Abstract
Aluminum has no defined biological function and it is potentially involved in the pathogenesis of neurodegenerative disorders. Furthermore, the presence of fluoride causes more aluminum to accumulate in the brain, resulting in increased neuronal damage. In recent years, resveratrol through its ameliorative effects was found to be a neuroprotectant. This study reports the protective effects of resveratrol on combined aluminum and fluoride induced neuronal damage through oxidative stress in rats. Protective effects of resveratrol (30 mg/kg b.w) on markers of oxidative stress were determined in rats exposed to aluminum chloride (100 mg/kg b.w) along with sodium fluoride (10 mg/kg b.w) for 8 weeks. The results showed a statistically significant (p<0.05) increase in lipid peroxidation (LPx) as well as a significant (p<0.05) decrease in superoxide dismutase and catalase activity. Enlarged cells, neurofibrillary tangles, and vacuolar spaces showing oxidative stress in the cerebral cortex were also observed in hematoxylin and eosin stained sections in aluminum and fluoride treated rats. Administration of resveratrol along with aluminum + fluoride showed significant reversal of oxidative stress and neuronal damage in rats. Thus resveratrol potentially acts as a neuroprotectant against aluminum chloride + sodium fluoride induced neuronal damage through its anti-oxidant efficacy.
Keywords: resveratrol, aluminium, fluoride, oxidative stress, neurodegeneration
Introduction
Aluminum is the commonest metal in the earth’s crust and the third most abundant metal in the biosphere, occurring either naturally or introduced anthropogenically, and it has no defined biological and neurochemical functions. It is widely used in many industries, such as packing, electronics and constructions, to ameliorate affordability due to its good strength, light weight and conductivity. With the increasing manufacture of products comprising aluminum, workers are highly exposed to aluminum in the workplace. Its bioavailability, the ability to cross the blood-brain barrier, and the relatively slow rate of aluminum elimination from the brain leads to its accumulation in the brain (Kawahara, 2007).
Schroeder and co-workers (1997) hypothesized that fluoride and aluminum would form fluoroaluminum complexes (AlF3) in the stomach that exhibit increased transport into the bloodstream and are likely to cross the blood–brain barrier. This has implicated neurotoxicity in several neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, etc. Many epidemiological studies have reported that chronic exposure to aluminum in drinking water may represent risk factors for aging-related neuronal damage (Armstrong et al., 1996; Neri & Hewitt, 1991; Walton, 1996). Aluminum is a known toxicant producing free radicals, which results in oxidative stress, damage of dendrites and synapses, resulting in neurodegeneration (Isokawa & Levesque, 1991; Mundy et al., 1997). Aluminum-induced neuronal degeneration in rats was enhanced when the animals were fed low doses of fluoride. The presence of fluoride caused more aluminum to cross the blood-brain barrier, causing reduction of neuronal density, damage to neuronal cells, alterations in neuronal architecture and in the cerebrovasculature (Varner et al., 1998). These reports have suggested that aluminum and fluoride exposure leads to neurodegeneration. Thus aluminum and fluoride induced neurodegeneration in the brain has been used as a model to assess the protective effects of resveratrol.
Resveratrol, a polyphenolic compound (trans-3, 4′, 5-trihydroxy stilbene), is a naturally occurring phytochemical, which has been found in many plant species including grapes, herbs, peanuts and berries. This polyphenol has been suggested to offer phytoalexin in plants and as a strong antioxidant to reduce oxidative damage induced by several toxicants in animals (Frankel, 1993; Tadolini et al., 2000). Several investigations reported resveratrol as a compound with antioxidant, anti-inflammatory and chemo preventive properties (Baur and Sinclair, 2006; Fremont, 2006; Granados-Soto, 2003). These considerations led us to investigate the protective effects of resveratrol against aluminum and fluoride induced neurodegeneration. In the present study, the protective effects of resveratrol were assessed by measuring lipid peroxidation, as well as superoxide dismutase and catalase activities in the cerebral hemisphere of rats exposed to AlCl3+NaF to show its role in neurodegeneration. To our knowledge, so far no studies have been reported concerning protective effects of resveratrol against aluminum and fluoride induced neurotoxicity. The aim of this paper is to report findings of ameliorative mechanisms of resveratrol against aluminum and fluoride induced neurodegeneration.
Materials and methods
Chemicals
Aluminum chloride, sodium fluoride and resveratrol were purchased from the Sigma Aldrich Company. All other chemicals used in the experiment were of analytical grade.
Healthy Sprague Dawley rats were procured from the National Institute of Nutrition, Hyderabad, India. The animals were 6 weeks old and their body weight was in the range of 180±10 g. All experiments were performed in accordance with the ethical guidelines of the ethical committee (CPCSEA). The rats were housed in plastic cages at hygienic conditions and maintained at room temperature of 25–27 °C, with 12 hrs light/dark cycle for one week prior to onset of the experiments to acclimatize them to the laboratory conditions. They were provided with water ad libitum and standard rat chow procured from the National Institute of Nutrition, Hyderabad, India. The animals were randomized and assigned to 4 groups of 5 animals each. Group-I (Control) was given free access to diet and water. Group-II (AlCl3+NaF) received a dosage of 100 mg/kg b.w. of aluminum chloride and 10 mg/kg b.w of sodium fluoride. Group III (AlCl3+NaF+Res) received a dosage of 100 mg/kg b.w. of aluminum chloride + 10 mg/kg b.w of sodium fluoride and 30 mg/kg b.w of resveratrol. Group IV (Res) received resveratrol alone in the dosage of 30 mg/kg b.w. The resveratrol dose was standardized and based on an earlier report (Kelsey et al., 2013). All the treatments were given orally with orogastric tube. The doses were administered between 08:00–09:00 a.m. daily for 8 weeks. For the measurements of biochemical parameters and histological analysis, the animals were sacrificed by decapitation. The brains were removed immediately for the study.
Biochemical analysis in the cerebrum
In the cerebrum samples of the four groups the following markers were assayed: lipid peroxidation, superoxide dismutase and catalase activity.
Lipid peroxidation
Lipid peroxide formation was assayed in accordance with the method of Wills (1966). The results were expressed as nmol of MDA/mg protein/3 mins. MDA is the end product of peroxidized polyunsaturated fatty acids. The pink color was taken as an index of lipid peroxidation measured at the absorption maximum at 532 nm.
Superoxide dismutase
Superoxide dismutase activity was determined by the method of Kono (1978). The method is designed to observe the inhibition of oxidation rate of nitroblue tetrazolium using hydroxylamine hydrochloride as electron donor. The result is expressed in units (U/mg protein). One unit of enzyme activity is the inverse amount of enzyme which is required for 50% inhibition.
Catalase activity
Catalase activity was measured according to the method of Luck (1971). In this method, hydrogen peroxide was used as substrate for the reaction with catalase. The UV absorption of hydrogen peroxide was spectrophotometrically recorded at 240 nm. The difference in the absorbance is a measure of catalase activity. The results were expressed as μ Mol of H2O2 decomposed/min/mg protein.
Histopathological studies
The animals were sacrificed by cervical dislocation and the brains were taken out immediately and fixed in 10% formaldehyde solution. Sections of the cortex were stained with hematoxylin and eosin to visualize the general cell morphology of the brain of the four animal groups (Luna, 1968).
Statistical analysis
The results were tested for significance using one-way analysis of variance. Post-hoc tests were also performed at the 5% level of significance and are depicted in the form of bar charts, which equivalently represent the data of mean ± standard error of the mean.
Results
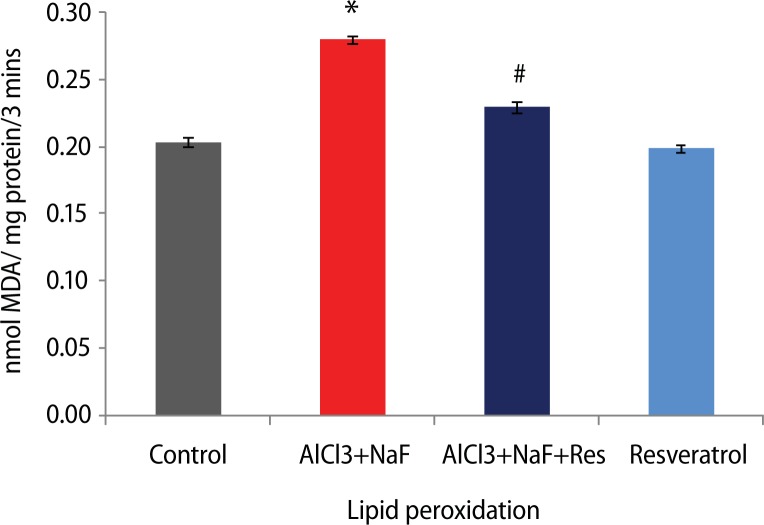
Following AlCl3+NaF treatment for 8 weeks, the malondialdehyde (MDA) levels (37.5%) were significantly elevated (p<0.05) as an index of lipid peroxidation, compared to the control group. The group treated with resveratrol along with AlCl3+NaF treatment showed a significant decrease (18%) in MDA levels compared to the AlCl3+NaF treated group. In addition, the group administered resveratrol alone showed insignificant difference compared to the control group (Figure 1).
Figure 1.
Effect of resveratrol treatment on lipid peroxidation in rats subjected to aluminum chloride along with sodium fluoride treatment. Height of histogram bar represents mean values with error bars showing SE values of 5 animals each. *p<0.05 as compared to Control group and #p<0.05 as compared to AlCl3+NaF treated group.
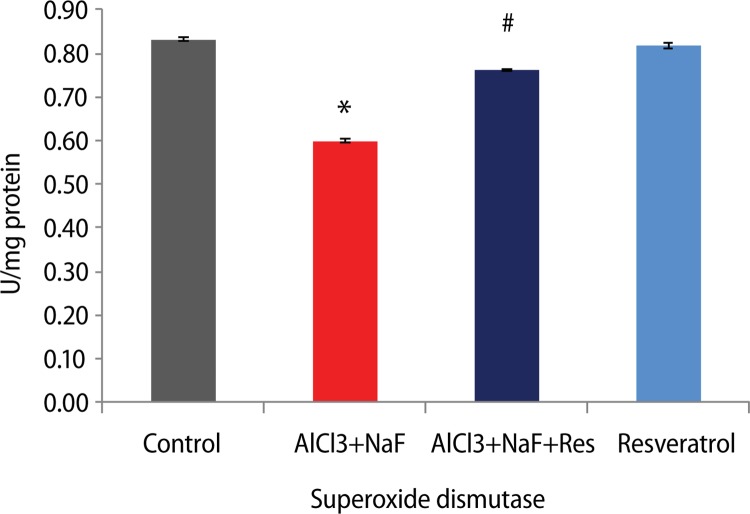
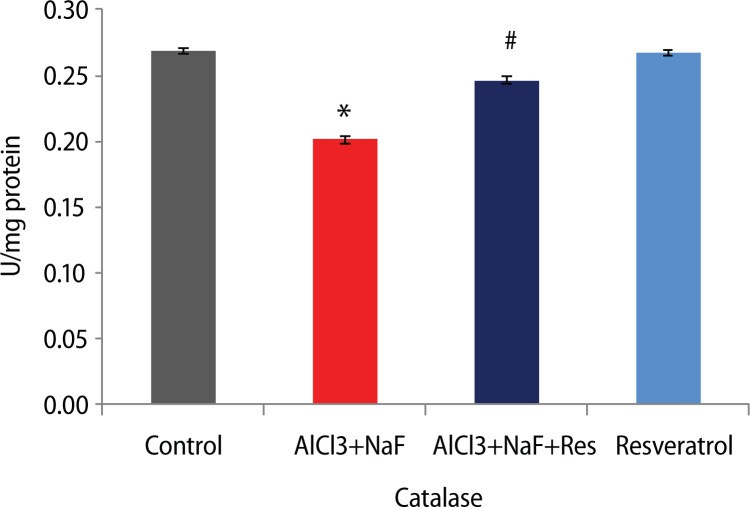
Furthermore, the activities of the enzymes superoxide dismutase and catalase were significantly decreased (p<0.05) with respective 27.9% and 25.2% in the AlCl3+ NaF treated group compared to the control group. The superoxide dismutase (27.2%) and catalase activities (22.5%) were significantly increased in the group administered resveratrol against AlCl3+NaF treatment. The group administered resveratrol alone showed insignificant difference compared to the control group (Figures 2 and 3).
Figure 2.
Effect of resveratrol treatment on superoxide dismutase activity in rats subjected to aluminum chloride along with sodium fluoride treatment. Height of histogram bar represents mean values with error bars showing SE values of 5 animals each. *p<0.05 as compared to Control group and #p<0.05 as compared to AlCl3+NaF treated group.
Figure 3.
Effect of resveratrol treatment on catalase activity in rats subjected to aluminum chloride along with sodium fluoride treatment. Height of histogram bar represents mean values with error bars showing SE values of 5 animals each. *p<0.05 as compared to Control group and #p<0.05 as compared to AlCl3+NaF treated group.
Histological analysis
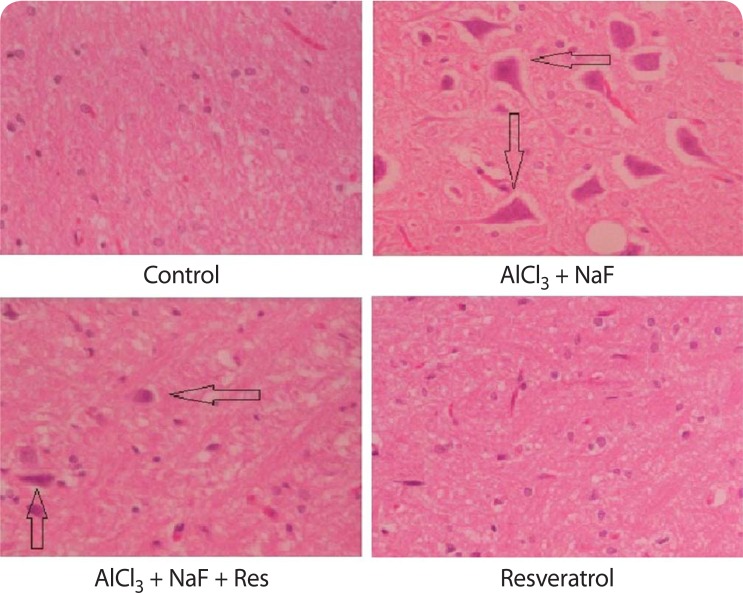
Damaged neurons, enlarged cells, and vacuolar spaces around the cells along with neuro fibrillary tangles were observed in the AlCl3+NaF treated group as compared to the control group. There was a significant decrease in alterations in the resveratrol treated group against AlCl3+NaF group of animals. In addition, there were no morphological changes in the resveratrol alone treated group as compared to the animals of the control group (Figure 4).
Figure 4.
AlCl3 + NaF treated group shows the appearance of neurofibrillary tangles, vacuolar spaces around the cells and enlarged cells as a measure of toxicity and their decrease in the AlCl3 + NaF + Resveratrol treated group. In addition, there were no morphological changes in the resveratrol alone treated group as compared to the control group.
Discussion
Though pathological effects of aluminum have been investigated extensively since decades, still the mechanism by which it causes neuronal damage has not yet been elucidated. One predictable mechanism is oxidative injury and its suggested contribution to some neuronal disorders (Nehru & Anand, 2005). The toxic effects of aluminum primarily on the cerebrum have been suggested to occur by damaging the neuronal cell membrane, which results in loss of neuronal cell membrane fluidity, an increase in permeability, differences in membrane potential and manifestations in receptor functions. There are several manifestations in which lipid peroxidation is also one of the factors causing oxidative damage and playing a vital role in the toxicity (Anane & Creppy, 2001). In the present study, elevation of lipid peroxidation in the brain was evidenced by increased production of MDA levels, which is supported by previous reports suggesting participation of free radical induced oxidative damage mediating the aluminum toxicity. The ameliorative effect on lipid peroxidation in this investigation was evidenced by the decrease of MDA levels in the group treated with resveratrol against AlCl3+NaF, as compared with the AlCl3+ NaF treated group (Figure 1). The increased lipid peroxidation is at least a part due to the decreasing superoxide dismutase activity in the brain. This results in a notable increase in the rate of peroxidation leading to membrane and neuronal damage.
SOD is the first line of defense and it dismutases peroxides into hydrogen peroxide and oxygen. Hydrogen peroxide is converted by catalase present in the peroxisomes of cells into oxygen and water and serves to remove hydrogen peroxide produced by oxidases. Moreover, the brain is prone to peroxide damage through various factors, such low antioxidant concentration as well as high oxygen turnover. These factors are suggestive of the toxic effects of aluminum on the brain. Superoxide dismutase and catalase are blockers of free radicals generated by toxicants. In the present investigation, aluminum chloride and sodium fluoride altered the cellular redox state by significantly decreasing the superoxide dismutase and catalase activities in the AlCl3+NaF treated group as compared to the control group (Figures 2 and 3). These results are in accordance with an early study showing a significant decrease in superoxide dismutase and catalase activities in the cerebrum, cerebellum and brainstem after aluminum treatment (Dua & Gill, 2001). The superoxide dismutase and catalase activities were significantly reversed in the resveratrol treated group against AlCl3+NaF treated group as compared with the AlCl3+NaF treated group, showing the protective effect of resveratrol.
In this investigation, neurofibrillary tangles, vacuolar spaces and ghost-like cells were observed in the cortex of the AlCl3+NaF group due to neuronal destruction. This finding suggests that aluminum and fluoride exerted their toxic effects through oxidative stress as a result of excitotoxicity, as compared to the control group, and the toxic effects were reversed in the resveratrol treated against aluminium and fluoride treated group (Figures 4). The present results also corroborate the thesis that both aluminum and fluoride may form complexes and accumulate in the brain, elevating free radicals and peroxidation levels and causing oxidative stress as well as prolonged neurotoxicity leading to neuronal damage. This result is in accordance with the previous report (Blaylock, 2012). The present results showed a protective effect of resveratrol when administered against AlCl3+NaF, as compared to the AlCl3+NaF treated group. The group administered resveratrol alone did not show any manifestations compared to the control group, suggesting that resveratrol does not affect healthy individuals.
Conclusion
In conclusion, this study reports toxic effects of aluminum along with fluoride in the brain as a result of free radical generation and neuronal damage. These findings were ameliorated by administration of resveratrol, playing a vital role in the cellular defense against oxidative damage by scavenging free radicals and providing protection from neuronal damage. Thus, resveratrol is suggested to be a potent neuroprotectant in preventing neuronal damage and formation of neurofibrillary tangles in the brain.
Acknowledgement
The authors would like to thank UGC, New Delhi for providing BSR-RFSMS (F.4-1/2006(BSR)/7-143/2007(BSR)) fellowship to NCR and BSR (F-19-118/2014 (BSR)) onetime grant, partial funds under DSA II- SAP (5-26/2015/DSA/SRA II) to KPR.
REFERENCES
- Anane R, Creppy EE. Lipid peroxidation as pathway of aluminium cytotoxicity in human skin fibroblast cultures: prevention by superoxide dismutase+catalase and vitamins E and C. Hum Exp Toxicol. 2001;20:477–481. doi: 10.1191/096032701682693053. [DOI] [PubMed] [Google Scholar]
- Armstrong RA, Winsper SJ, Blair JA. Aluminium and Alzheimer’s disease: review of possible pathogenic mechanisms. Dementia. 1996;7(1):1–9. doi: 10.1159/000106845. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bimla Nehru, Priya Anand. Oxidative damage following chronic aluminium exposure in adult and pup rat brains. Journal of Trace Elements in Medicine and Biology. 2005;19:203–208. doi: 10.1016/j.jtemb.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Blaylock RL. Aluminium induced immunoexcitotoxicity in neurodevelopmental and neurodegenerative disorders. Current Inorganic Chemistry. 2012;2(1):1–8. [Google Scholar]
- Dua R, Gill KD. Aluminium phospide exposure: implications on rat brain lipid Peroxidation and antioxidant defence system. Pharmacol Toxicol. 2001;89(6):315–319. doi: 10.1034/j.1600-0773.2001.d01-167.x. [DOI] [PubMed] [Google Scholar]
- Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. Lancet. 1993;341(8852):1103–4. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- Fremont L. Biological effects of resveratrol. Life Sci. 2000;66(8):663–673. doi: 10.1016/s0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Granados-Soto V. Pleiotropic effects of resveratrol. Drug News Perspect. 2003;16(5):299–307. doi: 10.1358/dnp.2003.16.5.829318. [DOI] [PubMed] [Google Scholar]
- Isokawa M, Levesque MF. Increased NMDA responses and dendritic degeneration in human epileptic hippocampal neurons in slices. Neurosci Lett. 1991;132(2):212–216. doi: 10.1016/0304-3940(91)90304-c. [DOI] [PubMed] [Google Scholar]
- Kawahara M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J of Alzheimers Dis. 2005;8(2):171–182. doi: 10.3233/jad-2005-8210. [DOI] [PubMed] [Google Scholar]
- Potter Kelsey A, Buck Amy C, Self Wade K, Callan Megan E, Sunil Smrithi, Capadona Jeffrey R. The effect of resveratrol on neurodegeneration and blood brain barrier stability surrounding intracortical microemectrodes. Biomaterials. 2013;34:7001–7015. doi: 10.1016/j.biomaterials.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186(1):189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- Luck H. Methods of enzymatic analysis. New York: Academic Press; 1971. pp. 885–93. [Google Scholar]
- Luna LG. Manual of Histological Staining Methods of the Armed Forces institute of Pathology. 3rd Edition. NY: McGraw-Hill; 1968. pp. 36–37. [Google Scholar]
- Mundy WR, Freudenrich TM, Kodavanti PR. Aluminum potentiates glutamate-induced calcium accumulation and iron-induced oxygen free radical formation in primary neuronal cultures. Molecular and Chemical Neuropathology. 1997;32(1–3):41–57. doi: 10.1007/BF02815166. [DOI] [PubMed] [Google Scholar]
- Neri LC, Hewitt D. Aluminium, Alzheimer’s disease, and drinking water. Lancet. 1991;338(8763):390. doi: 10.1016/0140-6736(91)90531-s. [DOI] [PubMed] [Google Scholar]
- Schroeder JC, Tolbert PE, Eisen EA, Monson RR, Hallock MF, Smith TJ, Woskie R, Hammond SK, Milton DK. Mortality studies of machining fluid exposure in the automobile industry. IV: A case-control study of lung cancer. Am J Ind Med. 1997;31(5):525–33. doi: 10.1002/(sici)1097-0274(199705)31:5<525::aid-ajim5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tadolini B, Juliano C, Piu L, Franconi F, Cabrini L. Resveratrol inhibition of lipid peroxidation. Free Radic Res. 2000;33(1):105–14. doi: 10.1080/10715760000300661. [DOI] [PubMed] [Google Scholar]
- Varner JA, Jensen KF, Horvath W, Isaacson RL. Chronic administration of aluminum-fluoride or sodium-fluoride to rats in drinking water: alterations in neuronal and cerebrovascular integrity. Brain Res. 1998;784(1–2):284–98. doi: 10.1016/s0006-8993(97)01336-x. [DOI] [PubMed] [Google Scholar]
- Walton JR. Amyloid, aluminium and the aetiology of Alzheimer’s disease. Med J Aust. 1996;164(6):382–383. [PubMed] [Google Scholar]
- Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99(3):667–676. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]