodley 1993 USA; Coodley 1996 USA; Humphrey 1999 USA; Kelly 1999 ZMB; Lawson 2010 NIG; McClelland 2004 KEN; Semba 1998 USA; Semba 2007 MWI).
All but 19 trials were funded either fully or partly from government sources (Allard 1998 CAN; Asdamongkol 2013 THA; Baeten 2002 KEN; Baum 2010 USA; Baum 2013 BWA; Burbano 2002 USA; Coodley 1993 USA; Coodley 1996 USA; Green 2005 SGP; Grigoletti 2013 BRA; Humphrey 1999 USA; Hurwitz 2007 USA; Isanaka 2012 TZA; Jiamton 2003 THA; Kelly 1999 ZMB; McClelland 2004 KEN; Semba 1998 USA; Stallings 2014 USA; Villamor 2008 TZA); five were fully or partly funded by pharmaceutical companies (Bang 2012 DEN; Coodley 1993 USA; Jiamton 2003 THA; Kelly 1999 ZMB; Overton 2015 USA); and one trial did not provide the source of funding (Zhao 2010 CHN).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Comparison 1: Multiple micronutrients versus placebo
Ten trials compared a daily multiple micronutrient supplement to placebo, given for between two weeks and two years (see Table 8). Most participants were ART‐naive HIV‐positive adults, and in three trials all participants were on treatment for active pulmonary tuberculosis. Only Guwatudde 2015 UG recruited people already taking ART (49.8% of trial participants), and the remaining participants were commenced on ART at the start of this trial. Trials were conducted in Africa (Baum 2013 BWA; Guwatudde 2015 UG; Kelly 1999 ZMB; Kelly 2008 ZMB; McClelland 2004 KEN; Range 2006 TZA; Semba 2007 MWI; Villamor 2008 TZA), Thailand (Jiamton 2003 THA), and China (Zhao 2010 CHN).
3. Characteristics of trials evaluating multiple micronutrients versus placebo.
| Trial ID | Country | Participants | Baseline HAART use (%) | Mean baseline CD4+ cell count (cells/mm3) |
Mean baseline viral load (copies/ml or log10 copies/mL) |
MMN dose1 | Duration of supplementation |
| Baum 2013 BWA | Botswana | HIV‐positive | 0 | 423 (median) | 11,800 (median) | High | 24 months |
| Guwatudde 2015 UG | Uganda | HIV‐positive | 49.82 | 145 (median) 137 (median) |
N/A | Standard | 18 months |
| Jiamton 2003 THA | Thailand | HIV‐positive | 0 | 244 (median) | 3.9 (1.0) | High | 48 weeks |
| Kelly 1999 ZMB | Zambia | HIV‐positive plus chronic diarrhoea | 0 | 291 (median) | N/A | High | 2 weeks |
| Kelly 2008 ZMB | Zambia | HIV‐positive | 03 | N/A | N/A | Standard | 1.9 years4 |
| McClelland 2004 KEN | Kenya | HIV‐positive | 0 | 294 (209) | 5.3 (0.9) | High | 6 weeks |
| Zhao 2010 CHN | China | HIV‐positive | Not stated | 417 (69) | Not stated | Standard | 6 months |
| Range 2006 TZA | Tanzania | HIV‐positive plus active TB | 0 | 363 (275) | 4.02 (0.98) | High | 8 months |
| Semba 2007 MWI | Malawi | HIV‐positive plus active TB | 0 | Not stated | 5.4 (median) | Standard | 24 months5 |
| Villamor 2008 TZA | Tanzania | HIV‐positive plus active TB | 0 | 305 (227) | 4.6 (1.0) | High | 24 months |
Abbreviations: HAART: highly active antiretroviral therapy; MMN: multiple micronutrient; TB: Tuberculosis
1Standard dose supplements provided most of the micronutrients at the level of the Dietary Recommended Intake (DRI). High‐dose supplements provided most of the micronutrients in multiples of the DRI. 2Guwatudde 2015 UG: participants who received ART for no longer than 6 months. The rest of the trial participants were initiated on ART at baseline. 3Kelly 2008 ZMB: we excluded participants taking HAART from the analysis of CD4 and viral load. 4Kelly 2008 ZMB was a cross‐over trial, with cross‐over at the end of 1.9 years. We did not include the outcome data for the period after cross‐over. 5Semba 2007 MWI: the median duration of follow‐up was 12.5 months, due to the introduction of ART programme.
Four trials evaluated multiple micronutrient supplements in doses consistent with the Recommended Daily Intake (RDA) (standard dose supplements), and six trials used substantially higher doses (high dose supplements). In summary, high dose supplements included: vitamin A (2 to 3 x RDA), B vitamins (6 to 20 x RDA), vitamin C (3 to 5 times x RDA), vitamin D (1 x RDA), Vitamin E (2 to 20 x RDA), selenium (2 to 7 x RDA), and zinc (2 to 4 x RDA) (see Table 9).
4. Composition of multiple micronutrient supplements.
| Micronutrient | RDA male aged 19 to 70 years | Standard doses1 | High doses2 | Standard dose | High dose | ||||||||
| Kelly 2008 ZMB | Zhao 2010 CHN | Semba 2007 MWI | Guwatudde 2015 UG | Baum 2013 BWA | Kelly 1999 ZMB | Jiamton 2003 THA | McClelland 2004 KEN | Range 2006 TZA | Villamor 2008 TZA | Isanaka 2012 TZA | |||
| Vitamin A | 900 µg (3000 IU) | — | 200 µg (660 IU) | 2424µg (8000 IU) | — | — | 3182 µg (10500 IU) | 3027 µg (9990 IU) | — | 1500 µg (5000 IU) | 1515 µg (5000 IU) | — | — |
| B‐carotene | — | 4.8 mg | — | — | — | — | — | 6 mg | — | — | — | — | — |
| Vitamin B1 (Thiamine) | 1.2 mg | 1.4 mg | 1 mg | 1.5 mg | 1.4 mg | 20 mg | — | 24 mg | 20 mg | 20 mg | 20 mg | 1.2 mg | 20 mg |
| Vitamin B2 (riboflavin) | 1.3 mg | 1.4 mg | 1 mg | 1.7 mg | 1.4 mg | 20mg | — | 15 mg | 20 mg | 20 mg | 20 mg | 1.2 mg | 20 mg |
| Vitamin B3 (niacin) | 16 mg | 18 mg | ‐ | 20 mg | 18 mg | 100 mg | — | 54 mg | 100mg | 40 mg | 100 mg | 15 mg | 100 mg |
| Vitamin B6 (pyridoxine) | 1.3 to 1.7 mg | 1.9 mg | 1 mg | 2 mg | 1.9 mg | 25 mg | — | 40mg | 25 mg | 25 mg | 25 mg | 1.3mg | 25 mg |
| Vitamin B9 (folinic acid) | 400 µg | 400 µg | 150 µg | 400 µg | 400 µg | 800 µg | 5000 µg | 100 µg | 800 µg | 800 µg | 800 µg | 400 µg | 800 µg |
| Vitamin B12 | 2.4 µg | 2.6 µg | 6 µg | 2.6 µg | 50 µg | — | 30µg | 50 µg | 50 µg | 50 µg | 2.4 µg | 50 µg | |
| Panthothenic acid | 5 mg | — | — | — | — | — | — | 40 mg | — | — | — | — | — |
| Vitamin E | 15 mg | 10 mg | 15 mg | 133 mg | 10 mg | 30 mg | 300 mg | 80 mg | 30 mg | 60 mg | 200 mg | 15 mg | 30 mg |
| Vitamin D | 5 to 15 µg (200 to 600 IU) | 5 µg (200 IU) | 5 µg (200 IU) | 10 µg (400 IU) | — | — | — | 20 µg (800 IU) | — | 5 µg (200 IU) | — | — | — |
| Vitamin K | 120 µg | — | — | — | — | — | — | 180 µg | — | — | — | — | — |
| Vitamin C | 90 mg | 70 mg | 100 mg | 500 mg | 70 mg | 500 mg | 300 mg | 400 mg | 500 mg | 200 mg | 500 mg | 80 mg | 500 mg |
| Selenium | 55 µg | 65 µg | 30 µg | 65 µg | — | 200 µg | 150 µg | 400 µg | 200 µg | 200 µg | 100 µg | — | — |
| Iron | 8 mg | 6 mg | — | — | — | — | 10 mg | — | — | — | — | — | |
| Zinc | 11 mg | 15 mg | 5 mg | 10 mg | — | — | 200 mg | 30 mg | — | 45 mg | — | — | — |
| Copper | 0.9 mg | — | — | — | — | — | — | 3 mg | — | 5 mg | — | — | — |
| Iodine | 150 µg | — | — | 175 µg | — | — | — | 300 µg | — | — | — | — | — |
| Chromium | 35 µg | — | — | — | — | — | — | 150 µg | — | — | — | — | — |
| Manganese | 2.3 mg | — | — | — | — | — | — | 8 mg | — | — | — | — | — |
| Calcium | 1000 mg | — | 400 mg | — | — | — | — | — | — | — | — | — | — |
Abbreviations: IU: International units; RDA:Recommended Daily Allowance
1Standard dose supplements provided most of the micronutrients at the level of the RDA. 2High‐dose supplements provided most of the micronutrients in multiples of the RDA.
Of the ten trials, we judged four to be at low risk of selection bias (Baum 2013 BWA; Guwatudde 2015 UG, Isanaka 2012 TZA; Jiamton 2003 THA), and we considered seven to be at low risk of performance and detection bias as they adequately described blinding (Baum 2013 BWA; Guwatudde 2015 UG; Jiamton 2003 THA; Kelly 2008 ZMB; Range 2006 TZA; Semba 2007 MWI; Villamor 2008 TZA).
Mortality
Overall, statistically significant differences on mortality were not demonstrated, but the trials were substantially underpowered to confidently detect or exclude small but clinically important effects (risk ratio (RR) 0.91, 95% confidence interval (CI) 0.72 to 1.15; 7 trials, 2897 participants, Analysis 1.1).
1.1. Analysis.
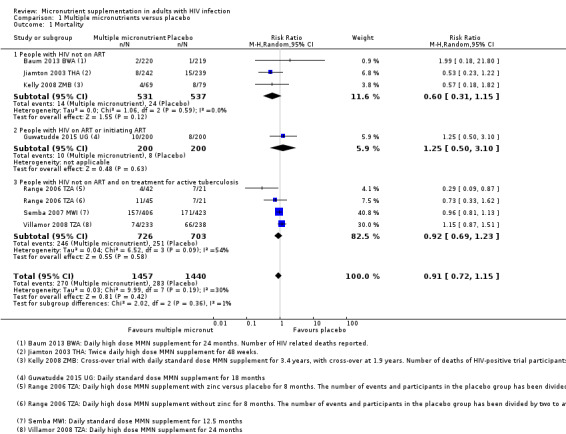
Comparison 1 Multiple micronutrients versus placebo, Outcome 1 Mortality.
In three trials in ART‐naive adults, although the proportion of deaths was lower with supplementation, the 95% confidence interval (CI) was wide and included the possibility of both clinically important effects and no effect (RR 0.60, 95% CI 0.31 to 1.15; 3 trials, 1068 participants; I² statistic = 0%). One additional small trial from Zambia, with only four weeks follow‐up, also reported no difference in mortality but did not present data that we could include in the meta‐analysis (Kelly 1999 ZMB).
In the only trial in adults on ART, Guwatudde 2015 UG, the proportion of deaths was similar in both treatment arms, and the 95% CI was very wide (RR 1.25, 95% CI 0.50 to 3.10; 1 trial, 400 participants).
In three trials in adults on treatment for active tuberculosis (and also not on ART), although the CI was narrower, there was no effect of supplementation on mortality (RR 0.92, 95% CI 0.69 to 1.23; 3 trials, 1429 participants; I² statistic = 54%). One small factorial trial from Tanzania, Range 2006 TZA, found a statistically significant effect in a subgroup of HIV‐positive participants who received multiple micronutrients plus zinc, but the CI was wide and the trial was underpowered (RR 0.29, 95% CI 0.09 to 0.87; 1 trial, 84 participants). A positive result in an underpowered study is not likely to reflect a true result (low positive predictive value (PPV)) and the magnitude of the effect estimate may also be exaggerated (Button 2013). Furthermore, the trial authors reported differential attrition between treatment groups.
Morbidity and clinical disease progression
Two trials reported the risk of hospital admission, and although this was lower with multiple micronutrient supplementation, the CIs were wide and included both clinically important benefits and harms (RR 0.86, 95% CI 0.61 to 1.22; 2 trials, 881 participants, Analysis 1.2). In one trial, Guwatudde 2015 UG, participants commenced ART at the start of the trial and in the second trial, Jiamton 2003 THA, participants were not on ART.
1.2. Analysis.
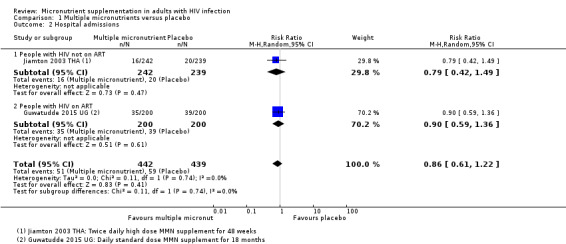
Comparison 1 Multiple micronutrients versus placebo, Outcome 2 Hospital admissions.
One additional trial in people on treatment for tuberculosis reported no difference in the risk of clinical disease progression (hazard ratio (HR) 1.08 95% CI 0.72 to 1.62; 1 trial, 313 participants, Analysis 1.3).
1.3. Analysis.
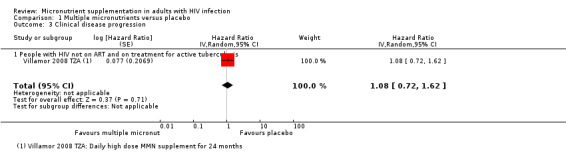
Comparison 1 Multiple micronutrients versus placebo, Outcome 3 Clinical disease progression.
Virological and immunological outcomes
Nine trials reported changes in CD4+ count over periods from four weeks to two years (see Table 10). We could not incorporate one additional trial from Botswana that reported time‐to‐event analyses (time to reach CD4+ count < 250 cells/mm³) into the meta‐analyses (Baum 2013 BWA).
5. Change in CD4 cell count (cells/mm3): multiple micronutrients versus placebo.
| Trial ID | Statistical measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | ||||
| Guwatudde 2015 UG | Median (IQR) | 145 (86 to 215) | Not reported | 200 | 137 (68 to 192) | Not reported | 200 | 18 months | MD ‐ 6.17 (95% CI ‐29.3 to 16.9)2 |
| Jiamton 2003 THA | Median (IQR) | 244 (52 to 544) | 200 (66 to 358) | 192 | 261 (50 to 550) | 232 (73 to 377) | 184 | 48 weeks | "Did not differ" |
| Kelly 1999 ZMB | Median (IQR) | 292 | Not reported | 66 | 282 | Not reported | 69 | 4 weeks | "Not different"3 |
| Kelly 2008 ZMB | Mean (SD) | 370 (190) | 415 (242) | 41 | 365 (212) | 409 (192) | 43 | 1.9 years3 | P = 0.55 |
| McClelland 2004 KEN | Mean (SD) | 294 (209) | 300 (205) | 179 | 262 (202) | 265 (189) | 178 | 6 weeks | Adjusted regression co‐efficient 23 (95% CI 3 to 43); P = 0.03 |
| Zhao 2010 CHN | Mean (SD) | 417 (69) | 589 (85) | 50 | 466 (72) | 483 (59) | 49 | 6 months | P < 0.05 |
| Range 2006 TZA | Mean (SD) | 460 (391) | 423 (373) | 48 | 460 (385) | 403 (460) | 48 | 8 weeks | P = 0.18 |
| Villamor 2008 TZA | Mean (SD) | 305 (277) | Not reported | 200 | 339 (256) | 340 (240) | 204 | 2 years5 | MD ‐5 (−37 to 26); P = 0.74 |
| Baum 2013 BWA | Median (IQR) | 428 (336 to 555) | Not reported | 220 | 411 (327 to 545) | Not reported | 217 | 2 years | Not reported7 |
Abbreviations: IQR: Interquartile range; MD: Mean difference; SD: Standard deviation
1The number of participants stated is the number assessed for end‐point data. 2Guwatudde 2015 UG: the trial authors reported a mean difference which is different to our calculation. The reasons for this are unclear. 3Kelly 1999 ZMB: the trial authors did not report data that we could include in a meta‐analysis. 4Kelly 2008 ZMB was a cross‐over trial, with cross‐over at the end of 1.9 years. CD4+ counts were recorded during the second year of follow‐up. The data for the period after cross‐over is not included in this table. 5Range 2006 TZA: data shown are for multiple micronutrients plus zinc versus placebo. There were also no differences for micronutrients without zinc versus placebo. 6Villamor 2008 TZA also reported outcomes at 8 months, with no significant difference between groups. 7Baum 2013 BWA: data shown are for multivitamins plus selenium versus placebo. The trial authors reported reductions in the risk of CD4+ falling to < 250 cells/µL for multivitamins plus selenium versus placebo (HR 0.48, 95% CI 0.26 to 0.88 ) and for multivitamins alone versus placebo (HR 0.54, 95% CI 0.3 to 0.98). Multivariate analysis showed that this effect was only apparent with supplementation of both multivitamins and selenium (HR 0.46, 95% CI 0.25 to 0.85).
Six trials reported data as means with standard deviation (SD) and the pooled effect had a wide 95% CI including modest benefits and harms (mean difference (MD) 24.79, 95% CI −23.54 to 73.12; 6 trials, 1581 participants; Analysis 1.4, Analysis 1.5). Only one small trial from China administering multiple micronutrients at around the daily recommended intake, reported a statistically significant effect with supplementation after six months (1 trial, 99 participants, Zhao 2010 CHN). The other much larger trials that administered higher doses of multiple micronutrients found no suggestion of effects; including 808 participants with HIV alone, and 674 participants with HIV plus active tuberculosis
Three trials reported data as medians and interquartile range (IQR), and neither found a statistically significant result (3 trials, 911 participants, data not pooled, Table 10)
One additional trial from Botswana reported data as the HR of reaching a CD4+ count of less than 250 cells/mm³. The hazard was lower with high dose supplements of multivitamins plus selenium for two years (HR 0.48, 95% CI 0.26 to 0.88; 1 trial, 439 participants) and with multivitamins alone (HR 0.54; 95% CI 0.3 to 0.98; 1 trial, 436 participants) However, the trial authors reported that this effect was only apparent with supplementation of both multivitamins and selenium after adjustment for multiple confounders (HR 0.46, 95% CI 0.25 to 0.85; 1 trial, 439 participants) (Baum 2013 BWA)
1.4. Analysis.
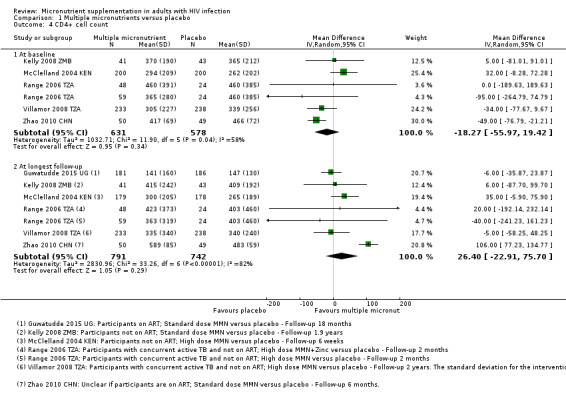
Comparison 1 Multiple micronutrients versus placebo, Outcome 4 CD4+ cell count.
1.5. Analysis.

Comparison 1 Multiple micronutrients versus placebo, Outcome 5 CD4+ cell count at longest follow‐up; subgrouped by participant characteristics.
Five trials of high dose multiple micronutrients reported changes in viral load at time points from six weeks to two years (see Table 11).
6. Change in viral load (log10 copies/mL): multiple micronutrients versus placebo.
| Trial ID | Statistical measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | ||||
| Baum 2013 BWA | Median (IQR) | 4.0 (3.3‐4.7) | Not reported2 | 220 | 4.3 (3.6 to 4.8) | Not reported2 | 217 | 24 months | P = 0.43 |
| Jiamton 2003 THA | Mean (SD) | 3.9 | 4.4 (1.4) | 714 | 4.2 | 4.5 (1.54) | 69 | 48 weeks | P = 0.4 |
| McClelland 2004 KEN | Mean (SD) | 5.3 (0.9) | 5.3 (0.9) | 179 | 5.4 (0.9) | 5.4 (0.9) | 178 | 6 weeks | P = 0.4 |
| Range 2006 TZA | Mean (SD) | 3.72 (1.18) | 3.85 (1.4) | 48 | 3.9 (1.33) | 4.1 (1.54) | 48 | 8 weeks | "Not significant"5 |
| Villamor 2008 TZA | Mean (SD) | 4.6 (1.0) | Not reported | 71 | 4.6 (0.9) | 4.74 (1.54) | 69 | 2 years6 | MD −0.08 (−0.22 to 0.05); P = 0.23 |
Abbreviations: CI: Confidence interval; IQR: Interquartile range; MD: Mean difference; SD: Standard deviation
1The number of participants stated is the number assessed for end‐point data. 2Baum 2013 BWA: multiple imputation of viral load data was performed. The trial authors did not provide details. 3Baum 2013 BWA: data shown are for multivitamins plus selenium versus placebo. There were also no differences for multivitamins without selenium versus placebo. 4Jiamton 2003 THA: viral load analyses was conducted on the first 140 consecutive participants (29% of participants). 5Range 2006 TZA: data shown are for multiple micronutrients plus zinc versus placebo. There were also no differences for micronutrients without zinc versus placebo. 6Villamor 2008 TZA also reported outcomes at 8 months, with no significant difference between groups.
Four trials reported data as means with SDs and the pooled estimate was close to no effect with a CI which included a modest benefit and no effect (MD −0.10, 95% CI −0.25 to 0.05; 4 trials, 1064 participants; Analysis 1.6,Analysis 1.7)
The fifth trial reported no effect on viral load in a multivariable random‐effects regression model (1 trial, 437 participants, P = 0.4) (Baum 2013 BWA)
1.6. Analysis.
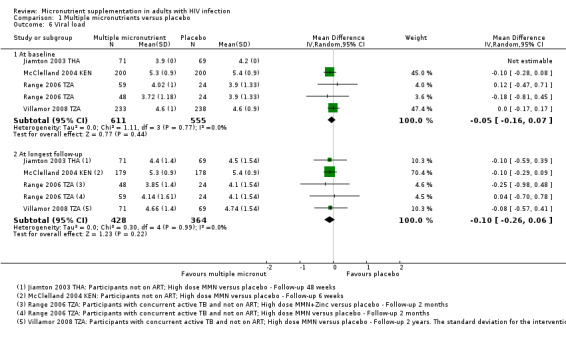
Comparison 1 Multiple micronutrients versus placebo, Outcome 6 Viral load.
1.7. Analysis.
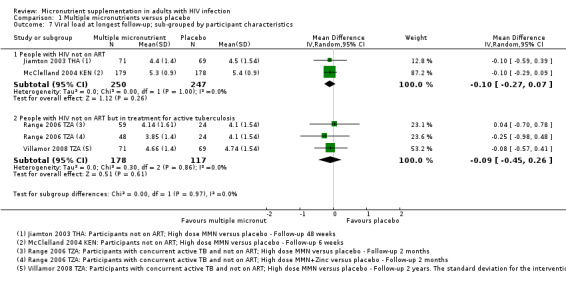
Comparison 1 Multiple micronutrients versus placebo, Outcome 7 Viral load at longest follow‐up; sub‐grouped by participant characteristics.
Nutritional status and blood micronutrient concentrations
Three trials reported changes in measures of body composition (BMI, weight, mid‐upper arm circumference (MUAC), fat mass or lean body mass) with no statistically significant differences between groups (see Table 12).
7. Change in nutritional status parameters: multiple micronutrients versus placebo.
| Trial ID | Nutritional parameter | Statistical measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | |||||
| Villamor 2008 TZA | BMI (kg/m2) | Mean (SD) | 19.3 (2.8) | Not reported | 233 | 19.6 (2.9) | 21.2 (3.3) |
238 | 2 years2 | MD ‐0.1 (−0.4 to 0.2); P = 0.37 |
| Guwatudde 2015 UG | Weight (kg) Haemoglobin (g/dL) |
Not reported Median (IQR) |
Not reported 12.2 (11.2 to 13.2) |
Not reported Not reported |
200 200 |
Not reported 12.3 (11.3 to 13.5) |
Not reported Not reported |
200 200 |
18 months 18 months |
MD 0.54 (−0.40 to 1.48); P = 0.691 MD 0.16 (−0.21 to 0.16); P = 0.977 |
| Jiamton 200312 | Blood vitamin E (µmol/L)3 | Mean (SD) | 22 (9) | Not reported | Not reported | 19 (7) | Not reported | Not reported | 48 weeks | MD 10.7 (7.0 to 14.3)4; P < 0.001 |
| Blood selenium (µmol/L)5 | Mean (SD) | 1.6 (0.2) | Not reported | Not reported | 1.6 (0.2) | Not reported | Not reported | 48 weeks | MD 0.16 (0.0 to 0.34)6; P = 0.04 | |
| Kelly 1999 ZMB12 | Blood vitamin A (µmol/L)7,8 | Mean | 0.63 | Not reported | 66 | 0.65 | Not reported | 69 | 4 weeks | P = 0.21 |
| Blood vitamin E (µmol/L)3,8 | Mean | 11.4 | Not reported | 66 | 11.7 | Not reported | 69 | 4 weeks | "No difference" | |
| Kelly 2008 ZMB | BMI (kg/m2) MUAC (cm) Fat mass (kg) Lean body mass (kg) Grip strength (kg) |
Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 1.9 years 9 | "No significant differences at any time point" |
| Semba 2007 MWI12 | Blood vitamin A (µmol/L)7,11 | Geometric mean | 0.59 | Reported in a graph | 383 | 0.59 | Reported in a graph | 397 | 8 months | "Significantly higher" |
| Blood selenium (µmol/L)10,11 | Geometric mean | 0.66 | Reported in a graph | 392 | 0.64 | Reported in graph | 405 | 8 months | ||
Abbreviations: BMI: Body Mass Index; IQR: Interquartile range; MUAC: Mid‐upper arm circumference; MD: Mean difference; SD: Standard deviation
1The number of participants stated is the number assessed for endpoint data. 2Villamor 2008 TZA also reported outcomes at 8 months, with no significant difference between groups (MD 0, 95% CI −0.2 to 0.3; P = 0.74). 3Reference value for vitamin E sufficiency ≥ 11.6 µmol/L. 4Jiamton 2003 THA: the trial authors reported endpoint data on a subset of 44 participants. The trial authors did not state the number of participants for each treatment group. Baseline vitamin E levels (µmol/L) reported for 112 participants. 5Reference value for selenium sufficiency: ≥ 0.95 µmol/L. 6Jiamton 2003 THA: the trial authors reported endpoint data on a subset of 54 participants. The number of participants for each treatment group is not stated. Baseline selenium levels (µmol/L) reported for 129 participants. 7Reference value for vitamin A deficiency: < 0.7 µmol/L. 8Kelly 1999 ZMB: the trial authors reported that 67% and 55% of participants were deficient in vitamins A and E, respectively, at baseline. 9Kelly 2008 ZMB was a cross‐over trial, with cross‐over at the end of 1.9 years. 10Reference value for selenium sufficiency: ≥ 0.75 µmol/L. 11Semba 2007 MWI: the trial authors reported that 60% and 75% of participants were deficient in vitamin A and selenium respectively, at baseline. 12Analysis of blood micronutrient concentrations did not include adjustment for biomarkers of inflammation.
Three trials reported changes in blood concentrations of vitamin A, vitamin E, or selenium (see Table 12), with statistically significant increases after 48 weeks supplementation in Thailand (Jiamton 2003 THA), and eight months supplementation in Malawi (Semba 2007 MWI). The third small trial from Zambia reported no change in serum vitamin A or vitamin E concentrations after four weeks supplementation, despite many being deficient in one or both at baseline (1 trial, 135 participants, Kelly 1999 ZMB).
Adverse events associated with supplementation
One trial with high dose micronutrient supplements reported no differences in serious adverse events such as acute diarrhoea, vomiting, or severely elevated ALT levels (Baum 2013 BWA). Genital HIV‐shedding was increased after six weeks, following high dose supplements in another trial (McClelland 2004 KEN). A third trial of high dose supplementation reported discolouration of urine more frequently in the intervention group, but no differences for other minor adverse events such as nausea, headache, dizziness, drowsiness, or rash (Jiamton 2003 THA). Three trials that involved high dose supplementation did not report any adverse events (Kelly 1999 ZMB; Range 2006 TZA; Villamor 2008 TZA).
High dose supplements was associated with a decrease in peripheral neuropathy in one trial, although this was not reported for just the subgroup with HIV (RR 57%, 95% CI 41% to 69%; 1 trial, 887 participants) (Villamor 2008 TZA). Participants in this trial were taking isoniazid for active tuberculosis which is a known vitamin B6 antagonist and may cause neuropathy without supplementation.
One trial with standard dose supplements reported no differences in adverse events such as nausea and vomiting (Guwatudde 2015 UG). A cluster‐randomized trial with standard dose supplements reported four cases of pellagra in the placebo group (three were associated with high ethanol intakes) (Kelly 2008 ZMB). Two trials that involved standard dose supplementation did not report any adverse events (Semba 2007 MWI; Zhao 2010 CHN).
Certainty of the evidence
For a critical appraisal of the summary of evidence, see the 'Summary of findings for the main comparison' table (Table 1) and Additional tables 8, 9, and 10 for the stratified analyses (participants taking ART or not, and whether they were on concurrent treatment for tuberculosis or not) (Table 13; Table 14; Table 15).
8. Multiple micronutrients compared to placebo for adults with HIV infection not currently taking ART.
| Multiple micronutrients compared to placebo for adults with HIV infection not currently taking ART | ||||||
| Participant or population: adults with HIV infection not currently taking ART Settings: all settings Intervention: multiple micronutrient supplementation (standard or high dose daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Micronutrients | |||||
|
Mortality Follow‐up: 12 to 24 months |
45 per 1000 | 26 per 1000 (14 to 52) |
RR 0.60 (0.31 to 1.15) |
1068 (3 trials) | ⊕⊕⊝⊝
low1,2,3,4 due to indirectness and imprecision |
Multiple micronutrients may reduce mortality |
|
Hospital admissions Follow‐up: 48 weeks |
84 per 1000 |
66 per 1000 (35 to 125) |
RR 0.79 (0.42 to 1.49) |
481 (1 trial) |
⊕⊝⊝⊝
very low1,4,5 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on hospital admissions |
|
CD4 cell count Follow‐up: 6 weeks to 2 years |
The mean in the placebo groups ranged from 265 to 409 cells/mm³ |
The mean in the multiple micronutrient group was 30.36 cells/mm³ higher (7.13 lower to 67.84 higher) |
— | 441 (2 trials) | ⊕⊕⊝⊝ low1,6,7 due to indirectness and inconsistency |
Multiple micronutrients may have little or no effect on CD4+ cell count |
|
Viral load Follow‐up: 6 to 48 weeks |
The mean in the placebo groups ranged from 4.4 to 5.3 log10copies/mL |
The mean in the multiple micronutrient groups was 0.10 log10copies/mL lower (0.27 lower to 0.07 higher) |
— | 497 (2 trials) |
⊕⊕⊕⊝
moderate1,8 due to indirectness |
Multiple micronutrients probably have little or no effect on viral load |
|
BMI (kg/m²) Follow‐up: 1.9 years |
— | — | — | 84 (1 trial) | ⊕⊝⊝⊝
very low1,9,10 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on BMI |
| The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ART: antiretroviral therapy; BMI: body mass index; CI: confidence interval; MUAC: mid‐upper arm circumference; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: all trials were at low risk of selection bias. Appropriate methods of blinding were used. 2No serious heterogeneity: none of the trials found statistically significant effects. 3Downgraded by 1 for serious indirectness: the three trials were conducted in Botswana (Baum 2013 BWA), Zambia (Kelly 2008 ZMB) and Thailand (Jiamton 2003 THA).The finding of no effect may not apply to all populations. 4Downgraded by 1 for serious imprecision: the 95% CI is wide and includes both clinically important effects and no effect. The overall meta‐analysis is substantially underpowered to confidently exclude effects. 5Downgraded by 2 for very serious indirectness: only a single trial is available from Thailand (Jiamton 2003 THA). The finding of no effect is not easily generalized to other settings. 6Downgraded by 1 for serious inconsistency: One trial in Botswana among ART‐naive adults (not included in the meta‐analysis) reported a reduced risk of reaching a CD4+ cell count of less than 250 cells/mm³ after two years of high dose supplementation. This finding is inconsistent with other trials that used similar combinations of micronutrients and selenium. 7Downgraded by 1 for serious indirectness: these two trials both used high‐dose multiple micronutrients and were conducted in Kenya (with 6 weeks follow‐up) and Zambia (with 1.9 years follow‐up). TThe finding of no effect may not apply to people on ART or other populations and settings. 8Downgraded by 1 for serious indirectness: these two studies both used high dose multiple micronutrients and were conducted in Kenya (with 6 weeks follow‐up) and Thailand (with 48 weeks follow‐up). The finding of no effect may not apply to people on ART or other populations and settings. 9Downgraded by 2 for very serious indirectness: only a single trial from Zambia (Kelly 2008 ZMB) reports measures of nutritional status. This does not exclude the possibility of effects in some populations. 10Downgraded by 1 for serious imprecision: this trial is underpowered to detect or exclude clinically important differences. The trial reported no difference in BMI, mid‐upper arm circumference (MUAC), lean body mass or fat mass but did not present data.
9. Multiple micronutrients compared to placebo for adults with HIV infection currently taking ART.
| Multiple micronutrients compared to placebo for adults with HIV infection currently taking ART | ||||||
| Participant or population: adults with HIV infection currently taking ART Settings: any setting Intervention: multiple micronutrient supplementation (standard dose daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Micronutrients | |||||
|
Mortality Follow‐up: 12 to 24 months |
40 per 1000 | 50 per 1000 (20 to 124) |
RR 1,25 (0.50 to 3.10) |
400 (1 trial) | ⊕⊝⊝⊝
very low1,2,3 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on mortality |
|
Hospital admissions Follow‐up: 48 weeks |
195 per 1000 | 176 per 1000 (115 to 265) |
RR 0.90 (0.59 to 1.36) |
400 (1 trial) | ⊕⊝⊝⊝
very low1,2,3 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on mortality |
|
CD4 cell count Follow‐up: 18 months |
The mean change in the placebo group was 147 cells/mm³ | The mean change in the multiple micronutrient group was 6.17 cells/mm³lower (29.3 lower to 16.9 higher) |
_ | 367 (1 trial) | ⊕⊝⊝⊝
very low1,2,4 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on CD4 cell count |
| Viral load | — | — | — | — | — | — |
|
Weight (kg) Follow‐up: 18 months |
The mean change in the placebo group was 3.3 kg | The mean change in the multiple micronutrient group was 0.54 kg higher (0.40 lower to 1.48 higher) |
400 (1 trial) | ⊕⊝⊝⊝
very low1,2,4 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on weight | |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: this trial was at low risk of selection bias. The trial authors used appropriate methods of blinding. 2Downgraded by 2 for serious indirectness: this single trial was conducted in Uganda and administered standard dose multiple micronutrients for two years. The finding of no effect may not be applicable to higher dose or the populations or settings. 3Downgraded by 2 for serious imprecision: this single trial is significantly underpowered to confidently detect or exclude effects. 4Downgraded by 1 for serious imprecision: the 95% CI is wide and includes what may be clinically important effects and no effect.
10. Multiple micronutrients compared to placebo for adults with HIV infection and concurrent active tuberculosis.
| Multiple micronutrients compared to placebo for adults with HIV infection and concurrent active tuberculosis not currently taking ART | ||||||
| Participant or population: adults with HIV infection and concurrent active tuberculosis not currently taking ART Settings: any setting Intervention: multiple micronutrient supplementation (standard or high dose daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Micronutrients | |||||
|
Mortality Follow‐up: 8 to 24 months |
357 per 1000 | 328 per 1000 (246 to 439) |
RR 0.92 (0.69 to 1.23) |
1429 (3 trials) | ⊕⊕⊝⊝
low1,2.3,4 due to indirectness and imprecision |
Multiple micronutrients may have little or no effect on mortality |
|
Clinical disease progression from stage 3 to stage 4 Follow‐up: 24 months |
— | — | HR 1.08 (0.72 to 1.62 | 313 (1 trial) |
⊕⊝⊝⊝ very low1,4,5 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on clinical disease progression |
|
CD4 cell count Follow‐up: 2 to 24 months |
The mean in the placebo groups ranged from 340 to 403 cells/mm³ |
The mean in the multiple micronutrient group was 5.77 cells/mm³ lower (55.8 lower to 44.25 higher) |
— | 674 (2 trials) | ⊕⊕⊝⊝ low1,3,4 due to indirectness and imprecision |
Multiple micronutrients may have no effect on CD4 cell count |
|
Viral load Follow‐up: 2 to 24 months |
The mean in the placebo groups ranged from 4.1 to 4.7 log10copies/mL |
The mean in the multiple micronutrient groups was 0.09 log10copies/mL lower (0.45 lower to 0.26 higher) |
— | 343 (2 trials) |
⊕⊕⊝⊝ low1,3,4 due to indirectness and imprecision |
Multiple micronutrients may have no effect on viral load |
|
BMI Follow‐up: 24 months |
The mean BMI in the placebo group was 21.2 kg/m2 | The mean BMI in the micronutrient group was 0.1 lower (0.4 lower to 0.2 higher) |
— | 471 (1 trial) | ⊕⊝⊝⊝ very low1,4,5 due to indirectness and imprecision |
We don't know if multiple micronutrients have any effect on BMI |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: ART: antiretroviral therapy; CI: confidence interval; HR: hazard ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1No serious risk of bias: the trials were at low risk of selection bias, except for two trials that recruited both HIV‐positive and HIV‐negative participants and did not stratify the randomization (Range 2006 TZA; Semba 2007 MWI). The trials used appropriate methods of blinding. 2No serious heterogeneity: one small subgroup of a trial in Tanzania did find a statistically significant difference (Range 2006 TZA), but larger trials did not. 3Downgraded by 1 for serious indirectness: the three trials were conducted in Tanzania and Malawi and most patients were not taking ART. The finding of no effect may not apply to people on ART or other populations and settings. 4Downgraded by 1 for serious imprecision: the 95% CI is wide and includes clinically important effects and no effect. 5Downgraded by 2 for serious indirectness: data is provided by a single trial from Tanzania and participants were not on antiretroviral therapy (ART).
Comparison 2: High‐dose versus standard dose multivitamins
One large Tanzanian trial, Isanaka 2012 TZA, investigated the effects of a standard versus a high‐dose daily multivitamin supplement for 24 months among participants starting HAART (see Table 9).
Mortality
There were no statistically significant differences in all‐cause mortality (RR 1.06, 95% CI 0.89 to 1.26; 1 trial, 3418 participants; Analysis 2.1), or AIDS‐related mortality (RR 1.14, 95% CI 0.82 to 1.58; 1 trial, 3418 participants; Isanaka 2012 TZA).
2.1. Analysis.
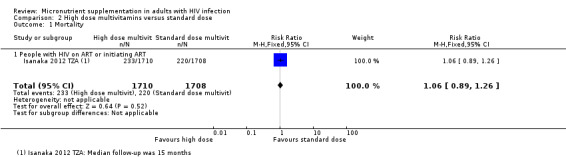
Comparison 2 High dose multivitamins versus standard dose, Outcome 1 Mortality.
Morbidity and clinical disease progression
There was no difference in clinical disease progression events combined with death from any cause (RR 1.00, 95% CI 0.96 to 1.04; 1 trial, 3418 participants).
Immunological and virological outcomes
There was a small difference between groups in mean CD4+ cell count at baseline (MD −7.00 cells/mm³, 95% CI −13.74 to −0.26), and a similar difference at 15 months (MD −12.00 cells/mm³, 95% CI −24.00 to −0.00; 1 trial, 3418 participants, Analysis 2.2). No differences were demonstrated in mean viral load at baseline or end of follow‐up (MD −0.20 log10copies/mL, 95% CI −0.51 to 0.11; 1 trial, 236 participants, Analysis 2.3; Isanaka 2012 TZA).
2.2. Analysis.
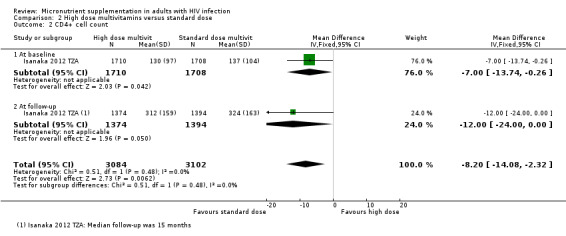
Comparison 2 High dose multivitamins versus standard dose, Outcome 2 CD4+ cell count.
2.3. Analysis.
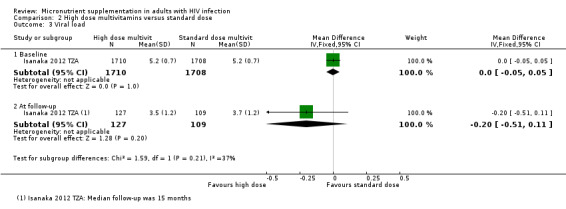
Comparison 2 High dose multivitamins versus standard dose, Outcome 3 Viral load.
Nutritional status and blood micronutrient concentrations
There was no difference in BMI between the two groups (MD 0 kg/m2; 95% CI −0.2 to 0.2; 1 trial, 3418 participants). Blood concentrations of micronutrients were not reported.
Adverse events associated with supplementation
This trial was stopped early with a median length of follow‐up of 15 months due to an increased risk of elevated ALT levels (greater than 40 IU/L) among trial participants who received high dose multivitamin supplements (incidence rate ratio (IRR) 1.44, 95% CI 1.11 to 1.87; one trial, 2921 participants). No differences were observed for other adverse events such as fatigue, nausea or vomiting, diarrhoea, severe anaemia, and rashes or lesions. The incidence of peripheral neuropathy was lower with high dose supplements (IRR 0.81, 95% CI 0.7 to 0.94; 1 trial, 3418 participants).
Comparison 3: Vitamin A versus placebo
Four trials compared a vitamin A supplement to placebo (see Table 16). Participants from two trials in the USA received a single high dose of vitamin A (200,000 IU to 300,000 IU) and were followed up for four to eight weeks (Humphrey 1999 USA; Semba 1998 USA). ART‐naive women in one trial from Kenya received a daily dose of vitamin A (10,000 IU) for six weeks (Baeten 2002 KEN). The trial authors of a small cross‐over trial of daily supplements containing a vitamin A precursor (β‐carotene) did not report data before cross‐over (Coodley 1993 USA).
11. Characteristics of trials evaluating vitamin A supplements versus placebo.
| Trial ID | Country | Participants | Baseline ART use | Mean baseline blood vitamin A concentration (µmol/L)1 | Mean baseline CD4+ cell count (cells/mm3) | Mean baseline viral load (log10 copies/mL) | Dose2 | Duration of supplementation |
| Baeten 2002 KEN | Kenia | HIV‐positive women | 0% | 0.097 (median) 0.095 (median) |
240 (median) 203 (median) |
5.34 (median) 5.54 (median) |
10,000 IU retinol daily | 6 weeks |
| Humphrey 1999 USA | USA | HIV‐positive women | 49% | 1.52 (0.42) 1.41 (0.31) |
Not reported | Not reported | 300,000 IU retinol | Single dose |
| Semba 2007 USA | USA | HIV‐positive IDUs | 46% | 1.61 1.37 |
296 (median) 259 (median) |
9.49 (median) 9.67 (median) |
200,000 IU retinol | Single dose |
| Coodley 1993 USA3 | USA | HIV‐positive | 94% | — | — | — | 180 mg β‐carotene | 4 weeks |
Abbreviations: IDUs: Injection drug users; IU: International units; RDA: Recommended Daily Allowance
1Reference value for vitamin A sufficiency: > 1.05 µmol/L. 2RDA for a male aged 19 to 70 years is 900 µg (3000 IU) daily. 3Coodley 1993 USA was a cross‐over trial, with cross‐over at the end of 4 weeks. The baseline and outcome data is not reported for the period before cross‐over and therefore we could not include it.
Of the four trials, we judged one to be at low risk of selection bias (Semba 1998 USA), and all four that adequately described blinding and were at low risk of performance and detection bias.
Mortality, morbidity, and clinical disease progression
The trials did not report these outcomes.
Immunological and virological outcomes
Two trials reported changes in CD4+ count at four and six weeks follow‐up, and neither reported statistically significant changes (2 trials, 464 participants, data not pooled, see Table 17). One trial did report that mean CD4+ count was higher in the supplemented group after six weeks (P = 0.04), but the difference was no longer statistically significant after multivariate linear regression analysis (Baeten 2002 KEN).
12. Change in CD4 cell count (cells/mm3): vitamin A versus placebo.
| Trial ID | Statistical measure | Intervention | Control | Timing of end‐point | Difference between groups at end‐point (as reported by trial authors) | ||||
| Baseline | End‐point | N1 | Baseline | End‐point | N1 | ||||
| Semba 1998 USA | Median | 296 | Reported in a graph | Not reported2 | 259 | Reported in a graph | Not reported2 | 4 weeks | P = 0.17 |
| Baeten 2002 KEN | Median | 240 | 272 | 176 | 203 | 225 | 178 | 6 weeks | P = 0.04 Adjusted regression coefficient 0.34 −0.22 to 0.90); P = 0.90 |
1The number of participants stated is the number assessed for endpoint data. 2Semba 1998 USA: the trial authors reported that 110 particpants completed the trial, but did not report the number of participants for each treatment group.
Three trials reported changes in viral load, with no statistically significant differences at four to eight weeks (3 trials, 495 participants, data not pooled, see Table 18).
13. Change in viral load (log10 copies/mL) : Vitamin A versus placebo.
| Trial ID | Statistical measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | ||||
| Semba 1998 USA | Median | 9.49 | Reported in a graph | Not reported2 | 9.67 | Not reported | Not reported2 | 4 weeks | P = 0.17 |
| Humphrey 1999 USA | Geometric mean | Reported in a graph | Reported in a graph | 19 | Reported in a graph | Reported in a graph | 12 | 8 weeks | P = 0.56 |
| Baeten 2002 KEN | Median | 5.34 | 5.34 | 176 | 5.54 | 5.49 | 178 | 6 weeks | P = 0.1 |
1The number of participants stated is the number assessed for endpoint data. 2Semba 1998 USA: the trial authors reported that 110 particpants completed the trial, but do not report the number of participants for each treatment group.
Nutritional status and blood micronutrient concentrations
Three trials reported changes in blood retinol concentrations (data not pooled, see Table 19). In the only trial with a significant proportion of participants with vitamin A deficiency at baseline (59% < 1.05 µmol/L), median serum concentrations were higher after six weeks of supplementation compared to placebo (P = 0.03) (Baeten 2002 KEN). In the other two trials, in which most participants were not deficient, average blood retinol concentrations remained unchanged after follow‐up periods of four and eight weeks, respectively (Humphrey 1999 USA; Semba 1998 USA).
14. Change in nutritional status parameters: vitamin A versus placebo.
| Trial ID | Nutritional parameter | Statistical Measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | |||||
| Baeten 2002 KEN3 | Vitamin A (µmol/L)2 | Median | 0.97 | 1.03 | Not reported | 0.95 | 0.94 | Not reported | 6 weeks | P = 0.03. "No effect" reported for those who were severely deficient at baseline |
| Semba 1998 USA3 | Vitamin A (µmol/L)3 | Median | 1.61 | Presented in a graph | Not reported | 1.37 | Presented in a graph | Not reported | 4 weeks | "Not different" |
| Humphrey 1999 USA3 | Vitamin A (µmol/L)3 | Median | 1.56 | 1.54 | 20 | 1.37 | 1.30 | 15 | 4 weeks | "No change" |
1The number of participants stated is the number assessed for endpoint data. 2Baeten 2002 KEN: Data converted from µg/dL to µmol/L. 3Analysis of blood micronutrient concentrations did not include adjustment for biomarkers of inflammation.
Note: one further trial from the USA evaluated supplementation with a vitamin A precursor, β‐carotene (60 mg) three times daily (Coodley 1996 USA). This trial reported an increase in blood concentrations of β‐carotene at three months but no statistically significant effects on CD4+ cell count.
Adverse events associated with supplementation
Signs or symptoms of toxicity (headache, nausea, vomiting, diarrhoea, fever) were similar in the intervention and control groups at 24 hours and one week after administration in one trial (Humphrey 1999 USA). No adverse events were reported in the other two trials of vitamin A supplementation (Baeten 2002 KEN; Semba 2007 USA). Slight skin discolouration was reported by the participants in the intervention group of a one small trial of β‐carotene supplementation (Coodley 1993 USA).
Certainty of the evidence
For a critical appraisal of the summary of evidence, see 'Summary of findings' table 2 (Table 2).
Comparison 4: Vitamin D versus placebo
Five trials compared a vitamin D supplement, with or without a calcium supplement, to placebo (see Table 20). Participants in two trials received a total of three or four doses of vitamin D (100,000 IU), given every three to five months (Giacomet 2013 ITA; Wejse 2009 GNB). Two trials from the USA used a daily supplement containing 7000 IU vitamin D or 4000 IU vitamin D plus calcium (1000 mg), respectively, for 12 months (Overton 2015 USA; Stallings 2014 USA). A fifth trial from Denmark combined a single dose of vitamin D (100,000 IU) at study entry with a daily vitamin D supplement (1200 IU) plus calcium (1200 mg) for 16 weeks (Bang 2012 DEN).
15. Characteristics of trials evaluating Vitamin D supplements versus placebo.
| Trial ID | Country | Participants | Baseline ART use | Mean baseline blood 25(OH)2 vitamin D concentration (ng/mL)1 | Mean baseline CD4+ cell count (cells/mm3) | Mean baseline viral load (log10 copies/mL) | Dose2 | Duration of supplementation |
| Bang 2012 DEN | Denmark | HIV‐positive men | 100% | 27.2 (11.5) 29.2 (12.4) |
507 (268) 463 (197) |
Not reported | 100,000 IU then 1200 IU | Single dose at baseline then daily for 16 weeks (plus 1200 mg calcium daily) |
| Giacomet 2013 ITA | Italy | HIV‐positive; ≤ 30 years | 86% | 15 (median)3 | 663 (median) 673 (median) |
Not reported | 100,000 IU | Single dose at baseline and at 3, 6, and 9 months |
| Overton 2015 USA | USA | HIV‐positive men and women | 0%4 | 26.7 (median) 25.1 (median) |
346 (median) 337 (median) |
4.5 | 4000 IU | Daily for 48 weeks (plus 100 mg calcium) |
| Stallings 2014 USA | USA | HIV‐positive; ≤ 25 years | 76% | 18.2 (8.4) 17.7 (9) |
Not reported5 | 3.17 (0.96)6 | 7000 IU | Daily for 12 months |
| Wejse 2009 GNB | Guinea‐Bissau | HIV‐positive plus active TB | 0% | Not reported for HIV‐positive participants | Not reported for HIV‐positive participants | Not reported | 100,000 IU | Single dose at baseline, 5, 8 months |
Abbreviations: ART: antiretroviral therapy; IU: International units; TB: Tuberculosis
1Reference value for vitamin D sufficiency: 25 (OH) vitamin D ≥ 30 ng/mL. 2RDA for a male aged 19 to 70 years ranges between 5 to 15 µg (200 to 600 IU) daily. 3Giacomet 2013 ITA: only participants with low blood vitamin D concentrations were included in the trial (25(OH)D < 30 ng/mL). 4Overton 2015 USA: all trial participants were intiated on ART at baseline. 5Stallings 2014 USA: 62% of partcipants had a CD4 cell count > 500 cells/mm3. 6Stallings 2014 USA: 44% of trial participants presented with a detectable viral load at baseline (vitamin D group:13, placebo group: 11).
In one trial from Guinnea‐Bissau participants did not receive ART and were on treatment for active pulmonary tuberculosis (Wejse 2009 GNB). In the other trials most participants were on ART (see Table 20).
We considered only one of the five trials to be at low risk of selection bias (Wejse 2009 GNB), but three trials adequately described blinding and we considered them to be at low risk of detection and performance bias (Bang 2012 DEN; Giacomet 2013 ITA; Wejse 2009 GNB).
Mortality, morbidity, and clinical disease progression
Only a single trial reported mortality in people with active tuberculosis, which was significantly underpowered to evaluate mortality (RR 1.15, 95% CI 0.65 to 2.02; 1 trial, 131 participants, Analysis 3.1). The effect estimate has wide CIs which include both important effects and no effect.
3.1. Analysis.
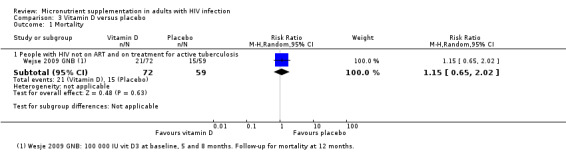
Comparison 3 Vitamin D versus placebo, Outcome 1 Mortality.
Immunological and virological outcomes
Four trials reported changes in the mean or median CD4+ cell counts, over periods from four to 12 months and found no statistically significant effects (4 trials, 288 participants, data not pooled; see Table 21).
16. Change in CD4 cell count (cells/mm3): vitamin D versus placebo.
| Trial ID | Statistical measure | Baseline | Endpoint | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Intervention | Control | N1 | Intervention | Control | N1 | ||||
| Bang 2012 DEN | Mean (SD) | 507 (268) | 463 (197) | 17 | Not reported | Not reported | 15 | 16 weeks | "No changes" in naïve or activated CD4+ cell counts |
| Giacomet 2013 ITA | Median | 15 | 15 | 25 | Not reported | Not reported | 25 | 12 months | MD 58.1 (−114.5 to 230.7)2 |
| Overton 2015 USA | Median | 346 | 342 | 79 | 5513 | 5263 | 86 | 48 weeks | P = 0.90 |
| Wejse 2009 GNB | Mean | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 8 months | MD −22 (P = 0.17)4 |
Abbreviations: MD: Mean difference; SD: Standard deviation
1The number of participants stated is the number assessed for endpoint data. 2Giacomet 2013 ITA: the trial authors also reported no difference in CD4+ cell count at 3, 6, and 9 months. 3Overton 2015 USA: the trial authors reported an increase in CD4+ cell count within both treatment groups (P > 0.001). 4Wejse 2009 GNB: subset of 41 HIV‐positive participants who had CD4+ cell counts at baseline and endpoint.
One very small study from the USA reported a reduction in viral load over time in those participants with a detectable viral load, using a multi‐level regression model (1 trial, 28 participants, P < 0.05) (Stallings 2014 USA).
Nutritional status and blood micronutrient concentrations
Four trials reported changes in serum concentrations of 25‐hydroxy vitamin D for periods that ranged from four to 12 months. Both single dose supplements and daily supplements resulted in a significant increase in mean or median blood concentrations (ng/mL) (4 trials, 305 participants, data not pooled; see Table 22).
17. Change in nutritional status parameters: vitamin D versus placebo.
| Trial ID | Nutritional parameter | Statistical Measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | |||||
| Bang 2012 DEN2 | 25(OH) vitamin D (ng/mL)2 | Mean (SD) | 27.2 (11.5) | 31.6 (9.9) | 17 | 29.2 (12.4) | 19.2 (13.9) | 15 | 16 weeks | P < 0.001 |
| Giacomet 2013 ITA2 | 25(OH) vitamin D (ng/mL)2 | Median | 15 | Not reported | 25 | 15 | Not reported | 25 | 12 months | MD 12.5 (5.9 to 19) P < 0.001 |
| Overton 2015 USA2 | 25(OH) vitamin D (ng/mL)2 | Median | 26.7 | 56.4 | 79 | 25.1 | 26.2 | 86 | 48 weeks | P < 0.001 |
| Stallings 2014 USA2 | 25(OH) vitamin D (ng/mL)2 | Mean (SD) | 10.3 (6.4) | 17 (13.1) | 30 | 11.3 (7.6) | 10.5 (6.2) | 28 | 12 months | P < 0.001 |
Abbreviations: MD: Mean difference; SD: Standard deviation
1The number of participants stated is the number assessed for end‐point data. 2Analysis of blood micronutrient concentrations did not include adjustment for biomarkers of inflammation.
Note: one further trial from the USA compared supplementation with 4000 IU vitamin D to 7000 IU vitamin D daily in participants on ART (Dougherty 2015 USA). This trial reported an increase in blood concentrations of 25‐hydroxy vitamin D with both doses at three months, but no statistically significant effects on viral load.
Adverse events associated with supplementation
One trial reported one case of hypercalcaemia in a trial participant who received a single high dose of vitamin D, followed by daily administration (Bang 2012 DEN). The other four trials of vitamin D supplementation did not report any cases of hypercalcaemia (Giacomet 2013 ITA; Overton 2015 USA; Stallings 2014 USA; Wejse 2009 GNB).
Certainty of the evidence
For a critical appraisal of the summary of evidence, see 'Summary of findings' table 3 (Table 3).
Comparison 5: Zinc versus placebo
Six trials compared a zinc supplement to placebo, given for between two weeks and 18 months (see Table 23). Two trials provided daily supplements at the level of the RDA (Asdamongkol 2013 THA; Baum 2010 USA), with one being a small trial from Thailand in participants with immunological discordance on ART (Asdamongkol 2013 THA).
18. Characteristics of trials evaluating zinc supplements versus placebo.
| Trial ID | Country | Participants | Baseline ART use | Mean baseline blood zinc concentration (µg/L)1 | Mean baseline CD4+ cell count (cells/mm3) | Mean baseline viral load (copies/mL or log10 copies/mL) | Dose2 | Duration of supplementation |
| Asdamongkol 2013 THA3 | Thailand | HIV‐positive | 100% with immunological discordance | 80 (median) 76 (median) |
183 (median) 162 (median) |
Not reported | 15 mg daily | 6 months |
| Baum 2010 USA | USA | HIV‐positive | 62%4 | 60 (10)5 | 373 (280) | 4.0 (1.0) | 12 mg (women) 15 mg (men) |
18 months |
| Cárcamo 2006 PER | Peru | HIV‐positive plus persistent diarrhoea | 0% | 66 (median) 65 (median) |
65 (median) 55 (median) |
Not reported | 50 mg twice daily | 14 days |
| Green 2005 SGP | Singapore | HIV‐positive | 95% | 86.9 (15.0) 92.2 (18.3) |
112 (62) 131 (65) |
26 338 (38 335) 28 093 (41 056 |
50 mg daily | 28 days |
| Lawson 2010 NIG | Nigeria | HIV‐positive plus active TB | 0% | Not reported | Not reported | Not reported | 90 mg weekly6 | 6 months |
| Range 2006 TZA | Tanzania | HIV‐positive plus active TB | 0% | Not reported | 406 (median) 460 (median) |
3.83 (median) 3.90 (median) |
45 mg daily | 8 months |
Abbreviations: ART: Antiretroviral therapy; RDA: Recommended Daily Allowance; TB: Tuberculosis
1Reference value for zinc sufficiency: > 70 µg/L. 2RDA for a male aged 18 to 70 years is 11 mg daily. 3Asdamongkol 2013 THA: the trial authors stratified participants with or without low blood zinc concentrations and randomized them to receive zinc or placebo. 4Baum 2010 USA: proportion of trial participants who were on ART and had an undetectable viral load at baseline: 30% 5Baum 2010 USA: the trial authors excluded participants with normal baseline blood zinc levels (≥ 75 µg/L) 6Lawson 2010 NIG: the trial authors randomized participants to receive either weekly doses of zinc (90 mg) and vitamin A (5000 IU), zinc (90 mg) and placebo, or a dual placebo.
Higher doses of zinc (50 mg to 100 mg) were given to ARV‐naive participants with persistent diarrhoea for 14 days in one trial in Peru (Cárcamo 2006 PER) and to participants on ART from Singapore for four weeks (Green 2005 SGP). In another two trials, participants were on treatment for active pulmonary tuberculosis, and received either a high daily dose of zinc (45 mg) or a weekly dose (90 mg) for six to eight months (Lawson 2010 NIG; Range 2006 TZA).
We considered only one of the six trials to be at low risk of selection bias (Cárcamo 2006 PER), but five trials adequately described blinding and we judged them to be at low risk of performance or detection bias.
Mortality
All three trials that reported deaths were substantially underpowered to confidently detect or exclude effects. None of the trials found statistically significant results and the 95% CI for the overall effect was wide, including both important effects and no effect (RR 1.24, 95% CI 0.53 to 2.86; 3 trials, 433 participants, Analysis 4.1).
4.1. Analysis.
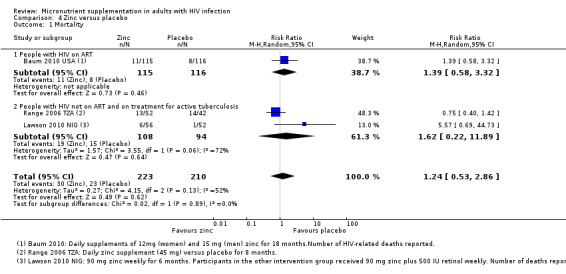
Comparison 4 Zinc versus placebo, Outcome 1 Mortality.
Morbidity and clinical disease progression
One trial from Peru in ART‐naive adults with persistent diarrhoea reported that a high daily dose of zinc (100 mg) had no effect on the persistence of diarrhoea after two weeks (HR 0.91, 95% CI 0.5 to 1.66; 1 trial, 104 participants, Analysis 4.2; Cárcamo 2006 PER).
4.2. Analysis.
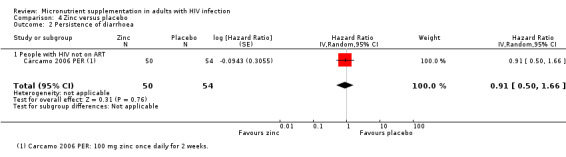
Comparison 4 Zinc versus placebo, Outcome 2 Persistence of diarrhoea.
One trial from the USA reported that daily zinc supplementation at the level of the DRI for 18 months significantly reduced the proportion of participants with diarrhoea over time (odds ratio (OR) 0.4, 95% CI 0.18 to 0.87; 1 trial, 231 participants, Analysis 4.3; Baum 2010 USA). However, the 95% CI was wide and the trial was also underpowered to have confidence in this result.
4.3. Analysis.
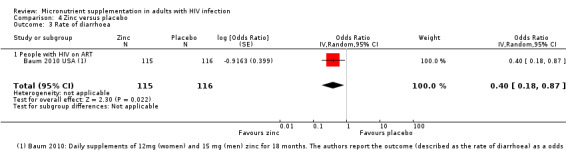
Comparison 4 Zinc versus placebo, Outcome 3 Rate of diarrhoea.
Immunological and virological outcomes
Three trials reported changes in CD4+ cell count over periods from 28 days to six months (3 trials, 192 participants, data not pooled, see Table 24) . One trial reported a statistically significant difference in one small subgroup (Asdamongkol 2013 THA). This subgroup is substantially underpowered and therefore a positive result is not likely to reflect a true result (low PPV) (Button 2013). However, one additional trial from the USA in adults on ART reported a statistically significant reduction in the risk of reaching a CD4+ count less than 200 cells/mm³ after supplementation for 18 months (RR 0.24, 95% CI 0.10 to 0.56; 1 trial, 231 participants; Table 24; Baum 2010 USA).
19. Change in CD4 count (cells/mm3) : zinc versus placebo.
| Trial ID | Statistical measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | ||||
| Asdamongkol 2013 THA | Median (IQR) |
183 (151 to 213) |
250 (190 to 286) |
13 | 162 (139 to 182) |
192 (162 to 254) |
17 | 6 months | Supplementation increased median CD4+ in those with low zinc at baseline (P = 0.042) but not those with normal zinc (P > 0.05) |
| Baum 2010 USA | Mean (SD) |
385 (285) |
Not reported | 104 | 361 (275) |
Not reported | 96 | 18 months | Reduced risk of CD4+ < 200 cells/µL2 with intervention (RR 0.24, 95% CI 0.10 to 0.56) |
| Cárcamo 2006 PER | Median | 66 | Not reported | — | 65 | Not reported | — | — | Not reported |
| Green 2005 SGP | Mean (SD) |
113 (61) |
127 (73) |
30 | 134 (63) |
156 (75) |
33 | 28 days | P = 0.91 |
| Range 2006 TZA | Mean (95% CI) |
406 (327 to 485) |
422 (331 to 512) |
58 | 460 (351 to 569) | 403 (309 to 569) |
48 | 2 months | "Not significant" |
Abbreviations: CI: Confidence interval; IQR: Interquartile range; RR: Relative risk; SD: Standard deviation
1The number of participants stated is the number assessed for endpoint data.
Three trials reported changes in viral load over periods from 28 days to 18 months, and all three trials (including Baum 2010 USA) reported no statistically significant differences with supplementation (3 trials, 400 participants, data not pooled, see Table 25).
20. Change in viral load (copies/mL or log10 copies/mL): zinc versus placebo.
| Trial ID | Statistical measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | ||||
| Baum 2010 USA | Mean (SD) |
4.0 (1.0) |
Not reported | 115 | 4.0 (1.1) |
Not reported | 116 | 18 months | "Not affected by supplementation" |
| Green 2005 SGP | Mean (SD) |
24,740 (36,856) |
27,652 (39,418) |
30 | 26,286 (40,297) |
24,551 (39,013) |
33 | 28 days | P = 0.26 |
| Range 2006 TZA | Mean (95% CI) | 3.83 (3.52 to 4.15) | 4.28 (3.86 to 4.71) | 58 | 3.9 (3.53 to 4.27) | 4.1 (3.67 to 4.54) | 48 | 2 months | "Not significant" |
Abbreviations: CI: Confidence interval; SD: Standard deviation; TB: Tuberculosis
1The number of participants stated is the number assessed for endpoint data.
Nutritional status and blood micronutrient concentrations
Two trials reported blood zinc concentrations at the trial endpoints of 28 days and 6 months, respectively (two trials, data not pooled, see Table 26) (Asdamongkol 2013 THA; Green 2005 SGP). No difference in zinc concentrations were reported, except for a small number of participants from one trial in Thailand who were deficient in zinc at baseline (Asdamongkol 2013 THA).
21. Changes in nutritional status parameters: zinc versus placebo.
| Trial ID | Nutritional parameter | Statistical Measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by trial authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | |||||
| Asdamongkol 2013 THA2,3 | Blood zinc (µg/L) | Median (IQR) |
80 (66 to 87) |
82 (71 to 100) |
13 | 76 (66 to 88) |
74 (69 to 82) |
17 | 6 months | "higher after zinc supplementation, particularly in patients with low plasma zinc levels at baseline" |
| Baum 2010 USA | Blood zinc (µg/L) | Mean (SD) |
40 (10) |
Not reported | Not reported | 40 (11) |
Not reported | Not reported | 18 months | Adjusted4 regression coefficient ß = 0.04; P = 0.0472 |
| Cárcamo 2006 PER5 | Blood zinc (µg/L) | Median | 66 | Not reported | Not reported | 65 | Not reported | Not reported | 14 days | Not reported5 |
| Green 2005 SGP5 | Blood zinc (µg/L) | Mean (SD) | 92.2 (18.3) | 120.3 (68.0) | 30 | 86.9 (15) | 111.8 (37.9) | 33 | 28 days | P = 0.67 |
Abbreviations: IQR: Interquartile range; SD: Standard deviation
1The number of participants stated is the number assessed for endpoint data. 2Asdamongkol 2013 THA: participants with or without low blood zinc concentrations were stratified and randomized to receive zinc or placebo. 3Analysis of blood micronutrient concentrations did not include adjustment for biomarkers of inflammation. 4Baum 2010 USA: regression coefficient adjusted for C‐reactive protein levels (biomarker for inflammation). 5Cárcamo 2006 PER: the trial authors reported a smaller proportion of participants in the supplemented group with low zinc levels after 14 days of follow‐up (65.6% versus 93.7%; P = 0.01).
In addition, the authors of one trial from Peru in adults with persistent diarrhoea reported a smaller proportion of supplemented participants with low blood zinc levels after 14 days (1 trial, 159 participants, P = 0.01, see Table 26) (Cárcamo 2006 PER). Another trial from the USA reported significantly higher blood zinc concentrations in the supplemented group at the study endpoint of 18 months, adjusted for C‐reactive protein levels (1 trial, 231 participants, ß = 0.04; P = 0.047) (Baum 2010 USA).
Adverse events associated with supplementation
In one trial one participant in the intervention group developed an erythematous rash after taking a standard dose supplement for one month, which resolved when the supplement was discontinued (Asdamongkol 2013 THA). Two trials of high dose supplementation reported similar numbers of participants in the intervention and control groups with gastrointestinal symptoms such as vomiting, diarrhoea or abdominal pain (Cárcamo 2006 PER; Green 2005 SGP).
Certainty of the evidence
For a critical appraisal of the summary of evidence, see 'Summary of findings' table 4 (Table 4).
Comparison 6: Selenium versus placebo
Four trials compared a daily selenium supplement (200 µg) to placebo, given for between nine and 24 months (see Table 27). Two trials recruited only ART‐naive participants (Baum 2013 BWA; Kamwesiga 2015 RWA), and two recruited both ART‐naive people and people on ART (Burbano 2002 USA; Hurwitz 2007 USA).
22. Characteristics of trials evaluating selenium supplements versus placebo.
| Trial ID | Country | Participants | Baseline ART use | Mean baseline blood selenium concentration (µg/l)1 | Mean baseline CD4+ cell count (cells/mm3) | Mean baseline viral load (copies/mL or log10 copies/mL) | Dose2 | Duration of supplementation (months) |
| Baum 2013 BWA | Botswana | HIV‐positive | 0 % | 65 (10) 70 (24)3 |
423 (median) | 18 500 (median) | 200 µg daily | 24 months |
| Burbano 2002 USA | USA | HIV‐positive IDUs | Combination therapy 21% HAART 46%3 |
Not reported5 | 427 (421) 378 (295) |
55,257 (147, 152) 60,905 (144, 292) |
200 µg daily | 12 months |
| Hurwitz 2007 USA | USA | HIV‐positive | 73% | Not reported6 | 417 (264) 441 (266) |
24,558 (87,051) 10,491 (20,251) |
200 µg daily | 9 months |
| Kamwesiga 2015 RWA | Rwanda | HIV‐positive | 0%7 | Not reported | 552 (median) 527 (median) |
3.8 (median) 3.9 (median) |
200 µg daily | 24 months |
Abbreviations: ART: antiretroviral therapy; HAART: Highly active antiretroviral therapy; IDUs: injection drug users.
1Reference values used to define selenium sufficiency: > 75 µg/L or > 85 µg/L 2RDA for a male aged 18 to 70 years is 55 µg daily. 3Sales 2010 BWA in Baum 2013 BWA: Baseline selenium concentrations reported for a sub‐sample of 79 trial participants. 4Burbano 2002 USA: the trial authors reported fewer ARV naive participants in the selenium group (24%) compared to the placebo group (37%) at baseline. 5Burbano 2002 USA: participants with low baseline blood selenium levels (≤ 85 µg/L) were excluded from the trial. 6Hurwitz 2007 USA: participants with low baseline blood selenium levels (≤ 75 µg/L) were excluded from the trial. 7Kamwesiga 2015 RWA: participants who were eligible for ART were excluded from the trial.
Of the four trials, we only judged one to be at low risk of selection bias (Baum 2013 BWA), and three to be at low risk of performance and detection bias as they adequately described blinding (Baum 2013 BWA; Burbano 2002 USA; Hurwitz 2007 USA).
Mortality
Not reported.
Morbidity and clinical disease progression
One trial in HIV‐positive injection drug users in the USA reported a statistically significant reduction in the risk of hospital admissions for opportunistic infections and HIV‐related conditions after supplementation for 12 months (RR 0.40, 95% CI 0.21 to 0.75; 1 trial, 186 participants, Analysis 5.1). However, the trial authors stated that fewer participants in the placebo group compared to the selenium group were on ART at baseline (P < 0.05) which may have influenced this result (Burbano 2002 USA).
5.1. Analysis.
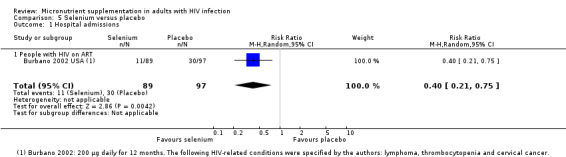
Comparison 5 Selenium versus placebo, Outcome 1 Hospital admissions.
Immunological and virological outcomes
All four trials reported measures of change in CD4+ cell count with mixed findings and poor reporting of baseline and end values (see Table 28). In people not taking ART we observed the following.
23. Change in CD4 cell count (cells/mm3): selenium versus placebo.
| Trial ID | Statistical measure | Intervention | Control | Timing of endpoint | Difference between groups at endpoint (as reported by study authors) | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | ||||
| Baum 2013 BWA | Median (IQR) |
423 (347 to 539) |
Not reported | 220 | 411 (327 to 545) |
Not reported | 217 | 2 years | Not reported2 |
| Burbano 2002 USA | Mean (SD) | 427(421) | Not reported | Not reported | 376(295) | Not reported | Not reported | 12 months | Not reported3 |
| Hurwitz 2007 USA | Mean (SD) | 417 (264) | Not reported | Not reported | 441 (266) | Not reported | Not reported | 9 months | Not reported4 |
| Kamwesiga 2015 RWA | Median (IQR) | 552 (470 to 636) | Not reported | 149 | 527 (465 to 610) | Not reported | 151 | 24 months | Not reported5 |
Abbreviations: CI: confidence interval; HR: hazard ratio; IQR: interquartile range; RR: risk ratio; SD: standard deviation 1The number of participants stated is the number assessed for endpoint data. 2Baum 2013 BWA: the trial authors reported no reduction in risk of CD4+ falling to < 250 cells/µL2 (HR 0.83, 95% CI 0.48 to 1.42). 3Burbano 2002 USA: the trial authors reported that 46% of participants in the placebo group versus 25% in the selenium group experienced a decline in CD4 cell count > 50 cells/mm3 (P = 0.01). 4Hurwitz 2007 USA: the trial authors reported in a multiple regression model that increased selenium levels predicted a greater decrease in viral load (P < 0.02), which predicted a greater increase in CD4 counts (P < 0.04). 5Kamwesiga 2015 RWA: the trial authors reported a 44 % reduction in the rate of CD4+ cell decline per month (MD 1.74, 95% CI 0.31 to 3.17). No reduction in risk of CD4+ falling to < 350 cells/µL2 (RR 0.81, 95% CI 0.61 to 1.09).
Baum 2013 BWA reported no significant reduction in the risk of reaching a CD4+ count < 250 cells/mm³ with selenium supplements for two years in people not on ART (HR 0.83 95% CI: 0.48 to 1.42; 1 trial, 437 participants), and Kamwesiga 2015 RWA reported no significant reduction in the risk of reaching a CD4+ count of less than 350 cells/mm³ (RR 0.81 95% CI 0.61 to 1.09; 1 trial, 300 participants)
However, Kamwesiga 2015 RWA reported a reduction in the monthly rate of CD4 cell depletion (MD 1.74, 95% CI 0.31 to 3.17; 1 trial, 300 participants)
In populations with mixed exposure to ART, we observed the following.
Burbano 2002 USA reported that fewer trial participants in the supplemented group experienced a CD4 cell decline of greater than 50 cells/mm³ (P = 0.01; authors' own figures)
Hurwitz 2007 USA reported a multiple regression model that found higher selenium levels predicted a greater increase in CD4+ cell counts at 9 months (P < 0.04; authors' own figures). However, this trial is at high risk of selective reporting as the statistically significant results are only for a subgroup of participants classified as 'selenium responders'. It is unclear if this classification or analysis was planned a priori
Only three trials reported effects on viral load and statistically significant benefits were only reported from the multiple regression model used by Hurwitz 2007 USA.
Nutritional status and blood micronutrient concentrations
Three trials reported statistically significant increases in blood selenium concentrations of participants after supplementation for 6 to 12 months (527 participants, data not pooled, see Table 29)
24. Change in nutritional status parameters: selenium versus placebo.
| Trial ID | Nutritional parameter | Statistical measure | Intervention | Control | Timing of endpoint | Comment | ||||
| Baseline | Endpoint | N1 | Baseline | Endpoint | N1 | |||||
| Burbano 2002 USA2 | Blood selenium (µg/L) | — | Not reported | Not reported | ‐ | Not reported | Not reported | Not reported | 12 months | Not reported 3 |
| Hurwitz 2007 USA2 | Blood selenium (µg/L) | — | Not reported | Not reported | 83 | Not reported | Not reported | 91 | 9 months | MD 31.7 (27.4 to 36); P<0.001 |
| Kamwesiga 2015 RWA | Blood selenium (µg/L) | — | Not reported | Not reported | ‐ | Not reported | Not reported | — | 24 months | — |
| Sales 20102 | Blood selenium (µg/L)4 | Mean (SD) | 65 (10) | 147 (15.3) | 33 | 70 (24) | 69 (12.1) | 46 | 6 months | P<0.001 |
Abbreviations: MD: Mean difference; SD: Standard deviation
1The number of participants stated is the number assessed for endpoint data. 2Analysis of blood micronutrient concentrations did not include adjustment for biomarkers of inflammation. 3Burbano 2002 USA: the trial authors reported proportions of participants with blood selenium levels < 135 µg/L at the end of the trial: 89% versus 47% (P = 0.001). 4Sales 2010: data reported on a subsample of trial participants from the trial by Baum 2013 BWA.
Adverse events associated with supplementation
One trial reported no differences in symptoms such as nausea, vomiting and skin and hair changes of participants, but those in the intervention group were more likely to report anxiety (P = 0.04) and sleep symptoms (P = 0.01). (Kamwesiga 2015 RWA). Another trial stated that all serious adverse events reported (which included acute diarrhoea, vomiting, or severely elevated ALT levels) were adjudicated as having a remote relationship to the intervention (Baum 2013 BWA). Two other selenium supplementation trials reported no adverse events (Burbano 2002 USA; Hurwitz 2007 USA).
Certainty of the evidence
For a critical appraisal of the summary of evidence, see 'Summary of findings' table 5 (Table 5).
Comparison 7: Vitamin E plus vitamin C versus placebo
One small Canadian trial compared high daily doses of vitamins E (800 IU) and C (1000 mg) to placebo in adults on combination ART for three months. Participants were followed up for six months because of a possible carry‐over effect of the intervention (Allard 1998 CAN).
Allocation concealment was not well described and so the risk of selection bias was unclear, but the trial was adequately blinded.
Mortality
Not reported.
Morbidity and clinical disease progression
Allard 1998 CAN reported that high daily doses of vitamin E and C for three months had no effect on the risk of new AIDS defining infections after six months (RR 3.54, 95% CI 0.43 to 29.43; 1 trial, 49 participants).
Immunological and virological outcomes
Allard 1998 CAN reported no effect on viral load (log10copies/mL) after three months supplementation of high daily doses of vitamin E and C (MD 0.95 log 10 copies/mL, 95% CI 0.14 to 2.04; 1 trial, 49 participants).
Nutritional status and blood micronutrient concentrations
This trial reported that high daily doses of vitamins E and C for three months increased blood concentrations of vitamin E (µmol/L) (MD 28.70, 95% CI 20.01 to 37.39; one trial, 49 participants) and vitamin C (µmol/L) (MD 27.30, 95% CI 12.88 to 41.72; 1 trial, 49 participants) of adults on ART (Allard 1998 CAN).
Adverse events associated with supplementation
Two participants in the intervention group reported epigastric discomfort (Allard 1998 CAN).
Comparison 8: Folinic acid versus placebo
One small Brazilian trial compared the effect of a daily folinic acid supplement (5 mg) to placebo on the vascular response of 30 HIV‐positive adults on ART (Grigoletti 2013 BRA). This trial was at low risk of selection bias, and detection and performance bias.
Mortality
Not reported.
Morbidity and clinical disease progression
Not reported.
Immunological and virological outcomes
This trial reported no difference in median CD4 cell counts after daily supplementation of folinic acid for four weeks (1 trial, 30 participants, P = 0.994) (Grigoletti 2013 BRA).
Nutritional status and blood micronutrient concentrations
This trial reported increases in blood concentrations of folate and vitamin B12 after supplementation of folinic acid for four weeks (1 trial, 30 participants, P < 0.001) (Grigoletti 2013 BRA).
Adverse events associated with supplementation
No adverse events were reported (Grigoletti 2013 BRA).
Comparison 9: Iron versus no iron
One trial in the USA compared the effect of a daily micronutrient supplement containing iron to a supplement without iron in female injection drug users for 12 months. Of the trial participants who were HIV‐positive, approximately one‐third were on HAART at baseline and during the study (Semba 2007 USA).
Mortality
Not reported.
Morbidity and clinical disease progression
Not reported.
Immunological and virological outcomes
This trial reported no difference in CD4 cell counts or viral load measurements in participants who received the micronutrient plus iron supplement compared to those who received the supplement without iron for 12 months (CD4 cell count (cells/mm³): MD 35, 95% CI −83.5 to 153.5; viral load (log10copies/mL): MD −0.4, 95% CI −0.99 to 0.19; 1 trial, 103 participants; Semba 2007 USA).
Adverse events associated with supplementation
No adverse events were reported (Semba 2007 USA).
Discussion
Summary of main results
Multiple micronutrients
Routine multiple micronutrient supplementation may have little or no effect on mortality in adults living with HIV, but the pooled analysis remains underpowered to confidently exclude small effects (low certainty evidence). Trials were conducted in antiretroviral therapy (ART)‐naïve adults (3 trials, 1068 participants, low certainty evidence), adults on ART (1 trial, 400 participants, very low certainty evidence), and adults with concurrent active tuberculosis (3 trials, 1429 participants, low certainty evidence).
Routine supplementation for up to two years, has also not been shown to have consistent benefits on either mean CD4+ cell count (low certainty evidence) or mean viral load (moderate certainty evidence). One recent trial in ART‐naïve adults reported a reduction in the risk of reaching a CD4+ cell count of less than 250 cells/mm³ after two years of high dose supplementation in Botswana. However, this effect was only robust in the trial arm receiving multiple micronutrients plus selenium (not either supplementation alone) and is inconsistent with the findings of other trials using similar combinations of micronutrients and selenium.
In one additional trial that compared high dose multiple micronutrient supplementation with standard doses in people on antiretroviral therapy (ART), peripheral neuropathy was reduced with high dose supplements compared to standard dose, but the trial was stopped early due to increased adverse events in the high dose group.
Single or dual micronutrients
None of the trials of single or dual micronutrient supplements were adequately powered to assess for effects on mortality or morbidity outcomes such as hospital admissions and persistence or rate of diarrhoea. Clinically important effects on CD4+ cell count or viral load were not reported. Supplementation probably does increase blood concentrations of vitamin D and zinc, and may also increase blood concentrations of vitamin A, especially in those who are deficient at baseline.
Overall completeness and applicability of evidence
The included trials of multiple micronutrient supplements were predominantly conducted in people who either were not taking ART or were on concurrent treatment for tuberculosis. In these populations, routine supplementation has not been shown to consistently improve disease progression as measured by average CD4+ cell count. One recent well‐conducted trial, from Botswana detected a benefit in the group receiving multivitamins with selenium but not with multivitamins alone. However, the lack of demonstrable benefit in other trials with similar selenium content, suggest that this effect should be repeated before reliable conclusions can be drawn.
The trials of single or dual micronutrient supplements included more participants on ART, and several were conducted in populations with proven micronutrient deficiencies. Despite this the only demonstrable benefits were improvements in serum levels of some micronutrients. However, these effects may be enough for some to recommend routine supplementation in similar populations.
There are several possible explanations for the lack of benefit seen in many of these trials.
The period of supplementation may have been insufficient to demonstrate effects, with benefits only accruing over prolonged periods of supplementation. Supplementation ranged from as little as four weeks up to two years.
The prevalence of micronutrient deficiencies may have been too low in some of these populations to demonstrate an effect. Baseline micronutrient status was poorly assessed in many of the included trials, particularly those evaluating multiple micronutrients, so it is difficult to determine which populations these negative results should be applied to. Only one trial reported adjustment of their analysis of blood micronutrient concentrations for the effect of inflammation, an important confounder.
The doses supplemented varied considerably. Many trials evaluated doses significantly higher than the daily recommended intake, and one trial of multiple micronutrients directly compared high doses with standard doses to investigate this. However, it should be noted that the high doses were not well‐tolerated in this trial.
The trials may simply be too small to demonstrate effects. Certainly there is insufficient evidence to say that micronutrients could never have effects.
Given the lack of benefit in those not on ART, it seems unlikely that large effects would be seen in those who are taking ART, but adequately powered trials may still be justifiable to explore this. There may be a number of ways by which specific micronutrients may impact on or interact with ART, including aspects of drug pharmacokinetics. However, the clinical significance of these interactions remains to be determined (Raiten 2011).
Quality of the evidence
We considered the certainty of the evidence for most of the outcomes in this review to be low or very low, meaning that we can have only minimal confidence in these effects. We downgraded the certainty of the evidence mainly for the following reasons.
Indirectness: since micronutrient deficiencies differ widely among populations it is difficult to generalize the findings of a single trial, or even a few trials, to all settings, and all populations.
Imprecision: most included trials were small and well below the optimal information size for the outcomes that were being measured and therefore not able to reliably detect or exclude an effect.
Potential biases in the review process
We tried to minimize any biases in the review process by performing a comprehensive search of the literature, and by independently selecting studies, appraising studies, and extracting data. Two review authors, MV and SD, assessed the risk of bias of the new included studies using the updated 'Risk of bias' tool (Higgins 2011).
This review included outcome data for HIV participants from four RCTs that included both HIV‐positive and HIV‐negative participants without stratified randomization (Kelly 2008 ZMB; Lawson 2010 NIG; Range 2006 TZA; Semba 2007 MWI).
Agreements and disagreements with other studies or reviews
In the previous version of this Cochrane Review, Irlam 2010 concluded that further trials of single supplements (vitamin D, zinc, and selenium) were required to build the evidence base for adults and that the long‐term clinical benefits, adverse effects, and optimal formulation of multiple micronutrient supplements required further investigation.
Forrester 2011 conducted a narrative review to investigate whether the 2003 WHO recommendations for micronutrient intake in HIV‐positive adults should change. This review focused primarily on the results of nine trials of multiple micronutrient supplementation; seven trials in non‐pregnant HIV‐positive adults, and two in pregnant HIV‐positive women. The authors noted that "five of the six trials that used high‐dose multiple micronutrients showed benefits in terms of either improved CD4 cell counts or survival", but also that "many of these trials were small and of short duration, and the long‐term risks and benefits of high‐dose multiple micronutrients are not established". Our analysis and appraisal of the evidence agrees that there is currently insufficient evidence to make firm conclusions about the effects of supplementation.
For the effects of micronutrient supplementation in pregnant women and children with HIV, see the separate Cochrane reviews by Siegfried 2012 and Irlam 2013.
Authors' conclusions
Implications for practice.
To date trials of routine multiple micronutrient supplementation have not demonstrated consistent clinically important benefits on HIV disease progression or mortality. However, the trials are generally too small to confidently exclude the possibility of important effects.
These findings should not be interpreted as a reason to deny supplementation where specific deficiencies have been demonstrated (such as vitamin D, zinc, and selenium), or where the person's diet is unlikely to meet the recommended daily allowance of vitamins and minerals.
Implications for research.
Furthermore, adequately powered studies with sufficient follow‐up periods are still required to confidently prove or exclude any long‐term clinical benefit of routine supplementation.
Such research should not be to the detriment of ART, as this remains the one intervention to date that has consistently been shown to reduce morbidity and mortality, and improve the nutritional status of adults living with HIV/AIDS.
What's new
| Date | Event | Description |
|---|---|---|
| 16 May 2017 | New search has been performed | We included 17 new trials in this review update, and assessed the certainty of the evidence using the GRADE approach. Nigel Rollins stepped down from the review author team. Solange Durao and David Sinclair joined as review authors. |
| 16 May 2017 | New citation required and conclusions have changed | The original protocol for this review included studies in both HIV‐positive children and pregnant women (Irlam 2002). Two separate reviews on the role of micronutrient supplementation for HIV‐positive pregnant women, Siegfried 2012, and children, Irlam 2013, have been published. The primary focus of this review update was therefore on the role of micronutrient supplementation in HIV‐positive men and women who were not pregnant. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 3 June 2011 | Amended | Paragraph added about the review being split into three reviews. |
| 19 January 2011 | Amended | External source of support added. |
| 9 November 2010 | New citation required and conclusions have changed | Substantial update of the review. |
| 9 November 2010 | New search has been performed | Substantial update. |
| 9 November 2010 | Feedback has been incorporated | External reviewers' feedback incorporated into update |
| 30 September 2010 | New search has been performed | Inclusion of 16 additional trials, assessment of Risk of Bias using new ROB tool, and extensive updating of text. |
Acknowledgements
We are grateful to Cochrane South Africa and the editorial base of the Cochrane Infectious Diseases Group, for assistance and support in preparing this review update. We also thank the following individuals.
Tamara Kredo, Cochrane SA, for providing advice and guidance throughout the review process
Joy Oliver, Cochrane SA, for conducting the searches for this update
Tonya Esterhuizen, Centre for Evidence‐based Health Care, Stellenbosch University, for providing statistical advice
Xuan Hui, a methodologist of the Cochrane Eyes and Vision Group, for the translation of the included Chinese article into English
Nigel Rollins, who was an author on the previously published versions of this review (Irlam 2002; Irlam 2005; Irlam 2010)
The editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). David Sinclair and Solange Durao were partly supported by the Effective Health Care Research Consortium. This Consortium is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this review do not necessarily reflect UK government policy.
Appendices
Appendix 1. CENTRAL search strategy
| Search | Query |
| #1 | MeSH descriptor: [HIV Infections] explode all trees |
| #2 | MeSH descriptor: [HIV] explode all trees |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or hiv infect* or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immuno‐deficiency virus or human immun* deficiency virus or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun* deficiency syndrome |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #6 | #1 or #2 or #3 or #4 or #5 |
| #7 | MeSH descriptor: [Micronutrients] explode all trees |
| #8 | micronutrient or micronutrients or trace element or trace elements or vitamin or vitamins |
| #9 | MeSH descriptor: [Carotenoids] explode all trees |
| #10 | carotenoids or carotenoid or carotene or carotenes |
| #11 | 24,25‐dihydroxyvitamin D 3 or "25‐hydroxyvitamin D 2" or "4‐aminobenzoic acid" or acetylcarnitine or alpha‐tocopherol or aminobenzoic acids or ascorbic acid or beta carotene or beta‐tocopherol or biotin or boron or cadmium or calcifediol or calcitriol or carnitine or cholecalciferol or chromium or cobalt or cobamides or cod liver oil or copper or dehydroascorbic acid or dihydrotachysterol or dihydroxycholecalciferols or ergocalciferols or flavin mononucleotide or folic acid or formyltetrahydrofolates or fursultiamin or gamma‐tocopherol or hydroxocobalamin or hydroxycholecalciferols or inositol or iodine or iron or leucovorin or manganese or magnesium or molybdenum or niacin or niacinamide or nickel or nicorandil or nicotinic acids or palmitoylcarnitine or pantothenic acid or pteroylpolyglutamic acids or pyridoxal or pyridoxal phosphate or pyridoxamine or pyridoxine or riboflavin or selenium or silicon or tetrahydrofolates or thiamine or thiamine monophosphate or thiamine pyrophosphate or thiamine triphosphate or thioctic acid or tin or tocopherols or tocotrienols or ubiquinone or vanadium or zinc |
| #12 | #7 or #8 or #9 or #10 or #11 |
| #13 | #6 and #12 from 2010 to 2013, in Trials |
Appendix 2. PubMed search strategy
| Search | Query |
| #7 | Search ((#1 AND #2 AND #5)) AND ("2010/01/01"[Date ‐ Publication] : "2013/07/05"[Date ‐ Publication]) |
| #6 | Search (#1 AND #2 AND #5) |
| #5 | Search (#3 OR #4) |
| #4 | Search (“24,25‐dihydroxyvitamin D 3” OR “25‐hydroxyvitamin D 2” OR “4‐aminobenzoic acid” OR acetylcarnitine OR alpha‐tocopherol OR aminobenzoic acids OR ascorbic acid OR beta carotene OR beta‐tocopherol OR biotin OR boron OR cadmium OR calcifediol OR calcitriol OR carnitine OR cholecalciferol OR chromium OR cobalt OR cobamides OR cod liver oil OR copper OR dehydroascorbic acid OR dihydrotachysterol OR dihydroxycholecalciferols OR ergocalciferols OR flavin mononucleotide OR folic acid OR formyltetrahydrofolates OR fursultiamin OR gamma‐tocopherol OR hydroxocobalamin OR hydroxycholecalciferols OR inositol OR iodine OR iron OR leucovorin OR manganese OR magnesium OR molybdenum OR niacin OR niacinamide OR nickel OR nicorandil OR nicotinic acids OR palmitoylcarnitine OR pantothenic acid OR pteroylpolyglutamic acids OR pyridoxal OR pyridoxal phosphate OR pyridoxamine OR pyridoxine OR riboflavin OR selenium OR silicon OR tetrahydrofolates OR thiamine OR thiamine monophosphate OR thiamine pyrophosphate OR thiamine triphosphate OR thioctic acid OR tin OR tocopherols OR tocotrienols OR ubiquinone OR vanadium OR zinc) |
| #3 | Search (micronutrients OR micronutrient OR “trace element” OR “trace elements” OR vitamins OR vitamin OR carotenoids OR carotenoid OR carotenes OR carotene) |
| #2 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) |
Appendix 3. Embase search strategy
| Search | Query |
| #12 | #1 AND #7 AND #10 AND [embase]/lim AND [1‐1‐2010]/sd NOT [5‐7‐2013]/sd |
| #11 | #1 AND #7 AND #10 |
| #10 | #8 OR #9 |
| #9 | '24,25‐dihydroxyvitamin d 3'/de OR '24,25‐dihydroxyvitamin d 3' OR '25‐hydroxyvitamin d 2'/de OR '25‐hydroxyvitamin d 2' OR '4‐aminobenzoic acid'/de OR '4‐aminobenzoic acid' OR 'acetylcarnitine' OR 'acetylcarnitine'/de OR acetylcarnitine OR 'alpha‐tocopherol'/de OR 'alpha‐tocopherol' OR 'aminobenzoic acids'/de OR 'aminobenzoic acids' OR 'ascorbic acid'/de OR 'ascorbic acid' OR 'beta carotene'/de OR 'beta carotene' OR 'beta‐tocopherol'/de OR 'beta‐tocopherol' OR 'biotin' OR 'biotin'/de OR biotin OR 'boron' OR 'boron'/de OR boron OR 'cadmium' OR 'cadmium'/de OR cadmium OR 'calcifediol' OR 'calcifediol'/de OR calcifediol OR 'calcitriol' OR 'calcitriol'/de OR calcitriol OR 'carnitine' OR 'carnitine'/de OR carnitine OR 'cholecalciferol' OR 'cholecalciferol'/de OR cholecalciferol OR 'chromium' OR 'chromium'/de OR chromium OR 'cobalt' OR 'cobalt'/de OR cobalt OR 'cobamides' OR 'cobamides'/de OR cobamides OR 'cod liver oil'/de OR 'cod liver oil' OR 'copper' OR 'copper'/de OR copper OR 'dehydroascorbic acid'/de OR 'dehydroascorbic acid' OR 'dihydrotachysterol' OR 'dihydrotachysterol'/de OR dihydrotachysterol OR 'dihydroxycholecalciferols' OR 'dihydroxycholecalciferols'/de OR dihydroxycholecalciferols OR 'ergocalciferols' OR 'ergocalciferols'/de OR ergocalciferols OR 'flavin mononucleotide'/de OR 'flavin mononucleotide' OR 'folic acid'/de OR 'folic acid' OR 'formyltetrahydrofolates'/de OR 'formyltetrahydrofolates' OR 'fursultiamin' OR 'fursultiamin'/de OR fursultiamin OR 'gamma‐tocopherol'/de OR 'gamma‐tocopherol' OR 'hydroxocobalamin' OR 'hydroxocobalamin'/de OR hydroxocobalamin OR 'hydroxycholecalciferols' OR 'hydroxycholecalciferols'/de OR hydroxycholecalciferols OR 'inositol' OR 'inositol'/de OR inositol OR 'iodine' OR 'iodine'/de OR iodine OR 'iron' OR 'iron'/de OR iron OR 'leucovorin' OR 'leucovorin'/de OR leucovorin OR 'manganese' OR 'manganese'/de OR manganese OR 'magnesium' OR 'magnesium'/de OR magnesium OR 'molybdenum' OR 'molybdenum'/de OR molybdenum OR 'niacin' OR 'niacin'/de OR niacin OR 'niacinamide' OR 'niacinamide'/de OR niacinamide OR 'nickel' OR 'nickel'/de OR nickel OR 'nicorandil' OR 'nicorandil'/de OR nicorandil OR 'nicotinic acids'/de OR 'nicotinic acids' OR 'palmitoylcarnitine' OR 'palmitoylcarnitine'/de OR palmitoylcarnitine OR 'pantothenic acid'/de OR 'pantothenic acid' OR 'pteroylpolyglutamic acids'/de OR 'pteroylpolyglutamic acids' OR 'pyridoxal' OR 'pyridoxal'/de OR pyridoxal OR 'pyridoxal phosphate'/de OR 'pyridoxal phosphate' OR 'pyridoxamine' OR 'pyridoxamine'/de OR pyridoxamine OR 'pyridoxine' OR 'pyridoxine'/de OR pyridoxine OR 'riboflavin' OR 'riboflavin'/de OR riboflavin OR 'selenium' OR 'selenium'/de OR selenium OR 'silicon' OR 'silicon'/de OR silicon OR 'tetrahydrofolates' OR 'tetrahydrofolates'/de OR tetrahydrofolates OR 'thiamine' OR 'thiamine'/de OR thiamine OR 'thiamine monophosphate'/de OR 'thiamine monophosphate' OR 'thiamine pyrophosphate'/de OR 'thiamine pyrophosphate' OR 'thiamine triphosphate'/de OR 'thiamine triphosphate' OR 'thioctic acid'/de OR 'thioctic acid' OR 'tin' OR 'tin'/de OR tin OR 'tocopherols' OR 'tocopherols'/de OR tocopherols OR 'tocotrienols' OR 'tocotrienols'/de OR tocotrienols OR 'ubiquinone' OR 'ubiquinone'/de OR ubiquinone OR 'vanadium' OR 'vanadium'/de OR vanadium OR 'zinc' OR 'zinc'/de OR zinc |
| #8 | 'micronutrient'/de OR micronutrient OR 'micronutrients'/de OR micronutrients OR 'trace element'/de OR 'trace element' OR 'trace elements'/de OR 'trace elements' OR 'trace mineral'/de OR 'trace mineral' OR 'vitamin'/de OR vitamin OR 'vitamins'/de OR vitamins OR 'carotenoid'/de OR carotenoid OR 'carotenoids'/de OR carotenoids OR 'carotene'/de OR carotene OR carotenes |
| #7 | #2 NOT #6 |
| #6 | #3 NOT #5 |
| #5 | #3 AND #4 |
| #4 | 'human'/de OR 'normal human'/de OR 'human cell'/de |
| #3 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de |
| #2 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus':ab,ti OR 'human immuno+deficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune+deficiency virus':ab,ti OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno+deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti OR 'acquired immune+deficiency syndrome':ab,ti |
Appendix 4. WHO ICTRP search strategy
hiv | vitamin OR micronutrient OR trace element OR carotenoid
Appendix 5. ClinicalTrials.gov search strategy
Interventional Studies | HIV | micronutrient OR "trace element" OR vitamin OR carotenoid OR carotene
Appendix 6. List of country codes
| ISO 3166‐1 alpha‐3 CODE | Country |
| BRA | Brazil |
| BWA | Botswana |
| CAN | Canada |
| CHN | China |
| DEN | Denmark |
| GNB | Guinnea‐Bissau |
| ITA | Italy |
| KEN | Kenia |
| MWI | Malawi |
| NIG | Nigeria |
| PER | Peru |
| RWA | Rwanda |
| SGP | Singapore |
| THA | Thailand |
| TZA | Tanzania |
| UGA | Uganda |
| USA | United States |
| ZMB | Zambia |
Source: International organization for Standardization (ISO 2016)
Appendix 7. Additional assessment of risk of bias in included cluster‐randomized trials
Domain 1: recruitment bias
Recruitment bias can occur when individuals are recruited to the trial after the clusters have been randomized (Higgins 2011). The types of participants recruited can be influenced by the knowledge of whether the specific cluster is an intervention or a control cluster.
Adequate: when no recruiting was done after randomization.
Inadequate: when additional recruiting was done after randomization.
Unclear: when no reporting was done regarding the timing of recruiting all participants.
Domain 2: baseline imbalance
Cluster‐randomized trials often randomize all clusters at once, therefore, a lack of allocation concealment should not usually be a problem (Higgins 2011). However, when there is only a small number of clusters, there is a possibility of chance baseline imbalances between the randomized groups. This may affect either the clusters or the individuals.
Adequate: when the baseline comparability of clusters is sufficient, or when statistical adjustment for baseline characteristics occurred (Higgins 2011).
Inadequate: when there are significant differences between clusters and no statistical adjustments for baseline characteristics were made accordingly.
Unclear: when no reporting was done regarding baseline characteristics, or when it is not clear whether the differences between the clusters were significant.
Domain 3: loss of clusters
It is possible that complete clusters may be lost from a trial, and have to be omitted from the analysis (Higgins 2011). In the same way as for missing outcome data in individually randomized trials, this may lead to bias in cluster‐randomized trials. In addition, missing outcomes for individuals within clusters may also lead to a risk of bias in cluster‐randomized trials.
Adequate: there were no missing data, or the missing data were addressed in the correct manner.
Inadequate: there were missing data and it was dealt with in a way that could have introduced bias.
Unclear: when no reporting was done regarding missing data (either complete clusters or individuals within clusters), or when it is unclear whether the authors of the primary study have dealt with the missing data adequately (for example, acceptable statistical adjustments).
Data and analyses
Comparison 1. Multiple micronutrients versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 7 | 2897 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.72, 1.15] |
| 1.1 People with HIV not on ART | 3 | 1068 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.31, 1.15] |
| 1.2 People with HIV on ART or initiating ART | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.50, 3.10] |
| 1.3 People with HIV not on ART and on treatment for active tuberculosis | 3 | 1429 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.23] |
| 2 Hospital admissions | 2 | 881 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.61, 1.22] |
| 2.1 People with HIV not on ART | 1 | 481 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.42, 1.49] |
| 2.2 People with HIV on ART | 1 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.59, 1.36] |
| 3 Clinical disease progression | 1 | Hazard Ratio (Random, 95% CI) | 1.08 [0.72, 1.62] | |
| 3.1 People with HIV not on ART and on treatment for active tuberculosis | 1 | Hazard Ratio (Random, 95% CI) | 1.08 [0.72, 1.62] | |
| 4 CD4+ cell count | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 At baseline | 5 | 1209 | Mean Difference (IV, Random, 95% CI) | ‐18.27 [‐55.97, 19.42] |
| 4.2 At longest follow‐up | 6 | 1533 | Mean Difference (IV, Random, 95% CI) | 26.40 [‐22.91, 75.70] |
| 5 CD4+ cell count at longest follow‐up; subgrouped by participant characteristics | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 People with HIV not on ART | 2 | 441 | Mean Difference (IV, Random, 95% CI) | 30.36 [‐7.13, 67.84] |
| 5.2 People with HIV on ART or initiating ART | 1 | 367 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐35.87, 23.87] |
| 5.3 People with HIV not on ART and on treatment for active tuberculosis | 2 | 626 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐55.80, 44.25] |
| 5.4 People with HIV ‐ Not stated if they are taking ART | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 106.0 [77.23, 134.77] |
| 6 Viral load | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 At baseline | 4 | 1166 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.16, 0.07] |
| 6.2 At longest follow‐up | 4 | 792 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.26, 0.06] |
| 7 Viral load at longest follow‐up; sub‐grouped by participant characteristics | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 People with HIV not on ART | 2 | 497 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 7.2 People with HIV not on ART but in treatment for active tuberculosis | 2 | 295 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.45, 0.26] |
Comparison 2. High dose multivitamins versus standard dose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 3418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.26] |
| 1.1 People with HIV on ART or initiating ART | 1 | 3418 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.89, 1.26] |
| 2 CD4+ cell count | 1 | 6186 | Mean Difference (IV, Fixed, 95% CI) | ‐8.20 [‐14.08, ‐2.32] |
| 2.1 At baseline | 1 | 3418 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐13.74, ‐0.26] |
| 2.2 At follow‐up | 1 | 2768 | Mean Difference (IV, Fixed, 95% CI) | ‐12.0 [‐22.00, ‐0.00] |
| 3 Viral load | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Baseline | 1 | 3418 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.05, 0.05] |
| 3.2 At follow‐up | 1 | 236 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.51, 0.11] |
Comparison 3. Vitamin D versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 People with HIV not on ART and on treatment for active tuberculosis | 1 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.65, 2.02] |
Comparison 4. Zinc versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.53, 2.86] |
| 1.1 People with HIV on ART | 1 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.58, 3.32] |
| 1.2 People with HIV not on ART and on treatment for active tuberculosis | 2 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.22, 11.89] |
| 2 Persistence of diarrhoea | 1 | 104 | Hazard Ratio (Random, 95% CI) | 0.91 [0.50, 1.66] |
| 2.1 People with HIV not on ART | 1 | 104 | Hazard Ratio (Random, 95% CI) | 0.91 [0.50, 1.66] |
| 3 Rate of diarrhoea | 1 | 231 | Odds Ratio (Random, 95% CI) | 0.40 [0.18, 0.87] |
| 3.1 People with HIV on ART | 1 | 231 | Odds Ratio (Random, 95% CI) | 0.40 [0.18, 0.87] |
Comparison 5. Selenium versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hospital admissions | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.75] |
| 1.1 People with HIV on ART | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.75] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allard 1998 CAN.
| Methods | Country: Canada Setting: primary care physicians Duration of recruitment: April 1995 to August 1996 Duration of follow‐up: 6 months Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: patients of participating physicians with stable HIV‐infection. Exclusion criteria: active opportunistic infection, smoking, prior antioxidant therapy, hyperlipidaemia, kidney/liver dysfunction, intractable diarrhoea (≥ 6 liquid stools/day), vomiting, gastrointestinal (GI) bleeding Participants randomized: 49; 47 males and 2 females; mean age = 39 years Loss to follow‐up/withdrawal: 0 Exclusions postrandomization: 0 |
|
| Interventions | Intervention: 800 IU vitamin E and 1000 mg vitamin C Control: placebo Duration: daily for 3 months |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | Two participants in the intervention group reported epigastric discomfort | |
| Notes | Number of participants on antiretroviral therapy (ART)
Controlled diet 2 weeks prior to randomization and throughout study period, and dietary counselling. Source of funding: Canadian Foundation for AIDS Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used a random number table to perform randomization. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not adequately describe allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial authors blinded the participants and investigators to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were high and unequal proportions of missing outcomes (3/23 intervention group versus 6/26 control group). |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information; the trial protocol was unavailable. |
| Other bias | Unclear risk | The trial authors did not declare their conflicts of interest, if any. |
Asdamongkol 2013 THA.
| Methods | Country: Bangkok, Thailand Setting: HIV Clinic, Ramathibodi Hospital, Mahidol University Duration of recruitment: May 2011 to April 2012 Duration of trial: 6 months Duration of follow‐up: 6 months Design: randomized placebo‐controlled trial, stratified according to baseline blood zinc levels Follow‐up: monitoring of clinical condition, adverse events, and adherence every 3 months |
|
| Participants | Inclusion criteria: HIV‐positive people aged ≥ 18 years who were on ART for at least 12 months, with complete viral suppression (HIV RNA 40 copies/mL), and having CD4+ cell count < 200 cells/mm³ that increased < 30% from baseline after achieving undetectable HIV RNA after 12 months of ART. Exclusion criteria: pregnancy or women planning to become pregnant, and participants who previously received zinc supplements (duration not stated). Participants screened: 70 Participants eligible for randomization: 31 Participants randomized: 31 (low blood zinc levels (N = 12); normal blood zinc levels (N = 19)) Mean age at randomization: 45 ± 11 years 21 female/10 male 62.3% (144/231) of participants were receiving ART at baseline. The trial authors reported that the distribution of the ART regimens was similar between the 2 groups at baseline. |
|
| Interventions | InterventionS: 15 mg chelated zinc daily Control: placebo daily Duration: 6 months Compliance: participants were asked to return any unused study medication every 3 months and pill counts were conducted. The trial authors did not report compliance rates. |
|
| Outcomes | Primary
Secondary
|
|
| Adverse events | One participant in the zinc group developed an erythematous rash after supplementation for 1 month, which resolved when the participants discontinued taking the zinc supplement. | |
| Notes | Links to other studies (study ID): Asdamongkol 2012 under Asdamongkol 2013 THA Source of funding: research grant of Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand Conflict of interest: None Ethics: Ramathibodi Hospital, Mahidol University Institutional Ethics committee but type of consent not stated Trial registration: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial randomized participants to receive zinc supplements or placebo in a 1:1 ratio in blocks of 4. However, the trial authors did not clearly describe the process of selecting the blocks. |
| Allocation concealment (selection bias) | Unclear risk | Although the trial authors stated that the pharmacy bottled the supplements, they did not provide any information on whether the bottles were prelabelled in a sequential order. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial authors stated that the zinc or placebo pills were indistinguishable in shape, size, and colour. Outcome measures (laboratory assays) were unlikely to have been affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant in the zinc group was withdrawn from the trial during the follow‐up period. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was unavailable. |
| Other bias | Low risk | The trial authors declared that they had no conflicts of interest. |
Baeten 2002 KEN.
| Methods | Country: Kenya Setting: hospital outpatient clinic Duration of recruitment: September 1998 to June 2000 Median duration of follow‐up: 42 days (32 to 445 days) Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: HIV‐1 seropositive women attending Coast Provincial General Hospital outpatient clinics in Mombasa, Kenya Exclusion criteria: age < 18 or > 45; pregnancy, or use of vitamin supplements or oral contraceptive pills Participants randomized: 400; 400 females; median age = 28 years Loss to follow‐up/withdrawal: 46 Exclusions postrandomization: 0 |
|
| Interventions | Intervention: vitamin A (10,000 IU retinyl palmitate) Control: placebo Duration: daily for 6 weeks |
|
| Outcomes | Primary outcomes:
Secondary outcomes
CD4 and CD8 counts |
|
| Adverse events | None reported | |
| Notes | Source of funding: research grants from NIH, University of Washington, and Fogarty International Center; International AIDS Research and Training Program scholarships; Gen‐Probe (reagents) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used computer‐generated block randomization. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe how they performed allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The participants and investigators were blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition in both treatment groups (vitamin A group: 12%; placebo group: 11%). The trial authors stated that those lost to follow‐up had more advanced HIV disease and were more likely to be vitamin A‐deficient. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information regarding selective reporting; the trial protocol was not available. |
| Other bias | Unclear risk | The trial authors did not declare whether or not they had conflicts of interest. |
Bang 2012 DEN.
| Methods | Country: Denmark Setting: outpatient clinic, Department of Infectious Diseases, Hvidovre Hospital Duration of recruitment: July 2008 to September 2009 Duration of trial: 15 months Duration of follow‐up: 16 weeks Design: randomized placebo‐controlled trial with 3 intervention arms Follow‐up: at baseline, a medical history and assessment of total calcium intake was performed for each participant. At baseline and at 16 weeks, bloods were taken to measure immunological parameters, HIV‐viral load, parathyroid hormone, calcium, vitamin D, and biochemical bone markers. The trial assessed quality of life of each participant at baseline and at 16 weeks (Medical Outcomes Study (MOS) 36‐item Short Form Health Survey (SF‐36) questionnaire) |
|
| Participants | Inclusion criteria: HIV‐positive males aged ≥ 18 years who were receiving highly active antiretroviral therapy (HAART) Exclusion criteria: previous bone disease, tuberculosis, sarcoidosis, active malignancy with bone metastasis, elevated serum calcium Participants randomized: 61 Mean age at randomization: 48 ± 9 years No reported baseline differences between treatment groups in terms of time since HIV diagnosis, type of HAART, CD4 and CD8 cell counts, HIV viral load, calcium intake Treatment assignment of 2 participants who used vitamin D supplementation prior to the study not reported |
|
| Interventions | InterventionS: calcitriol and vitamin D: 100,000 IU vitamin D at study entry; tablets containing 1200 mg calcium plus 1200 IU vitamin D and 0.5 µg to 1.0 µg calcitriol daily Vitamin D: 100,000 IU vitamin D at study entry; tablets containing 1200 mg calcium plus 1200 IU vitamin D and placebo daily Control: placebo at trial entry; tablets containing 1200 mg calcium and placebo daily Duration: 16 weeks Compliance: participants were asked to return any unused trial medication after 16 weeks. Compliance rates with the daily study tablets were not reported for each treatment group. According to the trial authors, 77% and 67% of participants achieved a satisfactory compliance (defined by the trial authors as ≥ 80% of the number of tablets dispensed) for the calcitriol/placebo tablet and cholecalciferol/placebo tablet, respectively. |
|
| Outcomes | Primary outcome
Secondary outcome:
|
|
| Adverse events | Hypercalcaemia (calcitriol + vitamin D group (2 events); vitamin D group (1 event), constipation (8 events)) 11 adverse events were only reported as unrelated to the study medication (calcitriol + vitamin D group (6 events); vitamin D group (3 events); control group (2 events)) |
|
| Notes | Source of funding: Pharma‐Vinci, Roche (sponsorship of trial supplements) Conflict of interest: nothing declared Ethics: regional ethics committee and the National Board of Health. Trial registered at clinicaltrials.gov (NCT00990678) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It is unclear how the trial generated randomization codes. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not provide any information regarding allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial authors reported that participants and investigators were blinded throughout the trial. For the outcomes of CD4/CD8 cell counts and viral loads, lack of blinding of outcome assessors was unimportant. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was not similar across treatment groups: (19/20 (95%) for calcitriol and vitamin D group; 17/19 (89%) for vitamin D group, and 15/22 (68%) for control group). The reasons for attrition in each group were unclear. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol from clinicaltrials.gov states the measurement of all outcomes at baseline and at 2, 4, 8, 12, and 16 weeks. However, the trial authors reported data for baseline and 16 weeks only. Although the trial authors reported T lymphocyte subsets (CD4, CD8) as the primary outcome, the trial protocol describes blood vitamin D concentrations as the primary outcome. |
| Other bias | Unclear risk | The trial authors did not include any statement on conflicts of interest. It was unclear whether study sponsors played any role in the study design or reporting of study findings. |
Baum 2010 USA.
| Methods | Country: USA Setting: primary health care clinic, Miami Duration of recruitment:March 2002 to December 2005 Duration of trial: 46 months Duration of follow‐up: 18 months Design: randomized placebo‐controlled trial Follow‐up: a nurse practitioner performed a physical examination, medical history, urine toxicology, and took bloods (CD4 count, HIV viral load, C‐Reactive protein, zinc) from participants at baseline and every 6 months. The trial assessed participants' morbidity during monthly visits to clinic by means of a questionnaire, which was confirmed by information recorded in medical charts. Cause of death was determined by means of authorized contacts, medical records, and death certificates. |
|
| Participants | Inclusion criteria: HIV‐positive people aged ≥ 18 years with low plasma zinc levels (< 0.75 mg/L) and no history of endocrine or psychiatric disorders Exclusion criteria: premenopausal women who were pregnant or had an intention to become pregnant; plasma zinc levels ≤ 0.35 mg/L at any time during the trial Participants screened: 557 Participants eligible for randomization: 246 Participants randomized: 231; 62 female and 169 male Mean age at randomization: 42.7 ± 7 years 62.3% (144/231) of participants were receiving ART at baseline. There were no reported baseline differences in demographic characteristics, clinical disease stage, CD4 cell count, HIV viral load, adherence to ART, drug or alcohol use, cigarette use, or plasma zinc levels |
|
| Interventions | Intervention: 12 mg of elemental zinc for women; 15 mg for men daily Control: placebo daily Duration: 18 months Compliance: assessed monthly with questionnaires and pill counts. 3.65 ± 0.31 pills returned out of a possible 4 pills per month After completion of the trial it is reported that participants who received zinc had higher blood zinc concentrations over time compared to those who received placebo, after controlling for C‐Reactive protein concentrations (data not provided). |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Adverse events | None | |
| Notes | Source of funding: National Institute on Drug Abuse Conflict of interest: none Ethics: Florida International University Institutional Review Board Trial registered at clinicaltrials.gov (NCT00149552) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was unclear how the trial generated randomization codes. |
| Allocation concealment (selection bias) | Low risk | A pharmacist bottled and precoded the trial supplements for each participant for the entire trial period according to the randomization code. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial authors reported that the clinical and study personnel and participants were blinded. Only the pharmacist and statistician were aware of treatment assignments during the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was low attrition. However, the uneven numbers, deaths, plasma zinc, and dropouts may be related to main outcome. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes as reported are consistent with the trial protocol from www.clinicaltrials.gov. |
| Other bias | Low risk | The trial authors declared that they had no conflicts of interest. An independent company that was not involved in the design or implementation of the study, or the analysis and reporting of the findings. manufactured the trial supplements. |
Baum 2013 BWA.
| Methods | Country: Botswana Setting:Princess Marina Hospital, Gaborone, Botswana Duration of recruitment: Duration of trial: 4 years and 7 months (December 2004 to July 2009) Duration of follow‐up: 24 months Design: randomized placebo‐controlled factorial trial with 3 intervention arms Follow‐up: a monthly questionnaire was administered about acceptability of the supplement, adherence, adverse effects, and intercurrent morbidity (health events occurring between the trial visits; confirmed by documentation in medical record) Every 3 months: physical examination and medical history performed by a nurse or physician, blood sample taken for CD4 cell count Every 6 months: HIV viral load, plasma micronutrient levels (20% subsample), and blood chemistries |
|
| Participants | Inclusion criteria: HIV‐positive participants aged 18 years and older, ART‐naive and CD4 cell count > 350 /µL Exclusion criteria: pregnancy Participants screened: 1003 Participants eligible for randomization: 922 Participants randomized: 878 Median age at randomization: 31 to 33 years The trial authors reported no statistically differences in baseline CD4 cell count, HIV viral load, Body Mass Index (BMI), haemoglobin, albumin, total cholesterol, and HDL‐cholesterol levels. |
|
| Interventions | Intervention (multivitamins group): thiamin 20 mg, riboflavin 20 mg, vitamin B6 25 mg, niacin 100 mg, vitamin B12 50 µg, folic acid 0.8 mg, vitamin C 500 mg, vitamin E 30 mg Intervention (selenium group): 200 µg daily Intervention (multivitamins plus selenium group): as above Control: placebo daily Administered as 1 pill daily. Pills were indistinguishable in shape, size, and colour. Duration: 24 months Compliance: pill counts at each follow‐up visit, Adherence reported as 96% (no standard deviation (SD) stated) The trial measured plasma micronutrient levels (subsample of participants) but the trial authors did not report this information All participants received isoniazid (INH) prophylaxis |
|
| Outcomes | Primary outcome
Secondary outcomes:
|
|
| Adverse events | The trial authors reported a total of 79 adverse events which they judged as having a remote relationship with the trial intervention. Acute diarrhoea/vomiting: 5 events (3 in multivitamins plus selenium group; 2 placebo group) Severely elevated alanine transaminase (ALT) (> 5 times normal range): 3 events (1 in multivitamins group; 2 in placebo group) |
|
| Notes | Links to other trials (trial ID): Sales 2010 Source of funding: National Institute on Drug Abuse Conflict of interest: none Ethics: Florida International University Institutional Review Board, Harvard School of Public Health IRB, Botswana Health Research Unit of the National Ministry of Health Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician generated block randomization in blocks of 20. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned into one of the trial groups using the next sequential number from the randomization list generated by the data centre. The pharmacist prelabelled the pills for the entire trial with the identification number according to the assignment list. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pills were indistinguishable in shape, size, and colour; also the trial authors stated that trial personnel and participants were blinded. Outcome measures (laboratory assays) were unlikely to be affected by detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The trial authors reported the number of participants who withdrew from the trial due to pregnancy; however, other reasons for lost‐to follow‐up were not stated. There were similar levels of attrition across treatment groups (selenium group: 17% (38/220); multivitamins plus selenium group: 19% (42/220); placebo group: 15% (21/219)) For the measures of viral load, the trial authors performed multiple imputation, but did not provide any details in terms of the proportion of data that was missing. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was unavailable. |
| Other bias | Low risk | The trial authors declared no conflicts of interest. The trial was funded by a non‐conflicting funding source. |
Burbano 2002 USA.
| Methods | Country: USA Setting: community‐based clinic Duration of recruitment: 1998 to 2000 Duration of follow‐up: 12 months Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: confirmed HIV, past or present use of illegal drugs, ≥ 18 years, adequate selenium status (> 85 µg/L) Exclusion criteria: selenium deficient (< 85 µg/L) Participants randomized: 259 112 female Median age = 40 years (range 24 to 54) Loss to follow‐up/withdrawal: 73 at 12 months Exclusions postrandomization: 0 |
|
| Interventions | 200 microgram selenium or placebo daily for 12 months. | |
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | None reported | |
| Notes | Number of participants on ART:
Selenium group: 64 (76%)
Control group: 60 (53%) Number, type, and duration of hospital admissions recorded 2 years prior and during study period. Medical records reviewed by team of physicians. Source of funding: research grant and commercial (materials) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe how they performed sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe how they performed allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusions from the analysis (73/259 (28%)) not reported by treatment group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information; the trial protocol was unavailable |
| Other bias | Unclear risk | The trial authors did not declare on any conflicts of interest, if any. |
Coodley 1993 USA.
| Methods | Country: USA Setting: hospital outpatient clinics Duration of recruitment: not stated Duration of follow‐up: 8 weeks Design: randomized cross‐over trial; no washout period |
|
| Participants | Inclusion criteria: HIV‐seropositive Exclusion criteria: on other forms of vitamin A supplementation; significant hepatic or renal dysfunction; active opportunistic infection or fever Participants randomized: 21 20 male and 1 female Median age: not stated Loss to follow‐up/withdrawal: 4 Exclusions postrandomization: 0 |
|
| Interventions | Intervention: 60 mg beta‐carotene Control: placebo Duration: 3 times daily for 4 weeks |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | No toxicity; skin discolouration in treatment group | |
| Notes | CD4 count data reported as means and ranges 16 participants received ART. Source of funding: Hoffman La Roche Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition at 1 month; the trial authors provided reasons. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information; the trial protocol was unavailable. |
| Other bias | Unclear risk | The trial authors did not declare on conflict of interest, if any. |
Coodley 1996 USA.
| Methods | Country: USA Setting: hospital outpatient clinic and private practice Duration of recruitment: not stated Duration of follow‐up: 3 months Design: randomized controlled trial (RCT) |
|
| Participants | Inclusion criteria: HIV‐seropositive; > 21 years Exclusion criteria: other forms of vitamin A supplementation 30 days prior to study; ART 60 days prior to study; significant hepatic or renal dysfunction; CD4 < 50 or > 600 Participants randomized: 72 63 male and 9 female Median age: not stated Loss to follow‐up/withdrawal: 4 at 1 month; 22 at 3 months Exclusions postrandomization: 0 |
|
| Interventions | Intervention: 60 mg beta‐carotene + multivitamins Control: placebo + multivitamins Duration: 3 times daily for 3 months |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | None reported | |
| Notes | Number of participants on ART: treatment group: 10 (28%); control group: 17 (47%) Source of funding: research grant and commercial (materials) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition at 3 months (22/72 participants were lost to follow‐up); the trial authors did not provide reasons. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information; the trial protocol was unavailable. |
| Other bias | Unclear risk | The trial authors did not declare on conflict of interest, if any. |
Cárcamo 2006 PER.
| Methods | Country: Peru Setting: tertiary hospitals Duration of recruitment: June 1998 to Jan 2000 Duration of follow‐up: 2 weeks Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: HIV‐seropositive, persistent diarrhoea (≥ 7 days) without prior treatment Exclusion criteria: none stated Participants randomized: 159 49 female and 110 male Median age = 30 years (range 19 to 57) in zinc group Median age = 31 years (range 19 to 64) in placebo group Loss to follow‐up/withdrawal: 51 Exclusions postrandomization: 0 |
|
| Interventions | Intervention: zinc sulphate (100 mg) Control: placebo Duration: daily for 14 days |
|
| Outcomes | Primary outcomes
Secondary outcomes:
|
|
| Adverse events | Gastrointestinal symptoms attributable to the medication (nausea, vomiting, abdominal pain) similar in both treatment groups | |
| Notes | Sulfamethoxazole‐trimethoprim prescribed for participants with enteric bacterial pathogens (23 in zinc group and 12 in placebo) Source of funding: Fogarty IARTP grant; University of Washington Center for AIDS Research; Centers for Disease Control and Prevention (CDC) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used computer‐generated blocked randomization. |
| Allocation concealment (selection bias) | Low risk | The trial authors stated that the treatment allocators were unable to access the assignment roll. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were high losses to follow‐up in both groups (34.6% intervention group versus 29.5% control group). |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information; the trial protocol was unavailable. |
| Other bias | Unclear risk | The trial authors did not declare on conflict of interest, if any. |
Dougherty 2015 USA.
| Methods | Country: USA Setting: 2 outpatient clinics, Philadelphia Design: RCT (safety trial) Duration of recruitment: January 2010 to January 2011 Duration of follow‐up: 12 weeks Follow‐up: participants were followed up at 6 and 12 weeks. Blood and urine measurements were also performed for vitamin D (25‐hydroxy vitamin D, 1,25‐dihydroxy vitamin D), calcium, metabolic parameters, and immunological parameters. |
|
| Participants | Inclusion criteria: participants with perinatally acquired HIV (PHIV) and behaviorally‐acquired HIV (BHIV) Exclusion criteria: participation in another study impacting 25(OH) vitamin D, pregnant or lactating females, and other conditions affecting growth, dietary intake, or nutritional status. People who were taking supplements that contained vitamin D were not eligible. Those willing to discontinue supplementation with approval of their medical provider were eligible after a 2‐month washout period. Participants screened: 240 Participants eligible for randomization: 146 Randomization was stratified by HIV acquisition (PHIV/BHIV) and season of the year Participants randomized: 44 Mean age at randomization: 18.4 ± 4.7 years (4000 IU vitamin D group) and 19.1 ± 5.0 yrs (7000 IU vitamin D group) 30 male and 14 female Clinical characteristics, growth status, ART regimen similar at baseline |
|
| Interventions | Intervention: 7000 IU vitamin D group One gelatin capsule containing one 2000 IU capsule and one 5000 IU softgel (over‐encapsulated) daily Control: 4000 IU vitamin D group One gelatin capsule containing two 2000 IU capsules (over‐encapsulated) daily Duration: 12 weeks Compliance: residual tablets or volumes recorded at the 12‐week visit. Adherence also assessed by questionnaire at 6,12 weeks and telephonically at weeks 1, 3, 5, 8, and 10. The trial authors did not report the mean adherence during the trial period. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | No evidence of any adverse biochemical, haematological, immunological, or virological event | |
| Notes | Source of funding: National Institutes of Health Conflict of interest: none Ethics: Children's Hospital of Philadelphia IRB Trial registration: NCT01092338 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was unclear how the randomization codes were generated. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not provide any information regarding the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not specified except for participants who received a single capsule which was identical in size, shape, and colour. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was due to migration (n = 1) and loss to follow‐up (n = 1). |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was not available. |
| Other bias | Low risk | The trial authors declared no conflicts of interest. A non‐conflicting funding source funded the trial. |
Giacomet 2013 ITA.
| Methods | Country: Italy Setting: outpatient clinic, Milan Duration of recruitment: April 2011‐ Duration of trial: 15 months Duration of follow‐up: 12 months Design: randomized placebo‐controlled trial Follow‐up: blood samples were taken from each participant (vitamin D, immunological parameters) at baseline and at 3, 6, 9, 12 months |
|
| Participants | Inclusion criteria: HIV‐positive people aged ≤ 30 years, low blood vitamin D concentrations (25(OH)D < 30 ng/mL) Exclusion criteria: participants of African descent, hyperparathyroidism, vitamin D supplementation during the 12 month period prior to study entry, use of any medication known to alter vitamin D (excluding ARV) in the previous 6 months, concomitant severe illness Participants screened: 90 Participants eligible for randomization: 57 Participants randomized: 52 Median age at randomization: vitamin D group: 20 (interquartile range (IQR) 18 to 23) yrs; placebo group: 18 (15 to 23) years 86% (43/50) of participants on HAART No reported baseline differences in clinical disease stage, type of antiretroviral therapy (ART), CD4 cell count, blood vitamin D, calcium, Parathyroid hormone (PTH) concentrations. Undetectable viral load (< 37 copies/mL) was recorded for 72% (18/25) and 84% (21/25) of participants in the vitamin D and placebo group, respectively at baseline. |
|
| Interventions | Intervention: single dose of 100,000 IU vitamin D (oral dose in oil suspension) at baseline and at 3, 6, and 9 months Control: single dose of placebo (oral dose in oil suspension) at baseline and at 3, 6, and 9 months Compliance: directly observed |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | Reported no adverse events during the trial period | |
| Notes | Source of funding: Italian Ministry of Health Conflict of interest: none Ethics: Luigi Sacco Hospital Ethical Committee Trial registered at clinicaltrialsregister.eu (2011‐00059354) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used block randomization in blocks of 4. |
| Allocation concealment (selection bias) | Unclear risk | The trial used matching sealed plastic syringes labelled with unique identification numbers, but it is unclear if these numbers were sequentially labelled or not. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All the trial participants, outcome assessors (laboratory technicians and immunologists) and personnel, except the paediatrician who administered the treatment, were blinded to it. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors did not provide any reasons for lost to follow‐up of 2 participants in the placebo group at 3 and 6 months. The trial authors performed ITT analyses. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes as reported are consistent with the trial protocol from www.clinicaltrialsregister.eu |
| Other bias | Low risk | The trial authors declared that they had no conflicts of interest. |
Green 2005 SGP.
| Methods | Country: Singapore Setting: outpatient clinic at national HIV referral centre, Tan Tock Seng Hospital Duration of trial: January 2003 to July 2003 (7 months) Duration of follow‐up: 28 days Design: randomized placebo‐controlled trial Follow‐up: medical history, physical examination, adverse events at 14 and 28 days. Bloods taken in fasted state at baseline and after 28 days for immunological parameters and zinc levels |
|
| Participants | Inclusion criteria: HIV‐positive participants > 18 years with CD4 count < 200 cells/mm³, no opportunistic infections 6 months prior and stable ART for 3 months prior to study entry Exclusion criteria: pregnancy, intravenous drug users, on immunomodulatory therapy, oral zinc supplementation Participants screened: 420 Participants eligible for randomization:189 Participants randomized: 66 Mean age at randomization: 40 ± 7.8 yrs (zinc group) versus 40 ± 8.3 yrs (placebo group) 61 male and 5 female 77% of participants were on HAART. Demographic, clinical characteristics, and antiretroviral drug regimes were similar in both treatment groups at baseline. |
|
| Interventions | Intervention: 220 mg zinc sulphate (50 mg elemental zinc) administered as a capsule daily along with ART Control: placebo Supplement and placebo capsules were identical in appearance Duration: 28 days Compliance: adherence was reported as the proportion of participants in each treatment group who took ≥ 90% of scheduled doses (zinc group: 93.5% (30/32); control group: 94% (32/34)). |
|
| Outcomes | Primary outcome: immune response to tuberculosis Secondary outcomes: CD4, CD8 cell counts, naive T cells, blood zinc levels |
|
| Adverse events | Nausea or vomiting (3 participants in both groups), diarrhoea (4 participants in intervention group versus 1 participant in control group) One participant from the zinc group developed Indinavir‐related renal colic on day 7. One participant from the placebo group developed fever on day 28 and was diagnosed with M. fortuitum infection. One participant from the placebo group developed a Staphylococcus aureus soft tissue abscess on day 23. |
|
| Notes | Conflict of interest: none Source of funding: National Health Group Singapore Ethics:Tan Tock Seng Hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a computer‐generated randomization sequence in blocks of 6. |
| Allocation concealment (selection bias) | Unclear risk | It was unclear how the trial organized the process of treatment allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was not specified, except for participants who received identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial withdrew one participant due to an adverse event. This was unlikely to have influenced the results. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was not available. |
| Other bias | Low risk | The trial authors included a statement regarding no conflicts of interest. |
Grigoletti 2013 BRA.
| Methods | Country: Brazil Setting: outpatient clinic, Hospital de Clínicas de Porto Alegre, Porto Alegre Duration of recruitment: August 2009 to September 2011 Duration of trial: 25 months Duration of follow‐up: 4 weeks Design: randomized placebo‐controlled trial Follow‐up: blood samples and vascular measurements taken at baseline and again at 4 weeks |
|
| Participants | Inclusion criteria: HIV‐positive adults on HAART (at least 6 months) with undetectable HIV viral load (<50 copies/mL) and CD4 count > 200 cells/mm³ Exclusion criteria: diabetes mellitus, active infection, liver or renal disease, history of cardiovascular disease, uncontrolled hypertension, pregnancy, use of illicit drugs, mental illness, current tobacco use, women on hormone replacement therapy and current intake of dietary supplements (such folic acid, antioxidants) Participants screened: 1332 Participants eligible for randomization: 175 Participants randomized: 30 participants stratified according to sex Mean age at randomization: 45 ± 2 years 14 male and 16 female All participants were on HAART. Clinical characteristics and antiretroviral drug regimes were similar at baseline. |
|
| Interventions | Intervention: 1 capsule containing 5 mg folinic acid daily (in the morning) Control: 1 placebo capsule, indistinguishable in appearance, daily (in the morning). Duration: 4 weeks Compliance: no details provided, but an increase in blood folic acid concentrations was demonstrated in the intervention group. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Adverse events | No adverse events were reported | |
| Notes | Conflict of interest: none Source of funding: research grants and scholarship (Hospital de Clınicas de Porto Alegre Fund for Research (FIPE‐ HCPA), Coordination for the Development of Higher Education (CAPES), Brazil) Ethics: Hospital de Clínicas de Porto Alegre Ethics review board |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used computer‐generated randomization in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | A person not affiliated to the trial precoded and sequentially numbered bottles. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial authors reported blinding of participants and investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up occurred. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information, and the trial protocol was not available |
| Other bias | Low risk | The trial authors declared that they had no conflicts of interest |
Guwatudde 2015 UG.
| Methods | Country: Uganda Setting: outpatient clinic, Mackerere University College, Kampala Design: placebo‐controlled trial Duration of recruitment: April 2010 to June 2012 Duration of follow‐up: 18 months Follow‐up: at baseline and at 3, 6, 12, and 18 months participants underwent a full clinical examination. In addition, assessments of the following was performed: complete blood counts, CD4 cell count, presence of syphilis, malaria, intestinal parasites, and nutritional status. |
|
| Participants | Inclusion criteria: HIV‐positive adults ≥ 18 yrs, initiation of ART at the time of randomization or on HAART for at least 6 months, intention to stay within 20 km of study site Exclusion criteria:Pregnancy, participants who were very ill Participants screened: 1134 Participants eligible for randomization: 421 Participants randomized: 400 Mean age at randomization: 36.9 ± 9.6 years (intervention group) 34.7 ± 8.1 years (control group) 123 male and 277 female Clinical characteristics, duration of ART, and multivitamin use similar at baseline. |
|
| Interventions | Intervention (multivitamin group): daily tablet providing the following vitamins at the RDA level 1.4 mg vitamin B1, 1.4 mg vitamin B2, 1.9 mg vitamin B6, 2.6 µg vitamin B12, 18 mg niacin, 0.4 mg folic acid, 70 mg vitamin C, 10 mg vitamin E Control: daily tablet consisting of placebo Duration: 18 months Compliance: assessed by research staff every month by recording participant self reported compliance and conducting pill counts. The trial authors did not report mean compliance. |
|
| Outcomes | Primary: changes in CD4 cell count and weight, quality of life Secondary: changes in haemoglobin, blood ALT concentrations, development of a new or recurrent disease progression event including all‐cause mortality, hospitalization events, changes in ART from first‐ to second‐line therapy |
|
| Adverse events | A total of 550 adverse events were reported mostly related to nausea and vomiting, with no differences between treatment arms. The trial authors also reported 1 event of severe anaemia (Hb < 7 g/dL) in the multivitamin group and 3 events of high ALT concentrations (> 200 IU/L) (1 in multivitamin arm, 2 in placebo arm). |
|
| Notes | Conflict of interest: none Source of funding: Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health research grant Ethics: Scientific Review Committee of the Infectious Diseases Institute at Makerere University College of Health Sciences and Institutional Review Boards of Harvard School of Public Health and Makerere University School of Public Health Trial registered at clinicaltrials.gov (NCT 1228578) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Prior to initiation of the trial, a staff member at Harvard School of Public Health (HSPH) who was not associated with trial implementation generated serial numbers from 1 to 400 and randomly assigned participants to intervention or placebo groups, in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Trial regimen bottles were labelled with serial numbers only. The trial pharmacist at the trial site dispensed the assigned regimen bottles to participants in sequential order of enrolment. The list that showed the trial arm linked to each serial number remained with the trial statistician at HSPH and was not accessible to trial staff in Uganda. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Trial participants, staff, and investigators were blinded. The size, colouring, and packaging of the placebo was identical to the multivitamin tablet. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was low (8%) and was due to death (18/400), migration (7/400), withdrawal of consent (2/400), and loss to follow‐up (6/400). |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes were as reported, and were consistent with the trial protocol (Guwatudde 2015 UG). |
| Other bias | Low risk | The trial authors declared they had no conflicts of interest. A non‐conflicting funding source funded the trial. |
Humphrey 1999 USA.
| Methods | Country: USA Setting: HIV clinic Duration of recruitment: January to July 1996 Duration of follow‐up: 8 weeks Design: randomized placebo‐controlled safety trial |
|
| Participants | Inclusion criteria: 18 to 45 years, CD4 > 200 cells/mm³ Exclusion criteria: pregnant or breastfeeding Participants randomized: 40 women Mean age (SD) in years = 36.2 (5.6) in vitamin A group and 33.2 (5.6) in placebo group Loss to follow‐up/withdrawal: 1 Exclusions postrandomization: 0 |
|
| Interventions | Intervention: 300,000 IU vitamin A Control: placebo Duration: single dose |
|
| Outcomes | Primary outcomes
Secondary outcomes:
|
|
| Adverse events | Signs or symptoms of toxicity (headache, nausea, vomiting, diarrhoea, fever) similar in the intervention and control groups at 24 hours and 1 week after administration. | |
| Notes | Number of participants on ART
Source of funding: Paediatric AIDS Foundation grant |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation used. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information; the trial protocol was not available. |
| Other bias | Unclear risk | The trial authors did not declare on conflicts of interest, if any |
Hurwitz 2007 USA.
| Methods | Country: USA Setting: university clinic Duration of recruitment: June 2001 to July 2005 Duration of follow‐up: 9 months Design: randomized placebo controlled trial |
|
| Participants | Inclusion criteria: aged 18 to 55 years; no history of major systemic disorders related to HIV; premenopausal and not pregnant Exclusion criteria: on treatment for chronic conditions; selenium deficient Participants randomized: 310 Mean age = 40.5 years 179 male and 86 female Loss to follow‐up/withdrawal: 88 Exclusions postrandomization: 48 pretreatment |
|
| Interventions | Intervention: selenium (200 µg) Control: placebo Duration: daily for 9 months |
|
| Outcomes | Primary outcomes
|
|
| Adverse events | None | |
| Notes | Participants on ART: 105/141 (74%) in selenium group; 87/121 (72%) in placebo group Preliminary analysis at 9 months of an 18‐month trial Source of funding: National Institutes of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used computerized block randomization. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment used. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were high unexplained losses to follow‐up, which were balanced between groups. The trial authors performed imputational analyses. |
| Selective reporting (reporting bias) | High risk | The trial only reported data on main study outcomes for subgroups of participants (selenium responding versus non‐responding participants). |
| Other bias | Low risk | The trial authors declared they had no conflicts of interest. |
Isanaka 2012 TZA.
| Methods | Country: Tanzania Setting: outpatient HAART clinics Duration of recruitment: November 2006 to November 2008 Median duration of follow‐up: 15 months (IQR 6 to 19) Design: RCT Follow‐up: full clinical examination and HIV disease staging (WHO staging criteria) of participants conducted at baseline and thereafter monthly by trial physicians. Occurrence of any illness in the previous month recorded by trial nurses at baseline and thereafter monthly. They also performed anthropometric measurements (height, weight, waist, hip, mid‐thigh, mid‐arm circumference) at baseline and monthly thereafter. Bloods were taken from participants at baseline and every 4 months for T cell counts, full blood count, and ALT. HIV viral load was determined at the same time intervals, but was subject to the availability of reagents. Dietary intake was assessed at baseline and every 12 months. Participants were followed up monthly until the date of death, loss to follow‐up, or early study closure (refer to section on adverse events). Cause of death was determined by the use of medical records and standard verbal autopsy techniques by 2 HIV clinicians who had to reach consensus. |
|
| Participants | Inclusion criteria: men and women with HIV‐infection initiating ART (WHO Stage 4 HIV disease and CD4 count < 200; WHO Stage HIV infection and CD4 count < 350), intention to stay in Dar es Salaam for at least 2 years Exclusion criteria: pregnant or lactating women Participants randomized: 3418 Mean age at randomization: 37.8 ± 8.6 years (high‐dose group) versus 38.4 ± 8.6 years (standard‐dose group) 1141 female, 569 male (high‐dose group) versus 1181 female, 527 male (standard‐dose group) |
|
| Interventions | Intervention (high‐dose group): micronutrient tablet daily containing 8 different micronutrients at multiple levels of the Recommended Daily Allowance (RDA): thiamine 20 mg, riboflavin 20 mg, vitamin B6 25 mg, niacin 100 mg, vitamin B12 50 µg, folic acid 0.8 mg, vitamin C 500 mg, vitamin E 30 mg Control (standard‐dose group): micronutrient tablet daily containing 8 different micronutrients at single level of the RDA: thiamin 1.2 mg, riboflavin 1.2 mg, vitamin B6 1.3 mg, niacin 15 mg, vitamin B12 2.4 µg, folic acid 0.4 mg, vitamin C 80 mg, vitamin E 15 mg The intervention and control tablets were indistinguishable in terms of appearance and taste. Duration: minimum of 24 months Compliance: determined by the number of tablets absent from the returned bottles every month divided by the number of the tablets the participant should have taken. Mean compliance was reported as 90% for both treatment groups (no variance reported). Co‐intervention: cotrimoxazole prophylaxis if CD4 count < 200 in all participants |
|
| Outcomes | Primary: all‐cause mortality, HIV disease progression (new or recurrent episode of HIV disease according to the WHO Clinical Staging system) Secondary: AIDS‐related mortality (due to Pjiroveci pneumonia, pulmonary tuberculosis, extrapulmonary tuberculosis, Kaposi sarcoma, wasting, HIV/AIDS with opportunistic infection, invasive cervical carcinoma), changes in CD4 count, HIV viral load, BMI, haemoglobin |
|
| Adverse events | Preliminary data analysis after 1 year after the start of the trial (November 2008) indicated an increased mortality risk with high‐dose supplementation. Subsequently, all participants received standard dose supplements up to March 2008 when it was determined that the increased mortality risk was restricted to severely malnourished participants (BMI < 16). These participants were subsequently excluded from enrolment and those who had been enrolled received standard dose supplementation. Since 612 participants enrolled in this period did not fulfil the eligibility criteria of the trial protocol, the sample size was increased. However, the trial was terminated prematurely in March 2009 because of evidence of increased ALT levels among participants receiving the high‐dose supplement. Other adverse events reported include during the trial included fatigue, nausea or vomiting, diarrhoea, severe anaemia, peripheral neuropathy, rashes or lesions and genital discharge or sores. |
|
| Notes | Source of funding: National Institute of Child Health Conflict of interest: none Ethics: Harvard School of Public Health, Muhimbili University of Health and Allied Sciences, Tanzania Food and Drugs Authority, and National Institute of Medical Research Trial registered at clinicaltrials.gov (NCT00383669) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a computer‐generated randomization sequence in blocks of 20. |
| Allocation concealment (selection bias) | Low risk | Independent pharmacists performed sequential numbering of trial supplements. At each research site participants were allocated to the next numbered bottle at that site. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The trial authors reported the participants and research staff as blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall loss to follow‐up was not reported. The trial authors only analysed viral load for a subset (7%) of trial participants and therefore this outcome was judged to be at high risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | The primary and secondary outcomes as reported, as well as the reported changes to the trial protocol, are consistent with the trial protocol at www.clinicaltrials.gov. |
| Other bias | High risk | Trial was stopped early due to evidence of increased levels of ALT in the high‐dose group. |
Jiamton 2003 THA.
| Methods | Country: Thailand Setting: outpatient clinic Duration of recruitment: March 2000 to January 2001 Duration of follow‐up; 48 weeks Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: older than 18 years; 50 < CD4 < 550 Exclusion criteria: taking ARV or micronutrients for during month prior to enrolment Participants randomized: 481 (stratified according to CD4 cell count < 200 cells/mm³ and ≥ 200 cells/mm³) 189 male and 292 female Mean age = 32 years Loss to follow‐up/withdrawal: 79 at 48 weeks Exclusions postrandomization: 0 |
|
| Interventions | Intervention: micronutrient supplement (3000 µg vitamin A, 6 mg beta‐carotene, 20 µg vitamin D, 80 mg vitamin E, 180 µg vitamin K, 400 mg vitamin C, 24 mg vitamin B1, 15 mg vitamin B2, 40 mg vitamin B6, 30 µg vitamin B12, 0.1 mg folic acid, 40 mg pantothenic acid, 10 mg iron, 200 mg magnesium, 8 mg manganese, 30 mg zinc, 300 µg iodine, 3 mg copper, 400 µg selenium, 150 µg chromium, 60 mg cysteine) Control: placebo Duration: twice daily for 48 weeks. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | A total of 137 minor adverse events such as dizziness, drowsiness, nausea, and rash reported, with more participants in the intervention arm who reported urine discolouration (P < 0.001) | |
| Notes | Source of funding: Nestle Foundation; Vitabiotics | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used centralized randomization in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Interventions were packaged in identical coded bottles. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition was low overall (12/242 in the treatment group and 3/239 in the placebo group). Similar baseline median CD4 cell counts among participants from both treatment groups who were lost to follow‐up. The trial authors used survival analysis to address missing outcome data. However, the trial authors only analysed viral load for a subset (29%) of trial participants and so we judged this trials to be at high risk of attrition bias for this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information; the trial protocol was not available. |
| Other bias | Low risk | The trial authors provide a declaration on no conflicts of interest. |
Kamwesiga 2015 RWA.
| Methods | Country: Rwanda Setting: 2 outpatient facilities Design: placebo‐controlled trial Duration of recruitment: not stated Duration of follow‐up: 24 months Follow‐up: trained nursing practitioners will collect clinical, nutritional, and psychosocial data at baseline and at 6, 12, 18, and 24 months. Blood samples will also be taken for CD4 cell count and viral load measurements at these time intervals. |
|
| Participants | Inclusion criteria: HIV‐positive adults aged ≥ 21 years, ART‐naive with CD4 cell counts 400 to 650 cells/mm³, HIV‐positive women willing to practice barrier method of birth control, intention to remain in clinic catchment area for study period Exclusion criteria: pregnancy Participants screened: 2680 Participants eligible for randomization: 300 Participants randomized: 300 Mean age at randomization: 33 years (median) IQR 28 to 39 years (intervention group); 35 years (median) IQR 28 to 41 years (control group) 98 male and 202 female Sociodemographic characteristics, CD4 cell count, HIV viral load, BMI similar at baseline. |
|
| Interventions | Intervention: 1 tablet containing 200 µg selenium (in the form of selenomethionine) daily Control: 1 placebo tablet daily Duration: 24 months Compliance: adherence counselling provided at baseline and every month. The trial authors did not report mean compliance data. |
|
| Outcomes | Primary: composite of the following: reduction in CD4 cell count < 350 cells/mm³ or start of ART or development of AIDS‐defining illness Secondary: viral load at 6, 12, 18, and 24 months, quality of life, weight gain, presence of opportunistic infections, mortality |
|
| Adverse events | Most participants did not report any symptoms. Self‐reported symptoms included nausea, vomiting, skin or hair changes or both, and changes in emotional status. Participants in the selenium group were more likely to report anxiety (41 events versus 16 events in the placebo group; P = 0.04) and sleep symptoms (36 events versus 15 events in the placebo group, P = 0.01). | |
| Notes | Source of funding: Global Benefit, Canada. Micronutrient supplement supplied by a nutraceutical company (CanAlt labs and Seroyal) Conflict of interest: none Ethics: institutional review boards of the Canadian College of Naturopathic Medicine and Wilfred Laurier University in Canada, and the National Ethics Committee (NEC) in Rwanda. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The research department of the manufacturing company prepared the randomization schedule using a simple randomization block design, but the trial authors did not specify the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors stated that each participant was assigned to a unique study identification number. Sequential numbering and allocation was not explicitly stated. They also stated that an unblinded allocation list was provided to the treatment provider and an independent statistician for the purpose of conducting an interim analyses. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial authors did not specify whether blinding was performed, except for participants who received identical supplements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was 6% in both treatment groups (9/151 in the selenium group and 9/149 in the placebo group). Reasons for attrition included death (n = 2), migration (n = 9) and loss to follow‐up (n = 7). The trial authors stated that 10 participants (4 in the selenium group and 6 in the placebo group) were not included in their primary analyses. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes are consistent with the trial protocol (Kamwesiga 2015 RWA). |
| Other bias | Low risk | Although the pharmaceutical industry provided the trial supplements, the trial authors included a statement of no conflicts of interest. |
Kelly 1999 ZMB.
| Methods | Country: Zambia Setting: home care service of tertiary hospital Duration of recruitment: not stated Duration of follow‐up: 4 weeks Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: adults with persistent diarrhoea for more than 1month Exclusion criteria: < 18 years, pregnancy, administration of antibiotics in the week prior to recruitment, Karnofsky scores > 80 or < 50. Participants randomized: 135 79 male and 56 female Median age = 32.5 years (micronutrient); 34 (placebo) Loss to follow‐up/withdrawal: 29 Exclusions postrandomization: 0 |
|
| Interventions | Intervention: micronutrient supplement (10,500 IU vitamin A, 300 mg vitamin C, 300 mg vitamin E, 150 µg selenium and 200 mg zinc sulphate)
Both treatment groups also received 5 mg folic acid and 800 mg albendazole twice daily. Control: placebo Duration: daily for 2 weeks |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | None | |
| Notes | Source of funding: Smithkline Beecham | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation used. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Micronutrient and placebo capsules were not identical; it was unclear whether providers and assessors were blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 25% of participants were lost to follow‐up due to death and the tradition of going back to the family home when terminally ill. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information; the trial protocol was not available |
| Other bias | Unclear risk | The trial authors did not provide a declaration on conflicts of interest. |
Kelly 2008 ZMB.
| Methods | Country: Zambia Setting: Misisi township, Lusaka, Zambia Duration of trial: August 2003 to December 2006 (41 months) Duration of follow‐up: 38 months Design: cluster randomized cross‐over trial (cluster randomization by household) Follow‐up: trained nurses recorded episodes of diarrhoea and cough every 2 weeks. Nutritional status (height, weight, MUAC, body impedance) and household hygiene (score) were assessed at 6, 14, 22, and 38 months |
|
| Participants | Inclusion criteria: all adult (≥ 18 years) residents HIV status: HIV‐positive (n = 136); HIV negative (n = 224), and unknown HIV‐status (n = 140) |
|
| Interventions | Members of the same household received the same treatment allocation Intervention: multiple micronutrient tablet once daily (β‐carotene 4.8 mg; 1.4 mg vitamin B1, 1.4 mg vitamin B2, 1.9 mg vitamin B6, 2.6 µg vitamin B12; 18 mg niacin; 70 mg vitamin C; 10 mg vitamin E; 0.4 mg folic acid; 30 mg iron; 15 mg zinc; 2 mg copper; 65 µg selenium and 150 µg iodine) Control: placebo tablet once daily (supplement and placebo tablets identical in appearance and taste Duration: 1.9 years to trial cross‐over; thereafter 1.5 years Adherence: unused trial supplements were retrieved monthly. Median compliance reported as > 95% (variance not reported) at the crossover point. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Adverse events | Four cases of pellagra occurred in the placebo group; 3 of whom were associated with high ethanol intakes | |
| Notes | Source of funding: Wellcome trust Ethics: University of Zambia Research Ethics Committee; London School of Hygiene and Tropical Medicine Research Ethics Committee Trial registration: ISRCTN 31173864 Conflict of interest: declared no conflict of interest. None of the authors had any financial interest in manufacturing or licensing of any micronutrient formulation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial statistician generated the random number sequence for the selection of households. |
| Allocation concealment (selection bias) | Low risk | Only the statistician and manufacturers had access to the trial code. The supplements were supplied in precoded, sealed plastic bottles. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, investigators, and assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were high rates of attrition (30% before cross‐over; 42% at trial completion); the trial authors did not state the attrition rates for each treatment group. |
| Selective reporting (reporting bias) | Low risk | All the outcomes as reported are consistent with the trial protocol from www.controlled‐trials.com, except for all‐cause mortality. |
| Other bias | Low risk | The trial authors declared that they had no conflicts of interest. Recruitment bias: households were randomized, all participating members of each household received the same treatment allocation. Baseline imbalance: randomization was stratified by household size Loss of clusters: attrition of clusters was not reported. |
Lawson 2010 NIG.
| Methods | Country: Nigeria Setting: 8 district hospitals, Abuja, Nigeria Duration of recruitment: September 2003 to June 2005 Duration of trial:Not stated Duration of follow‐up: 6 months Design: randomized placebo‐controlled factorial trial with three intervention arms Follow‐up :Blood samples (complete blood count, erythrocyte sedimentation rate (ESR) and biochemical tests) and three sputum samples and a chest X‐ray, were conducted at enrolment. Sputum specimens were collected weekly for the first 8 weeks of therapy and again at 12, 16, 20 and 24 weeks. Blood samples and chest X‐rays were repeated at 2 and 6 month follow‐up. |
|
| Participants | Inclusion criteria: HIV‐positive and HIV‐negative adults aged > 15 years with sputum positive pulmonary tuberculosis Exclusion criteria: previous antituberculous therapy, pregnancy, lactation, use of corticosteroids or zinc in the previous month, major surgery in the previous month, diabetes, severe cardiovascular or hepatic disease, currently taking oral contraceptives, unable to return Participants screened: 1321 (399 smear‐positive) Participants eligible for randomization: 350 Participants randomized: 350 (155 HIV‐positive participants not stratified) Mean age at randomization: 29 to 34 years The mean BMI was greater in the zinc group at baseline:21.3 ± 4.7 versus 19.6 ± 3.5 (zinc and vitamin A group) versus 19.8 ± 3.3 (placebo group) |
|
| Interventions | Intervention: 90 mg elemental zinc plus retinol (5000 IU) weekly or 90 mg elemental zinc plus placebo weekly Control: duel placebo (similar in appearance) weekly Duration: 6 months Adherence: all participants received their supplements under direct observation for the first 2 months together with standard antituberculous treatment (2RHZE/4HE). Monthly supplies were then given for the following 4 months. |
|
| Outcomes | Primary outcomes: sputum conversion, CXR scores Secondary outcomes: clinical symptoms, BMI, Karnofsky score, deaths, ESR, haemoglobin |
|
| Adverse events | Two major adverse events (participants withdrawn from trial) | |
| Notes | Source of funding: none stated Conflict of interest: no statement included Ethics: Liverpool School of Tropical Medicine Research Ethics Committee, Zankli Medical Centre Institutional Review Board Trial registration: ISRCTN36636609 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation into three supplement groups was performed at the Liverpool School of Tropical Medicine using permuted block randomization with 4 different block sizes. Random numbers generated by Minitab with block randomization using 4 different block sizes. |
| Allocation concealment (selection bias) | Low risk | An investigator who was not on site prepared the allocation sequence. Treatment assignments were prepared in serially numbered sealed envelopes. Sequentially numbered packets were assigned consecutively to participants according to allocation sequence. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators, participants, and the laboratory staff were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow‐up of HIV‐positive participants at 6 months was not balanced between treatment groups: zinc group 16 % (8/56); zinc and vitamin A group 23% (11/47); and placebo group: 30% (16/52). |
| Selective reporting (reporting bias) | Low risk | Primary outcomes reported were consistent with the trial protocol (ISRCTN36636609). |
| Other bias | Unclear risk | The trial authors did not provide any conflict of interest statement. |
McClelland 2004 KEN.
| Methods | Country: Kenya Setting: outpatient clinics at Coast Provincial General Hospital, Mombasa Duration of recruitment: September 1998 to June 2000 Duration of trial: 22 months Duration of follow‐up: 6 weeks Design: randomized placebo‐controlled trial Follow‐up: at the 6 week follow‐up visit participants underwent a physical examination and bloods and genital tract specimens were collected (as before at baseline) |
|
| Participants | Inclusion criteria: women (18 to 45 years) with HIV‐1 infection Exclusion criteria: women who were pregnant or the use of vitamin supplements or oral contraceptives during 3‐month period before study entry Participants screened: 2021 Participants eligible for randomization: 650 Number randomized: 400 (plus 200 participants in vitamin A arm) Mean age: 29 ± 7 years (micronutrient group) versus 29 ± 6 years (placebo group) No participant received ART. It is reported that CD4 cell count and vaginal HIV shedding were higher in the micronutrient group (statistical significance not shown). |
|
| Interventions | Intervention: micronutrient supplement (20 mg vitamin B1, 20 mg vitamin B2, 25 mg vitamin B6, 50 µg vitamin B12; 100 mg niacin; 500 mg vitamin C; 30 mg vitamin E; 0.8 mg folic acid; 200 µg selenium) administered as a hard gel capsule daily. Control: placebo (supplement and placebo capsules identical in appearance) Duration: daily for 6 weeks Compliance: reported as the proportion of participants in each treatment group who took 95% of scheduled doses (micronutrient group: 93.7% (168/179); control group: 92.1% (164/178). Supplements were dispensed with an electronic alarm vial. |
|
| Outcomes | Primary outcomes: vaginal and cervical HIV‐1 shedding Secondary outcomes: CD4, CD8 cell count, viral load |
|
| Adverse events | Multivitamin supplementation increased cervical and vaginal shedding of HIV‐positive cells | |
| Notes | Source of funding: National Institutes of Health and University of Washington Clinical Nutrition Research Unit Conflict of interest: statement not included Ethics: University of Nairobi, University of Washington Trial registration: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used computer‐generated randomization sequence in blocks. |
| Allocation concealment (selection bias) | Unclear risk | It was unclear who was responsible for the allocation of treatment (sequential numbering of medication bottles not specified). |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding was not specified except for participants who received identical capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Twenty‐one(10.5%) and 22(11%) participants were lost to follow‐up from the multivitamin and placebo groups respectively. However, the trial authors did not state the reasons for loss to follow‐up. Participants who were lost to follow‐up had lower CD4 cell counts compared to those who completed the trial, but cell counts were not reported for each treatment group. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was not available |
| Other bias | Unclear risk | The trial authors did not provide any statement regarding conflicts of interest. |
Overton 2015 USA.
| Methods | Country: USA Setting:39 AIDS Clinical trials network research units Duration of recruitment: September 2011 to February 2012 Duration of trial: 48 weeks Duration of follow‐up: 48 weeks Design: randomized placebo‐controlled trial Follow‐up: blood samples at enrolment, 24 and 48 weeks; DXA scan at enrolment and again at 48 weeks |
|
| Participants | Inclusion criteria: HIV‐positive people who were not on ART with viral load > 1000 copies/mL and blood 25 (OH) vitamin D level ≥ 10 and < 75 ng/mL, creatinine clearance ≥ 60 mL/min and serum calcium < 10.5 mg/dL. Exclusion criteria: participants taking daily supplements containing calcium and vitamin D exceeding 500 mg and 800 IU respectively, any biphosphonate therapy, recent steroid or chemotherapy treatment, thyroid disease, substance or alcohol abuse, a history of fragility fracture, osteoporosis or nephrolithiasis or weight > 300 lb. Pregnant and lactating women were also excluded. Participants screened: 218 Participants eligible for randomization: 183 Number randomized: 167 Mean age: 36 years (IQR 28 to 47) (vitamin D/calcium group) versus 31 years (IQR 25 to 44) (placebo group) Two participants from the vitamin D/calcium group had protocol violations due to the incorrect screening vitamin D assay being performed. The trial authors did not include this data in the analyses. |
|
| Interventions | Intervention: 4000 IU vitamin D3 daily plus 500 mg calcium carbonate twice daily with food Control: placebo daily plus placebo twice daily with food (identical in appearance) Duration: daily for 48 weeks Compliance: all participants were initiated on first line ART (EFV/FTC/TDF) |
|
| Outcomes | Primary: change in total hip bone mineral density (BMD) Secondary: changes in lumbar spine BMD, 25(OH) vitamin D levels, parathyroid hormone (PTH), markers of bone turnover, and other inflammatory biomarkers and CD4 cell counts at 24 and 48 weeks |
|
| Adverse events | No differences were observed between the treatment groups in terms of reported adverse events during the study period. Vitamin D/calcium group (33 Grade 1‐2 events, 15 Grade 3 events, and 2 Grade 4 events) and placebo group (33 Grade 1‐2 events, 15 Grade 3 events, and 5 Grade 4 events). No cases of hypercalcaemia were reported. One death in the Vitamin D/calcium group was reported in the context of rapid HIV disease progression. | |
| Notes | Source of funding: National Institute of Allergy and Infectious Diseases. The pharmaceutical industry provided supplements and placebos Conflict of interest: none Ethics: Institutional Review Boards of all participating research sites Trial registration: NCT 01403051 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors reported block randomization stratified for baseline 25(OH) vitamin D levels (≤ 20 and > 20 ng/mL), but the trial authors did not specify the method of generation randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report the details regarding the allocation of study supplements. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not specified except for participants who received identical supplements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was 9% (15/167). Reasons for attrition include death (n = 1), non‐adherence to treatment/study visits (n = 2), withdrew consent (n = 1), or lost to follow‐up (n = 11). |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes reported were consistent with trial protocol (NCT 01403051). |
| Other bias | High risk | The pharmaceutical industry sponsored ARTs and supplements, industry representatives served on the study team and reviewed manuscript prior to publication, thus there was a potential conflict of interest. |
Range 2006 TZA.
| Methods | Country: Tanzania Setting: 5 district health facilities Duration of recruitment: August 2001 to July 2002 Design: placebo‐controlled 2 x 2 factorial trial |
|
| Participants | Inclusion criteria: HIV‐positive and HIV‐negative people aged ≥15 years with sputum‐positive pulmonary tuberculosis (new or relapsed cases) Exclusion criteria: participants who defaulted tuberculosis chemotherapy or those who remained smear‐positive on chemotherapy (failure cases) and those with serious tuberculosis or other disease unlikely to survive; pregnant and lactating women. Participants randomized: 530 213 HIV‐positive 325 male and 205 female Mean age = 35.4 years Participants analysed: 499 Loss to follow‐up/withdrawal: 77 within 244 days post‐treatment Exclusions postrandomization: 31 |
|
| Interventions | InterventionS: micronutrient supplement contained vitamin A (1.5 mg), vitamin B1 (20 mg), vitamin B2 (20 mg), vitamin B6 (25 mg), vitamin B12 (50mg), folic acid (0·8 mg), niacin (40 mg), vitamin C (200 mg), vitamin E (60 mg), vitamin D3 (5 mg), selenium (0·2 mg) and copper (5 mg), and zinc tablets contained 45 mg elementary zinc Control: placebo (2 x 2 factorial) Duration: daily for 8 months. All participants received a standard 8 month tuberculosis chemotherapy regimen. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | None reported | |
| Notes | Source of funding: Danish International Development Assistance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized randomization. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes, codes unbroken until post‐analysis |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Differential attrition rates between treatment groups > 10% [Zinc group: 10.3% (6/58); Multivitamin/mineral group: 23.7% (14/59); Multivitamin/mineral plus zinc group: 12.5 % (6/48) Placebo group: 12.5% (6/48)] |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information; study protocol not available |
| Other bias | Unclear risk | Randomization was not stratified by HIV‐status of participants. Declared no conflict of interest |
Semba 1998 USA.
| Methods | Country: USA Setting: community‐based clinic Duration of recruitment: not stated Duration of follow‐up: 4 weeks Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: HIV‐positive intravenous drug users participating in ALIVE (AIDS Linked to Intravenous Experiences) Cohort (N = 630); ≥ 18 years; not taking vitamin A supplements Exclusion criteria: CD4 > 500 cells/mm³; pregnancy. Participants randomized: 120 89 male and 31 female Mean age = 38.2 years 50% treatment group versus 43% placebo group on ART Loss to follow‐up/withdrawal: 8.3% at 4 weeks Exclusions postrandomization: 0 |
|
| Interventions | Intervention: single dose of 200 000 IU vitamin A Control: placebo |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | None reported | |
| Notes | Source of funding: USAID | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a random number table in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | The trial used sequentially numbered envelopes to conceal allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information; the trial protocol was not available. |
| Other bias | Unclear risk | The trial authors did not provide any declaration regarding conflicts of interest. |
Semba 2007 MWI.
| Methods | Country: Malawi Setting: 8 community health centres Duration of recruitment: July 1999 to October 2004 Design: placebo‐controlled |
|
| Participants | Inclusion criteria: HIV‐positive and HIV‐negative adults with smear‐positive pulmonary tuberculosis (new cases) Exclusion criteria: prior or current tuberculosis chemotherapy, prior vitamin supplements Participants randomized: 1148 829 HIV‐positive 336 male and 493 female Mean age = 34 years Participants analysed: 1148 Loss to follow‐up: 103 in HIV‐positive group (50 and 53 in micronutrient and placebo groups, respectively) Exclusions postrandomization: 0 |
|
| Interventions | Intervention: micronutrient supplement (vitamin A, C, D, E, B6, B12, riboflavin, thiamine, niacin, folate, zinc, iodine, selenium) Control: placebo Duration: daily for 24 months. All participants received a standard 8‐month tuberculosis chemotherapy regimen. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | Not reported | |
| Notes | Source of funding: National Institutes of Health and the Fogarty International Centre. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used blocked randomization. |
| Allocation concealment (selection bias) | Low risk | The trial used prepacked sequentially numbered supplements. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The attrition rates were greater than 10% and the trial authors did not report the reasons for loss to follow‐up (supplement group 12.3%; placebo group 12.5%) |
| Selective reporting (reporting bias) | Low risk | The trial protocol was available; the trial authors reported on all outcomes of interest. |
| Other bias | Unclear risk | The trial authors did not stratify randomization by HIV status of participants. The trial authors did not declare their conflicts of interest, if any. |
Semba 2007 USA.
| Methods | Country: USA Setting: study clinic Duration of recruitment: September 2002 to August 2005 Design: controlled trial |
|
| Participants | Inclusion criteria: women ≥ 18 years; history of injection drug use (IDU) within past 10 years; hepatitis C (HCV) antibody‐positive; Karnofsky status > 80%; serum ferritin < 200 ng/mL Exclusion criteria: pregnant; history of liver failure, renal disease, interferon therapy for HCV; haemochromatosis; blood disorders Participants randomized: 458 Mean age = 40 years 138 (30.1%) HIV‐positive Participants analysed: 115 at 12 months Loss to follow‐up/withdrawal:151 (33%) Exclusions postrandomization: 0 |
|
| Interventions | Intervention: micronutrients with iron (18 mg) Control: micronutrients only Duration: daily for 12 months |
|
| Outcomes | Primary outcomes
|
|
| Adverse events | Not reported | |
| Notes | On HAART: 27/69 (intervention) and 23/69 (control) Trial stopped early due to slow recruitment. Source of funding: National Institute on Drug Abuse; National Institute on Nursing Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used computerized randomization. |
| Allocation concealment (selection bias) | Low risk | The trial used prepacked sequentially numbered study supplements. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was high loss to follow‐up (27.7%) in both groups, and the trial authors did not report this information by HIV status. |
| Selective reporting (reporting bias) | Unclear risk | There was insufficient information; the trial protocol was not available. |
| Other bias | Unclear risk | The trial authors did not provide a declaration on conflicts of interest. |
Stallings 2014 USA.
| Methods | Country: USA Setting: Children’s Hospital of Philadelphia, Philadelphia Duration of recruitment: July 2011 to June 2013 Duration of follow‐up: 12 months Design: randomized placebo‐controlled trial Follow‐up: participants were followed up at 3, 6, and 12 months. At each visit adverse events and compliance were recorded. Blood and urine measurements were also performed for vitamin D (25‐Hydroxy vitamin D, 1,25 Dihydroxy vitamin D), calcium, metabolic parameters, and immunological parameters. |
|
| Participants | Inclusion criteria: perinatally acquired HIV infection (PHIV), 5.0 to 24.9 year or behaviorally‐acquired HIV infection (BHIV), 15.0 to 24.9 years; usual state of good health 2 weeks before study entry Exclusion criteria: other adverse growth, dietary intake, or nutritional status conditions, pregnancy, lactation, and use of vitamin D3 supplements. If Vitamin D3 supplements were discontinued, participants underwent a 2‐week wash‐out period before trial entry. Participants screened: 121 Participants eligible for randomization: 58 Participants randomized: 58 (stratified by PHIV/BHIV) Mean age at randomization: 20.7 ± 3.7 years 76% of participants on HAART (vitamin D group:23/30 (77%); placebo group 21/28 (75%)) The trial authors reported no statistically differences in baseline disease characteristics, vitamin D status, dietary intake, or metabolic parameters. |
|
| Interventions | Intervention: 7000 IU vitamin D3 daily (those unable to swallow capsules took 0.49 mL daily of 400 IU vitamin D3 drops) Control: placebo capsules (those unable to swallow capsules took 0.49 mL daily of placebo drops) Duration: 12 months Compliance: residual capsules or volume (in the case of drops) were recorded at follow‐up visits. Mean adherence was 92 ± 8% over 12 months with no differences between groups. |
|
| Outcomes | Primary: blood 25‐Dehydroxy vitamin D levels Secondary: HIV load (among participants with a detectable viral load), CD4% |
|
| Adverse events | Four participants in the placebo group were withdrawn from the study after 6 months according to prespecified criteria (3 consecutive 25 (OH) vitamin D values < 11 ng/mL). No participant experienced the predefined serious adverse event of 25 (OH) vitamin D levels > 80 ng/mL at any time during the follow‐up period. Serum calcium levels increased from 9.5 ± 0.4 to 9.6 ± 0.4 mg/dL after 12 months in the vitamin D group. | |
| Notes | Source of funding: NIH/National Center for Complementary and Alternative Medicine,National Center for Research Resources, National Center for Advancing Translational Sciences Conflict of interest: the trial authors declared no conflict of interest. Ethics: Children's Hospital of Philadelphia Institutional Review Board Trial registration: clinicaltrials.gov (NCT 01475890) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors stated that participants were randomized in parallel (1:1 ratio) to receive the intervention of the placebo. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not provide any information in terms of how study supplements were numbered and allocated to participants. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The trial authors referred to a "double blind" study but it was unclear who was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were 10% (3/30) in vitamin D group versus 17% (5/28) in placebo group. Three participants were lost to follow‐up (without reasons) in the vitamin D group and 1 participant in the placebo group. Four participants from the placebo group were withdrawn from study (according to prespecified withdrawal rules). |
| Selective reporting (reporting bias) | Low risk | The primary outcomes as reported were consistent with the trial protocol from www.clinicaltrials.gov. |
| Other bias | Low risk | The trial authors declared no conflicts of interest. |
Villamor 2008 TZA.
| Methods | Country: Tanzania Setting: 5 outpatient tuberculosis clinics Duration of recruitment: April 2000 to April 2005 Median duration of follow‐up: 30 months (IQR 15 to 41) Design: randomized placebo‐controlled trial |
|
| Participants | Inclusion criteria: HIV‐positive and HIV‐negative adults aged 18 to 65 years with positive sputum smears for acid‐fast bacilli who planned to stay in Dar es Salaam for 2 years Exclusion criteria: pregnancy, antituberculosis treatment for > 4 weeks in previous year, Karnofsky score < 40% HIV‐positive participants randomized: 471 273 male and 198 female Mean age = 34 years Loss to follow‐up: 67 in HIV‐positive group (33 and 34 in micronutrient and placebo groups, respectively) Exclusions postrandomization: 0 |
|
| Interventions | Intervention: micronutrient supplement (retinol; vitamins B1, B2, B6, B12; niacin; vitamin C; vitamin E; folic acid; selenium) Control: placebo Duration: daily for 24 months. All participants received DOTS antituberculosis chemotherapy. |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
|
| Adverse events | None reported | |
| Notes | Source of funding: National Institute of Allergy and Infectious Diseases; US Department of Agriculture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used computer‐generated permuted blocks of 20. Randomization was stratified by HIV status of participants. |
| Allocation concealment (selection bias) | Unclear risk | All clinical and research staff were unaware of the participants’ treatment assignment, but the trial authors provided insufficient information. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The trial authors did not provide reasons for losses to follow‐up, although they used appropriate statistical analyses. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information; the trial protocol was not available. |
| Other bias | Low risk | The trial authors declared that they had no conflicts of interest. |
Wejse 2009 GNB.
| Methods | Country: Guinea‐Bissau Setting: tuberculosis clinics in urban disease surveillance site Duration of recruitment: November 2003 to December 2005 Duration of follow‐up: 12 months Design: placebo‐controlled, parallel group |
|
| Participants | Inclusion criteria: tuberculosis participants starting antituberculosis treatment, ≥ 15 years Exclusion criteria: none Participants randomized: 222 male and 143 female; mean age 37.5 yrs; 131 HIV‐positive Participants analysed: 365 Loss to follow‐up/withdrawal: 84 Exclusions postrandomization: 2 |
|
| Interventions | Intervention: 100,000 IU cholecalciferol (vitamin D) Control: placebo Duration: at inclusion; 5 and 8 months after inclusion |
|
| Outcomes | Primary outcomes:
Reduction in a clinical severity score (tuberculosis score) Secondary outcomes: 12‐month mortality |
|
| Adverse events | Minor adverse events reported; no difference between groups. There were no reported cases of hypercalcaemia. | |
| Notes | Source of funding: Aarhus University Hospital; Danish Research Council for Developmental Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors used a computer‐generated sequence when performing randomization. |
| Allocation concealment (selection bias) | Low risk | The trial used identical, sequentially numbered containers to perform allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, staff, and researchers were blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up was unknown in the HIV‐positive subgroup. |
| Selective reporting (reporting bias) | Low risk | The trial protocol was available; the trial authors reported on all outcomes of interest. |
| Other bias | Low risk | The trial authors did not prespecify the HIV subgroup analyses but proportions were equally distributed; the trial funder and provider had no role in trial design. |
Zhao 2010 CHN.
| Methods | Country:China Setting: village in Huaiyang county, Henan province Design: randomized placebo‐controlled trial Duration of recruitment: June 2008 to November 2008 Duration of follow‐up: 6 months |
|
| Participants | Inclusion criteria: HIV‐positive people aged 25 to 49 years with BMI 18‐25kg/m2 and CD4 count > 200 with no clinical symptoms of AIDS Exclusion criteria: not stated Number randomized: 102 Mean age at randomization: micronutrient group:37.8 ± 2.9 years; placebo group: 37.3 ± 2.3 years 50 male and 49 female No baseline differences between treatment groups in terms of gender, weight, height |
|
| Interventions | Intervention: tablet containing: vitamin A 200 µg, β‐carotene 200 µg, vitamin D 5 µg, thiamin 1 mg, riboflavin 1 mg, vitamin B6 1 mg, folic acid 0.15 mg, vitamin C 100 mg, vitamin E 15 mg, iron 6 mg, zinc 5 mg, selenium 30 µg, calcium 400 mg Control: identical placebo daily Duration: 6 months Compliance: not reported |
|
| Outcomes | Primary: change in absolute CD3, CD4, and CD8 counts and of markers of humoral immunity (IgA, IgG, IgM, and C3) | |
| Adverse events | Not reported | |
| Notes | Source of funding: not specified Conflict of interest: not specified Ethics: review board not specified Trial registration: not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | There was no information from the trial authors on blinding of participants, investigators, or outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants, out of a total number of 102 randomized, were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was not available. |
| Other bias | Unclear risk | The study authors did not provide conflict of interest statements. |
Abbreviations: ALT: alanine aminotransferase; ALP: alkaline phosphate; ART: antiretroviral therapy; AST: aspartate aminotransferase; BMI: Body Mass Index; CXR: Chest X‐Ray; ESR: erythrocyte sedimentation rate ; GFR: glomerular filtration rate; HAART: highly active antiretroviral therapy; IDU: injection drug user; HCV: Hepatitis C; INH: isoniazid; IQR: interquartile range; MUAC: mid‐upper arm circumference; NSAIDS: non‐steroidal anti‐inflammatory drugs; PTH:Parathyroid hormone; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghdassi 2010 | Trial participants had highly active antiretroviral therapy (HAART)‐related co‐morbidity |
| AIDS Policy Law 2012 | Not a randomized controlled trial (RCT) |
| Arpadi 2012 | Most trial participants were under 15 years of age |
| Austin 2006 | The trial intervention was irrelevant to this review |
| Balasubramanyam 2011 | Co‐intervention present |
| Balfour 2014 | The trial intervention was irrelevant to this review |
| Brown 2015 | This study did not report any relevant outcomes |
| Chow 2010 | Trial participants had HAART‐related co‐morbidity |
| Coelho 2015 | Not a RCT |
| Currier 2010 | Not a RCT |
| Daneshpajouhnejad 2011 | This study did not report any relevant trial outcomes |
| Etminani‐Esfahani 2012 | Not a RCT |
| Gharakhanian 2011 | Not a RCT |
| Groleau 2013 | This study did not report any relevant trial outcomes |
| Havens 2012a | This study did not report any relevant trial outcomes |
| Havens 2012b | This study did not report any relevant trial outcomes |
| Havens 2012c | This study did not report any relevant trial outcomes |
| Havens 2012d | This study did not report any relevant trial outcomes |
| Hemsworth 2012 | The trial intervention was irrelevant to this review |
| Hummelen 2011 | The trial intervention was irrelevant to this review |
| Kaiser 2006 | The trial intervention was irrelevant to this review |
| Kakalia 2011a | Most trial participants were less than 15 years of age |
| Kakalia 2011b | Most trial participants were less than 15 years of age |
| Lachmann 2014 | Not a RCT |
| Ladep 2010 | Co‐intervention present |
| Lange 2009 | The trial intervention was irrelevant to this review |
| Lange 2011 | The trial intervention was irrelevant to this review |
| Lescoat 2012 | Not a RCT |
| Lin 2013 | Trial participants had HAART‐related co‐morbidity |
| Longenecker 2011 | This study did not report any relevant trial outcomes |
| Madrid 2012 | Not a RCT |
| Mandal 2011 | This study did not report any relevant trial outcomes |
| Martineau 2013 | Not a RCT |
| Mascitelli 2011 | Not a RCT |
| Mburu 2010 | The trial intervention was irrelevant to this review |
| Mehta 2010 | Not a RCT |
| Morgan 2010 | Co‐intervention present |
| Motswagole 2013 | The trial intervention was irrelevant to this review |
| Pasquet 2011 | Not a RCT |
| PrayGod 2011 | The trial intervention was irrelevant to this review |
| Schall 2016 | The study did not report any relevant outcomes |
| Scrimgeour 2010 | The study did not report any relevant outcomes |
| Singhal 2010 | The trial intervention was irrelevant to this review |
| Steenhoff 2015 | More than 20% of the trial participants were less than 15 years of age |
| Stewart 2011 | Co‐intervention present |
| Sudarsanam 2011 | The trial intervention was irrelevant to this review |
| Visser 2011 | The study did not report any relevant trial outcomes |
| Welz 2011 | Not a RCT |
Abbreviations: HAART: highly active antiretroviral therapy; RCT: randomized controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Odunukwe 2016.
| Methods | Country: Nigeria Setting: HIV treatment centre, Lagos |
| Participants | Inclusion criteria: HIV‐positive men and women who were eligible for highly active antiretroviral therapy (HAART) and who were HBsAg positive (Hepatitis B virus or HBV) |
| Interventions | Intervention: 200 µg selenium daily plus HAART Control: HAART Duration: 18 months |
| Outcomes | Changes in HBV load, HIV load, CD4 cell count, and alanine transaminase (ALT) |
| Notes |
Abbreviations: HAART: highly active antiretroviral therapy; HBV: hepatitis B.
Characteristics of ongoing studies [ordered by study ID]
Lebouché 2014.
| Trial name or title | The role of extended‐release niacin on immune activation and neurocognition in HIV‐positive patients treated with antiretroviral therapy (CTN PT006) |
| Methods | Country: Canada Setting: Chronic Viral Illness Service, Montreal Chest Institute of the McGill University Health Centre (MUHC), and the Cliniquemédicale l’Actuel, Montreal Design: randomized cross‐over trial |
| Participants | Inclusion criteria: 21 years or older, viral load < 50 copies/mL for the last 3 months, CD4+ T‐cell count ≤ 350 cells/μL; and on stable ART (ART unchanged for treatment failure (rebound in viral load)) for more than 12 months. Exclusion criteria: prior history of hypersensitivity reaction to niacin or any other component of the study drug; prior history of flushing; liver disease (including coinfection with hepatitis B or C virus) or unexplained persistent elevations of serum transaminases; alanine aminotransferase (ALT) or aspartate aminotransferase (AST) or alkaline phosphatase > 2.5 times the upper limit of normal ; active duodenal or gastric peptic ulcer; active bleeding disorders; history of gout; active AIDS events in the last 3 months as determined by the treating physician; unstable angina or acute phase myocardial infarction; diabetic or potentially diabetic with hypercholesterolaemia; renal dysfunction; co‐enrolment in another study involving neurocognitive evaluation; or pregnant or nursing or planning to become pregnant. |
| Interventions | Immediate versus deferred use of ER niacin for 24 weeks. The administration of ER niacin will be titrated (weeks 0 to 4: 500 mg, weeks 5 to 12: 1000 mg, weeks 12 to 24: 2000 mg). All participants will receive ART |
| Outcomes | Primary outcome: T cell activation (change in percentage of CD8+ CD38+ HLA‐DR+ T‐cells) Secondary outcomes: change in total CD4 cell count |
| Starting date | February 2012 |
| Contact information | bertrand.lebouche@mcgill.ca |
| Notes |
NCT 01295034.
| Trial name or title | Vitamin D supplements for HIV‐positive patients on cART (NCT01295034) |
| Methods | Country: USA Setting: Mount Sinai Medical Center, New York Design: controlled trial |
| Participants | Inclusion criteria: HIV‐positive adults ≥ 18 to 70 yrs, stable highly active antiretroviral therapy (HAART) regimen (at least 12 months) with undetectable viral load (at least 6 months), not consuming more than 2 g calcium and 800 IU vitamin D daily Exclusion criteria: receiving vitamin D, current treatment for bone disease, receiving medications known to affect bone mineralization, medical conditions known to affect vitamin D, calcium and phosphate levels, kidney disease, unstable medical condition likely to preclude participation in a 12‐month trial, pregnancy |
| Interventions | Intervention: oral dose of 50,000 IU vitamin D2 weekly for 8 weeks, thereafter 1000 IU vitamin D2 daily for 48 weeks Control: oral dose of 2000 to 4000 IU vitamin D3 daily for 12 months, with dose titration as necessary Duration: 12 months |
| Outcomes | Primary: 25 (OH) vitamin D levels (% of participants who have levels in the range of 30 to 60 ng/mL) Secondary: change in CD4 cell count |
| Starting date | March 2011 |
| Contact information | andrea.branch@mssm.edu |
| Notes |
NCT 01798680.
| Trial name or title | Trial of Vitamin D in HIV progression (TOV4) |
| Methods | Country: Tanzania Setting: Dar es Salaam Design: placebo‐controlled trial |
| Participants | Inclusion criteria: HIV‐positive adults ≥ 18 yrs, initiation of HAART at the time of randomization, 25(OH) vitamin D concentration < 30 ng/mL Exclusion criteria:pregnancy, participation in another micronutrient trial |
| Interventions | Intervention: oral dose of 50000 IU vitamin D3 weekly for 4 weeks, thereafter 2000 IU vitamin D3 daily up to 12 months Control: oral dose of placebo weekly for 4 weeks, thereafter 2000 IU vitamin D3 daily up to 12 months Duration: 12 months |
| Outcomes | Primary: All‐cause death, pulmonary tuberculosis within 12 months of randomization Secondary: CD4 cell count, clinical diagnosis of co‐morbidities, weight, calcium, Parathyroid hormone (PTH) and alkaline phosphate (ALP) concentrations |
| Starting date | February 2014 |
| Contact information | mina@hsph.harvard.edu fmugusi@muhas.ac.tz |
| Notes |
NCT 02810275.
| Trial name or title | Folinic Acid: Supplementation and Therapy (NCT02810275) |
| Methods | Country: Brazil Setting: Hospital de Clinicas de Porto Alegre |
| Participants | Inclusion criteria: HIV infected and HIV‐HCV co‐infected men and women aged 18 to 50 years receiving HAART with undetectable viral load for more than 6 months Exclusion criteria: diabetes mellitus, previous CVD: acute myocardial infarction, myocardial revascularization, or stroke,creatinine > 1.5 mg/dL,clinical diagnosis or ultrasound, endoscopic, or laboratory evidence of liver cirrhosis, on treatment with: statins, fibrates, hormone replacement therapy, sulfonamides, vitamin supplements, or folinic acid in the last 30 days and pregnant women. |
| Interventions | Intervention: 5 mg folinic acid daily Control: placebo daily Duration: 4 weeks |
| Outcomes | Changes in flow mediated dilatation. serum homocysteine levels |
| Starting date | October 2012 |
| Contact information | Sandra Costa Fuchs, Hospital de Clinicas de Porto Alegre |
| Notes |
NCT 02827643.
| Trial name or title | Vitamin D and Calcium Supplement Attenuate Bone Loss Among HIV‐ Infected Patients Receiving Tenofovir Disoproxil Fumarate, Lamivudine or Emtricitabine and Efavirenz (NCT02827643) |
| Methods | Country: Thailand Setting: Ramathibodi Hospital, Mahidol University, Bangkok |
| Participants | Inclusion criteria: HIV‐1‐infected patients aged 18 to 50 years who start 3TC or FTC, TDF, and EFV within 3 months before enrolment Exclusion criteria: CrCl < 60 mL/min/1.73 m2, CaCO3 supplement > 500 mg/day or vitamin D supplement > 800 IU/day, steroid use (equivalent to prednisolone> 5 mg/day more than 3 months), osteoporosis treatment, serum calcium > 10.5 g/dL clinical history of fragility fracture, pregnancy, or breastfeeding, secondary amenorrhoea, hyperthyroidism, history of kidney stone or current active opportunistic infection |
| Interventions | Intervention: once daily calcium carbonate 1,250 mg (600 mg elemental calcium) and weekly vitamin D2 (20,000 IU) plus TDF/3TC or FTC/EFV therapy Control: TDF/3TC or FTC/EFV therapy Duration: 24 weeks |
| Outcomes | Changes in bone mineral density, 1,25 (OH) vitamin D concentrations |
| Starting date | June 2016 |
| Contact information | pataweeb44@gmail.com |
| Notes |
NCT 02856269.
| Trial name or title | Zinc Supplementation and Cardiovascular Risk in HIV (NCT02856269) |
| Methods | Country: USA Setting: University Hospitals Cleveland Medical Center |
| Participants | Inclusion criteria: HIV‐1 infected adults aged ≥ 18 years with blood zinc level ≤ 0.75 mg/L that are receiving a stable antiretroviral regimen with no plans to change during study with HIV‐1 RNA level of ≤ 400 copies/mL and no diarrhoea or nausea/vomiting for the last month Exclusion criteria: pregnancy/lactation,presence of inflammatory condition, regular use of agents that may affect inflammation in the last 3 months. regular use of NSAIDS, aspirin, or statins will be allowed as long as dose has been stable for the last 3 months and is not expected to change during the study. Presence of active neoplastic diseases requiring chemotherapy and use of immunosuppressive drugs, known cardiovascular disease, uncontrolled diabetes, allergy or intolerance to zinc sulfate. AST, and ALT > 2.5 x upper normal limit, haemoglobin < 9.0 g/dLor GFR < 50 mL/min |
| Interventions | Intervention: 45 mg zinc gluconate daily Control: 90 mg zinc gluconate daily Duration: 12 weeks |
| Outcomes | Changes in blood zinc concentrations |
| Starting date | September 2016 |
| Contact information | mccomsey.grace@clevelandactu.org |
| Notes |
Abbreviations: ALT: alanine aminotransferase; ALP: alkaline phosphate; ART: antiretroviral therapy; AST: aspartate aminotransferase; GFR: glomerular filtration rate; HAART: highly active antiretroviral therapy; NSAIDS: non‐steroidal anti‐inflammatory drugs; PTH: parathyroid hormone.
Differences between protocol and review
The original protocol for this review included studies in both HIV‐positive children and pregnant women (Irlam 2002). Two separate reviews on the role of micronutrient supplementation for HIV‐positive pregnant women, Siegfried 2012, and children, Irlam 2013, have been published. The primary focus of this review update was therefore on the role of micronutrient supplementation in HIV‐positive men and women who were not pregnant.
Contributions of authors
Marianne Visser (MV) initiated the review update and contributed to all stages of the review. Solange Durao (SD) contributed to all stages of the review update. David Sinclair (DS) contributed to the preparation of the review update for submission. James Irlam (JI) commented on the report of the review update. Nandi Siegfried (NS) assisted with study selection and commented on the review update.
Sources of support
Internal sources
SACC HIV/AIDS Mentoring Programme, South Africa.
South African Cochrane Centre, South Africa.
Medical Research Council, South Africa.
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
Marianne Visser (MV) has no known conflicts of interest. Solange Durao (SD) has no known conflicts of interest. David Sinclair (DS) has no known conflicts of interest. James Irlam (JI) has no known conflicts of interest. Nandi Siegfried (NS) has provided consultancies to several World Health Organization (WHO) guidelines processes within the HIV department including nutritional interventions.
Unchanged
References
References to studies included in this review
Allard 1998 CAN {published data only}
- Allard JP, Aghdassi E, Chau J, Tam C, Kovacs CM, Salit IE, et al. Effects of vitamin E and C supplementation on oxidative stress and viral load in HIV‐infected subjects. AIDS 1998;12(13):1653‐9. [DOI] [PubMed] [Google Scholar]
Asdamongkol 2013 THA {published data only}
- Asdamongkol N, Phanachet P, Sungkanoparph S. Low plasma zinc levels and immunological responses to zinc supplementation in HIV‐infected patients with immunological discordance after antiretroviral therapy. Journal of the International AIDS Society 2012;15(Suppl 4):152‐3. [DOI] [PubMed] [Google Scholar]
- Asdamongkol N, Phanachet P, Sungkanuparph S. Low plasma zinc levels and immunological responses to zinc supplementation in HIV‐infected patients with immunological discordance after antiretroviral therapy. Japanese Journal of Infectious Diseases 2013;66(6):469‐74. [DOI] [PubMed] [Google Scholar]
Baeten 2002 KEN {published data only}
- Baeten JM, McClelland RS, Overbaugh J, Richardson BA, Emery S, Lavreys L, et al. Vitamin A supplementation and human immunodeficiency virus type 1 shedding in women: results of a randomized clinical trial. Journal of Infectious Diseases 2002;185(8):1187‐91. [DOI] [PubMed] [Google Scholar]
Bang 2012 DEN {published data only}
- Bang U, Kolte L, Hitz M, Dam Nielsen S, Schierbeck LL, Andersen O, et al. Correlation of increases in 1,25‐dihydroxyvitamin D during vitamin D therapy with activation of CD4+ T lymphocytes in HIV‐1‐infected males. HIV Clinical Trials 2012;13(3):162‐70. [DOI] [PubMed] [Google Scholar]
- Bang UC, Kolte L, Hitz M, Schierbeck LL, Nielsen SD, Benfield T, et al. The effect of cholecalciferol and calcitriol on biochemical bone markers in HIV type 1‐infected males: results of a clinical trial. AIDS Research and Human Retroviruses 2013;29(4):658‐64. [DOI] [PubMed] [Google Scholar]
Baum 2010 USA {published data only}
- Baum MK, Lai S, Sales S, Page JB, Campa A. Randomized, controlled clinical trial of zinc supplementation to prevent immunological failure in HIV‐infected adults. Clinical Infectious Diseases 2010;50(12):1653‐60. [PUBMED: 20455705] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima C, Campa A, Sales S, Stewart T, Garces L, Baum MK. Zinc supplementation prevents increases in prevalence of hypertension among HIV infected adults in Miami. FASEB Journal 2010;24(1 Supplement):718.7. [Google Scholar]
Baum 2013 BWA {published data only}
- Baum MK, Campa A, Lai S, Sales Martinez S, Tsalaile L, Burns P, et al. Effect of micronutrient supplementation on disease progression in asymptomatic, antiretroviral‐naive, HIV‐infected adults in Botswana: a randomized clinical trial. JAMA 2013;310(20):2154‐63. [DOI: 10.1001/jama.2013.280923] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sales S, Tsalaile L, Burns PJ, Campa A, Makhema J, Marlink R, et al. Selenium (Se) supplementation for 6 months increases plasma selenium levels in HIV+ adults in Botswana. FASEB Journal 2010;24(1 Supplement):916.10. [Google Scholar]
Burbano 2002 USA {published data only}
- Burbano X, Miguez‐Burbano MJ, McCollister K, Zhang G, Rodriguez A, Ruiz P, et al. Impact of a selenium chemoprevention clinical trial on hospital admissions of HIV‐infected participants. HIV Clinical Trials 2002;3(6):483‐91. [DOI] [PubMed] [Google Scholar]
Cárcamo 2006 PER {published data only}
- Cárcamo C, Hooton T, Weiss NS, Gilman R, Wener MH, Chavez V, et al. Randomized controlled trial of zinc supplementation for persistent diarrhea in adults with HIV‐1 infection. Journal of Acquired Immune Deficiency Syndromes 2006;43(2):197‐201. [DOI] [PubMed] [Google Scholar]
Coodley 1993 USA {published data only}
- Coodley GO, Nelson HD, Loveless MO, Folk C. Beta‐carotene in HIV infection. Journal of Acquired Immune Deficiency Syndromes 1993;6(3):272‐6. [PubMed] [Google Scholar]
Coodley 1996 USA {published data only}
- Coodley GO, Coodley MK, Lusk R, Green TR, Bakke AC, Wilson D, et al. Beta‐carotene in HIV infection: an extended evaluation. AIDS 1996;10(9):967‐73. [DOI] [PubMed] [Google Scholar]
Dougherty 2015 USA {published data only}
- Dougherty KA, Schall JI, Zemel BS, Tuluc F, Hou X, Rutstein RM, et al. Safety and efficacy of high‐dose daily vitamin D3 supplementation in children and young adults infected with human immunodeficiency virus. Journal of the Paediatric Infectious Diseases Society 2014;3(4):294‐303. [DOI: 10.1093/jpids/piu012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giacomet 2013 ITA {published and unpublished data}
- Giacomet V, Vigano A, Manfredini V, Cerini C, Bedogni G, Mora S, et al. Cholecalciferol supplementation in HIV‐infected youth with vitamin D insufficiency: effects on vitamin D status and T‐cell phenotype: a randomized controlled trial. HIV Clinical Trials 2013;14(2):51‐60. [DOI] [PubMed] [Google Scholar]
Green 2005 SGP {published data only}
- Green JA, Lewin SR, Wightman F, Lee M, Ravindran S, Paton NI. A randomised controlled trial of oral zinc on the immune response to tuberculosis in HIV‐infected patients. International Journal of Tuberculosis and Lung Disease 2005;9(12):1378‐84. [PubMed] [Google Scholar]
Grigoletti 2013 BRA {published data only}
- Grigoletti SS, Guindani G, Moraes RS, Ribeiro JP, Sprinz E. Short‐term folinic acid supplementation improves vascular reactivity in HIV‐infected individuals: a randomized trial. Nutrition 2013;29(6):886‐91. [DOI] [PubMed] [Google Scholar]
Guwatudde 2015 UG {published data only}
- Guwatudde D, Ezeamama AE, Bagenda D, Kyeyune R, Wabwire‐Mangen F, Wamani H, et al. Multivitamin supplementation in HIV infected adults initiating antiretroviral therapy in Uganda: the protocol for a randomized double blinded placebo controlled efficacy trial. BMC Infectious Diseases 2012;12:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guwatudde D, Wang M, Exeamama AE, Bagenda D, Kyeyune R, Wamani H, et al. The effect of standard dose multivitamin supplementation on disease progression in HIV‐infected adults initiating HAART; a randomized double blind placebo‐controlled trial in Uganda. BMC Infectious Diseases 2015;15:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphrey 1999 USA {published data only}
- Humphrey JH, Quinn T, Fine D, Lederman H, Yamini‐Roodsari S, Wu LS, et al. Short‐term effects of large‐dose vitamin A supplementation on viral load and immune response in HIV‐infected women. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1999;20(1):44‐51. [DOI] [PubMed] [Google Scholar]
Hurwitz 2007 USA {published data only}
- Hurwitz BE, Klaus JR, Llabre MM, Gonzalez A, Lawrence PJ, Maher KJ, et al. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: a randomized controlled trial. Archives of Internal Medicine 2007;167(2):148‐54. [DOI] [PubMed] [Google Scholar]
Isanaka 2012 TZA {published data only}
- Isanaka S, Mugusi F, Fawzi WW. Standard‐dose vs high‐dose multivitamin supplements for HIV‐‐reply. JAMA 2013;309(6):546. [DOI] [PubMed] [Google Scholar]
- Isanaka S, Mugusi F, Hawkins C, Spiegelman D, Okuma J, Aboud S, et al. Effect of high‐dose vs standard‐dose multivitamin supplementation at the initiation of HAART on HIV disease progression and mortality in Tanzania: a randomized controlled trial. JAMA 2012;308(15):1535‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padayatty SJ, Levine M. Standard‐dose vs high‐dose multivitamin supplements for HIV. JAMA 2013;309(6):545‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jiamton 2003 THA {published data only}
- Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B, Hanshaoworakul W, et al. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV‐infected individuals living in Bangkok. AIDS 2003;17(17):2461‐9. [DOI] [PubMed] [Google Scholar]
Kamwesiga 2015 RWA {published data only}
- Kamwesiga J, Mutabazi V, Kayumba J, Tayari JK, Smyth R, Fay H, et al. Effect of selenium supplementation on CD4 T‐cell count recovery, viral suppression, morbidity and quality of life of HIV‐infected patients in Rwanda: study protocol for randomized controlled trial. Trials 2011;12:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamwesiga J, Mutabazi V, Kayumba J, Tayari JK, Uwimbabazi JC, Batanage G, et al. Effect of selenium supplementation on CD4+ T‐cell recovery, viral suppression and morbidity of HIV‐infected patients in Rwanda: a randomized controlled trial. AIDS 2015;29(9):1045‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 1999 ZMB {published data only}
- Kelly P, Musonda R, Kafwembe E, Kaetano L, Keane E, Farthing M. Micronutrient supplementation in the AIDS diarrhoea‐wasting syndrome in Zambia: a randomized controlled trial. AIDS 1999;13(4):495‐500. [DOI] [PubMed] [Google Scholar]
Kelly 2008 ZMB {published data only}
- Kelly P, Katubulushi M, Todd J, Banda R, Yambayamba V, Fwoloshi M, et al. Micronutrient supplementation has limited effects on intestinal infectious disease and mortality in a Zambian population of mixed HIV status: a cluster randomized trial. American Journal of Clinical Nutrition 2008;88(4):1010‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Shawa T, Mwanamakondo S, Soko R, Smith G, Barclay GR, et al. Gastric and intestinal barrier impairment in tropical enteropathy and HIV: limited impact of micronutrient supplementation during a randomised controlled trial. BMC Gastroenterology 2010;10:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Shawa T, Sanderson I. Gastric hypochlorhydria and intestinal barrier dysfunction in HIV infection is not dependent on nutrition: a randomised controlled trial of supplementation. Gut 2009;58:A82‐3. [Google Scholar]
Lawson 2010 NIG {published data only}
- Lawson L, Thacher TD, Yassin MA, Onuoha NA, Usman A, Emenyonu NE, et al. Randomized controlled trial of zinc and vitamin A as co‐adjuvants for the treatment of pulmonary tuberculosis. Tropical Medicine & International Health 2010;15(12):1481‐90. [DOI] [PubMed] [Google Scholar]
McClelland 2004 KEN {published data only}
- McClelland RS, Baeten JM, Overbaugh J, Richardson BA, Mandaliya K, Emery S, et al. Micronutrient supplementation increases genital tract shedding of HIV‐1 in women: results of a randomized trial. Journal of Acquired Immune Deficiency Syndromes 2004;37(5):1657‐63. [DOI] [PubMed] [Google Scholar]
Overton 2015 USA {published data only}
- Overton ET, Chan ES, Brown TT, Tebas P, McComsey GA, Melbourne KM, et al. High‐dose vitamin D and calcium attenuates bone loss with antiretroviral therapy initiation. Annals of Internal Medicine 2015;162(12):815‐24. [DOI: 10.7326/M14-1409] [DOI] [PMC free article] [PubMed] [Google Scholar]
Range 2006 TZA {published data only}
- Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi‐vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two‐by‐two factorial trial in Mwanza, Tanzania. British Journal of Nutrition 2006;95(4):762‐70. [DOI] [PubMed] [Google Scholar]
Semba 1998 USA {published data only}
- Semba RD, Lyles CM, Margolick JB, Caiaffa WT, Farzadegan H, Cohn S, et al. Vitamin A supplementation and human immunodeficiency virus load in injection drug users. Journal of Infectious Diseases 1998;177(3):611‐6. [DOI] [PubMed] [Google Scholar]
Semba 2007 MWI {published data only}
- Semba RD, Kumwenda J, Zijlstra E, Ricks MO, Lettow M, Whalen C, et al. Micronutrient supplements and mortality of HIV‐infected adults with pulmonary TB: a controlled clinical trial. International Journal of Tuberculosis and Lung Disease 2007;11(8):854‐9. [PubMed] [Google Scholar]
Semba 2007 USA {published data only}
- Semba RD, Ricketts EP, Mehta S, Netski D, Thomas D, Kirk G, et al. Effect of micronutrients and iron supplementation on hemoglobin, iron status, and plasma hepatitis C and HIV RNA levels in female injection drug users: a controlled clinical trial. Journal of Acquired Immune Deficiency Syndromes 2007;45(3):298‐303. [DOI] [PubMed] [Google Scholar]
Stallings 2014 USA {published data only}
- Stallings VA, Schall JI, Hediger ML, Zemel BS, Tuluc F, Dougherty KA, et al. High‐dose vitamin D3 supplementation in children and young adults with HIV: a randomized, placebo‐controlled trial. Paediatric Infectious Disease Journal 2014;34(2):e32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villamor 2008 TZA {published data only}
- Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K, et al. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. Journal of Infectious Diseases 2008;197(11):1499‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wejse 2009 GNB {published data only}
- Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double‐blind, randomized, placebo‐controlled trial. American Journal of Respiratory and Critical Care Medicine 2009;179(9):843‐50. [DOI] [PubMed] [Google Scholar]
Zhao 2010 CHN {published data only}
- Zhao F, Feng XL, Xu W, Ma YM, Wang Z, LI WJ. Effect of micronutrients on the immune status of human immunodeficiency virus‐positive individuals. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2010;32(3):340‐2. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aghdassi 2010 {published data only}
- Aghdassi E, Arendt BM, Salit IE, Mohammed SS, Jalali P, Bondar H, et al. In patients with HIV‐infection, chromium supplementation improves insulin resistance and other metabolic abnormalities: a randomized, double‐blind, placebo controlled trial. Current HIV Research 2010;8(2):113‐20. [DOI] [PubMed] [Google Scholar]
AIDS Policy Law 2012 {published data only}
- No authors listed. High‐dose vitamins may be harmful for those on HAART. AIDS Policy Law 2012;28(2):3. [PubMed] [Google Scholar]
Arpadi 2012 {published data only}
- Arpadi SM, McMahon DJ, Abrams EJ, Bamji M, Purswani M, Engelson ES, et al. Effect of supplementation with cholecalciferol and calcium on 2‐y bone mass accrual in HIV‐infected children and adolescents: a randomized clinical trial. American Journal of Clinical Nutrition 2012;95(3):678‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Austin 2006 {published data only}
- Austin J, Singhal N, Voigt R, Smaill F, Gill MJ, Walmsley S, et al. A community randomized controlled clinical trial of mixed carotenoids and micronutrient supplementation of patients with acquired immunodeficiency syndrome. European Journal of Clinical Nutrition 2006;60(11):1266‐76. [DOI] [PubMed] [Google Scholar]
Balasubramanyam 2011 {published data only}
- Balasubramanyam A, Coraza I, Smith EO, Scott LW, Patel P, Iyer D, et al. Combination of niacin and fenofibrate with lifestyle changes improves dyslipidemia and hypoadiponectinemia in HIV patients on antiretroviral therapy: results of "heart positive," a randomized, controlled trial. Journal of Clinical Endocrinology and Metabolism 2011;96(7):2236‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Balfour 2014 {published data only}
- Balfour L, Spaans JN, Fergusson D, Huff H, Mills EJ, Porte CJ, et al. Micronutrient deficiency and treatment adherence in a randomized controlled trial of micronutrient supplementation in ART‐naive persons with HIV. PLoS ONE 2014;9(1):e85607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2015 {published data only}
- Brown JC, Schall JI, Rutstein RM, Leonard MB, Zemel BS, Stallings VA. The impact of vitamin D3 supplementation on muscle function among HIV‐infected children and young adults: a randomized controlled trial. Journal of Musculoskeletal & Neuronal Interactions 2015;15(2):145‐53. [PMC free article] [PubMed] [Google Scholar]
Chow 2010 {published data only}
- Chow DC, Stein JH, Seto TB, Mitchell C, Sriratanaviriyakul N, Grandinetti A, et al. Short‐term effects of extended‐release niacin on endothelial function in HIV‐infected patients on stable antiretroviral therapy. AIDS 2010;24(7):1019‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coelho 2015 {published data only}
- Coelho L, Cardoso SW, Luz PM, Hoffman RM, Mendonça L, Veloso VG, et al. Vitamin D3 supplementation in HIV infection: effectiveness and associations with antiretroviral therapy. Nutrition Journal 2015;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Currier 2010 {published data only}
- Currier J. Report from the 11th International Workshop on Adverse Drug Reactions and Co‐morbidities in HIV. Treatment for patients with central fat accumulation. Journal Watch. AIDS Clinical Care 2010;22(3):21‐2. [PubMed] [Google Scholar]
Daneshpajouhnejad 2011 {published data only}
- Daneshpajouhnejad P. The effect of zinc supplementation on the number of lymphocytes in HIV‐infected patients: a randomized trial. European Journal of Medical Research 2011;16:167‐8. [Google Scholar]
Etminani‐Esfahani 2012 {published data only}
- Etminani‐Esfahani M, Khalili H, Soleimani N, Jafari S, Abdollahi A, Khazaeipour Z, et al. Serum vitamin D concentration and potential risk factors for its deficiency in HIV positive individuals. Current HIV Research 2012;10(2):165‐70. [DOI] [PubMed] [Google Scholar]
Gharakhanian 2011 {published data only}
- Gharakhanian S, Kotler DP. Diabetes mellitus, HIV infection, and vitamin D: time to act or time to think?. AIDS 2011;25(4):531‐3. [DOI] [PubMed] [Google Scholar]
Groleau 2013 {published data only}
- Groleau V, Herold RA, Schall JI, Wagner JL, Dougherty KA, Zemel BS, et al. Blood lead concentration is not altered by high‐dose vitamin D supplementation in children and young adults with HIV. Journal of Pediatric Gastroenterology and Nutrition 2013;56(3):316‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Havens 2012a {published data only}
- Havens PL, Mulligan K, Hazra R, Flynn P, Rutledge B, Loan MD, et al. Serum 25‐hydroxyvitamin D response to vitamin D3 supplementation 50,000 IU monthly in youth with HIV‐1 infection. Journal of Clinical Endocrinology Metabolism 2012;97(11):4004‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Havens 2012b {published data only}
- Havens PL, Mulligan K, Hazra R, Loan MD, Pan CG, Bethel J, et al. Safety and efficacy of once‐monthly dosing of vitamin D3 (50,000 IU) in HIV‐infected youth: Adolescent Trials Network Study 063. Antiviral Therapy 2011;16:A9‐10. [Google Scholar]
Havens 2012c {published data only}
- Havens PL, Mulligan K, Hazra R, Loan MD, Rutledge BN, Bethel J, et al. Increase in fibroblast growth factor 23 (FGF23) in response to vitamin D3 supplementation in HIV‐infected adolescents and young adults on tenofovir‐containing combination antiretroviral therapy (cART): Adolescent Trials Network (ATN) study 063. Antiviral Therapy 2012;17:A7‐8. [Google Scholar]
Havens 2012d {published data only}
- Havens PL, Stephensen CB, Hazra R, Flynn PM, Wilson CM, Rutledge B, et al. Vitamin D3 decreases parathyroid hormone in HIV‐infected youth being treated with tenofovir: a randomized, placebo‐controlled trial. Clinical Infectious Diseases 2012;54(7):1013‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemsworth 2012 {published data only}
- Hemsworth JC, Hekmat S, Reid G. Micronutrient supplemented probiotic yogurt for HIV‐infected adults taking HAART in London, Canada. Gut Microbes 2012;3(5):414‐9. [DOI] [PubMed] [Google Scholar]
Hummelen 2011 {published data only}
- Hummelen R, Hemsworth J, Changalucha J, Butamanya NL, Hekmat S, Habbema JD, et al. Effect of micronutrient and probiotic fortified yogurt on immune‐function of anti‐retroviral therapy naive HIV patients. Nutrients 2011;3(10):897‐909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaiser 2006 {published data only}
- Kaiser JD, Campa AM, Ondercin JP, Leoung GS, Pless RF, Baum MK. Micronutrient supplementation increases CD4 count in HIV‐infected individuals on highly active antiretroviral therapy: A prospective, double‐blinded, placebo‐controlled trial. Journal of Acquired Immune Deficiency Syndromes 2006;42(5):523‐8. [DOI] [PubMed] [Google Scholar]
Kakalia 2011a {published data only}
- Kakalia S, Sochett E, Assor E, Arneson C, Stephens D, Read S, et al. Vitamin D supplementation and CD4 count in HIV infected children. Canadian Journal of Infectious Diseases and Medical Microbiology 2010;21:53B. [Google Scholar]
Kakalia 2011b {published data only}
- Kakalia S, Sochett EB, Stephens D, Assor E, Read SE, Bitnun A. Vitamin D supplementation and CD4 count in children infected with human immunodeficiency virus. Journal of Pediatrics 2011;159(6):951‐7. [DOI] [PubMed] [Google Scholar]
Lachmann 2014 {published data only}
- Lachmann R, Bevan MA, Kim S, Patel N, Banga R, Chandra N, et al. A trial of vitamin D as an adjunct to HAART in HIV infection: potential to reduce progression and mortality. HIV Medicine 2014;15:47‐8. [Google Scholar]
Ladep 2010 {published data only}
- Ladep N, Shehu N, Muazu A, Ugoagwu P, Kakjing F, Badung B, et al. Efficacy of multivitamins containing phosphatidyl choline in the management of hepatotoxicity from antiretroviral and/or antituberculous drugs. International Journal of Infectious Diseases 2010;14:e245. [Google Scholar]
Lange 2009 {published data only}
- Lange J, Gazzard B, Diaz R, Gori A, Mourmans B, Raijmakers J, et al. Effect of a nutritional intervention on CD4+ T cell decline in HIV‐1‐positive adults not on antiretroviral therapy: A multicentre, randomized, controlled, double‐blind clinical trial (BITE), with 52 weeks of follow‐up. HIV Medicine 2009;10:42. [Google Scholar]
Lange 2011 {published data only}
- Lange JMA. The potential of nutritional interventions in HIV infection. European Journal of Pharmacology 2011;668:e3. [Google Scholar]
Lescoat 2012 {published data only}
- Lescoat A, Poinsignon Y, Dos Santos A, Cano Y, Floch F, Jardel H. Vitamin D supplementation: A iatrogenic hypercalcemia concerning an HIV‐infected patient with disseminated tuberculosis. Presse Medicale 2012;41(12 Pt 1):1299‐301. [DOI] [PubMed] [Google Scholar]
Lin 2013 {published data only}
- Lin C, Grandinetti A, Shikuma C, Souza S, Parikh N, Nakamoto B, et al. The effects of extended release niacin on lipoprotein sub‐particle concentrations in HIV‐infected patients. Hawai'i Journal of Medicine & Public Health 2013;72(4):123‐7. [PMC free article] [PubMed] [Google Scholar]
Longenecker 2011 {published data only}
- Longenecker CT, Hileman CO, Carman TL, Ross AC, Seydafkan S, Brown TT, et al. Vitamin D supplementation and endothelial function in vitamin D deficient HIV‐infected patients: a randomized placebo‐controlled trial. Antiviral Therapy 2012;17(4):613‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Madrid 2012 {published data only}
- Madrid L, Deyà À, Noguera‐Julian A, Falcón‐Neyra L, Valls A, Neth O, et al. Normal levels of vitamin D among HIV‐infected Catalan pediatric patients. Journal of Acquired Immune Deficiency Syndromes 2012;61(2):e18‐9. [DOI] [PubMed] [Google Scholar]
Mandal 2011 {published data only}
- Mandal P, Campa A, Sales Martinez S, Williams S, Barr S, Shin DH, et al. Vitamin D, cognition and depression in HIV + adults. FASEB Journal 2011;25(1):766.3. [Google Scholar]
Martineau 2013 {published data only}
- Martineau AR. Vitamin D: an adjunct to antiretroviral therapy?. Journal of Infectious Diseases 2013;207(3):373‐5. [DOI] [PubMed] [Google Scholar]
Mascitelli 2011 {published data only}
- Mascitelli L, Pezzetta F, Goldstein MR. Cholesterol, vitamin D and cardiovascular prevention in HIV patients treated with antiretroviral therapy. International Journal of Cardiology 2011;146(3):441‐2. [DOI] [PubMed] [Google Scholar]
Mburu 2010 {published data only}
- Mburu AS, Thurnham DI, Mwaniki DL, Muniu EM, Alumasa FM. The influence of inflammation on plasma zinc concentration in apparently healthy, HIV+ Kenyan adults and zinc responses after a multi‐micronutrient supplement. European Journal of Clinical Nutrition 2010;64(5):510‐7. [DOI] [PubMed] [Google Scholar]
Mehta 2010 {published data only}
- Mehta S, Fawzi WW. Micronutrient supplementation as adjunct treatment for HIV‐infected patients. Clinical Infectious Diseases 2010;50(12):1661‐3. [DOI] [PubMed] [Google Scholar]
Morgan 2010 {published data only}
- Morgan E, Wobeser W, Gambhir V, Iourtchenko M, Zhang P. Effect of micronutrient supplementation on lactate metabolism and mitochondrial respiratory chain protein expression in persons treated for HIV. Canadian Journal of Infectious Diseases and Medical Microbiology 2010;21:55B. [Google Scholar]
Motswagole 2013 {published data only}
- Motswagole BS, Mongwaketse TC, Mokotedi M, Kobue‐Lekalake RI, Bulawayo BT, Thomas TS, et al. The efficacy of micronutrient‐fortified sorghum meal in improving the immune status of HIV‐positive adults. Annals of Nutrition & Metabolism 2013;62(4):323‐30. [DOI] [PubMed] [Google Scholar]
Pasquet 2011 {published data only}
- Pasquet A, Viget N, Ajana F, Tribonniere X, Dubus S, Paccou J, et al. Vitamin D deficiency in HIV‐infected patients: associated with non‐nucleoside reverse transcriptase inhibitor or efavirenz use?. AIDS 2011;25(6):873‐4. [DOI] [PubMed] [Google Scholar]
PrayGod 2011 {published data only}
- PrayGod G, Range N, Faurholt‐Jepsen D, Jeremiah K, Faurholt‐Jepsen M, Aabye MG, et al. Daily multi‐micronutrient supplementation during tuberculosis treatment increases weight and grip strength among HIV‐uninfected but not HIV‐infected patients in Mwanza, Tanzania. Journal of Nutrition 2011;141(4):685‐91. [DOI] [PubMed] [Google Scholar]
Schall 2016 {published data only}
- Schall JI, Hediger ML, Zemel BS, Rutstein RM, Stallings VA. Comprehensive safety monitoring of 12‐month daily 7000‐IU vitamin D3 supplementation in human immunodeficiency virus–infected children and young adolescents. Journal of Parenteral and Enteral Nutrition 2016;40(7):1057‐63. [DOI] [PubMed] [Google Scholar]
Scrimgeour 2010 {published and unpublished data}
- Scrimgeour AG, Lukaski HC, Polhemus ME, Otieno L, McGraw SM, Young AJ, et al. Effect of zinc supplementation on diarrhea and malaria morbidity in adults in rural Kenya. FASEB Journal 2010;24(1):538.12. [Google Scholar]
Singhal 2010 {published data only}
- Singhal N, Fergusson D, Huff H, Mills EJ, Porte C, Walmsley S, et al. Design and methods of the MAINTAIN study: a randomized controlled clinical trial of micronutrient and antioxidant supplementation in untreated HIV infection. Contemporary Clinical Trials 2010;31(6):604‐11. [DOI] [PubMed] [Google Scholar]
Steenhoff 2015 {published data only}
- Steenhoff AP, Schall JI, Samuel J, Seme B, Marape M, Ratshaa B, et al. Vitamin D₃ supplementation in Batswana children and adults with HIV: a pilot double blind randomized controlled trial. PLoS ONE 2015;10(2):e0117123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2011 {published data only}
- Stewart T, Campa A, Shin DH, Sales Martinez S, Li Y, Hatsu I, et al. Antioxidant supplementation in HIV+ persons on antiretroviral therapy (ART): a pilot study. FASEB Journal 2011;25(1 Suppl):981.5. [Google Scholar]
Sudarsanam 2011 {published data only}
- Sudarsanam TD, John J, Kang G, Mahendri V, Gerrior J, Franciosa M, et al. Pilot randomized trial of nutritional supplementation in patients with tuberculosis and HIV‐tuberculosis coinfection receiving directly observed short‐course chemotherapy for tuberculosis. Tropical Medicine & International Health 2011;16(6):699‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visser 2011 {published data only}
- Visser ME, Grewal HM, Swart EC, Dhansay MA, Walzl G, Swanevelder S, et al. The effect of vitamin A and zinc supplementation on treatment outcomes in pulmonary tuberculosis: a randomized controlled trial. American Journal of Clinical Nutrition 2011;93(1):93‐100. [DOI] [PubMed] [Google Scholar]
Welz 2011 {published data only}
- Welz T, Childs K, Ibrahim F, Poulton M, Taylor CB, Moniz CF, et al. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS 2010;24(12):1923‐8. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Odunukwe 2016 {published data only}
- Odunukwe NN, Okwuzu JO, Okwuraiwe AP, Gbajabiamila TA, Musa ZA, Ezeobi PM, et al. Selenium as adjunct to HAART in the management of HIV/Hepatitis B virus coinfection: a randomized open label study. African Journal of Clinical and Experimental Microbiology 2016;17(3):197‐203. [Google Scholar]
References to ongoing studies
Lebouché 2014 {published data only}
- Lebouché B, Jenabian M, Singer J, Graziani GM, Engler K, Trottier B, et al. The role of extended‐release niacin on immune activation and neurocognition in HIV‐infectedpatients treated with antiretroviral therapy –CTN PT006: study protocol for a randomized controlled trial. Trials 2014;15:390. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT 01295034 {published data only}
- NCT 01295034. Vitamin D supplements for HIV‐positive patients on cART. clinicaltrials.gov/show/NCT01295034 (first received 10 February 2011).
NCT 01798680 {published data only}
- NCT 01798680. Trial of Vitamin D in HIV progression (TOV4). clinicaltrials.gov/show/NCT01798680 (first received 21 February 2013).
NCT 02810275 {published data only}
- NCT 02810275. Folinic Acid: Supplementation and Therapy. clinicaltrials.gov/show/NCT02810275 (first received 19 June 2016).
NCT 02827643 {published data only}
- NCT 02827643. Vitamin D and Calcium Supplement Attenuate Bone Loss Among HIV‐ Infected Patients Receiving Tenofovir Disoproxil Fumarate, Lamivudine or Emtricitabine and Efavirenz. clinicaltrials.gov/show/NCT02827643 (first received 6 July 2016).
NCT 02856269 {published data only}
- NCT 02856269. Zinc Supplementation and Cardiovascular Risk in HIV. clinicaltrials.gov/show/NCT02827643 (first received 29 July 2016).
Additional references
Aibibula 2016
- Aibibula W, Cox J, Hamelin A, Mamiya H, Klein MB, Brassard P. Food insecurity and low CD4 count among HIV‐infected people: a systematic review and meta‐analysis. AIDS Care 2016;28(12):1577‐85. [DOI] [PubMed] [Google Scholar]
Aziz 2013
- Aziz M, Livak B, Burke‐Miller J, French A, Glesby MJ, Sharma A, et al. Vitamin D insufficiency may impair CD4 recovery among Women's Interagency HIV Study (WIHS) participants with advanced disease on HAART. AIDS 2013;27(4):573‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baum 1997
- Baum MK, Shor‐Posner G, Lai S, Zhang G, Lai H, Fletcher MA, et al. High risk of HIV‐related mortality is associated with selenium deficiency. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1997;15(5):370‐4. [DOI] [PubMed] [Google Scholar]
Baum 2003
- Baum MK, Campa A, Lai S, Lai H, Page JB. Zinc status in human immunodeficiency virus type 1 infection and illicit drug use. Clinical Infectious Diseases 2003;37 Suppl 2:S117‐23. [PUBMED: 12942385] [DOI] [PubMed] [Google Scholar]
Button 2013
- Button KS, Loannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience 2013;14(5):365‐76. [DOI] [PubMed] [Google Scholar]
Chandra 1997
- Chandra RK. Nutrition and the immune system: an introduction. Journal of Nutrition 1997;66(2):460S‐3S. [DOI] [PubMed] [Google Scholar]
Collaborative Group on AIDS Incubation 2000
- Collaborative Group on AIDS Incubation and HIV Survival including the CASCADE EU Concerted Action. Time from HIV‐1 seroconversion to AIDS and death before widespread use of highly‐active antiretroviral therapy: a collaborative re‐analysis. Lancet 2000;355(9210):1131‐7. [PubMed] [Google Scholar]
DAIDS 2014
- Division of AIDS. Table for grading the severity of adult and pediatric adverse events. http://rsc.tech‐res.com/docs/default‐source/safety/daids_ae_grading_table_v2_nov2014.pdf (accessed 16 February 2017).
Forrester 2011
- Forrester JE, Sztam KA. Micronutrients in HIV/AIDS: is there evidence to change the WHO 2003 recommendations?. American Journal of Clinical Nutrition 2011;94(6):1683S‐9S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gebrehiwot 2014
- Gebrehiwot T, Veen A. Coping with food insecurity on a micro‐scale: evidence from Ethiopian rural households. Ecology of Food and Nutrition 2014;53(2):214‐40. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 01 March 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Graham 1991
- Graham NM, Sorensen D, Odaka N, Brookmeyer R, Chan D, Willett WC, et al. Relationship of serum copper and zinc levels to HIV‐1 seropositivity and progression to AIDS. Journal of Acquired Immune Deficiency Syndromes 1991;4(10):976‐80. [PUBMED: 1890606] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Version 5.1.0. The Cochrane Collaboration.
Irlam 2007
- Irlam J, Hussey G, Dhansay M. The effects of nutritional interventions in HIV/AIDS: Micronutrients. In: ASSAf Study Panel, editor(s). HIV/AIDS, TB and Nutrition: Scientific inquiry into the nutritional influences on human immunity with special reference to HIV infection and active TB in South Africa. Pretoria: Academy of Science of South Africa (ASSAf), 2007:143‐51. [Google Scholar]
Irlam 2013
- Irlam JH, Siegfried N, Visser ME, Rollins NC. Micronutrient supplementation for children with HIV infection. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010666] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISO 2016
- International Organization for Standardization. ISO 3166‐1:2013 Codes for the representation of names of countries and their subdivisions ‐‐ Part 1: Country codes. www.iso.org/iso/home/standards.htm (accessed 31 October 2016).
Kupka 2004
- Kupka R, Msamanga GI, Spiegelman D, Morris S, Mugusi F, Hunter DJ, et al. Selenium status is associated with accelerated HIV disease progression among HIV‐infected pregnant women in Tanzania. Journal of Nutrition 2004;134(10):2556‐60. [DOI] [PubMed] [Google Scholar]
Martí‐Carvajal 2010
- Martí‐Carvajal AJ, Cruciani M. Pharmacological interventions for treating dyslipidemia in patients with HIV infection. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008869] [DOI] [Google Scholar]
Mbuagbaw 2010
- Mbuagbaw LCE, Irlam JH, Spaulding A, Rutherford GW, Siegfried N. Efavirenz or nevirapine in three‐drug combination therapy with two nucleoside‐reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral‐naïve individuals. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD004246.pub3] [DOI] [PubMed] [Google Scholar]
Raiten 2011
- Raiten DJ. Nutrition and pharmacology: general principles and implications for HIV. American Journal of Clinical Nutrition 2011;94(6):1697S‐702S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raiten 2015
- Raiten DJ, Sakr Ashour FA, Ross AC, Meydani SN, Dawson HD, Stephensen CB, et al. Inflammation and Nutritional science for Programs/Policies and interpretation of research Evidence (INSPIRE). Journal of Nutrition 2015;145(5):1039S‐108S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ross 2011
- Ross AC, Judd S, Kumari M, Hileman C, Storer N, Labbato D, et al. Vitamin D is linked to carotid intima‐media thickness and immune reconstitution in HIV‐positive individuals. Antiviral Therapy 2011;16(4):555‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schneider 2008
- Schneider E, Whitmore S, Glynn KM, Dominguez K, Mitsch A, McKenna MT. Revised surveillance case definitions for HIV infection among adults, adolescents, and children aged <18 months and for HIV infection and AIDS among children aged 18 months to <13 years‐‐UnitedStates, 2008. Morbidity and Mortality Weekly Report. Recommendations and reports 2008;57(RR‐10):1‐12. [PubMed] [Google Scholar]
Semba 1993
- Semba RD, Graham NM, Caiaffa WT, Margolick JB, Clement L, Vlahov D. Increased mortality associated with vitamin A deficiency during human immunodeficiency virus type 1 infection. Archives of Internal Medicine 1993;153(18):2149‐54. [PubMed] [Google Scholar]
Semba 1999
- Semba RD, Tang AM. Micronutrients and the pathogenesis of human immunodeficiency virus infection. British Journal of Nutrition 1999;81(3):181‐9. [DOI] [PubMed] [Google Scholar]
Siegfried 2012
- Siegfried N, Irlam JH, Visser ME, Rollins NN. Micronutrient supplementation in pregnant women with HIV infection. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009755] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sudfeld 2012
- Sudfeld CR, Wang M, Aboud S, Giovanucci EL, Mugusi FM, Fawzi WW. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PLoS ONE 2012;7(6):e40036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 1997
- Tang AM, Graham NMH, Chandra RK, Saah AJ. Low serum vit B12 concentrations are associated with faster Human immunodeficiency virus type 1 (HIV‐1) disease progression. Journal of Nutritiom 1997;127(2):345‐51. [DOI] [PubMed] [Google Scholar]
Tang 2005
- Tang AM, Lanzelotti J, Hendricks K, Gerrior J, Ghosh M, Woods M, et al. Micronutrients: current issues for HIV care providers. AIDS 2005;19(9):847‐61. [DOI] [PubMed] [Google Scholar]
UNAIDS 2014
- Joint United Nations Programme on HIV/AIDS. Fact sheet 2014. www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/factsheet/2014/20140716_FactSheet_en.pdf (accessed 21 April 2015).
Viard 2011
- Viard JP, Souberbielle JC, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS 2011;25(10):1305‐15. [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV‐related diseases in adults and children. Geneva: World Health Organization, 2007. [Google Scholar]
WHO 2015
- World Health Organization. Guideline on when to start antiretroviral therapy and on pre‐exposure prophylaxis for HIV. September 2015. http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf (accessed 08 March 2016). [PubMed]
References to other published versions of this review
Irlam 2002
- Irlam JH, Visser ME, Rollins N, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003650] [DOI] [PubMed] [Google Scholar]
Irlam 2005
- Irlam J, Visser MME, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD003650.pub2] [DOI] [PubMed] [Google Scholar]
Irlam 2010
- Irlam J, Visser MME, Rollins NN, Siegfried N. Micronutrient supplementation in children and adults with HIV infection. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD003650.pub3] [DOI] [PubMed] [Google Scholar]


