Abstract
Background
Differentiating both typhoid (Salmonella Typhi) and paratyphoid (Salmonella Paratyphi A) infection from other causes of fever in endemic areas is a diagnostic challenge. Although commercial point‐of‐care rapid diagnostic tests (RDTs) for enteric fever are available as alternatives to the current reference standard test of blood or bone marrow culture, or to the widely used Widal Test, their diagnostic accuracy is unclear. If accurate, they could potentially replace blood culture as the World Health Organization (WHO)‐recommended main diagnostic test for enteric fever.
Objectives
To assess the diagnostic accuracy of commercially available rapid diagnostic tests (RDTs) and prototypes for detecting Salmonella Typhi or Paratyphi A infection in symptomatic persons living in endemic areas.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, MEDLINE, Embase, Science Citation Index, IndMED, African Index Medicus, LILACS, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) up to 4 March 2016. We manually searched WHO reports, and papers from international conferences on Salmonella infections. We also contacted test manufacturers to identify studies.
Selection criteria
We included diagnostic accuracy studies of enteric fever RDTs in patients with fever or with symptoms suggestive of enteric fever living in endemic areas. We classified the reference standard used as either Grade 1 (result from a blood culture and a bone marrow culture) or Grade 2 (result from blood culture and blood polymerase chain reaction, or from blood culture alone).
Data collection and analysis
Two review authors independently extracted the test result data. We used a modified QUADAS‐2 extraction form to assess methodological quality. We performed a meta‐analysis when there were sufficient studies for the test and heterogeneity was reasonable.
Main results
Thirty‐seven studies met the inclusion criteria and included a total of 5080 participants (range 50 to 1732). Enteric fever prevalence rates in the study populations ranged from 1% to 75% (median prevalence 24%, interquartile range (IQR) 11% to 46%). The included studies evaluated 16 different RDTs, and 16 studies compared two or more different RDTs. Only three studies used the Grade 1 reference standard, and only 11 studies recruited unselected febrile patients. Most included studies were from Asia, with five studies from sub‐Saharan Africa. All of the RDTs were designed to detect S.Typhi infection only.
Most studies evaluated three RDTs and their variants: TUBEX in 14 studies; Typhidot (Typhidot, Typhidot‐M, and TyphiRapid‐Tr02) in 22 studies; and the Test‐It Typhoid immunochromatographic lateral flow assay, and its earlier prototypes (dipstick, latex agglutination) developed by the Royal Tropical Institute, Amsterdam (KIT) in nine studies. Meta‐analyses showed an average sensitivity of 78% (95% confidence interval (CI) 71% to 85%) and specificity of 87% (95% CI 82% to 91%) for TUBEX; and an average sensitivity of 69% (95% CI 59% to 78%) and specificity of 90% (95% CI 78% to 93%) for all Test‐It Typhoid and prototype tests (KIT). Across all forms of the Typhidot test, the average sensitivity was 84% (95% CI 73% to 91%) and specificity was 79% (95% CI 70% to 87%). When we based the analysis on the 13 studies of the Typhidot test that either reported indeterminate test results or where the test format means there are no indeterminate results, the average sensitivity was 78% (95% CI 65% to 87%) and specificity was 77% (95% CI 66% to 86%). We did not identify any difference in either sensitivity or specificity between TUBEX, Typhidot, and Test‐it Typhoid tests when based on comparison to the 13 Typhidot studies where indeterminate results are either reported or not applicable. If TUBEX and Test‐it Typhoid are compared to all Typhidot studies, the sensitivity of Typhidot was higher than Test‐it Typhoid (15% (95% CI 2% to 28%), but other comparisons did not show a difference at the 95% level of CIs.
In a hypothetical cohort of 1000 patients presenting with fever where 30% (300 patients) have enteric fever, on average Typhidot tests reporting indeterminate results or where tests do not produce indeterminate results will miss the diagnosis in 66 patients with enteric fever, TUBEX will miss 66, and Test‐It Typhoid and prototype (KIT) tests will miss 93. In the 700 people without enteric fever, the number of people incorrectly diagnosed with enteric fever would be 161 with Typhidot tests, 91 with TUBEX, and 70 with Test‐It Typhoid and prototype (KIT) tests. The CIs around these estimates were wide, with no difference in false positive results shown between tests.
The quality of the data for each study was evaluated using a standardized checklist called QUADAS‐2. Overall, the certainty of the evidence in the studies that evaluated enteric fever RDTs was low.
Authors' conclusions
In 37 studies that evaluated the diagnostic accuracy of RDTs for enteric fever, few studies were at a low risk of bias. The three main RDT tests and variants had moderate diagnostic accuracy. There was no evidence of a difference between the average sensitivity and specificity of the three main RDT tests. More robust evaluations of alternative RDTs for enteric fever are needed.
2 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (4 Mar, 2016) were included
Plain language summary
The accuracy of rapid diagnostic tests for detecting typhoid and paratyphoid (enteric) fever
Cochrane researchers assessed the accuracy of commercially‐available rapid diagnostic tests and their prototypes (including TUBEX, Typhidot, Typhidot‐M, Test‐it Typhoid, and other tests) for detecting typhoid and paratyphoid (enteric) fever in people living in countries where the estimated number of individuals with the disease at any one time is greater than 10 per 100,000 population. If accurate, they could replace the current World Health Organization (WHO)‐recommended diagnostic test: culture (growing the bacteria that causes the infection from a patient’s blood or bone marrow).
Background
Typhoid fever and paratyphoid fever are infections caused by the bacteria Salmonella Typhi and Salmonella Paratyphi A respectively. The term ‘enteric fever’ is used to describe both infections. Enteric fever can be difficult to diagnose as the signs and symptoms are similar to those of other infectious diseases that cause fever such as malaria.
The recommended test to confirm if a person has enteric fever is to grow the Salmonella from their blood. It takes at least 48 hours to give a result, so cannot help healthcare workers make a diagnosis the same day the blood culture is taken. Blood cultures may give a negative result even though a person has enteric fever. The test also requires a laboratory and trained staff, which are often unavailable in communities where enteric fever is common.
Rapid diagnostic tests (RDTs) are designed to be easy to use, and to deliver a quick result without the need for a blood culture laboratory. The cost of an enteric fever RDT would be significantly less than a blood culture, and requires less training to perform.
Study characteristics
Cochrane researchers searched the available literature up to 4 March 2016 and included 37 studies. Most studies recruited participants from South Asia. Most participants were adults, with 22 studies including children. All of the RDTs evaluated detected Salmonella Typhi (typhoid fever) only.
Quality of the evidence
The Cochrane researchers evaluated the quality of the data for each study using a standardized checklist called QUADAS‐2. High quality studies that compared different types of RDT in the same patients were few in number. Two‐thirds of the included studies did not evaluate the RDTs in the context of patients who are typically tested for the disease. Many studies utilized a particular study design (a case control study) which risks overestimating RDT accuracy. In the studies evaluating the Typhidot RDT, it was often unclear how many test results were indeterminate, when the test cannot distinguish a current episode of infection from a previous disease episode. Overall, the certainty of the evidence in the studies that evaluated enteric fever RDTs was low.
Key results
Sensitivity indicates the percentage of patients with a positive test result who are correctly diagnosed with disease. Specificity indicates the percentage of patients who are correctly identified as not having disease. TUBEX showed an average sensitivity of 78% and specificity of 87%. Typhidot studies, grouped together to include Typhidot, Typhidot‐M, and TyphiRapid‐Tr02, showed an average sensitivity of 84% and specificity of 79%. When Typhidot studies with clear reporting of indeterminate results are considered, the average sensitivity and specificity of Typhidot was 78% and 77% respectively. Test‐It Typhoid and prototypes (KIT) showed an average sensitivity of 69% and specificity of 90%.
Based on these results, in 1000 patients with fever where 30% (300 patients) have enteric fever, we would expect Typhidot tests reporting indeterminate results or where tests do not produce indeterminate results to, on average, miss the diagnosis (give a false negative result) in 66 patients with enteric fever, TUBEX to miss 66, and Test‐It Typhoid and prototypes (KIT) to miss 93. In the 700 people without enteric fever, the number of people incorrectly given a diagnosis of enteric fever (a false positive result) would be on average 161 with these Typhidot tests, 91 with TUBEX, and 70 with the Test‐It Typhoid and prototypes (KIT). These differences in the number of false negative and false positive results in patients from the different tests are not statistically important. The RDTs evaluated are not sufficiently accurate to replace blood culture as a diagnostic test for enteric fever.
Summary of findings
Summary of findings'. ''Summary of findings' table 1.
|
Review question: to assess the diagnostic accuracy of rapid diagnostic tests (RDTs) for detecting enteric fever in persons living in endemic areas presenting to a healthcare facility with fever Patients/population: clinically‐suspected enteric fever patients or unselected febrile patients Role: first test for enteric fever in patients presenting to a healthcare facility with fever in endemic areas Index tests: all RDTs specifically designed to enteric fever cases applied to patient blood or urine samples Reference standards: bone marrow culture, peripheral blood culture, peripheral blood culture, and polymerase chain reaction (PCR) on blood Studies: prospective cohort, retrospective case control Setting: healthcare facility in enteric fever endemic areas | ||||||
| Index test | Effect (95% confidence interval (CI)) |
Participants Total number, number with disease, (number of studies) |
Test result | Number of results per 1000 participants tested 1 (95% CI) | ||
| Prevalence 1% | Prevalence 10% | Prevalence 30% | ||||
| Typhidot (all types) |
Sensitivity 84 (73 to 91) Specificity 79 (70 to 87) |
6928, 982 (22) | TP FN FP TN |
8 (7 to 9) 2 (1 to 3) 208 (129 to 297) 782 (693 to 861) |
84 (73 to 91) 16 (9 to 27) 189 (117 to 270) 711 (630 to 783) |
252 (219 to 273) 48 (27 to 81) 147 (91 to 210) 553 (490 to 609) |
| Typhidot indeterminants reported or not applicable | Sensitivity 78 (65 to 87) Specificity 77 (66 to 86) |
5555, 662 (13) | TP FN FP TN |
8 (7 to 9) 2 (1 to 3) 228 (139 to 337) 762 (653 to 851) |
78 (65 to 87) 22 (13 to 35) 207 (126 to 306) 693 (594 to 774) |
234 (195 to 261) 66 (39 to 105) 161 (98 to 238) 539 (462 to 602) |
| Typhidot indeterminate results reported | Sensitivity 66 (59 to 73) Specificity 81 (58 to 93) |
1721, 339 (6) | TP FN FP TN |
7 (6 to 7) 3 (3 to 4) 188 (69 to 416) 802 (574 to 921) |
66 (59 to 73) 34 (27 to 41) 171 (63 to 378) 729 (522 to 837) |
198 (177 to 219) 102 (81 to 123 ) 133 (49 to 294) 567 (406 to 651) |
| TUBEX | Sensitivity 78 (71 to 85) Specificity 87 (82 to 91) |
4885, 627 (14) | TP FN FP TN |
8 (7 to 9) 2 (2 to 3) 129 (89 to 178) 861 (812 to 901) |
78 (71 to 85) 22 (15 to 29) 117 (81 to 162) 783 (738 to 819) |
234 (213 to 255) 66 (45 to 87) 91 (63 to 126) 609 (574 to 637) |
| Test‐it Typhoid and KIT prototypes (threshold > 1+) | Sensitivity 69 (59 to 78) Specificity 90 (78 to 93) |
2828, 682 (9) | TP FN FP TN |
7 (6 to 8) 3 (2 to 4) 99 (69 to 218) 891 (772 to 921) |
69 (59 to 78) 31 (22 to 41) 90 (63 to 198) 810 (702 to 837) |
207 (177 to 234) 93 (66 to 123) 70 (49 to 154) 630 (546 to 651) |
| Attributes of tests contributing to benefits and risks | ||||||
| Rapid diagnostic tests (RDTs)2 | RDTs are designed to provide test results typically in less than 1 hour, whereas currently used blood culture tests require 48 hours. The technical ability needed to conduct these rapid tests is designed to be lower than typical laboratory based tests, meaning they have the potential to be delivered nearer to the patient, further reducing time to diagnosis. However, some variants of the Typhidot test requires additional laboratory equipment, whereas the TUBEX and Test‐it Typhoid test do not. The TUBEX tests and some variants of Typhidot require cold chain storage. The Test‐it Typhoid test does not. In this Cochrane Review all included rapid tests were used on blood samples. None of the included studies conducted tests on urine samples. | |||||
| Overall certainty of evidence | ||||||
|
Indeterminate results: for the Typhidot index test, there are concerns about studies which do not report indeterminate results (IgM negative and IgG positive). These results can frequently occur and if these results are not included in the analysis this biases study results to be overly‐optimistic. Case control studies: many of these studies use a case control design. This study design is at risk of overestimating both sensitivity and specificity. Reference standard: the highest grade of reference standard includes either bone marrow culture or PCR using blood, in addition to blood culture. However using bone marrow as a reference standard is invasive and more severe patients may be selected into these studies. Most included studies use only blood culture, and studies using more than 1 reference standard for example, PCR showed a reduction in RDT sensitivity by 20% to 25%. Precision: average estimates of both sensitivity and specificity have low precision, due to the heterogeneity between studies. Paired studies: there are few paired studies, where more than 1 test is used in the same patients. These studies provide the most direct evidence for comparing tests. Typhidot paired with TUBEX: Total 4245, 484 patients with disease. Typhidot paired with Test‐it Typhoid and KIT prototypes: no paired studies. Test‐it Typhoid and KIT prototypes paired with TUBEX: total 127, 64 patients with disease. It remains unclear if the tests were used in the same cohort of patients. | ||||||
Abbreviations: False Negatives (FN); False Positives (FP); immunoglobulin‐G (IgG); immunoglobulin‐M (IgM); Royal Tropical Institute, Amsterdam (KIT); polymerase chain reaction (PCR); True Negatives (TN); True Positives (TP).
1We used 2 systematic reviews of bacteraemia in Asia and Africa to inform prevalences of 30% (Asia); 10% (Africa: adults and children) and 1% (Africa: children) (Reddy 2010; Deen 2012). 2Keddy 2011.
Background
Target condition being diagnosed
Typhoid and paratyphoid (enteric) fever are diseases caused by Salmonella enterica serovar Typhi and Paratyphi A respectively. Typhoid, the more common infection, is an important infectious disease in low‐ and middle‐income countries (LMICs) with over 22 million new cases worldwide and an estimated 200,000 deaths annually (WHO 2003). South and South‐East Asia are the most affected areas of the world, with an estimated annual incidence in some areas of greater than 100 people per 100,000 population (Crump 2004). Enteric fever is common in areas with inadequate sanitation and hygiene, particularly regarding food, water, and disposal of human excrement, and only to this extent are these diseases tropical (Gill 2009). Despite advances in technology and public health strategies, enteric fever remains a major cause of morbidity in the developing world (Bhutta 2006). Urbanization, global warming, and traditional methods of waterside living have created even greater demands for clean water in developing countries (UNICEF 2006). We will use the term 'enteric fever' throughout this Cochrane Review to include both typhoid and paratyphoid fever, unless specified. The causative organisms are Gram‐negative bacilli that are transmitted by the faecal‐oral route when a person ingests food or water that is contaminated with infected human faeces. The most important reservoirs of infection are short‐term convalescents or chronic human carriers. Food handlers who are carriers are a particularly important source of transmission (Gill 2009; Andrews 2015).
The clinical presentation of enteric fever varies from a mild illness with a low‐grade fever, malaise, and slight dry cough to a severe clinical illness with multiple complications including intestinal perforation (Ismail 2006). Toxic apathy, blanching 'rose spots' on the trunk, abdominal organomegaly, and diarrhoea are also associated with enteric fever, but the clinical picture is highly variable between geographical location and age groups. Enteric fever can present in many different and non‐specific ways, thus posing a diagnostic challenge for the health professional. Enteric fever is usually diagnosed on clinical grounds and treated presumptively. The diagnosis may be delayed or missed, while other febrile illnesses are being considered (Parry 2002).
There is antimicrobial resistance to S. enterica serovar Typhi and Paratyphi A worldwide (Kariuki 2015). Health professionals in the tropics overprescribe antimicrobials for many reasons, including cultural factors and patient expectation (Okeke 2005). The purchase of drugs such as antimicrobials from untrained vendors and unlicensed pharmacists is commonplace in the developing world (Larsson 2008). A major challenge is the inability to confirm diagnoses in resource‐limited settings where traditional laboratory methods of diagnosing enteric fever are unavailable. Healthcare workers are therefore reliant on their clinical skills to make an educated guess of the cause of illness or to prescribe an antimicrobial that targets several bacteria, or both (Shetty 2008). This over treatment has contributed to increasing resistance to fluoroquinolones (for example, ciprofloxacin) and multiple drug resistance (resistance to chloramphenicol, ampicillin, and co‐trimoxazole) in S. enterica serovar Typhi and Paratyphi A in endemic Asian countries (Chuang 2009).
Index test(s)
Current enteric fever rapid diagnostic tests (RDTs) include a variety of different methods and formats. RDTs can be applied to blood or urine samples, with blood RDTs (using either venous or capillary samples, or both) most common. Test formats are based on lateral flow, flow‐through, agglutination, or solid phase methods (Pastoor 2008). RDTs may detect antigens (components of the causative Salmonella organism) or antibodies (markers of the person's immune response to the antigen). The type of antibody class or immunoglobulin detected could be either immunoglobulin‐M (IgM), which may be indicative of recent exposure, or immunoglobulin‐G (IgG), which can indicate recent or previous exposure. Examples of commercial RDTs for enteric fever that have been undergoing evaluation in recent years include Typhidot®, Typhidot‐M®, and TUBEXTM (Baker 2010; Thriemer 2013). Future RDTs are also likely to take a serological approach, although the identification of novel antigens that are free of cross‐reacting epitopes is a major challenge (Baker 2010).
Typhidot, TUBEX, and Test‐It Typhoid (KIT) RDTs
The three commercially available index tests that have most commonly been evaluated in published studies are: Typhidot (including Typhidot‐M, and TyphiRapid Tr‐02); TUBEX; and Test‐It Typhoid and its earlier prototypes developed by the Royal Tropical Institute (KIT), Amsterdam. The Typhidot test measures both IgM and IgG antibodies against a 50 kDa outer membrane protein (OMP) antigen in a miniaturized dot‐blot enzyme‐linked immunosorbent assay (ELISA) format. The test is considered positive if the IgM is positive, and indeterminate if the IgG is positive but IgM negative. The Typhidot‐M test measures IgM against the same 50 kDa antigen in the same dot‐blot format after removal of the total IgG. The TyphiRapid Tr‐02 test measures IgM antibodies against the 50 kD antigen in an immunochromatographic (ICT) format.
The TUBEX TF tests for antibodies against S. Typhi lipopolysaccharide (LPS) antigen by quantifying inhibition of binding between O9 monoclonal antibodies and LPS‐coupled magnetic particles. A visible decolourization of patient serum in the test reagent solution through magnetic particle separation indicates a positive result. Samples are graded as 0 to 10 according to the colour of the reaction mixture at the end of the procedure. Those with a grade greater than 2 are considered positive. Unlike the Typhidot test there has been a single version of the TUBEX test, although there may have been minor test modifications not made public by the manufacturer (Thriemer 2013).
The tests developed by KIT detect IgM antibodies against the S. Typhi LPS O9 antigen. The test has been applied in different formats as a prototype RDT using a dipstick and latex agglutination format, and an ICT lateral flow assay. The ICT lateral flow format is now commercially available as the Test‐it Typhoid test.
Other RDTs included
Enterocheck WB® detects S. Typhi‐specific antibodies to LPS antigen in an ICT lateral flow format. As the patient sample flows through the cassette, the antibody‐antigen complexes are immobilized by a coated membrane leading to the formation of a pink to pink‐purple coloured band. The absence of this coloured band in the test region indicates a negative test result (Anusha 2011; Anagha 2012).
SD Bioline similarly utilizes an ICT method to visually and qualitatively detect IgG and IgM antibodies to unspecified S. Typhi antigens which are indirectly labelled with colloidal gold (via an antibody). The immune complexes are captured by anti‐IgM or anti‐IgG antibodies immobilized on the test strip to give a qualitatively positive or negative result (Kawano 2007).
The Multi‐Test Dip‐S‐Tick is also a qualitative test, but in a dipstick format that detects IgG antibodies against S. Typhi O, H, and Vi antigens. It is part of a fever stick which tests for five other pathogens in addition to S. Typhi (Olsen 2004).
The PanBio test utilizes a direct ELISA format. Unspecified S. Typhi antigen‐coated microwell strips are incubated with a patient’s serum for 20 minutes. The absorbance readings at a wavelength of 450 nm are converted into 'PanBio units' with greater than 10 PanBio units considered positive, and less than 10 PanBio units as negative (Gopalakrishnan 2002).
With the Mega Salmonella test, patient antibodies bind to unspecified S. Typhi antigens insolubilized on microplates, and are quantitatively detected by ELISA with both an IgM and IgG‐specific peroxidase‐labelled reagent (Kawano 2007).
Clinical pathway
Prior test(s)
A RDT for enteric fever should be used in a patient who presents with fever who currently lives in, or has recently visited, an area of medium to high endemicity. It is likely that patients would not have received any prior testing. However, it is more likely that a patient may have been given a clinical diagnosis, or indeed empirical antimicrobial treatment, based on history and examination (Darton 2014). The setting could be primary, secondary, or even tertiary care, but more commonly in a setting that has limited diagnostic laboratory facilities. Unfortunately the clinical diagnosis of the disease is imprecise, so any patient with a fever from endemic regions should be subject to an enteric fever RDT, not just those with classical signs and symptoms of the target conditions (Parry 2011). In areas endemic for HIV, dengue, and malaria as well as enteric fever, patients may have had other point‐of‐care testing performed (Abba 2011).
Role of index test(s)
The definitive diagnosis of enteric fever requires confirmation with a laboratory test to distinguish it from other infections (such as dengue, malaria, rickettsial infections, leptospirosis, and melioidosis) that present with similar symptoms (Waddington 2014). The current recommendation is to use blood culture to diagnose enteric fever (WHO 2003). This test is specific, but lacks sensitivity and so will miss patients who actually have the disease (Mogasale 2016). A bone marrow culture, although more sensitive, is impractical for routine use (Wain 2001). Furthermore, bacterial culture requires a relatively sophisticated laboratory usually unavailable in areas where enteric fever is common (Parry 2011).
It is anticipated that in low‐resource settings endemic for enteric fever, a robust RDT could be utilized instead of blood or bone marrow cultures in a febrile patient, that is to replace the expensive reference standard test in daily clinical practice. A positive RDT result at the point‐of‐care would prompt treatment with appropriate antimicrobials. A negative result would prompt consideration of other illnesses as the cause of the patient’s fever (Parry 2011). Simple, accurate, and robust RDTs would be of considerable help to clinicians managing patients in areas where enteric fever is common (Baker 2010). In addition, an enteric fever RDT could be used as a triage tool to trigger further testing, such as blood culture, in settings where microbiological culture is less accessible. In secondary or tertiary care settings a positive RDT could warrant the collection of a peripheral blood culture prior to starting antimicrobial therapy (Parry 2011).
Alternative test(s)
Widal test
The Widal test (WT) is a serological test that detects agglutinating antibodies to LPS (O antigen) and flagella (H antigen). The WT is the principal alternative test and is widely used but is neither sensitive nor specific (Olopoenia 2000). In its original format the WT required both acute and convalescent‐phase serum samples taken approximately 10 days apart. The test has also been evaluated as a single, acute‐phase serum sample (Saha 1996). In people with enteric fever, titres often rise before the clinical onset, making it very difficult to demonstrate the diagnostic four‐fold rise between initial and subsequent samples (Gill 2009).
The role of the WT is controversial because the sensitivity, specificity, and predictive values vary considerably between geographical areas (Parry 2002). Test results need to be interpreted carefully in the light of previous history of enteric fever and vaccination. Interpretation of the result is also greatly helped by knowledge of the background levels of antibodies in the local healthy population (House 2001). The increasing use of enteric fever vaccines and the occurrence of infection with other Salmonella enterica serovars lower the specificity of the WT (Waddington 2014). Infection with non‐Salmonella organisms (for example, malaria, dengue, brucellosis) also leads to cross‐reactivity in the WT in enteric fever‐endemic regions (Olopoenia 2000). There is considerable variation in agglutinin levels among non‐infected populations. These levels are susceptible to change over time and depend on the degree of endemicity (Parry 2002). Despite these shortcomings of both sensitivity and specificity, because the WT is simple and inexpensive, it is still widely used as a diagnostic test (Fadeel 2004).
Nucleic acid amplification tests
Nucleic acid amplification tests (NAATs) for enteric fever diagnosis, such as polymerase chain reaction (PCR), and real‐time PCR are being explored. Theoretically, NAATs could amplify DNA from dead or unculturable bacteria, thus addressing the concern of poor culture positivity because of pre‐treatment with antimicrobials (Wain 2001). One study found that a novel three‐colour real‐time PCR technique had the same limitations in test sensitivity as culture and deemed it an unsuitable methodology for the routine diagnosis of enteric fever (Nga 2010). Methods that combine culture and PCR methods have been also been tested (Zhou 2010). The use of NAATs in developing countries will most likely be limited in the medium‐term because of high cost and the lack of laboratory infrastructure (Olsen 2004).
Metabolomics
A new group of diagnostic tests rely on the metabolites produced by the host in response to infection. Metabolites induced by specific infections could be measured in the blood and urine of affected patients (Baker 2010). By comparing the metabolite profiles from healthy patients to profiles of patients with typhoid and paratyphoid infections, thresholds could be determined to identify those with acute enteric fever (McKinnon 2014). Similar studies have used metabolomics to identify diagnostic markers of malaria and dengue fever (Andrews 2015). The use of metabolomic tests currently requires specialized laboratory infrastructure, so use of these tests in both developed and developing countries is likely to have very restricted applicability.
Rationale
RDTs have the potential to be useful to clinicians working in resource‐limited settings in LMICs. Differentiating the common causes of the febrile patient by clinical criteria is challenging without the laboratory support for blood films, serology, or blood cultures (Bhutta 2006). A diagnostic test in such a setting must be cheap, simple to perform, and able to quickly deliver a result. Such a test should correctly identify true enteric fever cases among febrile patients, ensuring prompt and specific treatment, allowing the avoidance of broad‐spectrum medication that cover all common causes of fever. In many endemic areas, treatment for enteric fever may be given to all patients with fever (Larsson 2008). The diagnosis of enteric fever by an RDT could reduce unnecessary prescription of antimicrobials, reduce drug expenditure, and limit the development of antimicrobial resistance (Andrews 2015). The role of an enteric fever RDT in practice is to identify those febrile patients who warrant anti‐Salmonella antibiotic treatment as opposed to conservative management, antimalarial treatment, or treatment for other bacterial infections (Parry 2011).
The reference standard for diagnosing enteric fever has been culture of S. Typhi or Paratyphi A from bone marrow, peripheral blood, or other sterile sites. The mainstay of diagnosis in clinical practice is a positive blood culture, although the test is only positive in 40% to 80% of cases, usually in the first two weeks of the disease (Parry 2002; WHO 2003). This lack of sensitivity is due to the low number of bacteria circulating in the blood, and may also be affected by: prior antimicrobial therapy (Wain 1998); the type of culture medium used; the ratio of blood to broth; stage of illness at the time of presentation; and the duration of incubation (Mogasale 2016). Bone marrow culture gives a higher culture‐positive rate, probably because the concentration of organisms is higher than in the blood, and may remain positive even after antibiotic therapy has been started (Wain 2001). Bone marrow culture is positive in 80% to 95% of patients with enteric fever, including in patients who have been taking antibiotics for several days regardless of the duration of the illness (Parry 2002). Although bone marrow culture is more sensitive, it is difficult to obtain, relatively invasive, and is of little use in public health settings (Wain 2001). Even with sophisticated laboratories, confirming the diagnosis of enteric fever can be difficult with negative blood or bone marrow cultures despite a patient actually having enteric fever (Baker 2010).
It is quite possible that RDTs are more sensitive than the current reference standards for enteric fever. If laboratory isolation of the causative organisms is neither cost‐effective nor reliable, then there is a potential role for RDTs to replace microbiological culture as the main diagnostic test (Parry 2011). If no single reference standard test exists, use of a composite reference standard (CRS) could improve estimation of diagnostic test accuracy (Storey 2015).
Objectives
To assess the diagnostic accuracy of commercially available rapid diagnostic tests (RDTs) and prototypes for detecting Salmonella Typhi or Paratyphi A infection in symptomatic persons living in endemic areas.
Secondary objectives
To identify which types and brands of commercial test best detect enteric fever.
To investigate the sources of heterogeneity between study results (see the 'Investigations of heterogeneity' section).
Methods
Criteria for considering studies for this review
Types of studies
We included the following types of studies.
Randomized controlled trials (RCTs) in which patients are randomized to one of several index tests and all receive a reference standard.
Paired comparative trials in which a series of patients receive two or more index tests and a reference standard.
Prospective cohort studies in which a series of patients from a given population are recruited and receive one or more index test and a reference standard.
Retrospective case control studies that compare a group of patients with laboratory‐confirmed enteric fever cases (positive reference standard) and a group of patients without enteric fever (negative reference standard). In case control design studies, we only extracted data relating to the index test(s) from control groups participants with fever, and not from healthy control participants without fever.
Participants
Patients living in enteric fever‐endemic areas attending a healthcare facility with fever were eligible. This may or may not have included patients with a clinical suspicion of enteric fever.
When only a subgroup of participants in a study was eligible for inclusion in the review, we included the study provided that we were able to extract relevant data specific to that subgroup. Subgroups included participants enrolled as separate groups, for example a clinical cohort subgroup without healthy control patient subgroup (Fadeel 2011).
Index tests
All rapid diagnostic tests (RDTs) specifically designed to detect enteric fever cases. We categorized the tests as follows.
RDTs that were applied to blood samples (venous or capillary) to detect antigens.
RDTs that were applied to blood samples (venous or capillary) to detect antibodies (IgG, IgM, or both).
RDTs that were applied to urine samples to detect antigens.
RDTs that were applied to urine samples to detect antibodies (IgG, IgM, or both).
We classified the RDTs further by format, for example, lateral flow, flow‐through, agglutination, or solid phase kits.
Studies may have compared one or more RDT against one or more reference standard.
Target conditions
Typhoid fever caused by Salmonella enterica serovar Typhi.
Paratyphoid fever caused by Salmonella enterica serovar Paratyphi A.
Reference standards
Studies were required to diagnose enteric fever using one of the following reference standards.
Bone marrow culture.
Peripheral blood culture, peripheral blood PCR, or both.
We defined a Grade 1 study as one that used both bone marrow culture and peripheral blood culture as the reference standard. In Grade 1 studies, we considered either bone marrow or peripheral blood culture positivity a positive reference standard.
We defined a Grade 2 study as one that used either peripheral blood culture only as the reference standard, or peripheral blood culture and peripheral blood PCR as the composite reference standard. In Grade 2 studies, we considered either blood culture or blood PCR positivity a positive composite reference standard.
As overall estimates of accuracy ignoring the use of different reference standards are difficult to interpret, we reported the results separately for each grade of reference standard (Reitsma 2009).
Search methods for identification of studies
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, or ongoing).
Electronic searches
We searched the following databases using the search terms and strategy described in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register (4 March 2016); MEDLINE (OVID, 1966 to 1 March 2016); Embase (OVID, 1974 to 4 March 2016); Science Citation Index‐expanded (Web of Science, 1900 to 4 March 2016), IndMED; African Index Medicus, and LILACS (1982 to 4 March 2016). We also searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch). for trials in progress, using "typhoid", "paratyphoid", "enteric fever", "rapid diagnostic test", "RDT", and "diagnostics" as search terms.
Searching other resources
We checked the reference lists of all studies identified by the above methods, and we manually searched World Health Organization (WHO) reports. In addition we manually searched papers from the 3rd (1997) to the 7th (2009) International Conferences on Typhoid Fever and other Salmonellosis. We contacted test manufacturers to identify ongoing or unpublished studies.
Data collection and analysis
Selection of studies
One review author (LW) screened the titles and abstracts of articles identified by the search strategy. We coded articles that did not fulfil the inclusion criteria as 'do not retrieve'. In the case of potentially eligible articles or if we were unclear whether the articles met the inclusion criteria or not, we coded these articles as 'retrieve'. We retrieved the full‐text texts of articles in the 'retrieve' category. Two review authors (LW and CMP) independently assessed the full‐text articles for inclusion and consulted a third review author (SM) in case of disagreement. We listed all studies excluded after full‐text assessments and their reasons for exclusion in the 'Characteristics of excluded studies' section. We presented the study selection process in a study flow diagram.
Data extraction and management
Two review authors (LW and CMP) independently extracted a standard set of data from each study article (see Appendix 2), using a pre‐piloted specifically designed data extraction form. A third review author (SM) cross checked the data extraction and resolved any discrepancies by discussion with the two review authors (LW and CMP). If information was missing or not clear, we contacted the study investigators.
We extracted the number of true positives, true negatives, false positives, and false negatives based only on the Salmonella enterica serovars the test was designed to detect (Typhi or Paratyphi A) as a 2 x 2 table for each study along with the corresponding threshold value. If data for multiple 2 x 2 tables were presented based on more than one threshold for a single study, we extracted each table and the threshold values. If this data (2 x 2 table) was also available for a subgroup of patients in the study, we extracted this data if the subgroup of patients was of interest (that is, grouped by patient age). For studies that we only included a subgroup of participants in the review, we only extracted this data and presented it for that particular subgroup. In case control design studies, we restricted negative controls to febrile participants, and we excluded healthy control participants from the 2 x 2 table data.
Where a study applied multiple index tests or reference standards, we extracted data for each test. Since blood culture, bone marrow culture, and blood PCR are imperfect reference standards, where possible we extracted the results of a composite reference standard (blood culture and bone marrow culture, or blood culture and blood PCR), such that we documented a negative result if bone marrow culture, blood culture, PCR, or all three, were negative (Reitsma 2009). We extracted the number of uninterpretable or invalid test results.
For Moore 2014 and Maude 2015, two review authors (LW and CMP) were the study authors, so one review author (SM) independently extracted data using individual participant data (from CMP) as we could not extract ideal data for review from the published articles. In Fadeel 2011, the article did not report results summarized across the cohort. For both Typhidot and TUBEX tests, for nested case control results within a cohort of patients, we back calculated 2 x 2 tables to reflect cohort composition (see the 'Strengths and weaknesses of the review' section).
Assessment of methodological quality
Two review authors (LW and CMP) independently assessed the quality of each individual study using a modified QUADAS‐2 tool (Whiting 2003; see Appendix 3). We answered each quality indicator on the checklist with a 'yes', 'no', or 'unclear' response for each study, and we provided the reason for our judgment.
Statistical analysis and data synthesis
We entered all 2 x 2 table data from all RDTs in included articles into Review Manager 5 (RevMan 5) (Review Manager 2014), which calculates sensitivity and specificity with 95% confidence intervals (CIs). We used forest plots and summary receiver operating characteristic (SROC) plots to present the variation in sensitivities and specificities between studies. In the description of studies we recorded the number of uninterpretable or invalid test results.
The statistical analysis focused on sensitivity and specificity at average operating points for the three main commercially‐available RDTs and their prototypes: TUBEX; Typhidot (including Typhidot‐M); and Test‐it Typhoid (and KIT prototypes). We included each test in a separate meta‐analysis. For other tests we identified fewer than four studies, so we did not complete any meta‐analysis summary. Where sufficient data were available, we performed meta‐analyses to estimate and compare the performance of the tests.
For Test‐It Typhoid and prototypes (KIT) studies, we performed a meta‐analysis for the threshold of > 1+ only as this was the manufacturer's recommendation. Data from the same study may contribute to different comparisons (for example, RDT versus blood culture; RDT versus bone marrow and blood culture), but we only combined one set of data from each study in an individual meta‐analysis.
For meta‐analysis we used the bivariate random‐effects models of sensitivity and specificity (Reitsma 2005; Chu 2006). We exported the data from RevMan 5 (Review Manager 2014) into STATA models fitted using xtmelogit with all three main test types included in a single model allowing for unequal variances between tests and allowing correlation of sensitivity and specificity for each test in the random effects. Within xtmelogit we calculated pairwise comparisons of the difference between sensitivity and difference in specificity with 95% CIs of the three tests. We also used xtmelogit for heterogeneity analyses to compare sensitivity and specificity for the subgroup of studies where the Typhidot test reported indeterminate test results or not. We entered meta‐analysis parameter estimates (bivariate model parameter estimates and confidence and prediction region parameters) into RevMan 5 (Review Manager 2014).
For PanBio Multi‐test Dip‐S‐Tick, Mega Salmonella, and SD Bioline tests, where the only included data is from comparisons of tests with fewer than four studies, we compared individual tests with results from Typhidot and TUBEX on the same participants as available. We based comparisons on conservative estimates from unpaired comparisons of proportions, as paired data were not available from articles. Where 95% CIs did not overlap between test estimates, we established statistical significance without formal testing. Where 95% CI overlapped, we reported the differences in unpaired proportions with 95% CIs for the differences.
Investigations of heterogeneity
As part of the Secondary objectives, we planned to investigate the sources of heterogeneity between study results, including the following.
Salmonella enterica serovars (Typhi or Paratyphi A).
Study design (see 'Types of studies').
Test population (patients with a clinically‐suspected infection of typhoid or paratyphoid, or unselected febrile patients).
Reference test (Grade 1 or Grade 2 ‐ see 'Reference standards').
Index test format (for example, lateral flow versus agglutination; IgM versus IgG versus IgM‐IgG combination).
Index test sample (blood versus urine participant sample).
Level of disease endemicity (for example, medium versus high) (Crump 2004).
Participant characteristics (for example, adults versus children).
Geographical location (by sub‐Saharan Africa versus the rest of the world).
The rationale for distinguishing sub‐Saharan Africa from the rest of the world was that non‐typhoidal Salmonellae (NTS) are an important cause of bacteraemia in sub‐Saharan Africa (Parry 2011), and may affect the performance of enteric fever RDTs in these settings.
Sensitivity analyses
There was insufficient data to carry out sensitivity analyses to assess the robustness of the meta‐analyses based on quality components.
Assessment of reporting bias
We did not attempt to assess reporting bias.
Results
Results of the search
We have summarized the study selection process in a PRISMA flow‐chart (Figure 1). We performed a literature search up to 4 March 2016 and identified a total of 2885 titles and abstracts. There were 2411 articles after we removed duplicates. We retrieved 95 full‐text articles for assessment. From the total number of 95 full‐text articles retrieved and assessed, we included a total of 37 studies for qualitative analysis in the Cochrane Review. We did not include two of the studies (Anagha 2012 and Anusha 2011) in the quantitative analysis as together they were not powered sufficiently for a meta‐analysis of the single index test (Enterocheck WB) they evaluated (Table 2; Figure 2). The number of included studies in the quantitative analysis after full‐text assessment was 35.
1.
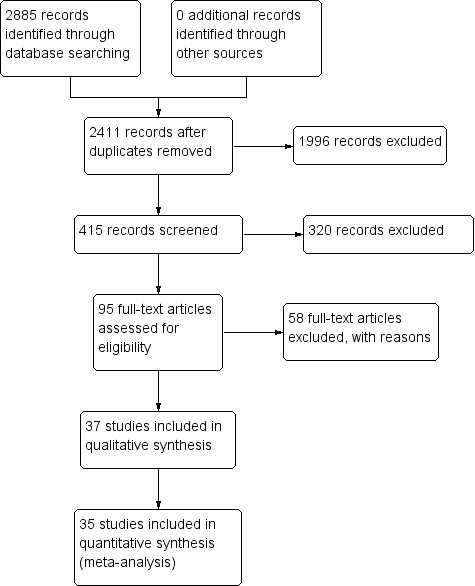
PRISMA flow diagram.
1. Summary of all index tests.
| Index Test Name | Manufacturer | Methods | Formats | Biological specimen | Threshold for positivity values | Number of evaluations |
| TUBEX® TF | IDL Biotech, Bromma, Sweden | Inhibition Binding Magnetic Immunoassay. Detects IgM to S. Typhi O9 antigen. Semi‐quantitative colorimetric. | Mix buffer/reagent into plastic well with patient specimen. 3 minutes for result. | Whole blood, plasma, or serum | Semi‐quantitative colour change scale (0 to 10) provided by manufacturer. Positive if colour change scale ≥ 3. | 14 |
| Typhidot® | Malaysian Bio‐Diagnostics Research, Selangor, Malaysia | Dot‐enzyme immunoassay. Detects IgG and IgM to 50 kdA S. Typhi Outer Membrane Protein (OMP) antigen. | Mix serum/whole blood plus reagent incubating commercially‐prepared pre‐dotted antigen filter paper strips. 60 minutes for result. | Whole blood, plasma, or serum | Qualitative: either positive or negative. A positive result is a visible reaction (IgG or IgM) of an intensity equal to or greater than that of the control reaction on the commercially prepared filter paper. | 17 |
| Typhidot‐M® | Malaysian Bio‐Diagnostics Research, Selangor, Malaysia | Dot‐enzyme immunoassay. Detects IgM to 50 kdA S. Typhi OMP antigen. | Mix serum/whole blood plus reagent incubating commercially‐prepared pre‐dotted antigen filter paper strips. 60 minutes for result. | Whole blood, plasma, or serum | Qualitative: either positive or negative. Positive as per Typhidot. The absence of any visible spot indicated a negative test result. | 6 |
| TyphiRapid Tr‐02 (Typhidot) | Reszon Diagnostics International, Malaysia | Prototype of Typhidot. Immunochromatography assay. Detects IgM to 50 kdA S. Typhi OMP antigen. |
Mix serum/whole blood plus buffer/reagent into a well. | Whole blood, plasma, or serum | We were unable to get hold of the manufacturer and are awaiting a response from the study author | 1 |
| KIT ICT Test‐It TyphoidTM | LifeAssay Diagnostics, Cape Town, South Africa | Lateral flow immunochromatographic (ICT) assay. Detects IgM to S. Typhi lipopolysaccharide (LPS) antigen. Semi‐quantitative. | Mix serum/whole blood plus buffer/reagent into lateral flow cassette. Two‐site (test and control) immunoassay on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Semi‐quantitative result line intensity scale (negative to +4) provided by manufacturer. A positive result is ≥ +1 | 3 |
| KIT Dipstick Assay | Royal Tropical Institute (KIT), Amsterdam | Detects IgM to S. Typhi LPS antigen. Simplified version of ELISA technique. | Strip of nitrocellulose membrane with immobilized antigen detection band. Serum plus reagent incubated on dipstick for 3 hours at room temperature. Dipsticks rinses with water and dried. >3 hours for result. | Serum | Semi‐quantitative result line intensity scale (negative to +4) provided by manufacturer. A positive result is ≥ +1 | 5 |
| KIT Dri‐Dot Assay (latex agglutination) |
Royal Tropical Institute (KIT), Amsterdam | Detects IgM to S. Typhi LPS antigen. White agglutination card. | Dot of dried detection reagent conjugated to blue latex reagent. Antigen‐activated latex stabilized by drying a drop of latex reagent onto card suspended in serum. Card rotated by hand in near‐horizontal position to further induce agglutination. 30 seconds for result. | Serum | Qualitative: positive or negative. Positive when agglutination was observed within 30 seconds. Negative when no agglutination was observed. | 1 |
| SD Bioline Salmonella typhi IgG/IgM Fast | Standard Diagnostics Inc., Gyeonggi, Korea | ICT flow method. Detects IgM and IgG antibodies to unspecified S. Typhi antigens. | 4 drops of reagent mixed well with patient specimen. Nitrocellulose strip suspended into with 3 sites (IgM, IgG, and control). 30 minutes for result. | Serum, plasma, or whole blood |
Qualitative: positive or negative. Positive if line appears in both control and 1 or both of IgM or IgG test zones. | 3 |
| Enterocheck WB® | Zephyr Biologicals, Goa, India | ICT Detects IgM antibodies to S. Typhi LPS antigen. |
Two‐site (IgM test, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Qualitative: positive or negative. Presence of a line in both the test and control zones indicates a positive result. | 2 |
| Enteroscreen ® | Zephyr Biologicals, Goa, India | ICT Detects IgM and IgG antibodies to S. Typhi LPS antigen. |
Three‐site (IgG, IgM, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, plasma, or serum | Qualitative: positive or negative. Presence of a line in both the test (IgG, IgM, or both) and control zones indicates a positive result. | 1 |
| Multi‐test Dip‐S‐Tick | PanBio Inc., Columbia, Maryland, USA | Tests for five pathogens, including S. Typhi. Dipstick format that detects anti‐O, anti‐H,anti‐Vi, IgM, or IgG antibodies. | Detailed information not available | Heparinized whole blood, serum, or plasma | Detailed information not available | 1 |
| Mega Salmonella | Mega Diagnostics, Los Angeles, California, USA | Detect IgG and IgM antibodies to unspecified Salmonella antigens. Quantitatively detected by ELISA with peroxidase‐labelled reagents. | Results read in a microplate ELISA reader. | Whole blood, serum, or plasma | Detailed information not available | 1 |
| OnSite Typhoid IgG/IgM Combo | CTK Biotech Inc., San Diego, California, USA | Lateral flow immunoassay. Detects IgG and IgM antibodies against recombinant O and H S. Typhi antigens. |
Three‐site (IgG, IgM, and control) immunoassay cassette on a porous nitrocellulose membrane. 15 minutes for result. | Whole blood, serum, or plasma | Qualitative: positive or negative. Presence of a line in both the test (IgG, IgM, or both) and control zones indicates a positive result. | 2 |
Abbreviations: immunochromatographic (ICT); immunoglobulin‐G (IgG); immunoglobulin‐M (IgM); Tropical Institute, Amsterdam (KIT); lipopolysaccharide (LPS); outer membrane protein (OMP).
2.
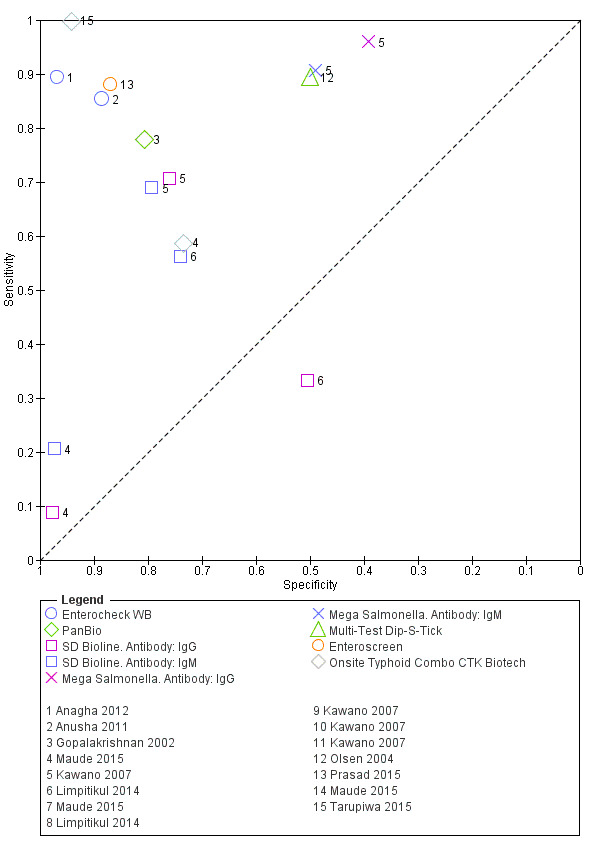
Summary receiver operating characteristic plot: Enterocheck WB, PanBio, SD Bioline, Mega Salmonella, Multi‐Test Dip‐S‐Tick.
Most included studies recruited participants from the Asia‐Pacific. The South Asian study locations included: India (10 studies); Bangladesh (five studies); and Pakistan (four studies). In South‐East Asia, the study locations included: Indonesia (five studies); Vietnam (two studies); Malaysia (one study); Cambodia (one study); Thailand (one study), and Papua New Guinea (one study). East Asian countries included China (one study) and the Philippines (one study). From Africa, two studies were from the north (Egypt), and five studies were from sub‐Saharan countries (Kenya, Tanzania, Zimbabwe, and South Africa) where non‐typhoidal Salmonellae (NTS) are also an important cause of bacteraemia. Six studies recruited patients from areas of medium enteric fever endemicity (Crump 2004). Most study participants were from areas considered highly endemic for enteric fever (Crump 2004).
Eighteen of the studies included both adults and children, and seven studies included children only. The age distribution of recruited patients was not clear in 14 of the included studies. Thirty‐three studies included participants attending a tertiary healthcare facility, 15 studies included secondary (district) healthcare attendees, and seven studies included primary healthcare attendees. Twenty studies recruited inpatients, 12 studies recruited outpatients, while 10 studies did not state the point of recruitment.
All of the RDTs evaluated were antibody tests on blood designed to detect S. Typhi infection. None of the included studies evaluated a RDT that detected S. Paratyphi A infection. All the RDTs evaluated used venous blood as the biological sample with one study additionally using capillary blood samples (Anusha 2011). There were no suitable studies that evaluated RDTs using other biological samples such as saliva or urine.
The included studies evaluated 13 index tests in total (Table 2). The most commonly evaluated RDTs were Typhidot and its variants (Typhidot; Typhidot‐M; TyphiRapid Tr‐02; Malaysian Biodiagnostic Research SDN BHD, Malaysia) in 22 studies, and TUBEX TF (IDL Biotech, Sollentuna, Sweden) in 14 studies. An index test created by the Royal Tropical Institute, Amsterdam (KIT), and now commercially available as the Test‐it‐Typhoid test (LifeAssay Diagnostics, South Africa) was evaluated in three different test formats in nine studies (dipstick assay; latex agglutination assay; lateral flow immunochromatographic test (ICT)). Other index tests evaluated included: Enterocheck WB (Zephyr Biomedicals, Tulip Group, Goa, India) in two studies; Enteroscreen (Zephyr Biomedicals, Tulip Group, Goa, India); SD Bioline (Standard Diagnostics, Kyonggi‐do, Korea); Mega Salmonella (Mega Diagnostics, Los Angeles,USA); Multi‐Test Dip‐S‐Tick (PANBIO INDX Inc., Baltimore, USA); and Onsite Typhoid IgG/IgM combo (CTK Biotech Inc., San Diego, California, USA) in one study each.
Methodological quality of included studies
We have summarized the methodological quality of the 37 included studies in Figure 3. We extracted this data using a modified QUADAS‐2 criteria proforma (Appendix 3) that focused on four domains of methodological quality: patient selection; index test; reference standard; and flow and timing. The domain with the highest level of risk for bias across all studies was that of patient selection (> 50%). We have summarized the risk of bias and the review authors' judgements about the applicability concerns of these domains for each included study in Figure 4.
3.
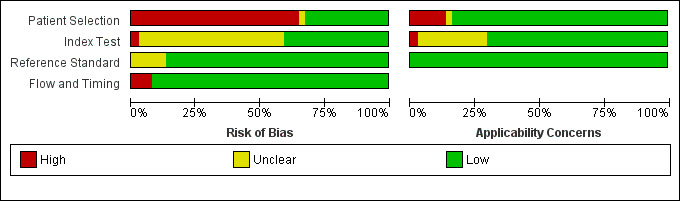
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
4.
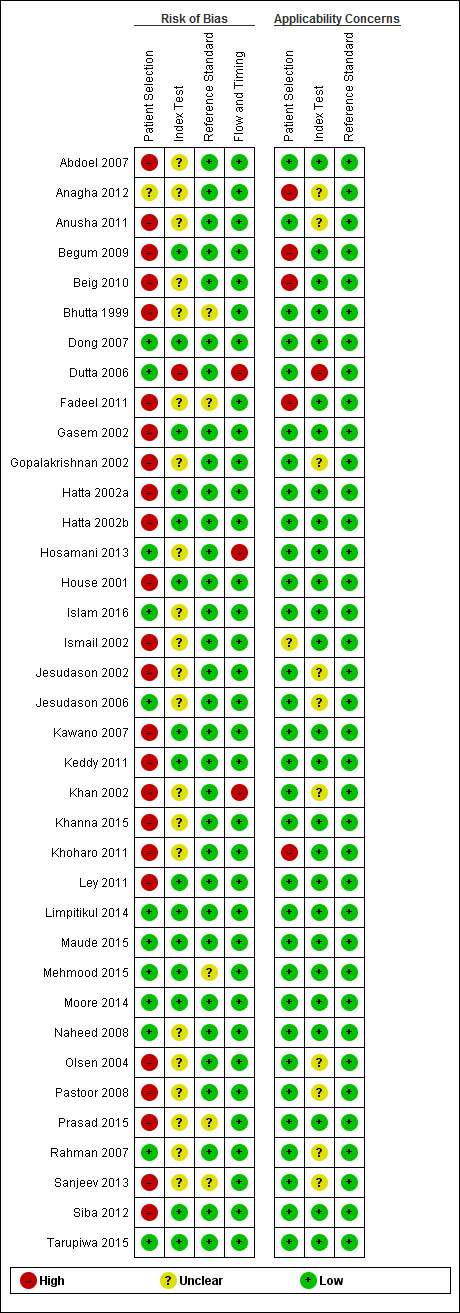
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
Only 11 studies recruited unselected febrile patients. Most included studies selected patients on the basis of a clinical suspicion of enteric fever, although the criteria for suspecting enteric fever were usually not stated. Only three studies employed the Grade 1 reference standard, with blood and bone marrow culture (Bhutta 1999; Gasem 2002; Khan 2002). All studied used peripheral blood culture. Three studies also used blood PCR (Siba 2012; Moore 2014; Maude 2015). One study used stool culture, and another used the Widal Test in a composite reference standard (Gopalakrishnan 2002; Pastoor 2008). Only half of the included studies reported that the index test results were interpreted without knowledge of the reference standard results. Patients were recruited prospectively in 26 of the 37 included studies. Index tests were performed retrospectively on stored samples in 18 studies. Twenty‐three studies reported enrolling a consecutive or random group of patients (see the 'Characteristics of included studies' section). Sixteen studies used a case control design where diagnostic accuracy results can be overestimated, although all these studies reported results separately for control groups from febrile patients. Nineteen studies used cohort (not case control) designs, and in two studies the reporting was unclear.
Findings
Typhidot and its variants
Three variants of the Typhidot test were studied: Typhidot (17 studies); Typhidot‐M (six studies); and TyphiRapid Tr‐02 (one study).
For the Typhidot test, indeterminate results can be produced which are classified as both IgM test negative but IgG test positive (Olsen 2004; Naheed 2008). Some studies explicitly classified indeterminate results, where others did not clearly report indeterminate results (Siba 2012), or only presented the IgM data without the IgG data (Khan 2002). We attempted to separately extract the IgM and IgG positive data from each study and, where possible, used the IgM data only to allow comparison of results between all three types of Typhidot test by classifying the indeterminate results as negative (see the 'Differences between protocol and review' section).
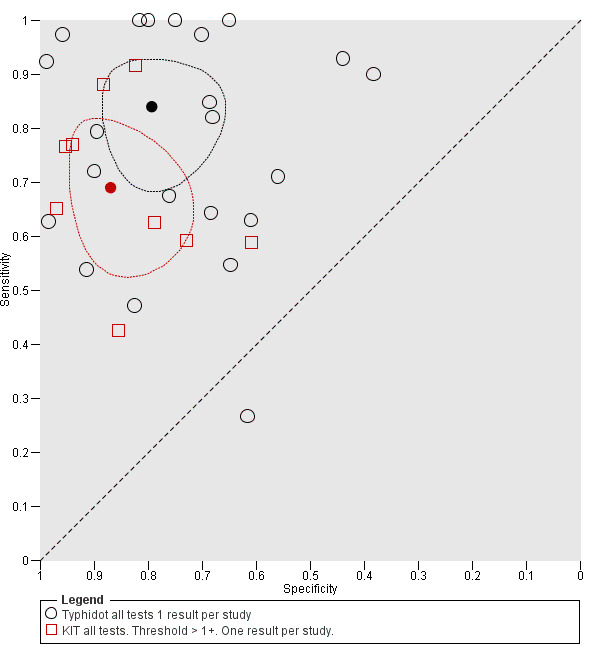
The study results plotted in receiver operating characteristic (ROC) space are shown in Figure 5. The Typhidot variant studies did not perform consistently across studies. Figure 6 shows the forest plots of studies evaluating Typhidot RDTs by various test type, and by whether indeterminate results were reported or not. There is no obvious visually distinguishable trend in test performance with prevalence across non‐case control studies.
5.
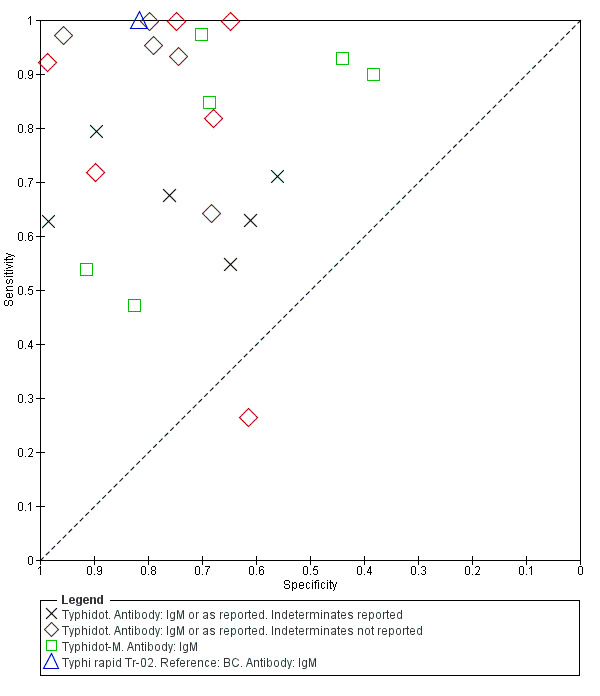
Summary ROC Typhidot all test types.
6.
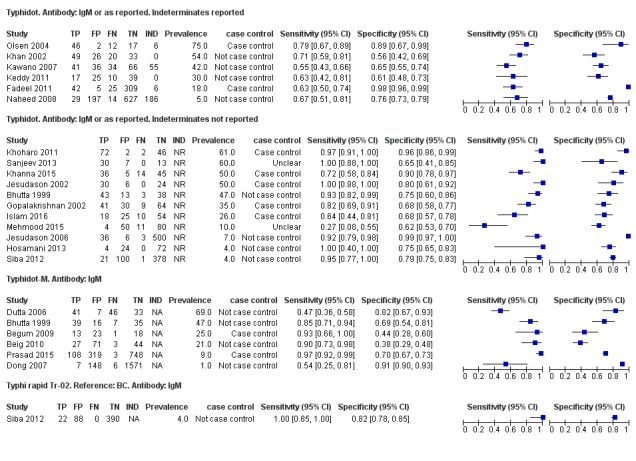
Forest plots for Typhidot all test types.
The included studies used three different grades of reference test: Grade 1 (peripheral blood culture or bone marrow culture, or both); Grade 2 (peripheral blood culture only); and Grade 2 (peripheral blood culture, nucleic acid amplification (blood PCR), or both). To determine the impact of the reference test on accuracy, we plotted the study results in ROC space according to the reference test used in Figure 7. In the study that used both blood culture alone, and blood culture combined with blood PCR on the same patients (Siba 2012), use of the composite reference standard of PCR and blood culture lowered test sensitivity results by about 25%.
7.
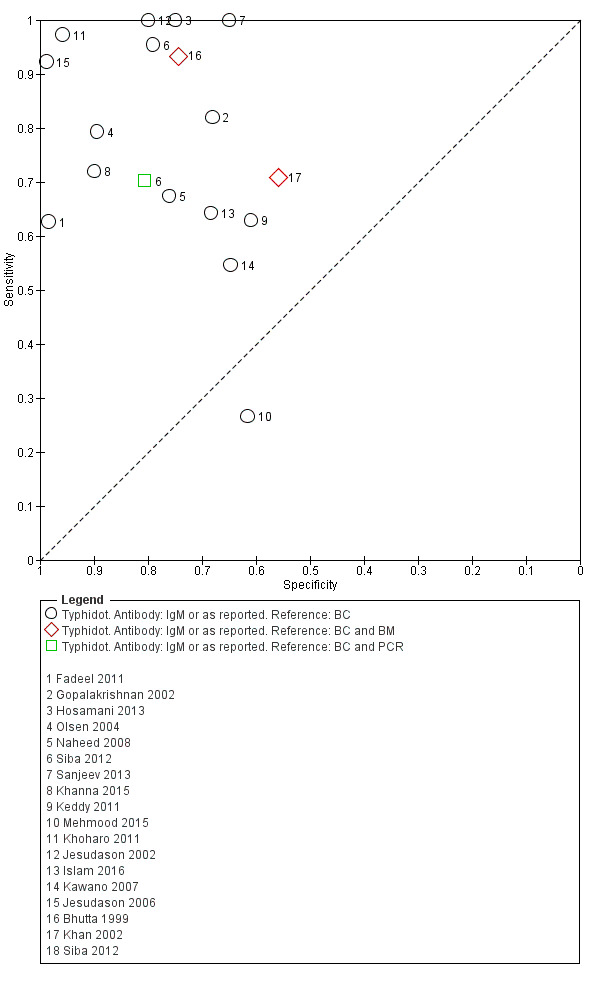
Summary receiver operating characteristic plot of tests: Typhidot and Typhidot‐M by reference test.
Abbreviations: BC: blood culture; BM: bone marrow; BC & PCR: blood culture and polymerase chain reaction.
The median sample size of all studies of Typhidot and its variants was 127 (range 50 to 1732). The earliest study was published in 1999, with the remainder being published in the 2000s. The latest study was published in 2016. Sensitivities ranged from 27% to 100%, and specificities ranged from 38% to 99% (Figure 6). The meta‐analytical average sensitivity and specificity for all three Typhidot test types were 84% (95% confidence interval (CI) 73% to 91%) and 79% (70% to 87%) respectively based on 22 studies (Table 1). However, based on the 13 Typhidot studies where indeterminates were reported or were not produced by the test (Typhidot‐M and TyphiRapid Tr‐02) which have a lower risk of bias, the average sensitivity was 78% (95% CI 65% to 87%) and specificity was 77% (95% CI 66% to 86%). Comparing the 13 studies at lower risk of bias with the nine studies that did not report indeterminates, the difference in sensitivity was −9.8% (95% CI −26.1% to 6.4%) and specificity of −8.0% (95% CI −24.2% to 8.3%). Studies where indeterminates were not reported are at a higher risk of bias and have both higher average sensitivity and specificity, although neither difference is statistically significant.
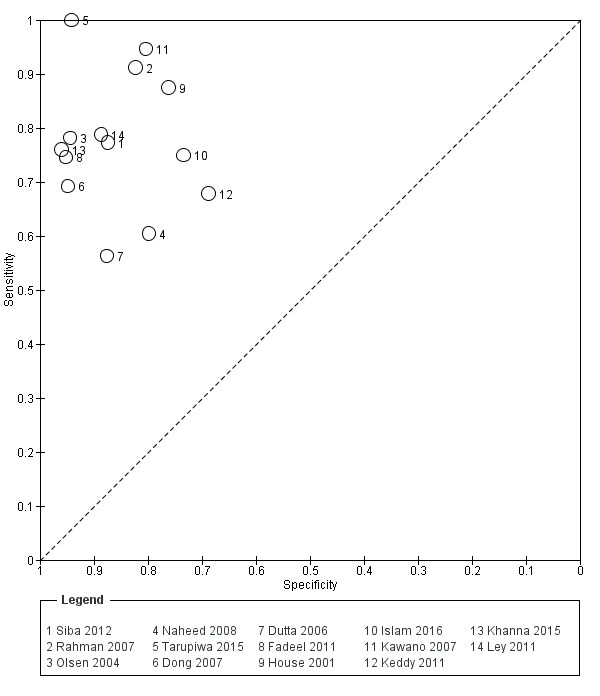
TUBEX
Fourteen studies evaluated TUBEX. We have presented the study results plotted in ROC space and as a forest plot in Figure 8 and Figure 9, which illustrate heterogeneity in test performance between studies. All included studies were Grade 2 (peripheral blood culture only as reference standard), with one study using both blood culture and blood PCR (Siba 2012). This heterogeneity is mirrored when the TUBEX test results are presented by those with and without a case control study design (Figure 10). One study used two different reference tests (Figure 11). As with the Typhidot studies, the composite reference standard of blood culture and PCR lowered sensitivity by around 25%.
8.
Summary receiver operating characteristic plot of test: TUBEX. Reference test: Blood culture. One result per study.
9.
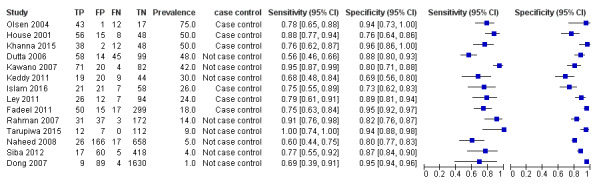
Forest plot of TUBEX. Reference test blood culture.
10.
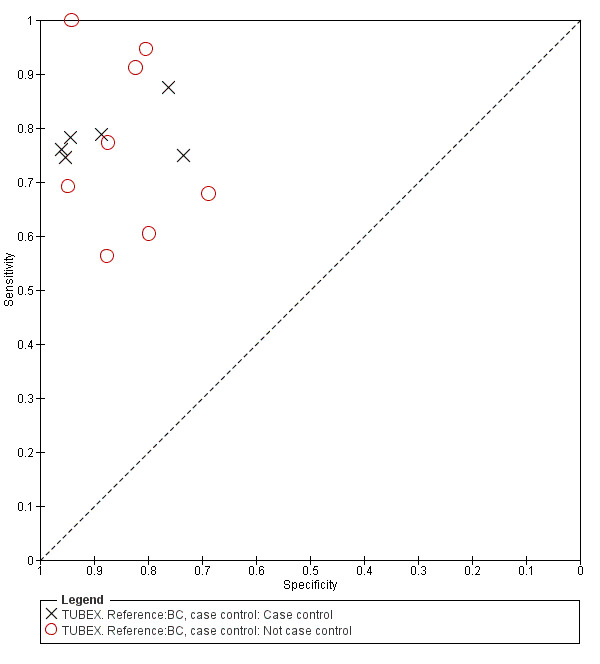
Summary receiver operating characteristic plot: TUBEX by case control design.
Abbreviation: BC: blood culture.
11.
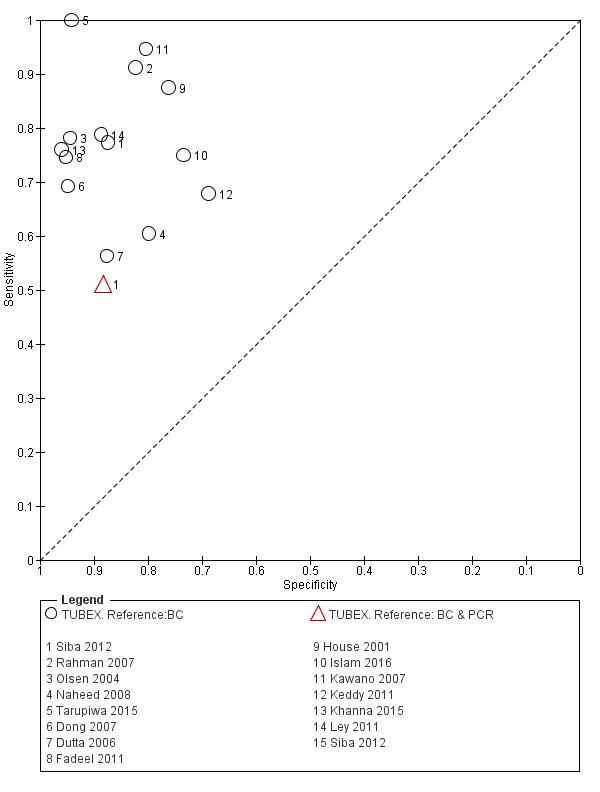
Summary receiver operating characteristic plot: TUBEX by reference test
Abbreviations: BC: blood culture; BC & PCR: blood culture and polymerase chain reaction.
The median sample size was 158 (range 73 to 1732). The earliest study was published in 2001, and the most recent study published in 2016. Sensitivities ranged from 56% to 100%, and specificities ranged from 69% to 96% (Figure 9). The meta‐analytical average sensitivity and specificity (95% CI) were 78% (71% to 85%) and 87% (82% to 91%) respectively (Table 1).
Test‐It Typhoid and Royal Tropical Institute (KIT) prototypes
Nine studies evaluated the performance of the Test‐it Typhoid index test and its earlier KIT prototype formats: five as a dipstick assay; one as a latex agglutination test; and three as the ICT lateral flow assay. The KIT ICT lateral flow assay is now commercially available as Test‐It Typhoid (LifeAssay) and two studies evaluated this (Moore 2014; Maude 2015). In the dipstick and lateral flow assay formats, the test gives a semi‐quantitative result scored as 1+, 2+, 3+, or 4+ dependent on the intensity of the band on the test strip. The manufacturer's recommended threshold that is considered positive is 1+ or more. A few studies have additionally evaluated a threshold of 2+ or more.
All studies evaluating this test plotted in ROC space by different test types (1+ result classified as positive) are presented in Figure 12. Although the dipstick and ICT RDTs appear to perform better with higher average sensitivities, most studies adopted a case control design (Figure 13).
12.
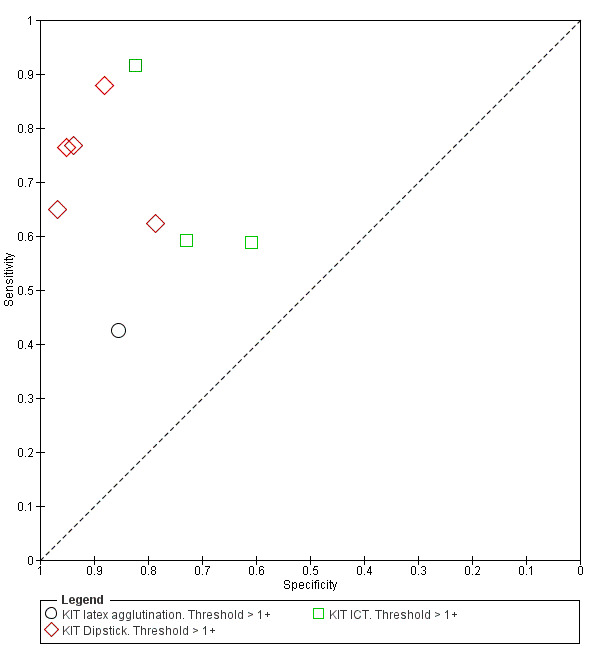
Summary receiver operating characteristic plot: KIT all test types. Threshold > 1+.
13.
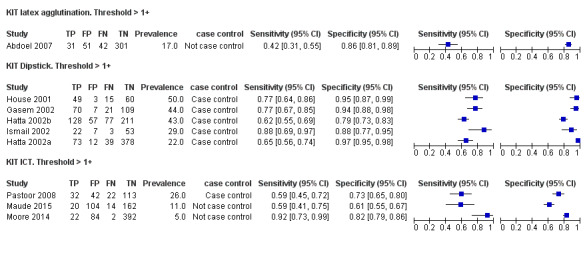
Forest plot of tests: KIT Threshold > 1+ by test type. Reference test: blood culture.
The results for both thresholds (1+ versus 2+ when we could extract these results from the same study) are illustrated in Figure 14. Increasing the threshold to greater or equal to 2 (≥ 2+) decreases the sensitivity of the index test but increases the specificity. One study suggested the diagnostic accuracy was improved by using a threshold of 2+ or more (Moore 2014).
14.
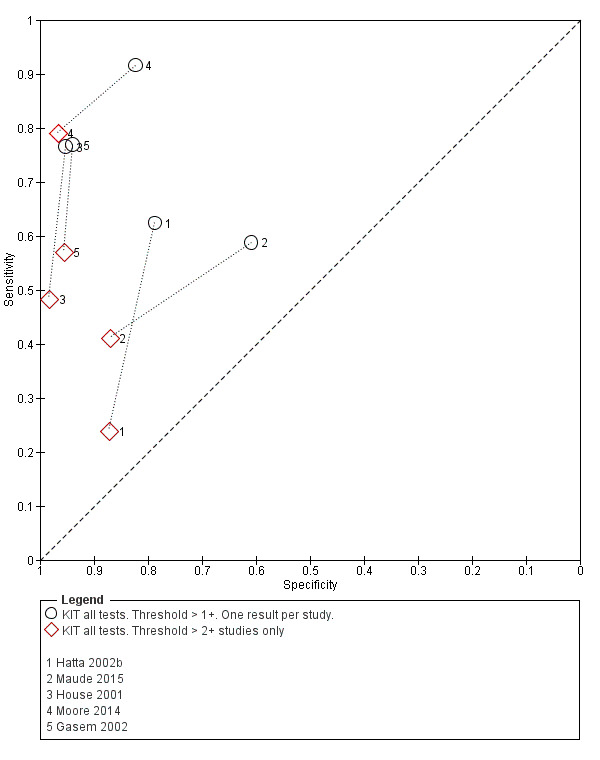
Summary receiver operating characteristic plot: KIT test by threshold > 1+ and > 2+.
Included studies evaluated these assays against different reference standards: Grade 2 (peripheral blood culture only); and Grade 2 (peripheral blood culture and blood PCR) (Moore 2014; Maude 2015). One study was a Grade 1 study (peripheral blood culture, or bone marrow culture, or both) although less than half (61/127) had a bone marrow culture performed, with the remainder using blood culture only as the reference standard (Gasem 2002). Figure 15 illustrates the performance of the ICT lateral flow assay by these different reference standards. Figure 16 present study results according to case control or non‐case control design.
15.
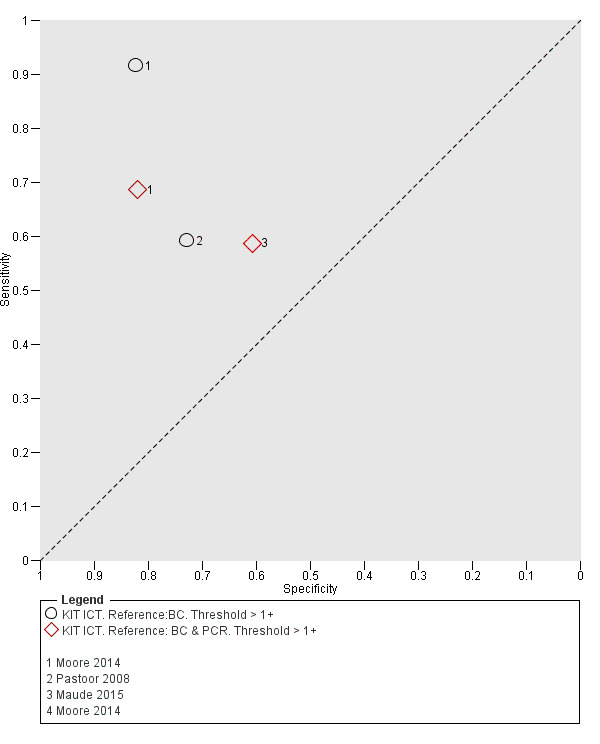
Summary receiver operating characteristic plot: KIT ICT by reference test.
Abbreviations: BC: blood culture; BC & PCR: blood culture and polymerase chain reaction.
16.
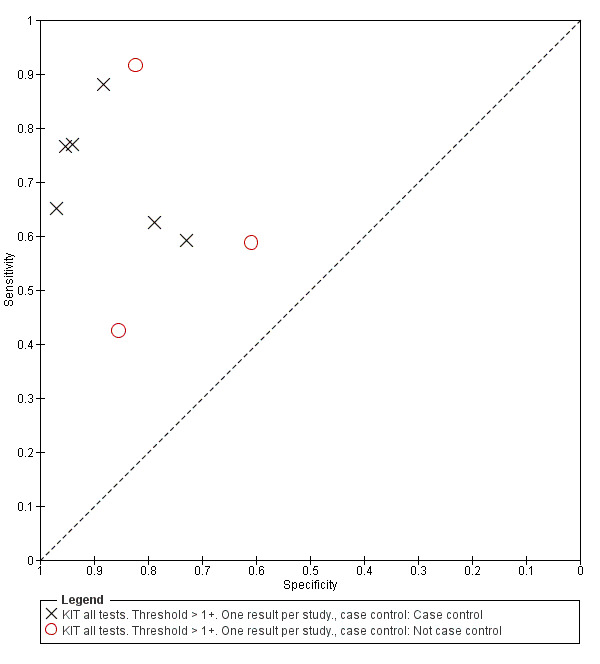
Summary receiver operating characteristic plot: KIT by case control (All test types. Threshold >1+).
Combining all different formats, the median sample size was 300 (range 85 to 502). Studies were published from 2001 to 2015. Sensitivities ranged from 42% to 92%, and specificities ranged from 61% to 97% (Figure 13). The meta‐analytical average sensitivity and specificity across all nine studies of KIT RDTs based on a threshold of > +1 was 69% (95% CI 59% to 78%) and 90% (95% CI 78% to 93%) respectively (Table 1).
Comparisons between index tests
When comparing the three main tests (Typhidot, TUBEX, and Test‐it Typhoid (KIT ICT)) we used two different groups of comparator Typhidot test because of the risk of bias introduced when studies at risk of indeterminates do not report whether indeterminates were present or how they were treated in study results. Our primary analysis related to all Typhidot tests (based on 22 studies) with a sensitivity analysis based on restricting to the 13 Typhidot studies with lower risk of bias due to clear reporting of indeterminates.
Using all 37 studies including all 22 studies with Typhidot results to compare Typhidot, TUBEX, and Test‐It Typhoid (KIT) tests, TUBEX had a 10% higher average sensitivity than Test‐It Typhoid (KIT) (95% CI −1.6% to 21.7%) although this was not a statistically significant difference. The specificity was similar between tests with TUBEX having a slightly lower average specificity of 0.5% (95% CI −7.7% to 8.9%). This also was not a statistically significant difference.
Comparing Typhidot to Test‐It Typhoid (KIT), there was a statistically significant difference in average sensitivity when compared to all Typhidot tests (Typhidot higher sensitivity 15.0%, 95% CI 2.0% to 28.1%) but the difference in sensitivity was not statistically significant when Test‐It Typhoid was compared to Typhidot tests with a lower risk of bias, due to clear reporting of indeterminates (9.3%, 95% CI −5.2% to 23.7%). The differences in average specificity were not statistically significant for either comparison (22 Typhidot studies: lower Typhidot specificity of −7.6%, 95% CI −18.6% to 3.4%; 13 Typhidot studies: lower Typhidot specificity of −9.5%, 95% CI −21.5% to 2.4%).
Comparing Typhidot to TUBEX, Typhidot had a slightly higher average sensitivity when all studies were compared to TUBEX but this was not statistically significant (5.0%, 95% CI −6.1% to 16.1%). When TUBEX was compared to Typhidot tests with a lower risk of bias due to clear reporting of indeterminates, Typhidot had a slightly lower, but not significant, average sensitivity (−0.7%, 95% CI −13.6% to 12.0%). The average specificity was lower for Typhidot compared with TUBEX based on all studies (−8.2%, 95% CI −17.7% to 1.4%) and based on Typhidot studies with lower risk of bias due to clear reporting of indeterminates (−10.1%, 95% CI −20.6% to 0.5%). In neither case was the difference in specificity statistically significant.
Paired comparisons between index tests
Direct comparison of diagnostic tests in the same patients in the same study provides the highest level of evidence to compare tests (Rutter 2001; Takwoingi 2013).
Eleven studies compared different RDTs within the same study. There were 10 paired comparisons of Typhidot/Typhidot‐M and TUBEX (Figure 17), and one study compared TUBEX and Test‐It Typhoid (and KIT prototypes) (House 2001), although it is unclear whether or not these were on the same patients (Figure 18). There were no paired comparisons of Test‐It Typhoid (and KIT prototypes) and Typhidot tests. There was no statistically significant difference in either average sensitivity nor average specificity between Typhidot and TUBEX tests, with a lower sensitivity in Typhidot (−7.6%, 95% CI −19.8% to 4.6%) and a lower specificity in Typhidot (−3.7%, 95% CI −13.9% to 6.5%). This is supported by Figure 17, where no consistent direction is evident for differences between these tests.
17.
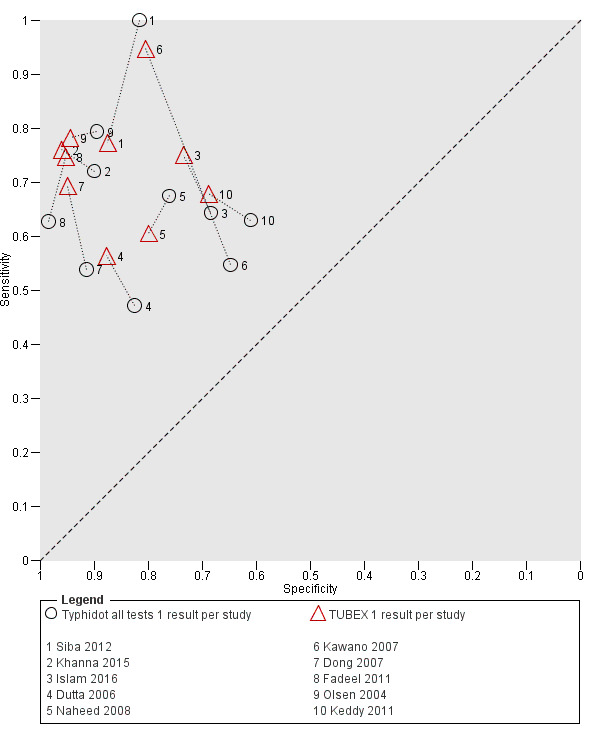
Summary receiver operating characteristic plot: Typhidot versus TUBEX. Paired studies only. One result per index test per study.
18.
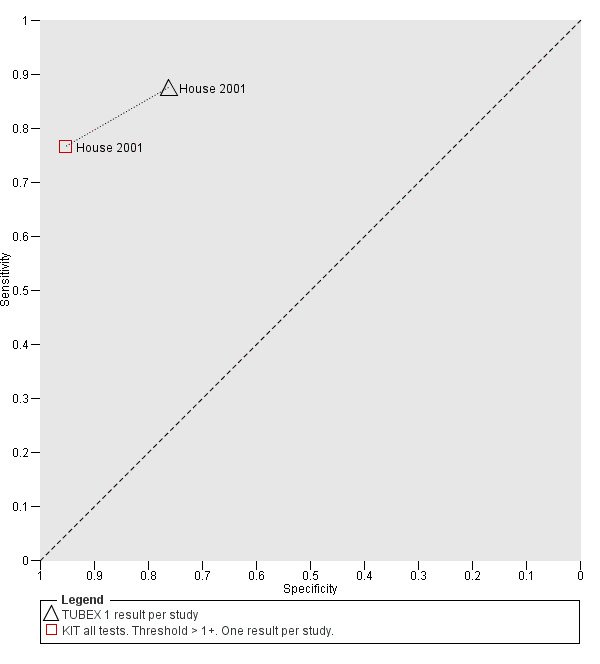
Summary receiver operating characteristic: TUBEX versus KIT. Paired results. One result per index per study.
Other RDT evaluations
There were seven other commercial RDTs that were evaluated by only 1, 2, or 3 studies, and therefore we did include them in the meta‐analyses ('Methodological quality of included studies' section). We have presented the results of these individual studies and tests in the 'Data and analyses' section and Figure 2. Further research is needed before there is sufficient data to recommend these tests. From the current studies, the most promising tests are Enterocheck WB, Enteroscreen, and PanBio.
Enterocheck WB was not compared with any other index tests in the two included studies (Anusha 2011; Anagha 2012), so only lower quality indirect evidence is available to compare test performance to other tests (Figure 2). For both studies, both sensitivity and specificity were reasonably high (Anagha 2012: sensitivity 89%, 95% CI 67% to 99%; specificity 97%, 95% CI 89% to 100%; Anusha 2011: sensitivity 85%, 95% CI 73 to 94%; specificity 89%, 95% CI 85% to 92%).
Enteroscreen was only tested in one case control study (Prasad 2015), where it was compared to Typhdot in overlapping participants. In this single case control study, Enteroscreen had a significantly lower sensitivity (Typhidot higher sensitivity based on conservative estimate of unpaired proportions; difference in sensitivity 9%, 95% CI 3% to 16%) but a significantly higher specificity (Typhidot lower specificity; difference 17%, 95% CI 14% to 20%).
Gopalakrishnan 2002 tested both PanBio and Typhidot in the same study. While the sensitivity of the tests was similar (78% and 82% respectively), the specificity of PanBio was superior in this study (81% versus 68%; 13% difference in conservative unpaired proportions with 95% CI 0.6% to 25%. We noted that there was insufficient data for more appropriate paired comparison).
Multi‐test Dip‐S‐Tick was tested in the same study participants as TUBEX and Typhidot (Olsen 2004). There was no significant difference in sensitivity between the tests, but a clinically and statistically inferior specificity in Multi‐test‐Dip‐S‐Tick (specificity: 50%, 95% CI 26% to 74%) compared in the same participants with both TUBEX (TUBEX higher specificity; difference in specificity of 44%, 95% CI 19% to 69%) and Typhidot (Typhidot higher specificity; difference in specificity of 39% (95% CI 12% to 66%).
A single study compared Mega Salmonella to Typhidot, TUBEX, and SD Bioline using the same participants (Kawano 2007). Mega Salmonella had superior sensitivity to Typhidot and SD Bioline but significantly lower specificity (the 95% CI for specificity did not overlap with those from TUBEX or SD Bioline). In this study TUBEX has similar sensitivity to Mega Salmonella (95% and 91% respectively) and significantly higher specificity (80%, 95% CI 71 to 88) versus 49% (95% CI 39 to 59) respectively). Mega Salmonella had an inferior performance to TUBEX, SD Bioline, and Typhidot, although this was only based on evidence from one included study.
Three included studies evaluated SD Bioline (Kawano 2007; Limpitikul 2014; Maude 2015), and all three studies reported the preferred IgM test format. In Kawano 2007, SD Bioline IgM had an inferior performance to TUBEX when tested on the same participants. SD Bioline had significantly lower sensitivity to TUBEX (51% (95% CI 58% to 72%) versus 95% (95% CI 87% to 99%) respectively) and similar specificity (76% versus 80% respectively). In Maude 2015, SD Bioline IgM had significantly lower sensitivity at 21% (95% CI 9% to 38%) compared to both Test‐It Typhoid (Life Assay) and Onsite Typhoid (CTK Biotech), both with a reported sensitivity of 59% (95% CI 41% to 75%), indicated as the 95% CIs did not overlap.
Two included studies assessed Onsite Typhoid (CTK Biotech). In Maude 2015, it was compared with both Test‐It Typhoid (Life Assay) and the SD Bioline test. Onesite Typhoid had similar results to the Test‐It Typhoid test, which were superior in sensitivity to SD Bioline. However, SD Bioline had significantly higher specificity (97%, 95% CI 95% to 99%) than both Test‐It Typhoid test (61%, 95% CI 55% to 67%) and Onsite Typhoid (74%, 95% CI 68% to 79%). Tarupiwa 2015 evaluated Onsite Typhoid alongside TUBEX, where the performances of both tests were closely comparable. We note that these results are based on two studies and further research is needed.
Heterogeneity
There were insufficient studies for formal heterogeneity analysis using meta‐analysis of test subgroups, except for a comparison of Typhidot test studies at lower risk of bias due to clear reporting of indeterminate results. For other potential sources of heterogeneity ('Investigations of heterogeneity' and 'Secondary objectives' sections) where individual study characteristics could be investigated, such as study design, prevalence, and study reference standard, we presented results for visual examination of heterogeneity in summary ROC (SROC) plots and forest plots.
Discussion
The principal findings of this systematic review were that the diagnostic accuracy of the three main groups of commercially available rapid diagnostic tests (RDTs) for enteric fever (Typhidot and its variants, TUBEX, Test‐It Typhoid and prototype (KIT) tests) was moderate. There was no statistically significant difference in the average sensitivity between Typhidot, TUBEX, or Test‐It Typhoid tests, except when we compared all Typhidot tests to Test‐It Typhoid (84% all Typhidot studies, 78% Typhidot studies with low risk of bias due to clear reporting of indeterminates, 78% TUBEX, 69% Test‐It Typhoid). There was no statistically significant difference for average specificity between these tests (79% all Typhidot studies, 77% Typhidot with low risk of bias due to clear reporting of indeterminates, 87% TUBEX, 90% Test‐It Typhoid); see 'Summary of findings' table 1 (Table 1).
A clinically useful test requires high values for both sensitivity and specificity. There was no statistical evidence to demonstrate that one group of tests was significantly better than the other (Figure 17; Figure 18; Figure 19; Figure 20; Figure 21). The quality of studies that evaluated the diagnostic accuracy of RDTs for enteric fever was generally low. Only three of the 37 included studies used the Grade 1 reference standard requiring a bone marrow and blood culture result, and less than one‐third of studies recruited unselected febrile patients.
19.
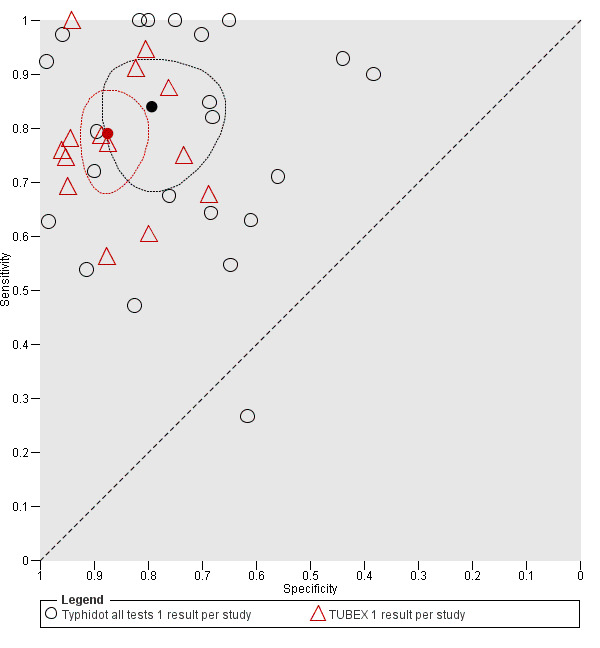
Summary receiver operating characteristic plot: Typhidot versus TUBEX tests. One result per index test per study.
20.
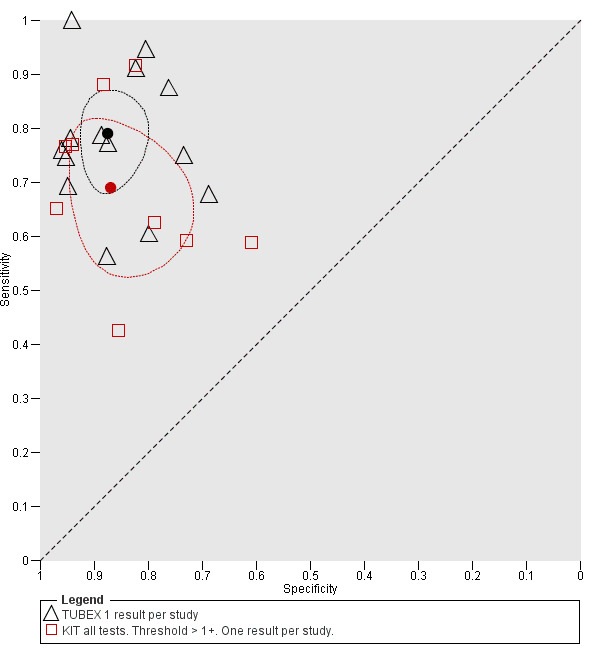
Summary receiver operating characteristic plot: TUBEX versus Test‐it Typhoid (KIT) tests. One result per index test per study.
21.
Summary receiver operating characteristic: Typhidot versus KIT. No paired studies. One result per index per study.
In a hypothetical cohort of 1000 patients presenting with fever where 30% (300 patients) have enteric fever: on average, and based on all the test results, Typhidot will miss the diagnosis in 48 of the 300 patients with enteric fever (66 missed based on Typhidot studies with low risk of bias due to clear reporting of indeterminates); TUBEX will miss 66; and Test‐It Typhoid and prototype (KIT) tests will miss 93. In the 700 people without enteric fever the average number of patients with a false positive diagnosis of enteric fever would be 147 with Typhidot tests, (161 in Typhidot tests with low risk of bias due to clear reporting of indeterminates), 91 with TUBEX, and 70 with Test‐It Typhoid and prototype (KIT) tests. The target product profile of an enteric fever RDT has not been defined. A sensitivity of > 90% and specificity of > 95% are probably minimum targets. In our hypothetical cohort of patients a test with our minimum target product profile would miss on average 30 of 300 enteric fever patients and give a false positive diagnosis in 35 of 700 without enteric fever.
RDTs for other febrile illnesses, such as malaria and dengue, already have been tested extensively in standardized evaluations that have provided an evidence base for World Health Organization (WHO) guidance and for the diagnostic algorithms used in endemic regions (WHO 2009; Abba 2011). The diagnostic tests for acute enteric fever have not been evaluated with the same rigorous methods. A diagnostic test to detect chronic (asymptomatic) carriers and individuals who have had prior exposure to the causative pathogens may also be of considerable epidemiological value. Such tests could potentially strengthen surveillance programmes aimed at identifying populations with a high‐burden of enteric fever that might benefit from vaccination initiatives (Andrews 2015). The lack of such diagnostics obscures the true burden and impact of the disease; crucial information needed for policymakers, Ministries of Health, and others (Baker 2010; Crump 2014).
It is important to highlight the heterogeneity among the included studies. Patient selection (unselected febrile patients versus those suspected to have enteric fever) is a major source of heterogeneity. The variation in how indeterminate results in evaluations of Typhidot (IgG positivity, IgM positivity, or both) were treated and reported was also considerable (see the 'Strengths and weaknesses of the review' section). Most included studies took place in tertiary centres in South‐Asian settings highly endemic for enteric fever. There were also studies set in medium‐endemic regions but relatively few in sub‐Saharan Africa (Crump 2004).
Thriemer review
Thriemer and colleagues published a systematic review of TUBEX and Typhidot for the diagnosis of acute enteric fever (Thriemer 2013). They reported a meta‐analysis average sensitivity and specificity of TUBEX of 69% (95% CI 45% to 85%) and 88% (95% CI 83% to 91%) respectively. The Thriemer review authors also reported Typhidot sensitivity and specificity estimates of between 56% and 84% and 31 and 97% respectively (Thriemer 2013). They did not perform a meta‐analysis for Typhidot due to the limited data available. These results are comparable to the findings of this Cochrane Review: TUBEX sensitivity of between 71% to 85% and specificity 82% to 91%; Typhidot sensitivity 73% to 91% and specificity 70% to 87% (Table 1). There are however a number of methodological differences between the two reviews.
Thriemer 2013 only included studies that used a commercial blood culture system with automated detection of positive cultures, and excluded studies using an 'in‐house' blood culture system with manual detection of positive cultures. The number of studies of these tests using commercial blood culture systems was limited, which meant a meta‐analysis was not possible. Commercial blood culture systems ensure that the reference test has been performed in a consistent and quality assured manner. If the 'in‐house' blood culture system employs accepted media formulations and is subjected to appropriate quality control testing, it should be as sensitive as commercial systems (Wilson 1994). The major difference between the commercial automated and 'in‐house' manual blood culture systems relates to the speed of result, with the automated systems detecting bacterial growth earlier.
Thriemer 2013 did not include test accuracy data for the Typhidot‐M test. The Thriemer review authors explored various classifications of how to treat the indeterminate results when describing the statistical approach to analysing the Typhidot test data. In our Cochrane Review we have included studies that looked at Typhidot‐M and classified indeterminate results as negative. To allow a clearer comparison between the Typhidot and Typhidot‐M test results, we extracted the IgM antibody data from the Typhidot studies when given in the report.
The Thriemer review only included commercially available RDTs at the time of the literature search. We included the Test‐it Typhoid ICT lateral flow assay (LifeAssay Diagnostics), which is now commercially available. This test was developed from several prototype RDTs by the Royal Tropical Institute (KIT) in Amsterdam. The Test‐it Typhoid test and the KIT protypes all measure IgM antibodies against an lipopolysaccharide (LPS) antigen in various formats. In this review we have evaluated both the KIT prototypes and the commercial RDT.
Reference standard
The evaluation of RDTs in enteric fever is complicated by the lack of a suitable reference standard (Baker 2010). The quality of the reference standard used in these studies affects the diagnostic accuracy results of each RDT. Combinations of peripheral blood culture, bone marrow culture, and blood PCR positivity have been used to indicate a true positive result (enteric fever case). If these reference tests are negative then we have described these as a non‐enteric fever case. Blood culture lacks sensitivity (WHO 2003; Mogasale 2016), so it is likely some of the culture‐negative patients will actually have enteric fever. It must be acknowledged that culture‐negative patients with a positive RDT result may actually be true positives rather than false positives. Most Grade 2 studies used blood culture only as the reference standard (Figure 7; Figure 11). The stronger studies were those where index tests were evaluated against more than one different reference test (Siba 2012; Moore 2014). Studies with more robust reference standards demonstrated reduced RDT sensitivity. The Grade 1 studies using bone marrow culture were conducted in higher prevalence populations (Khan 2002: 54%; Bhutta 1999: 47%), and perhaps in those with more severe disease. This correlates with the reduced index test performance in other high prevalence studies (Olsen 2004: 75%). In the TUBEX (Figure 7) and Typhidot (Figure 11) studies, there seem to be a common 20% to 25% reduction in sensitivity when the blood polymerase chain reaction (PCR) result was combined with blood culture as a composite reference standard. PCR has the potential ability to increase the number of typhoid cases identified by detecting dead bacteria or bacteria that cannot be cultured (Massi 2005; Nga 2010). It appears that these patients are less likely to be antibody positive in the RDTs, which explains the decrease in sensitivity when a PCR reference test is used.
Study design
The identification of studies that use or avoid a case control design formed part of the assessment of methodological quality (Whiting 2003). Case control designs can introduce bias and increase apparent accuracy as more severe disease is often compared to healthy patients. Studies that avoid a case control design by recruiting a cohort of unselected febrile patients have a lower risk of bias relating to patient selection. Over a third (16) of the 37 studies used a case control study design. Figure 22, Figure 10, and Figure 16 are receiver operating characteristic (ROC) plots for Typhidot, TUBEX, and Test‐It Typhoid and KIT prototypes respectively. Each study is plotted indicating whether they adopted or avoided a case control design. Across all three index test groups, case control studies had higher apparent accuracy, with results having a higher combination of sensitivity and specificity. This highlights the importance of robust study designs in the evaluation of diagnostic test accuracy.
22.
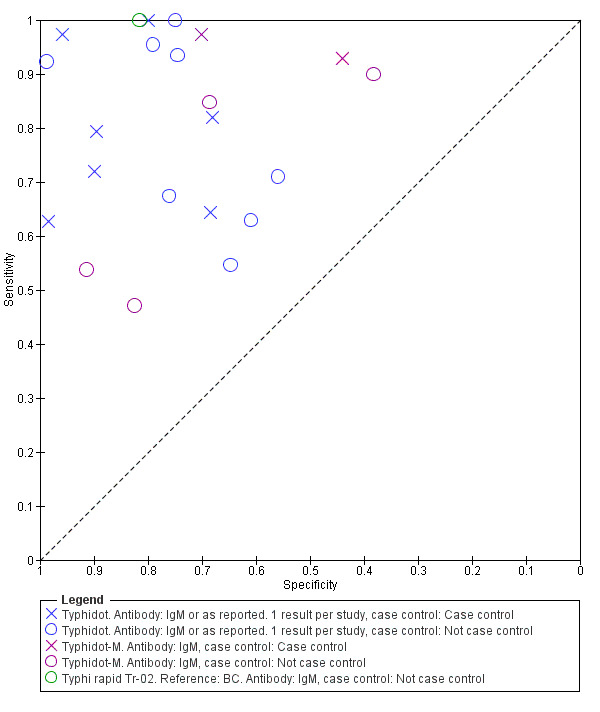
Summary receiver operating characteristic plot: Typhidot tests by case control design.
Only 11 of the 37 included studies recruited unselected febrile patients. Most of the other studies used a clinical suspicion of enteric fever as the major entry criteria, but rarely specified the precise clinical criteria used to suspect the disease. The choice of the optimum non‐disease control group is also difficult. Unselected febrile patients with another confirmed diagnosis are the optimum control group, but difficult to recruit. Thriemer 2013 also discussed this control group issue (Type 1 control). Patients with suspected enteric fever or non‐specific fever but who are blood culture negative are less satisfactory as a non‐disease control group (Type 2 control) and will decrease the apparent specificity of the test (Thriemer 2013). Cases in this group may actually have enteric fever despite testing negative on both index and reference tests. In addition to this, studies that analysed index tests in healthy afebrile controls are likely to have overestimated specificity.
Comparisons between tests
Comparisons of diagnostic tests are typically based on a combination of both direct comparisons where the tests are compared in the same patients, and indirect comparisons, where the tests being compared are conducted on different patients. Direct comparisons are at lower risk of bias as when the same patients at the same time point are tested as patients are tested with the same disease severity and comorbidities, and other features of study design that may give rise to potential for bias are also the same.
We compared Typhidot, TUBEX, and Test‐It Typhoid based on a combination of direct and indirect test comparisons. We did not detect any statistically significant difference between these tests when the comparisons were based on Typhidot tests at lower risk of bias due to clear reporting of indeterminates.
There were 11 studies with direct comparisons of different RDTs within the same study (Figure 17; Figure 18). TUBEX and Typhidot/Typhidot‐M were the most common comparisons. There was no statistical difference detected and no consistent direction of difference found between these two groups of index tests (Typhidot and variants versus TUBEX).
Summary of main results
We have summarized the main quantitative diagnostic test accuracy results in 'Summary of findings' table 1 (Table 1).
The number of high quality studies that evaluated the diagnostic accuracy of RDTs for enteric fever was low, as many studies adopted a case control study design.
Only 3/37 included studies used the Grade 1 reference standard of bone marrow culture.
Less than one‐third of the included studies (11/37) recruited unselected febrile patients. Most used a clinical suspicion of enteric fever as the major inclusion criterion.
Most included studies (86%) recruited patients from the Asia‐Pacific region, and 50% of studies recruited from South Asia.
The three main groups of RDTs for enteric fever evaluated were: Typhidot and its variants; TUBEX; and the Test‐it Typhoid test with its earlier dipstick/latex agglutination/lateral flow assays prototypes developed by the Royal Tropical Institute (KIT), Amsterdam.
The diagnostic accuracy for enteric fever of the three main RDT groups was moderate. TUBEX performed the most consistently with moderate average sensitivity (78%) and better specificity (87%), but when compared to Typhidot there was no evidence to suggest that one was better than the other.
The Test‐it Typhoid tests and KIT protypes demonstrated moderate sensitivity, but higher levels of specificity (average 90%).
For Enterocheck WB, Enteroscreen, PanBio Multi‐test Dip‐S‐Tick, Mega Salmonella, SD Bioline,and Onsite Typhoid, there is insufficient evidence to recommend these tests, as there are only results from 1, 2, or 3 included studies. Several of these RDTs had inferior performance to either Typhidot or TUBEX, based on comparison of sensitivity in the same participants in single studies.
We did not find any statistically significant differences in sensitivity or specificity between Typhidot tests evaluated with low risk of bias due to clear reporting of indeterminates and the TUBEX and Test‐It Typhoid tests, based on combined data from both direct and indirect test comparisons (comparisons of test on either the same patients or different patients).
Analysis of direct paired (comparative) data was possible across 10 studies comparing Typhidot and TUBEX, but we did not find any statistically significant difference between the two tests. It is not possible to state that one group of index tests has higher accuracy than another. Within individual studies data was available to compare other commercial tests, and further studies are needed to substantiate findings from single studies.
There was insufficient data to formally investigate sources of heterogeneity as listed in the 'Secondary objectives' and 'Investigations of heterogeneity' sections.
There were no eligible studies that evaluated RDTs exclusively for detecting paratyphoid disease.
Strengths and weaknesses of the review
A major problem with most included studies was the use of a relatively weak reference standard. Blood culture has an estimated sensitivity of between 40% to 80% (WHO 2003), with a more recent systematic review estimating sensitivity to be around 60% (Mogasale 2016). Only three studies used the best reference standard currently available (blood culture and bone marrow culture). Bone marrow culture is estimated to increase the number of true positives by an additional 10% over blood culture alone (WHO 2003; Mogasale 2016). The additional benefit of a blood PCR result is undefined, and the testing methodology has not yet been standardized (Smits 2013). A weak reference standard means that a number of true positive results were classified as false negatives (Reitsma 2009). There was a great variation in the reporting of the accreditation and quality of microbiology laboratories where the cultures were processed.
Statistical analysis of Typhidot and its variants was complicated, given the evolution of the product target from measuring both IgM and IgG antibodies to just IgM alone. This was compounded by the inadequate clarity of the reported results. Many of the included studies were not well reported, and did not perform well under the scrutiny of the modified QUADAS‐2 tool. The data for a number of studies was incomplete, and could not be clarified despite contacting corresponding authors. Only a few studies reported blinding of the index and reference tests.
A weakness of the review related to the classification and subsequent analysis of indeterminate results for Typhidot tests. When we could extract both IgG and IgM data for Typhidot, we classified a case that was IgG positive and IgM negative as indeterminate. This differed from the treatment of indeterminate results of some included studies (Fadeel 2011; Olsen 2004).
Thriemer 2013 described the differences in sensitivity and specificity from one study (Kawano 2007) in three different ways: when indeterminate results were excluded; when indeterminate results were considered negative; and when indeterminate results were included in the denominator. In our Cochrane Review, this is illustrated in Figure 5 and Figure 6. These demonstrate a roughly 20% decrease in sensitivity when we included indeterminate results in the analysis. It is important to acknowledge variation in the classification of indeterminate results as a limitation in the analysis of results for Typhidot.
Data extraction from certain case control studies, Fadeel 2011, required careful recalculation where different categories of negative patients were described, for example, blood culture negative and Widal Test positive, versus known negatives. Index tests were then tested against different sub‐groups within the cohort. This change in sampling meant that the prevalence of disease changed depending on which subgroup the index test was used in.
This review covers both typhoid and paratyphoid fever, but there were no suitable studies related to paratyphoid alone. Another weakness of this review was the variability in the treatment of paratyphoid cases as part of the diagnostic test accuracy data between studies. In one study, authors excluded cases of blood culture positive Salmonella Paratyphi A (Jesudason 2006). A number of studies classified blood culture positive cases of paratyphoid as true negatives (Gasem 2002; Dutta 2006; Hosamani 2013; Sanjeev 2013). In contrast, paratyphoid fever was classified as a target condition along with typhoid fever in two studies (Dong 2007; Prasad 2015).
Applicability of findings to the review question
A low number of studies have evaluated the diagnostic test accuracy of enteric fever RDTs. Furthermore, the number of good quality studies was low. The main issues relating to quality include: utility of a second‐class reference standard; recruitment of clinically suspected enteric fever patients as opposed to unselected febrile patients; poor reporting of whether investigators were blinded to reference test results when interpreting the index tests; and frequent use of a case control design. The sensitivity and specificity of TUBEX, Typhidot and its variants, and Test‐it Typhoid test and its KIT protypes are not robust enough to replace existing diagnostic tools in enteric fever.
Authors' conclusions
Implications for practice.
The moderate sensitivity and specificity of the evaluated RDTs does not support their use as a replacement for blood culture for diagnosing enteric fever. The performance of the RDTs might be improved by combination with a transparent clinical algorithm for suspected enteric fever, but such algorithms do not exist. RDTs can only influence clinical practice if healthcare professionals trust the result. Although the specificity of the TUBEX and Test‐it Typhoid test and KIT prototypes were fairly good, if the RDT delivers a negative result in a patient believed to have enteric fever, the clinician is still likely to prescribe antimicrobials. If a febrile patient from an endemic region with a positive enteric fever RDT result also has an alternative febrile illness diagnostic positive (for example, dengue or malaria RDT) this further complicates management.
Although this Cochrane Review treated typhoid and paratyphoid fever as separate target conditions, in clinical practice the distinction is not clear. Paratyphoid fever is often milder as a clinical syndrome compared to typhoid (Waddington 2014), although in some reports the two syndromes have been indistinguishable (Maskey 2006). In some geographical areas, the levels of multi drug‐resistance in S. Paratyphi A is lower than in S. Typhi, but nalidixic acid resistance is more common (Darton 2014). Despite these differences in antimicrobial susceptibility patterns between typhoid and paratyphoid (McKinnon 2014), an RDT that detects both typhoid and paratyphoid infections is the most clinically relevant in terms of prompting the commencement of antimicrobials. An RDT that distinguishes the two serovars should not alter management (Andrews 2015).
Implications for research.
The cornerstone of diagnostic test accuracy studies is the reference standard. Research into developing a better reference standard for the diagnosis of enteric fever in both adults and children is needed (Mogasale 2016). This could help the diagnosis of enteric fever in well‐resourced settings, and significantly raise the quality of future evaluations of RDTs and other diagnostic tests (Reitsma 2009). The formulation of a composite reference standard for enteric fever could be one such strategy (Storey 2015). RDTs that detect both paratyphoid and typhoid fever on the same test are necessary given the similarities in treatment, and the increasing similarities in clinical presentation in some settings (Maskey 2006).
Current enteric fever RDTs rely on detecting immuno‐serological responses. Alternative biomarkers of acute enteric fever, such as metabolomic profiles (Baker 2010; McKinnon 2014), could form the basis of new groups of RDTs. The unique host genomic signatures during bacterial versus viral infections could also lead to novel RDTs in the future (Herberg 2016).
Combining an RDT within a transparent clinical algorithm for the febrile patient could potentially improve diagnostic test accuracy. Further research on combining clinical prediction rules for febrile illnesses in typhoid endemic with disease‐specific RDTs could be a potential route in a community‐based setting (Parry 2011). Qualitiative research on how healthcare professionals view RDTs will be needed to guide larger‐scale implementation programmes.
Acknowledgements
The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this review do not necessarily reflect UK government policy. We thank Sarah Donegan (SD) who contributed to the statistical integrity of the protocol and review planning; Vittoria Lutje (VL) who was responsible for the strategy and conduct of the literature search; Christianne Esparza (CE) who co‐ordinated the retrieval of papers for the review; and Anne‐Marie Stephani (AMS) who co‐ordinated administrative and technical support.
The Contact Editors for this review were Dr Karen Steingart and Dr Olalekan Uthman.
Appendices
Appendix 1. Search strategy
Ovid MEDLINE® In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE®
1 typhoid fever/
2 exp Salmonella enterica/
3 exp paratyphoid fever/
4 "typhoid fever".mp.
5 "paratyphoid fever".mp.
6 "enteric fever".mp.
7 (typhi or paratyphi or "salmonella enterica").ab. or (typhi or paratyphi or "salmonella enterica").ti.
8 1 or 2 or 3 or 4 or 5 or 6 or 7
9 "rapid diagnostic test*".ab. or "rapid diagnostic test*".ti.
10 RDT*.ab. or RDT*.ti.
11 "serodiagnostic test*".ab. or "serodiagnostic test*".ti.
12 (Widal or "DOT enzyme immunoassay" or typhiDOT or TUBEX).ab. or (Widal or "DOT enzyme immunoassay" or typhiDOT or TUBEX).ti.
13 ("solid‐phase" or "DOT blot").ab. or ("solid‐phase" or "DOT blot").ti.
14 serodiagnosis/
15 immunoblotting/
16 "immunochromatographic lateral flow assay*".ab. or "immunochromatographic lateral flow assay*".ti.
17 (typhirapid or "latex agglutination" or "test‐it‐typhoid" or enterocheck or "SD bioline" or "dip‐s‐tick" or panbio or "mega salmonella" or naats or "nucleid acid amplication test*").ab. or (typhirapid or "latex agglutination" or "test‐it‐typhoid" or enterocheck or "SD bioline" or "dip‐s‐tick" or panbio or "mega salmonella" or naats or "nucleid acid amplication test*").ti.
18 ("antigen detection" or "antibody detection").ab. or ("antigen detection" or "antibody detection").ti.
19 ("blood culture*" or "bone marrow culture*").ab. or ("blood culture*" or "bone marrow culture*").ti.
20 Reagent Kits, Diagnostic/
21 Serologic Tests/
22 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21
23 8 and 22
Embase
1 typhoid fever/
2 exp Salmonella enterica/
3 exp paratyphoid fever/
4 "typhoid fever".mp.
5 "paratyphoid fever".mp.
6 "enteric fever".mp.
7 (typhi or paratyphi or "salmonella enterica").ab. or (typhi or paratyphi or "salmonella enterica").ti.
8 1 or 2 or 3 or 4 or 5 or 6 or 7
9 "rapid diagnostic test*".ab. or "rapid diagnostic test*".ti.
10 RDT*.ab. or RDT*.ti.
11 "serodiagnostic test*".ab. or "serodiagnostic test*".ti.
12 (Widal or "DOT enzyme immunoassay" or typhiDOT or TUBEX).ab. or (Widal or "DOT enzyme immunoassay" or typhiDOT or TUBEX).ti.
13 antigen detection/
14 antibody detection/
15 blood culture/
16 bone marrow culture/
17 ("solid‐phase" or "DOT blot").ab. or ("solid‐phase" or "DOT blot").ti.
18 serodiagnosis/
19 immunoblotting/
20 "immunochromatographic lateral flow assay*".ab. or "immunochromatographic lateral flow assay*".ti.
21 (typhirapid or "latex agglutination" or "test‐it‐typhoid" or enterocheck or "SD bioline" or "dip‐s‐tick" or panbio or "mega salmonella" or naats or "nucleid acid amplication test*").ab. or (typhirapid or "latex agglutination" or "test‐it‐typhoid" or enterocheck or "SD bioline" or "dip‐s‐tick" or panbio or "mega salmonella" or naats or "nucleid acid amplication test*").ti.
22 typhoid rapid test/
23 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
24 8 and 23
Web of ScienceTM Core Collection
Indexes=SCI‐EXPANDED
#2 AND #1
# 2 TOPIC: ("rapid diagnostic test*" OR RDT*) OR TOPIC: ("serodiagnostic test*" OR Widal or "DOT enzyme immunoassay" or typhiDOT or TUBEX) OR TOPIC: ("solid‐phase" or "DOT blot" OR serodiagnosis OR immunoblotting) OR TOPIC: (typhirapid or "latex agglutination" or "test‐it‐typhoid" or enterocheck or "SD bioline" or "dop‐s‐tick" or panbio or "mega salmonella" or naats or "nucleid acid amplication test*") OR TOPIC: ("antigen detection" or "antibody detection" OR "blood culture*" OR "bone marrow culture*")
# 1 TOPIC: ("typhoid fever" OR "paratyphoid fever" OR "enteric fever") OR TOPIC: ("salmonella typhi" OR "salmonella paratyphi")
LILACS
Search on : typhoid OR paratyphoid OR salmonella typhi OR salmonella enterica [Words] and "rapid diagnostic test$" OR RDT$ OR widal OR typhidot OR tubex OR serological test$ OR immunoblotting OR DOT [Words]
IndMED, African index Medicus
'typhoid", "paratyphoid", "enteric fever", and "rapid diagnostic test*", RDT.
Appendix 2. Data extraction
| Study ID | First author, year of publication |
| Clinical features and setting | Clinical features: presenting signs and symptoms; index of suspicion for enteric fever (that is, suspected versus unselected febrile); and recent prior antimicrobial treatment. Setting: healthcare facility; country; endemicity; and endemic subspecies. |
| Participants | Sample size; age; gender; comorbidities; point of recruitment (in‐patients/ out‐patients); and pregnancy. |
| Study design | Whether patients enrolled prospectively or retrospectively. Whether sampling methods were consecutive or random. If the study enrolled more than 1 rapid diagnostic test (RDT), how were tests allocated to individuals or did individuals receive all the tests? Were RDTs used on suspected typhoid/paratyphoid cases or unselected febrile patients? |
| Target condition | Typhoid fever or paratyphoid fever, or both |
| Reference standard | Which reference standard was used (bone marrow/blood culture/PCR/combination)? Who performed the reference standard test(s)? Where was the test performed? How many repeats were used? Number of observers/operators. Methods of inter‐observer discrepancy resolution. Has the laboratory received quality accreditation by an external agency? |
| Index tests |
Salmonella enterica serovars designed to detect Typhi (typhoid), Paratyphi A (paratyphoid), or both. Commercial name. Blood or urine. If blood RDT, capillary or venous blood. Antigen or antibody detection. If antibody detection, subclass detected (that is, IgG/IgM). Format. Transport and storage conditions. Details of test operators, including any special training provided. Where was the test performed? Number of observers/operators and methods of inter‐observer discrepancy resolution. Threshold, that is, what constituted a positive result? |
| Data | Numbers of true positives, false positives, true negative, and false negatives. |
| Notes | Source(s) of funding |
Abbreviations: Rapid diagnostic test (RDT); Immunoglobulin‐G (IgG), Immunoglobulin‐M (IgM); Polymerase chain reaction (PCR).
Appendix 3. Assessment of methodological quality
| Quality indicator | Notes |
| 1. Patient selection | |
| Was a consecutive or random sample of patients enrolled? | Yes: if the study recruited a consecutive or random sample of eligible patients No: if the study selected patients by convenience Unclear: if the study did not report the method of patient selection, or this was not clearly reported |
| Was a case control design avoided? | Yes: if the study recruited unselected febrile patients No: if the study recruited confirmed or suspected cases of enteric fever, or both as a case group Unclear: for all other scenarios or if this was not clearly reported |
| Did the study avoid inappropriate exclusions? | Yes: if there were no participants excluded from the analysis, or if exclusions were adequately described. No: if there were unexplained exclusion of participants Unclear: if insufficient information was given to assess whether any participants were excluded from the analysis |
| Could the selection of patients introduced bias? | Low risk: inclusion and exclusion criteria clearly described, for example, patients with fever, patients suspected to have enteric fever, or both High risk: inclusion and exclusion criteria not included Unclear risk: If selection criteria were partially reported |
| Are there concerns that the included patients and setting do not match the review question? | Low concern: patients with fever and recruited from an area of high or medium endemicity for enteric fever as defined by Crump 2004 High concern: patients without fever or recruited from an area of low endemicity for enteric fever (Crump 2004) Unclear concern: if the location or clinical characteristics of participants were not adequately described |
| 2. Index test | |
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes: person undertaking the index test did not know the results of the reference tests, or if the tests were carried out in different places No: if the same person performed both tests, or the results of the reference tests were known to the person undertaking the index tests Unclear: if insufficient information provided |
| If a threshold was used, was it pre‐specified? | Yes: if the threshold's pre‐specified by the respective manufacturers were described and followed No: if the manufacturer's thresholds were described but not followed Unclear: if this is not clearly described or there were no thresholds for the evaluated RDT |
| Could the conduct or interpretation of the index test have introduced bias? | Low risk: if the index test was utilized according to manufacturers' instructions High risk: if the use of index tests(s) deviated from manufacturers' instructions Unclear risk: if insufficient information provided |
| Are there concerns that the index test, its conduct, or interpretation differ from the review question? | Low concern: if the index test was used to diagnose enteric fever in symptomatic patients from areas of high or medium enteric fever endemicity (Crump 2004) High concern: if the index test was used to diagnose enteric fever in patients from areas of low endemicity for enteric fever (Crump 2004), or those who are asymptomatic Unclear concern: if the location or clinical characteristics of participants were not described |
| 3. Reference standard | |
| Is the reference standard likely to correctly identify the target condition? | Yes: if bone marrow and blood culture (Grade 1 Reference standard) are performed at an externally accredited laboratory and adequate blood/marrow volumes were taken (Wain 1998; Wain 2001) No: If inadequate blood/marrow volumes were taken (Wain 1998; Wain 2001) Unclear: if blood culture alone (Grade 2 Reference standard) is performed, or if external quality assurance accreditation of the relevant laboratory or blood/marrow volumes were not described |
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes: person undertaking the reference test did not know the results of the index tests, or if the tests were carried out in different places No: if the same person performed both tests, or the results of the index tests were known to the person undertaking the reference tests Unclear: if insufficient information provided |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk: if the reference standard results and index tests were analysed separately High risk: if the reference standard results and index tests results were analysed together Unclear risk: if insufficient information was provided |
| Are there concerns that the target condition as defined by the reference standard does not match the question? | We will judge this to be 'low risk' for all studies that use isolation of Salmonella Typhi, or Paratyphi A, or both from blood,bone marrow, or both. |
| 4. Flow and timing | |
| Was there an appropriate interval between index test and reference standard? | Yes: if the index test and reference standard(s) were collected on the same patients at the same time or within 24 hours of each other No: if the time period between index test and reference standard(s) collection was > 24 hours Unclear: if the time period between index test and reference standard collection was not described |
| Did all patients receive the same reference standard? | Yes: if the same reference test(s) was/were used in all participants No: if different reference test(s) was/were used depending on index test results Unclear: if insufficient information was provided |
| Were all patients included in the analysis? | Yes: if the number of participants in the two‐by‐two table matched the number of participants recruited into the study or if sufficient explanation was provided for any discrepancy. No: number of participants in the two‐by‐two table did not match the number of participants recruited into the study and insufficient explanation was provided for any discrepancy Unclear: if insufficient information was given to permit judgement |
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.
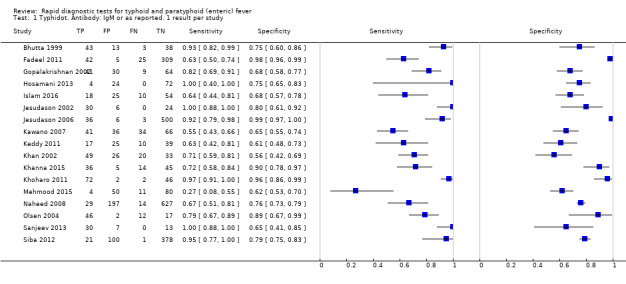
Typhidot. Antibody: IgM or as reported. 1 result per study.
2. Test.
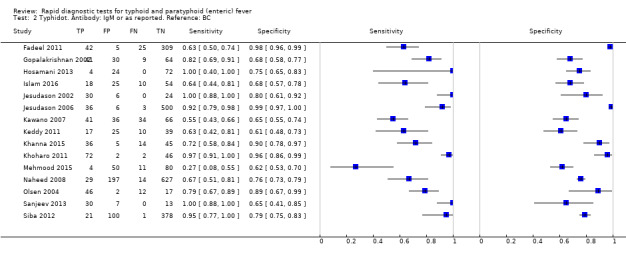
Typhidot. Antibody: IgM or as reported. Reference: BC.
3. Test.
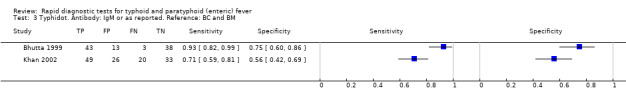
Typhidot. Antibody: IgM or as reported. Reference: BC and BM.
4. Test.

Typhidot. Antibody: IgM or as reported. Reference: BC and PCR.
5. Test.
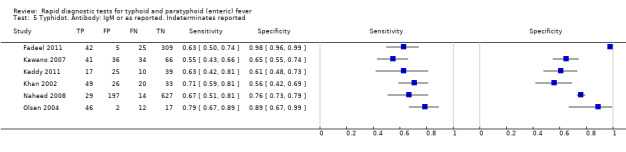
Typhidot. Antibody: IgM or as reported. Indeterminates reported.
6. Test.
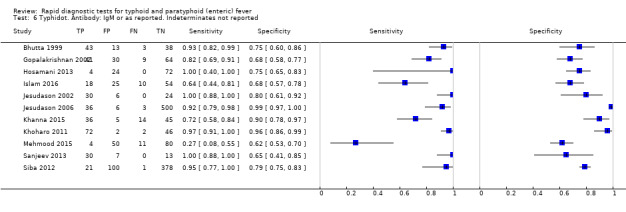
Typhidot. Antibody: IgM or as reported. Indeterminates not reported.
7. Test.
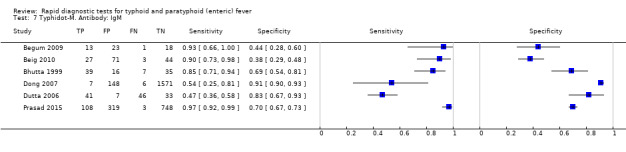
Typhidot‐M. Antibody: IgM.
8. Test.

Typhi rapid Tr‐02. Reference: BC. Antibody: IgM.
9. Test.

Typhi rapid Tr‐02. Reference: BC & PCR. Antibody: IgM.
10. Test.
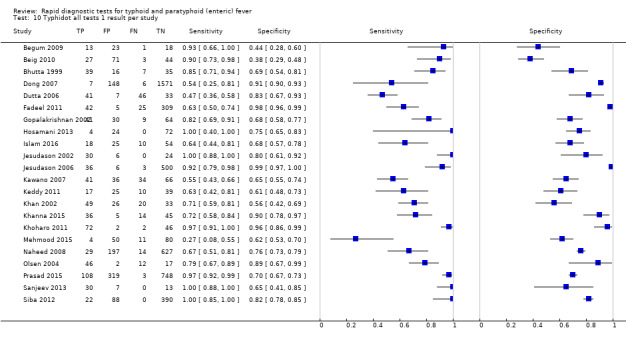
Typhidot all tests 1 result per study.
11. Test.
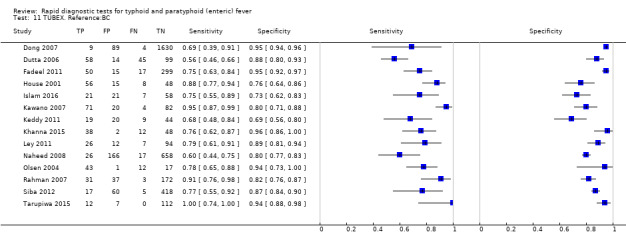
TUBEX. Reference:BC.
12. Test.

TUBEX. Reference: BC & PCR.
13. Test.
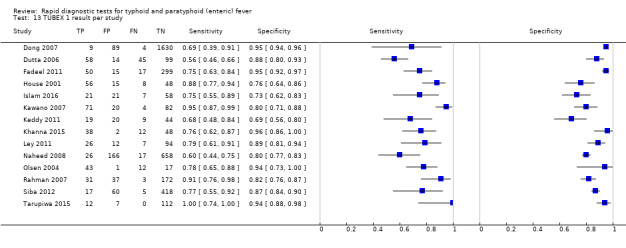
TUBEX 1 result per study.
14. Test.
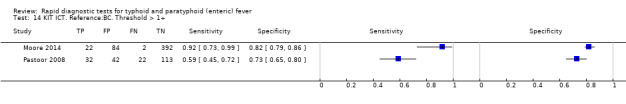
KIT ICT. Reference:BC. Threshold > 1+.
15. Test.
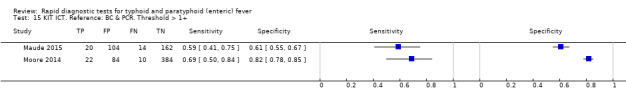
KIT ICT. Reference: BC & PCR. Threshold > 1+.
16. Test.

KIT latex agglutination. Threshold > 1+.
17. Test.
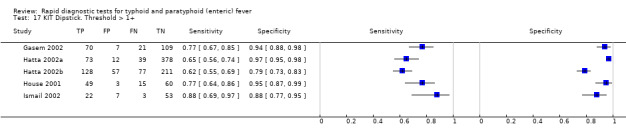
KIT Dipstick. Threshold > 1+.
18. Test.
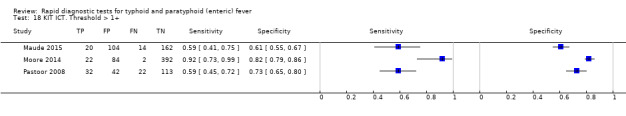
KIT ICT. Threshold > 1+.
19. Test.
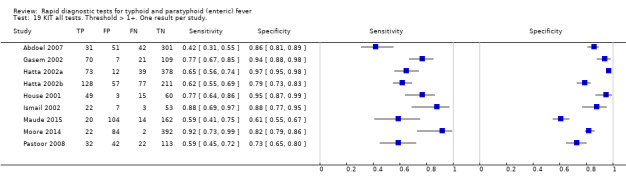
KIT all tests. Threshold > 1+. One result per study..
20. Test.
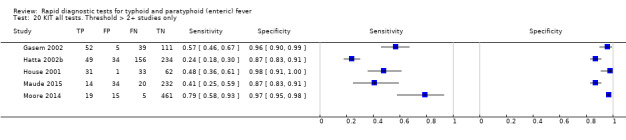
KIT all tests. Threshold > 2+ studies only.
21. Test.
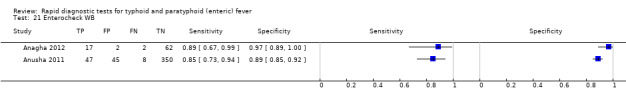
Enterocheck WB.
22. Test.

PanBio.
23. Test.
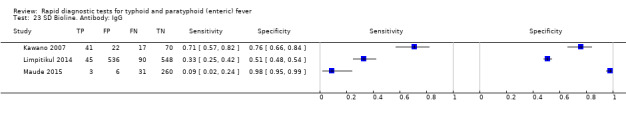
SD Bioline. Antibody: IgG.
24. Test.
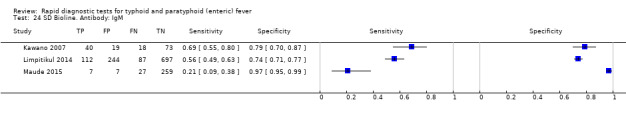
SD Bioline. Antibody: IgM.
25. Test.
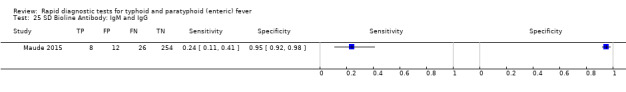
SD Bioline Antibody: IgM and IgG.
26. Test.

Mega Salmonella. Antibody: IgG.
27. Test.

Mega Salmonella. Antibody: IgM.
28. Test.

Multi‐Test Dip‐S‐Tick.
29. Test.

Enteroscreen.
30. Test.
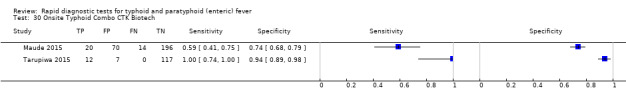
Onsite Typhoid Combo CTK Biotech.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abdoel 2007.
| Study characteristics | |||
| Patient sampling | Prospective multi‐centre study Healthcare setting: primary, secondary, and tertiary healthcare centres Point of recruitment: inpatients and outpatients |
||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: not stated Entry criteria: clinical suspicion of typhoid Sample size: 425 |
||
| Index tests | Name: latex agglutination assay, Royal Tropical Institute (KIT), Netherlands Biological sample: venous blood |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Retrospective analysis. Index tests performed on stored serum samples. Time interval not stated. | ||
| Comparative | |||
| Notes | The study authors report that two raters evaluated the reproducibility of 123 of the index tests. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Anagha 2012.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: secondary Point of recruitment: not specified whether inpatient or outpatient |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: fever > 4 days and clinical suspicion of typhoid Sample size: 83 |
||
| Index tests | Enterocheck WB | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective analysis.Time interval not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Anusha 2011.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary paediatric hospital. Point of recruitment: not specified whether inpatients or outpatients |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: mean age 6.25 years, SD 3.86 years Gender distribution: male 52% female 48% Entry criteria: children between 6 months and 18 years of age, and fever ≥ 3 days, and clinical features of typhoid Sample size: 450 |
||
| Index tests | Enterocheck WB | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective study. | ||
| Comparative | |||
| Notes | Index tests were used on whole blood or serum, but the study authors did not specify the numbers of each. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Begum 2009.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: not specified whether inpatient or outpatient |
||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: clinical suspicion of typhoid fever, and febrile non‐typhoid controls, and healthy controls Data extraction was based on febrile non‐typhoid controls only Sample size: 100 |
||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective study. Timing not stated. | ||
| Comparative | |||
| Notes | Healthy (afebrile) controls also recruited. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Beig 2010.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: paediatric inpatient |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: children (not formally stated) Gender distribution: not stated Entry criteria: 6 months to 12 years, and fever > 4 days, and clinical suspicion of typhoid Sample size: 145 |
||
| Index tests | Typhidot‐M | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective study. Timing not stated. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Bhutta 1999.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: paediatric inpatients |
||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: children (not formally stated) Gender distribution: male 41% female 49% Entry criteria: clinical suspicion of typhoid fever Sample size: 97 |
||
| Index tests | Typhidot and Typhidot‐M | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: Peripheral blood culture and/or bone marrow culture |
||
| Flow and timing | Prospective study. Timing unclear. | ||
| Comparative | |||
| Notes | Malaysian Biodiagnostic Research (Kuala Lumpur, Malaysia) donated rapid diagnostic tests (RDTs) | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Dong 2007.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study as part of a vaccine surveillance programme Healthcare settings: primary, secondary, and tertiary centres (85 in total) Point of recruitment: inpatient and outpatient |
||
| Patient characteristics and setting | Countries: China Level of typhoid endemicity (Crump 2004): medium Age: not specified Gender distribution: not specified Entry criteria: aged between 5 and 60 years with a history of fever ≥ 3 days Sample size: 1874 |
||
| Index tests | Typhidot‐M TUBEX |
||
| Target condition and reference standard(s) | Target condition: both Salmonella Typhi and Salmonella Paratyphi A Reference standard: peripheral blood culture (8 mL) |
||
| Flow and timing | Prospective multicentre study as part of a vaccine surveillance programme. Index tests performed in real time during patient recruitment. | ||
| Comparative | |||
| Notes | Reported diagnostic test accuracy for detecting cases of Salmonella Paratyphi A as well as Salmonella Typhi. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Dutta 2006.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study as part of a community‐based typhoid surveillance study and mass vaccination programme Healthcare setting: primary, secondary, and tertiary (7 health outposts in total) Point of recruitment: inpatient and outpatient |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: fever ≥ 3 days Sample size: 6697 plus 172 healthy controls. Only a subset of participants had TUBEX or Typhidot testing. Control participants for 2x2 were based on febrile participants and did not include healthy controls. |
||
| Index tests | TUBEX Typhidot |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Community‐based typhoid surveillance study and mass vaccination programme. Timing of sample testing unclear. | ||
| Comparative | |||
| Notes | Not all patients received the same index test. If Salmonella Paratyphi was isolated, study authors classified this as a true negative. If a participant was both blood culture‐positive and malaria film‐positive, the study authors excluded them from the analysis (n = 1). Study authors only included a small number or participants in the analysis. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| High | High | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Fadeel 2011.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary (5 fever hospitals) Point of recruitment: inpatients |
||
| Patient characteristics and setting | Countries: Egypt Level of typhoid endemicity (Crump 2004): medium Age: over the age of 4 years Gender distribution: not stated Entry criteria: fever lasting for at least 2 days, or febrile ≥ 38.5°C on admission, with a clinical suspicion of typhoid fever or brucellosis Sample size: 2897 |
||
| Index tests | TUBEX Typhidot‐M |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standards: peripheral blood culture |
||
| Flow and timing | Divided into 3 main groups of 'typhoid' (cases), 'febrile non‐typhoid' (controls), and healthy controls. Timing unclear. | ||
| Comparative | |||
| Notes | Case: control design. Excluded febrile cases of diarrhoea and pneumonia. Study authors classified a Widal Test titre of > 320 as a typhoid case |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Gasem 2002.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary (3) and tertiary (1) Point of recruitment: inpatient |
||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: not stated Gender distribution: not stated Entry criteria: clinical suspicion of typhoid (127) and 80 febrile 'non‐typhoids' Sample size: 207 |
||
| Index tests | Dipstick assay from the Royal Tropical Institute, Netherlands (KIT) | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standards: peripheral blood culture or bone marrow culture, or both |
||
| Flow and timing | Prospective multi‐centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Not all patients had both bone marrow culture and blood culture. Study authors classified Isolation of Salmonella Paratyphi as a non‐typhoid case. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Gopalakrishnan 2002.
| Study characteristics | |||
| Patient sampling | Retrospective single‐centre study Healthcare setting: tertiary Point of recruitment: not specified whether inpatient or outpatient |
||
| Patient characteristics and setting | Countries: Malaysia Level of typhoid endemicity (Crump 2004): medium Age: not specified Gender distribution: not specified Entry criteria: Widal test titres greater than 640 Sample size: 144 |
||
| Index tests | Typhidot PanBio |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standards: peripheral blood culture or stool culture, or both |
||
| Flow and timing | Retrospective analysis of stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Inclusion criteria based on Widal Test titres ‐ limiting. Reference standard included isolation of Salmonella Typhi from stool Index tests were performed retrospectively on stored samples. Typhidot‐M performed on only small subset of samples. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hatta 2002a.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: primary, secondary, and tertiary Point of recruitment: inpatient and outpatient |
||
| Patient characteristics and setting | Countries: Indonesia and Kenya Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: clinical suspicion of typhoid, and other febrile illnesses (controls), and healthy afebrile controls Sample size: 504 |
||
| Index tests | Dipstick Assay, Royal Tropical Institute (KIT), Netherlands | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective recruitment at multiple sites. Timing unclear. | ||
| Comparative | |||
| Notes | Case‐control study design from 2 geographical locations, including controls from a non‐endemic area (Netherlands). | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hatta 2002b.
| Study characteristics | |||
| Patient sampling | Propspective multicentre study Healthcare setting: Primary, secondary, and tertiary Point of recruitment: inpatient and outpatient |
||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: not specified Gender distribution: not specified Entry criteria: clinical suspicion of typhoid Sample size: 473 |
||
| Index tests | Dipstick assay, Royal Tropical Institute (KIT) Netherlands | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard: peripheral blood culture (5 mL) |
||
| Flow and timing | Prospective multi‐centre study. Timing unclear. | ||
| Comparative | |||
| Notes | There is a potential overlap of patients/data between the paper by Hatta 2002a. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Hosamani 2013.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: not stated |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 58% Male 42% Female Entry criteria: history of fever more than 2 to 3 days duration and a clinical diagnosis of enteric fever |
||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | No sources of funding declared. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| High | |||
House 2001.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary Point of recruitment: inpatients |
||
| Patient characteristics and setting | Countries: Vietnam Level of typhoid endemicity (Crump 2004): high Age: adults and children Gender distribution: not specified Entry criteria: Salmonella Typhi on blood culture, and febrile controls, and healthy controls Sample size: 290 |
||
| Index tests | TUBEX Dipstick Assay, Royal Tropical Institute (KIT), Netherlands |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | Mostly children recruited. Sample size 290 but only 127 analysed. Case control design. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Islam 2016.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary international reference centre Point of recruitment: not stated |
||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 52% male 48% female Entry criteria: non‐pregnant, 1 to 59 years of age, fever ≥ 39.0°C for 3 to 7 days duration, lacking obvious alternative diagnosis |
||
| Index tests | TUBEX Typhidot TPTest |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (3 to 5 mL) |
||
| Flow and timing | Prospective study at a tertiary reference centre. Timing unclear. | ||
| Comparative | |||
| Notes | Unable to clarify whether patients in Group VI (visceral leishmaniasis/tuberculosis) also received a blood culture. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ismail 2002.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: tertiary (5 infectious diseases hospitals) Point of recruitment: inpatients |
||
| Patient characteristics and setting | Countries: Egypt Level of typhoid endemicity (Crump 2004): medium Age: not specified Gender distribution: not specified Entry criteria: febrile in‐patients meeting pre‐determined case definitions Sample size: 85 |
||
| Index tests | Dipstick assay, Royal Tropical Institute (KIT), Netherlands | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective multicentre study. Samples tested retrospectively 2 to 3 months after recruitment. | ||
| Comparative | |||
| Notes | Part of a brucellosis diagnostic study. Samples tested retrospectively 2 to 3 months later. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Jesudason 2002.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Tertiary healthcare setting Point of recruitment: unclear whether inpatient, outpatient, or both |
||
| Patient characteristics and setting | Country: India Level of typhoid endemicity (Crump 2004): high Age(s): unclear Gender distribution: unclear Four pre‐determined groups for entry into the study:
Sample size: 150 recruited (60 analysed) |
||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Indian Association Medical Microbiology External Quality Assurance Scheme laboratory accreditation | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Jesudason 2006.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: both inpatients and outpatients |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Ages: unclear Gender distribution: unclear Entry criteria: clinical suspicion of typhoid fever Sample size: 563 |
||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors excluded one case of Salmonella paratyphi A. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Kawano 2007.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary infectious diseases hospital Point of recruitment: inpatients |
||
| Patient characteristics and setting | Countries: Philippines Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: 53.6% (male) 46.4% (female) Entry criteria: febrile patients with a clinical suspicion of typhoid fever Sample size: 177 |
||
| Index tests | TUBEX Typhidot SD Bioline Mega Salmonella |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Keddy 2011.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary hospitals Point of recruitment: inpatient |
||
| Patient characteristics and setting | Countries: South Africa and Tanzania Level of typhoid endemicity (Crump 2004): medium Age: both adults and children Gender distribution: 54.3% (male) 45.7% (female) Entry criteria: South Africa ‐ clinically suspected typhoid fever with no pre‐treatment with antibiotics Tanzania ‐ unselected febrile illnesses, but only those with clinical suspicion of typhoid fever were recruited Sample size: 92 |
||
| Index tests | TUBEX Typhidot |
||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard: peripheral blood culture |
||
| Flow and timing | Prospective multicentre study | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Khan 2002.
| Study characteristics | |||
| Patient sampling | Retrospective single centre study Healthcare setting: tertiary hospital Point of recruitment: both inpatient and outpatient |
||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: unclear Gender distribution: unclear Entry criteria: patients with clinical suspicion of typhoid who went on to have the index RDT Sample size: 1760 (128 analysed) |
||
| Index tests | Typhidot‐M | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard: peripheral blood culture, or bone marrow culture, or both |
||
| Flow and timing | Retrospective analysis on stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Unable to distinguish which cases were bone marrow positive. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | No | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Khanna 2015.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: unclear |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: not stated Entry criteria: cases were febrile patients with a positive blood culture for Salmonella Typhi. Healthy afebrile controls |
||
| Index tests | TUBEX Typhidot |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (5 mL) |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Case control study | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Khoharo 2011.
| Study characteristics | |||
| Patient sampling | Prospective single‐centre study Healthcare setting: tertiary Point of recruitment: not stated |
||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: adults (> 18 years) Gender distribution: not stated Entry criteria: aged 18 to 40 years; fever < 14 days; clinical features suggesting typhoid fever; no history of antimicrobial therapy or typhoid immunization in the recent past |
||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | No declaration of funding. Entry criteria could exclude numerous cases of typhoid. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ley 2011.
| Study characteristics | |||
| Patient sampling | Retrospective multi‐centre study Healthcare settings: secondary Point of recruitment: both inpatient and outpatient |
||
| Patient characteristics and setting | Countries: Tanzania Level of typhoid endemicity (Crump 2004): medium Age: children between the ages of 2 months and 14 years Gender distribution: unclear Entry criteria: selected samples from a fever surveillance study Surveillance study entry criteria: fever > 3 days or those matching set clinical severity criteria |
||
| Index tests | TUBEX | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture |
||
| Flow and timing | Retrospective analysis on stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Only blood culture positive patients included. Samples from 2 different patient populations | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Limpitikul 2014.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study (3 hospitals within a single province) Healthcare setting: secondary Point of recruitment: both inpatients and outpatients |
||
| Patient characteristics and setting | Countries: Thailand Level of typhoid endemicity (Crump 2004): high Age: children under 15 years of age Gender distribution: not recorded Entry criteria: any febrile illness in children under 15 years of age |
||
| Index tests | SD Bioline | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) |
||
| Flow and timing | Prospective recruitment with a retrospective analysis of stored samples. | ||
| Comparative | |||
| Notes | Outbreak situation in Songkhla Province. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Maude 2015.
| Study characteristics | |||
| Patient sampling | Prospective single‐centre study Healthcare setting: tertiary Point of recruitment: inpatient |
||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 173 males; 127 females Entry criteria: > 6 months of age with < 2 weeks fever and a documented fever > 38 |
||
| Index tests | Test‐It‐Typhoid (KIT immunochromatographic lateral flow assay) SD Bioline CTK Biotech Onsite |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (1 to 12 mL in children, 5 to 12mL in adults) or blood nucleic acid amplification (polymerase chain reaction (PCR)), or both |
||
| Flow and timing | Prospective recruitment with retrospective testing of stored samples. | ||
| Comparative | |||
| Notes | Two review authors (LW and CMP) are authors on this study. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Mehmood 2015.
| Study characteristics | |||
| Patient sampling | Retrospective single centre analysis study Healthcare setting: tertiary Point of recruitment: not stated |
||
| Patient characteristics and setting | Countries: Pakistan Level of typhoid endemicity (Crump 2004): high Age: mixed Gender distribution: 59 males/86 females Entry criteria: unselected fever of greater than 3 days |
||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not specified) |
||
| Flow and timing | Retrospective analysis of stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Moore 2014.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: inpatient |
||
| Patient characteristics and setting | Countries: Cambodia Level of typhoid endemicity (Crump 2004): high Age: children over 6 months and under 16 years Gender distribution: unclear Entry criteria: documented fever of > 38°C Sample size: 500 |
||
| Index tests | Immunochromatographic lateral flow assay, KIT (Test‐It‐Typhoid prototype) | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture |
||
| Flow and timing | Prospective single centre study. Retrospective testing of stored samples. | ||
| Comparative | |||
| Notes | Score of 2+ or more considered positive. We contacted the study authors for further details based on the abstract. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Naheed 2008.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: primary community clinics Point of recruitment: outpatients |
||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: 51% (male) 49% (female) Entry criteria: fever for any duration in < 5 years / > 3 days in > 5years and a documented fever of 38.0°C Sample size: 867 |
||
| Index tests | TUBEX Typhidot |
||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture |
||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors classified 139 results that were indeterminate as negative. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Olsen 2004.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary Point of recruitment: inpatients |
||
| Patient characteristics and setting | Countries: Vietnam Level of typhoid endemicity (Crump 2004): high Age: both adults and children Gender distribution: 56.9% (male) 43.1% (female) Entry criteria: > 4 days of fever, and greater than 3 years old and controls with other febrile illnesses Sample size: 79 (59 patients and 20 controls) |
||
| Index tests | TUBEX Typhidot Multi‐Test Dip‐S‐Tick |
||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture |
||
| Flow and timing | Prospective multicentre study. Samples processed at a different site. Timing unclear. | ||
| Comparative | |||
| Notes | Different processing sites for blood culture, that is not in the same laboratory | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Pastoor 2008.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: inpatient |
||
| Patient characteristics and setting | Countries: Indonesia Level of typhoid endemicity (Crump 2004): high Age: unclear Gender distribution: unclear Entry criteria: clinical suspicion of typhoid fever Sample size: 209 |
||
| Index tests | Immunochromatographic lateral flow assay, Royal Tropical Institute (KIT), Netherlands | ||
| Target condition and reference standard(s) | Target condition:Salmonella Typhi Reference standard(s): peripheral blood culture and Widal Test |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors compared diagnostic test results of the ICT with both blood culture and the Widal Test. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Prasad 2015.
| Study characteristics | |||
| Patient sampling | Single centre retrospective analysis study Healthcare setting: tertiary Point of recruitment: both inpatients and outpatients |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: unclear Gender distribution: unclear Entry criteria: clinical suspicion of enteric fever |
||
| Index tests | Typhidot‐M Enteroscreen‐IgM |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not stated) |
||
| Flow and timing | Retrospective analysis of stored samples. Timing unclear. | ||
| Comparative | |||
| Notes | Study authors classified Salmonella Paratyphi blood culture positive cases as disease‐negative. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | No | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Rahman 2007.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: outpatients |
||
| Patient characteristics and setting | Countries: Bangladesh Level of typhoid endemicity (Crump 2004): high Age: children Gender distribution: unclear Entry criteria: fever > 3 days but < 7 days Sample size: 243 |
||
| Index tests | TUBEX | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Sanjeev 2013.
| Study characteristics | |||
| Patient sampling | Prospective single centre study Healthcare setting: tertiary Point of recruitment: not stated |
||
| Patient characteristics and setting | Countries: India Level of typhoid endemicity (Crump 2004): high Age: not clear Gender distribution: not stated Entry criteria: clinical suspicion of typhoid fever |
||
| Index tests | Typhidot | ||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (volume not specified) |
||
| Flow and timing | Prospective single centre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Siba 2012.
| Study characteristics | |||
| Patient sampling | Prospective multicentre study Healthcare setting: secondary and tertiary hospitals Point of recruitment: outpatients |
||
| Patient characteristics and setting | Country: Papua New Guinea Level of typhoid endemicity (Crump 2004): high Age: adults and children Gender distribution: 51% (male) 49% (female) Entry criteria: febrile patients with axillary temp > 37.5°C and > 2 days of fever (or clinical suspicion of typhoid fever) Sample size: 530 (500 analysed) |
||
| Index tests | TUBEX Typhidot TyphiRapid‐Tr02 |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture and PCR |
||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | |||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Tarupiwa 2015.
| Study characteristics | |||
| Patient sampling | Prospective multi‐centre study Healthcare setting: primary Point of recruitment: outpatient |
||
| Patient characteristics and setting | Countries: Zimbabwe Level of typhoid endemicity (Crump 2004): medium Age: mixed Gender distribution: not stated Entry criteria: 'typical signs and symptoms of typhoid' |
||
| Index tests | TUBEX On‐Site Typhoid IgG/IgM Combo |
||
| Target condition and reference standard(s) | Target condition: Salmonella Typhi Reference standard(s): peripheral blood culture (3 to 5 mL) |
||
| Flow and timing | Prospective multicentre study. Timing unclear. | ||
| Comparative | |||
| Notes | Diagnostic test accuracy data not provided in published paper but supplied separately by the corresponding authors. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | No | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Low | |||
Abbreviations: PCR: polymerase chain reaction; RDT: rapid diagnostic test.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alejandria 2012 | Meta‐analysis from an International Congress on Infectious Diseases (ICID) poster abstract |
| Bakr 2011 | 4 different types of Widal Test used, that is, not a new rapid diagnostic test (RDT) |
| Banchuin 1987 | Antigen detection was neither a commercially‐available rapid diagnostic test or a prototype. |
| Banerjee 1984 | We were unable to extract specificity and sensitivity data |
| Boomsma 1988 | We were unable to extract sensitivity and specificity data |
| Cardona‐Castro 2000 | Not a commercially available test ('Dot Blot' Test from Bio‐Rad Laboratories, Richmond, CA) |
| Castonguay‐Vanier 2013 | We could only extract data for patients with Gram‐negative rod positive blood cultures. The study authors did not present data on RDT performance on culture negative patients, therefore we could not perform analyses. |
| Chaicumpa 1992 | Not a commercially available test (an unspecified Indirect dot blot ELISA) |
| Chart 2007 | Not a commercially available RDT. A range of Salmonella serodiagnostic tests were performed at a UK reference laboratory on sera from UK residents returning from travelling abroad. |
| Chatterjee 1988 | Not a commercially available test. "COAG" co‐agglutination test produced in‐house by Indian tertiary hospital laboratory. |
| Choo 1994 | We were unable to extract data about performance of test in blood culture positive patients. DOT EIA (early Typhidot‐M). |
| Choo 1997 | We were unable to extract relevant sensitivity and specificity data. DOT‐EIA (early Typhidot‐M). |
| Chua 2012 | Evaluates a test for detecting chronic carriage rather than acute typhoid (enteric) fever |
| Coovadia 1986 | Not a commercially available test (passive haemagglutination). |
| Das 2013 | Not a commercially available test ‐ candidate created by SPAN Diagnostics (India) |
| Dhanalakshmi 1986 | We were unable to determine which blood culture positive patients were also positive on the urinary COAG tests. |
| el‐Falaky 1970 | We were unable to extract sensitivity and specificity data as no cut‐offs mentioned for haemagglutination. |
| Fadeel 2004 | Not a commercial test: ELISA antibody detection from urine |
| Felezsko 2004 | Letter outlining use of TUBEX to detect non‐typoidal Salmonella infections (e.g. S. enteritidis) |
| Gorelov 1988 | Comparison of two types of Widal Test |
| Handojo 2004 | Evaluation of a Widal slide agglutination test, a variant of an existing diagnostic test. |
| Hoffman 1986 | Evaluation of a slide agglutination Widal Test |
| House 2005 | Paired serum samples rather than a single use RDT |
| Jackson 1995 | Dot Enzyme Immmunoassay (EIA) ‐ early Typhidot‐M. We were unable to extract sensitivity or specificity data. |
| John 1984 | Not a commercial test: passive bacterial agglutination |
| Kalhan 1998 | Not a commercial test: reverse passive haemagglutination assay (possible RDT candidate) |
| Kalhan 1999 | Not a commercial test: Latex Agglutination Test |
| Kariuki 2004 | No actual RDT evaluated. Study compared blood culture with the Widal Test. |
| Kaur 1988a | Not commercially‐available rapid diagnostic tests. In‐house latex agglutination (LAT) and coagglutination (COAG) tests which are not prototypes. |
| Kaur 1988b | The serodiagnostic tests evaluated were not commercially available point‐of‐care tests. |
| Khanam 2013 | The TPTest is not a commercially‐available RDT |
| Khanam 2015 | The study detailed the assessment of the human immune response rather than diagnostic test accuracy |
| Kollaritsch 1988 | Letter to the editor about a single case ‐ not a diagnostic study |
| Korbsrisate 1998 | Not a commercial test: Indirect ELISA IgM antibody detection |
| Kuchuloria 2016 | No commercial RDTs were used in the febrile illness study, only laboratory serology for Salmonella Typhi |
| Lim 1998 | Reference standard inadequately described, and not all patients received any form of reference standard. TUBEX. |
| Lutterloh 2012 | Use of TUBEX to determine cases as part of active surveillance during an outbreak. We were unable to extract any data regarding diagnostic test accuracy. |
| Malik 2001 | No data of index test (Typhidot) positivity in non‐culture positive patients. |
| Mukherjee 1993 | Not a commercial test. In‐house co‐agglutination test |
| Munir 2015 | This study only included clinical typhoid or conifrmed typhoid cases. Study authors excluded patients currently receiving or who had recently received antimicrobials. We were unable to extract data related to diagnostic test accuracy. |
| Narayanappa 2010 | We were unable to extract data index test data (Typhidot‐M) from control (non‐typhoid fever) group |
| Neil 2012 | Variety of serological diagnostic tests used during investigation of an acute outbreak in Uganda. No specific RDT used. |
| Nguyen 1997 | The monoclonal antibody‐based dot‐blot ELISA evaluated is not a commercially‐available rapid diagnostic test. |
| Ong 1989 | Test based on adherence IgM "capture" ‐ not commercially available. Confirmed typhoid case was blood or stool culture positive, or both. |
| Pandya 1995 | Not a commercially available RDT: latex agglutination to a) Typhi Vi; and b) Barber protein |
| Petchclai 1987 | Not a commercial test: passive haemagglutination test (PHA) We were unable to extract sensitivity and specificity data |
| Peterson 2010 | Evaluation of general bacterial microarray/genetics rather than point‐of‐care testing |
| Preechakasedkit 2012 | RDT development rather than evaluation of test accuracy |
| Rai 1989 | Non‐commercial tests. We were unable to extract sensitivity and specificity data. |
| Shrivastava 2011 | Repeat publication of data published by Olsen 2004 from Vietnam. |
| Surachmanto 2011 | TUBEX in asthmatics. We were unable to extract diagnostic test data. |
| Tantivanich 1984 | Not a commercial test: latex agglutination. |
| Thevanesam 1992 | Widal Test evaluation, not a commercial RDT |
| Watt 2005 | We were unable to extract sensitivity and specificity data |
| West 1989 | Not a commercial test: urinary co‐agglutination technique |
| Wijedoru 2012 | Data from this study had already been included in Moore 2014 |
| Yan 2011 | We were unable to extract specificity data |
| Zaka‐ur‐Rab 2012 | Not a commercial test: Salivary IgA to lipopolysaccharide (LPS) |
Abbreviations: RDT: rapid diagnostic test.
Differences between protocol and review
We amended the reference test definition when it became apparent that some studies had used a PCR test to detect Salmonella Typhi or Salmonella Paratyphi A DNA in blood samples. We included peripheral blood PCR in addition to peripheral blood culture as a Grade 2 reference standard. In the studies that used a blood PCR in addition to blood culture, a positive blood culture or blood PCR represented a positive reference test.
During the interval between protocol and full review publication, a modified tool assessment of methodological quality was ratified and released (QUADAS‐2). We used this newer tool for the full review instead of QUADAS‐1 as originally intended in the protocol (Appendix 3).
The major differences between the protocol and the review relate to the intended statistical analysis. Some of the studies of the Test‐it Typhoid test and its KIT prototypes used two test thresholds. We were able to use bivariate analysis to focus on test operating points instead of hierarchical summary receiver operating characteristic (HSROC) analysis. Typhidot and TUBEX tests results did not use different test thresholds. A number of the planned statistical analyses of subgroups were underpowered due to the low number of available studies. The main subgroup analysis performed was by test manufacturer (Typhidot/Typhidot‐M, TUBEX and Test‐it Typhoid and KIT prototype RDTs) as there were sufficient available studies to potentially allow robust comparisons. We did not perform the following planned subanalyses: Salmonella enterica serovars (Typhi, Paratyphi A, or both); reference standard test applied (bone marrow and blood culture [Grade 1] versus blood culture alone [Grade 2]); study design (case control, prospective cohort, randomized controlled trial, paired comparative trial); test population (clinically‐suspected enteric fever versus unselected febrile patients); and index test biological sample type (blood versus urine). Where possible we have replaced these subanalyses with graphical presentation of subgroups in SROC plots.
For the Typhidot test and its variants we decided to extract the IgM data alone from each study. Typhidot detects both IgG and IgM antibodies, while Typhidot‐M detects IgM antibodies only. A detectable IgG result may indicate current or recent acute but also previous infection whereas IgM indicates current or recent acute infection. In order to compare the data of Typhidot with the data of Typhidot‐M, if the IgM data was not recorded separately from the IgG data, we excluded the results.
Contributions of authors
LW and CMP conceived the review. LW wrote the protocol and SD and CMP edited the protocol (Wijedoru 2010). LW and CMP assessed abstracts, selected studies for inclusion, extracted data, and assessed methodological quality. Susan Mallett (SM) led the statistical analysis and interpretation of statistical results. LW and CMP led clinical interpretation of results. LW wrote the report with editing by CMP and SM. All review authors have seen and approved the final version of this Cochrane Review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development (DFID), UK.
Grant: 5242
Declarations of interest
LW and CMP are authors of Moore 2014 and Maude 2015. SM has no known conflicts of interest.
Unchanged
References
References to studies included in this review
Abdoel 2007 {published data only}
- Abdoel TH, Pastoor R, Smits HL, Hatta M. Laboratory evaluation of a simple and rapid latex agglutination assay for the serodiagnosis of typhoid fever. Transactions of the Royal Society of Tropical Medicine and Hygiene 2007;101(10):1032‐8. [DOI] [PubMed] [Google Scholar]
Anagha 2012 {published data only}
- Anagha K, Deepika B, Shahriar R, Sanjeev K. The easy and early diagnosis of typhoid fever. Journal of Clinical and Diagnostic Research 2012;6(2):198‐9. [Google Scholar]
Anusha 2011 {published data only}
- Anusha R, Ganesh R, Lalitha J. Comparison of a rapid commercial test, Enterocheck WB®, with automated blood culture for diagnosis of typhoid fever. Annals of Tropical Paediatrics 2011;31(3):231‐4. [DOI] [PubMed] [Google Scholar]
Begum 2009 {published data only}
- Begum Z, Hossain MA, Musa AK, Shamsuzzaman AK, Mahmud MC, Ahsan MM, et al. Comparison between DOT EIA IgM and Widal Test as early diagnosis of typhoid fever. Mymensingh Medical Journal 2009;18(1):13‐7. [PubMed] [Google Scholar]
Beig 2010 {published data only}
- Beig FK, Ahmad F, Ekram M, Shukla I. Typhidot M and Diazo test vis‐à‐vis blood culture and Widal Test in the early diagnosis of typhoid fever in children in a resource poor setting. Brazilian Journal of Infectious Diseases 2010;14(6):589‐93. [DOI] [PubMed] [Google Scholar]
Bhutta 1999 {published data only}
- Bhutta ZA, Mansurali N. Rapid serologic diagnosis of pediatric typhoid fever in an endemic area: a prospective comparative evaluation of two dot‐enzyme immunoassays and the Widal Test. American Journal of Tropical Medicine and Hygiene 1999;61(4):654‐7. [DOI] [PubMed] [Google Scholar]
Dong 2007 {published data only}
- Dong B, Galindo CM, Shin E, Acosta CJ, Page AL, Wang M, et al. Optimizing typhoid fever case definitions by combining serological tests in a large population study in Hechi City, China. Epidemiology and Infection 2007;135(6):1014‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dutta 2006 {published data only}
- Dutta S, Sur D, Manna B, Sen B, Deb AK, Deen JL, et al. Evaluation of new‐generation serologic tests for the diagnosis of typhoid fever: data from a community‐based surveillance in Calcutta, India. Diagnostic Microbiology and Infectious Disease 2006;56(4):359‐65. [DOI] [PubMed] [Google Scholar]
Fadeel 2011 {published data only}
- Fadeel MA, House BL, Wasfy MM, Klena JD, Habashy EE, Said MM, et al. Evaluation of a newly developed ELISA against Widal, TUBEX‐TF and Typhidot for typhoid fever surveillance. Journal of Infection in Developing Countries 2011;5(3):169‐75. [DOI] [PubMed] [Google Scholar]
Gasem 2002 {published data only}
- Gasem MH, Smits HL, Goris MGA, Dolmans WMV. Evaluation of a simple and rapid dipstick assay for the diagnosis of typhoid fever in Indonesia. Journal of Medical Microbiology 2002;51(2):173‐7. [DOI] [PubMed] [Google Scholar]
Gopalakrishnan 2002 {published data only}
- Gopalakrishnan V, Sekhar WY, Soo EH, Vinsent RA, Devi S. Typhoid fever in Kuala Lumpur and a comparative evaluation of two commercial diagnostic kits for the detection of antibodies to Salmonella Typhi. Singapore Medical Journal 2002;43(7):354‐8. [PubMed] [Google Scholar]
Hatta 2002a {published data only}
- Hatta M, Goris MGA, Heerkens E, Gooskens J, Smits HL. Simple dipstick assay for the detection of Salmonella Typhi‐specific IgM antibodies and the evolution of the immune response in patients with typhoid fever. American Journal of Tropical Medicine and Hygiene 2002;66(4):416‐21. [DOI] [PubMed] [Google Scholar]
Hatta 2002b {published data only}
- Hatta M, Mubin H, Abdoel T, Smits HL. Antibody response in typhoid fever in endemic Indonesia and the relevance of serology and culture to diagnosis. South East Asian Journal of Tropical Medicine and Public Health 2002;33(4):742‐51. [PubMed] [Google Scholar]
Hosamani 2013 {published data only}
- Hosamani MA, Patil AB, Nadagir SD, Madhusudhan NS, Sambrani P. Diagnosis of enteric fever by Widal and two dot‐enzyme immunoassays: utility and difficulties. Journal of Pure and Applied Microbiology 2013;7(3):2378‐83. [Google Scholar]
House 2001 {published data only}
- House D, Wain J, Ho VA, Diep TS, Chinh NT, Bay PV, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. Journal of Clinical Microbiology 2001;39(3):1002‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Islam 2016 {published data only}
- Islam K, Sayeed MA, Hossen E, Khanam F, Charles RC, Andrews J, et al. Comparison of the performance of the TPTest, Tubex, Typhidot and Widal immunodiagnostic assays and blood cultures in detecting patients with typhoid fever in Bangladesh, including using a Bayesian latent class modeling approach. PLoS Neglected Tropical Diseases 2016;10(4):e0004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 2002 {published data only}
- Ismail TF, Smits H, Wasfy MO, Malone JL, Fadeel MA, Mahoney F. Evaluation of dipstick serologic tests for diagnosis of brucellosis and typhoid fever in Egypt. Journal of Clinical Microbiology 2002;40(9):3509‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jesudason 2002 {published data only}
- Jesudason M, Esther E, Mathai E. Typhidot test to detect IgG and IgM antibodies in typhoid fever. Indian Journal of Medical Research 2002;116:70‐2. [PubMed] [Google Scholar]
Jesudason 2006 {published data only}
- Jesudason MV, Sivakumar S. Prospective evaluation of a rapid diagnostic test Typhidot® for typhoid fever. Indian Journal of Medical Research 2006;123(4):513‐6. [PubMed] [Google Scholar]
Kawano 2007 {published data only}
- Kawano L, Leano SA, Agdamag DMA. Comparison of serological test kits for diagnosis of typhoid fever in the Philippines. Journal of Clinical Microbiology 2007;45(1):246‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keddy 2011 {published data only}
- Keddy KH, Sooka A, Letsoalo ME, Hoyland G, Chaignat CL, Morrissey AB, et al. Sensitivity and specificity of two typhoid fever rapid antibody tests for laboratory diagnosis at two sub‐Saharan African sites. Bulletin of the World Health Organization 2011;89(9):640‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2002 {published data only}
- Khan E, Azam F, Ahmed S, Hassan R. Diagnosis of typhoid fever by Dot Enzyme Immunoassay in an endemic region. Journal of the Pakistan Medical Association 2002;52(9):415‐7. [PubMed] [Google Scholar]
Khanna 2015 {published data only}
- Khanna A, Khanna M, Gill KS. Comparative evaluation of Tubex TF (inhibition magnetic binding immunoassay) for typhoid fever in endemic area. Journal of Clinical and Diagnostic Research 2015;9(11):DC14‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khoharo 2011 {published data only}
- Khoharo HK. A comparative study of the typhidot (Dot‐EIA) and Widal tests in blood culture positive cases of typhoid fever. Tropical Doctor 2011;41(3):136‐8. [DOI] [PubMed] [Google Scholar]
Ley 2011 {published data only}
- Ley B, Thriemer K, Ame SM, Mtove GM, Seidlein L, Amos B, et al. Assessment and comparative analysis of a rapid diagnostic test (TUBEX®) for the diagnosis of typhoid fever among hospitalized children in rural Tanzania. BMC Infectious Diseases 2011;11:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Limpitikul 2014 {published data only}
- Limpitikul W, Henpraserttae N, Saksawad R, Laoprasopwattana K. Typhoid outbreak in Songkhla, Thailand 2009–2011: clinical outcomes, susceptibility patterns, and reliability of serology tests. PLoS ONE 2014;9(11):e111768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maude 2015 {published data only}
- Maude RR, Jong HK, Wijedoru L, Fukushima M, Ghose A, Samad R, et al. The diagnostic accuracy of three rapid diagnostic tests for typhoid fever at Chittagong Medical College Hospital, Chittagong, Bangladesh. Tropical Medicine and International Health 2015;20(10):1376‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehmood 2015 {published data only}
- Mehmood K, Sundus A, Naqvi IH, Ibrahim MF, Siddique O, Ibrahim NF. Typhidot: a blessing or a menace. Pakistan Journal of Medical Sciences 2015;31(2):439‐43. [DOI: 10.12669/pjms.312.5934] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014 {published data only}
- Moore CE, Pan‐Ngum W, Wijedoru LPM, Sona S, Nga TVT, Duy PT, et al. Evaluation of the diagnostic accuracy of a typhoid IgM flow assay for the diagnosis of typhoid fever in Cambodian children using a Bayesian latent class model assuming an imperfect gold standard. American Journal of Tropical Medicine and Hygiene 2014;90(1):114‐20. [DOI: 10.4269/ajtmh.13-0384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naheed 2008 {published data only}
- Naheed A, Ram PK, Brooks WA, Mintz ED, Hossain MA, Parsons MM, et al. Clinical value of TUBEX™ and Typhidot® rapid diagnostic tests for typhoid fever in an urban community clinic in Bangladesh. Diagnostic Microbiology and Infectious Disease 2008;61(4):381‐6. [DOI] [PubMed] [Google Scholar]
Olsen 2004 {published data only}
- Olsen SJ, Pruckler J, Bibb N, Nguyen TM, Tran MT, Nguyen TM, et al. Evaluation of rapid diagnostic tests for typhoid fever. Journal of Clinical Microbiology 2004;42(5):1885‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pastoor 2008 {published data only}
- Pastoor R, Hatta M, Abdoel TH, Smits HL. Simple, rapid, and affordable point‐of‐care test for the serodiagnosis of typhoid fever. Diagnostic Microbiology and Infectious Disease 2008;61(2):129‐34. [DOI] [PubMed] [Google Scholar]
Prasad 2015 {published data only}
- Prasad KJ, Oberoi JK, Goel N, Wattal C. Comparative evaluation of two rapid Salmonella‑IgM tests and blood culture in the diagnosis of enteric fever. Indian Journal of Medical Microbiology 2015;33(2):237‐42. [DOI] [PubMed] [Google Scholar]
Rahman 2007 {published data only}
- Rahman M, Siddique AK, Tam FCH, Sharmin S, Rashid H, Iqbal A, et al. Rapid detection of early typhoid fever in endemic community children by the TUBEX® O9‐antibody test. Diagnostic Microbiology and Infectious DIsease 2007;58(3):275‐81. [DOI] [PubMed] [Google Scholar]
Sanjeev 2013 {published data only}
- Sanjeev H, Nayak S, Pai Asha KB, Rai R, Karnaker V, Ganesh HR. A systematic evaluation of a rapid DOT‐EIA, blood culture, and Widal Test in the diagnosis of typhoid fever. Nitte University Journal of Health Science 2013;3(1):21‐4. [Google Scholar]
Siba 2012 {published data only}
- Siba V, Horwood PF, Vanuga K, Wapling J, Sehuko R, Siba PM, et al. Evaluation of serological diagnostic tests for typhoid fever in Papua New Guinea using a composite reference standard. Clinical and Vaccine Immunology 2012;19(11):1833‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tarupiwa 2015 {published data only}
- Tarupiwa A, Tapera S, Mtapuri‐Zinyowera S, Gumbo P, Ruhanya V, Gudza‐Mugabe M, et al. Evaluation of TUBEX‐TF and OnSite Typhoid IgG/IgM Combo rapid tests to detect Salmonella enterica serovar Typhi infection during a typhoid outbreak in Harare, Zimbabwe. BioMed Research Notes 2015;8:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Alejandria 2012 {published data only}
- Alejandria M, Concepcion AO, Li RJ, Gutierrez J. The sensitivity and specificity of serologis tests in the diagnosis of typhoid fever in adults: a meta‐analysis. International Journal of Infectious Diseases 2012;16(Suppl 1):e393. [DOI: ] [Google Scholar]
Bakr 2011 {published data only}
- Bakr WMK, Aktar LA, Ashour MS, Toukhy AM. The dilemma of Widal Test ‐ which brand to use? A study of four different Widal brands: a cross sectional comparative study. Annals of Clinical Microbiology and Antimicrobials 2011;10:7. [10.1186/1476‐0711‐10‐7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Banchuin 1987 {published data only}
- Banchuin N, Appassakij H, Sarasombath S, Manatsathit S, Rungpitarangsi B, Komolpit P, et al. Detection of Salmonella typhi protein antigen in serum and urine: a value for diagnosis of typhoid fever in an endemic area. Asian Pacific Journal of Allergy and Immunology 1987;5(2):155‐9. [PubMed] [Google Scholar]
Banerjee 1984 {published data only}
- Banerjee V, Mukherjee A. Comparative evaluation of microtitre and tube agglutination technique for serodiagnosis of enteric fever. Journal of Communicable Diseases 1984;16(1):82‐3. [PubMed] [Google Scholar]
Boomsma 1988 {published data only}
- Boomsma LJ. Clinical aspects of typhoid fever in two rural Nigerian hospitals: a prospective study. Tropical and Geographical Medicine 1988;40(2):97‐102. [PubMed] [Google Scholar]
Cardona‐Castro 2000 {published data only}
- Cardona‐Castro N, Gotuzzo E, Rodriguez M, Guerra H. Clinical application of a dot blot test for diagnosis of enteric fever due to Salmonella entrica serovar Typhi is patients with typhoid fever from Colombia and Peru. Clinical and Diagnostic Laboratory Immunology 2000;7(2):312‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castonguay‐Vanier 2013 {published data only}
- Castonguay‐Vanier J, Davong V, Bouthasavong L, Sengdetka D, Simmalavong M, Seupsavith A, et al. Evaluation of a simple blood culture amplification and antigen detection method for diagnosis of Salmonella enterica serovar Typhi bacteraemia. Journal of Clinical Microbiology 2013;51(1):142‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaicumpa 1992 {published data only}
- Chaicumpa W, Ruangkunaporn Y, Burr D, Chongsa‐Nguan M, Echeverria P. Diagnosis of typhoid fever by detection of Salmonella Typhi antigen in urine. Journal of Clinical Microbiology 1992;30(9):2513‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chart 2007 {published data only}
- Chart H, Cheasty T, Pinna E, Siorvanes L, Wain J, Alam D, et al. Serodiagnosis of Salmonella enterica serovar Typhi and S. enterica serovars Paratyphi A, B and C human infections. Journal of Medical Microbiology 2007;56(Pt 9):1161‐6. [DOI: 10.1099/jmm.0.47197-0] [DOI] [PubMed] [Google Scholar]
Chatterjee 1988 {published data only}
- Chatterjee PP, Mohan M, Talwar V, Rawat S. Evaluation of co‐agglutination test for diagnosis of typhoid fever in children. Indian Journal of Medical Research 1988;87:157‐60. [PubMed] [Google Scholar]
Choo 1994 {published data only}
- Choo KE, Oppenheimer SJ, Ismail AB, Ong KH. Rapid serodiagnosis of typhoid fever by dot enzyme immunoassay in an endemic area. Clinical Infectious Diseases 1994;19(1):172‐6. [DOI] [PubMed] [Google Scholar]
Choo 1997 {published data only}
- Choo KE, Davis TME, Ismail A, Ong KH. Longevity of antibody responses to a Salmonella Typhi‐specific outer membrane protein: interpretation of a dot enzyme immunosorbent assay in an area of high typhoid fever endemicity. American Journal of Tropical Medicine and Hygiene 1997;57(6):656‐9. [DOI] [PubMed] [Google Scholar]
Chua 2012 {published data only}
- Chua AL, Aziah I, Balaram P, Bhuvanendran S, Anthony AAA, Mohmad SN, et al. Identification of carriers among individuals recruited in the Typhoid Registry in Malaysia using stool culture, polymerase chain reaction, and dot enzyme immunoassay as detection tools. Asia Pacific Journal of Public Health 2012;27(2):NP2740‐8. [DOI: 10.1177/1010539512458521; PUBMED: 23000800] [DOI] [PubMed] [Google Scholar]
Coovadia 1986 {published data only}
- Coovadia YM, Singh V, Bhana RH, Moodley N. Comparison of passive haemagglutination test with Widal agglutination test for serological diagnosis of typhoid fever in an endemic area. Journal of Clinical Pathology 1986;39(6):680‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Das 2013 {published data only}
- Das S, Rajendran K, Dutta P, Taha SK, Dutta S. Validation of a new serology‐based dipstick test for rapid diagnosis of typhoid fever. Diagnostic Microbiology and Infectious Disease 2013;76(1):5‐9. [DOI: 10.1016/j.diagmicrobio.2013.01.012] [DOI] [PubMed] [Google Scholar]
Dhanalakshmi 1986 {published data only}
- Dhanalakshmi D, Mallika M, Kumaravel K, Bhavani M, Lakshminarayana CS. Detection of Salmonella Typhi antigens by slide coagglutination in urine from patients with typhoid fever. Indian Journal of Pathology and Microbiology 1984;27(1):33‐5. [PubMed] [Google Scholar]
el‐Falaky 1970 {published data only}
- el‐Falaky IH, Hassan A, Wahab MF, el‐Kholy S. Diagnosis of Typhoid Fever by Haemagglutination. Journal of the Egyptian Public Health Association 1970;45(1):109‐18. [PubMed] [Google Scholar]
Fadeel 2004 {published data only}
- Fadeel MA, Crump JA, Mahoney FJ, Nakhla IA, Mansour AM, Reyad B, et al. Rapid diagnosis of typhoid fever by enzyme‐linked immunosorbent assay detection of Salmonella serovar Typhi antigens in urine. American Journal of Tropical Medicine and Hygiene 2004;70(3):323‐8. [PubMed] [Google Scholar]
Felezsko 2004 {published data only}
- Feleszko W, Maksymiuk J, Oracz G, Golicka D, Szajewska H. The TUBEX™ typhoid test detects current Salmonella infections. Journal of Immunological Methods 2004;285(1):137‐8. [DOI: 10.1016/j.jim.2003.10.020] [DOI] [PubMed] [Google Scholar]
Gorelov 1988 {published data only}
- Gorelov AL, Levina GA, Kulyakina MN, Pavlova IP, Shakkanina KL, Prozorovsky SSV. Development and employment of an enzyme immuno‐assay test system for the detection of Salmonella typhi antigens in the blood typhoid fever patient. Laboratornoe Delo 1988;1:79‐83. [PubMed] [Google Scholar]
Handojo 2004 {published data only}
- Handojo I, Edijanto SP, Probohoesodo MY, Mahartini NN. Comparison of the diagnostic value of local Widal slide test with imported Widal slide test. South East Asian Journal of Tropical Medicine and Public Health 2004;35(2):366‐70. [PubMed] [Google Scholar]
Hoffman 1986 {published data only}
- Hoffman SL, Flanigan TP, Klaucke D, Leksana B, Rockhill RC, Punjabi NH, et al. The Widal slide agglutination test: a valuable rapid diagnostic test in typhoid fever patients at the Infectious Disease Hospital of Jakarta. American Journal of Epidemiology 1986;123(5):869‐75. [DOI] [PubMed] [Google Scholar]
House 2005 {published data only}
- House D, Chinh NT, Diep TS, Parry CM, Wain J, Dougan G, et al. Use of paired serum samples for serodiagnosis of typhoid fever. Journal of Clinical Microbiology 2005;43(9):4889‐90. [DOI: 10.1128/JCM.43.9.4889-4890.2005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 1995 {published data only}
- Jackson AA, Ismail A, Ibrahim TA, Kader ZS, Nawi NM. Retrospective review of dot enzyme immunoassay test for typhoid fever in an endemic area. South East Asian Journal of Public Health 1995;26(4):625‐30. [PubMed] [Google Scholar]
John 1984 {published data only}
- John TJ, Sivadasan K, Kurien B. Evaluation of passive bacterial agglutination for the diagnosis of typhoid fever. Journal of Clinical Microbiology 1984;20(4):751‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalhan 1998 {published data only}
- Kalhan R, Kaur I, Singh RP, Gupta HC. Rapid diagnosis of typhoid fever. Indian Journal of Paediatrics 1998;65(4):561‐4. [DOI] [PubMed] [Google Scholar]
Kalhan 1999 {published data only}
- Kalhan R, Kaur I, Singh RP, Gupta HC. Latext agglutination test (LAT) for the diagnosis of typhoid fever. Indian Journal of Paediatrics 1999;36(1):65‐8. [PubMed] [Google Scholar]
Kariuki 2004 {published data only}
- Kariuki S, Mwituria J, Munyalo A, Revathi G, Onsongo J. Typhoid is over‐reported in Embu and Nairobi, Kenya. African Journal of Health Science 2004;11(3‐4):103‐10. [PubMed] [Google Scholar]
Kaur 1988a {published data only}
- Kaur IR, Talwar V, Gupta HC, Rawat S. Comparative evaluation of latex agglutination (LAT) and coagglutination (COAG) tests for rapid diagnosis of typhoid fever. Journal of Communicable Diseases 1998;20(4):344‐8. [PUBMED: 3268601] [PubMed] [Google Scholar]
Kaur 1988b {published data only}
- Kaur I, Talwar V, Rawat S, Anwar M, Gupta HC. Comparison of rapid serodiagnostic tests for antigen detection in typhoid fever. Indian Journal of Pathology and Microbiology 1998;31(3):245‐7. [PUBMED: 3235132] [PubMed] [Google Scholar]
Khanam 2013 {published data only}
- Khanam F, Sheikh A, Sayeed MA, Bhuiyan MS, Choudhury FK, Salma U, et al. Evaluation of a typhoid/paratyphoid diagnostic assay (TPTest) detecting anti‐Salmonella IgA in secretions of peripheral blood lymphocytes in patients in Dhaka, Bangladesh. PLoS Neglected Tropical Diseases 2013;7(7):e2316. [DOI: 10.1371/journal.pntd.0002316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khanam 2015 {published data only}
- Khanam F, Sayeed MA, Choudhury FK, Sheikh A, Ahmed D, Goswami D, et al. Typhoid fever in young children in Bangladesh: clinical findings, antibiotic susceptibility pattern and immune responses. PLoS Neglected Tropical Diseases 2015;9(4):e0003619. [DOI: 10.1371/journal.pntd.0003619] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kollaritsch 1988 {published data only}
- Kollaritsch H, Hirschl A, Stanek G, Rotter ML. Agglutination test versus ELISA in the diagnosis of typhoid fever. European Journal of Epidemiology 1988;4(1):127‐8. [DOI] [PubMed] [Google Scholar]
Korbsrisate 1998 {published data only}
- Korbsrisate S, Sarasombath S, Praaporn N, Iamkamala P, Hossain M, Mckay S. Detection of IgM antibody against region IV flagellin of Salmonella paratyphi A. Southeast Asian Journal of Tropical Medicine and Public Health 1998;29(4):864‐71. [PubMed] [Google Scholar]
Kuchuloria 2016 {published data only}
- Kuchuloria T, Imnadze P, Mamuchishvili N, Chokheli M, Tsertsvadze T, Endeladze M, et al. Hospital‐based surveillance for infectious etiologies among patients with acute febrile illness in Georgia, 2008–2011. American Journal of Tropical Medicine and Hygiene 2016;94(1):236‐42. [DOI: 10.4269/ajtmh.15-0400] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 1998 {published data only}
- Lim PL, Tam FCH, Cheong YM, Jegathesan M. One‐step 2‐minute test to detect typhoid‐specific antibodies based on particle separation in tubes. Journal of Clinical Microbiology 1998;36(8):2271‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lutterloh 2012 {published data only}
- Lutterloh E, Likaka A, Sejvar J, Manda R, Naiene J, Monroe SS, et al. Multidrug‐resistant typhoid fever with neurologic findings on the Malawi‐Mozambique border. Clinical Infectious Diseases 2012;54(8):1100‐6. [DOI: 10.1093/cid/cis012] [DOI] [PubMed] [Google Scholar]
Malik 2001 {published data only}
- Malik AS, Malik RH. Typhoid fever in Malaysian children. Medical Journal of Malaysia 2001;56(4):478‐90. [PubMed] [Google Scholar]
Mukherjee 1993 {published data only}
- Mukherjee C, Malik A, Khan HM, Malik A. Rapid diagnosis of typhoid fever for coagglutination in an Indian hospital. Journal of Medical Microbiology 1993;39(1):74‐7. [DOI] [PubMed] [Google Scholar]
Munir 2015 {published data only}
- Munir T, Lodhi M, Ali S, Hussain Zaidi SB, Razak S. Early diagnosis of typhoid By PCR for FliC‐d gene of Salmonella Typhi in patients taking antibiotics. Journal of the College of Physicians and Surgeons‐‐Pakistan 2015;25(9):662‐6. [PubMed] [Google Scholar]
Narayanappa 2010 {published data only}
- Narayanappa D, Sripathi R, Jagdishkumar K, Rajani HS. Comparative study of dot enzyme immunoassay (Typhidot‐M) and Widal test in the diagnosis of typhoid fever. Indian Pediatrics 2010;47(4):331‐3. [DOI] [PubMed] [Google Scholar]
Neil 2012 {published data only}
- Neil KP, Sodha SV, Lukwago L, O‐Tipo S, Mikoleit M, Simington SD, et al. A large outbreak of typhoid fever associated with a high rate of intestinal perforation in Kasese District, Uganda, 2008‐2009. Clinical Infectious Diseases 2012;54(8):1091‐9. [DOI: 10.1093/cid/cis025] [DOI] [PubMed] [Google Scholar]
Nguyen 1997 {published data only}
- Nguyen NQ, Tapchaisri P, Chongsa‐nguan M, Cao VV, Doan TT, Sakolvaree Y, et al. Diagnosis of enteric fever caused by Salmonella spp. in Vietnam by a monoclonal antibody‐based dot‐blot ELISA. Asian Pacific Journal of Allergy and Immunology 1997;15(4):205‐12. [PubMed] [Google Scholar]
Ong 1989 {published data only}
- Ong LY, Pang T, Lim SH, Tan EL, Puthucheary SD. A simple adherence test for detection of IgM antibodies in typhoid. Journal of Medical Microbiology 1989;29(3):195‐8. [DOI] [PubMed] [Google Scholar]
Pandya 1995 {published data only}
- Pandya M, Pillai P, Deb M. Rapid diagnosis of typhoid fever by detection of Barber protein and Vi antigen of Salmonella serotype Typhi. Journal of Medical Microbiology 1995;43(3):185‐8. [DOI] [PubMed] [Google Scholar]
Petchclai 1987 {published data only}
- Petchclai B, Ausavarungnirun R, Manatsathit S. Passive hemagglutination test for enteric fever. Journal of Clinical Microbiology 1987;25(1):138‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peterson 2010 {published data only}
- Peterson G, Bai J, Nagaraja TG, Narayanan S. Diagnostic microarray for human and animal bacterial diseases and their virulence and antimicrobial resistance genes. Journal of Microbiological Methods 2010;80(3):223‐30. [DOI: 10.1016/j.mimet.2009.12.010] [DOI] [PubMed] [Google Scholar]
Preechakasedkit 2012 {published data only}
- Preechakasedkit P, Pinwattana K, Dungchai W, Siangproh W, Chaicumpa W, Tongtawe P, et al. Development of a one‐step immunochromatographic strip test using gold nanoparticles for the rapid detection of Salmonella Typhi in human serum. Biosensors & Bioelectronics 2012;31(1):562‐6. [DOI: 10.1016/j.bios.2011.10.031] [DOI] [PubMed] [Google Scholar]
Rai 1989 {published data only}
- Rai GP, Zachariah K, Shrivastava S. Comparative efficacy of indirect haemagglutination test, indirect fluorescent antibody test and enzyme linked immunosorbent assay in serodiagnosis of typhoid fever. Journal of Tropical Medicine and Hygiene 1989;92(6):431‐4. [PubMed] [Google Scholar]
Shrivastava 2011 {published data only}
- Shrivastava B, Shrivastava V, Shrivastava A. Comparative study of diagnostic procedures in Salmonella infection, causative agent of typhoid fever ‐ an overview study. International Research Journal of Pharmacy 2011;2(9):127‐9. [Google Scholar]
Surachmanto 2011 {published data only}
- Surachmanto E. Prevalance of co‐morbidity of asthma acute exacerbation with typhoid fever. European Journal of Allergy and Clinical Immunology 2011;66(Suppl 94):141. [Google Scholar]
Tantivanich 1984 {published data only}
- Tantivanich S, Chongsanguan M, Sangpetchsong V, Tharavanij S. A simple and rapid diagnostic test for typhoid fever. Southeast Asian Journal of Tropical Medicine and Hygiene 1984;15(3):317‐22. [PubMed] [Google Scholar]
Thevanesam 1992 {published data only}
- Thevanesam V. An evaluation of the SAT in the diagnosis of typhoid. Ceylon Medical Journal 1992;37(2):48‐51. [PubMed] [Google Scholar]
Watt 2005 {published data only}
- Watt G, Jongsakul K, Ruangvirayuth R, Kantipong P, Silpapojakul K. Short report: prospective evaluation of a multi‐test strip for the diagnoses of scrub and murine typhus, leptospirosis, dengue fever and Salmonella Typhi infection. American Journal of Tropical Medicine and Hygiene 2005;72(1):10‐2. [PubMed] [Google Scholar]
West 1989 {published data only}
- West B, Richens JE, Howard PF. Evaluation in Papua New Guinea of a urine coagglutination test and a Widal slide agglutination test for rapid diagnosis of typhoid fever. Transactions of the Royal Society of Tropical Medicine and Hygiene 1989;83(5):715‐7. [DOI] [PubMed] [Google Scholar]
Wijedoru 2012 {published data only}
- Wijedoru LPM, Kumar V, Chanpheaktra N, Chheng K, Smits HL, Pastoor R, et al. Typhoid fever among hospitalised children in Siem Reap, Cambodia. Journal of Tropical Pediatrics 2012;58(1):68‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2011 {published data only}
- Yan M, Tam FCH, Kan B, Lim PL. Combined rapid (TUBEX) test for Typhoid‐Paratyphoid A fever based on strong anti‐012 response: design and critical assessment of sensitivity. PLoS ONE 2011;6(9):e24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zaka‐ur‐Rab 2012 {published data only}
- Zaka‐ur‐Rab Z, Abqari S, Shahab T, Islam N, Shukla I. Evaluation of salivary and anti‐Salmonella Typhi lipopolysaccharide IgA ELISA for serodiagnosis of typhoid fever in children. Archives of Diseases in Childhood 2012;97(3):236‐8. [DOI: 10.1136/adc.2011.300622] [DOI] [PubMed] [Google Scholar]
Additional references
Abba 2011
- Abba K, Deeks JJ, Olliaro PL, Naing CM, Jackson SM, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated Plasmodium falciparum malaria in endemic countries. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008122.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andrews 2015
- Andrews JR, Ryan ET. Diagnostics for invasive Salmonella infections: Current challenges and future directions. Vaccine 2015;33(Supplement 3):C8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baker 2010
- Baker S, Favorov M, Dougan G. Searching for the elusive typhoid diagnostic. BMC Infectious Diseases 2010;10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhutta 2006
- Bhutta ZA. Current concepts in the diagnosis and treatment of typhoid fever. BMJ 2006;333(7558):78‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta‐analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331‐2. [DOI] [PubMed] [Google Scholar]
Chuang 2009
- Chuang CH, Su LH, Perera J, Carlos C, Tan BH, Kumarasinghe G, et al. Surveillance of antimicrobial resistance of Salmonella enteric serotype Typhi in seven Asian countries. Epidemiology and Infection 2009;137(2):266‐9. [DOI] [PubMed] [Google Scholar]
Crump 2004
- Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bulletin of the World Health Organization 2004;82(4):346‐53. [PMC free article] [PubMed] [Google Scholar]
Crump 2014
- Crump JA. Updating and refining estimates of typhoid fever burden for public health action. Lancet Global Health 2014;2(10):e551‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darton 2014
- Darton TC, Blohmke CJ, Pollard AJ. Typhoid epidemiology, diagnostics and the human challenge model. Current Opinion in Gastroenterology 2014;30(1):7‐17. [DOI: 10.1097/MOG.0000000000000021; PUBMED: 24304980] [DOI] [PubMed] [Google Scholar]
Deen 2012
- Deen J, Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. Community‐acquired bacterial bloodstream infections in developing countries in South and Southeast Asia: a systematic review. Lancet Infectious Diseases 2012;12(6):480‐7. [DOI] [PubMed] [Google Scholar]
Gill 2009
- Gill GV, Beeching NJ. Typhoid and paratyphoid fevers. Lecture Notes in Tropical Medicine. 6th Edition. West Sussex: Wiley‐Blackwell, 2009:280‐6. [Google Scholar]
Herberg 2016
- Herberg JA, Kaforou M, Wright VJ, Shailes H, Eleftherohorinou H, Hoggart CJ, et al. Diagnostic test accuracy of a 2‐transcript host RNA signature for discriminating bacterial versus viral infection in febrile children. JAMA 2016;316(8):835‐45. [DOI: 10.1001/jama.2016.11236] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 2006
- Ismail TF. Rapid Diagnosis of Typhoid Fever. Indian Journal of Medical Research 2006;123(4):489‐92. [PubMed] [Google Scholar]
Kariuki 2015
- Kariuki S, Gordon MA, Feasey N, Parry CM. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 2015;33(Suppl 3):C21‐9. [DOI: 10.1016/j.vaccine.2015.03] [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsson 2008
- Larsson M, Kronvall G, Chuc NT, Karlsson I, Lager F, Hanh HD, et al. Antibiotic medication and bacterial resistance to antibiotics: a survey of children in a Vietnamese community. Tropical Medicine & International Health 2008;5(10):711‐21. [DOI] [PubMed] [Google Scholar]
Maskey 2006
- Maskey AP, Day JN, Phung QT, Thwaites GE, Campbell JI, Zimmerman M, Farrar JJ, Basnyat B. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clinical Infectious Diseases 2006;42(9):1247‐53. [DOI] [PubMed] [Google Scholar]
Massi 2005
- Massi MN, Shirakawa T, Gotoh A, Bishnu A, Hatta M, Kawabata M. Quantitative detection of Salmonella enteric serovar Typhi from blood of suspected typhoid fever patients by real‐time PCR. International Journal of Medical Microbiology 2005;295(2):117‐20. [DOI] [PubMed] [Google Scholar]
McKinnon 2014
- McKinnon LR, Abdool Karim Q. Honing in on enteric fever. eLife 2014;3:e03545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mogasale 2016
- Mogasale V, Ramani E, Mogsale VV, Park JY. What proportion of Salmonella Typhi cases are detected by blood culture? A systematic literature review. Annals of Clinical Microbiology and Antimicrobials 2016;15(1):32. [DOI: 10.1186/s12941-016-0147-z; PUBMED: 27188991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nga 2010
- Nga TVT, Karkey A, Dongol S, Thuy HN, Dunstan S, Holt K, et al. The sensitivity of real‐time PCR amplification targeting invasive Salmonella serovars in biological specimens. BMC Infectious Diseases 2010;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okeke 2005
- Okeke IN, Klugman KP, Bhutta ZA, Duse AG, Jenkins P, O'Brien TF, et al. Antimicrobial resistance in developing countries. Part II: strategies for containment. Lancet Infectious Diseases 2005;5(9):568‐80. [DOI] [PubMed] [Google Scholar]
Olopoenia 2000
- Olopoenia LA, King AL. Widal Agglutination Test ‐ 100 years later: still plagued by controversy. Postgraduate Medical Journal 2000;76(892):80‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parry 2002
- Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. Typhoid Fever. New England Journal of Medicine 2002;347(22):1770‐82. [DOI] [PubMed] [Google Scholar]
Parry 2011
- Parry CM, Wijedoru L, Arjyal A, Baker S. The utility of diagnostic tests for enteric fever in endemic locations. Expert Review of Anti‐Infective Therapy 2011;9(6):711‐25. [DOI: 10.1586/eri.11.47.; PUBMED: 21692675] [DOI] [PubMed] [Google Scholar]
Reddy 2010
- Reddy EA, Shaw AV, Crump JA. Community‐acquired bloodstream infections in Africa: a systematic review and meta‐analysis. Lancet Infectious Diseases 2010;10(6):417‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
Reitsma 2009
- Reitsma JB, Rutjes AWS, Khan KS, Coomarasamy A, Bossuyt PM. A review of solutions for diagnostic accuracy studies with an imperfect or missing reference standard. Journal of Clinical Epidemiology 2009;62(8):797‐806. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutter 2001
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [DOI] [PubMed] [Google Scholar]
Saha 1996
- Saha SK, Ruhulamin M, Hanif M, Islam M, Khan WA. Interpretation of the Widal test in the diagnosis of typhoid fever in Bangladeshi children. Annals of Tropical Paediatrics 1996;16(1):75‐8. [DOI] [PubMed] [Google Scholar]
Shetty 2008
- Shetty P. Antibiotic resistance: frequently asked questions. 26 March 2008. www.scidev.net/global/health/feature/antibiotic‐resistance‐frequently‐asked‐questions.html (accessed 10 June 2009).
Smits 2013
- Smits HL. Limitations of typhoid fever diagnostics and the need for prevention. Expert Review of Molecular Diagnostics 2013;13(2):147‐9. [DOI: 10.1586/ERM.12.145] [DOI] [PubMed] [Google Scholar]
Storey 2015
- Storey HL, Huang Y, Crudder C, Golden A, Santos T, Hawkins K. A meta‐analysis of typhoid diagnostic accuracy studies: a recommendation to adopt a standardized composite reference. PLoS ONE 2015;10(11):e0142364. [DOI: 10.1371/journal.pone.0142364] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takwoingi 2013
- Takwoingi Y, Leeflang MM, Deeks JJ. Empirical evidence of the importance of comparative studies of diagnostic test accuracy. Annals of Internal Medicine 2013;158(7):544‐54. [DOI: 10.7326/0003-4819-158-7-201304020-00006; PUBMED: 23546566] [DOI] [PubMed] [Google Scholar]
Thriemer 2013
- Thriemer K, Ley B, Menten J, Jacobs J, Ende J. A systematic review and meta‐analysis of the performance of two point of care typhoid fever tests, TUBEX TF and Typhidot, in endemic countries. PLOS ONE 2013;8(12):e81263. [DOI: 10.1371/journalpone.0081263] [DOI] [PMC free article] [PubMed] [Google Scholar]
UNICEF 2006
- UNICEF. Progress for Children. A Report Card on Water and Sanitation. Number 6, September 2006. https://www.unicef.org/publications/files/Progress_for_Children_No._5_‐_English.pdf (accessed 11 September 2010).
Waddington 2014
- Waddington CS, Darton TC, Pollard AJ. The challenge of enteric fever. Journal of Infection 2014;68(Supplement 1):S38‐50. [DOI: 10.1016/j.jinf.2013.09.013] [DOI] [PubMed] [Google Scholar]
Wain 1998
- Wain J, Diep TS, Ho VA, Walsh AM, Nguyen TT, Parry CM, et al. Quantitation of bacteria in blood of typhoid fever patients and relationship between counts and clinical features, transmissibility, and antibiotic resistance. Journal of Clinical Microbiology 1998;36(6):1683‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wain 2001
- Wain J, Pham JB, Ha V, Nguyen NM, To SD, Walsh AL, et al. Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. Journal of Clinical Microbiology 2001;39(4):1571‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Background document: the diagnosis, treatment and prevention of typhoid fever. WHO/V&B/03.07. 2003. http://apps.who.int/iris/bitstream/10665/68122/1/WHO_V‐B_03.07_eng.pdf (accessed 11 September 2010).
WHO 2009
- World Health Organization. Evaluation of commercially available anti‐dengue virus immunoglobulin M tests. Diagnostic Evaluation Series No. 3. http://www.who.int/tdr/publications/documents/diagnostics‐evaluation‐3.pdf?ua=1. Special Programme for Research and Training in Tropical Diseases (accessed 11 September 2010). [ISBN 978 92 4 1597753]
Wilson 1994
- Wilson ML. Blood cultures. Introduction. Clinics in Laboratory Medicine 1994;14(1):1‐7. [PubMed] [Google Scholar]
Zhou 2010
- Zhou L, Pollard AJ. A fast and highly sensitive blood culture PCR method for clinical detection of Salmonella enterica serovar Typhi. Annals of Clinical Microbiology and Antimicrobials 2010;9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Wijedoru 2010
- Wijedoru L, Donegan S, Parry C. Rapid Diagnostic Tests for Typhoid and Paratyphoid (Enteric) Fever. Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008892] [DOI] [PMC free article] [PubMed] [Google Scholar]


