Abstract
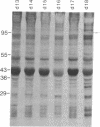
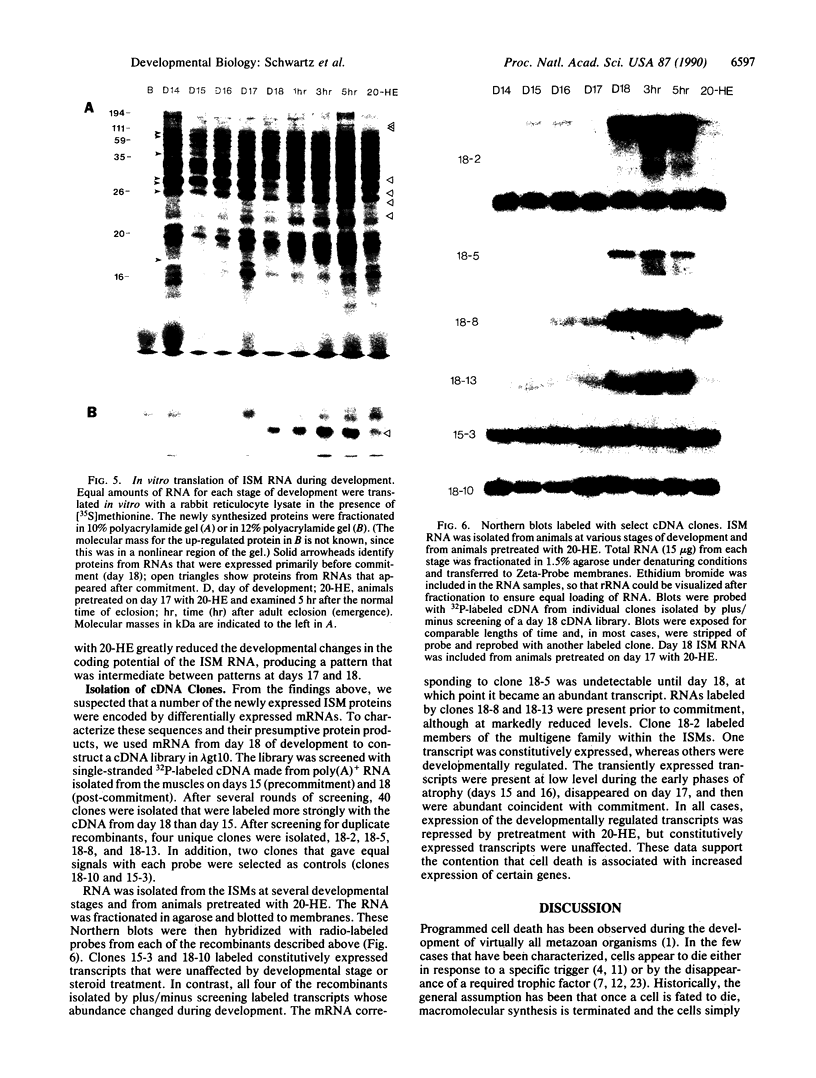
The intersegmental muscles of the tobacco hawkmoth Manduca sexta die during the 36-hr period after metamorphosis. The trigger for cell death is a fall in the ecdysteroid titer. Commitment of the intersegmental muscles to degenerate involves selective repression and activation of ecdysteroid-responsive genes. When the pattern of gene expression is altered after injection of either 20-hydroxyecdysone or actinomycin D, the muscles persist. cDNA clones have been isolated for four genes that become abundantly expressed coincident with the commitment to degenerate. The data presented here indicate that programmed cell death is not due to the cessation of macromolecular synthesis in condemned cells but rather is due to the activation of a differentiative pathway.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaulaton J., Lockshin R. A. Ultrastructural study of the normal degeneration of the intersegmental muscles of Anthereae polyphemus and Manduca sexta (Insecta, Lepidoptera) with particular reference of cellular autophagy. J Morphol. 1977 Oct;154(1):39–57. doi: 10.1002/jmor.1051540104. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Garrels J. I. Quantitative two-dimensional gel electrophoresis of proteins. Methods Enzymol. 1983;100:411–423. doi: 10.1016/0076-6879(83)00070-1. [DOI] [PubMed] [Google Scholar]
- HAMBURGER J., RICHET G. Enseignements tirés de la pratique du rein artificiel pour l'interprétation des désordres électrolytiques de l'urémie aiguë. Rev Fr Etud Clin Biol. 1956 Jan;1(1):39–55. [PubMed] [Google Scholar]
- Hershko A. Ubiquitin-mediated protein degradation. J Biol Chem. 1988 Oct 25;263(30):15237–15240. [PubMed] [Google Scholar]
- Jacobson A. Purification and fractionation of poly(A)+ RNA. Methods Enzymol. 1987;152:254–261. doi: 10.1016/0076-6879(87)52028-6. [DOI] [PubMed] [Google Scholar]
- Kimura K. I., Truman J. W. Postmetamorphic cell death in the nervous and muscular systems of Drosophila melanogaster. J Neurosci. 1990 Feb;10(2):403–401. doi: 10.1523/JNEUROSCI.10-02-00403.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Manetto V., Perry G., Tabaton M., Mulvihill P., Fried V. A., Smith H. T., Gambetti P., Autilio-Gambetti L. Ubiquitin is associated with abnormal cytoplasmic filaments characteristic of neurodegenerative diseases. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4501–4505. doi: 10.1073/pnas.85.12.4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. M. Nick translation. Methods Enzymol. 1987;152:91–94. doi: 10.1016/0076-6879(87)52012-2. [DOI] [PubMed] [Google Scholar]
- Mori H., Kondo J., Ihara Y. Ubiquitin is a component of paired helical filaments in Alzheimer's disease. Science. 1987 Mar 27;235(4796):1641–1644. doi: 10.1126/science.3029875. [DOI] [PubMed] [Google Scholar]
- Nishikawa B. K., Fowlkes D. M., Kay B. K. Convenient uses of polymerase chain reaction in analyzing recombinant cDNA clones. Biotechniques. 1989 Jul-Aug;7(7):730–735. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W., Haverkamp L. J., Prevette D., McManaman J. L., Appel S. H. Reduction of naturally occurring motoneuron death in vivo by a target-derived neurotrophic factor. Science. 1988 May 13;240(4854):919–922. doi: 10.1126/science.3363373. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Tytell M., Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev Biol. 1990 Mar;138(1):104–113. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- Perdew G. H., Schaup H. W., Selivonchick D. P. The use of a zwitterionic detergent in two-dimensional gel electrophoresis of trout liver microsomes. Anal Biochem. 1983 Dec;135(2):453–455. doi: 10.1016/0003-2697(83)90711-x. [DOI] [PubMed] [Google Scholar]
- Saunders J. W., Jr Death in embryonic systems. Science. 1966 Nov 4;154(3749):604–612. doi: 10.1126/science.154.3749.604. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Truman J. W. Hormonal control of muscle atrophy and degeneration in the moth Antheraea polyphemus. J Exp Biol. 1984 Jul;111:13–30. doi: 10.1242/jeb.111.1.13. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Truman J. W. Hormonal control of rates of metamorphic development in the tobacco hornworm Manduca sexta. Dev Biol. 1983 Sep;99(1):103–114. doi: 10.1016/0012-1606(83)90257-9. [DOI] [PubMed] [Google Scholar]
- Schwartz L. M., Truman J. W. Peptide and steroid regulation of muscle degeneration in an insect. Science. 1982 Mar 12;215(4538):1420–1421. doi: 10.1126/science.6278594. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Edgar D. Neurotrophic factors. Science. 1985 Jul 19;229(4710):238–242. doi: 10.1126/science.2409599. [DOI] [PubMed] [Google Scholar]
- Weber R. Inhibitory effect of actinomycin D on tail atrophy in Xenopus larvae at metamorphosis. Experientia. 1965 Nov 15;21(11):665–666. doi: 10.1007/BF02144069. [DOI] [PubMed] [Google Scholar]
- Yuan J. Y., Horvitz H. R. The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol. 1990 Mar;138(1):33–41. doi: 10.1016/0012-1606(90)90174-h. [DOI] [PubMed] [Google Scholar]