Abstract
Background:
Selective augmentation of hippocampal activity in ways similar to that caused by ketamine may have therapeutic advantages over ketamine, which has psychotomimetic and reinforcing effects likely due to effects outside the hippocampus (i.e., off-target effects).
Methods:
Here we evaluated the antidepressant-like response to a negative allosteric modulator of α5 subunit- containing gamma aminobutyric acid subtype A receptors, L-655,708, as these receptors are expressed to a much greater extent in the hippocampus than in other brain areas.
Results:
Systemic administration of L-655,708 produced a sustained antidepressant-like effect in the forced swim test that was comparable with that of ketamine and was blocked by hippocampal inactivation with lidocaine. However, in contrast to ketamine, L-655,708 did not affect prepulse inhibition of startle, nor did it maintain responding in rats trained to self-administer i.v. ketamine.
Conclusion:
Taken together, these findings suggest that activation of the hippocampus by L-655,708 produces an antidepressant-like effect in the absence of any psychotomimetic or abuse-related effects.
Keywords: antidepressant, α-5-containing GABAA receptors, L-655, 708, abuse
Significance Statement
The therapeutic utility of ketamine in depression has stimulated intense research into finding novel drugs that would mimic its beneficial effects, but be devoid of its abuse potential. In this report, effects of a negative allosteric modulator of α5 subunit-containing GABAA receptors, namely L-655-708, were studied. This receptor was targeted as it is expressed in the hippocampus, a key site of action for the antidepressant-like effect of ketamine, to a much greater extent than in other brain regions. L-655,708 produced a ketamine-like antidepressant effect but unlike ketamine it was not self-administered. This drug then, or others like it, might be a useful antidepressant but not have abuse potential.
Introduction
Although promising, the use of ketamine to treat refractory depression has been limited by adverse effects, including abuse potential and psychotomimetic effects (Krystal et al., 1994). It has been hypothesized that, as a glutamatergic NMDA receptor antagonist, ketamine enhances glutamatergic signaling of pyramidal neurons in the hippocampus through preferential inhibition of glutamatergic excitation of GABAergic interneurons (Sanacora and Schatzberg, 2015). We recently demonstrated preclinically that a pathway from the ventral hippocampus (vHipp) to the medial prefrontal cortex is both necessary and sufficient for the sustained antidepressant-like effects of ketamine (Carreno et al., 2016). Thus, targeting the vHipp selectively might produce ketamine-like antidepressant effects without the off-target actions that limit the clinical utility of ketamine.
The α5-subunit of the gamma aminobutyric acid subtype A (GABAA) receptor is primarily expressed within the hippocampus, with significantly lower expression observed throughout cortical layers and ventral striatum (Sur et al., 1999). These receptors are largely extrasynaptic and localized to the base of dendritic spines of pyramidal neurons (Brunig et al., 2002). Thus, α5-GABAA receptors are positioned to provide an inhibitory input to glutamatergic hippocampal projection neurons (Brunig et al., 2002). L-655,708 is a selective negative allosteric modulator of the benzodiazepine site of α5-GABAA receptor (Quirk et al., 1996). Recent preclinical data from Thompson and colleagues (2015) support the use of such drugs as antidepressants (Fischell et al., 2015). Here, we used it to test the hypothesis that the sustained antidepressant-like effects of L-655,708 are mediated by the hippocampus and produces fewer side effects than ketamine.
Methods
Experiments were carried out using adult male Sprague-Dawley rats, 300 to 350g (Harlan). Rats were group housed and maintained in a temperature-controlled environment on a 14:10 hour light-dark cycle and had access to food and water ad libitum. Experimental protocols were approved by the Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio in accordance with the guidelines of the Public Health Service, American Physiological Society, and the Society for Neuroscience.
Drugs
L-655,708 was purchased from Tocris. It is a potent, selective inverse agonist for the benzodiazepine site of GABAA receptors containing the α5 subunit (Ki = 0.45 nM) displaying 50- to 100-fold selectivity over other subtypes of GABAA receptors (Quirk et al., 1996). Ketamine and lidocaine were purchased from Sigma. L-655,708 was dissolved in 50% (v/v) propylene glycol and saline or 20% DMSO and saline, whereas ketamine and lidocaine were dissolved in saline. For self-administration studies, ketamine was also evaluated when dissolved in 10% propylene glycol.
Behavioral Assessment
Forced Swim Test (FST)
As described previously (Carreno et al., 2016), rats were placed into a Plexiglas cylinder (21 x 46 cm) filled with water (25oC), and behavior was digitally recorded by a video camera placed above the tank for a period of 6 minutes. Rats were tested at either 30 minutes or 1 week following a single administration of L-655,708 (3 mg/kg, i.p.). The dose was chosen based on a previous study (Samardzic et al., 2014). A time sampling technique was utilized where the most prominent behavior (immobility, swimming or climbing) observed in each 5-second bin was recorded for the first 5 minutes of the test (Cryan et al., 2005). The behavioral raters were blind to the treatment.
I.V. Self-Administration
Rats were surgically prepared with an indwelling catheter in the left femoral vein under isoflurane anesthesia as previously described (Collins and France, 2015). Ketamine self-administration was conducted in standard operant conditioning chambers (Med Associates, St. Albans, VT). Rats were allowed to acquire responding for 1 mg/kg/infusion ketamine during daily 90-minute sessions with responding reinforced according to a fixed ratio (FR) 1: timeout (TO) 5-sec schedule as described previously (Collins and Woods, 2007). Subsequently, responding was maintained under an FR5: TO 5-second schedule. The dose of L-655,708 (0.32 mg/kg/infusion) was chosen based on its relative potency to produce antidepressant-like effects in the FST, i.e., 3-fold more potent that ketamine. The potential reinforcing effects of L-655,708 were evaluated by substitution from a ketamine baseline, with 0.32 mg/kg/infusion L-655,708 and its vehicle (50% v/v propylene glycol) evaluated over 7 consecutive days. Between substitution tests, rats were returned to baseline conditions (1 mg/kg/infusion ketamine) for at least 3 sessions and until responding stabilized (no more than 20% difference in the number of infusions earned).
Prepulse Inhibition (PPI) of the Startle Response
Sensorimotor gating is often evaluated using the PPI paradigm. Rats were administered L-655,708 (3mg/kg i.p.), ketamine (10mg/kg i.p.), or vehicle (50% propylene glycol in saline). These are doses that produce positive effects in the FST. Fifteen minutes following drug administration, rats were placed into an SR-LAB Startle Response System where they habituated to a background white noise (65 dB) for 5 minutes, followed by 10 startle trials where 120-dB (40 millisecond) pulses were administered at an average inter-trial interval of 15 seconds. Following habituation to the startle stimulus, the effect of 3 distinct prepulse intensities (69, 73, and 81 dB, 20 milliseconds) preceding the startle stimulus by 100 milliseconds (peak-to-peak) were examined to determine PPI.
Elevated Plus Maze (EPM)
The EPM test was carried out as described previously (Bondi et al., 2008), but without white noise in the room. Rats were administered L-655,708 (3 mg/kg i.p.) or vehicle (50% propylene glycol in saline), a dose that produced positive effects in the FST. thirty minutes following drug administration, rats were placed into the center platform, right at the junction between a closed and an open arm, and allowed to explore the EPM for 5 minutes. Measures of time spent and number of entries made in each of the arms were taken by infrared sensor beams positioned at the entry of each arm. Data were analyzed using the ANY-MAZE program.
Stereotaxic Survival Surgeries
All stereotaxic survival surgical procedures were performed under general anesthesia in a semisterile environment. Briefly, male Sprague Dawley rats were pretreated with atropine (0.1 mg/kg i.p.), anesthetized with sodium pentobarbital (60 mg/kg i.p.), and placed into a stereotaxic apparatus using blunt atraumatic ear bars followed by canulae implantation.
Transient Pharmacological Inactivation of the Ventral Hippocampus with Lidocaine
As described previously (Carreno et al., 2016), bilateral cannulae (Plastics One: C317G(2)- C/C distance of 10.4 mm, D/V -5.5 mm below plate) were implanted 2 mm dorsal to the vHipp (A/P -5.3, M/L ±5.2) and fixed in place with dental cement and 4 anchor screws. The wound was sutured and the rats were allowed to recover for at least 1 week before behavioral experiments. On the day of L-655,708 administration, rats (n = 8/group) were transferred into the behavior facility and allowed to acclimatize for at least 1 hour prior to the administration of either lidocaine (2% w/v, 0.5 μL, Sigma-Aldrich) or Dulbecco’s PBS (0.5 μL) directly into each vHipp via an injector that extended 2 mm past the end of the indwelling cannula. The intracerebral injection was immediately followed by the systemic administration of L-655,708 (3 mg/kg i.p.) or 50% propylene glycol in saline (1 mL/kg i.p.). Rats were tested in the FST 30 minutes or 7 days following injection.
Statistical Analysis
The FST and EPM data involving vehicle and L-655,708 were analyzed by Student’s t test. The effect of lidocaine on the L-655,708 response was analyzed by 2-way ANOVA followed by Holm-Sidak posthoc test. PPI data were analyzed by 2-way ANOVA followed by Holm-Sidak posthoc test. For self-administration studies, a 2-way (substitution drug x day) repeated-measures ANOVA followed by a Holm-Sidak posthoc test was used to determine if the number of infusions earned during each substitution test (as well as the first session in which ketamine was replaced) differed from the number of ketamine infusions (1.0 mg/kg) maintained under baseline conditions. P < .05 was considered significant. All data are presented as the mean ± SEM.
Results
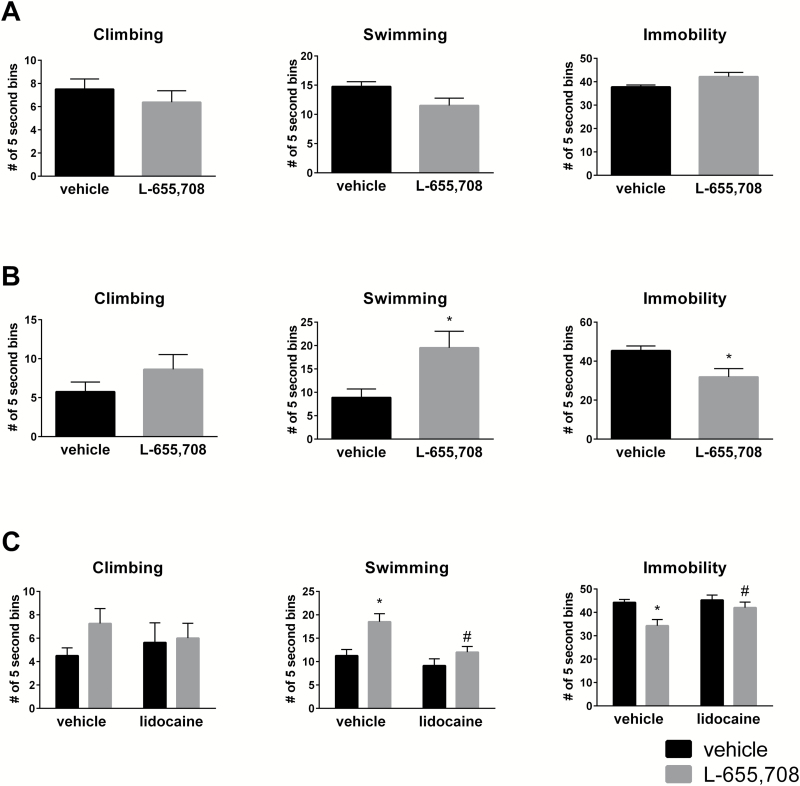
Similar to ketamine (Carreno et al., 2016), a single systemic administration of L-655,708 produced an antidepressant-like effect in the FST that persisted for 1 week (Figure 1B). No effect of L-655,708 was produced in the FST 30 minutes following drug administration (Figure 1A). To evaluate if activation of the vHipp was responsible for this sustained antidepressant response, the Na+ channel blocker lidocaine was microinjected into the vHipp to produce a reversible inactivation of hippocampal activity lasting ~10 to 30 minutes (Sandkuhler et al., 1987; Floresco et al., 1996; Pereira de Vasconcelos et al., 2006). The main effects and interaction for climbing were F (1, 31) L-655,708=1.48 (P=.23); F (1, 31) lidocaine=0.00 (P=.96); F (1, 31) L-655,708x lidocaine=0.85 (P=.36). The main effects and interaction for swimming were F (1, 31) L-655,708=12.00 (P=.002); F (1, 31) lidocaine=8.70 (P=.006); F (1, 31) L-655,708x lidocaine=2.24 (P=.14). The main effects and interaction for immobility were F (1, 31) L-655,708=9.01 (P=.006); F (1, 31) lidocaine=3.93 (P=.057); F (1, 31) L-655,708x lidocaine=2.34 (P=.13). The posthoc analysis revealed that the transient inactivation of the vHipp with lidocaine, at the time of L-655,708 administration, completely prevented the decrease in immobility (t=2.48, P<.05) and increase in swimming (t=3.14, P<.05) observed 1 week after its administration (Figure 1C).
Figure 1.
L-655,708 produced a sustained antidepressant-like effect that is mediated by the vHipp. Two separate cohorts of rats were injected with L-655,708 (3 mg/kg, i.p.) or vehicle (50% propylene glycol). The FST was used to detect antidepressant-like efficacy 30 minutes (A) or 1 week (B) following drug treatment. *P < .05, Student’s t test. (C) Lidocaine into the vHipp blocked antidepressant-like effects of L-655,708 at 1 week. Rats were implanted with stainless steel cannulae targeting the vHipp and administered either vehicle (aCSF) or lidocaine (2%, 5 μL) directly into the vHipp followed by the systemic administration of L-655,708 (3 mg/kg, i.p.) or vehicle. The FST was used to detect antidepressant-like effects 1 week following drug treatment. *P<.05 vs vehicle-vehicle; #P<.05 vs L-655,708-vehicle, Holm-Sidak’s posthoc analysis. n=8 rats/group.
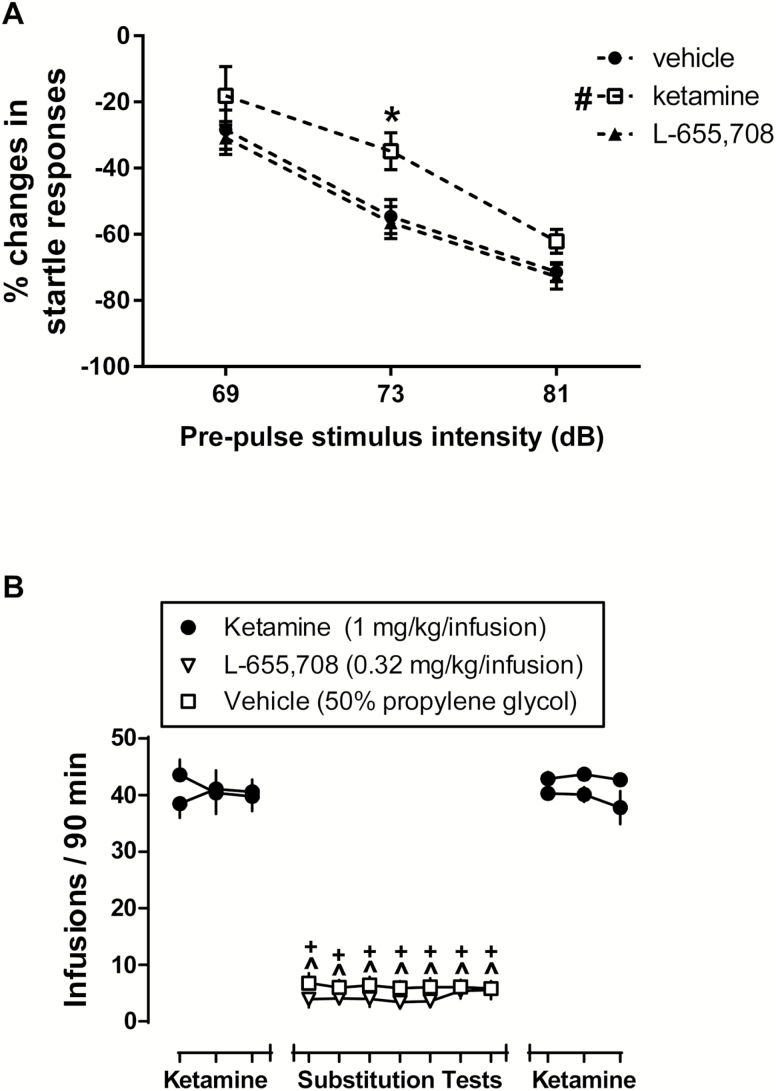
PPI of startle is a test of sensorimotor gating where a weak pre-stimulus inhibits the startle response to a stronger stimulus. It has been used as a putative measure of psychotomimetic effects as a wide variety of drugs that induce psychosis also disrupt PPI in both animal patient populations (Geyer et al., 2001). The main effects and interaction for PPI were F (1, 95) treatment=6.59 (P=.02); F (1, 95) intensity=47.24 (P<.001); F (1, 95) treatment x intensity=0.37 (P=.82). Increasing prepulse intensities significantly attenuated the startle response to a 120-dB stimulus in control rats. Consistent with its reported psychotomimetic effects, ketamine significantly disrupted PPI as evidenced by an exaggerated startle response during prepulse trials. The posthoc analysis revealed that there was a significant treatment effect of ketamine vs vehicle (t=2.06, P<.05) and a significant effect between ketamine and vehicle at 73 db (t= 2.68, P<.05) (Figure 2A). It is important to note that an initial study failed to show an effect of ketamine in disrupting PPI in rats (Mansbach and Geyer, 1989). However, subsequent studies have shown that depending upon the stimulus parameters, dose, and injection-to-test interval, ketamine disrupted PPI in rats (Mansbach and Geyer, 1991; Johansson et al., 1995; Swerdlow et al., 1998). By contrast, L-655,708 did not affect PPI at a dose that produced a sustained antidepressant-like effect (Figure 2A).
Figure 2.
L-655,708 did not produce the reinforcing or psychotomimetic effects of ketamine. (A) Prepulse inhibition (PPI) of startle response. Ketamine (10 mg/kg i.p.) induced deficits in PPI, particularly at 73 dB. L-655,708 (3 mg/kg i.p.) did not affect PPI. n=9–13 rats/group. (B) Self-administration maintained by ketamine (1 mg/kg/infusion) under a fixed ratio (FR) 5 schedule of reinforcement. Substitution of L-655,708 (0.32 mg/kg/infusion) for ketamine resulted in low levels of responding that did not differ from vehicle (50% propylene glycol). Replacing L-655,708 (or vehicle) with ketamine resulted in a rapid recovery of self-administration to presubstitution levels. #P<.05 denotes significant treatment effect of ketamine vs vehicle; *P<.05 represents significant effect between ketamine and vehicle at 73 db. ^P<.05 denotes a significant difference between the number of self-administered infusions of ketamine and L-655,708. +P<.05 denotes a significant difference between the number of self-administered infusions of ketamine and vehicle.
I.v. self-administration procedures are the gold-standard for evaluating the abuse liability of candidate medications, as it has high levels of both face and predictive validity (O’Connor et al., 2011). Consistent with previous reports (Collins and Woods, 2007), rats readily self-administered ketamine (1 mg/kg/infusion) under an FR5 schedule of reinforcement (Figure 2B), earning on average 40.7 (±2.4) infusions per session. By contrast, there was a main effect of day F (8, 56) day=215.4 (P<.001), but not substitute drug F (1, 7) substitute drug=3.06 (P=.12) when L-655,708 (0.32 mg/kg/infusion) or its vehicle was substituted for ketamine. Indeed, posthoc tests revealed that both L-655,708 (0.32 mg/kg/infusion) and its vehicle maintained similarly low levels of responding that were significantly lower than ketamine throughout the 7-day substitution (P<.05). Importantly, high rates of responding were recovered immediately when ketamine replaced either L-655,708 or vehicle (Figure 2B). Although smaller doses of ketamine and L-655,708 were evaluated in self-administration, it is important to note that the same dose ratio was used for all assays, with the dose of L-655,788 always being 3-fold smaller than the dose of ketamine (3 vs 10 mg/kg for FST and PPI, and 0.32 vs 1 mg/kg/infusion for self-administration, respectively).
In the EPM test, the open arm entries were not significantly different between vehicle-treated and L-655,708-treated rats (6.57 ± 1.08 for vehicle, n=7 and 7.25 ± 1.27 for L-655,708, n=8). In the same test, the numbers of entries amongst open and closed arms were also not significantly different among groups (16.86 ± 1.48 for vehicle, n=7 and 13.75 ± 2.06 for L-655,708, n=8).
Discussion
Here we demonstrate that activation of the hippocampus by a negative allosteric modulator of the α5- containing subtype of the GABAA receptor, L-655,708, produces a sustained antidepressant-like effect comparable with that observed with ketamine. This is consistent with recent work by Thompson and colleagues (Fischell et al., 2015) who reported a rapid antidepressant-like effect of L-655,708 in the sucrose preference and social interaction tests following chronic restraint stress in rats. We now extend this observation by demonstrating that L-655,708 is capable of producing a rapid and sustained antidepressant-like effect without also producing the psychotomimetic and reinforcing effects that limit the clinical utility of drugs such as ketamine.
Since L-655,708 demonstrates selectivity for the benzodiazepine site of α5-GABAA receptors (Quirk et al., 1996), which are primarily expressed in the hippocampus, L-655,708 likely produces its sustained antidepressant-like response by activating this region. Evidence for this is that inactivation of the vHipp with lidocaine at the time of L-655,708 administration prevented its sustained antidepressant-like effects, consistent with our previously published findings with ketamine (Carreno et al., 2016). The finding that L-655,708 produces an antidepressant-like response 1 week following its administration suggests that the inhibition of GABAA receptors by L-655,708 induces plastic alterations within the vHipp and its afferent targets (Paulsen and Moser, 1998); however, the precise molecular mechanisms contributing to this require further elucidation. In this respect, it is of interest that L-655,708 did not cause an antidepressant-like effect at 30 minutes, although this has been found with ketamine (Autry et al., 2011; Carreno et al., 2016).
The capacity of a drug to maintain i.v. self-administration in animals is highly predictive of its potential for abuse by humans. We and others have demonstrated that ketamine is a highly effective reinforcer in rats (Collins and Woods, 2007; Hiranita et al., 2013), consistent with its misuse and abuse by humans. Unlike ketamine, which reliably maintained self-administration, L-655,708 failed to maintain responding, strongly suggesting that it would have low or no abuse liability in humans. In addition and in contrast to ketamine, L-655,708 did not alter sensorimotor gating, suggesting that it is also devoid of the psychotomimetic effects of ketamine.
In addition to regulating medial prefrontal cortex activity, the ventral subregions of the hippocampus also significantly modulate ascending dopamine neurotransmission via a polysynaptic projection whereby the vHipp excites neurons in the nucleus accumbens that in turn inhibit ventral pallidal activity. As the ventral pallidal provides tonic inhibition of VTA dopamine neurons, activation of the vHipp will increase tonic dopamine neuronal activity at the level of the nucleus accumbens (Floresco et al., 2003; Lodge and Grace, 2006). Because of this, it has been hypothesized that the psychotomimetic and abuse-related effects of drugs such as ketamine result from their augmentation of vHipp activity and the subsequent enhancement of dopamine transmission. However, we do not believe this to be so. Rather, we hypothesized that the psychotomimetic and reinforcing effects of ketamine are due to activation of phasic dopamine neuron activity that is independent of the vHipp (Lodge and Grace, 2006). That L-655,708 produces a sustained antidepressant-like response without abuse-related or psychotomimetic effects is consistent with this view, but needs to be addressed in future studies
Given that benzodiazepine sites on GABAA receptors are localized throughout the brain, it is possible that large doses of L-655,708 could produce off-target effects, such as sedation, anxiety, or seizures, which are common to nonselective negative allosteric modulators of the benzodiazepine site (Atack et al., 2006; Dawson et al., 2006; Braudeau et al., 2011). It appears as though selective modulation of the α5-GABAA receptor does not produce any of these adverse effects (Atack et al., 2006; Dawson et al., 2006; Braudeau et al., 2011), although one study suggested that L-655,708 may have some anxiogenic effects in mice (Navarro et al., 2002). Using the EPM test, we found no anxiogenic-like effect at a dose of L-655,708 that produces sustained AD-like effects.
Taken together, we demonstrate here that selective activation of the hippocampus by a negative allosteric modulator of the α5-GABAA receptor, L-655,708, produces a sustained antidepressant-like effect comparable with that observed with ketamine without its psychotomimetic-like, abuse-related, or anxiogenic-like effects. Identification of pharmacological interventions that recapitulate the therapeutic effects of ketamine without its psychotomimetic and abuse-related effects provides a novel, safe, and effective treatment for patients suffering from refractory depression.
Statement of Interest
None.
Acknowledgments
This work was supported by NIMH grants (MH082933 to A.F. and MH090067 to D.L.), NARSAD award from the Maltz Family Foundation to D.L., and Pilot Project Grant from Center for Biomedical Neuroscience to F.R.C.
References
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. (2006) L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABAA receptors. Neuropharmacology 51:1023–1029. [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, Morilak DA. (2008) Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology 33:320–331. [DOI] [PubMed] [Google Scholar]
- Braudeau J, Delatour B, Duchon A, Pereira PL, Dauphinot L, de Chaumont F, Olivo-Marin JC, Dodd RH, Herault Y, Potier MC. (2011) Specific targeting of the GABA-A receptor alpha5 subtype by a selective inverse agonist restores cognitive deficits in Down syndrome mice. J Psychopharm 25:1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunig I, Scotti E, Sidler C, Fritschy JM. (2002) Intact sorting, targeting, and clustering of gamma-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J Comp Neurol 443:43–55. [DOI] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, Lodge DJ. (2016) Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Mol Psychiatry 21:1298–1308. [DOI] [PubMed] [Google Scholar]
- Collins GT, France CP. (2015) Determinants of conditioned reinforcing effectiveness: dopamine D2-like receptor agonist-stimulated responding for cocaine-associated stimuli. Eur J Pharmacol 769:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Woods JH. (2007) Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther 323:599–605. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. (2005) Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharm 182:335–344. [DOI] [PubMed] [Google Scholar]
- Dawson GR, Maubach KA, Collinson N, Cobain M, Everitt BJ, MacLeod AM, Choudhury HI, McDonald LM, Pillai G, Rycroft W, Smith AJ, Sternfeld F, Tattersall FD, Wafford KA, Reynolds DS, Seabrook GR, Atack JR. (2006) An inverse agonist selective for alpha5 subunit-containing GABAA receptors enhances cognition. J Pharmacol Exp Ther 316:1335–1345. [DOI] [PubMed] [Google Scholar]
- Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM. (2015) Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of alpha5-containing GABAA receptors. Neuropsychopharm 40:2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. (1996) Differential effects of lidocaine infusions into the ventral CA1/subiculum or the nucleus accumbens on the acquisition and retention of spatial information. Behav Brain Res 81:163–171. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. (2003) Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6:968–973. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. (2001) Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology 156:117–154. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Soto PL, Tanda G, Kopajtic TA, Katz JL. (2013) Stimulants as specific inducers of dopamine-independent sigma agonist self-administration in rats. J Pharmacol Exp Ther 347:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. (1995) Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav 52:649–654. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. (1994) Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51:199–214. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. (2006) The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology 31:1356–1361. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. (1989) Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacology 2:299–308. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. (1991) Parametric determinants in pre-stimulus modification of acoustic startle: interaction with ketamine. Psychopharmacology 105:162–168. [DOI] [PubMed] [Google Scholar]
- Navarro JF, Buron E, Martin-Lopez M. (2002) Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABA(A) receptors which contain the alpha-5 subunit, in the elevated plus-maze test. Prog Neuropsychopharmacol Biol Psychiatry 26:1389–1392. [DOI] [PubMed] [Google Scholar]
- O’Connor EC, Chapman K, Butler P, Mead AN. (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912–938. [DOI] [PubMed] [Google Scholar]
- Paulsen O, Moser EI. (1998) A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci 21:273–278. [DOI] [PubMed] [Google Scholar]
- Pereira de Vasconcelos A, Klur S, Muller C, Cosquer B, Lopez J, Certa U, Cassel JC. (2006) Reversible inactivation of the dorsal hippocampus by tetrodotoxin or lidocaine: a comparative study on cerebral functional activity and motor coordination in the rat. Neuroscience 141:1649–1663. [DOI] [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. (1996) [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the alpha 5 subunit. Neuropharmacology 35:1331–1335. [DOI] [PubMed] [Google Scholar]
- Samardzic J, Puskas L, Obradovic M, Lazic-Puskas D, Obradovic ID. (2014) Antidepressant effects of an inverse agonist selective for α5 GABA-A receptors in the rat forced swim test. Acta Veterinaria-Beograd 64:52–60. [Google Scholar]
- Sanacora G, Schatzberg AF. (2015) Ketamine: promising path or false prophecy in the development of novel therapeutics for mood disorders? Neuropsychopharmacology 40:259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J, Maisch B, Zimmermann M. (1987) The use of local anaesthetic microinjections to identify central pathways: a quantitative evaluation of the time course and extent of the neuronal block. Exp Brain Res 68:168–178. [DOI] [PubMed] [Google Scholar]
- Sur C, Fresu L, Howell O, McKernan RM, Atack JR. (1999) Autoradiographic localization of alpha5 subunit-containing GABAA receptors in rat brain. Brain Res 822:265–270. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bakshi V, Waikar M, Taaid N, Geyer MA. (1998) Seroquel, clozapine and chlorpromazine restore sensorimotor gating in ketamine-treated rats. Psychopharmacology 140:75–80. [DOI] [PubMed] [Google Scholar]