Abstract
Human immunodeficiency virus type 1 (HIV-1) infection is associated with B cell activation and exhaustion, and hypergammaglobulinemia. How these changes influence B cell responses to coinfections such as malaria is poorly understood. To address this, we compared B cell phenotypes and Abs specific for the Plasmodium falciparum vaccine candidate apical membrane Ag-1 (AMA1) in HIV-infected and uninfected adults living in Kenya. Surprisingly, HIV-1 infection was not associated with a difference in serum AMA1-specific Ab levels. HIV-infected individuals had a higher proportion of total atypical and total activated memory B cells (MBCs). Using an AMA1 tetramer to detect AMA1-specific B cells, HIV-infected individuals were also shown to have a higher proportion of AMA1-specific atypical MBCs. However, this proportional increase resulted in large part from a loss in the number of naive and resting MBCs rather than an increase in the number of atypical and activated cells. The loss of resting MBCs and naive B cells was mirrored in a population of cells specific for an Ag to which these individuals were unlikely to have been chronically exposed. Together, the data show that changes in P. falciparum Ag–specific B cell subsets in HIV-infected individuals mirror those in the overall B cell population, and suggest that the increased proportion of atypical MBC phenotypes found in HIV-1–infected individuals results from the loss of naive and resting MBCs.
Introduction
In the last three decades, there have been considerable advances in our understanding of HIV-mediated B cell dysfunction. HIV infection has been associated with increased rates of B cell lymphomas, autoimmune diseases, and hypergammaglobulinemia. Functional B cell impairments include decreased vaccine-derived Ig responses as well as increased vulnerability to pathogens known to depend on humoral immune responses including Streptococcus pneumonia, Haemophilus influenza, and malaria (1–8). These declines in Ag-specific Ab responses are paired with a rise in total Ig production and production of polyreactive self-antibodies attributed to nonspecific polyclonal B cell activation (9).
Recent research has sought to understand the mechanism behind humoral immune dysfunction in HIV, focusing on understanding the phenotypic abnormalities seen in the B cell pool. Studies have documented a proportional expansion of peripheral plasmablasts and activated B cells, as well as a decline in the normal adult resting memory B cell pool (10). There has also been considerable interest in a group of cells coined atypical or exhausted memory B cells (10, 11). These are Ag-experienced B cells that have undergone somatic hypermutation, but do not express CD27. They also express inhibitory receptors such as Fc-receptor–like-4 and are hypo-responsive to immune stimuli in vitro (10). Overall, current research suggests that HIV leads to hyperactivated yet dysfunctional memory B cells (12). However, there are critical knowledge gaps between the recently described phenotypic B cell abnormalities and clinically observed B cell disease in HIV-infected adults.
We sought to address one of these gaps in knowledge by answering the following question. When comparing HIV-infected and uninfected individuals, do changes in the phenotypes of circulating B cells that target a specific Ag correlate with a change in Ig level for that same Ag? In malaria-endemic regions, where there are high rates of HIV infection, there is often regular exposure to P. falciparum parasites, allowing for evaluation of B cell responses to a non-HIV Ag to which all study participants are likely to have regular exposure.
There is evidence that HIV-infected individuals have increased vulnerability to malaria and that B cell responses are a critical component of the immune response to blood-stage infection in immunocompetent hosts (7, 8, 13, 14). It is unclear whether increased malaria vulnerability in HIV is the result of alterations in the B cell response to malaria. Some reports have suggested HIV infection has no effect on malaria Ab production (15), whereas others have found a decrease in the breadth of malaria Ag reactivity (16). Assessment of P. falciparum Ag–specific memory B cells has typically been performed with B cell ELISpots, which allow for some assessment of the Ag-specific memory B cell population. However, B cell ELISpots cannot reliably capture all memory B cell subsets including the activated and atypical B cells of interest in HIV, which are suspected to have high rates of apoptosis in cell culture and do not readily differentiate into Ab-secreting cells in vitro, respectively. To address this limitation, we developed a B cell tetramer assay to directly detect P. falciparum Ag-specific B cells. We used this novel assay to phenotype B cells that are specific for a key malaria blood-stage Ag, apical membrane Ag-1 (AMA1), to which Ab responses have been correlated with malaria disease protection (17, 18). We assessed AMA1-specific B cell populations (subset proportion and concentration) in HIV-positive and negative individuals living in Kenya to determine if these phenotypes are correlated with Ab responses to the same Ag.
Materials and Methods
Human enrollment, clinical testing, and specimen collection
Kenyan venous blood samples were collected from HIV-negative individuals aged 18 y or older in a cross-sectional study at the Bondo Sub County Hospital, in Bondo, Western Kenya at the time of HIV testing. All individuals who were seen in the voluntary testing and counseling clinic on enrollment dates between May and October 2012 were offered participation in the study. Individuals with chronic medical conditions or acute systemic illness including fever (≥37.5°C), who were on immunosuppressants, were pregnant, or taking any antimalarials were excluded. Patients were assessed for active malaria infection and helminth infection at the time of the collection with commercial rapid diagnostic testing (CareStart; Access Bio, Somerset, NJ) and fecal Kato Katz examination, respectively. CD4 counts were measured in the Bondo Sub County Hospital. Viral load testing was done by the Centers for Disease Control and Prevention laboratory in Kisumu, Kenya from dried blood spots. PBMCs were separated from venous blood by polysucrose and sodium diatrizoate density gradient centrifugation (Sigma-Aldrich, St. Louis, MO) and cryopreserved in liquid nitrogen in heat-inactivated FBS (Sigma) and 10% dimethyl sulfoxide (Sigma). Plasma samples were stored at −20°C. Plasma was also collected from healthy North American volunteers who had never traveled to malaria-endemic regions. All subjects underwent informed consent. The Ethics Committee at the Kenyan Medical Research Institute and the University of Minnesota Institutional Review Board approved all procedures conducted.
Malaria-specific Ab testing
Ag-specific serum IgG levels were determined for all study participants using a multiplex suspension array technology assay (19). Uniquely dyed microspheres were coupled to 11 malaria Ags, AMA1 (FVO and 3D7), merozoite surface protein (MSP) 1-42 (FVO and 3D7), MSP3 (FVO), erythrocyte binding Ag 175, glutamine rich protein (R2 and R0), erythrocyte binding protein 2, and liver stage Ag 1 (3D7). Ag-coupled beads were incubated with sample sera in a multiplex format. Beads were washed and incubated with the secondary Ab-fluorophore conjugate. Beads were then washed again and counted on the Bio-Plex reader to determine the median fluorescence intensity (MFI) of each sample for each bead set. Arbitrary units were generated and used to standardize results by comparing reactivity of samples to malaria naive North American controls. One arbitrary unit was defined as the mean MFI of the 12 North American samples plus three standard deviations. An individual was considered to be serologically reactive to an Ag if their Ab level was greater than one arbitrary unit.
C-reactive protein measurement
C-reactive protein (CRP), a measurement of acute inflammation often used to evaluate immune activation, was measured using a commercial ELISA assay (Millipore, Darmstadt, Germany). A total of 10% of samples were run in duplicate. The kit used for CRP reports intra- and interassay coefficients of variation of up to 4.6 and 6.0%, respectively. Median interassay coefficients of variation for duplicate measurements of CRP was 20.5%. For samples assayed in duplicate, the average value was used in the final data analysis.
AMA1 B cell tetramer production
AMA1 B cell tetramers were produced using methods described previously (20, 21). Briefly, malaria antigenic tetramer reagent was synthesized using the well-characterized purified recombinant protein AMA1 (FVO) produced using the Pichia pastoris expression system. The recombinant AMA1 (FVO) was biochemically and biophysically comparable to clinical material (22). AMA1 was chemically biotinylated and made tetrameric through binding to a PE-labeled streptavidin core. The biochemical structure of AMA1 is shown in the supplemental data to demonstrate the potential biotinylation sites on the surface of domains 1 and 2 of the recombinant protein (Supplemental Fig. 1) (23). There is evidence that a significant number of B cells will bind PE, which limits many studies utilizing fluorescently labeled B cell Ags (20). To ensure that P. falciparum–specific B cells could be distinguished from PE, streptavidin, and biotin-specific B cells, a second fluorescent reagent was produced by conjugating the fluorochrome Alexa Fluor 680 (AF680) for human staining panel or Alexa Fluor 647 (AF647) for mouse staining panel to streptavidin-PE bound to biotin alone as previously described (24). A histidine (HIS) tag was added to the biotin-PE*AF680 and biotin-PE*AF647 tetramer because the AMA1 Ag contained a HIS tag for purification. From this point on, Ag-specific enriched populations will be described in the following way. The term AMA1-specific B cells will be used to describe B cells that bind the AMA1 tetramer but not the HIS-PE*AF680 tetramer (Supplemental Fig. 1). B cells that bind the HIS-PE*AF680 tetramer and the AMA1 tetramer, which contains, HIS, PE and streptavidin, will be called decoy-specific B cells. Of note, the low-level nonspecific background cannot be completely excluded from these populations.
AMA1 tetramer validation in mice
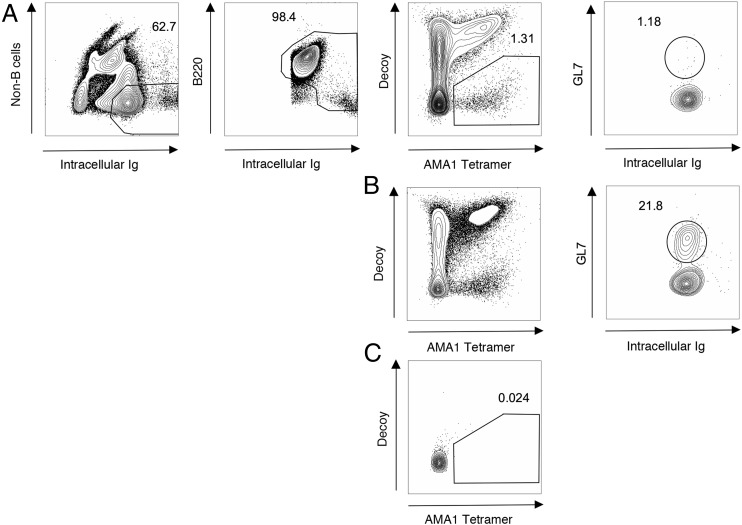
Pooled spleen and lymph nodes were harvested from 1) naive C57BL/6 (B6) mice, 2) B6 mice 7 d after immunization with the AMA1 adjuvanted with CFA, and 3) MD4 RAG–deficient (RAG1−/−) mice. MD4 RAG1−/− mice served as a negative control and have only B cells with B cell receptors specific for hen egg lysozyme. Samples were stained with biotin-PE*AF647 tetramer followed by biotinylated AMA1-PE tetramer. Cells were enriched using a magnetic column and anti-PE magnetic microbeads (Miltenyi Biotec, San Diego, CA). Samples were centrifuged and the column bound, and flow-through fractions were resuspended in sorter buffer (PBS with 2% FBS 0.1% ± Sodium Azide). Cells were incubated on ice for 30 min with a mixture of fluorescently labeled Abs to several mouse lymphocyte surface markers including eF450 labeled B220 (pan B cell marker; eBioscience, San Diego, CA), FITC-labeled GL7 (germinal center B cell marker; BD Biosciences, San Jose, CA), and APC-eF780 labeled surface anti-CD90.2 (anti-Thy1.2), Gr-1, CD11c, and F4/80 (non-B cells; eBioscience). Pelleted samples were resuspended in Cytofix/Cytoperm (BD Biosciences) and incubated for 25 min on ice. They were then washed with permeabilization buffer (BD Biosciences), incubated with Pacific Orange labeled Ig G (intracellular goat anti-mouse IgG H+L; BioLegend, San Diego, CA) on ice for 30 min, then washed again with permeabilization buffer. Cells were then resuspended in sorter buffer. AccuCheck counting beads (Invitrogen, Carlsbad, CA) added to both fractions were used to determine the total live lymphocyte count as previously described (20). All mouse experiments were done utilizing a specific pathogen–free facility under an approved University of Minnesota Institutional Animal Care and Use Committee protocol and in accordance with National Institutes of Health guidelines.
P. falciparum Ag-specific B cell subset flow assay in human PBMCs
Approximately 6–12 million human PBMCs from a subset of participants were thawed and resuspended in 90% RPMI 1640 with 10% heat inactivated FBS. Cells were incubated with the biotin-PE*AF680 tetramer followed by biotinylated AMA1-PE tetramer. Human samples did not undergo magnetic column enrichment. Cells were then incubated with a mixture of fluorescently labeled Abs. This panel included a selection of the following markers: BV421 labeled IgM (BioLegend), FITC, or BV421 labeled IgD (BD Biosciences or BioLegend), PE-Cy-7 labeled CD10 (BioLegend), APC or FITC labeled CD21 (BD Biosciences or BioLegend), BV650 or APC labeled CD27 (BioLegend), V500 labeled CD19 (BD Biosciences), and APC-eF780 labeled CD4, CD14, CD16, and CD8 (eBioscience).
Flow cytometry
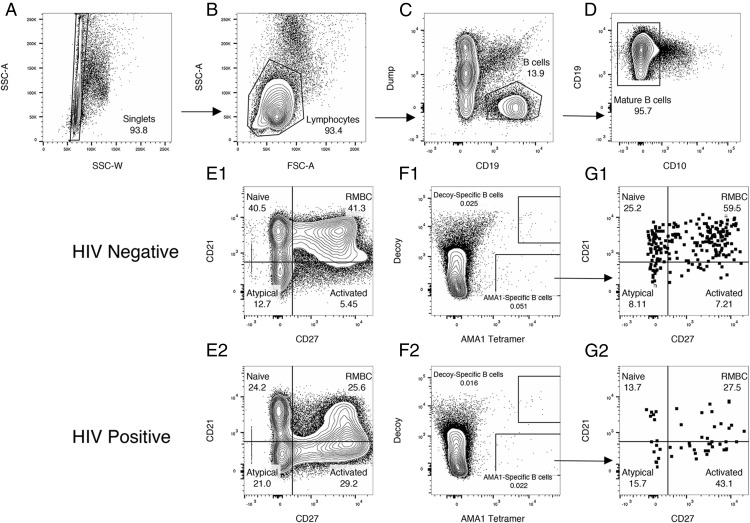
For both mouse and human studies, data were acquired on an LSR II cytometer with either four (355, 405, 488, and 633 nm) or five lasers (355, 405, 488, 561, and 640 nm). FlowJo Software (Tree Star, Ashland, OR) was used for the analysis. The gating strategies for mouse and human samples are shown in Figures 2 and 3, respectively. In human samples, B cells were identified in the following way. Lymphocytes were identified among single cells by forward and side scatter. B cells were identified by expression of CD19 and a lack of CD4, CD14, CD16, and CD8. Mature B cells were identified by a lack of CD10 expression. Among mature B cells, the subsets that have previously been described by Moir and Fauci (12) were identified by CD27 and CD21 expression. Note that human samples were not stained for CD20 so the CD27+CD21low subset contains peripheral plasmablasts. AMA1 and decoy-specific B cells were identified using the gating shown in Fig. 3F1 and 3F2 and in Supplemental Fig. 1. AMA1-specific B cells were those that bound the AMA1 tetramer but not the HIS-PE*AF680 tetramer. Decoy-specific B cells were those that bound the HIS-PE*AF680 tetramer and the AMA1 tetramer and are likely binding epitopes contained in both regents, specifically the PE, streptavidin biotin, and HIS tag. Those that bound the decoy but not the AMA1 tetramer were not included in the decoy-specific B cell counts because there was not sufficient separation between these cells and the dual negative population. Flow cytometry data were collected for human studies over six different experiments.
FIGURE 2.
Representative flow plots for AMA1 tetramer studies in mice. AMA1-specific B cell detection and germinal center reaction in Row (A) Naive B6 mouse and Row (B) B6 mouse 7 d post immunization with AMA1+CFA Row (C) Naive MD4 (anti-HEL BCR Tg) mouse. Numbers indicate percentage of total number of events on that graph that fall within gate.
FIGURE 3.
Identification and phenotyping of AMA1-specific B cells with an AMA1 tetramer in human samples. Representative human flow plots. B cells were identified by flow cytometry in PBMCs. (A) Single cells are selected using side scatter area and width. (B) Lymphocytes were selected based on side and forward scatter. (C) B cells were selected by choosing cells that expressed CD19 and did not express CD14, CD16, CD4, or CD8 (Dump). (D) Mature B cells were selected based on expression of CD10. (E1 and E2) Among mature B cells, naive (CD21hi,CD27−), resting memory (CD21hi,CD27+), activated and plasma cells/blasts (CD21low, CD27+), and atypical (CD21low,CD27−) memory B cells were identified based on CD27 and CD21 expression. (F1 and F2) Among mature B cells, AMA1 and decoy-specific cells are identified using the tetramer and AF680-PE labeled streptavidin. (G1 and G2). Among AMA1-specific B cells, naive (CD21hi,CD27−), resting memory (CD21hi,CD27+), activated and plasma cells/blasts (CD21low, CD27+) and atypical (CD21low,CD27−) memory B cells were identified based on CD27 and CD21 expression, using the same gates as applied to the whole mature B cell population. Numbers indicate percentage of total number of events on that graph that fall within gate.
Statistical analysis
Continuous variables were compared between HIV-infected and uninfected individuals with a Student t test for normally distributed data or with Wilcoxon rank-sum testing if not normally distributed. The distribution of data was examined using a histogram. If data were normal, means are displayed. Medians are reported for non-normal data. Categorical variables were compared between groups with χ2 testing. Correlations between Ab levels, HIV1 viral load, CRP levels, and CD4 counts were assessed with Spearman rank correlation.
Results
HIV infection is not associated with differences in the prevalence or level of P. falciparum–specific Abs
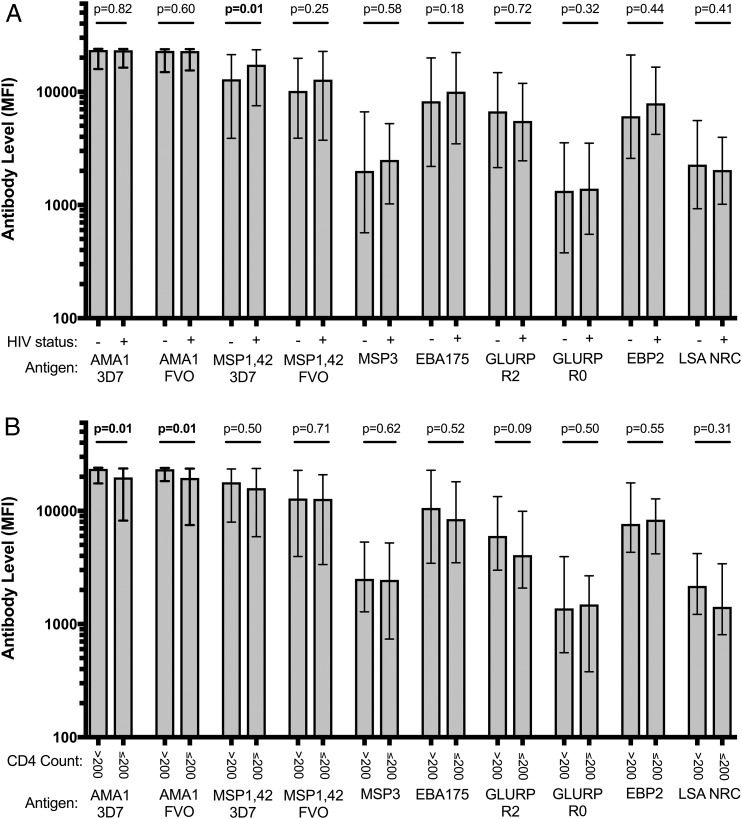
To determine whether HIV infection is associated with differences in the Ab response to P. falciparum, we analyzed the serum from 138 HIV-infected and 52 HIV-uninfected individuals from Kenya (Table I). Overall, no statistically significant differences were detected in the level or prevalence of AMA1 Abs between HIV-positive and negative individuals (Fig. 1, Supplemental Table I). Similar results were found when Abs specific to nine other P. falciparum Ags were assessed, with the exception of MSP1-42 (3D7). Interestingly, there were actually higher IgG levels to MSP1-42 (3D7) in HIV-infected individuals (p = 0.01). Within the HIV-infected group, individuals with CD4 counts below 200 cells per μl had significantly lower Ab levels to both the AMA1 FVO (p = 0.01) and 3D7 strains (p = 0.01, Fig. 1, Supplemental Table I). However, IgG levels to the other P. falciparum Ags did not differ significantly by CD4 count. Of note, there was no significant difference in the age, sex, bednet use, or malaria/helminth infection status between HIV-infected participants with CD4 counts above or below 200. AMA1 Ab levels did not correlate with HIV type 1 (HIV-1) viral load (Spearman rho = −0.13, p = 0.12) or serum C reactive protein (Spearman rho = −0.11, p = 0.19). Together, these data suggest that HIV infection does not lead to a substantial loss of circulating Abs that target malaria Ags in a region with holoendemic malaria.
Table I. Clinical characteristics of study participants in an area of stable malaria transmission in western Kenya.
| HIV Negative, n = 52 | HIV Positive, n = 138 | p Valuea | |
|---|---|---|---|
| Age, y, median (p25, p75)b | 24.6 (21.6, 32.2) | 29.4 (25.3, 36.2) | <0.01 |
| Female sex, n (%) | 25 (48.1) | 84 (60.9) | 0.11 |
| Helminth infection, n (%) | 9 (17.6)c | 31 (22.6)d | 0.46 |
| Malaria blood smear positive, n (%) | 3 (5.8) | 11 (8.0) | 0.61 |
| Bednet use, n (%) | 43 (84.3) | 115 (84.6) | 0.97 |
| CRP, mg/l, median (p25, p75)b | 0.52 (0.27, 1.15) | 4.72 (0.87, 26.12)d | <0.001 |
| WBC × 109 per liter, mean (SD) | 6.31 (1.66)c | 5.95 (2.22)e | 0.30 |
| Lymphocytes, % of WBC, mean (SD) | 32.3 (13.7)c | 33.2 (13.2)f | 0.68 |
| CD4 count (cells per ml), mean, (SD) | 328.6 (228.5)d | ||
| Participants with CD4 < 200, n (%) | 42 (30.7)d | ||
| HIV-1 viral load (copies/ml), median (p25, p75)b | 50370 (14546, 198155)d |
p value comparing HIV-negative to HIV-positive participants using χ2 for proportions and Wilcoxon rank sum test for medians.
p25, p75 = 25th percentile,75th percentile.
n = 51.
n = 137.
n = 128.
n = 129.
FIGURE 1.
Median Ab levels to 10 P. falciparum Ags or antigenic variants by HIV status and CD4 count. Ab concentration (MFI) to 10 P. falciparum Ags or antigenic variants by (A) HIV status and (B) CD4 count among adults living in an area of stable malaria transmission in western Kenya. MSP, merozoite surface protein; EBA, erythrocyte binding Ag; GLURP, glutamine rich protein; EBP, erythrocyte binding protein; LSA-NRC, recombinant liver stage Ag 1. The data are displayed as MFI. Bars represent median MFI and error bars represent interquartile range. The p values were generated using Wilcoxon rank-sum testing.
Validation of an AMA1 tetramer to detect AMA1-specific B cells by flow cytometry
Previous studies have found an increase in the frequency of atypical memory B cells in HIV-infected individuals. In that work, enrichment of this atypical population was seen in HIV-specific B cells, but the distribution of these B cells among these subsets for non-HIV targeting memory B cells is not known (10). In light of the minimal effect that HIV infection appeared to have on Plasmodium-specific Abs in our study, we hypothesized that B cells specific for P. falciparum might similarly be unaffected by HIV-1 infection. To assess the phenotype and frequency of B cells specific for AMA1, using murine samples we generated and validated an AMA1 tetramer for use with flow cytometry. The same tetramer was recently described in the literature for describing human B cells, generated using the similar protocol and AMA1 Ag (21).
In mouse samples, among Ig positive and CD90.2 (T cell marker), Gr-1 (macrophage marker), F4/80 (macrophage marker), and CD11c (dendritic cells marker) negative cells, cells that expressed B220 (B cell marker) or high levels of intracellular Ig were selected. Within this population, AMA1-specific B cells were detected as cells that bind the AMA1-PE tetramer but not a HIS-PE*AF647 tetramer (see Materials and Methods). AMA1-specific B cells were detected in naive B6 mice and were expanded in B6 mice following immunization with AMA1 in CFA (Fig. 2A, 2B). In the vaccinated B6 mice, a large population of AMA1-specific germinal center B cells were detectable as cells expressing GL7, which was absent in naive mice (Fig. 2A, 2B). AMA1 tetramer staining was done in MD4 RAG-1−/− mice, which lack RAG-1 and only have B cells specific for hen egg lysozyme. A significant AMA1 or AF647-specific cell population was not seen in the MD4 mouse, suggesting that the tetramers were binding to the BCR in an Ag-specific manner (Fig. 2C).
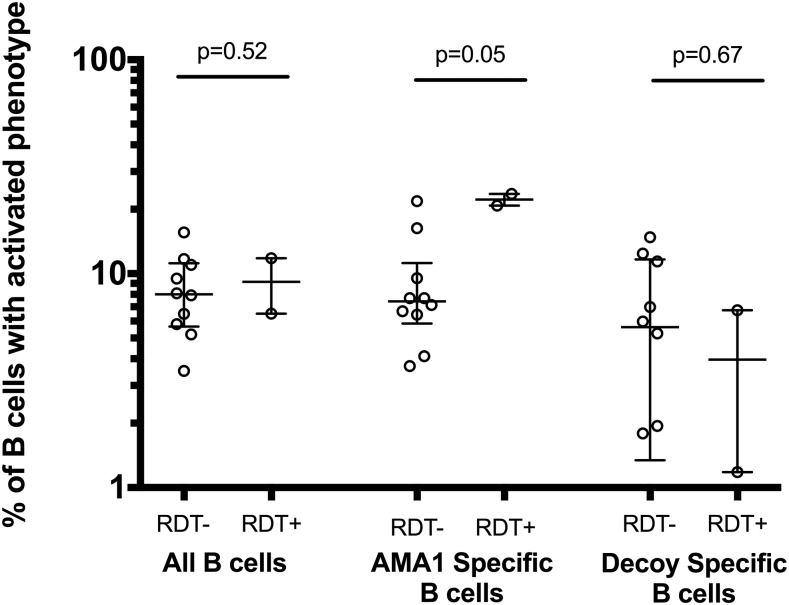
AMA1 tetramers were also used to detect AMA1-specific B cells in human blood. For each of the 34 study participants (22 HIV infected, 12 HIV uninfected, selected by the order of enrollment), 6–12 million PBMCs were incubated with AMA1 tetramers and enriched as described above. AMA1-specific B cells were detected within the CD19+ CD14− CD16− CD4− CD8− B cell population of all individuals assessed. AMA1-specific B cells were rare, accounting for only 0.01–0.15% of total CD19+ CD10− mature B cells in samples tested (Fig. 3). Among all samples, a median of 39 mature AMA1-specific B cells was detected (median of 81 in HIV-negative individuals and 23 in HIV-positive individuals). Two HIV-negative individuals whose samples were among those phenotyped by flow cytometry had asymptomatic malaria infection as determined by a P. falciparum-specific rapid diagnostic test. Among these individuals, we saw an expansion in the percentage of AMA1-specific B cells with the activated phenotype (CD10− CD21low CD27+). There was no corresponding increase in activated B cells in the total B cell pool, or the B cells specific for the HIS-PE*AF680 tetramer (Fig. 4). Together, these data suggest that AMA1 tetramers are able to identify AMA1-specific B cells.
FIGURE 4.
Percentage of activated B cells by malaria infection status among HIV-negative participants as determined by rapid diagnostic test. Left side of the chart shows the percentage of activated B cells among all B cells. The middle of the chart shows the percentage of B cells among B cells that specifically bind to the AMA1 tetramer. The right of the chart shows the degree of activation among decoy-specific cells. Medians are shown with error bars that display the interquartile range. Proportions of B cells between malaria-infected and uninfected individuals were compared with Wilcoxon rank-sum testing.
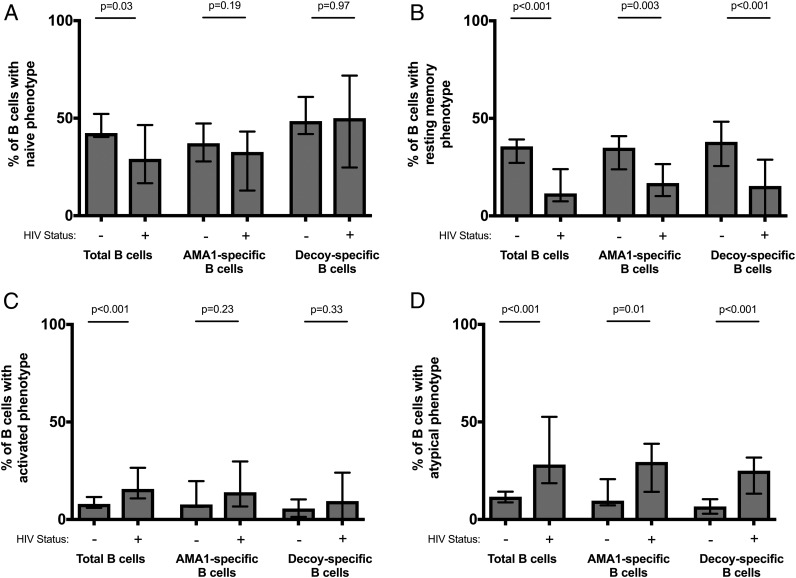
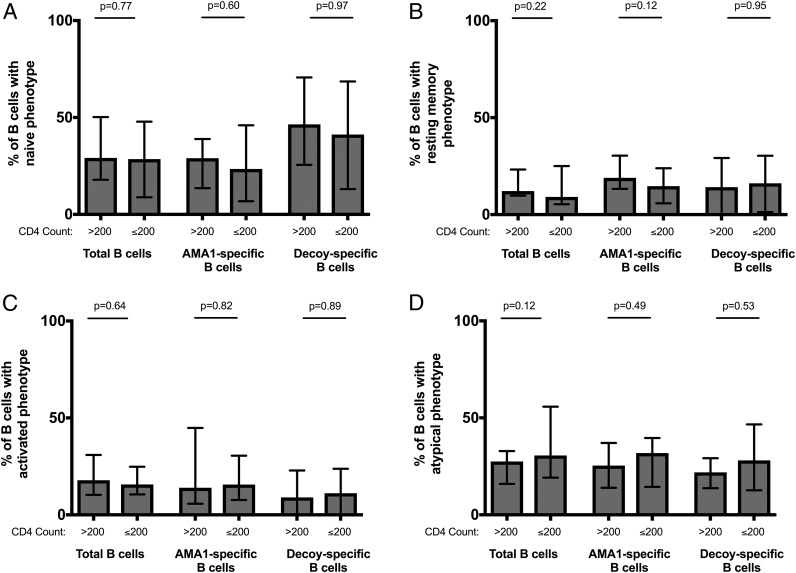
HIV-infected individuals have fewer AMA1-specific memory B cells, but higher proportions of activated and atypical memory B cells
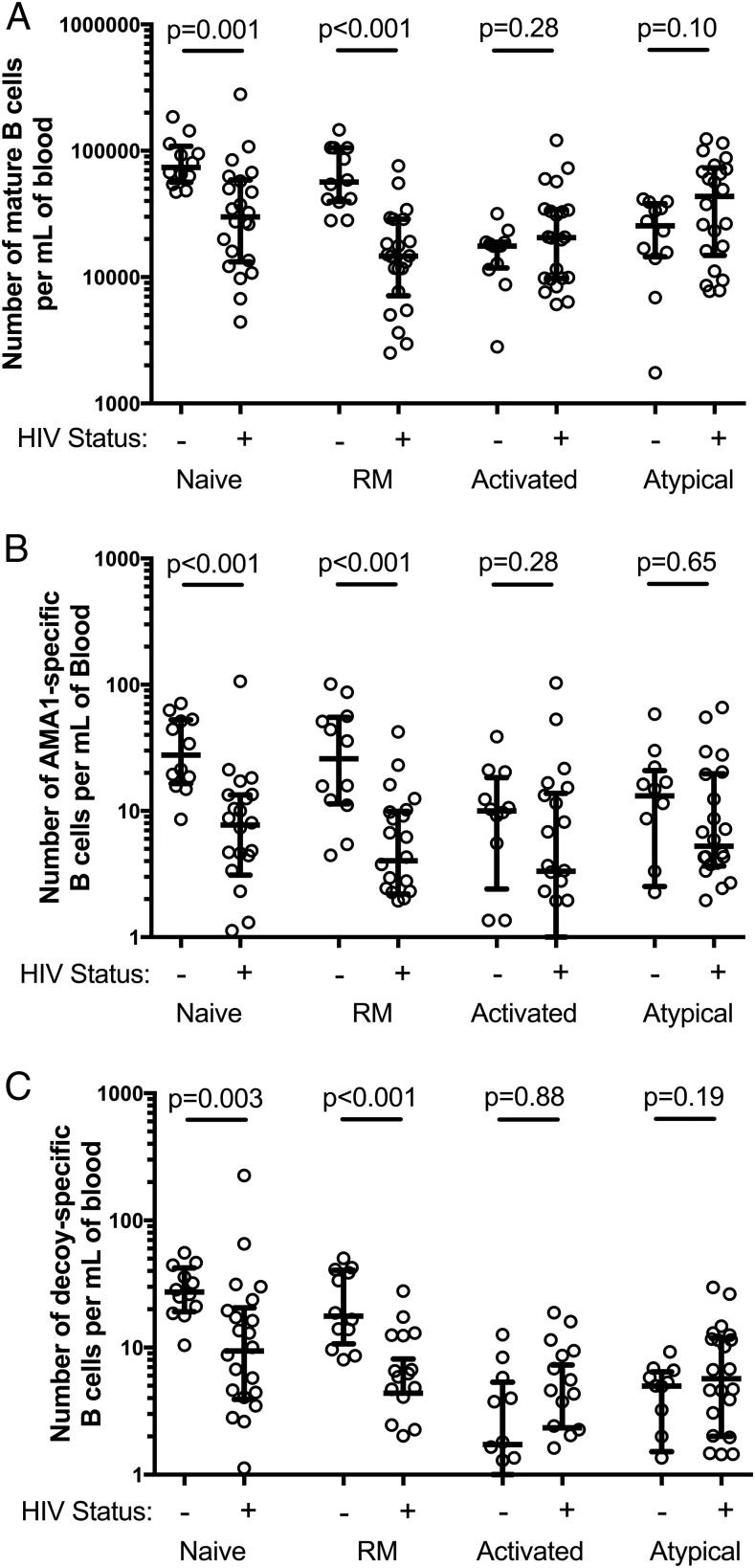
Consistent with previous reports, we found that HIV-infected individuals had increased proportions of total activated and total atypical (CD10− CD21low CD27−) memory B cells and decreased proportions of resting memory B cells (CD10− CD21+ CD27+) (Fig. 5, Supplemental Table II) (10). There was no significant difference in the proportion of total naive, resting memory, activated, and atypical subsets between HIV infected individuals with CD4 counts above and below 200 cells per μl. (Fig. 6, Supplemental Table II). Among AMA1-specific cells, a similar pattern was seen as for total B cells, i.e., in HIV-infected individuals, there was a higher proportion of AMA1-specific cells with an atypical phenotype (p = 0.01) and a contraction of AMA1-specific resting memory B cells (p = 0.003) (Fig. 5, Supplemental Table II). Using the lymphocyte count from the complete blood count taken at the time of blood collection to calculate a standardized concentration of AMA1-specific memory B cells, HIV-uninfected individuals had a median value of 108 AMA1-specific cells per milliliter of blood whereas HIV-infected individuals had a median value of 26 AMA1-specific cells per milliliter of blood (p = 0.01). When examining frequencies of B cell subsets in blood (number of cells per milliliter of blood), HIV-infected individuals had a significant decrease in the number of total and AMA1-specific naive and resting memory B cells (MBCs) per milliliter of blood when compared with HIV-uninfected individuals (Fig. 7). The number of total or AMA1-specific activated or atypical B cells per milliliter of blood did not differ significantly between HIV-infected and uninfected individuals. Overall, these data suggest that HIV infection leads to a significant loss of naive and resting MBCs, and that this loss leads to a higher proportion of atypical and activated B cells in HIV-infected individuals, rather than an actual increase in their frequency. These changes are evident not only in the total B cell population, but also B cells specific to the P. falciparum Ag AMA1.
FIGURE 5.
Percentage of mature (CD19+ CD10−) B cells of each phenotypic subset in HIV-infected and uninfected individuals. (A) Naive, (B) resting memory, (C) activated, and (D) atypical phenotype. Each chart shows the distribution of these cells among mature B cells by their Ag specificity (total B cell pool, AMA1-specific B cells, or decoy-specific B cells). Bars represent the median percentage and error bars show the interquartile range. HIV-infection status is indicated by − and + on the x-axis. Proportions of B cells between HIV-infected and uninfected individuals were compared with Wilcoxon rank-sum testing.
FIGURE 6.
Percentage of mature (CD19+ CD10−) B cells of each phenotypic subset in HIV-infected individuals with high (>200 cells per ml) and low (≤200 cells per ml) CD4 counts. (A) Naive, (B) resting memory, (C) activated, and (D) atypical phenotype. Each chart shows the distribution of these cells among mature B cells by their Ag specificity (total B cell pool, AMA1-specific B cells or decoy-specific B cells). Bars represent the median percentage and error bars show the interquartile range. Proportions of B cells between HIV-infected individuals with high and low CD4 counts were compared with Wilcoxon rank-sum testing.
FIGURE 7.
Concentration of mature (CD19+ CD10−) B cells by phenotypic subset (cells per milliliter of blood). (A) Median concentration of total B cells, (B) median concentration of AMA1-specific B cells, and (C) median concentration of decoy-specific B cells are displayed. HIV-infection status is indicated by − and + on the x-axis. The B cell concentration was compared between HIV-infected and uninfected individuals using Wilcoxon rank-sum testing. Error bars show the interquartile range. RM, resting memory. The p values were generated using Wilcoxon rank-sum testing.
Relationship of CRP to total B cell–subset proportion and concentration
To determine the extent to which HIV and inflammation independently affect B cell populations, we conducted regression analyses where the outcomes were the proportion or number of total B cell populations, and predictor variables were HIV infection status, age, and CRP concentration (Supplemental Table III). CRP concentrations and cell population frequency and numbers were log transformed for this analysis. For changes in the frequency of the naive, resting memory, and activated B cell populations, HIV infection was the primary predictor, suggesting that HIV may drive these changes in part through inflammation, as represented by CRP or other biomarkers. Interestingly, CRP independently predicted the concentration of atypical B cells.
Naive B cells specific for an irrelevant Ag are depleted during HIV-1 infection
Considering the association between HIV infection and naive AMA1-specific and total B cell concentration (Fig. 7A, 7B), we further examined the role of chronic Ag exposure in these observations. It is possible that with repeated exposure to an Ag like AMA1, there is loss of atypical and activated cells from BCR-mediated apoptosis. Our flow cytometry assay also allows for the phenotyping of B cells specific for the Ags present in both the AMA1 tetramer and the decoy, specifically, PE, streptavidin, biotin, and the HIS tag. Although it is possible that humans are exposed to B cell epitopes present in these Ags through natural sources, there is no known natural chronic source of exposure. If chronic Ag-driven apoptosis is the primary mechanism behind the observed total and malaria-specific B cell phenotypic changes with HIV infection, we would expect that these decoy-specific cells should not share these same phenotypic derangements. However, among our HIV-negative samples there was a significantly higher proportion of decoy-specific B cells with a naive phenotype that in the total B cell population (Fig. 5A, Wilcoxon rank sign test, p = 0.05). In HIV-infected individuals, there was a significantly lower number of decoy-specific B cells with a naive or resting memory phenotype than compared with HIV-uninfected individuals (Fig. 7C). This decrease resulted in a statistically significant rise in the proportion of decoy-specific cells that had atypical phenotypes (Fig. 5D). This finding among B cells that target an irrelevant Ag supports our hypothesis that the phenotypic abnormalities result from a loss of CD21 expressing B cells (naive and resting memory B cells).
Discussion
In the current study we found that in a malaria-endemic region of Kenya, HIV infection is associated with a decrease in P. falciparum Ag AMA1-specific resting memory B cells, but an increase in the proportion of AMA1-specific B cells with an activated or atypical phenotype. Interestingly, when evaluating the frequency of B cells in each of these subsets per milliliter of blood, HIV infection was associated with decreased frequencies of naive and resting MBCs, whereas HIV-infected and uninfected individuals had similar frequencies of atypical and activated B cells. This finding was observed among total B cells, AMA1-specific B cells, and B cells specific for an irrelevant Ag. Finally, the changes in Ag-specific memory B cell populations did not correlate with Ab levels to AMA1, which remained similar in HIV-infected and uninfected individuals. The significant decline in naive B cell frequency among decoy-specific B cells, an Ag to which the study population should not have been chronically exposed, further suggests that the process is polyclonal and may develop in the absence of Ag exposure. Together, these data suggest that the phenotypic characteristics of B cell exhaustion described in HIV infection does not affect baseline Ab production to P. falciparum Ags in adults and that a numerical loss in the CD21-expressing subsets (naive and resting memory B cells) may be a primary driver of the widely described phenomenon of atypical and activated B cell expansion.
A critical question that should be addressed is whether the phenotypic changes that we and others have described lead to an impairment of generation or production of high affinity Abs in response to Ag exposure in vivo. In our study, we measured Ab levels in asymptomatic and for the most part, nonmalaria–infected hosts. Therefore, Ab production for most individuals may be from long-lived plasma cells. Our participants came from a geographic area where it is likely they had many P. falciparum infections in childhood. Accordingly, this Ab production may reflect primary B cell responses from earlier in life, even prior to HIV infection. Perhaps HIV infection and immune activation has a greater effect on the generation of novel Abs rather than the previously existing repertoire. This would be consistent with our observations of a numerical loss in naive B cells in HIV infection. Observations in vaccination studies of people infected with HIV that support the hypothesis that novel exposures are more highly affected than recall responses. Abs levels to childhood immunizations such as measles, mumps, and rubella vaccines are maintained in HIV infection (25, 26). In contrast, Abs to Ags from recent vaccination such as hepatitis B vaccine are quickly lost, even with boosting and high dosing strategies (27). A model in which the pre-HIV Ab repertoire is maintained would be consistent with observations that risk of HIV associated severe malaria disease is greatest in areas of low malaria transmission (7). It may also explain why highly immunogenic Ag Ab levels are maintained with HIV infection, but a decreased breadth of malaria Ab responses is observed when measuring hundreds of P. falciparum Abs by protein microarray (16). Finally, this hypothesis is consistent with the observation that pregnant women with HIV do not develop immunity to placental malaria with repeated pregnancies as is seen in HIV-uninfected women. Development of an Ab to the chondroitin sulfate A–binding P. falciparum VAR2CSA protein is associated with decreased risk of subsequent malaria in pregnancy and placental malaria (28). This is thought to be the mechanism underlying the observation that primigravida women are more susceptible to placental malaria than multigravida women. HIV-infected women remain vulnerable to malaria in later pregnancies and have decreased Ab responses to VAR2CSA on placental P. falciparum isolates, suggesting impairment in the primary B cell response (29, 30). Studies of B cell populations in HIV-infected children and pregnant women in malaria-endemic areas could help to clarify the impact of HIV on primary versus secondary B cell responses.
We found that Ab levels to multiple P. falciparum Ags did not differ in HIV-infected and uninfected adults. However, even with similar levels of total IgG, it is possible that Abs produced by the HIV-infected host are not functionally equivalent to those of the HIV-uninfected host. There is evidence in HIV-infected individuals that although Ab reactions to influenza vaccination are maintained, there are specific deficiencies in IgG2 responses (31). There are also data demonstrating higher levels of IgG3 and lower levels of IgG2 and IgG4 from atypical versus classical memory B cells (32). If defects in B cell immunity underlie increased malaria susceptibility in HIV-infected individuals, it may be that overall serologic measurement may be insufficient to assess the ability of these Abs to protect a host from infection or disease. An increasing understanding of secondary lymphoid tissue pathology in HIV is emerging, characterized by changes in T follicular helper populations, the primary cell supporting affinity maturation among B cells (33). Future studies should assess whether the pathology of T cell help to B cells influences the affinity and functional characteristics (subclass, Fc posttranslational modification) of the resultant Abs produced in the HIV-infected host.
Our study may also have implications for malaria-driven B cell activation unrelated to HIV. Prior studies of memory B cells in malaria-endemic populations with a low prevalence of HIV have shown expansions in total atypical and activated memory B cells populations (34, 35). Our results add to those studies by directly measuring the proportions and concentrations of atypical and activated B cells in malaria-specific B cells in HIV-uninfected individuals. Our study findings lend further weight to the hypothesis that malaria may contribute to B cell abnormalities in HIV-uninfected African populations as has been previously described in Rwanda, Kenya, and Mali (10, 16, 34, 35). Future studies in malaria B cell immunity as well as other diseases with significant phenotypic B cell abnormalities should seek to evaluate whether these proportions are a true representation of the concentration of each B cell subset as well. Whether these phenotypic abnormalities affect B cell function in P. falciparum immunity is currently under investigation. There is evidence that atypical B cells in malaria may not represent an exhausted and hypo-functional B cell as has been described in HIV infection, but a functional Ab-secreting cell (32, 36). Finally, further studies should also attempt to address the role of other major copathogens in these regions that may affect B cell development and maintenance. With evidence that both HIV and malaria impair EBV immunity, the role of EBV in B cell phenotypic abnormalities should be specifically addressed (37).
In conclusion, the current study shows that HIV is correlated with an increase in the proportion of atypical B cells among all B cells and among B cells that target non-HIV Ags (P. falciparum AMA1 and irrelevant Ags: PE, streptavidin, biotin, and HIS tag), but also shows that the increase in the proportion of atypical B cells is primarily driven by a loss of naive and resting memory cells. This observation highlights a key barrier in interpreting immune responses that are analyzed with a set number of cells, a standard method for analyzing human immune responses with peripheral blood samples. When analyzing cell subsets using proportions of the parent population, a decline in one population may appear as an expansion in another. It is critical we measure not only the proportion of cells that express a marker interest, but also the overall frequency of these cells in the blood, which is likely a more accurate reflection of the overall number in the host. The study findings suggest that the primary way in which HIV infection affects the memory B cell population is to decrease overall and Ag-specific naive and resting memory B cells. Future studies should evaluate the effect of HIV infection on B cell population expansion, affinity maturation, Ab production, and Ab function in response to a defined immune stimulus.
Supplementary Material
Acknowledgments
We thank the study participants for involvement in this study. We thank Arthur Ajwang and the team at the Bondo Sub County Hospital for work in the field collection of these samples. We thank Dr. Peter Crompton for valuable discussions. This work is published with the permission of the Office of the Director of the Kenya Medical Research Institute.
This work was supported by the American Society of Tropical Medicine and Hygiene Centennial Award, National Center for Advancing Translational Sciences of the National Institutes of Health Award UL1TR000114, and National Institute of Allergy and Infectious Diseases Awards 2T32AI055433-06A1 and F32 AI109808-01. This research was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The online version of this article contains supplemental material.
- AMA1
- apical membrane Ag-1
- CRP
- C-reactive protein
- HIS
- histidine
- HIV-1
- HIV type 1
- MBC
- memory B cell
- MFI
- median fluorescence intensity
- MSP
- merozoite surface protein.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Titanji K., De Milito A., Cagigi A., Thorstensson R., Grützmeier S., Atlas A., Hejdeman B., Kroon F. P., Lopalco L., Nilsson A., Chiodi F. 2006. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood 108: 1580–1587. [DOI] [PubMed] [Google Scholar]
- 2.Ballet J. J., Sulcebe G., Couderc L. J., Danon F., Rabian C., Lathrop M., Clauvel J. P., Seligmann M. 1987. Impaired anti-pneumococcal antibody response in patients with AIDS-related persistent generalized lymphadenopathy. Clin. Exp. Immunol. 68: 479–487. [PMC free article] [PubMed] [Google Scholar]
- 3.Miotti P. G., Nelson K. E., Dallabetta G. A., Farzadegan H., Margolick J., Clements M. L. 1989. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA 262: 779–783. [PubMed] [Google Scholar]
- 4.Opravil M., Fierz W., Matter L., Blaser J., Lüthy R. 1991. Poor antibody response after tetanus and pneumococcal vaccination in immunocompromised, HIV-infected patients. Clin. Exp. Immunol. 84: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malaspina A., Moir S., Orsega S. M., Vasquez J., Miller N. J., Donoghue E. T., Kottilil S., Gezmu M., Follmann D., Vodeiko G. M., et al. 2005. Compromised B cell responses to influenza vaccination in HIV-infected individuals. J. Infect. Dis. 191: 1442–1450. [DOI] [PubMed] [Google Scholar]
- 6.von Gottberg A., de Gouveia L., Madhi S. A., du Plessis M., Quan V., Soma K., Huebner R., Flannery B., Schuchat A., Klugman K. 2006. Impact of conjugate Haemophilus influenzae type b (Hib) vaccine introduction in South Africa. Bull. World Health Organ. 84: 811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimwade K., French N., Mbatha D. D., Zungu D. D., Dedicoat M., Gilks C. F. 2004. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS 18: 547–554. [DOI] [PubMed] [Google Scholar]
- 8.Laufer M. K., van Oosterhout J. J., Thesing P. C., Thumba F., Zijlstra E. E., Graham S. M., Taylor T. E., Plowe C. V. 2006. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J. Infect. Dis. 193: 872–878. [DOI] [PubMed] [Google Scholar]
- 9.De Milito A., Nilsson A., Titanji K., Thorstensson R., Reizenstein E., Narita M., Grutzmeier S., Sönnerborg A., Chiodi F. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103: 2180–2186. [DOI] [PubMed] [Google Scholar]
- 10.Moir S., Ho J., Malaspina A., Wang W., DiPoto A. C., O’Shea M. A., Roby G., Kottilil S., Arthos J., Proschan M. A., et al. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205: 1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiss G. E., Crompton P. D., Li S., Walsh L. A., Moir S., Traore B., Kayentao K., Ongoiba A., Doumbo O. K., Pierce S. K. 2009. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J. Immunol. 183: 2176–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moir S., Fauci A. S. 2009. B cells in HIV infection and disease. Nat. Rev. Immunol. 9: 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewitt K., Steketee R., Mwapasa V., Whitworth J., French N. 2006. Interactions between HIV and malaria in non-pregnant adults: evidence and implications. AIDS 20: 1993–2004. [DOI] [PubMed] [Google Scholar]
- 14.Cohen S., McGREGOR I. A., Carrington S. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192: 733–737. [DOI] [PubMed] [Google Scholar]
- 15.Nnedu O. N., O’Leary M. P., Mutua D., Mutai B., Kalantari-Dehaghi M., Jasinskas A., Nakajima-Sasaki R., John-Stewart G., Otieno P., Liang X., et al. 2011. Humoral immune responses to Plasmodium falciparum among HIV-1-infected Kenyan adults. Proteomics Clin. Appl. 5: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramaniam K. S., Skinner J., Ivan E., Mutimura E., Kim R. S., Feintuch C. M., Portugal S., Anastos K., Crompton P. D., Daily J. P. 2015. HIV malaria co-infection is associated with atypical memory B cell expansion and a reduced antibody response to a broad array of Plasmodium falciparum antigens in Rwandan adults. PLoS One 10: e0124412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhouse B., Ho B., Hubbard A., Njama-Meya D., Narum D. L., Lanar D. E., Dutta S., Rosenthal P. J., Dorsey G., John C. C. 2011. Antibodies to Plasmodium falciparum antigens predict a higher risk of malaria but protection from symptoms once parasitemic. J. Infect. Dis. 204: 19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thera M. A., Doumbo O. K., Coulibaly D., Laurens M. B., Ouattara A., Kone A. K., Guindo A. B., Traore K., Traore I., Kouriba B., et al. 2011. A field trial to assess a blood-stage malaria vaccine. N. Engl. J. Med. 365: 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ondigo B. N., Park G. S., Gose S. O., Ho B. M., Ochola L. A., Ayodo G. O., Ofulla A. V., John C. C. 2012. Standardization and validation of a cytometric bead assay to assess antibodies to multiple Plasmodium falciparum recombinant antigens. Malar. J. 11: 427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pape K. A., Taylor J. J., Maul R. W., Gearhart P. J., Jenkins M. K. 2011. Different B cell populations mediate early and late memory during an endogenous immune response. Science 331: 1203–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishnamurty A. T., Thouvenel C. D., Portugal S., Keitany G. J., Kim K. S., Holder A., Crompton P. D., Rawlings D. J., Pepper M. 2016. Somatically hypermutated Plasmodium-specific IgM(+) memory B cells are rapid, plastic, early responders upon Malaria rechallenge. Immunity 45: 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis R. D., Wu Y., Martin L. B., Shaffer D., Miura K., Aebig J., Orcutt A., Rausch K., Zhu D., Mogensen A., et al. 2012. Phase 1 study in malaria naïve adults of BSAM2/Alhydrogel®+CPG 7909, a blood stage vaccine against P. falciparum malaria. PLoS One 7: e46094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones D. S., Rowe C. G., Chen B., Reiter K., Rausch K. M., Narum D. L., Wu Y., Duffy P. E. 2016. A method for producing protein nanoparticles with applications in vaccines. PLoS One 11: e0138761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor J. J., Martinez R. J., Titcombe P. J., Barsness L. O., Thomas S. R., Zhang N., Katzman S. D., Jenkins M. K., Mueller D. L. 2012. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J. Exp. Med. 209: 2065–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemper C. A., Zolopa A. R., Hamilton J. R., Fenstersheib M., Bhatia G., Deresinski S. C. 1992. Prevalence of measles antibodies in adults with HIV infection: possible risk factors of measles seronegativity. AIDS 6: 1321–1325. [DOI] [PubMed] [Google Scholar]
- 26.Sha B. E., Harris A. A., Benson C. A., Atkinson W. L., Urbanski P. A., Stewart J. A., Williams W. W., Murphy R. L., Phair J. P., Levin S. A., et al. 1991. Prevalence of measles antibodies in asymptomatic human immunodeficiency virus-infected adults. J. Infect. Dis. 164: 973–975. [DOI] [PubMed] [Google Scholar]
- 27.Kernéis S., Launay O., Turbelin C., Batteux F., Hanslik T., Boëlle P. Y. 2014. Long-term immune responses to vaccination in HIV-infected patients: a systematic review and meta-analysis. Clin. Infect. Dis. 58: 1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duffy P. E., Fried M. 2003. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect. Immun. 71: 6620–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricke C. H., Staalsoe T., Koram K., Akanmori B. D., Riley E. M., Theander T. G., Hviid L. 2000. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J. Immunol. 165: 3309–3316. [DOI] [PubMed] [Google Scholar]
- 30.Dembo E. G., Mwapasa V., Montgomery J., Craig A. G., Porter K. A., Meshnick S. R., Molyneux M. E., Rogerson S. J. 2008. Impact of human immunodeficiency virus infection in pregnant women on variant-specific immunity to malaria. Clin. Vaccine Immunol. 15: 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crum-Cianflone N. F., Collins G., Defang G., Iverson E., Eberly L. E., Duplessis C., Maguire J., Ganesan A., Agan B. K., Lalani T., et al. 2012. Immunoglobulin G subclass levels and antibody responses to the 2009 influenza A (H1N1) monovalent vaccine among human immunodeficiency virus (HIV)-infected and HIV-uninfected adults. Clin. Exp. Immunol. 168: 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muellenbeck M. F., Ueberheide B., Amulic B., Epp A., Fenyo D., Busse C. E., Esen M., Theisen M., Mordmüller B., Wardemann H. 2013. Atypical and classical memory B cells produce Plasmodium falciparum neutralizing antibodies. J. Exp. Med. 210: 389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colineau L., Rouers A., Yamamoto T., Xu Y., Urrutia A., Pham H. P., Cardinaud S., Samri A., Dorgham K., Coulon P. G., et al. 2015. HIV-infected spleens present altered follicular helper T cell (Tfh) subsets and skewed B cell maturation. PLoS One 10: e0140978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Illingworth J., Butler N. S., Roetynck S., Mwacharo J., Pierce S. K., Bejon P., Crompton P. D., Marsh K., Ndungu F. M. 2013. Chronic exposure to Plasmodium falciparum is associated with phenotypic evidence of B and T cell exhaustion. J. Immunol. 190: 1038–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ayieko C., Maue A. C., Jura W. G., Noland G. S., Ayodo G., Rochford R., John C. C. 2013. Changes in B cell populations and merozoite surface protein-1-specific memory B cell responses after prolonged absence of detectable P. falciparum infection. PLoS One 8: e67230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sullivan R. T., Kim C. C., Fontana M. F., Feeney M. E., Jagannathan P., Boyle M. J., Drakeley C. J., Ssewanyana I., Nankya F., Mayanja-Kizza H., et al. 2015. FCRL5 delineates functionally impaired memory B cells associated with Plasmodium falciparum exposure. PLoS Pathog. 11: e1004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moormann A. M., Chelimo K., Sumba O. P., Lutzke M. L., Ploutz-Snyder R., Newton D., Kazura J., Rochford R. 2005. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J. Infect. Dis. 191: 1233–1238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.