Abstract
Background
Human immunodeficiency virus (HIV) continues to be a leading cause of morbidity and mortality, particularly in sub‐Saharan Africa. Although antiretroviral drugs have helped to improve the quality of life and life expectancy of HIV‐positive individuals, there is still a need to explore other interventions that will help to further reduce the disease burden. One potential strategy is the use of interleukin‐2 (IL‐2) in combination with antiretroviral therapy (ART). IL‐2 is a cytokine that regulates the proliferation and differentiation of lymphocytes and may help to boost the immune system.
Objectives
To assess the effects of interleukin‐2 (IL‐2) as an adjunct to antiretroviral therapy for HIV‐positive adults.
Search methods
We searched the following sources up to 26 May 2016: the Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library; MEDLINE; Embase; the Web of Science; LILACS; the World Health Organization (WHO) International Clinical Trial Registry Platform (ICTRP); and ClinicalTrials.gov. We also checked conference abstracts, contacted experts and relevant organizations in the field, and checked the reference list of all studies identified by the above methods for any other potentially eligible studies.
Selection criteria
Randomized controlled trials (RCTs) that evaluated the effects of IL‐2 as an adjunct to ART in reducing the morbidity and mortality in HIV‐positive adults.
Data collection and analysis
Two review authors independently screened records and selected trials that met the inclusion criteria, extracted data, and assessed the risk of bias in the included trials. Where possible, we compared the effects of interventions using risk ratios (RR), and presented them with 95% confidence intervals (CI). We assessed the overall certainty of the evidence using the GRADE approach.
Main results
Following a comprehensive literature search up to 26 May 2016, we identified 25 eligible trials. The interventions involved the use of IL‐2 in combination with ART compared with ART alone. There was no difference in mortality apparent between the IL‐2 group and the ART alone group (RR 0.97, 95% CI 0.80 to 1.17; 6 trials, 6565 participants, high certainty evidence). Seventeen of 21 trials reported an increase in the CD4 cell count with the use of IL‐2 compared to control using different measures (21 trials, 7600 participants). Overall, there was little or no difference in the proportion of participants with a viral load of less than 50 cells/mL or less than 500 cells/mL by the end of the trials (RR 0.97, 95% CI 0.81 to 1.15; 5 trials, 805 participants, high certainty evidence) and (RR 0.96, 95% CI 0.82 to 1.12; 4 trials, 5929 participants, high certainty evidence) respectively. Overall there may be little or no difference in the occurrence of opportunistic infections (RR 0.79, 95% CI 0.55 to 1.13; 7 trials, 6141 participants, low certainty evidence). There was probably an increase in grade 3 or 4 adverse events (RR 1.47, 95% CI 1.10 to 1.96; 6 trials, 6291 participants, moderate certainty evidence). None of the included trials reported adherence.
Authors' conclusions
There is high certainty evidence that IL‐2 in combination with ART increases the CD4 cell count in HIV‐positive adults. However, IL‐2 does not confer any significant benefit in mortality, there is probably no difference in the incidence of opportunistic infections, and there is probably an increase in grade 3 or 4 adverse effects. Our findings do not support the use of IL‐2 as an adjunct to ART in HIV‐positive adults. Based on our findings, further trials are not justified.
23 April 2019
No update planned
Other
This is not a current research question.
Plain language summary
Interleukin‐2 as an adjunct to antiretroviral therapy for HIV‐positive adults
Why did we do this review?
HIV is still a major cause of death worldwide, particularly in Africa. HIV multiplies in the blood and damages the immune system. Therefore if HIV‐positive, one is more vulnerable to contract infections. The current drug treatment, antiretroviral therapy (ART), stops the virus from multiplying thereby allowing the body's immune system to recover. Interleukin‐ 2 (IL‐2) is a protein in the body which helps the process of multiplication of white blood cells which are the cells that fight infections. Although IL‐2 increases the amount of white cells we do not know if by increasing these we can add additional benefits to the use of ART alone. The aim of this Cochrane Review was to find out if using an extra treatment with antiretroviral therapy (ART), namely IL‐2, compared to using ART alone can reduce illness and death in HIV‐positive adults.
Key messages
We found that IL‐2 causes an increase in the CD4 immune cells (high certainty evidence). However, there is no difference in important effects such as death and other infections (high certainty evidence). There is probably an increase in side‐effects for those people using IL‐2 (moderate certainty evidence). Our findings do not support further use of IL‐2 as an add‐on treatment to ART in HIV‐positive adults.
Main results
After conducting a comprehensive search on 26 May 2016, we included 25 eligible trials conducted in six countries. There was no difference in the number of deaths between the IL‐2 group and those that got ART alone (6 trials, 665 participants, high certainty evidence). Seventeen of 21 trials reported an increase in the CD4 cell count with the use of IL‐2 compared to ART alone using different measures. Overall, there was no difference in the proportion of participants with a suppressed viral load of less than 50 cells/mL (5 trials, 805 participants, high certainty evidence) or less than 500 cells/mL by the end of the trials (4 trials, 5029 participants, high certainty evidence). Overall there may be little or no difference in the incidence of opportunistic infections (7 trials, 6141 participants, low certainty evidence). There was probably an increase in grade 3 or 4 adverse events (6 trials, 6291 participants, moderate certainty evidence). None of the included trials reported on adherence.
Summary of findings
Summary of findings for the main comparison. 'Summary of findings' table 1.
| Interleukin‐2 compared to control for HIV‐positive adults | ||||||
| Patient or population: HIV‐positive adults Settings: high‐ and middle‐income settings Intervention: interleukin‐2 (IL‐2) plus antiretroviral therapy (ART) Comparison: ART alone | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | IL‐2 | |||||
| All‐cause mortality | 60 per 1000 | 58 per 1000 (48 to 70) | RR 0.97 (0.80 to 1.17) | 6565 (6 trials) | ⊕⊕⊕⊕ high | There is little or no effect on all cause mortality |
| CD4 cell count | Tended to increase in all but one study | 7600 (21 trials) |
— | Tended to increase in all but one study | ||
| HIV RNA levels less than 50 cells/mL | 636 per 1000 | 617 per 1000 (515 to 732) | RR 0.97 (0.81 to 1.15) | 805 (5 trials) | ⊕⊕⊕⊕ high | There is little or no effect on viral suppression |
| HIV RNA levels less than 500 cells/mL | 81 per 1000 | 77 per 1000 (66 to 90) | RR 0.96 (0.82 to 1.12) | 5929 (4 trials) | ⊕⊕⊕⊕ high | |
| Opportunistic infections | 46 per 1000 | 39 per 1000 (26 to 54) | RR 0.79 (0.55 to 1.13) | 6141 (7 trials) | ⊕⊕⊝⊝ low1 | There may be little or no effect on opportunistic infections |
| Adverse events (grade 3 or 4) | 197 per 1000 | 242 per 1000 (193 to 303) | RR 1.47 (1.10 to 1.96) | 6291 (6 trials) | ⊕⊕⊕⊝ moderate2 | There is probably an increase in adverse events |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1Downgraded by 2 for imprecision due to low event rate resulting in a wide 95% CI is wide. The overall meta‐analysis remains underpowered to confidently exclude effects. 2Downgraded by 1 for imprecision.
Background
Description of the condition
Human immunodeficiency virus (HIV) continues to be a major cause of morbidity and mortality globally (UNAIDS 2016). In 2015 there were 2.1 million people newly diagnosed as HIV‐positive with almost half of those from Southern and Eastern Africa (UNAIDS 2016). In addition to the decrease in life expectancy caused by the disease, there are substantial health costs that may impact on the economy of affected countries. This has all led to strategic efforts by world leaders and researchers to discover effective treatments for the condition and thus curtail loss of life and the related social and economic burden (UNAIDS 2016).
HIV harms the body's immune system, particularly the CD4 lymphocytes. It destroys the host immune system, making it susceptible to opportunistic infections (Grimwade 2009; Harari 2004). Though various interventions have helped to improve the quality of life and life expectancy of HIV‐positive individuals, interventions are needed that will alleviate the effects of the disease by restoring the immune system (Harari 2004). For instance, following the introduction of antiretroviral therapy (ART) including at least three antiretroviral agents, the treatment of HIV infection is highly potent and fairly well tolerated but not without limitations (Nachega 2011). ART, which is currently the mainstay of treatment, inhibits viral replication and does not reconstitute the immune system directly (Blankson 2000; Piliero 2003). Many HIV‐positive adults do not achieve normal CD4 counts despite suppressing viral replication (Pett 2010).
Long‐term ART use is associated with drug‐resistant HIV strains, as well as cumulative drug‐related toxicities, including abnormalities in substrate metabolism (Piliero 2003). In addition, prolonged ART exposure may result in adherence fatigue and increased morbidity. This has encouraged the exploration of novel strategies to reduce the infection by augmenting the immune system and if possible completely reconstituting the immune system (Horn 2002). One such novel potential strategy has been the use of interleukin‐2 (IL‐2) as an adjunct with ART (Horn 2002).
Description of the intervention
IL‐2 is a cytokine that regulates the proliferation and differentiation of lymphocytes. Cytokines are immunological proteins produced by lymphocytes which work to expand the pool of immunological cells and mobilize latent reservoirs of such cells in people with HIV and other infections (Pett 2001). IL‐2 is a T‐cell growth factor produced predominantly by CD4+ T‐cells(Pett 2001). Its production is decreased in HIV‐positive participants (Abrams 2009). A synthetic version of the protein has been produced as proleukin. It is an important factor in the proliferation of CD4 T lymphocytes, which is a major target of HIV (Pett 2010). It is also useful in the differentiation of CD4 and CD8 cells, natural killer cells, and macrophages (Horn 2002). These cells are depleted in HIV‐positive participants, and therefore there has been this interest in the use of IL‐2 as an adjuvant therapy in the treatment of HIV‐positive individuals (Horn 2002). The low dose formulation of proleukin is rarely known to cause side effects and appears to be well‐tolerated (Horn 2002). Earlier studies reported that, by helping the reconstitution of the immune system, IL‐2 may help to defer the commencement of ART in certain participants by up to 48 weeks (Molina 2007). However, little is known about its interaction with ART and the potential toxicities in adults, children, and unborn babies (Horn 2002).
How the intervention might work
IL‐2 may work by increasing the CD4 cell count and therefore assisting to reconstitute the immune system, and help in the control of viral replication thereby boosting the effect of ART (Horn 2002). By priming the immune system it might help protect it from the damage caused by HIV and lead to lower susceptibility to opportunistic infections (Horn 2002).
Why it is important to do this review
If there is proven benefit of using IL‐2 as an adjunct in terms of decreased viral load and adverse effects, increased CD4 counts, and other patient‐related important outcomes, there will be value in introducing the use of IL‐2 more systematically as a treatment adjunct aiming for an overall improvement in morbidity and mortality. This review aims to summarize the available evidence from randomized controlled trials (RCTs) on the use of IL‐2 as an adjunct to ART in the treatment of HIV‐positive participants.
Objectives
To assess the effects of interleukin‐2 (IL‐2) as an adjunct to antiretroviral therapy (ART) for HIV‐positive adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
Adults who were 18 years old and above, diagnosed as seropositive for HIV on finger prick or laboratory blood testing and eligible to receive antiretroviral treatment (ART).
These included ART‐naive (no prior ART exposure) and ART‐experienced (previously treated or currently on ART) participants. The safety of interleukin‐2 (IL‐2) in children is not yet proven.
Types of interventions
IL‐2 and any combination of ART.
Variations of interest included IL‐2 co‐administered with antiretroviral monotherapy or with dual therapy or with the standard recommended three drug regimens.
We included any dose of IL‐2 for this review.
Types of outcome measures
Primary outcomes
All‐cause mortality.
Secondary outcomes
Change in CD4 cell count.
Proportion of participants with undetectable viral load at any time point after initiation of IL‐2.
Opportunistic infections.
Adherence (as measured by the trial authors).
Adverse events.
Search methods for identification of studies
Electronic searches
We formulated a comprehensive and exhaustive search strategy in order to identify all relevant studies regardless of language or publication status (published, unpublished, in press, and in progress).
Journals and trial databases
We searched the following electronic databases from 1980 up to 26 May 2016.
The Cochrane Central Register of Controlled Trials (CENTRAL), published in the Cochrane Library (Appendix 1).
MEDLINE (Appendix 2).
Embase (Appendix 3).
Along with MeSH terms and relevant keywords, we used the Cochrane Highly Sensitive Search Strategy for identifying reports of RCTs in MEDLINE (Higgins 2008a). We also searched references of included studies for other potentially relevant studies. Using a variety of relevant terms, we also searched ClinicalTrials.gov (www.clinicaltrials.gov; Appendix 4) and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (http://apps.who.int/trialsearch/) for any ongoing trials.
Searching other resources
Conference abstract databases
We planned to search Aegis archive of HIV/AIDS conference abstracts (www.aegis.org). However, this database is no longer functional and was not searched.
We did search the CROI and International AIDS Society websites for abstracts presented at conferences subsequent to those listed above using different combinations of relevant search terms, such as antiretroviral, interleukin‐2, HIV, viral load, therapy, CD4 count, and other terms in combination. We contacted experts and relevant organizations in the field to identify any other potentially eligible studies, including unpublished and ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (JO and CO) independently screened the titles and abstracts of the literature search results to identify potentially eligible studies. We resolved any discrepancies through discussion. We obtained the full‐text articles of all potentially eligible articles in order to formally assess eligibility using the prespecified eligibility criteria. If there was ambiguity we sought clarification from the study authors. We listed all excluded studies and their reasons for exclusion in a 'Characteristics of excluded studies' table. We also presented the study selection process in a PRISMA diagram.
Data extraction and management
Two review authors (JO and CO) independently extracted data from the included trials using a detailed data extraction form. We extracted the following information.
Study details: citation, start and end dates, location, study design, and details.
Participant details: study population eligibility (inclusion and exclusion) criteria, ages, population size, and attrition rate.
Details about the interventions: dose, duration of treatment, concomitant antiretroviral treatment (ART) regimens.
Details of the outcomes: CD4 cell count, viral load, death, adverse effects, and adherence.
For each dichotomous outcome, we extracted the number of participants experiencing the event and the number of participants in each treatment group. For each continuous outcome we extracted the mean or geometric mean values and standard deviations (SDs) (or information to estimate the SDs) for each treatment group, together with the numbers of participants in each group. We also extracted the median and range values if these were reported in place of mean and SDs values.
Assessment of risk of bias in included studies
Two review authors (JO and CO) performed the 'Risk of bias' assessments independently using the Cochrane 'Risk of bias' assessment tool (Higgins 2008b). The Cochrane approach assesses risk of bias in individual studies across the following six domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other potential biases.
We resolved any differences in opinion through discussion. We presented the 'Risk of bias' assessments for individual trials in the 'Risk of bias' tables, and also in a 'Risk of bias' summary and 'Risk of bias' graph.
Measures of treatment effect
For dichotomous outcomes, we used risk ratios (RRs) to measure treatment effect. For continuous outcomes, we presented the mean or median and SD values or ranges. We presented RRs and mean differences with 95% confidence intervals (CIs).
Unit of analysis issues
All included trials were RCTs and we analysed the data at the level of the individual.
Dealing with missing data
We did not apply any imputation measures for missing data as there were no missing data. we planned to contact authors for missing data, but this was not required.
Assessment of heterogeneity
We assessed statistical heterogeneity by visually inspecting the forest plots to detect overlapping confidence intervals, applying the Chi2 test (P value < 0.10 considered statistically significant), and also by using the I2 test statistic to evaluate the degree of heterogeneity.
Assessment of reporting biases
Funnel plots describe the relationship between the standard error and the effect size and provide a graphic display of potential reporting bias. We had planned to evaluate reporting bias by assessing the symmetry of a funnel plot. However, as the recommended 10 study minimum was not met for any of the outcomes, we did not proceed with the funnel plot assessment.
Data synthesis
We analysed data using Review Manager 5 (RevMan 5) software (Review Manager 5), and conducted meta‐analysis using the random‐effects model. We assessed the certainty of the evidence across each outcome measure by using the GRADE approach. The certainty rating across studies has four levels: high, moderate, low, or very low certainty but can be downgraded after assessment of five criteria: risk of bias, consistency, indirectness, imprecision, and publication bias. Similarly, observational studies are initially categorized as low certainty and can be downgraded by these same criteria. In exceptional circumstances they may be upgraded by three further criteria: large effect size, all plausible confounders would act to reduce the effect size, and evidence of a dose‐response effect (Guyatt 2008)
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis based on whether the participants were ART experienced or ART naive.
Sensitivity analysis
Several studies had unclear risk of bias due to unclear reporting on allocation concealment, but this was not adequate to prompt a sensitivity analysis based on the trial quality.
Results
Description of studies
Results of the search
We performed electronic literature searches up to 26 May 2016. We identified a total of 1007 records, which we screened by title/abstract. We identified 35 potentially eligible studies and obtained the full‐text articles of these studies. We excluded 10 studies, which we listed along with their reasons for exclusion in the 'Characteristics of excluded studies' table. Twenty‐five trials met the inclusion criteria of the review. We have presented the study selection process in a PRISMA flow diagram (Figure 1).
1.
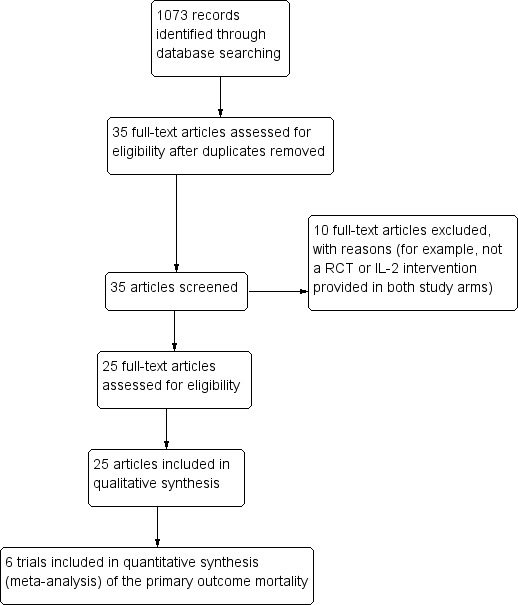
Study flow diagram
Included studies
See the 'Characteristics of included studies' and Table 2, which further describe the populations and interventions in the included trials.
1. Details of the interleukin‐2 intervention regimen.
| Number | Trial ID | Follow‐up duration | Dosing regimen for interleukin‐2 (IL‐2) | Comparisons | Outcomes | ART experienced or naive |
| 1 | Abrams 2002 | 16 months | Dose: 2 doses (4.5 and 7.5 miu) Route: subcutaneous Duration: twice daily for 5 days every 8 weeks |
ART not specified | Viral load CD4 cell count |
ART experienced |
| 2 | Abrams 2009b | 7 years | Dose: 4.5 miu Route: subcutaneous Duration: twice daily, 6 cycles |
ART not specified | Opportunistic infections Death from any cause Adverse events |
Not specified |
| 3 | Abrams 2009a | 7 years | Dose: 7.5 miu Route: subcutaneous Duration: twice daily, 3 cycles |
ART not specified | Opportunistic infections Death from any cause Adverse events |
Not specified |
| 4 | Amendola 2000 | 28 weeks | Dose: 1 miu Route: subcutaneous Duration: daily for 5 days/week every alternate week for 3 months |
Indinavir, stavudine, and lamivudine | CD4 cell count Viral load |
ART naive |
| 5 | de Boer 2003 | 12 months | Dose: 12 miu Route: intravenous Duration: for 3, 4, or 5 days every 8 weeks for 6 cycles |
ART not specified | CD4 cell count Viral load Adverse events and serious adverse events AIDS defining complex |
ART experienced |
| 6 | Caggiari 2001 | 12 months | Dose: 6 miu Route: subcutaneous Duration: from days 1 to 5 and days 8 to 12 of a 28‐day cycle for 6 cycles |
2 nucleoside reverse transcriptase inhibitors (NRTIs) or 2 NRTIs and indinavir | CD4 cell count Viral load |
ART naive |
| 7 | Carr 1998 | 12 months | Dose: 1 miu Route: subcutaneous and intravenous Duration: Group A: 12 miu daily for 5 days every 8 weeks (27 participants) Group B: 1 miu per cycle in equal divided doses in day 1 and 3 every 8 weeks (58 participants) |
Zidovudine + didanosine + zalcitabine | CD4 cell count Adverse events Viral load Opportunistic infections |
ART experienced |
| 8 | Davey 2000 | 48 weeks | Dose: 7.5 miu Route: subcutaneous Duration: 6 cycles every 12 hours for 5 days every 8 weeks |
ART not specified | CD4 cell count Viral load Adverse events |
ART experienced |
| 9 | Dybul 2002 | 12 months | Dose: 7.5 miu Route: subcutaneous Duration: 3 cycles for 5 days every 8 week |
ART not specified | CD4 cell count Viral load |
Not specified |
| 10 | Hengge 1998 | 12 months | Dose: 9.6 miu Route: subcutaneous Duration: 5 cycles were given. One cycle was given every 6 weeks over a period of 52 weeks. Treatment group A : subcutaneous administered daily in cycles consisting of 5 days. Treatment group B: subcutaneous administered at a dose of 9.6 miu daily whenever CD4 counts dropped to below 1.25 fold of individual's baseline value |
Saquinavir, lamivudine, and zidovudine | CD4 cell count Viral load Opportunistic infections |
ART experienced |
| 11 | Katlama 2002 | 24 weeks with outcomes measured at weeks 1, 6, 12, 18, and 24 | Dose: 4.5 miu Route: subcutaneous Duration: every 6 weeks for 4 cycles, every 12 hours for 5 days |
2 nucleoside analogues and one PI | CD4 cell count Viral load Adverse events ART experienced |
Not specified |
| 12 | Kelleher 1998 | 48 weeks | Dose: 12 miu Route: intravenous Duration: Group A: 12.6 miu as continuous intravenous infusions for 5 days every 8 weeks for 6 cycles. Group B (IL‐2 linked to polyethylene glycol plus ART): subcutaneous injections on days 1 and 3 of each 8‐week cycles |
ART included nucleoside analogues such as lamivudine | CD4 cell count Viral load |
ART experienced |
| 13 | Kovacs 1996 | 14 months | Dose: 18 miu Route: intravenous Duration: daily for 5 days every other month for 6 cycles from month 0 to 10 |
ART included didanosine, zidovudine, zalcitabine, or stavudine | CD4 cell count Plasma HIV RNA |
Not specified |
| 14 | Lalezari 2000 | 6 months | Dose: 1.2 miu, and then increased by 0.3 miu every 2 weeks for 6 months until a participant experienced grade 2 or greater toxicity. Route: subcutaneous Duration: once daily for 2 weeks |
ART not specified | CD4 cell count Viral load Adverse events |
ART experienced |
| 15 | Levy 1999 | 14 months | Dose: 12 miu and 3 miu Route: 12 miu intravenous and 3 miu subcutaneous intravenously (12 miu/day, N = 22) or subcutaneously (3 miu/m² twice daily, N = 24) for 5 days, or 2 miu/m² intravenous bolus, N = 22) administered every 2 months from week 2 to week 50 (7 cycles). |
Zidovudine (600 mg/day) plus didanosine (400 mg/day) | CD4 cell count Viral load Adverse events |
ART naive |
| 16 | Levy 2003 | 18 months | Dose: 5 miu Route: subcutaneous Duration: twice daily for a 5 day cycle given every 4 weeks for the first 3 cycles and then subsequently every 8 weeks for the next 7 cycles |
ART included lamivudine (300 mg/day), stavudine (60 to 80mg/day) and indinavir (2400 mg/day) | CD4 cell counts Viral load AIDS defining events |
ART naive or naive to PIs alone |
| 17 | Losso 2000 | 24 weeks | Dose: escalating doses of 1.5 miu, 4.5 miu, 7.5 miu Route: subcutaneous Duration: twice daily for 5 consecutive days every 8 weeks |
ART not specified | CD4 cell counts . Viral load |
Both naive and experienced participants were included in the study. |
| 18 | Marchetti 2002 | 48 weeks | Dose: 3 miu Route: subcutaneous Duration: administered as a single subcutaneous injection at days 1 to 5 and 8 to 12 of a 4‐week cycle, for a total of 3 cycles |
ART was either 2 nucleoside reverse transcriptase inhibitor and one PI or at least one non nucleoside reverse transcriptase inhibitor | CD4 cell count Viral load Adverse events |
ART experienced |
| 19 | Marchetti 2004 | 48 weeks | Dose: 3 miu Route: subcutaneous Duration: administered at day 1 to 5 and 8 to 12 for 10 weeks |
ART not specified | CD4 cell count | ART experienced |
| 20 | Mitsuyasu 2007 | 84 weeks | Dose: Group A 9 miu and Group B 7.5miu Route: intravenous and subcutaneous Duration: Group A: intravenous infusions 5 days every 8 weeks. Group B: subcutaneous injections 7.5 miu twice daily for 5 days every 8 weeks |
Received ART alone, 2 nucleosides and a PI | CD4 cell count Viral load |
Not specified |
| 21 | Ruxrungtham 2000 | 24 weeks | Dose: Group A 1.5 miu, Group B 4.5 miu, and Group C 7.5 miu Route: subcutaneous Duration: twice daily for 5 days, every 8 weeks for three cycles 8‐weekly |
ART not specified | CD4 cell count Viral load |
ART experienced |
| 22 | Stellbrink 2002 | 601 days | Dose: 9 miu Route: subcutaneous Duration: once daily (with an option to switch to 4.5 miu twice daily) for 5 consecutive days per cycle administered at 6‐weekly intervals |
ART consisting of stavudine 30 to 40 mg twice daily, and lamivudine 150 mg twice daily, nelfinavir 750 mg 3 times daily and saquinavir 600 mg 3 times daily. | CD4 cell count Viral load |
ART naive |
| 23 | Tambussi 2001 | 12 months | Dose: 3 regimens of IL‐2 Route: intravenous and subcutaneous Duration:differed by group see details below Group A: 12 miu by continuous intravenous infusion followed by subcutaneous 7.5 miu twice a day for 5 days every 8 weeks for the remaining 4 cycles. Group B: subcutaneous 7.5 miu twice a day for 5 days every 8 weeks for 6 cycles. Group C: subcutaneous 3 miu twice a day every 4 weeks |
2 NRTIs and saquinavir | CD4 cell count Viral load |
ART experienced |
| 24 | Tavel 2003 | 12 months | Dose: 7.5 miu Route: subcutaneous Duration: Group A: 7.5 miu twice daily for 5 days versus placebo plus ART. Group B: 7.5 miu twice a day for 5 days |
Nucleosides analogue reverse transcriptase inhibitor and either a non‐nucleosides analogue reverse transcriptase inhibitor or PI | CD4 cell count Viral load |
ART experienced |
| 25 | Vogler 2004 | 24 weeks | Dose: 1 miu Route: subcutaneous Duration: once daily |
2 nucleoside reverse transcriptase inhibitors | CD4 cell count Viral load |
ART experienced |
Abbreviations: ART antiretroviral therapy; IL‐2 Interleukin 2; NRTI nucleoside reverse transcriptase inhibitors; PI protease inhibitor
Study design and setting
We included 25 parallel‐design RCTs in the review (Abrams 2002; Abrams 2009a; Abrams 2009b; Amendola 2000; Caggiari 2001; Carr 1998; Davey 2000; de Boer 2003; Dybul 2002; Hengge 1998; Katlama 2002; Kelleher 1998; Kovacs 1996; Lalezari 2000; Levy 1999; Levy 2003; Losso 2000; Marchetti 2002; Marchetti 2004; Mitsuyasu 2007; Ruxrungtham 2000; Stellbrink 2002; Tambussi 2001; Tavel 2003; Vogler 2004).
Eleven trials were conducted in academic centres in the USA (Abrams 2002; Davey 2000; Dybul 2002; de Boer 2003; Abrams 2009a; Abrams 2009b; Kovacs 1996; Lalezari 2000; Mitsuyasu 2007; Tavel 2003; Vogler 2004). The other 14 included trials were conducted in Argentina (Losso 2000), France (Katlama 2002; Levy 1999; Levy 2003), Italy (Amendola 2000; Caggiari 2001; Marchetti 2002; Marchetti 2004; Tambussi 2001), Australia (Carr 1998; Kelleher 1998), Germany (Hengge 1998; Stellbrink 2002), and Thailand (Ruxrungtham 2000).
Participants
All participants were HIV‐positive adults either ART experienced or who were commenced on ART during the trial, with CD4 cell counts of at least 50 cells/mm³. The number of participants per trial ranged from nine participants (Dybul 2002), to 4111 participants (Abrams 2009a).
Interventions
In all included trials, participants in the intervention group received IL‐2 and ART, while those in the control group received ART alone. The dose of IL‐2 and the ART regimen varied across the included trials. Some trials compared doses of either 4.5 miu of IL‐2 , 7.5 miu of IL‐2 with ART, or different subgroups of both doses with the control group (Abrams 2002; Abrams 2009a; Abrams 2009b; Davey 2000). Some trials had three trial arms that compared different routes of administration, including subcutaneous versus intravenous administration with the control group (ART alone) (Carr 1998; Mitsuyasu 2007; Tambussi 2001). Other included trials had three trial arms that compared IL‐2, a control group, and different modified forms of IL‐2, such as polyethylene glycol (PEG) modified IL‐2 (Carr 1998; Kelleher 1998; Levy 1999), and granulocyte stimulating factor‐modified IL‐2 (Amendola 2000), and prednisone‐modified IL‐2 (Vogler 2004).
Outcomes
Of the outcomes of interest in this Cochrane Review, the included trials reported the following outcomes: all‐cause mortality, change in CD4 cell, viral load, opportunistic infections, and adverse effects. However, none of the included trials reported on adherence.
Excluded studies
After considering the full‐text articles, we excluded 10 potentially eligible studies that did not meet our inclusion criteria. We have provided the reasons for excluding these trials in the 'Characteristics of excluded studies' table.
Risk of bias in included studies
We have provided a graphical summary of the 'Risk of bias' assessment results (Figure 2; Figure 3).
2.
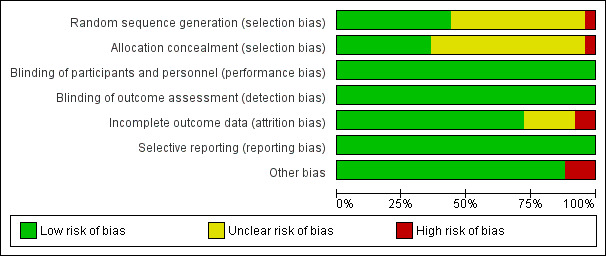
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included trials
3.
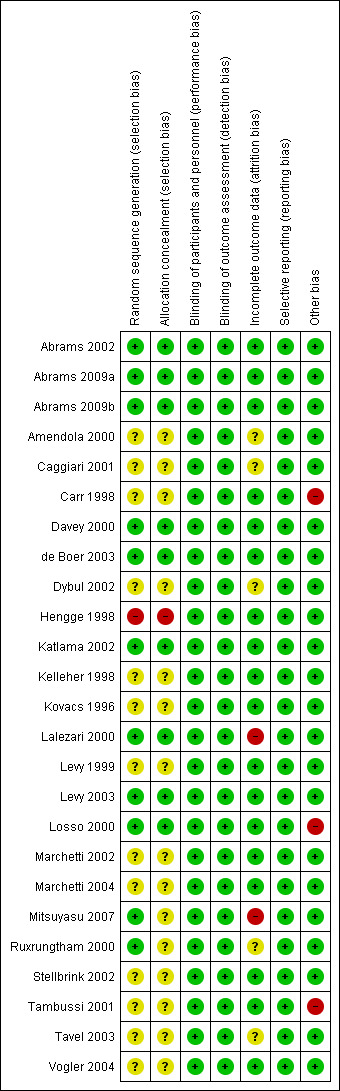
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included trial
Allocation
Random sequence generation
There was adequate sequence generation in 11 of the 25 included trials (Abrams 2002; Abrams 2009a; Abrams 2009b; Davey 2000; de Boer 2003; Katlama 2002; Lalezari 2000; Levy 2003; Losso 2000; Mitsuyasu 2007; Ruxrungtham 2000). There was high risk of selection bias in Hengge 1998. The remaining 13 included trials poorly reported the method of sequence generation.
Allocation concealment
More than half of included studies did not report allocation concealment clearly and were judged as having unclear risk of bias. One study, Hengge 1998, had high risk of allocation concealment bias due to the manner in which participant selection was conducted. .
Blinding
The included trials were open label trials with no blinding of participants. However, all of the reported outcome measures are objective. Therefore we judged each of the included trials as at low risk of bias regarding blinding.
Incomplete outcome data
We considered the following trials to have a low risk of attrition bias with low or minimal loss to follow‐up: Abrams 2002; Abrams 2009a; Abrams 2009b; Carr 1998; Davey 2000; de Boer 2003; Hengge 1998; Katlama 2002; Kelleher 1998; Kovacs 1996; Levy 1999; Levy 2003; Losso 2000; Marchetti 2002; Marchetti 2004; Stellbrink 2002; Tambussi 2001; and Vogler 2004. There was high risk of attrition bias in Lalezari 2000 and Mitsuyasu 2007. Five trials had unclear risk of attrition bias (Amendola 2000; Caggiari 2001; Dybul 2002; Ruxrungtham 2000; Tavel 2003).
Selective reporting
All included trials were at low risk of selective reporting bias. The trials reported all outcomes that they described in the methods in the results.
Other potential sources of bias
We identified other potential sources of bias in the following three trials (Carr 1998; Losso 2000; Tambussi 2001). In Carr 1998, there was potential for both detection bias or performance bias due to the fact that the IL‐2 group were hospitalized for five to six days longer than the control group. However, as the outcomes reported are considered objective (i.e. CD4 count and viral load) the potential risk is probably low. Losso 2000 had more monitoring in the IL‐2 group than in the control group, which caused a potential for detection bias and performance bias since some adverse effects could be subjective. There was a high risk of performance bias in Tambussi 2001 due to differential treatment. Participants who were randomized to the continuous intravenous high dose and subcutaneous high dose groups received the first two cycles of IL‐2 as inpatients and the following cycles on an outpatient basis, whereas participants in the low dose and control groups were followed up as outpatients from the beginning of the trial.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
All‐cause mortality
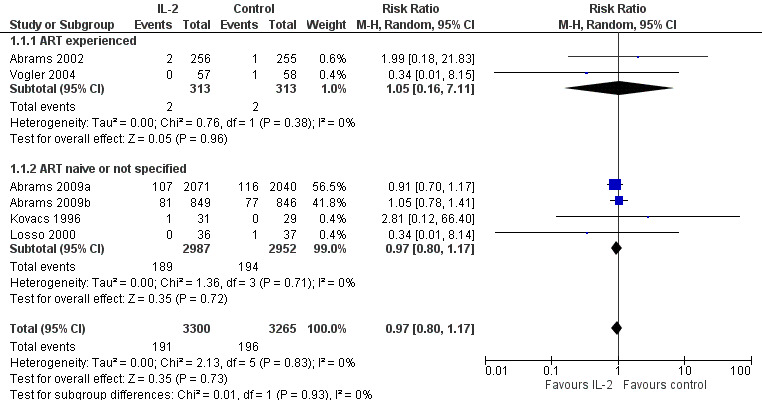
Eight trials reported on all‐cause mortality (Abrams 2002; Abrams 2009a; Abrams 2009b; Kovacs 1996; Levy 1999; Losso 2000; Mitsuyasu 2007; Vogler 2004). Trials reported mortality at six months (Vogler 2004), 12 months (Abrams 2002), 13 months (Levy 1999), 14 months (Kovacs 1996), 20 months (Mitsuyasu 2007), and seven years (Abrams 2009b; Abrams 2009a). Levy 1999 and Mitsuyasu 2007 had more than two trial arms, which we did not include in the pooled analysis. Therefore, we pooled results from six trials (Abrams 2002; Abrams 2009a; Abrams 2009b; Kovacs 1996; Losso 2000; Vogler 2004) (risk ratio (RR) 0.97, 95% confidence interval (CI) 0.80 to 1.17; 6 trials, 6565 participants, high certainty evidence; Analysis 1.1; Figure 4). There was no significant difference in the test for subgroup differences looking at ART experienced participants and others (ART naive or experienced or unclear ART status). We also did not find any significant subgroup differences with trials that reported the outcome at seven years and those that reported the outcome at less than 24 months.
1.1. Analysis.
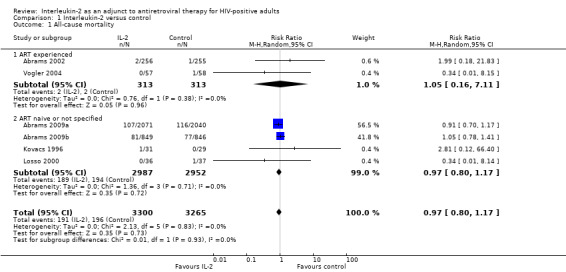
Comparison 1 Interleukin‐2 versus control, Outcome 1 All‐cause mortality.
4.
Secondary outcomes
Change in CD4 cell count
Twenty‐one trials reported on change in CD4 cell count: (Abrams 2002; Abrams 2009a; Abrams 2009b; Amendola 2000; Carr 1998; Davey 2000; de Boer 2003; Dybul 2002; Hengge 1998; Katlama 2002; Kovacs 1996; Lalezari 2000; Levy 1999; Levy 2003; Losso 2000; Marchetti 2002; Mitsuyasu 2007; Tambussi 2001; Tavel 2003; Ruxrungtham 2000; Vogler 2004).
We did not pool the results because the included trials reported either means or median values differently (see Table 3 which describes the different reporting on CD4 count changes by the included trials).
2. Effect of intervention: change in CD4 count.
| Increase in CD4 cell count with statistically significant difference | |
|
Abrams 2002 (at 12 months follow‐up) n = 511 |
The average difference change in CD4 cell count between the IL‐2 group and control group was 217.1 cells/mm³ (95% CI 188.6 to 245.5; P < 0.001) measured at 12 months. |
|
Carr 1998 (at 12 months follow‐up) n = 115 |
Median CD4 cell count increases of 359 and 44 cells/mm³ and a decline of 46 cells/mm³ in the cyclical continuous intravenous IL‐2, subcutaneous IL‐2, and ART alone group, respectively, over 12 months (P < 0.0001 for each intergroup comparison). |
|
Davey 2000 (at 12 months follow‐up) n = 82 |
The median increase in CD4 count at 12 months was 279 cells/mm³ in the IL‐2 group compared with 50 cells/mm³ in the control (P < 0.001). |
|
de Boer 2003 n = 81 |
The mean per cent increase in CD4 cell counts was 24.5% for IL‐2 recipients compared to a mean per cent decrease of 30.5% for control participants (P < 0.005). |
|
Hengge 1998 (at 12 months follow‐up) n = 64 |
The median CD4 cell counts increased from 363 to 485 (+ 33.6% standard deviation) in the IL‐2 group Group A (P < 0.01) and from 358 to 462 (+ 29.1%) in Group B (P < 0.01) and from 350 to 375 (+ 6.9% in the control group (not significant), respectively. |
|
Katlama 2002 (at 24 weeks, that is 6 months follow‐up) n = 72 |
The median increase in CD4 cells at week 24 was significantly higher in the IL‐2 group than in the control group (65 versus 18 cells/mm³; P < 0.0001). |
|
Kovacs 1996 (at 12 months follow‐up) n = 60 |
There was an increase in the mean (± SE) CD4 count from 428 ± 25 cells/mm³ at baseline to 916 ± 128 in the IL‐2 group, compared to a decreased from 406 ± 29 cells/mm3 to 349 ± 41 cells/mm³ in the control group (P < 0.001). |
|
Lalezari 2000 (at 26 weeks follow‐up) n = 115 |
The percentage increase in CD4 count from baseline of 3.59% in the IL‐2 group compared to 1.33% in the control group (P < 0.001). |
|
Levy 1999 (at 56 weeks follow‐up) n = 94 |
The median increase in CD4 count from baseline at 56 weeks was 564 cells/mm³ (P > 0.0001), 105 cells/mm³ (P = 0.58), and 676 cells/mm³ (P = 0.0002) in the subcutaneous (SC), polyethylene glycol modified, and intravenous (IV) IL‐2 group respectively, compared to 55 cells/mm³ in the control group |
|
Levy 2003 (at 74 weeks follow‐up) n = 118 |
The median increase in CD4 count from baseline at week was 865 cells/mm³ in the IL‐2 group compared to 262 cells/mm³ in the control group (P < 0.00001). |
|
Losso 2000 (at 24 weeks follow‐up) n = 73 |
The mean increase in CD4 count from baseline at week 24 of 27 cells/mm³ (P = 0.105), 105 cells/mm³ (P = 0.006), and 492 cells/mm³ (P < 0.001) in the 1.5, 4.5, and 7.5 miu dose groups of IL‐2. Overall 14 out of 36 (41%) of the IL‐2 group and 3 out of 37 (8%) of the controls had a magnitude increase of ≥ 1000 cells/mm³. |
|
Marchetti 2002 (48 weeks follow‐up) n = 22 |
IL‐2 treated participants had mean absolute CD4 T cell counts (S.E) significantly increase at the end of the IL‐2 treatment (week 48) from 147 (18) cells/mm³ at baseline to 298 (43.3) cells/mm³ (P=0001). The control participants also had a significant increase was observed 16 weeks 228 (29) cells/mm³ (P = 0.002). |
|
Mitsuyasu 2007 (at 48 weeks follow‐up) n = 159 |
Reported median increases of CD4 cell count were 459, 312, and 102 cells/mm³ in the intravenous, SC Il‐2, and control groups respectively at 48 weeks (P < 0.001 for both). |
|
Tavel 2003 (at 12 months follow‐up) n = 19 |
Reported a mean increase in CD4 count from baseline of 452 cells/mm3 in the IL‐2 group compared to 135 cells/mm3 in the control group (P < 0.05). |
|
Ruxrungtham 2000 n = 82 |
Reported an increase in the time weighted mean CD4 cell count 252 x 106 cells/mm³ over 24 weeks for the overall scIL‐2 group compared with 42 x 106 cell/mm³. |
| Increase in CD4 cell count but statistical significance not reported in the trials | |
|
Abrams 2009a (at 12 months follow‐up) (at 6 years follow‐up) n = 4111 |
Six trials measured at 1 year; median CD4 increase of 206 versus 21 cells/mm³ in the IL‐2 group versus the control group and reported an average median increase of 109 more in the IL‐2 group more than the control group over the entire 7 years (95% CI 40 to 60 over 6 years) |
|
Abrams 2009b (at 12 months follow‐up) n = 1695 |
Six trials measured at 1 year; median CD4 increase of 131 versus 32 cells/mm³ in the IL‐2 group versus the control group over 12 months and reported an average median increase of 53 more in the IL‐2 group more than the control group over the entire 7 years (95% CI 40 to 60 over 6 years) |
|
Dybul 2002 (at 12 months follow‐up) n = 9 |
Four participants treated with HAART plus 3 cycles of intermittent IL‐2 had an increase in median absolute CD4+ T cell count from 529 cells/mm³ (range: 502 to 738 cells/mm³) at enrolment to 1995 cells/mm³ (range: 1112 to 3064 cells/mm³; 268% increase) after 12 months of treatment (Figure 1A). Five participants treated with HAART alone had an increase in median CD4+ T cell count from 580 cells/mm³ (range: 416 to 662 cells) at enrolment to 712 cells/mm³ (range: 667 to 1160 cells/mm³; 52% increase) after 12 months of treatment |
| No significant increase in CD4 cell count | |
|
Vogler 2004 (at 24 weeks) n = 115 |
Mean change in CD4 count in the IL‐2 group and the control group was 40 and −1 respectively |
|
Tambussi 2001 n = 61 |
Reports that there was a progressive increase in circulating CD4 cells, determined at the beginning of each IL‐2 cycle, was observed in all participants receiving ART plus IL‐2, in comparison with those receiving ART alone but gave the values for the within subgroup variation |
|
Amendola 2000 (at 6 months follow‐up) n = 22 |
No significant difference between changes in CD4 counts in both groups |
Abbreviations: ART: antiretroviral therapy; ESPIRIT: Evaluation of Subcutaneous Proleukin in a Randomised International Trial; IL‐2: interleukin‐2; SILICAAT: subcutaneous recombinant human interleukin‐2 in HIV‐infected patients low CD4 counts under active antiretroviral therapy
Significant increase in CD4 cell count with IL‐2
Fifteen trials reported a significant increase in CD4 cell count in the group assigned to IL‐2 treatment (Abrams 2002; Carr 1998; Davey 2000; de Boer 2003; Hengge 1998; Katlama 2002; Kovacs 1996; Lalezari 2000; Levy 1999; Levy 2003; Losso 2000; Marchetti 2002; Mitsuyasu 2007; Tavel 2003; Ruxrungtham 2000).
Increase in CD4 cell count but statistical significance not reported
Five trials provided results for a relative increase in CD4 count in the groups receiving IL‐2. Abrams 2009a reported this outcome at seven years; Abrams 2009b and Amendola 2000 reported at six months; Dybul 2002 and Tambussi 2001 reported this at 84 weeks. However, these trials did not provide further details of whether the difference was statistically significant.
No significant increase in CD4 cell count
Two trials reported that there was no significant difference in the CD4 cell count between groups over 24 weeks, Ruxrungtham 2000 and Vogler 2004; however in the Ruxrungtham 2000 trial the lack of difference depended on the dosing of IL‐2 with higher doses resulting in a significant difference in a dose‐response manner.
Proportion of participants with undetectable viral load at any time point
Plasma viral load less than 50 copies/mL
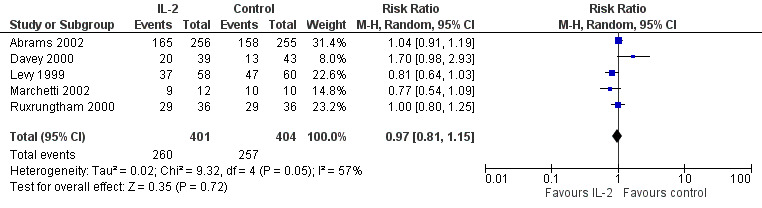
Seven trials reported on viral load of less than 50 copies/mL (Abrams 2002; Davey 2000; Lalezari 2000; Levy 2003; Marchetti 2002; Mitsuyasu 2007; Tavel 2003). None of the included trials found any significant difference between the two groups irrespective of the time when the viral load was measured. Overall, in the pooled analysis, there was no significant difference in the proportion of participants with a viral load of less than 50 copies/mL by the end of the trials (RR 0.97, 95% CI 0.81 to 1.15; 5 trials, 805 participants, high certainty evidence; Analysis 1.2; Figure 5).
1.2. Analysis.
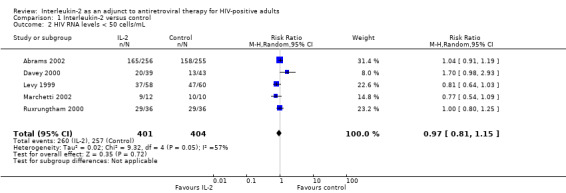
Comparison 1 Interleukin‐2 versus control, Outcome 2 HIV RNA levels < 50 cells/mL.
5.
Plasma viral load level less than 500 copies/mL
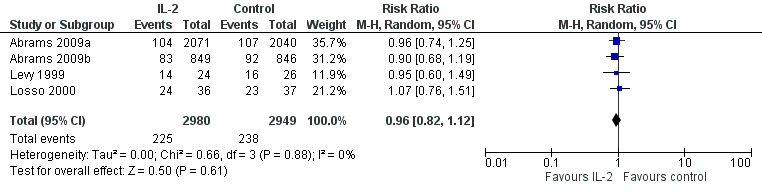
Four trials reported on plasma viral load of less that 500 copies/mL (Abrams 2009a; Abrams 2009b; Levy 1999; Losso 2000). None of the included trials found any significant difference between the two groups in viral load of less than 500 copies/mL irrespective of the time when the viral load was measured. The overall results did not show any significant difference in the two groups (RR 0.96, 95% CI 0.82 to 1.12; 4 trials, 5929 participants, high certainty evidence; Analysis 1.3; Figure 6).
1.3. Analysis.
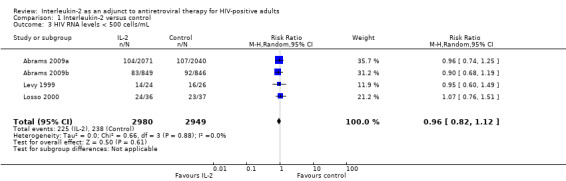
Comparison 1 Interleukin‐2 versus control, Outcome 3 HIV RNA levels < 500 cells/mL.
6.
Undetectable viral loads
In Amendola 2000, participants in both groups had HIV load levels below detection limit at the end of the study. Caggiari 2001 reported undetectable viral loads in six out of seven participants both the IL‐2 and ART only group. Carr 1998 did not report any difference in the mean viral load in any of the study arms. In Kovacs 1996, there were no significant differences between the groups in serial measurements of the plasma viral load or p24 antigen concentration during the 12 months of treatment.
Other viral load measurements
Six included trials found no significant difference in viral load (Hengge 1998; Katlama 2002; Levy 2003; Marchetti 2002; Tambussi 2001; Vogler 2004).
Opportunistic infections
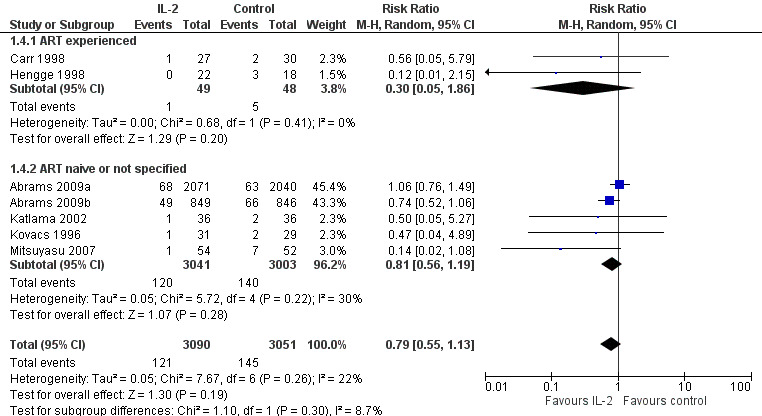
Seven included trials reported the incidence of opportunistic infections (Abrams 2009a; Abrams 2009b; Carr 1998; Hengge 1998; Katlama 2002; Kovacs 1996; Mitsuyasu 2007). Overall there was no significant difference between the two groups (RR 0.79, 95% CI 0.55 to 1.13; 7 trials, 6141 participants, low certainty evidence; Analysis 1.4; Figure 7).
1.4. Analysis.
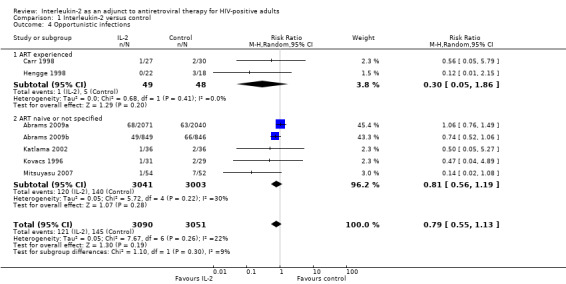
Comparison 1 Interleukin‐2 versus control, Outcome 4 Opportunistic infections.
7.
Adherence
None of the included trials reported on adherence.
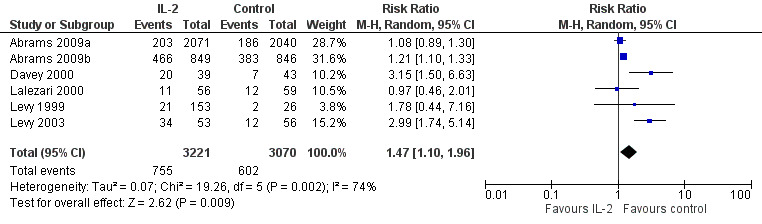
Adverse events
Nine included trials reported on adverse events (Abrams 2009a; Abrams 2009b; Davey 2000; de Boer 2003; Katlama 2002; Lalezari 2000; Levy 2003; Marchetti 2002; Tavel 2003).
GRADE 3 or higher adverse events
In Abrams 2009a, a total of 203 participants receiving IL‐2 and 186 participants in the control group had a grade 4 adverse event. In Abrams 2009b, a total of 203/849 participants receiving IL‐2 and 186/846 participants in the control group had a grade 4 adverse event. Davey 2000 reported grade 3 or higher adverse events in 20/39 participants in the IL‐2 group and in 7/43 adverse events in the control group (RR 3.13, 95% CI 1.50 to 6.63). Lalezari 2000 reported grade 3 adverse events in 10/56 participants (18%) in the IL‐2 group and in 9/59 (15%) of the control group while grade 4 adverse events were 1 (2%) and 3 (5%) respectively. Levy 1999 reported that severe adverse effects, such as aspartate transaminase deficiency, were reported in 2/26 of the participants in the control group and 16 participants (25%), 2 participants (5%), and 4 participants (9%), in the subcutaneous, PEG‐modified, and intravenous IL‐2 groups respectively. Severe neutropenia (less than 1 x 109/mL) was also seen in 2/26 (8%) participants in the control group and 9 participants (8%), 2 participants (9%), 3 participants (4.5%) in the subcutaneous, PEG‐modified, and intravenous IL‐2 groups. Levy 2003 reported that grade 3 or 4 adverse effects were noted in 34/53 participants (64%) in the IL‐2 group compared to 12/56 participants (22%) in the control group (P < 0.001).
In Mitsuyasu 2007, both IL‐2 arms were associated with significantly more grade 3 or 4 clinical toxic effects usually associated with IL‐2 treatment (with values of 30%, 53%, and 67% for 57, 58, and 59 participants) in the ART only, intravenous IL‐2 group, and subcutaneous IL‐2 group respectively. Tavel 2003 reported episodes of severe toxicities, neutropenia, and orthostatic blood pressure respectively in 2/5 participants compared to 0/4 in the control participants. Vogler 2004 reported no statistical significant difference between both groups in grade 3 or worse adverse effects (P ≥ 0.12). By the end of the trial at 24 weeks, two grade 4 events had occurred: one case of grade 4 hypertriglyceridaemia, one case of agitation in the ART plus IL‐2 group, and none in the control. Overall there were greater adverse effects in those participants receiving IL‐2 (RR 1.47, 95% CI 1.10 to 1.96; six trials, 6291 participants, moderate certainty evidence; Analysis 1.5; Figure 8).
1.5. Analysis.
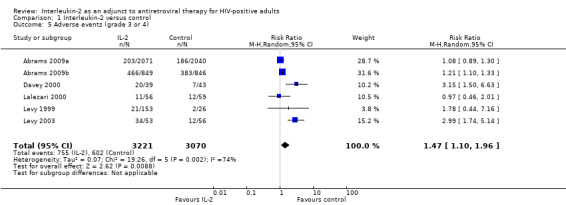
Comparison 1 Interleukin‐2 versus control, Outcome 5 Adverse events (grade 3 or 4).
8.
GRADE 2 or lower adverse events
In Lalezari 2000, grade 2 or lower adverse events were reported in 43/56 participants in the IL‐2 group and in 47/59 of the control group. Katlama 2002 reported that all participants receiving IL‐2 experienced at least one mild‐to‐moderate side‐effect, mainly constitutional symptoms such as fever, fatigue, malaise, and myalgias. Marchetti 2002 reported lower than grade 3 events in a total of 11 participants. Mild constitutional symptoms, such as fever (grade 1 to 2), fatigue, and myalgia were experienced by 10/12 participants receiving IL‐2, a reversible localized erythematous nodule at the site of injection was observed in 11/12 participants.
Discussion
Summary of main results
We identified 25 trials that met our inclusion criteria. The number of participants in the included trials varied from nine to 4111 participants. Interleukin‐2 (IL‐2) doses and the duration of follow‐up varied across the included trials. We judged the risk of bias due to methodological quality of the included studies to be low. There was no significant difference in mortality whether IL‐2 was added to the ART regimen or not (high certainty evidence). There was a significant increase in CD4 cell count in the IL‐2 group in most of the included trials (high certainty evidence). There was no statistically significant difference between viral load in both groups for measures less than 50 copies/mL or 500 copies/mL in most trials (high certainty evidence). IL‐2 probably causes an increase in adverse effects, particularly grade 3 or 4 adverse effects (moderate certainty evidence). Most of the included trials reported similar adverse events, neutropenia, and myalgia were most commonly reported. There is probably no difference in the incidence of opportunistic infections in the IL‐2 and control groups (low certainty evidence). Adherence was not reported in any of the included trials.
Overall completeness and applicability of evidence
We conducted a comprehensive search and included all relevant trials regardless of whether they reported the reviews outcomes of interest. Most included trials excluded participants who were previously on immunomodulators or steroids, or with an autoimmune disease, or with malignancy requiring them to be on immunomodulators. The trials were conducted in different settings: including high‐ and middle‐income countries. However, there is no plausible biological reason why the findings may not be applicable to low‐income settings.
Quality of the evidence
We assessed the certainty of the evidence using the GRADE methodology, and presented the basis for the judgements in a 'Summary of findings' table. The overall certainty of evidence on the effects of IL‐2 as an adjunct to ART for reducing morbidity and mortality in HIV‐infected adults individuals can be described as high, which means that we are confident in this result and further research is unlikely to change the direction of the effect. This finding was consistent across all the included trials that reported on the outcome. In addition, IL‐2 increases the CD4 cell count significantly and there is no difference in the proportion of participants with undetectable viral loads (high certainty evidence). IL‐2 probably does not cause any important difference in the rates of opportunistic infections (low certainty evidence). However, it probably causes increased grade 3 or 4 adverse effects (moderate certainty evidence).
Potential biases in the review process
We conducted a comprehensive search to ensure that we identified all relevant completed or ongoing studies. There were no language or publication restrictions. We also reduced the potential bias in the conduct of this review: two review authors independently screened the search output, extracted data, and assessed the methodological certainty of each included trial.
Agreements and disagreements with other studies or reviews
The findings of this review are similar to those of a literature review by Pett 2001, which showed that IL‐2 adjunctive therapy can significantly increase the CD4 pool of HIV‐positive participants compared to ART alone, however it has no significant effect on viral load, and has an increase in adverse effects, particularly grade 4 adverse effects in some trials and an acceptable adverse effect profile in others. Pett 2010 concluded that IL‐2 adjunctive therapy confers no clinical benefit on HIV‐positive participants and has no place in the therapeutic treatment of HIV. Three trials that we included in this Cochrane Review were also included in Pett 2010 (Abrams 2009a; Abrams 2009b; Stellbrink 2002).
The findings of this Cochrane Review differ from those of a pooled meta‐analysis of three randomized controlled trials (RCTs) by Emery 2000, which showed a significant decrease in viral load in participants on IL‐2 with ART compared to ART alone. However, Emery 2000 also showed no significant increase in mortality and concluded that despite substantial improvement in CD4 cell count and viral load there was no significant improvement in clinical outcomes.
Authors' conclusions
Implications for practice.
Interleukin‐2 (lL‐2) as an adjunct to ART leads to increases in CD4 cell counts in HIV‐infected adults on ART. However, IL‐2 (irrespective of the dose or duration) has no important effect on other clinically important positive outcomes, such as mortality, viral load reduction, and rates of opportunistic infections, but probably results in increased adverse effects. Our findings do not support the use of IL‐2 as an adjunct to ART in HIV‐infected adults.
Implications for research.
Further RCTs on the use of IL‐2 as adjunct to ART in HIV‐infected adults are not justifiable based on the findings of this Cochrane review. However, further basic research may be helpful to explore why IL‐2 causes increases in CD4 cell count.
Acknowledgements
We thank colleagues at Cochrane South Africa, South African Medical Research Council, Cape Town for technical and methodological support, particularly Joy Oliver for conducting the literature searches for this review. Tamara Kredo is partly supported by the Effective Health Care Research Consortium. This Consortium and the editorial base of the Cochrane Infectious Diseases Group is funded by UK aid from the UK Government for the benefit of developing countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Appendices
Appendix 1. CENTRAL search strategy
Date: 26 May 2016
| ID | Search | Hits |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | 8930 |
| #2 | MeSH descriptor: [HIV] explode all trees | 2820 |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | 16171 |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | 23 |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | 25 |
| #6 | #1 or #2 or #3 or #4 or #5 | 16256 |
| #7 | MeSH descriptor: [Antiretroviral Therapy, Highly Active] this term only | 1161 |
| #8 | MeSH descriptor: [Anti‐HIV Agents] explode all trees | 3013 |
| #9 | MeSH descriptor: [Antiviral Agents] this term only | 3778 |
| #10 | MeSH descriptor: [AIDS Vaccines] this term only | 371 |
| #11 | anti hiv or antiretroviral* or anti retroviral* or AIDS vaccin* | 7521 |
| #12 | #7 or #8 or #9 or #10 or #11 | 11280 |
| #13 | #6 and #12 | 7996 |
| #14 | MeSH descriptor: [Interleukin‐2] explode all trees | 848 |
| #15 | "interleukin 2":ti,ab or IL2:ti,ab or IL‐2:ti,ab aldesleukin:ti,ab or proleukin:ti,ab or "interleukin II":ti,ab or interleukin2:ti,ab or "interleukine 2":ti,ab (Word variations have been searched) | 1526 |
| #16 | #14 or #15 | 1794 |
| #17 | #13 and #16 | 144 |
Appendix 2. MEDLINE search strategy
Date: 26 May 2016
| Search | Query | Items found |
| #8 | Search (#3 AND #4 AND #7) | 634 |
| #7 | Search (#5 OR #6) | 69303 |
| #6 | Search (interleukin 2[tiab] OR interleukin2[tiab] OR IL2[tiab] OR IL‐2[tiab] OR aldesleukin[tiab] OR proleukin[tiab] OR interleukin II[tiab] OR interleukine 2[tiab]) | 62977 |
| #5 | Search interleukin‐2[mh] | 36542 |
| #4 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | 3285304 |
| #3 | Search (#1 AND #2) | 99540 |
| #2 | Search (antiretroviral therapy, highly active[MeSH] OR anti‐retroviral agents[MeSH] OR antiviral agents[MeSH:NoExp] OR ((anti[tiab]) AND (hiv[tiab])) OR antiretroviral*[tiab] OR ((anti[tiab]) AND (retroviral*[tiab])) OR HAART[tiab] OR ((anti[tiab]) AND (acquired immunodeficiency[tiab])) OR ((anti[tiab]) AND (acquired immuno‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immune‐deficiency[tiab])) OR ((anti[tiab]) AND (acquired immun*[tiab]) AND (deficiency[tiab])) | 157998 |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp]) | 344877 |
Appendix 3. Embase search strategy
Date: 26 May 2016
| No. | Query | Results |
| #11 | #3 AND #9 AND #10 | 318 |
| #10 | 'interleukin 2'/syn OR interleukin2:ab,ti OR 'aldesleukin'/syn OR 'proleukin'/syn OR il2 OR 'il+2'/syn OR 'interleukin ii'/syn OR 'interleukine 2' | 117687 |
| #9 | #4 NOT #8 | 1615894 |
| #8 | #5 NOT #7 | 5350278 |
| #7 | #5 AND #6 | 1470137 |
| #6 | 'human'/de OR 'normal human'/de OR 'human cell'/de | 17154813 |
| #5 | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | 6820415 |
| #4 | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti | 1811081 |
| #3 | #1 AND #2 | 140652 |
| #2 | 'human immunodeficiency virus vaccine'/exp OR 'human immunodeficiency virus vaccine' OR 'human immunodeficiency virus vaccine':ab,ti OR 'anti human immunedeficiency':ab,ti OR 'anti human immunodeficiency':ab,ti OR 'anti human immuno‐deficiency':ab,ti OR 'anti human immune‐deficiency':ab,ti OR 'anti acquired immune‐deficiency':ab,ti OR 'anti acquired immunedeficiency':ab,ti OR 'anti acquired immunodeficiency':ab,ti OR 'anti acquired immuno‐deficiency':ab,ti OR 'anti hiv':ab,ti OR antiretrovir*:ab,ti OR 'anti retroviral':ab,ti OR 'anti retrovirals':ab,ti OR 'anti retrovirus':ab,ti OR haart:ab,ti OR 'aids vaccine':ab,ti OR 'aids vaccines':ab,ti OR 'anti human immunodeficiency virus agent'/exp OR 'anti human immunodeficiency virus agent' OR 'anti human immunodeficiency virus agent':ab,ti OR 'antiretrovirus agent'/exp OR 'antiretrovirus agent' OR 'antiretrovirus agent':ab,ti OR 'highly active antiretroviral therapy'/exp OR 'highly active antiretroviral therapy' OR 'highly active antiretroviral therapy':ab,ti | 196031 |
| #1 | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'human immunodeficiency virus:ab,ti' OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'human immunodeficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune‐deficiency virus':ab,ti OR 'human immuno‐deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno‐deficiency syndrome':ab,ti OR 'acquired immune‐deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti | 446717 |
Appendix 4. ClinicalTrials.gov search strategy
Search strategy: HIV AND ("interleukin‐2" OR "interleukin 2" OR aldesleukin OR proleukin OR "interleukin II") | Interventional Studies | received from 11/14/2014 to 05/26/2016
Data and analyses
Comparison 1. Interleukin‐2 versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 6 | 6565 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 1.1 ART experienced | 2 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.16, 7.11] |
| 1.2 ART naive or not specified | 4 | 5939 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 2 HIV RNA levels < 50 cells/mL | 5 | 805 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.81, 1.15] |
| 3 HIV RNA levels < 500 cells/mL | 4 | 5929 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.82, 1.12] |
| 4 Opportunistic infections | 7 | 6141 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.13] |
| 4.1 ART experienced | 2 | 97 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.05, 1.86] |
| 4.2 ART naive or not specified | 5 | 6044 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.56, 1.19] |
| 5 Adverse events (grade 3 or 4) | 6 | 6291 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.10, 1.96] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abrams 2002.
| Methods | Open‐label randomized controlled trial (RCT) | |
| Participants | Eligibility criteria
Exclusion criteria
The trial included a total of 511 (256 in the interleukin group and 255 in the control group) HIV‐1 infected adults
|
|
| Interventions | Intervention group: intermittent administration of 2 doses (4.5 and 7.5 miu) of subcutaneous plus antiretroviral treatment (ART) Control group: ART alone. |
|
| Outcomes |
|
|
| Notes | The trial was conducted in the USA. Duration of follow‐up: minimum of 12 months. Median duration of follow‐up was 16.2 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used permuted block randomization with stratification by the CPCRA unit. |
| Allocation concealment (selection bias) | Low risk | The trial obtained random allocation of participants by calling the CPCRA Statistical Centre. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 15% of the participants were excluded from the final analysis or lost to follow‐up, and it was by intention‐to‐treat (ITT) analysis. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of bias from other sources. |
Abrams 2009a.
| Methods | Open‐label RCT | |
| Participants | Eligibility criteria: HIV‐infected adult Exclusion criteria: not specified |
|
| Interventions | Intervention group: 3 cycles and a dose of 7.5 miu of IL‐2 twice daily plus ART Control group: ART alone . |
|
| Outcomes |
|
|
| Notes | The trial was conducted in the USA. The median duration of follow‐up was 7.0 years. Ths trial was funded and sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial stratified randomization by individual clinical site. |
| Allocation concealment (selection bias) | Low risk | The central coordinating facility prepared all randomizations schedules. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The analysis was based on an ITT principle and less than 15% were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other forms of bias. |
Abrams 2009b.
| Methods | Open‐label RCT | |
| Participants | Eligibility criteria: HIV‐positive adults with CD4 cell count between 50 and 299 cells/mm³ Exclusion criteria: not specified |
|
| Interventions | Intervention group: 1 cycle of a dose of 4.5 miu twice daily for 5 consecutive days Control group: ART alone |
|
| Outcomes |
|
|
| Notes | The trial was conducted in the USA. The median duration of follow‐up was 7.6 years. The NIAID provided regulatory sponsorship, and Chiron, and subsequently Novartis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial stratified randomization by individual clinical site. |
| Allocation concealment (selection bias) | Low risk | The central co‐ordinating facility prepares all randomization schedules. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The analysis was based on an ITT principle and less than 15% were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other forms of bias. |
Amendola 2000.
| Methods | Open‐label RCT | |
| Participants | 22 HIV‐infected adults (12 males and 10 females) Inclusion criteria
Exclusion criteria
|
|
| Interventions | The participants were enrolled in 3 randomized groups.
All participants were treated with ART for 1 month before receiving differentiated therapies (ART; ART 1rIL‐2; (G‐CSF) ART 1rIL‐2) for an additional 12/24 weeks. |
|
| Outcomes |
|
|
| Notes | The trial was conducted in Italy. Duration of follow‐up: 24 weeks. Outcomes were analysed at baseline, 12, and 24 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe whether this was done or not. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe whether this was done or not. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We do not have enough information from the trial to make a judgement. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Caggiari 2001.
| Methods | Paralell single centred RCT | |
| Participants | 14 HIV‐infected adults Inclusion criteria
Exclusion criteria
|
|
| Interventions | Intervention group: 6 miu of IL‐2 from days 1 to 5 and 8 to 12 of a 28 day cycle for 6 cycles plus ART(2 reverse transcriptase inhibitors and indinavir) Control group: ART alone |
|
| Outcomes |
|
|
| Notes | This trial was conducted in Italy. Duration of follow‐up: 12 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no mention of the specific method of sequence generation or randomization but in the discussion section it was stated that randomization was done to ensure comparability of both groups |
| Allocation concealment (selection bias) | Unclear risk | There was no mention of the specific method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and not likely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We do not have enough information from the study to make a judgement. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Carr 1998.
| Methods | RCT | |
| Participants | 115 HIV‐infected adults Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | There were 3 trial arms
ART consisted of zidovudine + didanosine + zalcitabine |
|
| Outcomes |
|
|
| Notes | The trial was conducted in Australia. Duration of follow‐up: 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial not describe the method for sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial did not described the method for allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors analysed all participants included in the trial. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | High risk | There was a high risk of selection bias and detection bias. Participants were randomized on a 1:2:1 basis for the continuous intravenous IL‐2, PEG‐IL‐2 ( Polyethylene glycol ) modified IL‐2, and control groups respectively. The trial authors rationalized that the unequal randomization allowed for determination of the maximally tolerated dose significance levels of PEG IL‐2 as well as its efficacy. This was bound to cause selection bias. Secondly, IL‐2 participants were hospitalized the first 5 to 6 days of the cycle causing possible detection bias |
Davey 2000.
| Methods | Multicentred RCT | |
| Participants | Inclusion criteria: HIV‐infected adults Exclusion criteria: not specified |
|
| Interventions | Intervention: 6 cycles of IL‐2, 7.5 miu + ART Control: ART alone |
|
| Outcomes | CD4 cell count Viral load Adverse events |
|
| Notes | The trial was conducted in the USA. Duration of follow‐up was 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial randomly assigned participants to treatment groups by a computer generated block randomization with block sizes of 4 for the first 2 blocks and subsequently block sizes of 2. |
| Allocation concealment (selection bias) | Low risk | Central randomization by a biostatistician who was not part of the data analysis. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biostatisticians were blinded from knowing which participants were in which treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It is unlikely that there was attrition bias since < 15% withdrew or were lost to follow‐up. There was no differential loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective outcome reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
de Boer 2003.
| Methods | Multi‐centred RCT | |
| Participants | Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment group: participants received intravenous recombinant IL‐2 12 miu/day for 3, 4, or 5 days + ART every 8 weeks for 6 cycles Control group: ART alone |
|
| Outcomes |
|
|
| Notes | The trial was conducted in USA. Duration of follow‐up was 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There is likely to be a low risk of selection bias as participants were assigned in equal proportions of 1 to 4 treatments and participants were stratified by treatment centre. |
| Allocation concealment (selection bias) | Low risk | Randomization was done centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial addressed loss to follow‐up and was less than 15%. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of potential reporting bias from selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
Dybul 2002.
| Methods | RCT | |
| Participants | 9 participants Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment group: intermittent IL‐2 administered subcutaneously in 3 cycles for 5 days every 8 week plus ART Control group: ART alone. regimen: stavudine 30 mg to 40 mg twice daily, lamivudine 150 mg twice daily, indinavir 800 mg twice daily |
|
| Outcomes |
|
|
| Notes | This was a pilot study conducted in the USA. Duration of follow‐up: 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not provide any details. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not provide any details. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | We do not have enough information from the trial to make a judgement. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Hengge 1998.
| Methods | This was a prospective RCT | |
| Participants | There was a total of 64 participants. Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment group A (22): ART plus subcutaneous IL‐2 administered at a dose of 9.6 miu daily in cycles consisting of 5 days. A total of 5 cycles were given. One cycle was given every 6 weeks over a period of 52 weeks. Treatment group B (22): ART plus subcutaneous IL‐2 administered at a dose of 9.6 miu daily whenever CD4 counts dropped to below 1.25 fold of individual's baseline value. Control group: ART alone participants were followed up for a duration of 12 months |
|
| Outcomes |
|
|
| Notes | The trial was conducted in Germany. Duration of follow‐up: 12 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | There was no true randomization as controls were chosen from participants fulfilling the inclusion criteria who did not wish to experience the potentially adverse effects of lL‐2. Secondly, the trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | High risk | There is unlikely to be allocation concealment based on the support for judgement for sequence generation above. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There is unlikely to have been attrition bias as the trial excluded less than 15% of the participants. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting of outcomes. |
| Other bias | Low risk | There were no other potential sources of bias. |
Katlama 2002.
| Methods | Multicentred open label RCT | |
| Participants | A total of 72 participants. Inclusion criteria
Exclusion criteria: participants on cytotoxic chemotherapy or corticosteroids within 3 months prior to the trial |
|
| Interventions | Intervention: 4.5 miu of IL‐2 administered subcutaneously plus ART every 6 weeks for 4 cycles, every 12 hours for 5 days Control: ART alone |
|
| Outcomes |
|
|
| Notes | The trial was conducted in 18 clinical centres in France. Duration of follow‐up: 24 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial performed randomization of participants by using computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | The trial performed allocation centrally. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors used ITT for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Kelleher 1998.
| Methods | This was a single centred pilot study which was planned as a pilot study of a multi‐centred RCT to be conducted. | |
| Participants | 18 participants consecutively enrolled into 3 groups Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment group A (IL‐2 plus ART only)
as continuous intravenous infusions for 5 days every 8 weeks for 6 cycles Treatment group B (IL‐2 linked to polyethylene glycol plus ART)
Control group
Each group had 150 mg of lamivudine twice daily added to their regimen at 30 weeks. |
|
| Outcomes |
|
|
| Notes | This was a multicentred trial conducted in Australia. Duration of follow‐up: 48 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial excluded less than 15% of the participants. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Kovacs 1996.
| Methods | This was a single‐centred RCT | |
| Participants | A total of 60 participants were randomized Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment group: intravenous IL‐2 given at intermittent infusions of 18 miu plus ART Control group: ART alone consisting of zidovudine, zalcitabine, or stavudine with didanosine |
|
| Outcomes |
|
|
| Notes | Study was conducted in the USA. The duration of follow‐up was 14 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 15% were lost to follow‐up and the trial authors described withdrawals. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Lalezari 2000.
| Methods | Multicentred phase 2 RCT | |
| Participants | A total of 115 participants Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment group (IL‐2 plus ART): 51 participants
Control group: ART alone
|
|
| Outcomes |
|
|
| Notes | This trial was conducted in the USA. Duration of follow‐up: 26 weeks |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence generation was by block randomization stratified by study site. |
| Allocation concealment (selection bias) | Low risk | The trial authors used a central randomization process. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcomes measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More than 15% withdrew from the trial due to adverse effects and the trial authors did not analyse their results. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective outcome reporting. |
| Other bias | Low risk | There was no evidence of any other sources of bias. |
Levy 1999.
| Methods | Multicentred an open label RCT | |
| Participants | A total of 94 participants Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This study was conducted in 14 university clinics in France. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no evidence of incomplete outcome data. Less than 15% were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting bias. |
| Other bias | Low risk | There was no evidence of other sources of bias. |
Levy 2003.
| Methods | This was a prospective RCT. | |
| Participants | There were a total of 118 participants Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment group: ART started 4 weeks before plus subcutaneous IL‐2 administered at a dose of 5 miu twice daily for 5 days given every 4 weeks for the first 3 cycles and then subsequently every 8 weeks for the next 7 cycles. Control group: ART alone consisting of lamivudine (300 mg/day), stavudine (60 to 80mg/day), and indinavir (2400 mg/day). |
|
| Outcomes |
|
|
| Notes | This was a multicentred study conducted in 14 university clinics in France. Duration of follow‐up was 18 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors did not describe the method of sequence generation. However, the trial authors mentioned that randomization was centralized and stratified according to ART status. |
| Allocation concealment (selection bias) | Low risk | The trial authors used a centralized method of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial excluded less than 15% (2 out of 118 participants) from the analysis. There was no differential loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other sources of bias. |
Losso 2000.
| Methods | Study design was a prospective open‐labelled RCT | |
| Participants | There were a total of 73 participants Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This was a multi‐centred trial conducted in 6 clinical centres in Buenos Aires, Argentina. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used a stratified block randomization method, using blocks of 24 stratified according to ART history (naive or experienced) and clinical centres. |
| Allocation concealment (selection bias) | Low risk | The trial used a centralized method of randomization. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial excluded less than 15% (2 out of 73 participants) from the analysis. There was no differential loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | High risk | There was more monitoring in the treatment group compared to the control group. |
Marchetti 2002.
| Methods | Open labelled parallel RCT | |
| Participants | 22 participants were randomized: Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group (12): they were commenced on IL‐2 for a 4 week cycle for 3 cycles plus ART Control group (10): ART consisting of 2 nucleoside reverse transcriptase inhibitors (NRTI) and 1 PI |
|
| Outcomes |
|
|
| Notes | This trial was an explanatory trial conducted at the University of Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial followed up all participants to the end of the trial, and even included those who were lost to follow‐up afterwards in the results. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting bias. |
| Other bias | Low risk | There was no evidence of other potential sources bias. |
Marchetti 2004.
| Methods | This was a RCT | |
| Participants | There were a total of 15 participants Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group (8): participants received 3 cycles of low dose subcutaneous IL‐2 over a 48‐week period. Each cycle consisted of 3 miu IL‐2 administered at days of 1 to 5 and days 8 to 12 of a 10‐week duration plus ART Control group (7): ART alone |
|
| Outcomes |
|
|
| Notes | This was a small immunological trial conducted to investigate the long‐term kinetics of CD4 and CD8 cells when low dose IL‐2 is administered in Milan, Italy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting of outcomes. |
| Other bias | Low risk | We did not identify any other potential sources of bias. |
Mitsuyasu 2007.
| Methods | This was a multi‐arm parallel RCT | |
| Participants | There were a total of 159 participants Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group A (intravenous IL‐2 plus ART): received continuous infusions of IL‐2 at doses of 9 miu for 5 days every 8 weeks plus ART Treatment group B (subcutaneous IL‐2 plus ART): received subcutaneous injections of IL‐2 7.5 miu twice daily for 5 days every 8 weeks plus ART Control group: ART alone, 2 NRTIs, and a PI |
|
| Outcomes |
|
|
| Notes | This was a multicentred trial conducted at 26 AIDS Clinical Trials Group (ACTG) sites in the USA. Duration of the trial was 84 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | There is likely to be a low risk of selection bias because although the trial authors did not describe the method of sequence generation, participants were randomized in proportions of 1:1:1 and stratified by participation in a previous trial ACTG 928 and nucleosides. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The trial excluded more than 15% of participants from the analysis. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
Ruxrungtham 2000.
| Methods | Multicentred parallel RCT | |
| Participants | Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment groups (A, B, and C): received 1.5 miu, 4.5 miu, and 7.5 miu of IL‐2 administered twice daily for 5 days, every 8 weeks for three cycles. Control groups: ART alone. |
|
| Outcomes |
|
|
| Notes | This was a multi‐arm pragmatic trial conducted in Thailand. Duration of follow‐up was 24 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors did not clearly specify the method of random sequence generation but it appeared to be low risk. Randomization was stratified by clinical centre and ART treatment history, that is naive or pretreated. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The trial authors did not describe withdrawals or missing data. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
Stellbrink 2002.
| Methods | Prospective RCT | |
| Participants | There were 56 participants. Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | This was an open label RCT conducted in Germany. Mean follow‐up duration ranged from 582 ‐ 601 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors performed analysis was ITT and there were no losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
Tambussi 2001.
| Methods | This was a phase 2 RCT. | |
| Participants | There was a total of 61 participants. Inclusion criteria
Exclusion criteria: not specified |
|
| Interventions | Treatment arm A (high dose intravenous arm): ART plus 12 miu of IL‐2 by continuous intravenous infusion followed by subcutaneous 7.5 miu IL‐2 twice a day for 5 days every 8 weeks for the remaining 4 cycles. Treatment arm B (high dose subcutaneous arm): ART+subcutaneous 7.5 miu IL‐2 twice a day for 5 days every 8 weeks for 6 cycles. Treatment arm C (low dose arm): ART plus subcutaneous IL‐2 +3 miu twice a day every 4 weeks Control: ART alone |
|
| Outcomes |
|
|
| Notes | This was an phase 2 RCT conducted in Milan Italy. The duration of follow‐up was a 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not report the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not report the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial authors performed analysis by ITT principle and less than 15% of participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | High risk | There were differential treatment participants in the 2 groups. Participants were followed up as outpatients in 1 group and as inpatients in the other group. |
Tavel 2003.
| Methods | This was a double blinded randomized placebo controlled trial | |
| Participants | There were a total of 19 participants. Inclusion criteria
Exclusion criteria
|
|
| Interventions | Group A: subcutaneous IL‐2 (7.5 miu IL‐2 twice daily for 5 days) and placebo) plus ART Group B: IL‐2 (7.5 miu IL‐2 twice daily for 5 days) and 0.5 mg prednisone/kg/day for 7 days every 8 weeks plus ART Group C: 0.5 mg prednisone/ kg/day for 7 days every 8 weeks plus ART Group D: placebo orally for 7 days (1 cycle ) every 8 weeks plus ART |
|
| Outcomes |
|
|
| Notes | This study was conducted in the USA. Duration of follow‐up was 12 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no loss to follow‐up and ITT. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
Vogler 2004.
| Methods | This was a phase 2 multi‐centred randomized open label trial | |
| Participants | There was a total of 115 participants Inclusion criteria
Exclusion criteria
|
|
| Interventions | Treatment group: self administered subcutaneous IL‐2 1 miu daily in 0.2 mL volume at rotating skin sites in combination with continued ART Control: ART alone. |
|
| Outcomes |
|
|
| Notes | This trial was conducted in the USA. The duration of follow‐up was 24 weeks. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The trial was not blinded. However, the outcome measures are objective and unlikely to have been influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The trial excluded less than 15% from the analysis. |
| Selective reporting (reporting bias) | Low risk | There was no evidence of selective reporting. |
| Other bias | Low risk | There was no evidence of other potential sources of bias. |
Abbreviations: AIDS: acquired immunodeficiency virus; ART: antiretroviral therapy; CNS: central nervous system; PEG: polyethylene glycol; HIV: human immunodeficiency virus; ITT: intention to treat; IL‐2: interleukin‐2; NRTI: nucleoside reverse transcriptase inhibitors; PI: protease inhibitor; RCT: randomized controlled trial; USA: United States of America.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bosch 2010 | Both the intervention and control group received interleukin‐2 (IL‐2) |
| Chun 1999 | This was a cross‐sectional study |
| Crespo 2008 | This was a cohort study |
| Jacobson 2002 | This was a study of interleukin‐12 (IL‐12) and not IL‐2 |
| Kilby 2006 | Participants in the intervention group received both IL‐2 and a vaccine |
| Lafeuillade 2001 | Participants in the intervention group received both IL‐2 and interferon‐gamma |
| Martin 2005 | The study did not report any outcomes relevant to this review |
| Pett 2001 | This was a review of randomized controlled trials |
| Tavel 2010 | The study compared participants receiving no treatment, IL‐2 alone, or IL‐2 with antiretroviral treatment (ART) |
| Witzke 1998 | Here the comparison was between subcutaneous IL‐2 and ART and intravenous IL‐2 and ART. The control group was not relevant to this review |
Abbreviations: IL‐2: interleukin‐2.
Contributions of authors
Jennifer Onwumeh and Charles Okwundu wrote the draft protocol and review, independently performed the study selection and data extraction. Tamara Kredo contributed to the protocol, gave input into the manuscript, analysis and the final review.
Sources of support
Internal sources
Centre for Evidence‐Base Health Care, Stellenbosch University, South Africa.
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
Jennifer Onwumeh, Charles Okwundu, and Tamara Kredo have no known conflicts of interest with respect to this review.
Unchanged
References
References to studies included in this review
Abrams 2002 {published data only}
- Abrams DI, Bebchuk JD, Denning ET, Davey RT, Fox L, Lane HC, et al. Randomized, open‐label study of the impact of two doses of subcutaneous recombinant interleukin‐2 on viral burden in patients with HIV‐1 infection and CD4+ cell counts of > or = 300/mm3: CPCRA 059. Journal of Acquired Immune Deficiency Syndromes 2002;29(3):221‐31. [PUBMED: 11873071] [DOI] [PubMed] [Google Scholar]
Abrams 2009a {published data only}
- Abrams D, Emery S, Cooper DA, Darbyshire JH, Lane HC, Lundgren JD, et al. The evaluation of subcutaneous proleukin (interleukin‐2) in a randomized international trial: rationale, design, and methods of ESPRIT. Controlled Clinical Trials 2002;23(2):198‐220. [PUBMED: 11943448] [DOI] [PubMed] [Google Scholar]
Abrams 2009b {published data only}
- INSIGHT‐ESPRIT Study Group, SILCAAT Scientific Committee, Abrams D, Lévy Y, Losso MH, Babiker A, et al. Interleukin‐2 therapy in patients with HIV infection. New England Journal of Medicine 2009;361(16):1548‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amendola 2000 {published data only}
- Amendola A, Poccia F, Martini F, Gioia C, Galati V, Pierdominici M, et al. Decreased CD95 expression on naive T cells from HIV‐infected persons undergoing highly active anti‐retroviral therapy (HAART) and the influence of IL‐2 low dose administration. Irhan Study Group. Clinical and Experimental Immunology 2000;120(2):324‐32. [PUBMED: 10792383] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caggiari 2001 {published data only}
- Caggiari L, Zanussi S, Crepaldi C, Bortolin MT, Caffau C, D’Andrea M, et al. Different rates of CD41 and CD81 T‐cell proliferationin interleukin‐2–treated human immunodeficiency virus‐positive subjects. Cytometry (Communications in Clinical Cytometry) 2001;46:233‐7. [DOI] [PubMed] [Google Scholar]
Carr 1998 {published data only}
- Carr A, Emery S, Lloyd A, Hoy J, Garsia R, French M, et al. Outpatient continuous intravenous interleukin‐2 or subcutaneous, polyethylene glycol‐modified interleukin‐2 in human immunodeficiency virus‐infected patients: a randomized, controlled, multicenter study. Australian IL‐2 Study Group. Journal of Infectious Diseases 1998;178(4):992‐9. [PUBMED: 9806026] [DOI] [PubMed] [Google Scholar]
Davey 2000 {published data only}
- Davey RT Jr, Murphy RL, Graziano FM, Boswell SL, Pavia AT, Cancio M, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: A randomized controlled trial. JAMA 2000;284(2):183‐9. [PUBMED: 10889591] [DOI] [PubMed] [Google Scholar]
de Boer 2003 {published data only}
- Boer AW, Markowitz N, Lane HC, Saravolatz LD, Koletar SL, Donabedian H, et al. A randomized controlled trial evaluating the efficacy and safety of intermittent 3‐, 4‐, and 5‐day cycles of intravenous recombinant human interleukin‐2 combined with antiretroviral therapy (ART) versus ART alone in HIV‐seropositive patients with 100‐300 CD4+ T cells. Clinical Immunology 2003;106(3):188‐96. [PUBMED: 12706405] [DOI] [PubMed] [Google Scholar]
Dybul 2002 {published data only}
- Dybul M, Hidalgo B, Chun TW, Belson M, Migueles SA, Justement JS, et al. Pilot study of the effects of intermittent interleukin‐2 on human immunodeficiency virus (HIV)‐specific immune responses in patients treated during recently acquired HIV infection. Journal of Infectious Diseases 2002;185(1):61‐8. [PUBMED: 11756982] [DOI] [PubMed] [Google Scholar]
Hengge 1998 {published data only}
- Hengge UR, Goos M, Esser S, Exner V, Dötterer H, Wiehler H, et al. Randomized, controlled phase II trial of subcutaneous interleukin‐2 in combination with highly active antiretroviral therapy (HAART) in HIV patients. AIDS (London, England) 1998;12(17):F225‐34. [PUBMED: 9863864] [PubMed] [Google Scholar]
Katlama 2002 {published data only}
- Katlama C, Carcelain G, Duvivier C, Chouquet C, Tubiana R, Sa M, et al. Interleukin‐2 accelerates CD4 cell reconstitution in HIV‐infected patients with severe immunosuppression despite highly active antiretroviral therapy: the ILSTIM study‐‐ANRS 082. AIDS (London, England) 2002;16(15):2027‐34. [PUBMED: 12370501] [DOI] [PubMed] [Google Scholar]
Kelleher 1998 {published data only}
- Kelleher AD, Roggensack M, Emery S, Carr A, French MA, Cooper DA. Effects of IL‐2 therapy in asymptomatic HIV‐infected individuals on proliferative responses to mitogens, recall antigens and HIV‐related antigens. Clinical and Experimental Immunology 1998;113(1):85‐91. [PUBMED: 9697988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kovacs 1996 {published data only}
- Kovacs JA, Vogel S, Albert JM, Falloon J, Davey RT Jr, Walker RE, et al. Controlled trial of interleukin‐2 infusions in patients infected with the human immunodeficiency virus. New England Journal of Medicine 1996;335(18):1350‐6. [PUBMED: 8857018] [DOI] [PubMed] [Google Scholar]
Lalezari 2000 {published data only}
- Lalezari JP, Beal JA, Ruane PJ, Cohen CJ, Jacobson EL, Sundin D, et al. Low‐dose daily subcutaneous interleukin‐2 in combination with highly active antiretroviral therapy in HIV+ patients: a randomized controlled trial. HIV Clinical Trials 2000;1(3):1‐15. [PUBMED: 11590500] [DOI] [PubMed] [Google Scholar]
Levy 1999 {published data only}
- Levy Y, Capitant C, Houhou S, Carriere I, Viard JP, Goujard C, et al. Comparison of subcutaneous and intravenous interleukin‐2 in asymptomatic HIV‐1 infection: a randomised controlled trial. ANRS 048 study group. Lancet 1999;353(9168):1923‐9. [PUBMED: 10371571] [DOI] [PubMed] [Google Scholar]
Levy 2003 {published data only}
- Levy Y, Durier C, Krzysiek R, Rabian C, Capitant C, Lascaux AS, et al. Effects of interleukin‐2 therapy combined with highly active antiretroviral therapy on immune restoration in HIV‐1 infection: a randomized controlled trial. AIDS (London, England) 2003;17(3):343‐51. [PUBMED: 12556688] [DOI] [PubMed] [Google Scholar]
Losso 2000 {published data only}
- Losso MH, Belloso WH, Emery S, Benetucci JA, Cahn PE, Lasala MC, et al. A randomized, controlled, phase II trial comparing escalating doses of subcutaneous interleukin‐2 plus antiretrovirals versus antiretrovirals alone in human immunodeficiency virus‐infected patients with CD4+ cell counts >/=350/mm3. Journal of Infectious Diseases 2000;181(5):1614‐21. [PUBMED: 10823761] [DOI] [PubMed] [Google Scholar]
Marchetti 2002 {published data only}
- Marchetti G, Meroni L, Varchetta S, Terzieva V, Bandera A, Manganaro D, et al. Low‐dose prolonged intermittent interleukin‐2 adjuvant therapy: results of a randomized trial among human immunodeficiency virus‐positive patients with advanced immune impairment. Journal of Infectious Diseases 2002;186(5):606‐16. [PUBMED: 12195347] [DOI] [PubMed] [Google Scholar]
Marchetti 2004 {published data only}
- Marchetti G, Meroni L, Molteni C, Bandera A, Franzetti F, Galli M, et al. Interleukin‐2 immunotherapy exerts a differential effect on CD4 and CD8 T cell dynamics. AIDS (London, England) 2004;18(2):211‐6. [PUBMED: 15075538] [DOI] [PubMed] [Google Scholar]
Mitsuyasu 2007 {published data only}
- Mitsuyasu R, Gelman R, Cherng DW, Landay A, Fahey J, Reichman R, et al. The virologic, immunologic, and clinical effects of interleukin 2 with potent antiretroviral therapy in patients with moderately advanced human immunodeficiency virus infection: a randomized controlled clinical trial‐‐AIDS Clinical Trials Group 328. Archives of Internal Medicine 2007;167(6):597‐605. [PUBMED: 17389292] [DOI] [PubMed] [Google Scholar]
Ruxrungtham 2000 {published data only}
- Ruxrungtham K, Suwanagool S, Tavel JA, Chuenyam M, Kroon E, Ubolyam S, et al. A randomized, controlled 24‐week study of intermittent subcutaneous interleukin‐2 in HIV‐1 infected patients in Thailand. AIDS (London, England) 2000;14(16):2509‐13. [PUBMED: 11101062] [DOI] [PubMed] [Google Scholar]
Stellbrink 2002 {published data only}
- Stellbrink HJ, Lunzen J, Westby M, O'Sullivan E, Schneider C, Adam A, et al. Effects of interleukin‐2 plus highly active antiretroviral therapy on HIV‐1 replication and proviral DNA (COSMIC trial). AIDS (London, England) 2002;16(11):1479‐87. [PUBMED: 12131185] [DOI] [PubMed] [Google Scholar]
Tambussi 2001 {published data only}
- Tambussi G, Ghezzi S, Nozza S, Vallanti G, Magenta L, Guffanti M, el al. Efficacy of low‐dose intermittent subcutaneous interleukin (IL)‐2 in antiviral drug‐experienced human immunodeficiency virus‐infected persons with detectable virus load: a controlled study of 3 IL‐2 regimens with antiviral drug therapy. Journal of Infectious Diseases 2001;183(10):1476–84. [DOI] [PubMed] [Google Scholar]
Tavel 2003 {published data only}
- Tavel JA, Sereti I, Walker RE, Hahn B, Kovacs JA, Jagannatha S, et al. A randomized, double‐blinded, placebo‐controlled trial of intermittent administration of interleukin‐2 and prednisone in subjects infected with human immunodeficiency virus. Journal of Infectious Diseases 2003;188(4):531‐6. [PUBMED: 12898439] [DOI] [PubMed] [Google Scholar]
Vogler 2004 {published data only}
- Vogler MA, Teppler H, Gelman R, Valentine F, Lederman MM, Pomerantz RJ, et al. Daily low‐dose subcutaneous interleukin‐2 added to single‐ or dual‐nucleoside therapy in HIV infection does not protect against CD4+ T‐cell decline or improve other indices of immune function: results of a randomized controlled clinical trial (ACTG 248). Journal of Acquired Immune Deficiency Syndromes 2004;36(1):576‐87. [PUBMED: 15097300] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bosch 2010 {published data only}
- Bosch RJ, Pollard RB, Landay A, Aga E, Fox L, Mitsuyasu R. Continuing or adding IL‐2 in patients treated with antiretroviral therapy (ACTG Protocol A5051, a rollover trial of ACTG Protocol A328). AIDS Research and Therapy 2010;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chun 1999 {published data only}
- Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, et al. Effect of interleukin‐2 on the pool of latently infected, resting CD4+ T cells in HIV‐1‐infected patients receiving highly active anti‐retroviral therapy. Nature Medicine 1999;5(6):651‐5. [PUBMED: 10371503] [DOI] [PubMed] [Google Scholar]
Crespo 2008 {published data only}
- Crespo M, Caragol I, Falcó V, Ribera E, Urban S, Pahissa A. Efficacy of recombinant interleukin‐2 (rIL‐2) in patients with advanced HIV‐1 infection and blunted immune response to HAART. Enfermedades Infecciosas y Microbiologia Clinica 2008;26(1):27‐31. [DOI] [PubMed] [Google Scholar]
Jacobson 2002 {published data only}
- Jacobson MA, Spritzler J, Landay A, Chan E, Katzenstein D, Schock B, et al. A Phase I, placebo‐controlled trial of multi‐dose recombinant human interleukin‐12 in patients with HIV infection. AIDS (London, England) 2002;16(8):1147‐54. [PUBMED: 12004273] [DOI] [PubMed] [Google Scholar]
Kilby 2006 {published data only}
- Kilby JM, Bucy RP, Mildvan D, Fischl M, Santana‐Bagur J, Lennox J, et al. A randomized, partially blinded phase 2 trial of antiretroviral therapy, HIV‐specific immunizations, and interleukin‐2 cycles to promote efficient control of viral replication (ACTG A5024). Journal of Infectious Diseases 2006;194(12):1672‐6. [PUBMED: 17109338] [DOI] [PubMed] [Google Scholar]
Lafeuillade 2001 {published data only}
- Lafeuillade A, Poggi C, Chadapaud S, Hittinger G, Chouraqui M, Pisapia M, et al. Pilot study of a combination of highly active antiretroviral therapy and cytokines to induce HIV‐1 remission. Journal of Acquired Immune Deficiency Syndromes 2001;26(1):44‐55. [PUBMED: 11176268] [DOI] [PubMed] [Google Scholar]
Martin 2005 {published data only}
- Martin BK, Wu AW, Gelman R, Mitsuyasu RT, Adult AIDS Clinical Trials Group. Quality of life in a clinical trial of highly active antiretroviral therapy alone or with intravenous or subcutaneous interleukin‐2 administration. Journal of Acquired Immune Deficiency Syndromes 2005;40(4):428‐33. [PUBMED: 16280697] [DOI] [PubMed] [Google Scholar]
Pett 2001 {published data only}
- Pett SL, Emery S. Immunomodulators as adjunctive therapy for HIV‐1 infection. Journal of Clinical Virology 2001;22(3):289‐95. [PUBMED: 11564594] [DOI] [PubMed] [Google Scholar]
Tavel 2010 {published data only}
- Tavel JA1, INSIGHT STALWART Study Group, Babiker A, Fox L, Gey D, Lopardo G, et al. Effects of intermittent IL‐2 alone or with peri‐cycle antiretroviral therapy in early HIV infection: the STALWART study. PLoS ONE 2010;5(2):e9334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witzke 1998 {published data only}
- Witzke O, Winterhagen T, Reinhardt W, Heemann U, Grosse‐Wilde H, Kreuzfelder E, et al. Comparison between subcutaneous and intravenous interleukin‐2 treatment in HIV disease. Journal of Internal Medicine 1998;244(3):235‐40. [PUBMED: 9747746] [DOI] [PubMed] [Google Scholar]
Additional references
Abrams 2009
- INSIGHT‐ESPRIT Study Group, SILCAAT Scientific Committee, Abrams D, Lévy Y, Losso MH, Babiker A, et al. Interleukin‐2 therapy in patients with HIV infection. New England Journal of Medicine 2009;361(16):1548‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blankson 2000
- Blankson J, Siliciano RF. Interleukin 2 treatment for HIV infection. JAMA 2000;284(2):236‐8. [DOI: 10.1001/jama.284.2.236] [DOI] [PubMed] [Google Scholar]
Castro 1992
- Castro KG, Ward JW, Slutsker L, Buehler JW, Jaffe HW, Berkelman RL, et al. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Morbidity and Mortality Weekly Report. Recommendations and Reports 1992;41(RR‐17):1‐19. [PubMed] [Google Scholar]
Emery 2000
- Emery S, Capra WB, Cooper DA, Mitsuyasu RT, Kovacs JA, Vig P, et al. Pooled analysis of 3 randomized controlled trials of interleukin 2 therapy in adult human immunodeficiency virus type 1 disease. Journal of Infectious Diseases 2000;182(2):428‐34. [DOI] [PubMed] [Google Scholar]
Grimwade 2009
- Grimwade K, Swingler G. Cotrimoxazole prophylaxis for opportunistic infections in adults with HIV. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003108] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Harari 2004
- Harari A, Petitpierre S, Vallelian F, Pantaleo G. Skewed representation of functionally distinct populations of virus‐specific CD4 T cells in HIV‐1‐infected subjects with progressive disease: changes after antiretroviral therapy. Blood 2004;103(3):966‐72. [DOI: 10.1182/blood-2003-04-1203] [DOI] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Higgins 2008b
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008:187‐241. [Google Scholar]
Horn 2002
- Horn T. Proleukin (aldeseukin, Interleukin‐2 or IL‐2). www.aidsmed.com (accessed 23 August 2011).
Molina 2007
- Molina JM, Levy Y, Fournier I, Hamonic S, Bentata M, Beck‐Wirth G, et al. Abstract H‐718: Intermittent interleukin‐2 therapy to defer antiretroviral therapy in patients with human immuno deficiency virus infection. ICAAC. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2007 Sept 17‐20; Chicago (IL). Chicago (IL), 2007.
Nachega 2011
- Nachega J, Mugavero MJ, Zeier M, Vitória M, Gallant J. Treatment simplification in HIV‐infected adults as a strategy to prevent toxicity, improve adherence, quality of life and decrease healthcare costs. Patient Preference and Adherence 2011;5:357‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pett 2010
- Pett S, Kelleher AD, Emery S. Role of interleukin 2 in patients with HIV infection. Drugs 2010;70(9):1115‐30. [DOI] [PubMed] [Google Scholar]
Piliero 2003
- Piliero PJ. Long‐term toxicities associated with HIV and antiretroviral therapy. June 2003. http://www.thebody.com/content/art12984.html (accessed 26 December 2011).
Review Manager 5 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
UNAIDS 2016
- UNAIDS. Global AIDS Update. 2016. http://www.unaids.org/sites/default/files/media_asset/global‐AIDS‐update‐2016_en.pdf. UNAIDS, (accessed 18 August 2016).


