Abstract
Dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) plays a central role in medium spiny neurons in the neostriatum in the integration of various neurotransmitter signaling pathways. In its Thr-34-phosphorylated form, it acts as a potent protein phosphatase-1 inhibitor, and, in its Thr-75-phosphorylated form, it acts as a cAMP-dependent kinase inhibitor. Here, we investigated glutamate-dependent signaling cascades in mouse neostriatal slices by analyzing the phosphorylation of DARPP-32 at Thr-34 and Thr-75. Treatment with glutamate (5 mM) caused a complex change in DARPP-32 Thr-34 phosphorylation. An initial rapid increase in Thr-34 phosphorylation was NMDA/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/metabotropic glutamate-5 receptor-dependent and was mediated through activation of a neuronal nitric oxide synthase/nitric oxide/cGMP/cGMP-dependent kinase signaling cascade. A subsequent decrease in phosphorylation was attributable to activation of an NMDA/AMPA receptor/Ca2+/protein phosphatase-2B signaling cascade. This decrease was followed by rephosphorylation via a pathway involving metabotropic glutamate-5 receptor/phospholipase C and extracellular receptor kinase signaling cascade. Treatment with glutamate initially decreased Thr-75 phosphorylation through activation of NMDA/AMPA receptor/Ca2+/protein phosphatase-2A signaling. Thereafter, glutamate slowly increased Thr-75 phosphorylation through activation of metabotropic glutamate-1 receptor/phospholipase C signaling. Our analysis of DARPP-32 phosphorylation in the neostriatum revealed that glutamate activates at least five different signaling cascades with different time dependencies, resulting in complex regulation of protein kinase and protein phosphatase activities.
Keywords: dopamine, striatum, nitric oxide, metabotropic glutamate receptor, protein phosphatase
DARPP-32, a dopamine- and cAMP-regulated phosphoprotein of 32 kDa, is a signal transduction molecule that is selectively enriched in medium spiny neurons in the neostriatum (1). Mice lacking DARPP-32 exhibit profound deficits in their molecular, electrophysiological, and behavioral responses to dopamine, drugs of abuse, and antipsychotic medication (2), indicating an essential role for DARPP-32 in dopamine signaling. DARPP-32 is phosphorylated at Thr-34 by cAMP-dependent protein kinase (PKA), resulting in its conversion into a potent inhibitor of protein phosphatase-1 (PP-1). DARPP-32 is phosphorylated at Thr-75 by cyclin-dependent kinase 5 (Cdk5) (3). DARPP-32 phosphorylated at Thr-75 inhibits PKA activity and thereby reduces the efficacy of dopamine signaling. Dopamine, by means of dopamine D1 receptors, activates PKA, which directly stimulates DARPP-32 Thr-34 phosphorylation and indirectly stimulates DARPP-32 Thr-75 dephosphorylation (4, 5). By regulating the activity of PKA and PP-1, dopamine controls the state of phosphorylation of various intracellular targets.
Glutamate is the major excitatory neurotransmitter in the brain. The excitation of medium spiny neurons is regulated by a balance of glutamatergic inputs from corticostriatal and thalamostriatal pathways and dopaminergic inputs from the nigrostriatal pathway (6, 7). Through the use of various selective agonists and antagonists, glutamate receptor activation has been shown to modulate dopaminergic signaling. Several different signal transduction pathways initiated by NMDA/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and metabotropic glutamate (mGlu) receptors have been described. Activation of NMDA and AMPA receptors decreases DARPP-32 phosphorylation at Thr-34 and Thr-75 through Ca2+-dependent activation of protein phosphatase-2B (PP-2B) (calcineurin) and protein phosphatase-2A (PP-2A), respectively (8). Activation of group I mGlu-5 (mGlu5) receptors increases DARPP-32 Thr-34 phosphorylation by potentiating cAMP formation coupled to adenosine A2A receptors in an extracellular signal-regulated kinase (ERK)-dependent manner (9). Activation of group I mGlu receptors also increases DARPP-32 phosphorylation at Thr-75 as well as Ser-130 [casein kinase-1 (CK1)-site] (10). Group I mGlu receptors stimulate phospholipase C (PLC) and CK1 (11), resulting in activation of Cdk5 by an unknown mechanism, leading to phosphorylation of Thr-75. However, the signal transduction pathways mediating the biological effects of glutamate per se have not received much attention. Here, we have investigated the effects of various signaling pathways, activated by glutamate, on the phosphorylation of DARPP-32 at Thr-34 and Thr-75.
Materials and Methods
Preparation and Incubation of Striatal Slices. Neostriatal slices were prepared from male C57BL/6 mice (6–8 weeks old), as described in ref. 8. Slices were treated with drugs as specified in each experiment, and the following drugs were obtained: glutamate, MK-801, and SCH23390 from Sigma; 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), LY367385, 2-methyl-6-(phenylethynyl)pyridine (MPEP), 7-nitroindazole monosodium salt (7-NINA), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), U0126, U73122, and ZM241385 from Tocris Cookson (Bristol, U.K.); and calyculin A, cyclosporin A, and okadaic acid from LC Laboratories (Woburn, MA). After drug treatment, slices were transferred to Eppendorf tubes, frozen on dry ice, and stored at –80°C until assayed.
Immunoblotting. Frozen tissue samples were sonicated in boiling 1% SDS. Equal amounts of protein (100 μg) were processed by using 12% acrylamide gels as described in ref. 8. Immunoblotting was carried out by using a monoclonal antibody raised against a DARPP-32 peptide containing phospho-Thr-34, the site phosphorylated by PKA (mAb-23; 1:750 dilution) (30), and an affinity-purified polyclonal antibody raised against a DARPP-32 peptide containing phospho-Thr-75, the site phosphorylated by Cdk5 (1:6,000 dilution) (3). A monoclonal antibody (C24-5a; 1:7,500 dilution) generated against DARPP-32 (12), which is not phosphorylation state-specific, was used for reblotting the membranes to determine the total amount of DARPP-32 in samples. None of the experimental manipulations used in the present study altered the total amount of DARPP-32.
In some experiments, the membranes were immunoblotted by using an affinity-purified antibody that selectively detected the phosphorylated form of neuronal nitric oxide synthase (nNOS) at Ser-847 (1:500 dilution) (13) and then were reblotted by using an nNOS antibody (Santa Cruz Biotechnology) (1:2,000 dilution).
Antibody binding was detected by the ECL immunoblotting detection system (Amersham Pharmacia). Phospho-DARPP-32 and phospho-nNOS bands were quantified by densitometry using nih image 1.61 software (http://rsb.info.nih.gov/nih-image/). Samples from control and drug-treated slices were routinely analyzed on individual immunoblots. For each experiment, values obtained for treated slices were calculated relative to the value for the control slices. Normalized data from multiple experiments were averaged, and statistical analysis was carried out by using one-way ANOVA and Newman–Keuls test, unless specified.
Results
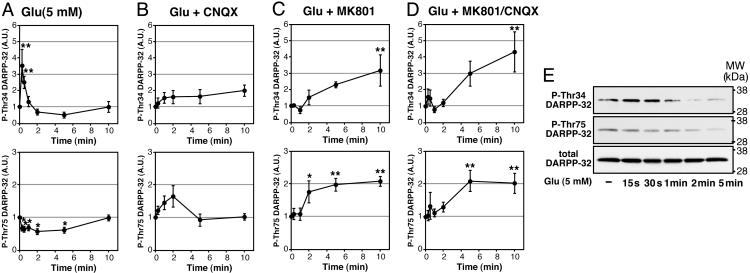
Effect of Glutamate on DARPP-32 Phosphorylation at Thr-34 and Thr-75 in Neostriatal Neurons. Treatment of neostriatal slices with glutamate (5 mM) rapidly increased the level of phospho-Thr-34 DARPP-32 by 3.5-fold within 15 s of incubation (Fig. 1A). The increase in DARPP-32 Thr-34 phosphorylation was transient, and phosphorylation returned to the basal level at 1 min of incubation. At 2 and 5 min of incubation time, the level of phospho-Thr-34 DARPP-32 had a tendency to be below the basal level, but the decrease was not statistically significant. Treatment with glutamate (5 mM) decreased the level of phospho-Thr-75 DARPP-32 to 60% of control by 5 min of incubation. The level of phospho-Thr-75 DARPP-32 returned to the basal level at 10 min of incubation.
Fig. 1.
Regulation of DARPP-32 phosphorylation at Thr-34 and Thr-75 by glutamate and the role of ionotropic glutamate receptors in neostriatal slices. Neostriatal slices were incubated in the absence (A) or presence (B–D) of an AMPA receptor antagonist, CNQX (20 μM) (B), an NMDA receptor antagonist, MK801 (100 μM) (C), or CNQX plus MK801 (D) for a total of 20 min, with addition of l-glutamic acid HCl (5 mM) for the indicated times at the end of incubation. (Upper) Phosphorylation at Thr-34. (Lower) Phosphorylation at Thr-75. A.U., arbitrary units. Data represent means ± SEM for 4–10 experiments. *, P < 0.05; **, P < 0.01, compared with 0 min. (E) Typical immunoblots for detection of phospho-Thr-34, phospho-Thr-75, and total DARPP-32 in the same membrane.
Role of Ionotropic and mGlu Receptors in the Regulation of DARPP-32 Phosphorylation. To investigate the role of ionotropic NMDA and AMPA receptors, the effect of glutamate was examined in the presence of an NMDA receptor antagonist, MK801 (100 μM); an AMPA receptor antagonist, CNQX (20 μM); or MK801 plus CNQX (Fig. 1 B–D). Pretreatment with MK801, CNQX, or MK801 plus CNQX did not affect the basal level of phospho-Thr-34 DARPP-32 but abolished the glutamate-induced, rapid increase in DARPP-32 Thr-34 phosphorylation (15- to 30-s incubation) and the glutamate-induced tendency to decrease Thr-34 phosphorylation below the basal level at 2 and 5 min of incubation. Furthermore, in the presence of MK801 alone or MK801 plus CNQX, glutamate increased the level of phospho-Thr-34 DARPP-32 at 10 min of incubation by 3- or 4-fold, respectively. In the presence of CNQX, glutamate slightly increased the level of phospho-Thr-34 DARPP-32 at 10 min of incubation, but the effect was not statistically significant. These results suggest that the rapid increase and the subsequent decrease in DARPP-32 Thr-34 phosphorylation induced by glutamate is mediated through activation of NMDA and AMPA receptors. The results also revealed a third delayed phase of glutamate-dependent signaling that was masked by the actions of NMDA and AMPA receptor activation.
Pretreatment with MK801, CNQX, or MK801 plus CNQX did not affect the basal level of phospho-Thr-75 DARPP-32 but abolished the glutamate-induced decrease in Thr-75 phosphorylation. In the presence of MK801 or MK801 plus CNQX, glutamate (5 mM) increased the level of phospho-Thr-75 DARPP-32 by 2-fold at 5 and 10 min of incubation. These results suggest that the decrease in DARPP-32 Thr-75 phosphorylation induced by glutamate is mediated through activation of NMDA and AMPA receptors.
To identify mGlu receptors involved in the regulation of DARPP-32 Thr-34 and Thr-75 phosphorylation by glutamate, the effect of glutamate was examined in the presence of an mGlu5 receptor antagonist, MPEP (10 μM); an mGlu1 receptor antagonist, LY341495 (100 μM); or MPEP plus LY367385 (compare Fig. 2 with Fig. 1A). Pretreatment with MPEP, LY341495, or MPEP plus LY341495 did not affect the basal level of phospho-Thr-34 or phospho-Thr-75 DARPP-32. Pretreatment with MPEP or with MPEP plus LY341495, but not LY341495, abolished the rapid increase in DARPP-32 Thr-34 phosphorylation induced by glutamate. Pretreatment with MPEP and/or LY341495 did not affect the glutamate-induced decrease in Thr-75 phosphorylation.
Fig. 2.
Role of mGlu receptors in the regulation of DARPP-32 phosphorylation by glutamate. Neostriatal slices were preincubated in the presence of an mGlu5 receptor antagonist, MPEP (10 μM) (A), an mGlu1 receptor antagonist, LY367385 (100 μM) (B), or MPEP plus LY367385 (C) for a total of 20 min, with addition of glutamate (5 mM) for the indicated times. Data represent means ± SEM for 6–12 experiments. *, P < 0.05; **, P < 0.01, compared with 0 min.
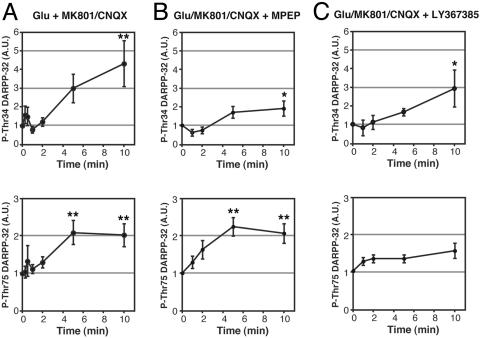
We next examined the role of mGlu receptors on the glutamate-induced regulation of DARPP-32 Thr-34 and Thr-75 phosphorylation, when glutamate effects on both NMDA and AMPA receptors were blocked (Fig. 3). The glutamate-induced increase in Thr-34 phosphorylation observed in the presence of MK801 plus CNQX was attenuated by MPEP (10 μM) (two-way ANOVA, P < 0.01). LY341495 (100 μM) slightly attenuated the delayed increase in Thr-34 phosphorylation, but the effect was not statistically significant. These results suggest that mGlu5 receptors are involved in glutamate-induced regulation of DARPP-32 Thr-34 phosphorylation in two ways: (i) a rapid increase in Thr-34 phosphorylation and (ii) a delayed increase in Thr-34 phosphorylation that is counteracted by the effects of NMDA and AMPA receptors.
Fig. 3.
Role of mGlu receptors in the glutamate-induced increase in DARPP-32 phosphorylation under conditions of NMDA and AMPA receptor blockade. Neostriatal slices were preincubated in the presence of MK801 (100 μM)/CNQX (20 μM) (A), MK801/CNQX plus MPEP (10 μM) (B), or MK801/CNQX plus LY367385 (100 μM) (C) for a total of 20 min, with addition of glutamate (5 mM) for the indicated times. Data represent means ± SEM for 4–7 experiments. *, P < 0.05; **, P < 0.01, compared with 0 min. A is reproduced from Fig. 1D for comparison.
The glutamate-induced increase in Thr-75 phosphorylation in the presence of MK801 plus CNQX was attenuated by LY341495 (100 μM) (two-way ANOVA, P < 0.01) but was not affected by MPEP (10 μM). Thus, under these conditions, activation of mGlu1 receptors by glutamate stimulates the phosphorylation of DARPP-32 at Thr-75.
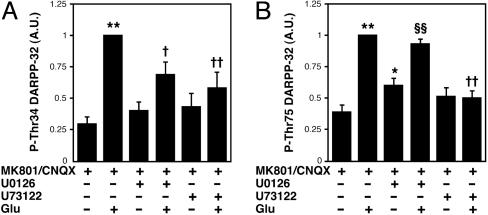
Role of ERK and PLCβ Signaling in mGlu Receptor-Mediated Regulation of DARPP-32 Phosphorylation. To further investigate the mechanism by which mGlu5 and mGlu1 receptors regulate DARPP-32 phosphorylation, the effects of an ERK inhibitor, U0126, and a PLCβ inhibitor, U73122, were examined on the glutamate-induced increase in DARPP-32 Thr-34 and Thr-75 phosphorylation in the presence of MK801 and CNQX (Fig. 4). Both U0126 (40 μM) and U73122 (25 μM) attenuated the stimulatory effect of glutamate on Thr-34 phosphorylation. In contrast, the stimulatory effect of glutamate on Thr-75 phosphorylation was attenuated by U73122 but not U0126. These results, together with those presented in Fig. 3, suggest that the delayed increase in Thr-34 phosphorylation is mediated through PLCβ and ERK signaling, activated by mGlu5 receptors, and that the delayed increase in Thr-75 phosphorylation is mediated through PLCβ signaling, activated by mGlu1 receptors.
Fig. 4.
Role of ERK and PLCβ in the glutamate-induced increase in DARPP-32 phosphorylation under conditions of NMDA and AMPA receptor blockade. Neostriatal slices were preincubated in the presence of MK801 (100 μM)/CNQX (20 μM), MK801/CNQX plus U0126 (40 μM), or MK801/CNQX plus U73122 (25 μM) for a total of 70 min, with addition of glutamate (5 mM) for 10 min. The quantified data were normalized to values obtained from slices treated with glutamate in the presence of MK801/CNQX. Data represent means ± SEM for 5–8 experiments. *, P < 0.05; **, P < 0.01, compared with MK801/CNQX alone; †, P <0.05; ‡, P < 0.01, compared with glutamate in the presence of MK801/CNQX; §§, P < 0.01, compared with MK801/CNQX plus U0126.
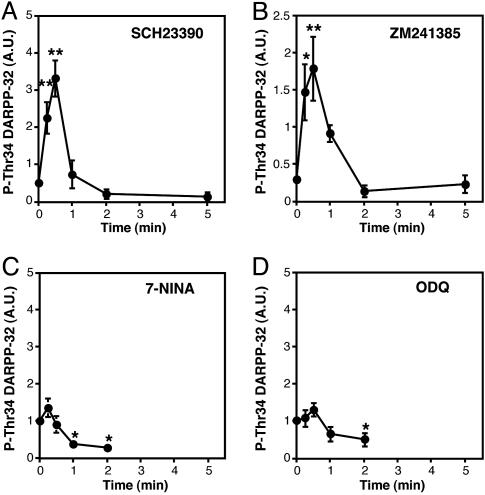
Role of nNOS/NO/cGMP/cGMP-Dependent Protein Kinase (PKG) Signaling in Glutamate-Induced Rapid Increase in DARPP-32 Thr-34 Phosphorylation. We next investigated the mechanisms by which activation of ionotropic NMDA and AMPA glutamate receptors rapidly increased DARPP-32 Thr-34 phosphorylation in neostriatal slices. Because dopamine (14) and adenosine (15), which stimulate DARPP-32 Thr-34 phosphorylation, are released in response to activation of NMDA and AMPA receptors, the effect of glutamate was examined in the presence of a dopamine D1 receptor antagonist, SCH23390, or an adenosine A2A receptor antagonist, ZM241385. Neither SCH23390 nor ZM241385 affected the rapid increase in DARPP-32 Thr-34 phosphorylation induced by glutamate (compare Fig. 5 A and B with Fig. 1A).
Fig. 5.
Role of nNOS/NO/cGMP/PKG signaling in the regulation of DARPP-32 phosphorylation at Thr-34 by glutamate. Neostriatal slices were preincubated in the presence of a dopamine D1 receptor antagonist, SCH23390 (1 μM) (A), an adenosine A2A receptor antagonist, ZM241385 (1 μM) (B), an nNOS inhibitor, 7-NINA (10 μM) (C), or a soluble guanylyl cyclase inhibitor, ODQ (30 μM) (D), for a total of 15 min, with addition of glutamate (5 mM) for the indicated times. Data represent means ± SEM for 3–5 experiments. *, P < 0.05; **, P < 0.01, compared with 0 min.
Glutamate, by activating NMDA and AMPA receptors, stimulates the synthesis of NO in nNOS- and somatostatin-positive interneurons in the neostriatum (16), and the synthesized NO regulates the function of medium spiny neurons (17). DARPP-32 at Thr-34 also is an excellent substrate for PKG as well as PKA (18, 19). We therefore examined the possible involvement of nNOS/NO signaling in the glutamate-induced increase in DARPP-32 Thr-34 phosphorylation. Pretreatment of slices with an nNOS inhibitor, 7-NINA (10 μM), or a soluble guanylyl cyclase inhibitor, ODQ (30 μM), did not affect the basal level of phospho-Thr-34 DARPP-32 but abolished the glutamate-induced increase in DARPP-32 Thr-34 phosphorylation at 15 and 30 s of incubation (Fig. 5 C and D). The results with 7-NINA and ODQ indicate that glutamate activates nNOS/NO/soluble guanylyl cyclase/cGMP/PKG signaling, leading to the rapid and transient phosphorylation of DARPP-32 at Thr-34.
Role of PPs in the Regulation of DARPP-32 Phosphorylation by Glutamate. To investigate the involvement of PPs, the effect of glutamate was examined in the presence of a PP-2B inhibitor, cyclosporin A (10 μM), or a PP-2A/PP-1 inhibitor, okadaic acid (1 μM), in neostriatal slices (20). Pretreatment of neostriatal slices with cyclosporin A and okadaic acid increased the basal level of phospho-Thr-34 by 4.5- and 6.2-fold, respectively. In the presence of cyclosporin A, treatment with glutamate (5 mM) increased the level of phospho-Thr-34 DARPP-32 by 5.2-fold over the cyclosporin A-stimulated level at 30 s of incubation (Fig. 6A Upper). Moreover, the stimulatory effect of glutamate was prolonged, compared with that in the absence of cyclosporin A. These results, together with those shown Fig. 1, suggest that, following the rapid increase in Thr-34 phosphorylation through activation of nNOS/NO/cGMP/PKG signaling, glutamate, through activation of AMPA and NMDA receptors, stimulates the dephosphorylation of DARPP-32 at Thr-34 by PP-2B.
Fig. 6.
Role of protein phosphatases in the regulation of DARPP-32 phosphorylation by glutamate. Neostriatal slices were preincubated in the presence of cyclosporin A (1 μM) (A) or okadaic acid (1 μM) (B) for a total of 65 min, with addition of glutamate (5 mM) for 5 min. Data represent means ± SEM for 4–7 experiments. *, P < 0.05; **, P < 0.01, compared with 0 min.
Okadaic acid abolished the glutamate-induced, rapid increase in DARPP-32 Thr-34 phosphorylation (Fig. 6B Upper). The results indicate that the ability of the glutamate-activated nNOS/NO/cGMP/PKG signaling cascade to induce a rapid increase in Thr-34 phosphorylation requires active PP-2A and/or PP-1.
Pretreatment of neostriatal slices with cyclosporin A did not affect the basal level of phospho-Thr-75 DARPP-32. In the presence of cyclosporin A, glutamate (5 mM) slightly increased the level of phospho-Thr-75 DARPP-32 at 15 and 30 s and decreased the level at 5 min of incubation (Fig. 6A Lower). The small increase in Thr-75 phosphorylation at early time points is presumably mediated by an indirect effect of cyclosporin A, because phospho-Thr-75 DARPP-32 is not a substrate for PP-2B in vitro or in slices (5). Pretreatment with okadaic acid increased the basal level of phospho-Thr-75 DARPP-32 by 8.1-fold and abolished any effect of glutamate (Fig. 6B Lower). In agreement with a previous report (8), these results suggest that the decrease in DARPP-32 Thr-75 phosphorylation is mediated through NMDA and AMPA receptor-dependent activation of PP-2A.
Regulation of nNOS Ser-847 Dephosphorylation by PP-2A. It has been reported that, in vitro or in cells, nNOS is phosphorylated at Ser-847 by Ca2+/calmodulin-dependent protein kinase II and dephosphorylated by PP-2A (13, 21). Furthermore, the phosphorylation of nNOS at Ser-847 results in the inhibition of nNOS activity (21). We therefore studied the effects of PP-2A/PP-1 inhibitors, okadaic acid and calyculin A, and a PP-2B inhibitor, cyclosporin A, on nNOS Ser-847 phosphorylation in neostriatal slices. We have previously reported that okadaic acid inhibits PP-2A/PP-1 activity by 80%/5% at 200 nM and by 95%/35% at 1 μM, and that calyculin A inhibits by 60%/65% at 200 nM (20). Treatment with calyculin A or okadaic acid, each at 200 nM, increased the level of phospho-Ser-847 nNOS by ≈11-fold, and treatment with okadaic acid at 1 μM increased the level by 18-fold (Fig. 7). Treatment with cyclosporin A did not affect nNOS Ser-847 phosphorylation. The results, showing the similar sensitivity to calyculin A and okadaic acid, suggest that nNOS at Ser-847 is mainly dephosphorylated by PP-2A in neostriatal slices.
Fig. 7.
Regulation of nNOS Ser-847 dephosphorylation by PP inhibitors in neostriatal slices. (Right) Neostriatal slices were incubated with calyculin A (CalyA, 200 nM), okadaic acid (OKA, 200 nM and 1 μM), or cyclosporin A (CyA, 10 μM) for 60 min. Immunoblots for detection of phospho-Ser-847 nNOS and total nNOS in the same membrane are shown (Left). Data represent means ± SEM for 4–7 experiments. **, P < 0.01, compared with control; ††, P < 0.01, compared with CalyA (200 nM); §§, P < 0.01, compared with OKA (200 nM).
Discussion
Glutamate is the major excitatory neurotransmitter in the brain and regulates multiple signal transduction cascades. Analysis of the regulation of DARPP-32 phosphorylation at Thr-34 (PKA-site) and Thr-75 (Cdk5-site) in neostriatal neurons revealed that glutamate, through activation of ionotropic (NMDA/AMPA) and/or mGlu receptors, modulates dopamine/DARPP-32 signaling by activating five different signal transduction cascades (Fig. 8): (i) NMDA/AMPA and mGlu5 receptor-mediated nNOS/NO/cGMP/PKG signaling (Thr-34↑), (ii) NMDA/AMPA receptor-mediated Ca2+/PP-2B signaling (Thr-34↓), (iii) NMDA/AMPA receptor-mediated Ca2+/PP-2A signaling (Thr-75↓), (iv) mGlu5 receptor-mediated PLC and ERK signaling (Thr-34↑), and (v) mGlu1 receptor-mediated PLC/CK1/Cdk5 signaling (Thr-75↑). These signal transduction pathways are activated with three different time courses: (i) rapid (15–30 s) activation of nNOS/NO/cGMP/PKG signaling, (ii) intermediate (1–5 min) activation of Ca2+/PP-2B and Ca2+/PP-2A signaling, and (iii) slow (5–10 min) activation of mGlu5 receptor/PLC and ERK and mGlu1 receptor/PLC signaling. Three signal transduction pathways, nNOS/NO/cGMP/PKG (Thr-34↑), mGlu5 receptor/PLC and ERK (Thr-34↑), and Ca2+/PP-2A (Thr-75↓), contribute positively, whereas two signal transduction pathways, Ca2+/PP-2B (Thr-34↓) and mGluR1 receptor/PLC (Thr-75↑), are antagonistic to dopamine/D1 receptor/phospho-Thr-34 DARPP-32 signaling.
Fig. 8.
Signaling cascades activated by glutamate NMDA/AMPA and mGlu receptors. Glutamate activates multiple signaling cascades by activation of NMDA/AMPA and mGlu1/5 receptors (A), leading to the modulation of DARPP-32 phosphorylation at Thr-34 (B) and Thr-75 (C) and the regulation of PP-1 and PKA activities. Pathways leading to increased phospho-Thr-34 DARPP-32 and increased PP-1 inhibition are shown in blue, and those leading to decreased phospho-Thr-34 DARPP-32 and decreased PP-1 inhibition are shown in red. Inhibition of PP-1 by phospho-Thr-34 DARPP-32 and inhibition of PKA by phospho-Thr-75 DARPP-32 are shown in orange. (B) In the initial 15–30 s of incubation, activation of NMDA/AMPA and mGlu5 receptors in nNOS-positive interneurons stimulates the synthesis and release of NO, leading to activation in medium spiny neurons of PKG and DARPP-32 Thr-34 phosphorylation. At 1–5 min of incubation, activation of NMDA/AMPA receptors induces Ca2+-dependent activation of PP-2B, resulting in the dephosphorylation of Thr-34. At 5–10 min of incubation, there is a gradual increase in DARPP-32 Thr-34 phosphorylation. This increase is due, in part, to activation of a pathway involving mGlu5 receptor/PLC/CK1/phospho-Ser-130 DARPP-32, resulting in inhibition of dephosphorylation of phospho-Thr-34 by PP-2B, and, in part, to activation of signaling through the adenosine A2A receptor pathway. A synergistic activation of adenosine A2A receptor signaling involves (i) activation of an mGlu5 receptor/ERK pathway and, through some yet unknown mechanism, increased efficiency of A2A receptor-induced cAMP formation, and (ii) increased extracellular adenosine levels arising from both medium spiny neurons and presynaptic terminals (15). (C) In the initial 5 min of incubation, activation of NMDA/AMPA receptors induces Ca2+-dependent activation of PP-2A, resulting in the dephosphorylation of Thr-75. Subsequently, activation of a pathway involving mGlu1 receptor/PLC/CK1/Cdk5 increases the phosphorylation of Thr-75.
Regulation of DARPP-32 Thr-34 Phosphorylation by Glutamate NMDA/AMPA and mGlu5 Receptor/nNOS/NO/cGMP/PKG Signaling. Glutamate induces a rapid and transient increase in DARPP-32 Thr-34 phosphorylation. The increase in Thr-34 phosphorylation is NMDA/AMPA- and mGlu5-receptor-dependent and is abolished by inhibitors of nNOS and soluble guanylyl cyclase, indicating that glutamate activates nNOS in somatostatin-positive, GABAergic interneurons, where nNOS is expressed in the neostriatum (16) and stimulates the synthesis of NO. The NO diffuses into medium spiny neurons and activates soluble guanylyl cyclase, resulting in the formation of cGMP and activation of PKG. DARPP-32 Thr-34 is an excellent substrate for PKG as well as PKA (18, 19), and therefore the activated PKG induces the phosphorylation of DARPP-32 at Thr-34. An increase in intracellular Ca2+, induced by Ca2+ influx from extracellular space through activation of NMDA and AMPA receptors (22) and by Ca2+ release from intracellular pools through activation of mGlu receptors (23), has been shown to activate nNOS and stimulate the formation of NO. Activation of nNOS/NO/cGMP/PKG signaling by glutamate and the subsequent increase in DARPP-32 Thr-34 phosphorylation likely play a physiological role, because both signaling components are required for the induction of long-term depression of excitatory synaptic potentials in the neostriatum (17, 24).
The state of phosphorylation of nNOS at Ser-847 is regulated by the balance of phosphorylation by Ca2+/calmodulin-dependent protein kinase and dephosphorylation by PP-2A, and the phosphorylated form of nNOS at Ser-847 has lower catalytic activity (13, 21). Our analysis revealed that dephosphorylation of nNOS at Ser-847 is mainly regulated by PP-2A in the neostriatum. In slices treated with okadaic acid (1 μM), glutamate failed to stimulate nNOS/NO/cGMP/PKG/DARPP-32 signaling, possibly due to high phosphorylation of nNOS Ser-847 (by ≈20-fold over basal), supporting the inhibitory role of Ser-847 phosphorylation in nNOS activity.
NMDA/AMPA Receptor/Ca2+/PP-2B Signaling. Activation of NMDA and AMPA receptors by glutamate results in an increase in intracellular Ca2+, leading to the stimulation of PP-2B. The dephosphorylation of DARPP-32 at Thr-34 by PP-2B counteracts the stimulatory effect of nNOS/NO/cGMP/PKG signaling on DARPP-32 Thr-34 phosphorylation and moreover reduces DARPP-32 Thr-34 phosphorylation below basal levels. When PP-2B was inhibited by cyclosporin A, the stimulatory effect of nNOS/NO/cGMP/PKG signaling was sustained for a longer time.
mGlu5 Receptor/PLC and ERK Signaling. Activation of mGlu5 receptors by glutamate induces DARPP-32 Thr-34 phosphorylation through activation of PLC and ERK signaling. The results are in agreement with our previous report showing that activation of mGlu5 receptors increases DARPP-32 Thr-34 phosphorylation by potentiating the action of adenosine A2A receptor/cAMP/PKA signaling in an ERK-dependent manner (9). Moreover, activation of group I mGlu receptors stimulates the phosphorylation of DARPP-32 at Ser-130 (CK1-site) through activation of PLC/PP-2B/CK1 signaling (10). In fact, glutamate, under the conditions of NMDA and AMPA receptor blockade, stimulated DARPP-32 Ser-130 phosphorylation in mouse neostriatal slices (A.N., unpublished data). Because the form of DARPP-32 phosphorylated at Ser-130 is resistant to the dephosphorylation of Thr-34 by PP-2B, activation of group I mGlu receptors and the subsequent increase in Ser-130 phosphorylation probably contributes to the increase in DARPP-32 Thr-34 phosphorylation.
Regulation of DARPP-32 Thr-75 Phosphorylation by Glutamate NMDA/AMPA Receptor/Ca2+/PP-2A Signaling. The present results indicate that activation of NMDA and AMPA receptors by glutamate reduces DARPP-32 Thr-75 phosphorylation by stimulating the dephosphorylation by PP-2A, in agreement with our previous studies (8). Activation of PP-2A is mediated through an increase in intracellular Ca2+ (8), but the mechanism by which Ca2+ activates PP-2A is not yet fully understood. It has been reported that PP-2A activity may be influenced by the direct binding of Ca2+ to an EF-hand motif in a PP-2A regulatory B subunit (B″/PR72) (25) and by the interaction with calmodulin-binding proteins such as striatin and S/G2 nuclear autoantigen (26). Because the phosphorylated form of DARPP-32 at Thr-75 inhibits PKA, the decrease in Thr-75 phosphorylation results in an increase in PKA activity by disinhibition (3).
mGlu1 Receptor/PLC Signaling. Group I mGlu receptors activate PLCβ and stimulate the generation of inositol trisphosphate, leading to an increase in intracellular Ca2+. The increased intracellular Ca2+ activates PP-2B, causing dephosphorylation of inhibitory autophosphorylation sites of CK1ε, which results in its activation (11). Cdk5 is subsequently activated by CK1 (10) by an unknown mechanism and stimulates the phosphorylation of DARPP-32 at Thr-75. In the present study, we found that glutamate stimulated DARPP-32 Thr-75 phosphorylation by selectively activating mGlu1 receptor/PLCβ signaling.
In contrast with the regulation of Thr-34 phosphorylation by mGlu5 receptors, Thr-75 phosphorylation was regulated by mGlu1 receptors. The mechanisms of the selective regulation of Thr-34 and Thr-75 phosphorylation by mGlu5 and mGlu1 receptors, respectively, during the delayed phase are not clearly understood. Group I mGlu (mGlu1 and mGlu5) receptors preferentially activate a PLC signaling cascade but also are known to activate other signaling cascades (27). mGlu5 receptors directly interact with adenosine A2A receptors (28) and potentiate adenosine A2A-receptor signaling in an ERK-dependent manner in indirect pathway neurons (9). Furthermore, functions of mGlu1 and mGlu5 receptors are differentially regulated by PKC, G protein-coupled receptor kinase, regulator of G protein signaling, and Src-family protein tyrosine kinase (27, 29). Signaling cascades, differentially activated by mGlu1 or mGlu5 receptors, might be factors in the selective regulation of DARPP-32 at Thr-34 and Thr-75.
In summary, glutamate, through regulation of the activity of at least five signaling pathways with distinct time dependencies, contributes to a complex pattern of phosphorylation of Thr-34 and Thr-75 DARPP-32. Phosphorylation of these two sites, through modulation of PP-1 and PKA activities, controls in a coordinated fashion the state of phosphorylation of a vast number of neuronal phosphoproteins and, thereby, many physiological parameters. These studies highlight the role of DARPP-32 in the interaction of dopamine and glutamate in the neostriatum under normal conditions. Future studies of these pathways also should result in a greater understanding of pathophysiological conditions such as Parkinson's disease and schizophrenia. It will be important in the future to elucidate the precise patterns of glutamate-induced signaling cascades in the two types of medium spiny neurons, the direct/striatonigral and the indirect/striatopallidal pathway neurons.
Acknowledgments
We thank Y. Terasaki, K. Fujisaki, and M. Koga for excellent technical assistance. This research was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (to A.N.), Public Health Service Grants MH40899 and DA10044 (to A.C.N. and P.G.), and Department of Defense Army Grant DAMD17-02-1-00705 (to P.G.).
Author contributions: A.N., H.H., M.T., A.C.N., and P.G. designed research; A.N. performed research; Y.W. contributed new reagents/analytic tools; A.N. and A.C.N. analyzed data; and A.N., A.C.N., and P.G. wrote the paper.
Abbreviations: CK, casein kinase; Cdk5, cyclin-dependent kinase 5; DARPP-32, dopamine- and cAMP-regulated phosphoprotein of 32 kDa; ERK, extracellular signal-regulated kinase; mGlu, metabotropic glutamate; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; nNOS, neuronal NO synthase; PKA, cAMP-dependent kinase; PKG, cGMP-dependent kinase; PLC, phospholipase C; PP, protein phosphatase; PP-1, PP-2A, and PP-2B, protein phosphatases 1, 2A, and 2B; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; MPEP, 2-methyl-6-(phenylethynyl)pyridine; 7-NINA, 7-nitroindazole monosodium salt; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one.
References
- 1.Svenningsson, P., Nishi, A., Fisone, G., Girault, J. A., Nairn, A. C. & Greengard, P. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 269–296. [DOI] [PubMed] [Google Scholar]
- 2.Fienberg, A. A., Hiroi, N., Mermelstein, P., Song, W.-J., Snyder, G. L., Nishi, A., Cheramy, A., O'Callaghan, J. P., Miller, D., Cole, D., et al. (1998) Science 281, 838–842. [DOI] [PubMed] [Google Scholar]
- 3.Bibb, J. A., Snyder, G. L., Nishi, A., Yan, Z., Meijer, L., Fienberg, A. A., Tsai, L. H., Kwon, Y. T., Girault, J. A., Czernik, A. J., et al. (1999) Nature 402, 669–671. [DOI] [PubMed] [Google Scholar]
- 4.Nishi, A., Snyder, G. L. & Greengard, P. (1997) J. Neurosci. 17, 8147–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishi, A., Bibb, J. A., Snyder, G. L., Higashi, H., Nairn, A. C. & Greengard, P. (2000) Proc. Natl. Acad. Sci. USA 97, 12840–12845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Chiara, G., Morelli, M. & Consolo, S. (1994) Trends Neurosci. 17, 228–233. [DOI] [PubMed] [Google Scholar]
- 7.Kaneko, T. & Fujiyama, F. (2002) Neurosci. Res. (N.Y.) 42, 243–250. [DOI] [PubMed] [Google Scholar]
- 8.Nishi, A., Bibb, J. A., Matsuyama, S., Hamada, M., Higashi, H., Nairn, A. C. & Greengard, P. (2002) J. Neurochem. 81, 832–841. [DOI] [PubMed] [Google Scholar]
- 9.Nishi, A., Liu, F., Matsuyama, S., Hamada, M., Higashi, H., Nairn, A. C. & Greengard, P. (2003) Proc. Natl. Acad. Sci. USA 100, 1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu, F., Ma, X. H., Ule, J., Bibb, J. A., Nishi, A., DeMaggio, A. J., Yan, Z., Nairn, A. C. & Greengard, P. (2001) Proc. Natl. Acad. Sci. USA 98, 11062–11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, F., Virshup, D. M., Nairn, A. C. & Greengard, P. (2002) J. Biol. Chem. 277, 45393–45399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemmings, H. C., Jr., & Greengard, P. (1986) J. Neurosci. 6, 1469–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Komeima, K., Hayashi, Y., Naito, Y. & Watanabe, Y. (2000) J. Biol. Chem. 275, 28139–28143. [DOI] [PubMed] [Google Scholar]
- 14.Desce, J. M., Godeheu, G., Galli, T., Artaud, F., Cheramy, A. & Glowinski, J. (1992) Neuroscience 47, 333–339. [DOI] [PubMed] [Google Scholar]
- 15.Latini, S. & Pedata, F. (2001) J. Neurochem. 79, 463–484. [DOI] [PubMed] [Google Scholar]
- 16.Dawson, T. M., Bredt, D. S., Fotuhi, M., Hwang, P. M. & Snyder, S. H. (1991) Proc. Natl. Acad. Sci. USA 88, 7797–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calabresi, P., Gubellini, P., Centonze, D., Picconi, B., Bernardi, G., Chergui, K., Svenningsson, P., Fienberg, A. A. & Greengard, P. (2000) J. Neurosci. 20, 8443–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hemmings, H. C., Jr., Williams, K. R., Konigsberg, W. H. & Greengard, P. (1984) J. Biol. Chem. 259, 14486–14490. [PubMed] [Google Scholar]
- 19.Tsou, K., Snyder, G. L. & Greengard, P. (1993) Proc. Natl. Acad. Sci. USA 90, 3462–3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishi, A., Snyder, G. L., Nairn, A. C. & Greengard, P. (1999) J. Neurochem. 72, 2015–2021. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi, Y., Nishio, M., Naito, Y., Yokokura, H., Nimura, Y., Hidaka, H. & Watanabe, Y. (1999) J. Biol. Chem. 274, 20597–20602. [DOI] [PubMed] [Google Scholar]
- 22.Crespi, F. & Rossetti, Z. L. (2004) J. Pharmacol. Exp. Ther. 309, 462–468. [DOI] [PubMed] [Google Scholar]
- 23.Bhardwaj, A., Northington, F. J., Martin, L. J., Hanley, D. F., Traystman, R. J. & Koehler, R. C. (1997) J. Cereb. Blood Flow Metab. 17, 153–160. [DOI] [PubMed] [Google Scholar]
- 24.Calabresi, P., Gubellini, P., Centonze, D., Sancesario, G., Morello, M., Giorgi, M., Pisani, A. & Bernardi, G. (1999) J. Neurosci. 19, 2489–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janssens, V., Jordens, J., Stevens, I., Van Hoof, C., Martens, E., De Smedt, H., Engelborghs, Y., Waelkens, E. & Goris, J. (2003) J. Biol. Chem. 278, 10697–10706. [DOI] [PubMed] [Google Scholar]
- 26.Moreno, C. S., Park, S., Nelson, K., Ashby, D., Hubalek, F., Lane, W. S. & Pallas, D. C. (2000) J. Biol. Chem. 275, 5257–5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermans, E. & Challiss, R. A. (2001) Biochem. J. 359, 465–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferre, S., Karcz-Kubicha, M., Hope, B. T., Popoli, P., Burgueno, J., Gutierrez, M. A., Casado, V., Fuxe, K., Goldberg, S. R., Lluis, C., et al. (2002) Proc. Natl. Acad. Sci. USA 99, 11940–11945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Blasi, A., Conn, P. J., Pin, J. & Nicoletti, F. (2001) Trends Pharmacol. Sci. 22, 114–120. [DOI] [PubMed] [Google Scholar]
- 30.Snyder, G. L., Girault, J.-A., Chen, J. Y. C., Czernik, A. J., Kebabian, J. W., Nathanson, J. A. & Greengard, P. (1992) J. Neurosci. 12, 3071–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]