Abstract
In late mitosis and G1, Mcm2–7 are assembled onto replication origins to ‘license' them for initiation. At other cell cycle stages, licensing is inhibited, thus ensuring that origins fire only once per cell cycle. Three additional factors—the origin recognition complex, Cdc6 and Cdt1—are required for origin licensing. We examine here how licensing is regulated in Xenopus egg extracts. We show that Cdt1 is downregulated late in the cell cycle by two different mechanisms: proteolysis, which occurs in part due to the activity of the anaphase-promoting complex (APC/C), and inhibition by a protein called geminin. If both these regulatory mechanisms are abrogated, extracts undergo uncontrolled re-licensing and re-replication. The extent of re-replication is limited by checkpoint kinases that are activated as a consequence of re-replication itself. These results allow us to build a comprehensive model of how re-replication of DNA is prevented in Xenopus, with Cdt1 regulation being the key feature. The results also explain the original experiments that led to the proposal of a replication licensing factor.
Keywords: Cdt1, DNA replication, geminin, replication licensing, Xenopus
Introduction
The precise replication of chromosomal DNA during S phase of every cell cycle is crucial for the maintenance of genetic integrity. To ensure that no segment of DNA replicates more than once in a single cell cycle, the replication process is divided into two nonoverlapping steps (reviewed in Blow and Hodgson, 2002; Nishitani and Lygerou, 2002). In the first stage, which occurs in late mitosis and early G1 phase, origins of replication are ‘licensed' for use in the upcoming S phase. This is achieved by the sequential loading of the origin recognition complex (ORC), Cdc6, Cdt1 and finally the heterohexameric Mcm2–7 complex onto the origins (Gillespie et al, 2001). Once loaded with Mcm2–7 complexes, origins are licensed and form a ‘pre-replicative complex'. Mcm2–7 are essential for subsequent DNA replication, and at initiation are displaced from the origins, probably moving along with the replication forks. This behaviour is consistent with a variety of data suggesting that they form a replicative helicase that unwinds DNA ahead of the replication fork. To prevent the re-replication of DNA, further origin licensing (Mcm2–7 reloading) must be prevented before the cell enters S phase.
A number of different mechanisms have been described that block licensing late in the cell cycle (Blow and Hodgson, 2002; Nishitani and Lygerou, 2002). One major pathway involves the cyclin-dependent kinases (CDKs), which are active from late G1 until the end of mitosis and negatively regulate different components of the licensing system. In the yeast Saccharomyces cerevisiae, CDKs apparently play a major role in preventing re-licensing by phosphorylation-dependent degradation of Cdc6, nuclear export of Mcm2–7 and inactivation of ORC. Mutations that block all three modes of CDK regulation resulted in partial re-replication of DNA (Nguyen et al, 2001). Metazoans have an additional licensing inhibitor: a small protein called geminin that specifically binds and inhibits Cdt1 (McGarry and Kirschner, 1998; Wohlschlegel et al, 2000; Tada et al, 2001; Lee et al, 2004). No geminin orthologue has been identified in either yeast or in Caenorhabditis elegans. Like CDKs, geminin is active throughout S phase, G2 and mitosis. At the end of mitosis, geminin is polyubiquitinated by the anaphase-promoting complex (APC/C), which targets it for inactivation and/or proteasome-mediated degradation (McGarry and Kirschner, 1998; Bastians et al, 1999; Li and Blow, 2004), allowing the licensing system to be activated. In Xenopus egg extracts, geminin is re-activated following its nuclear import in late G1 (Hodgson et al, 2002). In the Xenopus system, geminin is the major trans-acting inhibitor of licensing, both in metaphase (Tada et al, 2001) and late interphase (Hodgson et al, 2002). Removal of geminin from Drosophila cells using RNAi resulted in rapid downregulation of Cdt1/Dup and led to extensive but incomplete re-replication (Mihaylov et al, 2002). Similar results have been shown recently in human cells (Melixetian et al, 2004; Zhu et al, 2004).
Cdt1 activity can also be abolished by cell cycle-dependent proteolysis, as observed in Schizosaccharomyces pombe (Gopalakrishnan et al, 2001), humans (Wohlschlegel et al, 2000; Nishitani et al, 2001; Li et al, 2003), Drosophila (Quinn et al, 2001) and Xenopus (Hodgson et al, 2002). In humans, CDK-dependent phosphorylation targets Cdt1 for degradation at the start of S phase via SCF-mediated ubiquitination (Liu et al, 2004; Sugimoto et al, 2004). In C. elegans, ubiquitination of Cdt1 is mediated by the CUL-4 ubiquitin ligase, and inactivation of CUL-4 induces massive re-replication (Zhong et al, 2003). Overexpression of Cdt1 along with Cdc18/Cdc6 causes enhanced re-replication in S. pombe (Nishitani et al, 2000; Gopalakrishnan et al, 2001) and in p53-deficient human cells (Vaziri et al, 2003), whereas overexpression of a stabilised Cdt1 mutant induced re-replication in humans (Nishitani et al, 2004) and Drosophila (Thomer et al, 2004). In the present work, we have investigated the role of Cdt1 regulation in preventing re-replication of DNA in Xenopus. We show that both geminin activity and Cdt1 proteolysis can prevent re-replication of DNA late in the cell cycle. When geminin is removed and Cdt1 degradation is prevented, re-licensing and re-replication of DNA occur. However, this triggers checkpoint activation, which limits the amount of re-replication that actually takes place.
Results
Cdt1 proteolysis prevents re-replication
Figure 1A shows that, consistent with previous reports (Hodgson et al, 2002), Cdt1 levels in Xenopus egg extracts decline after release from metaphase. Degradation was largely unaffected by the presence of sperm DNA in the extract, although there was minor variation in the rate of degradation between different experiments. The profile of Cdt1 degradation resembled that of cyclin B, which is polyubiquitinated by the APC/C and degraded by the proteasome. Figures 1B and C show that degradation of Cdt1, like cyclin B and geminin, was inhibited by D-box peptide (a competitive inhibitor of the APC/C), MG132 (a proteasome inhibitor), Emi1 (a direct inhibitor of the APC/C; Reimann et al, 2001) and roscovitine (an inhibitor of CDKs that are required for APC/C activation). These results suggest that Cdt1 degradation on exit from metaphase is substantially dependent on APC/C-mediated ubiquitination.
Figure 1.
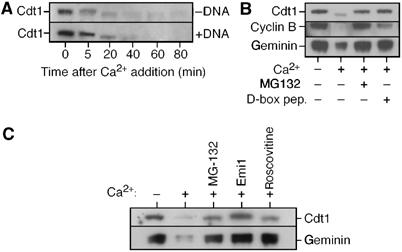
Cdt1 levels decline during interphase. (A) Metaphase-arrested Xenopus extracts were supplemented plus or minus 3 ng DNA/μl sperm nuclei, and then released into interphase by adding CaCl2. At the indicated times, aliquots of whole extract were subjected to SDS–PAGE and immunoblotted for Cdt1. (B, C) Metaphase-arrested extracts were supplemented plus or minus CaCl2, MG132, D-box peptide, Emi1 or roscovitine and incubated for 60 min before being immunoblotted for Cdt1, cyclin B and geminin (B) or Cdt1 and geminin (C).
We next examined the significance of Cdt1 degradation. On incubation of Xenopus egg extract without added DNA, licensing activity declines due to loss of Cdt1/RLF-B activity (Mahbubani et al, 1997). To test whether this decline is due to Cdt1 degradation, licensing activity was assayed after a 2 h preincubation. In this experiment (and all others here), cyclin B synthesis was blocked by cycloheximide, thus preventing progression into mitosis. Figure 2A shows that during the 2 h preincubation, the ability to license sperm DNA was lost. As previously reported (Mahbubani et al, 1997), this was due to a loss of Cdt1 activity as it was reversed by addition of Cdt1. When the preincubation was repeated with extract supplemented with MG132, the decline in licensing activity was substantially prevented.
Figure 2.
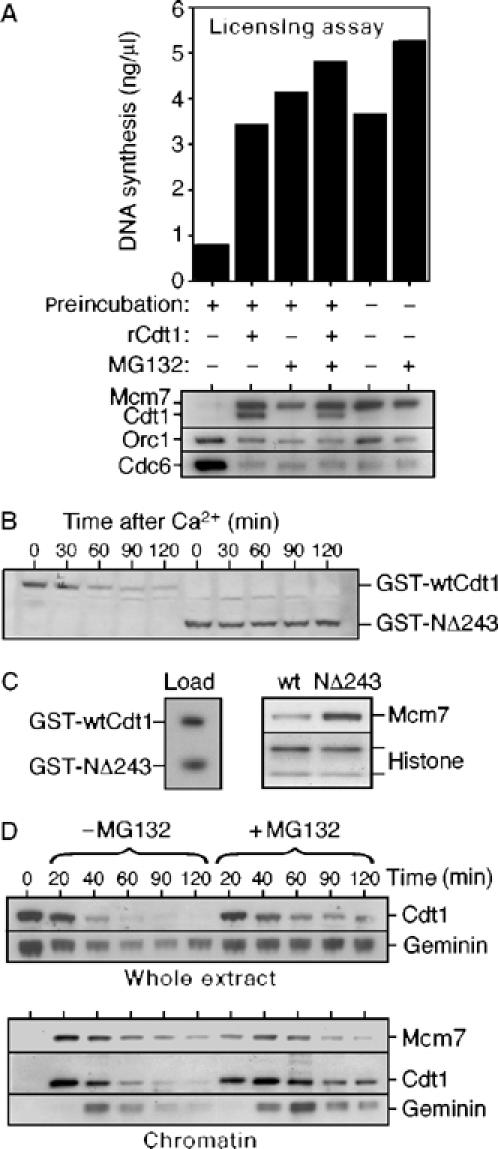
Degradation of Cdt1 results in the loss of licensing activity. (A) Metaphase-arrested extracts were supplemented with CaCl2 plus or minus MG132 and optionally preincubated for 2 h at 23°C. Sperm nuclei were then added (10 ng DNA/μl) plus or minus recombinant Cdt1 and extracts were incubated for a further 20 min. An aliquot was then transferred to fresh geminin-treated extract and assayed for its ability to support DNA synthesis (upper chart). Chromatin was isolated from the remaining sample and immunoblotted for Mcm7, Cdt1, Orc1 and Cdc6 (lower panel). (B, C) Metaphase-arrested extracts were supplemented with GST-wtCdt1 or GST-NΔ243 constructs, released into interphase with CaCl2 and incubated for 2 h. (B) At the indicated times, aliquots of whole extract were subjected to SDS–PAGE and immunoblotted for GST (4 ng/μl GST-Cdt1 constructs). (C) After 2 h, sperm nuclei were added (10 ng DNA/μl) and after additional 30 min incubation, chromatin was isolated and blotted for Mcm7 (0.3 ng/μl GST-Cdt1 construct). The left panel shows 3 ng of each construct blotted for GST. (D) Metaphase-arrested extracts were supplemented with sperm nuclei (10 ng DNA/μl) plus or minus MG132 and then released into interphase with CaCl2. At the indicated times, aliquots of whole extract (upper panels) or isolated chromatin (lower panels) were subjected to SDS–PAGE and immunoblotted for Cdt1, geminin or Mcm7.
Degradation of human and Drosophila Cdt1 is dependent on its N-terminus (Liu et al, 2004; Nishitani et al, 2004; Sugimoto et al, 2004; Thomer et al, 2004). We compared the stability of full-length Xenopus Cdt1 with a mutant lacking 243 N-terminal amino acids (NΔ243). Equal quantities of recombinant Cdt1, comparable to endogenous, were added to metaphase-arrested extracts, which were then released into interphase by addition of Ca2+ and incubated for 2 h (Figure 2B). Wild-type (wt) Cdt1 was efficiently degraded, whereas NΔ243 was stable. When sperm DNA was added to extracts preincubated for 2 h with either wt Cdt1 or NΔ243, only NΔ243 was able to efficiently load Mcms onto chromatin (Figure 2C), although before preincubation, wt Cdt1 was more efficient at supporting licensing than NΔ243 (data not shown). Together, these results suggest that degradation of Cdt1 during interphase can prevent re-licensing late in the cell cycle.
We next looked at licensing when endogenous Cdt1 was protected from proteolysis by MG132 (Figure 2D). In untreated extract, Mcm2–7 chromatin binding reaches a maximum by ∼20 min and then declines over the next 30–40 min as the DNA replicates (Kubota et al, 1997; Thömmes et al, 1997). Cdt1 is also lost from the chromatin with similar kinetics (Maiorano et al, 2000). Following nuclear assembly, geminin is activated (Hodgson et al, 2002) and is recruited to chromatin by Cdt1 (Gillespie et al, 2001) (Figure 2D, 40 min chromatin samples). When Cdt1 degradation was slowed by MG132, Cdt1 remained bound to the chromatin throughout S phase and into G2 (Figure 2D, right-hand lanes). Under these conditions, a faint ladder suggestive of Cdt1 polyubiquitination was seen on chromatin at ∼60 min (Figure 2D and data not shown). However, MG132 caused no obvious reloading of Mcm7 onto the replicated DNA. Instead, a prolonged recruitment of geminin to chromatin was observed, which plausibly explains why no re-licensing occurred.
Together with previous results (Hodgson et al, 2002), this suggests that two different pathways negatively regulate Xenopus Cdt1: Cdt1 proteolysis during interphase and inhibition by geminin after nuclear assembly. We therefore asked whether replicated DNA would be re-licensed if both mechanisms were suppressed. Sperm nuclei were incubated in extract immunodepleted of geminin or mock-depleted with nonimmune antibody, and supplemented plus or minus MG132. At 90 min, when replication is typically complete in normal extract, aphidicolin was added to prevent any further DNA synthesis and consequent displacement of Mcm2–7 from chromatin. Incubation was then continued for a further 60 min. Figure 3A shows the Mcm7 present on the chromatin at 90 and 150 min. In the geminin-depleted extract, an increase in Mcm7 on chromatin between these two times suggests that re-licensing of replicated DNA had occurred. This increase in Mcm7 binding was enhanced by the presence of MG132 in the geminin-depleted extract, due to stabilisation of Cdt1. In contrast, no increase in chromatin-bound Mcm7 between these two times was seen in the control depletion, irrespective of whether MG132 was present, because the Cdt1 was inhibited by geminin.
Figure 3.
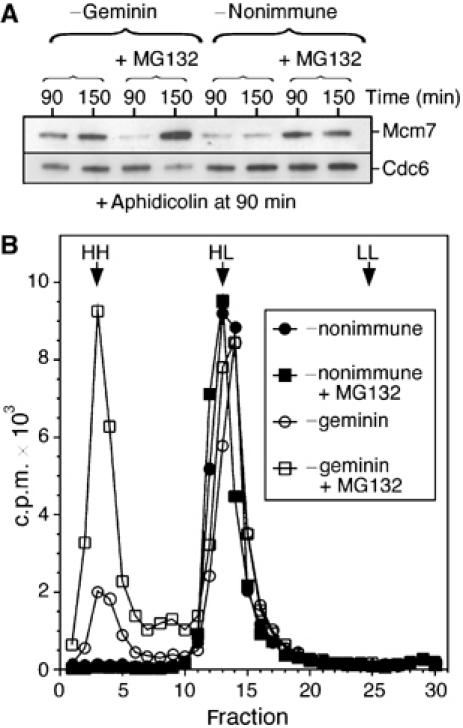
Geminin depletion and MG132 treatment permits efficient re-replication. Interphase Xenopus extracts were optionally supplemented with MG132 and then immunodepleted with either anti-geminin or nonimmune antibodies. (A) Sperm nuclei were incubated at 10 ng DNA/μl in the extracts for 90 min; 40 μM aphidicolin was then added and the incubation continued for a further 60 min. Chromatin was isolated at either 90 or 150 min and immunoblotted for Mcm7 and Cdc6. (B) The extracts were supplemented with sperm nuclei (5 ng DNA/μl), [α-32P]dATP and BrdUTP, and incubated for 180 min. DNA was then isolated and fractionated on CsCl equilibrium density gradients. The expected densities of heavy–heavy (HH), heavy–light (HL) and light–light (LL) DNA are indicated.
To determine whether this re-licensing could support re-replication, DNA was replicated in the four extracts in the presence of [α-32P]dATP and BrdUTP; nascent DNA was then fractionated on CsCl gradients (Figure 3B). Consistent with the immunoblotting results, no re-replicated (heavy–heavy (HH)) DNA was produced in nonimmune-depleted extracts, whether or not MG132 was present. In geminin-depleted extract without MG132, a small amount of re-replicated DNA was produced, while a combination of geminin depletion and MG132 allowed >50% of the replicated DNA to re-replicate. Taken together, these experiments suggest that the regulation of Cdt1 by a combination of proteolysis and geminin inhibition is essential to prevent re-replication of DNA in a single cell cycle.
Nuclear transfer experiments
The original experiments suggesting the existence of a replication licensing factor involved transferring replicated (G2) nuclei into fresh Xenopus extract and monitoring the degree of re-replication (Blow and Laskey, 1988). Only if G2 nuclei were permeabilised prior to transfer could they support re-replication in the fresh extract. We therefore examined how nuclear permeabilisation affects geminin and Cdt1 in similar nuclear transfer experiments. Figure 4A shows that nuclei assembled in vitro are extremely leaky when isolated in this procedure (Blow and Laskey, 1988). In G2 nuclei, geminin, Mcm7 and nucleoplasmin are predominantly soluble nucleoplasmic proteins (Hodgson et al, 2002) but are lost from nuclei after isolation (Figure 4A). When the isolated nuclei were reincubated in fresh extract, Cdt1 was rapidly recruited to the chromatin, irrespective of whether the nuclei had been permeabilised with lysolecithin. By 20 min, the Cdt1 on chromatin in the intact nuclei dropped slightly, possibly reflecting increased degradation in the nucleoplasm. Interphase extract also contains inactive geminin, which can be re-activated following import into nuclei (Hodgson et al, 2002; Li and Blow, 2004). Consistent with this, the intact nuclei accumulated geminin on chromatin after reincubation in fresh extract (Figure 4B). However, if the G2 nuclei had been permeabilised with lysolecithin prior to reincubation, significantly less geminin was recruited. This is presumably due to the permeabilised nuclei being unable to re-activate geminin until proper nuclear import could occur following nuclear envelope resealing and the establishment of a RanGTP/GDP gradient. Efficient reloading of Mcm7 was only observed in the permeabilised nuclei.
Figure 4.
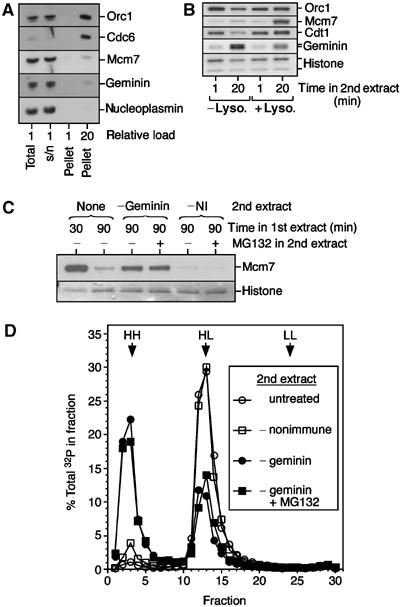
Efficient re-replication of G2 nuclei in geminin-depleted extract. Metaphase-arrested extracts were supplemented with sperm nuclei (10 ng DNA/μl) and released into interphase with CaCl2. After incubation for 90 min, extracts were diluted with buffer and nuclei were centrifuged through a sucrose cushion. (A) Aliquots of the whole extract (total), the pelleted nuclei (pellet) and the supernatant (s/n) after centrifugation were immunoblotted for Orc1, Cdc6, Mcm7, geminin and nucleoplasmin. In the first three lanes, 2.5% of each sample was loaded; in the last lane, 50% of the pellet was loaded. (B) Isolated nuclei were optionally treated with lysolecithin and then transferred to fresh extract. After 1 or 20 min, chromatin was re-isolated and immunoblotted for the indicated proteins. (C) Nuclei were isolated as in (A), and were transferred to fresh interphase extracts containing aphidicolin and optionally supplemented with MG132. After a further 60 min, chromatin was isolated and immunoblotted for Mcm7. As a control, chromatin was isolated from the first extract at either 30 or 90 min. (D) G2 nuclei were prepared as in (A), except that the extract was supplemented with BrdUTP and [α-32P]dATP. Intact nuclei were transferred to fresh untreated extract, extract immunodepleted with nonimmune antibodies, or extract immunodepleted of geminin plus or minus MG132. The second extract was also supplemented with BrdUTP. After a further 90 min incubation, DNA was isolated and fractionated on density gradients.
If re-replication is normally prevented solely by a combination of Cdt1 proteolysis and geminin inhibition, then intact G2 nuclei should be re-licensed if transferred to extract containing Cdt1 but lacking geminin. We therefore transferred intact G2 nuclei into extract depleted of geminin or depleted with nonimmune antibodies, plus or minus MG132. The second extract was also supplemented with aphidicolin to prevent DNA synthesis and consequent displacement of Mcm2–7 from chromatin. Figure 4C shows that after 60 min in geminin-depleted extract, Mcm7 had been loaded onto replicated chromatin, while no detectable Mcm7 was loaded in the nonimmune-depleted extract. As expected, MG132 had no significant effect on the degree of re-licensing in this experiment. To confirm that the re-licensing observed in geminin-depleted extract can support re-replication, we performed BrdUTP density substitution. Figure 4D shows that, consistent with previous reports (Blow and Laskey, 1988; Leno et al, 1992; Coverley et al, 1993), intact G2 nuclei did not re-replicate on transfer into fresh untreated extracts. Nuclei transferred into nonimmune-depleted extracts showed a very similar profile with a small additional peak of re-replicated (HH) DNA, probably caused by a slight defect in nuclear envelope integrity caused by the depletion. In contrast, nuclei incubated in geminin-depleted extracts underwent quantitative re-replication, as evidenced by the large peak of HH DNA. As expected, the presence of MG132 in the second extract did not significantly alter the degree of re-replication.
Effect of Cdt1 expression in G2
We have shown above that geminin depletion coupled with MG132-mediated stabilisation of Cdt1 permits re-replication of DNA. However, it could be argued that although Cdt1 is the main activity stabilised by MG132 (Figure 1), it may also stabilise some other factors required for re-replication. To address this, we tested whether addition of excess Cdt1 to extract in G2 can also induce re-licensing and re-replication. Sperm nuclei were incubated in Xenopus extract, which led to efficient replication and the displacement of Mcm7 from chromatin (Figure 5A). This G2 extract was then supplemented with recombinant Cdt1 (sufficient to titrate out the geminin) and incubated for a further 30 min. Addition of Cdt1 induced Mcm7 to be reloaded onto the replicated chromatin. The levels of Mcm7 reloaded were directly dependent on the amount Cdt1 added (Figure 5B). Figure 5C shows that the G2 nuclei treated with recombinant Cdt1 were efficiently replicated when transferred to geminin-containing extract, suggesting that they had been functionally re-licensed.
Figure 5.
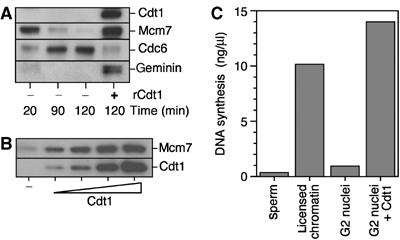
Cdt1 addition in G2 induces functional re-licensing. Sperm nuclei were incubated at 10 ng DNA/μl in metaphase-arrested extracts supplemented with CaCl2. After 90 min, extracts were optionally supplemented with recombinant Cdt1. (A) After 120 min, chromatin was isolated and immunoblotted for Cdt1, Mcm7, geminin and Cdc6. The concentration of added Cdt1 was 10 ng/μl. (B) As (A), with 0, 1.25, 2.5, 5 and 10 ng/μl Cdt1. (C) After 120 min, nuclei were isolated and transferred to interphase extract containing [α-32P]dATP and recombinant geminin. After a further 90 min incubation, DNA synthesis was assessed. As controls, naïve sperm nuclei and licensed chromatin (the 20 min sample from (A)) were incubated in the second extract. The concentration of added Cdt1 was 10 ng/μl.
We next investigated whether addition of recombinant Cdt1 to G2 extract could directly induce re-replication without nuclear transfer. Initial experiments gave variable degrees of re-replication in response to added Cdt1. In searching for the cause of this variability, we noticed that addition of Cdt1 to G2 extract induced phosphorylation of the checkpoint kinase Chk1 at Ser342 (equivalent to Ser345 in human Chk1) (Figure 6A). This site is phosphorylated by the ATM/ATR kinases in response to replication stress (Guo et al, 2000; Liu et al, 2000; Capasso et al, 2002). Consistent with this idea, the Cdt1-induced phosphorylation was abolished by caffeine, a potent inhibitor of ATM/ATR. We have recently shown that in response to treatment with aphidicolin, Xenopus extracts activate an ATR-dependent intra-S-phase checkpoint, which strongly represses replication initiation (Luciani et al, 2004). Since this checkpoint is caffeine sensitive and leads to the phosphorylation of Chk1 at Ser342, we investigated the effect of adding caffeine along with recombinant Cdt1 to G2 extracts. Figure 6B shows that when increasing amounts of recombinant Cdt1 were titrated into G2 extract (which resulted in increasing levels of Mcm7 loaded; Figure 5B), DNA synthesis first increased, then fell again. When caffeine was added with the Cdt1, however, DNA synthesis steadily increased with increasing Cdt1 concentration. The effect of caffeine on replication kinetics is shown in Figure 6C. Sperm chromatin was incubated for 90 min in interphase extract at which time S phase was complete. The addition of recombinant Cdt1 at this time induced further DNA synthesis. The subsequent addition of caffeine at 120 min caused a significant increase in the rate of DNA synthesis induced by recombinant Cdt1, without affecting the lack of DNA synthesis seen in the absence of added Cdt1. Together, these results suggest that addition of Cdt1 to G2 extract can activate a checkpoint signal that represses further initiation.
Figure 6.
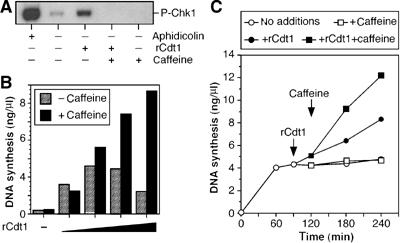
Cdt1 addition in G2 induces DNA synthesis and checkpoint activation. (A) Sperm nuclei were incubated at 10 ng DNA/μl in metaphase-arrested extracts supplemented with CaCl2. After 90 min, extracts were supplemented with 10 ng/μl recombinant Cdt1 and/or 5 mM caffeine. After a further 30 min, nuclei were isolated and immunoblotted for phospho-Chk1 (Ser342). As a positive control, 40 μM aphidicolin was added to the extracts at the start of the incubation. (B) Sperm nuclei were incubated at 3 ng DNA/μl in metaphase-arrested extracts supplemented with CaCl2. After 90 min, extracts were supplemented with [α-32P]dATP and various concentrations of recombinant Cdt1 (0, 1.25, 2.5, 5 and 10 ng/μl); at 120 min, the extract was optionally supplemented with 5 mM caffeine. At 240 min, additional DNA synthesis was assessed. (C) Sperm nuclei were incubated at 4 ng DNA/μl in metaphase-arrested extracts supplemented with [α-32P]dATP and CaCl2. After 90 min, extracts were optionally supplemented with 10 ng/μl recombinant Cdt1 (filled symbols) and, at 120 min, extracts were optionally supplemented with 5 mM caffeine (squares). Open circles show samples with no addition. At the indicated times, DNA synthesis was assessed.
To show that the extra DNA synthesis induced by recombinant Cdt1 represented re-replication, BrdUTP density substitution experiments were performed. For the experiment in Figure 7A, sperm nuclei were incubated for 90 min in extract containing [α-32P]dATP and BrdUTP; the extract was then optionally supplemented with recombinant Cdt1 and/or caffeine and the incubation continued for a further 90 min. Density fractionation showed that addition of Cdt1 induced some re-replication (HH DNA), and that the amount of re-replication was strongly enhanced by addition of caffeine. Interestingly, the combination of caffeine and Cdt1 produced a significant quantity of DNA with a density intermediate between heavy–light and heavy–heavy, as though replication forks were stalling.
Figure 7.
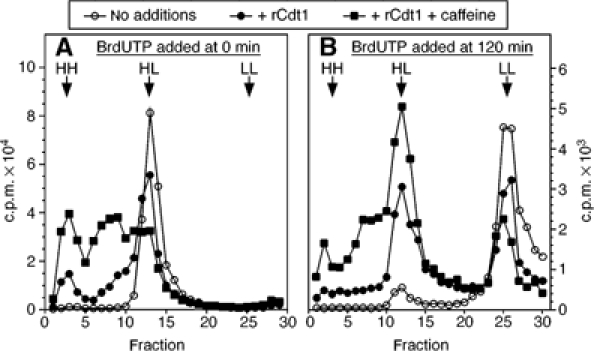
Cdt1 addition in G2 induces second- and third-round replication. (A) Sperm nuclei were incubated at 5 ng DNA/μl in metaphase-arrested extracts supplemented with BrdUTP, [α-32P]dATP and CaCl2. After 90 min, extracts were optionally supplemented with 10 ng/μl recombinant Cdt1 and 5 mM caffeine. After a further 90 min incubation, DNA was isolated and fractionated on CsCl equilibrium density gradients. (B) As (A), except that BrdUTP, Cdt1 and caffeine were all added to extract at 120 min, and the total incubation time before DNA isolation was 300 min.
We next asked whether excess Cdt1 caused any DNA to be replicated more than twice (re-re-replication), as would be expected if initiation had become completely deregulated. To do this, the experimental protocol was changed, so that sperm nuclei were first incubated for 120 min in extract containing [α-32P]dATP, to create replicated DNA with a light–light density. The extract was then supplemented with BrdUTP plus or minus recombinant Cdt1 and caffeine and the incubation continued for a further 180 min. In the absence of added Cdt1, this gave a major peak of light–light DNA, with a small heavy–light peak, which probably represents some first-round DNA synthesis occurring after 120 min. Addition of recombinant Cdt1 created a large peak of heavy–light DNA, which trailed over to the heavy–heavy position, consistent with a small amount of third-round replication. The combination of recombinant Cdt1 and caffeine significantly increased the amount of DNA with a density greater than heavy–light. This is consistent with the idea that excess Cdt1 can induce replication origins to fire multiple times, but that this causes increasing difficulties in completing each round of DNA replication.
Discussion
We show here that in Xenopus extracts, re-replication of DNA in a single cell cycle is largely prevented by downregulation of Cdt1 activity. This occurs as a consequence of cell cycle-regulated proteolysis and inhibition by geminin. When Cdt1 activity is maintained, efficient re-licensing and re-replication occurs. However, this also leads to activation of a checkpoint signal that ultimately restricts the degree of re-replication.
Cdt1 degradation in Xenopus
The degradation of Cdt1 late in the cell cycle is widespread in eukaryotes (Wohlschlegel et al, 2000, #8; Gopalakrishnan et al, 2001, #18; Nishitani et al, 2001, #21; Quinn et al, 2001, #22; Hodgson et al, 2002, #11; Li et al, 2003, #17; Zhong et al, 2003, #24). A previous study in Xenopus showed that in the absence of added DNA, Cdt1/RLF-B activity in Xenopus egg extracts declines over time, independently of CDK activity (Mahbubani et al, 1997). Since geminin is not re-activated in the absence of nuclei (Hodgson et al, 2002), this decline in activity is likely to be largely dependent on Cdt1 proteolysis. We show here that the degradation of Cdt1 during interphase plays a role in preventing re-replication of DNA in a single cell cycle. The kinetics of Cdt1 degradation, its insensitivity to the presence of DNA and its inhibition by the D-box peptide, Emi1 and roscovitine all suggest that a significant amount of this degradation occurs as a consequence of APC/C-mediated ubiquitination. In our experiments, we have used metaphase-arrested Xenopus extracts prepared from unactivated eggs, which are then released into interphase by the addition of CaCl2. We suspect that as these extracts exit metaphase, the APC/C remains active for longer than occurs in vivo, potentially overemphasising the normal role of the APC/C in Cdt1 degradation. It should also be noted that the Cdt1 degradation shown here could be an indirect consequence of APC activation. For example, it is possible that, as has been described in human cells (Ballabeni et al, 2004), Cdt1 is stabilised by interaction with geminin and becomes destabilised on exit from metaphase as a consequence of APC-dependent inactivation of geminin.
There is, however, some indication that Cdt1 degradation may be mediated by more than one pathway. First, the decline of Cdt1/RLF-B activity occurs over >30 min (Mahbubani et al, 1997). This is sufficiently slow to allow efficient origin licensing. Cdt1 can be immunoblotted on chromatin during early S phase (Maiorano et al, 2000), where it recruits geminin to the chromatin (Gillespie et al, 2001; Oehlmann et al, 2004) and is then subsequently lost. A distinct ladder suggestive of Cdt1 polyubiquitination is seen on chromatin during S phase. By this time, however, the APC/C should largely be inactive, while CDKs have been activated within the nuclei. Interestingly, the ladder of polyubiquitinated forms of Cdt1 was only observed on the chromatin but not in whole extract, suggesting that either only chromatin-bound Cdt1 is ubiquitinated or that Cdt1 on chromatin is protected from degradation.
Second, the degradation of Cdt1 on chromatin can be slowed (though not abolished) by the addition of aphidicolin (Maiorano et al, 2000), which is not consistent with an APC/C-mediated reaction but instead suggests a checkpoint- and CDK-dependent process. Indeed, two other labs have recently observed a CDK-dependent pathway for the degradation of Cdt1 during interphase in Xenopus (J Walter and H Takisawa, personal communication). CDK-dependent ubiquitination of Cdt1 by SCF resulting in Cdt1 degradation has been shown in human cells (Li et al, 2003; Liu et al, 2004; Sugimoto et al, 2004). We therefore propose that degradation of Cdt1 in Xenopus is started by the APC/C and then continued by a distinct CDK-dependent mechanism. Multiple mechanisms of Cdt1 degradation have also been proposed in Drosophila (Thomer et al, 2004).
A comprehensive model for re-replication control in Xenopus
The data presented here allow us to construct a model for how the re-replication of DNA is prevented in the early Xenopus embryo (Figure 8). Once template DNA has decondensed, four factors are essential for licensing in Xenopus: ORC, Cdc6, Cdt1 and Mcm2–7 (Gillespie and Blow, 2000; Gillespie et al, 2001). ORC remains bound to chromatin throughout interphase, and with a reduced affinity during mitosis (Rowles et al, 1996). Cdc6 binds to ORC-containing chromatin (Coleman et al, 1996) during early interphase and rebinds in S phase and G2 when the origins are unlicensed (Oehlmann et al, 2004). Mcm2–7 is potentially able to license new origins throughout interphase (Mahbubani et al, 1997). This leaves Cdt1 as the main activity whose downregulation late in the cell cycle prevents the re-licensing of replicated DNA.
Figure 8.
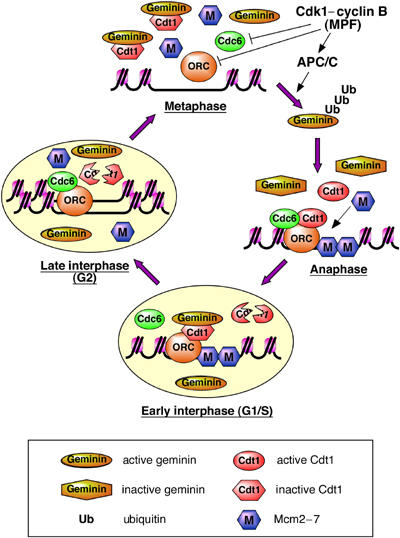
Model for re-replication control in the early Xenopus embryo. A small region of chromatin surrounding a single replication origin is shown. During metaphase, geminin is active and binds Cdt1, keeping it inactive. An excess of geminin over Cdt1 ensures complete Cdt1 inhibition. Cdk1 plays a subsidiary role by reducing the affinity of ORC and Cdc6 for the origin. On exit into anaphase,Cdk1-dependent APC/C activation occurs. The APC/C polyubiquitinates geminin, a significant proportion of which is deubiquitinated without being degraded, leaving it unable to inhibit Cdt1. Combined with the loss of Cdk1 activity during anaphase, this rapidly activates the licensing system and Mcm2–7 are loaded onto origins, displacing Cdc6. In early interphase (G1/S), chromosomal DNA is assembled into a nucleus. Geminin is imported into the nucleus and is re-activated, being recruited to chromatin with Cdt1. In addition, Cdt1 is degraded during early interphase. During S phase, initiation takes place at licensed origins, resulting in displacement of Mcm2–7 from origins, which allows Cdc6 to rebind ORC. Because of the presence of active geminin and the lack of Cdt1, replicated origins cannot be re-licensed and so DNA is not re-replicated.
During metaphase, geminin is active and inhibits Cdt1 (Wohlschlegel et al, 2000; Tada et al, 2001). This inhibition is essential for preventing licensing in metaphase, while the effect of Cdk1 in reducing the affinity of ORC and Cdc6 for chromatin provides only partial inhibition (Tada et al, 2001). In human cells, CDKs appear to play a more important role in preventing licensing during mitosis (Ballabeni et al, 2004). On exit into anaphase, geminin is ubiquitinated by the APC/C, whose activation depends on Cdk1 (McGarry and Kirschner, 1998; Li and Blow, 2004). Some of the ubiquitinated geminin is degraded, but a significant proportion is deubiquitinated, leaving the geminin unable to inhibit Cdt1 (Hodgson et al, 2002; Li and Blow, 2004). Activation of Cdt1 combined with the loss of cyclin B rapidly activates the licensing system, and Mcm2–7 is loaded onto origins. The licensing of origins reduces their affinity for Cdc6 (Oehlmann et al, 2004). After the end of mitosis, chromosomal DNA is assembled into interphase nuclei. The transport of inactive geminin into nuclei re-activates geminin (Hodgson et al, 2002). Throughout this period, Cdt1 degradation has been occurring, but there is typically enough Cdt1 remaining so that it can be observed recruiting geminin to chromatin early in S phase (Gillespie et al, 2001; Oehlmann et al, 2004). The initiation of DNA replication then occurs at licensed origins, a process that depends on prior nuclear assembly (Blow, 2001). Mcm2–7 are displaced from origins, probably moving ahead of the replication fork. The removal of Mcm2–7 from the vicinity of ORC allows Cdc6 to rebind to the origin (Oehlmann et al, 2004). Despite the presence of chromatin-bound ORC and Cdc6, and the presence of active soluble Mcm2–7, re-licensing of replicated origins does not take place because of a lack of Cdt1 activity, enforced by a combination of Cdt1 proteolysis and inhibition by geminin. Dual downregulation of Cdt1 activity can explain previous observations that removal of geminin alone does not induce significant re-replication in Xenopus (McGarry and Kirschner, 1998; McGarry, 2002).
The behaviour of the system conforms well to the original model proposing the existence of a ‘licensing factor' (Blow and Laskey, 1988). This proposal was based on the observation that transient nuclear envelope permeabilisation was required for replicated G2 nuclei to undergo a second round of DNA synthesis after transfer to fresh extract. A range of subsequent observations supported this hypothesis and led to the identification of Mcm2–7 as the replication licence (Leno et al, 1992; Blow, 1993; Coverley et al, 1993; Kubota and Takisawa, 1993; Chong et al, 1995; Kubota et al, 1995; Madine et al, 1995). The original paper (Blow and Laskey, 1988) characterised licensing factor as having four essential features: (1) it must stably bind chromatin; (2) licensing factor binding is essential for each origin to initiate replication; (3) each chromatin-bound licensing factor supports only a single initiation event, after which it is inactivated; (4) licensing factor is unable to cross into an intact nucleus in an active form. The first two features refer to the stable binding of Mcm2–7 to origins, which is essential for DNA replication. The third point is explained by Mcm2–7 being displaced from DNA as it replicates. Until the present work, the fourth point has been unexplained. We now show that nuclear envelope permeabilisation is required to block the re-activation of geminin that normally occurs when it is imported into nuclei (Hodgson et al, 2002). This also explains the previous observation that if the nuclear envelope of G2 nuclei is permeabilised and then resealed prior to addition to Xenopus egg extract, no re-replication occurs (Coverley et al, 1993).
One notable feature of this model is that CDKs do not play an essential role in preventing re-replication, as they do in yeast (Blow and Hodgson, 2002; Nishitani and Lygerou, 2002). It has previously been shown that addition of the CDK inhibitor p21Cip1 to Xenopus egg extracts in G2 does not induce re-licensing (Sun et al, 2000). This can be explained because neither geminin re-activation nor Cdt1 proteolysis is strictly dependent on interphase CDK activity (Mahbubani et al, 1997; Hodgson et al, 2002; this paper, Figure 1C). Similarly, human tissue culture cells can be induced to re-replicate by overexpressing Cdt1 and Cdc6 (Vaziri et al, 2003) or by ablating geminin (Melixetian et al, 2004; Zhu et al, 2004) without the need for downregulating CDKs. Instead, all metazoans studied so far appear to depend on downregulation of Cdt1 activity to prevent re-replication (Vaziri et al, 2003; Zhong et al, 2003; Melixetian et al, 2004; Nishitani et al, 2004; Zhu et al, 2004).
Checkpoint activation as a consequence of re-replication
When recombinant Cdt1 is added to G2 extract, a caffeine-sensitive phosphorylation of Chk1 occurs. The effect of the checkpoint is to limit the amount of re-replication, and resembles an ATR-dependent checkpoint that prevents initiation in Xenopus extract (Luciani et al, 2004). Previous work using mammalian cells has shown activation of checkpoint pathways when re-replication is induced by overexpression of Cdt1 (Vaziri et al, 2003; Nishitani et al, 2004) or by ablation of geminin (Melixetian et al, 2004; Zhu et al, 2004). Our results more resemble those of Vaziri et al, who showed that re-replication activated a p53-dependent checkpoint that inhibited further replication. In contrast, the checkpoint of Melixetian et al and Zhu et al predominantly blocked entry into mitosis, so that abrogation of the checkpoint reduced, rather than increased, the amount of DNA replication. Despite the similarity, the effect we see in Xenopus is unlikely to involve p53, since p53 exerts its major effects through modulating transcription, while the Xenopus early embryo is transcriptionally quiescent.
It is unclear what is responsible for activating this checkpoint in Xenopus. The effects are clearest when high levels of re-replication occur, possibly involving third-round (re-re-) replication. Under these conditions, density substitution experiments suggest that partially replicated DNA molecules accumulate. One possibility is that the checkpoint is activated as a consequence of forks running into the back of others: replication forks normally meet head-on during the course of termination, but should never collide with forks moving in the same direction. Another possibility is that the checkpoint is activated as a consequence of replication forks moving through DNA, which is already involved in sister chromatid cohesion. The cohesin molecule responsible for cohesion is thought to encircle the sister strands, and presumably there is a limit to how much DNA each molecule can accommodate. Xenopus egg extracts represent a powerful system for studying these potential effects of re-replication and understanding them at a biochemical level.
Materials and methods
Preparation and use of egg extracts
Metaphase-arrested and interphase low-speed Xenopus egg extracts were prepared as described (Chong et al, 1997). All extracts were supplemented with 250 μg/ml cycloheximide, 25 mM phosphocreatine and 10 μg/ml creatine phosphokinase before use, and incubations were performed at 23°C. Metaphase extracts were released into interphase by 0.3 mM CaCl2. Lysolecithin-permeabilised Xenopus sperm nuclei were prepared as described (Chong et al, 1997). DNA synthesis was assessed in extracts supplemented with [α32P]dATP by TCA precipitation as described (Chong et al, 1997). As appropriate, extracts were supplemented with 800 μM MG132, 2 mM D-box peptide (Peter et al, 2001), 1 μM Emi1, 0.5 mM roscovitine or 40 μM aphidicolin.
Immunodepletion of geminin from extract was performed essentially as described (Chong et al, 1997). Immunoblotting of the depleted extract showed no geminin. In some experiments, MG132 was included in the extract during depletion.
Recombinant protein and antibodies
Recombinant 6xHis-tagged Xenopus Cdt1 (Maiorano et al, 2000) was produced as described (Gillespie et al, 2001). Additionally, GST fusions of full-length Cdt1 (GST-wtCdt1) and a fragment lacking the first 243 amino acids Cdt1 (GST-NΔ243) were created in pGEX-2T (Pharmacia) (Ferenbach et al, submitted). Primer sequences are available on request. GST fusion proteins were solubilised from inclusion bodies and purified as described (Jares et al, 2004).
Recombinant 6xHis-tagged Xenopus gemininDEL was produced as described (McGarry and Kirschner, 1998). Antibodies against Xenopus geminin, Cdt1, Cdc6 and Mcm7 were as previously described (Tada et al, 1999; Prokhorova and Blow, 2000; Tada et al, 2001). Antibody against Xenopus cyclin B2 was a kind gift from Tim Hunt. Recombinant Xenopus Emi1 was a kind gift from Peter Jackson. Monoclonal antibody PA3C5 against nucleoplasmin (Dilworth et al, 1987) was prepared from a cell line kindly provided by Dr S Dilworth. Anti-phospho-Chk1 (Ser345) was from Cell Signalling Technology. The D-box peptide (RRTALGDVTNKVSE) (Peter et al, 2001) was a kind gift from Jim Hutchins and Paul Clarke. Immunoblots were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford, IL) and preflashed film.
Chromatin isolation
For immunoblotting experiments, 20 μl aliquots of extract were supplemented with sperm nuclei to 10 ng DNA/μl extract. After incubation, reactions were diluted in 500 μl NIBA (50 mM KCl, 50 mM Hepes–KOH pH 7.6, 5 mM MgCl2, 2 mM DTT, 0.5 mM spermidine, 0.15 mM spermine, 2.5 mM Mg-ATP, 1 μg/ml each leupeptin, pepstatin and aprotinin) supplemented with 0.1% Triton X-100 and underlayered with 100 μl of the same buffer containing 15% (w/v) sucrose. The chromatin was pelleted at 6000 g in a swinging bucket rotor at 4°C followed by SDS–PAGE and immunoblotting.
Licensing assay
Licensing assays were performed essentially as described (Li and Blow, 2004) by addition of chromatin samples to 6 μl interphase extract containing 1.5 ng/μl gemininDEL and [α-32P]dATP and incubation for a further 90 min; total DNA synthesis was measured by TCA precipitation as described above.
Density substitution
The extracts were supplemented with 400 μM BrdUTP and [α-32P]dATP. Reactions (20 μl) were stopped by dilution in 300 μl Stop N (20 mM Tris–HCl pH 8, 200 mM NaCl, 5 mM EDTA, 0.5% SDS, 1 μg/ml RNase). After 10 min at 37°C, 200 μg/ml proteinase K was added and incubated for a further 30 min. DNA was extracted with phenol/chloroform and ethanol precipitated. Nonincorporated [α-32P]dATP was removed by desalting on disposable 2.4 ml Sephadex G-50 columns. Samples were diluted in 500 μl of TE buffer (10 mM Tris–HCl, 1 mM EDTA, pH 7.5), mixed with 5.5 ml of 109% CsCl in TE and centrifuged at 35 000 r.p.m. in a 70.1 Ti rotor for 40 h at 20°C. Fractions (200 μl) were collected and radioactivity was measured by Cerenkov counting.
Nuclear transfer
Nuclear transfer experiments were performed essentially as described (Blow and Laskey, 1988). Sperm nuclei were incubated at 10 ng DNA/μl extract for 90 min in interphase extracts (20 μl). Samples were diluted in 500 μl buffer A (60 mM KCl, 15 mM Tris–HCl pH 7.4, 15 mM NaCl, 2 mM DTT, 0.5 mM spermidine, 0.15 mM spermine, 1 μg/ml leupeptin, pepstatin and aprotinin), underlayered with 100 μl of the same buffer containing 15% sucrose (w/v) and spun for 5 min at 2000 g in a swinging bucket rotor at 4°C. The supernatant was removed and the nuclear pellet resuspended in 20 μl fresh extract (depleted where appropriate) followed by incubation for a further 60 min. Optionally, after isolation, G2 nuclei were permeabilised by incubation with 100 μg/ml lysolecithin at room temperature for 10 min before addition to fresh extract.
Acknowledgments
We thank Tomo Tanaka and Anna Woodward for comments on the manuscript, Ben Hodgson for technical developments that supported this work and Peter Jackson for the gift of Emi1 protein. This work was supported by Cancer Research UK grant C303/A3135.
References
- Ballabeni A, Melixetian M, Zamponi R, Masiero L, Marinoni F, Helin K (2004) Human Geminin promotes pre-RC formation and DNA replication by stabilizing CDT1 in mitosis. EMBO J 23: 3122–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastians H, Topper LM, Gorbsky GL, Ruderman JV (1999) Cell cycle-regulated proteolysis of mitotic target proteins. Mol Biol Cell 10: 3927–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ (1993) Preventing re-replication of DNA in a single cell cycle: evidence for a replication licensing factor. J Cell Biol 122: 993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ (2001) Control of chromosomal DNA replication in the early Xenopus embryo. EMBO J 20: 3293–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Hodgson B (2002) Replication licensing—defining the proliferative state? Trends Cell Biol 12: 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Laskey RA (1988) A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature 332: 546–548 [DOI] [PubMed] [Google Scholar]
- Capasso H, Palermo C, Wan S, Rao H, John UP, O'Connell MJ, Walworth NC (2002) Phosphorylation activates Chk1 and is required for checkpoint-mediated cell cycle arrest. J Cell Sci 115: 4555–4564 [DOI] [PubMed] [Google Scholar]
- Chong JP, Mahbubani HM, Khoo CY, Blow JJ (1995) Purification of an MCM-containing complex as a component of the DNA replication licensing system. Nature 375: 418–421 [DOI] [PubMed] [Google Scholar]
- Chong JP, Thommes P, Rowles A, Mahbubani HM, Blow JJ (1997) Characterization of the Xenopus replication licensing system. Methods Enzymol 283: 549–564 [DOI] [PubMed] [Google Scholar]
- Coleman TR, Carpenter PB, Dunphy WG (1996) The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts. Cell 87: 53–63 [DOI] [PubMed] [Google Scholar]
- Coverley D, Downes CS, Romanowski P, Laskey RA (1993) Reversible effects of nuclear membrane permeabilization on DNA replication: evidence for a positive licensing factor. J Cell Biol 122: 985–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth SM, Black SJ, Laskey RA (1987) Two complexes that contain histones are required for nucleosome assembly in vitro: role of nucleoplasmin and N1 in Xenopus egg extracts. Cell 51: 1009–1018 [DOI] [PubMed] [Google Scholar]
- Gillespie PJ, Blow JJ (2000) Nucleoplasmin-mediated chromatin remodelling is required for Xenopus sperm nuclei to become licensed for DNA replication. Nucleic Acids Res 28: 472–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PJ, Li A, Blow JJ (2001) Reconstitution of licensed replication origins on Xenopus sperm nuclei using purified proteins. BMC Biochem 2: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Simancek P, Houchens C, Snaith HA, Frattini MG, Sazer S, Kelly TJ (2001) Redundant control of rereplication in fission yeast. Proc Natl Acad Sci USA 98: 13114–13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Kumagai A, Wang SX, Dunphy WG (2000) Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev 14: 2745–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson B, Li A, Tada S, Blow JJ (2002) Geminin becomes activated as an inhibitor of Cdt1/RLF-B following nuclear import. Curr Biol 12: 678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jares P, Luciani MG, Blow JJ (2004) A Xenopus Dbf4 homolog is required for Cdc7 chromatin binding and DNA replication. BMC Mol Biol 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Mimura S, Nishimoto S, Masuda T, Nojima H, Takisawa H (1997) Licensing of DNA replication by a multi-protein complex of MCM/P1 proteins in Xenopus eggs. EMBO J 16: 3320–3331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Mimura S, Nishimoto S, Takisawa H, Nojima H (1995) Identification of the yeast MCM3-related protein as a component of Xenopus DNA replication licensing factor. Cell 81: 601–609 [DOI] [PubMed] [Google Scholar]
- Kubota Y, Takisawa H (1993) Determination of initiation of DNA replication before and after nuclear formation in Xenopus egg cell free extracts. J Cell Biol 123: 1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Hong B, Choi JM, Kim Y, Watanabe S, Ishimi Y, Enomoto T, Tada S, Cho Y (2004) Structural basis for inhibition of the replication licensing factor Cdt1 by geminin. Nature 430: 913–917 [DOI] [PubMed] [Google Scholar]
- Leno GH, Downes CS, Laskey RA (1992) The nuclear membrane prevents replication of human G2 nuclei but not G1 nuclei in Xenopus egg extract. Cell 69: 151–158 [DOI] [PubMed] [Google Scholar]
- Li A, Blow JJ (2004) Non-proteolytic inactivation of geminin requires CDK-dependent ubiquitination. Nat Cell Biol 6: 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E, Li X, Yan F, Zhao Q, Wu X (2004) Cyclin-dependent kinases phosphorylate human Cdt1 and induce its degradation. J Biol Chem 279: 17283–17288 [DOI] [PubMed] [Google Scholar]
- Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, Luo G, Carattini-Rivera S, DeMayo F, Bradley A, Donehower LA, Elledge SJ (2000) Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev 14: 1448–1459 [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhao Q, Liao R, Sun P, Wu X (2003) The SCF(Skp2) ubiquitin ligase complex interacts with the human replication licensing factor Cdt1 and regulates Cdt1 degradation. J Biol Chem 278: 30854–30858 [DOI] [PubMed] [Google Scholar]
- Luciani MG, Oehlmann M, Blow JJ (2004) Characterization of a novel ATR-dependent, Chk1-independent, intra-S-phase checkpoint that suppresses initiation of replication in Xenopus. J Cell Sci 117: 6019–6030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madine MA, Khoo C-Y, Mills AD, Laskey RA (1995) MCM3 complex required for cell cycle regulation of DNA replication in vertebrate cells. Nature 375: 421–424 [DOI] [PubMed] [Google Scholar]
- Mahbubani HM, Chong JP, Chevalier S, Thömmes P, Blow JJ (1997) Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J Cell Biol 136: 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano D, Moreau J, Mechali M (2000) XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis. Nature 404: 622–625 [DOI] [PubMed] [Google Scholar]
- McGarry TJ (2002) Geminin deficiency causes a Chk1-dependent G2 arrest in Xenopus. Mol Biol Cell 13: 3662–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW (1998) Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Melixetian M, Ballabeni A, Masiero L, Gasparini P, Zamponi R, Bartek J, Lukas J, Helin K (2004) Loss of Geminin induces rereplication in the presence of functional p53. J Cell Biol 165: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylov IS, Kondo T, Jones L, Ryzhikov S, Tanaka J, Zheng J, Higa LA, Minamino N, Cooley L, Zhang H (2002) Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol Cell Biol 22: 1868–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VQ, Co C, Li JJ (2001) Cyclin-dependent kinases prevent DNA re-replication through multiple mechanisms. Nature 411: 1068–1073 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z (2002) Control of DNA replication licensing in a cell cycle. Genes Cells 7: 523–534 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z, Nishimoto T (2004) Proteolysis of DNA replication licensing factor Cdt1 in S-phase is performed independently of Geminin through its N-terminal region. J Biol Chem 279: 30807–30816 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z, Nishimoto T, Nurse P (2000) The Cdt1 protein is required to license DNA for replication in fission yeast. Nature 404: 625–628 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Taraviras S, Lygerou Z, Nishimoto T (2001) The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J Biol Chem 276: 44905–44911 [DOI] [PubMed] [Google Scholar]
- Oehlmann M, Score AJ, Blow JJ (2004) The role of Cdc6 in ensuring complete genome licensing and S phase checkpoint activation. J Cell Biol 165: 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter M, Castro A, Lorca T, Le Peuch C, Magnaghi-Jaulin L, Doree M, Labbe JC (2001) The APC is dispensable for first meiotic anaphase in Xenopus oocytes. Nat Cell Biol 3: 83–87 [DOI] [PubMed] [Google Scholar]
- Prokhorova TA, Blow JJ (2000) Sequential MCM/P1 subcomplex assembly is required to form a heterohexamer with replication licensing activity. J Biol Chem 275: 2491–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn LM, Herr A, McGarry TJ, Richardson H (2001) The Drosophila Geminin homolog: roles for Geminin in limiting DNA replication, in anaphase and in neurogenesis. Genes Dev 15: 2741–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann JD, Freed E, Hsu JY, Kramer ER, Peters JM, Jackson PK (2001) Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105: 645–655 [DOI] [PubMed] [Google Scholar]
- Rowles A, Chong JP, Brown L, Howell M, Evan GI, Blow JJ (1996) Interaction between the origin recognition complex and the replication licensing system in Xenopus. Cell 87: 287–296 [DOI] [PubMed] [Google Scholar]
- Sugimoto N, Tatsumi Y, Tsurumi T, Matsukage A, Kiyono T, Nishitani H, Fujita M (2004) Cdt1 phosphorylation by cyclin A-dependent kinases negatively regulates its function without affecting geminin binding. J Biol Chem 279: 19691–19697 [DOI] [PubMed] [Google Scholar]
- Sun W, Hola M, Pedley K, Tada S, Blow JJ, Todorov IT, Kearsey SE, Brooks RF (2000) The replication capacity of intact mammalian nuclei in Xenopus egg extracts declines with quiescence, but the residual DNA synthesis is independent of Xenopus MCM proteins. J Cell Sci 113: 683–695 [DOI] [PubMed] [Google Scholar]
- Tada S, Chong JPJ, Mahbubani HM, Blow JJ (1999) The RLF-B component of the replication licensing system is distinct from Cdc6 and functions after Cdc6 binds to chromatin. Curr Biol 9: 211–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada S, Li A, Maiorano D, Mechali M, Blow JJ (2001) Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol 3: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomer M, May NR, Aggarwal BD, Kwok G, Calvi BR (2004) Drosophila double-parked is sufficient to induce re-replication during development and is regulated by cyclin E/CDK2. Development 131: 4807–4818 [DOI] [PubMed] [Google Scholar]
- Thömmes P, Kubota Y, Takisawa H, Blow JJ (1997) The RLF-M component of the replication licensing system forms complexes containing all six MCM/P1 polypeptides. EMBO J 16: 3312–3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri C, Saxena S, Jeon Y, Lee C, Murata K, Machida Y, Wagle N, Hwang DS, Dutta A (2003) A p53-dependent checkpoint pathway prevents rereplication. Mol Cell 11: 997–1008 [DOI] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A (2000) Inhibition of eukaryotic replication by geminin binding to Cdt1. Science 290: 2309–2312 [DOI] [PubMed] [Google Scholar]
- Zhong W, Feng H, Santiago FE, Kipreos ET (2003) CUL-4 ubiquitin ligase maintains genome stability by restraining DNA-replication licensing. Nature 423: 885–889 [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen Y, Dutta A (2004) Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol Cell Biol 24: 7140–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]


