Abstract
Background:
Patients on the selective serotonergic reuptake inhibitors like citalopram report emotional blunting. We showed previously that citalopram reduces resting-state functional connectivity in healthy volunteers in a number of brain regions, including the dorso-medial prefrontal cortex, which may be related to its clinical effects. Bupropion is a dopaminergic and noradrenergic reuptake inhibitor and is not reported to cause emotional blunting. However, how bupropion affects resting-state functional connectivity in healthy controls remains unknown.
Methods:
Using a within-subjects, repeated-measures, double-blind, crossover design, we examined 17 healthy volunteers (9 female, 8 male). Volunteers received 7 days of bupropion (150 mg/d) and 7 days of placebo treatment and underwent resting-state functional Magnetic Resonance Imaging. We selected seed regions in the salience network (amygdala and pregenual anterior cingulate cortex) and the central executive network (dorsal medial prefrontal cortex). Mood and anhedonia measures were also recorded and examined in relation to resting-state functional connectivity.
Results:
Relative to placebo, bupropion increased resting-state functional connectivity in healthy volunteers between the dorsal medial prefrontal cortex seed region and the posterior cingulate cortex and the precuneus cortex, key parts of the default mode network.
Conclusions:
These results are opposite to that which we found with 7 days treatment of citalopram in healthy volunteers. These results reflect a different mechanism of action of bupropion compared with selective serotonergic reuptake inhibitors. These results help explain the apparent lack of emotional blunting caused by bupropion in depressed patients.
Keywords: bupropion, antidepressant, resting-state, functional connectivity, depression
Significance Statement
These results are opposite to that which we found with 7 days treatment of citalopram in healthy volunteers. These results reflect a different mechanism of action of bupropion compared with SSRIs. These results help explain the apparent lack of emotional blunting caused by bupropion in depressed patients.
Introduction
Resting state functional connectivity (RSFC) has been found dysfunctional in patients with major depressive disorder (MDD) in networks such as the Salience Network (SN), the Central Executive Network (CEN), and the Default Mode Network (DMN) (Sheline et al., 2010).
The SN and the CEN are described as task-positive networks, activated during tasks and less active at rest in healthy controls. The SN involving the anterior insula, pregenual anterior cingulate (pgACC) and amygdala is implicated in the processing of various aspects of salient stimuli, whereas the CEN, which consists of regions such as the dorsolateral, dorsal medial prefrontal cortex, and the posterior parietal cortex, is involved in cognitive functioning including attention and working memory (Bressler and Menon, 2010a). The DMN, more active at rest, involves brain regions such as the posterior cingulate cortex, precuneus cortex, and medial prefrontal cortex and is implicated in self-referential activity and emotion regulation (Bressler and Menon, 2010b).
Abnormalities in these networks have been found in depressed patients and are thought to reflect difficulties with the suppression of unwanted thoughts, deficits in attentional control over emotional stimuli, and difficulties with emotion recognition. However there have been inconsistencies in literature regarding the direction of effects with some studies finding increased SN and CEN (Horn et al., 2010; Sheline et al., 2010; Whitfield-Gabrieli and Ford, 2012; Manoliu et al., 2014; Ramasubbu et al., 2014) whilst others find reduced connectivity (Ye et al., 2012; Tahmasian et al., 2013; Liston et al., 2014) in these regions in MDD. These inconsistencies might be related to heterogeneity in the MDD population studied and also differences in medication history. To combat problems with medication history, we recently examined young people with high depression symptomatology but no medication history. We examined seed regions in the SN and the CEN and found decreased RSFC in the symptomatic group between the amygdala and the pgACC and hippocampus and precuneus. We also found decreased RSFC in the symptomatic group between the pgACC and the putamen and between the dorso-medial prefrontal cortex (dmPFC) and the precuneus (Rzepa and McCabe, 2016). We thus suggest that networks such as the SN and CEN may be targets for normalization with treatment.
Examining the effects of pharmacological treatments on neural processes, Fu et al. report that MDD patients showed increased connectivity in the anterior DMN with 12 weeks duloxetine (selective noradrenergic reuptake inhibitor) treatment (Fu et al., 2015). However, it has recently been shown that 8 weeks treatment of escitalopram (selective serotonergic reuptake inhibitors [SSRI]) significantly reduced dmPFC activity in MDD patients after treatment, which additionally correlated with depression symptom improvement (Wang et al., 2015). The inconsistencies may be related to the change in symptoms following treatment. Thus, investigating the effects of antidepressants outside of mood changes is a useful first step in understanding their mechanism of action. We and others have examined the effects of 7 days treatment with SSRIs in healthy volunteers and found decreased functional connectivity between the prefrontal cortex and the DMN (McCabe and Mishor, 2011; McCabe et al., 2011; van de Ven et al., 2013) and decreased functional connectivity over many networks with just a single dose of an SSRI (Klaassens et al., 2015). Furthermore, 2 weeks of duloxetine (SNRI) was also found to reduce DMN and task positive network connectivity in healthy volunteers (van Wingen et al., 2014). Taken together, this suggests the direction of effects on the resting state may be different based on the mechanism of action of the antidepressants examined. Moreover, reports suggest that SSRIs can contribute to emotional blunting in patients, where experiences both positive and negative are flattened (Price et al., 2009), whereas catecholamine antidepressants like bupropion (dopamine and noradrenaline reuptake inhibitor) (Stahl et al., 2004; Dwoskin et al., 2006) might be more efficacious at improving anhedonia in depression (Dunlop and Nemeroff, 2007; Nutt et al., 2007; McCabe et al., 2009). In light of this, we recently examined the effects of 7 days treatment with bupropion and found, unlike with citalopram (SSRI), enhanced neural activity in healthy controls during the anticipation, effort, and consummation of rewarding and aversive stimuli (Dean et al., 2016). However, how bupropion affects RSFC remains as-yet unknown.
Thus, our current study is the first to investigate the effects of 7 days bupropion administration on the RSFC in healthy volunteers using a double-blind, placebo-controlled, crossover design. Based on previous literature, we selected regions of interest (ROIs) that have been shown to be dysfunctional in depression (amygdala, pgACC, and the dmPFC). We hypothesized that bupropion, with its opposite effects to citalopram during our fMRI tasks, might also have opposite effects on RSFC compared with citalopram. That is, we expected that bupropion might increase RSFC, compared with the placebo control condition, in line with our recent study showing increased neural activity under bupropion (Dean et al., 2016).
Methods and Materials
Participants
Seventeen healthy, right-handed, Caucasian volunteers (mean 24 years, 9 female) were randomized to receive 7 days oral treatment with bupropion (150 mg/d) and 7 days oral treatment with placebo separated by a 2-week washout phase in a double-blind, within-subjects design. The study was located at the Centre for Neuroscience and Neurodynamics in the Department of Psychology at the University of Reading. Volunteers were recruited via advertisement and, after reading study information, provided written consent prior to screening. Ethical approval was obtained from the University of Reading.
The exclusion criteria included current/previous psychiatric disorder (including alcohol or drug dependency) using the DSM-IV Structured Clinical Interview (First et al., 2002), pregnancy, and any contraindications to MRI and bupropion (including family history of bipolar disorder and seizures/epilepsy). Volunteers were medication free for the past 3 months (excluding the contraceptive pill) before starting the study and underwent a physical examination. Volunteers had a healthy BMI, and their liking and craving for chocolate was measured using a questionnaire (Rolls and McCabe, 2007). Eleven volunteers were nonsmokers, 4 smoked <1 cigarette/wk, 1 smoked 5 cigarettes/wk, and 1 smoked 1 to 2 cigarettes/d on average. Baseline measures of mood and anhedonia were taken using the Beck Depression Inventory (Beck et al., 1961), Snaith-Hamilton Pleasure Scale (Snaith et al., 1995), Fawcett-Clarke Pleasure Capacity Scale (FCPS) (Fawcett et al., 1983), Temporal Experience of Positive Mood (TEP) (Gard et al., 2007), and Behavioral Inhibition/Activation Scales (BIS/BAS) (Carver and White, 1994). The BIS is hypothesized to be sensitive to cues of threat or punishment and is associated with avoidant or withdrawal behaviors (Zinbarg and Lira Yoon, 2008; Bijttebier et al., 2009). The BAS is hypothesized to be sensitive to cues of reward or appetite and is associated with approach behaviors (Fowles, 1988). Given that we used taste stimuli, including chocolate, volunteers also completed the Eating Attitudes Questionnaire (Garner et al., 1982) to assess eating attitudes.
Experimental Design
The study used a randomized, double blind, within-subjects, counterbalanced, crossover design. Volunteers received 7 days (1 tablet each morning) of bupropion treatment (150 mg/d) and 7 days of placebo treatment, separated by a 2-week washout phase. Treatment order was randomized, with 9 volunteers receiving bupropion first and 8 receiving placebo first. Volunteers underwent an fMRI scan on the 7th day of each treatment at approximately 3 hours after last dose. One volunteer had a scan after 6 days treatment (drug) due to experiencing adverse side-effects. Medication was provided by the Oxford Health NHS Foundation Trust and the Royal Free London NHS Foundation Trust. Participants were allowed only one caffeinated drink on the scan morning. Before scans, volunteers completed the Patient Rated Inventory of Side Effects (Sequenced Treatment Alternatives to Relieve Depression) to record any adverse side-effects. Mood was measured before and after scans using the befindlichkeit scale of mood and energy (von Zerssen et al., 1974) and a mood visual analogue scale (VAS).
MRI-derived measures of brain function, based on blood-oxygenation-level-dependent (BOLD) contrast, were used to compare brain responses at rest in the treatment and the placebo groups. The resting-state data were acquired before any other scans, including the structural scan. Subjects were instructed to lie in dimmed light with their eyes open, think of nothing in particular, and not to fall asleep, similar to our previous studies (McCabe and Mishor, 2011; McCabe et al., 2011; Cowdrey et al., 2012; Rzepa et al., 2015; Rzepa and McCabe, 2016) and a method found to have higher reliability than eyes closed (Patriat R et al., 2013).
Image Acquisition
A Siemens Magnetom Trio 3T whole body MRI scanner and a 32-channel head coil were used. Multi-band accelerated echo planar imaging sequencing (Center for Magnetic Resonance Research) was used with an acceleration factor of 6 and iPAT acceleration factor of 2. T2*-weighted EPI slices were obtained every 0.7 seconds (TR=0.7, TE=0.03), these parameters were optimized given our scanner capability and used to increase sampling rates and increase our power to detect resting state networks as has been shown previously with multiband (Xu et al., 2013) (Filippini et al., 2014). Fifty-four transverse slices with in-plane resolution of 2.4 x 2.4mm were attained and slice thickness was 2.4 mm. The matrix size was 96x96 and the field of view were 230x230 mm. Acquisition was performed during resting-state scan, yielding 400 volumes in total. Sagittal 3D MPRAGE images were also acquired with an isotropic in-plane resolution of 1x1x1 (TI=0.9 seconds, TR=2.02, flip angle 9°, FOV=250x250 mm) yielding 192 slices.
fMRI Data Analysis
Preprocessing
Imaging data were preprocessed and analyzed using FSL tools (www.fmrib.ox.ac.uk/fsl) (Smith et al., 2004). fMRI data preprocessing was carried out using FEAT (FMRI Expert Analysis Tool, version 6.0, a part of FSL software) and included the following steps: non-brain removal (Smith, 2002), motion correction using MCFLIRT (Jenkinson and Smith, 2001), spatial smoothing using a Gaussian kernel of full-width at half maximum of 5 mm, grand mean intensity normalization of the entire 4D dataset by a single multiplicative factor, and high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=64.0 s). fMRI volumes were registered to the individual’s structural scan and the MNI-152 standard space image (Montreal Neurological Institute, Montreal, QC, Canada) using FMRIB’s Linear Image Registration Tool (Jenkinson et al., 2001).
Time Series Extraction and Higher Level Analysis
To study RSFC, a seed-based correlation approach was used. Using the Harvard-Oxford subcortical structural atlas (Kennedy et al., 1998), we created a structural bilateral amygdala. To maximize the exact coverage, the masks of these seed regions were threshold by 20% to include voxels having at least 80% of probability of being in these particular regions. We also created seeds for the dmPFC (18 34 29; -24 35 28, 6-mm sphere) coordinates from (Sheline et al., 2010) and a pgACC seed (0 38 0, 8 mm sphere) coordinates from the center of the region in the Harvard-Oxford Structural atlas. Spheres were checked that they did not cross into other brain regions. The seeds were created with Wake Forest University Pickatlas tool in SPM8 similar to our recent paper (Rzepa and McCabe, 2016) and our previous RSFC paper with citalopram (McCabe et al., 2011).
The mean time course within the left and right seeds of each ROI (except for the pgACC, only comprising one medial seed) was calculated and used as a single regressor added to the general linear model. In addition, white matter signal, cerebrospinal fluid signal, 6 motion parameters (3 translations and 3 rotations), and the global mean time-series over whole brain were used as nuisance regressors. We have obtained white matter and cerebrospinal fluid masks using FSL’s FAST segmentation program. The resulting segmented images were then thresholded to ensure 80% tissue type probability. For each individual, the general linear model was analysed by using the FMRI Expert Analysis Tool [version 5.4, part of FMRIB’s Software Library (Smith et al., 2004)]. The resulting parameter estimate maps were then analyzed using higher level 1-sample t tests for group averages and between-samples t tests for group differences. Images were corrected for multiple comparisons using clusters with Z>2.3 voxel-wise thresholding and a family-wise error-corrected cluster significance threshold of P<.05 (Worsley, 2001). We also included FSLs FLAME1, which also considers the variance of the subject-specific parameter estimates and has been found by Eklund et al. (2016) to be a clusterwise inference that stands out as having much lower FWE, often being valid (<5%, i.e., reduced false positives) compared with other fMRI packages. From the results, we then only reported those that met the further correction for number of ROIs examined, which gave P < .016 (i.e., P<.05 Bonferroni corrected for the 3 networks of interest: amygdala, dmPFC, and pgACC) (Davidson et al., 2003). The percent BOLD signal change in the graphs is the PE/COPE values converted to mean percent BOLD signal change via Featquery (FSL; www.fmrib.ox.ac.uk/fsl;Smith et al., 2004) for the regions that had significant correlations with the seeds (Table 2).
Table 2.
Resting State Functional Connectivity for the dmPFC Seed Region
| MNI coordinates | ||||||
|---|---|---|---|---|---|---|
| Brain Region | X | Y | Z | Cluster size | z-Score | P value |
|
dmPFC seed
bupropion > placebo |
||||||
| PCC Precuneus |
4 8 |
-40 -58 |
14 32 |
449 449 |
3.47 3.1 |
<0.001
<0.001 |
P values cluster corrected family wise error P < .05, further corrected for multiple comparisons across 3 seed networks resulting in P = .016.
PCC, posterior cingulate cortex.
Correlational Analyses
To examine the relationship between the scores on behavioral questionnaires and RSFC, we extracted the percent BOLD signal change using FSL Featquery and correlated with scores on the following questionnaires: FCPS, BIS/BAS, and TEPS.
Results
Demographic Details and Mood Ratings
Demographic data (Table 1) indicated that participants had low depression and anhedonia scores, as measured on range of mood and anhedonia questionnaires. A repeated-measures ANOVA was performed to examine the effect of treatment (bupropion/placebo) and time (pre-/postscan) on mood and affect, as measured by the BFS and VAS (supplementary Table 1). Results revealed that there was no significant effect of treatment [F(1,16)=.483, P=.497], time [F(1,16)=.822, P=.378], treatment by time [F(1,16)=1.922, P=.185], treatment by VAS [F(1,16)=2.472, P=.084] or treatment by time by VAS interactions [F(1,16)=.689, P=.545]. There was also no significant effect of treatment [F(1,14)=1.61, P=.225] or treatment by time interaction [F(1,14)=2.176, P=.162] on total BFS scores. However, there was a significant main effect of time on overall BFS score [F(1,14)=5.879, P=.029].
Table 1.
Group Demographic and Psychosocial Measures
| Measure | |
|---|---|
| Age (y) | 24 (4.26) |
| Ethnicity | 100% Caucasian |
| BMI | 23.29 (2.38) |
| BDI | 1.71 (3.14) |
| FCPS | 136.76 (14.48) |
| SHAPS | 20.65 (5.67) |
| TEPS anticipatory | 47.53 (7.75) |
| TEPS consummatory | 37.59 (4.95) |
| BAS drive | 11.06 (2.49) |
| Fun seeking | 11.75 (3.11) |
| Reward responsiveness | 17.53 (1.87) |
| BIS | 20.41 (4.24) |
Abbreviations: BMI, Body Mass Index; BDI, Beck Depression Inventory; FCPS, Fawcett Clarke Pleasure Scale; SHAPS, Snaith-Hamilton Pleasure Scale; TEPS, Temporal Experience of Pleasure Scale; BAS/BIS, Behavioral Activation/Behavioral Inhibition Scale.
Data are means (SD) except for ethnicity, which is percentage.
Adverse Effects
Supplementary table 2 reports the number of adverse effects experienced on each treatment, as measured on the Patient Rated Inventory of Side Effects. The most commonly reported adverse effects across both treatment phases were headache (n= 5 per treatment), difficulty sleeping (n= 3 per treatment), and fatigue (n= 3 placebo, n = 5 bupropion). Dizziness (n= 4) was the most commonly reported adverse effect in the bupropion condition that was not reported in the placebo condition.
Main Effects of Stimuli on Blood Oxygen Level-Dependent Responses
Supplementary Tables 3 and 4 provide a summary of main effect of 1-sample t test on seed ROI functional connectivity (baseline) for the placebo and bupropion. Overall, the patterns of connectivity associated with each of the seed regions are consistent with resting-state functional connectivity experiments investigating effects of antidepressants in healthy individuals and depressed patients (Lai and Wu, 2012; Li et al., 2013; Posner et al., 2013; Wang et al., 2014, 2015; Shen et al., 2015; Sikora et al., 2016).
Within-Subjects Analysis Examining the Treatment Effect
Right dmPFC Seed
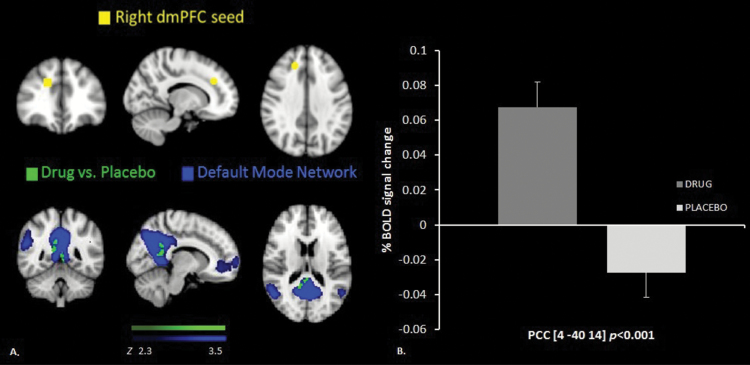
There was increased RSFC between the right dmPFC seed and the right posterior cingulate (Table 2; Figure 1) and precuneus cortex in the bupropion group compared with the placebo group. Both these results survived the correction for the number of seeds examined (P<.016; Bonferroni corrected for multiple comparisons). We also found increased functional connectivity between the left dmPFC seed region and the precuneus (-4 -46 48, P<.0394), yet this did not survive the multiple comparisons Bonferroni correction for the number of seeds examined.
Figure 1.
(A) Resting state functional connectivity (RSFC) between the right dorso-medial prefrontal cortex (dmPFC) seed region (yellow) and the posterior cingulate cortex, higher in the bupropion treatment than the placebo treatment (green) overlaid on the Default Mode Network (DMN). (B) Percent BOLD signal change extracted for both of the groups.
Correlations with Behavioral Measures
We found a negative correlation between the decreased RSFC of the dmPFC seed and the precuneus and increased BIS under the drug condition (r=-.494, P=.044; however, this did not survive multiple correction for the number of questionnaires examined) and no correlation in the placebo condition (r=.404, P=.108).
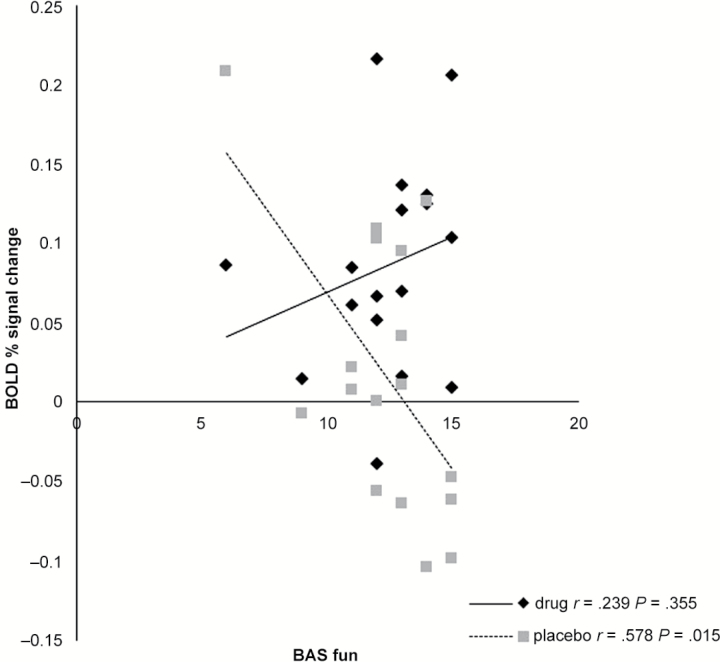
We also found a negative correlation between decreased RSFC of the dmPFC seed and the precuneus and decreased behavioral activation subtype of fun (BAS fun) under the placebo condition (r=-.578, P=.015; this also survived multiple correction for the number of questionnaires) but no correlation in the drug condition (r = .239, P=.355) (Figure 2). We also examined the difference in these correlations with the Fishers r to z transformation and found z= -2.68, P<.007 (2-tailed). This means that there was decreased connectivity in the placebo group as levels of fun reported decreased.
Figure 2.
Decreased Resting state functional connectivity (RSFC) between the dorso-medial prefrontal cortex (dmPFC) region and the precuneous correlates with decreasing Behavioral Activation Scale (BAS) fun scores under placebo only.
Discussion
The main aim of our study was to investigate the effects of 7 days bupropion treatment on RSFC in healthy volunteers. Our previous work found that the serotonin reuptake inhibitor citalopram reduced not only the brain’s activity during reward and aversive processing but also reduced RSFC (McCabe et al., 2010, 2011; McCabe and Mishor, 2011). We suggested that this profile of action might be related to citalopram’s positive clinical effects but perhaps also emotional blunting. Drugs like bupropion, which are dopaminergic noradrenergic reuptake inhibitors, are thought less likely to cause emotional blunting. In keeping with this notion, we have recently shown that bupropion (unlike citalopram) can increase the brain’s activity during the processing of positive and negative stimulation (Dean et al., 2016). Therefore, we hypothesized that perhaps bupropion would also increase the brain’s RSFC in key regions such as the dmPFC that we found modulated by citalopram (McCabe et al., 2011).
Our main findings revealed that bupropion increased RSFC between the dmPFC seed region and the PCC and between the dmPFC and the precuneus cortex compared with placebo. The dmPFC is implicated in many cognitive and emotional processes, while the precuneus and the PCC are thought to be involved in self-referential and memory processes (Bressler and Menon, 2010b; Jaworska et al., 2015; Janssen et al., 2015). Studies of RSFC in depressed patients have revealed abnormalities in similar regions (Abbott et al., 2013; Manoliu et al., 2013; Zhong et al., 2016), with the authors suggesting that these abnormalities might represent patients’ difficulty in disengaging from self-referential processes, which might lead to increased negative thoughts (Manoliu et al., 2013). Furthermore, van Tol et al., 2014 report decreased cortical thickness related to decreased RSFC between the dmPFC and the PCC/precuneus cortex in depressed patients. The authors suggest this might lead to maladaptive emotional regulation. Thus, our finding of increased RSFC between the dmPFC and the precuneus, key regions found dysfunctional in depression, might help explain the mechanism of action of treatments like bupropion. Moreover, we also found that our results are consistent with our previous data in young, unmedicated participants with depression symptoms that revealed reduced functional connectivity between the dmPFC and the precuneus (Rzepa and McCabe, 2016). Having found the same functional connectivity now increased under the bupropion condition suggests that perhaps these networks are involved in the putative mechanism of action of bupropion. Further studies should clarify this in depressed patients before and after treatment.
Interestingly, we also found a relationship between the BIS and the dmPFC precuneus RSFC in the bupropion group and the BAS in the placebo group. The BIS is hypothesized to be sensitive to cues of threat or punishment and is associated with avoidant or withdrawal behaviors (Zinbarg and Lira Yoon, 2008; Bijttebier et al., 2009). The BAS is hypothesized to be sensitive to cues of reward or appetite and is associated with approach behaviors (Fowles, 1988). Studies in depressed patients have shown high levels of BIS activation and lower levels of BAS activation (Fowles, 1988; Gray, 1994; Davidson, 1998;Meyer et al., 2001; Kasch et al., 2002; Johnson et al., 2003; Campbell-Sills et al., 2004; Pinto-Meza et al., 2006).
In this study, we found increased RSFC under the drug condition (between the dmPFC and the precuneus) that correlated with reduced behavioral inhibition scores. However, this result did not survive the further Bonferroni correction for the number of questionnaires examined. Nonetheless, we believe it would be of interest to examine in future larger studies how bupropion might affect the avoidance of negative information. Interestingly though, a recent study examining internet gaming addiction found that 6 weeks of bupropion treatment had a greater improvement on BIS scores than the SSRI escitalopram (Song et al., 2016).
As mentioned above, we also found that as RSFC under the placebo condition was increased (between the dmPFC and the precuneus), behavioral activation (Fun) levels were also higher. This result suggests that increased RSFC between these regions is correlated with increased feelings of pleasure and supports the notion that increasing connectivity under bupropion would be unlikely to cause emotional blunting (the flattening of emotional experiences). However, as there was no significant effect under the bupropion condition, this is purely speculative and would need to be examined with a larger sample size in healthy volunteers and then again in a depressed patient sample both before and after treatment.
Taken together, our results find that bupropion increases RSFC between the dmPFC seed region and the posterior cingulate cortex and the precuneus. The RSFC with the precuneus also correlates with increased experience of fun in the placebo condition. The RSFC results are opposite to that which we found previously with citalopram using similar methodology and seed regions and opposite to that found in young unmedicated people with depression symptoms. Thus, our results may indicate a mechanism by which bupropion might work as an antidepressant by increasing dmPFC RSFC.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Funding
This work was supported by the University of Reading fund for Dr McCabe.
Statement of Interest
Dr. McCabe has acted as a consultant to P1Vital, Givaudan, GWpharma, the British Broadcasting Company, and Channel 4. Zola Dean, Ewelina Rzepa, and Zola Dean report no biomedical financial interests or potential conflicts of interest.
Supplementary Material
Acknowledgments
We thank Dr Horndasch for clinical supervision.
References
- Abbott CC, Lemke NT, Gopal S, Thoma RJ, Bustillo J, Calhoun VD, Turner JA. (2013) Electroconvulsive therapy response in major depressive disorder: a pilot functional network connectivity resting state FMRI investigation. Front Psychiatr 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571. [DOI] [PubMed] [Google Scholar]
- Bijttebier P, Beck I, Claes L, Vandereycken W. (2009) Gray’s reinforcement sensitivity theory as a framework for research on personality–psychopathology associations. Clin Psychol Rev 29:421–430. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. (2010a) Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14:277–290. [DOI] [PubMed] [Google Scholar]
- Bressler SL, Menon V. (2010b) Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14:277–290. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Liverant GI, Brown TA. (2004) Psychometric evaluation of the behavioral inhibition/behavioral activation scales in a large sample of outpatients with anxiety and mood disorders. Psychol Assessment 16:244–254. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL. (1994) Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. J Pers Soc Psychol 67:319. [Google Scholar]
- Cowdrey FA, Filippini N, Park RJ, Smith SM, McCabe C. (2012) Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ. (1998) Affective style and affective disorders: perspectives from affective neuroscience. Cognition Emotion 12:307–330. [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. (2003) The neural substrates of affective processing in depressed patients treated with venlafaxine. Am J Psychiatry 160:64–75. [DOI] [PubMed] [Google Scholar]
- Dean Z, Horndasch S, Giannopoulos P, McCabe C. (2016) Enhanced neural response to anticipation, effort and consummation of reward and aversion during bupropion treatment. Psychol Med 46:2263–2274. [DOI] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64:327–337. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Rauhut AS, King‐Pospisil KA, Bardo MT. (2006) Review of the pharmacology and clinical profile of bupropion, an antidepressant and tobacco use cessation agent. CNS Drug Rev 12:178–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H. (2016) Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates (vol 113, pg 7900, 2016). Proc Natl Acad Sci U S A 113:E4929–E4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett J, Clark DC, Scheftner WA, Gibbons RD. (1983) Assessing anhedonia in psychiatric patients. Arch Gen Psychiatry 40:79–84. [DOI] [PubMed] [Google Scholar]
- Filippini N, Zsoldos E, Haapakoski R, Sexton CE, Mahmood A, Allan CL, Topiwala A, Valkanova V, Brunner EJ, Shipley MJ. (2014) Study protocol: the Whitehall II imaging sub-study. BMC Psychiatry 14:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. (2002) Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fowles DC. (1988) Psychophysiology and psychopathology: a motivational approach. Psychophysiology 25:373–391. [DOI] [PubMed] [Google Scholar]
- Fu CH, Costafreda SG, Sankar A, Adams TM, Rasenick MM, Liu P, Donati R, Maglanoc LA, Horton P, Marangell LB. (2015) Multimodal functional and structural neuroimaging investigation of major depressive disorder following treatment with duloxetine. BMC Psychiatry 15:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. (2007) Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res 93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner DM, Olmsted MP, Bohr Y, Garfinkel PE. (1982) The eating attitudes test: psychometric features and clinical correlates. Psychol Med 12:871–878. [DOI] [PubMed] [Google Scholar]
- Gray JA. (1994) Framework for taxonomy of psychiatric disorders. In: (Van Goozen , Van del Pool , Sergeant eds), pp. 29–59. [Google Scholar]
- Hobi V. (1985) Basler Befindlichkeitsskala. Manual Weinheim: Beltz. [Google Scholar]
- Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M. (2010) Glutamatergic and resting-state functional connectivity correlates of severity in major depression—the role of pregenual anterior cingulate cortex and anterior insula. Front Syst Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen TW, Heslenfeld DJ, Mourik RV, Logan GD, Oosterlaan J. (2015) Neural correlates of response inhibition in children with attention-deficit/hyperactivity disorder: a controlled version of the stop-signal task. Psychiatr Res 233:278–284. [DOI] [PubMed] [Google Scholar]
- Jaworska N, Yang XR, Knott V, MacQueen G (2015) A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry 16:448–471. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Turner RJ, Iwata N. (2003) BIS/BAS levels and psychiatric disorder: an epidemiological study. J Psychopathol Behav 25:25–36. [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. (2002) Behavioral activation and inhibition systems and the severity and course of depression. J Abnorm Psychol 111:589–597. [DOI] [PubMed] [Google Scholar]
- Kennedy DN, Lange N, Makris N, Bates J, Meyer J, Caviness VS., Jr (1998) Gyri of the human neocortex: an MRI-based analysis of volume and variance. Cereb Cortex 8:372–384. [DOI] [PubMed] [Google Scholar]
- Klaassens BL, van Gorsel HC, Khalili-Mahani N, van der Grond J, Wyman BT, Whitcher B, Rombouts SARB, van Gervend JA. (2015) Single-dose serotonergic stimulation shows widespread effects on functional brain connectivity. Neuroimage 122:440–450. [DOI] [PubMed] [Google Scholar]
- Lai CH, Wu YT. (2012) Frontal regional homogeneity increased and temporal regional homogeneity decreased after remission of first-episode drug-naive major depressive disorder with panic disorder patients under duloxetine therapy for 6 weeks. J Affect Disord 136:453–458. [DOI] [PubMed] [Google Scholar]
- Li B, Liu L, Friston KJ, Shen H, Wang L, Zeng LL, Hu D. (2013) A treatment-resistant default mode subnetwork in major depression. Biol Psychiatry 74:48–54. [DOI] [PubMed] [Google Scholar]
- Liston C, Chen AC, Zebley BD, Drysdale AT, Gordon R, Leuchter B, Voss HU, Casey BJ, Etkin A, Dubin MJ. (2014) Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 76:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthoffer D, Zimmer C, Forstl H, Bauml J, Riedl V, Wohlschlager AM, Sorg C. (2013) Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci 7:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Meng C, Brandl F, Doll A, Tahmasian M, Scherr M, Schwerthoffer D, Zimmer C, Forstl H, Bauml J, Riedl V, Wohlschlager AM, Sorg C. (2014) Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front Hum Neurosci 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Cowen PJ, Harmer CJ. (2009) Neural representation of reward in recovered depressed patients. Psychopharmacology (Berl) 205:667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z. (2011) Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage 57:1317–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Cowen PJ, Harmer CJ. (2010) Diminished neural processing of aversive and rewarding stimuli during selective serotonin reuptake inhibitor treatment. Biol Psychiatry 67:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe C, Mishor Z, Filippini N, Cowen PJ, Taylor MJ, Harmer CJ. (2011) SSRI administration reduces resting state functional connectivity in dorso-medial prefrontal cortex. Mol Psychiatry 16:592–594. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. (2001) Responsiveness to threat and incentive in bipolar disorder: relations of the BIS/BAS scales with symptoms. J Psychopathol Behav 23:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Carrasco JL, Stahl S. (2007) The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol 21:461–471. [DOI] [PubMed] [Google Scholar]
- Patriat R, Molloy EK, Meier TB, Kirk GR, Nair VA, Meyerand ME, Prabhakaran V, Birn RM. (2013) The effect of resting condition on resting-state fMRI reliability and consistency: a comparison between resting with eyes open, closed, and fixated. Neuroimage 78:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Meza A, Caseras X, Soler J, Puigdemont D, Perez V, Torrubia R. (2006) Behavioural inhibition and behavioural activation systems in current and recovered major depression participants. Pers Indiv Differ 40:215–226. [Google Scholar]
- Posner J, Hellerstein DJ, Gat I, Mechling A, Klahr K, Wang Z, McGrath PJ, Stewart JW, Peterson BS. (2013) Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry 70:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J, Cole V, Goodwin GM. (2009) Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry 195:211–217. [DOI] [PubMed] [Google Scholar]
- Ramasubbu R, Konduru N, Cortese F, Bray S, Gaxiola-Valdez I, Goodyear B. (2014) Reduced intrinsic connectivity of amygdala in adults with major depressive disorder. Front Psychiatry 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, McCabe C. (2007) Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur J Neurosci 26:1067–1076. [DOI] [PubMed] [Google Scholar]
- Rzepa E, McCabe C. (2016) Decreased anticipated pleasure correlates with increased salience network resting state functional connectivity in adolescents with depressive symptomatology. J Psychiatr Res 82:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzepa E, Tudge L, McCabe C. (2015) The CB1 neutral antagonist tetrahydrocannabivarin reduces default mode network and increases executive control network resting state functional connectivity in healthy volunteers. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. (2010) Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc Natl Acad Sci U S A 107:11020–11025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Yao J, Jiang X, Zhang L, Xu L, Feng R, Cai L, Liu J, Wang J, Chen W. (2015) Sub-hubs of baseline functional brain networks are related to early improvement following two-week pharmacological therapy for major depressive disorder. Hum Brain Mapp 36:2915–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora M, Heffernan J, Avery ET, Mickey BJ, Zubieta JK, Pecina M. (2016) Salience network functional connectivity predicts placebo effects in major depression. Biol Psychiatry Cogn Neurosci Neuroimaging 1:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–219. [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. (1995) A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry 167:99–103. [DOI] [PubMed] [Google Scholar]
- Song J, Park JH, Han DH, Roh S, Son JH, Choi TY, Lee H, Kim TH, Lee YS. (2016) A comparative study of the effects of bupropion and escitalopram on Internet gaming disorder. Psychiatr Clin Neurosci 70:527–535. [DOI] [PubMed] [Google Scholar]
- Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S. (2004) A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Primary care companion to the Journal of clinical psychiatry 6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahmasian M, Knight DC, Manoliu A, Schwerthoffer D, Scherr M, Meng C, Shao J, Peters H, Doll A, Khazaie H, Drzezga A, Bauml J, Zimmer C, Forstl H, Wohlschlager AM, Riedl V, Sorg C. (2013) Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front Hum Neurosci 7:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven V, Wingen M, Kuypers KPC, Ramaekers JG, Formisano E. (2013) Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tol MJ, Li M, Metzger CD, Hailla N, Horn DI, Li W, Heinze HJ, Bogerts B, Steiner J, He H, Walter M. (2014) Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psycholog Med 44:2053–2065. [DOI] [PubMed] [Google Scholar]
- van Wingen GA, Tendolkar I, Urner M, van Marle HJ, Denys D, Verkes RJ, Fernandez G. (2014) Short-term antidepressant administration reduces default mode and task-positive network connectivity in healthy individuals during rest. Neuroimage 88:47–53. [DOI] [PubMed] [Google Scholar]
- von Zerssen D, Strian F, Schwarz D. (1974) Evaluation of depressive states, especially in longitudinal studies. Modern Problems of Pharmacopsychiatry 7:189–202. [DOI] [PubMed] [Google Scholar]
- Wang L, Li K, Zhang Q, Zeng Y, Dai W, Su Y, Wang G, Tan Y, Jin Z, Yu X, Si T. (2014) Short-term effects of escitalopram on regional brain function in first-episode drug-naive patients with major depressive disorder assessed by resting-state functional magnetic resonance imaging. Psycholog Med 44:1417–1426. [DOI] [PubMed] [Google Scholar]
- Wang L, Xia M, Li K, Zeng Y, Su Y, Dai W, Zhang Q, Jin Z, Mitchell PB, Yu X, He Y, Si T. (2015) The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum Brain Mapp 36:768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. (2012) Default mode network activity and connectivity in psychopathology. Ann Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Worsley K. (2001) Statistical analysis of activation images. Functional MRI: an introduction to methods 14:251–270. [Google Scholar]
- Xu J, Moeller S, Auerbach EJ, Strupp J, Smith SM, Feinberg DA, Yacoub E, Uğurbil K. (2013) Evaluation of slice accelerations using multiband echo planar imaging at 3T. Neuroimage 83:991–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye T, Peng J, Nie BB, Gao J, Liu JT, Li Y, Wang G, Ma X, Li KC, Shan BC. (2012) Altered functional connectivity of the dorsolateral prefrontal cortex in first-episode patients with major depressive disorder. Eur J Radiol 81:4035–4040. [DOI] [PubMed] [Google Scholar]
- Zhong X, Pu W, Yao S. (2016) Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naive patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J Affect Disord 206:280–286. [DOI] [PubMed] [Google Scholar]
- Zinbarg RE, Lira Yoon K (RST and clinical disorders: Anxiety and depression. In: Corr PJ, ed), Cambridge University Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.