Abstract
Background:
Z-guggulsterone, an active compound extracted from the gum resin of the tree Commiphora mukul, has been shown to improve animal memory deficits via activating the brain-derived neurotrophic factor signaling pathway. Here, we investigated the antidepressant-like effect of Z-guggulsterone in a chronic unpredictable stress mouse model of depression.
Methods:
The effects of Z-guggulsterone were assessed in mice with the tail suspension test and forced swimming test. Z-guggulsterone was also investigated in the chronic unpredictable stress model of depression with fluoxetine as the positive control. Changes in hippocampal neurogenesis as well as the brain-derived neurotrophic factor signaling pathway after chronic unpredictable stress/Z-guggulsterone treatment were investigated. The tryptophan hydroxylase inhibitor and the tyrosine kinase B inhibitor were also used to explore the antidepressant-like mechanisms of Z-guggulsterone.
Results:
Z-guggulsterone (10, 30 mg/kg) administration protected the mice against the chronic unpredictable stress-induced increases in the immobile time in the tail suspension test and forced swimming test and also reversed the reduction in sucrose intake in sucrose preference experiment. Z-guggulsterone (10, 30 mg/kg) administration prevented the reductions in brain-derived neurotrophic factor protein expression levels as well as the phosphorylation levels of cAMP response element binding protein, extracellular signal-regulated kinase 1/2, and protein kinase B in the hippocampus and cortex induced by chronic unpredictable stress. Z-guggulsterone (10, 30 mg/kg) treatment also improved hippocampal neurogenesis in chronic unpredictable stress-treated mice. Blockade of the brain-derived neurotrophic factor signal, but not the monoaminergic system, attenuated the antidepressant-like effects of Z-guggulsterone.
Conclusions:
Z-guggulsterone exhibits antidepressant activity via activation of the brain-derived neurotrophic factor signaling pathway and upregulation of hippocampal neurogenesis.
Keywords: Z-guggulsterone, BDNF, CREB, CUS, major depression
Significance Statement
The impairment of the CREB-BDNF signal as well as the overproduction of proinflammatory factors contributes to the formation and/or development of major depression. Our previous findings showed that Z-guggulsterone, an active compound extracted from the gumresin of the tree Commiphora mukul, prevents neuroinflammation-triggered behavioral abnormalities and scopolamine-induced memory impairment via attenuation of the dysfunction of the CREB-BDNF signaling pathway. Within this context, our findings showed that Z-guggulsterone exerts antidepressant-like effects via activation of the CREB-BDNF signaling pathway, providing a new insight into the pharmacological role of Z-guggulsterone in the central nervous system and shedding light on the development of new antidepressants. Our results also extend the pharmacological role of Z-guggulsterone beyond a metabolic regulator to a potential modulator of major depression.
Introduction
Depression is one of the common public health problems facing the world today. In clinical practice, several different antidepressants have been developed and confirmed to alleviate the symptoms of major depression. However, the application of these drugs is always accompanied with many limitations (Fava, 2010; Fabbri et al., 2013; Sanchez et al., 2015). For instance, the first application of antidepressants is effective in only about one-third of patients, and approximately two-thirds of patients fail to achieve clinical improvements, even after several times trying (Schwartz et al., 2016). Thus, there is a strong need to explore new antidepressants.
The cAMP response element binding protein (CREB)-brain-derived neurotrophic factor (BDNF) signal is an attractive topic in research into the pathophysiological mechanism of major depression and new antidepressants. Chronic stresses have been reported to impair the function of the CREB-BDNF signal in the hippocampus and medial prefrontal cortex (mPFC) (Jiang et al., 2015; Xu et al., 2015), and administration of antidepressants can normalize the CREB-BDNF signal (Mizuki et al., 2014; Li et al., 2015). The impairment of the CREB-BDNF signal has also been observed in the postmortem hippocampus and mPFC of depressive individuals (Pandey et al., 2007). Most evidence proving the protective effect of the CREB-BDNF signal in major depression comes from the rodent experiments in which direct infusion of CREB or BDNF into hippocampus or mPFC exhibits obvious antidepressant-like effects through activation of the molecule downstream of the CREB-BDNF signal, such as extracellular-signal related kinase 1/2 (ERK1/2) and protein kinase B (Akt) (Gass and Riva, 2007; Castren and Rantamaki, 2010). Moreover, knockout of the brain BDNF has been confirmed to make rodents more susceptible to chronic stresses (Burke et al., 2013). Therefore, enhancement of the CREB-BDNF signal should be helpful for the therapy of major depression.
Guggulsterone, an active compound extracted from the gumresin of the tree Commiphora mukul, has been used for thousands of years to cope with obesity, arthritis, hypothyroidism, and lipid metabolism disorders. To date, little information has been reported regarding the role of guggulsterone in the central nervous system. For example, Z-guggulsterone has been reported to regulate the respiratory rhythm in the brainstem medulla slice of neonatal Sprague-Dawley rats in vitro (Zhao et al., 2014). Guggulsterone also improves the streptozocin-induced impairment of mouse memory (Saxena et al., 2007). Recently, our group reported that Z-guggulsterone can attenuate the behavioral abnormalities induced by neuroinflammation in the forced swimming test (FST) and tail suspension test (TST) (Huang et al., 2016a) and also prevents memory impairment in a scopolamine-induced memory impairment model through activation of the CREB-BDNF signal (Chen et al., 2016). The pathophysiological process of depression is associated with working memory impairment (Richter et al., 2013; Moreno et al., 2015) and neuroinflammation (Lisi et al., 2013; Couch et al., 2016). The impairment of the CREB-BDNF signal in the hippocampus and mPFC in depressive animals (Calabrese et al., 2014; Daniele et al., 2015) has also been shown to be mediated by the overproduction of proinflammatory factors. Thus, together with a recently reported antidepressant-like effect of a standardized hydroalcoholic extract of Commiphora mukul in olfactory bulbectomized rats (Kalshetti et al., 2015), we raised the possibility that Z-guggulsterone may have antidepressant-like activity. In the present study, we systematically investigated the antidepressant-like effect of Z-guggulsterone as well as its underlying mechanisms using the experiments of sucrose preference, TST, and FST in a mouse model of chronic unpredictable stress (CUS).
Materials and methods
Animals
Eight- to 10-week-old male C57BL/6J mice were housed 5/cage under standard conditions (12-h-light/-dark cycle; lights on from 7:00 am to 7:00 pm; 23 ± 1°C ambient temperature; 55 ± 10% relative humidity) for 1 week with free access to food and water. Each experimental group consisted of 10 mice. Behavioral experiments were carried out during the light phase. Animal experiments were conducted in accordance with internationally accepted guidelines for the use of animals in toxicology as adopted by the Society of Toxicology in 1999 and approved by the University Animal Ethics Committee of Nantong University (permit no. 2110836).
Experimental materials and procedures are available online in the supplementary Materials and Methods.
Statistical Analysis
All analyses were performed using SPSS 13.0 software, and data are presented as mean ± SEM. Differences between mean values were evaluated using 1-way or 2-way ANOVA, as appropriate. For 1-way ANOVA, posthoc tests were performed using LSD test. For 2-way ANOVA, Bonferroni’s posthoc tests were used to assess isolated comparisons. P<.05 was considered statistically significant.
Results
Antidepressant-Like Effects of Z-Guggulsterone in the FST and TST of Mice
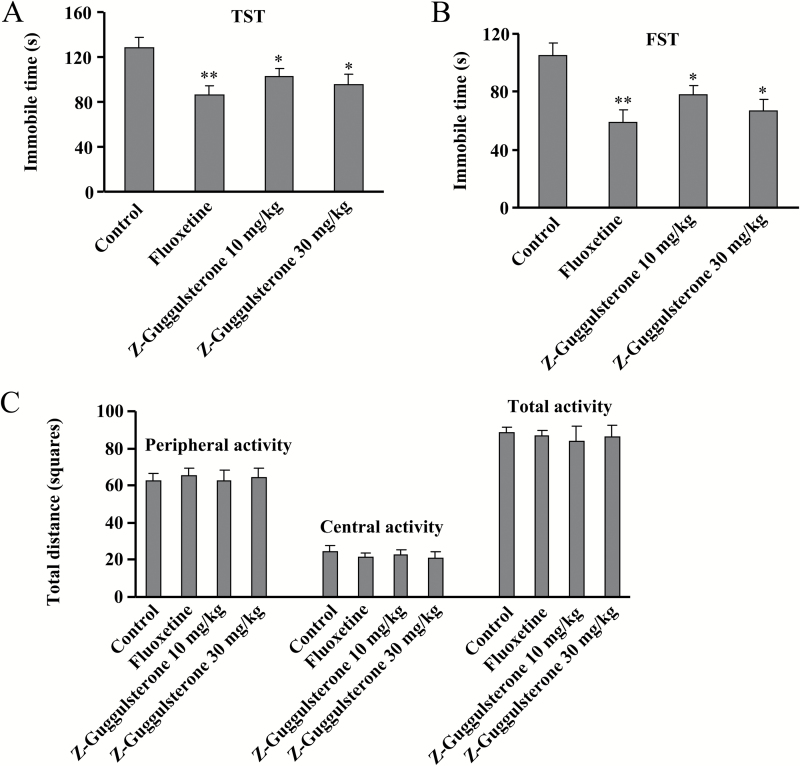
TST and FST, with a high predictive validity for antidepressant activity, have been widely used to detect the potential antidepressant activity of different compounds (Porsolt et al., 1977; Steru et a., 1985). Here, we first evaluated the effect of Z-guggulsterone (supplementary Figure 1) on potential antidepressant-like activity in TST assay. Results showed that similar with the effect of fluoxetine (20 mg/kg), a single injection of Z-guggulsterone (10 or 30 mg/kg, i.p., 30 minutes) produced an obvious antidepressant-like effect in the TST (Figure 1A). The data were subjected to a 1-way ANOVA with drug treatment as the factor and showed a significant main effect for Z-guggulsterone treatment [F(3, 36) = 4.31, P<.05] (Figure 1A). Subsequent posthoc analysis indicated that similar with fluoxetine, Z-guggulsterone administration (10, 30 mg/kg) decreased the immobile time of mice in the TST (n = 10, P<.05 vs control) (Figure 1B). Data from the FST revealed a significant main effect for Z-guggulsterone treatment [F(3, 36) = 6.46, P<.01], and posthoc analysis indicated that Z-guggulsterone treatment (10 or 30 mg/kg, i.p., 30 min) markedly reduced the immobile time compared with vehicle-treated mice (n = 10, P<.05 vs control), and fluoxetine also decreased the immobile time as expected in the FST (n = 10, P<.01 vs control) (Figure 1B).
Figure 1.
Z-Guggulsterone produces antidepressant-like effects in the tail suspension test (TST) and forced swimming test (FST). (A) Z-Guggulsterone (10, 30 mg/kg) treatment significantly decreased the immobile time of C57BL/6J mice in the TST. (B) Z-Guggulsterone (10, 30 mg/kg) treatment decreased the immobile time of C57BL/6J mice in the FST. (C) Z-Guggulsterone (10, 30 mg/kg) treatment had no effects on the spontaneous locomotor activity of C57BL/6J mice in the open-field test. Fluoxetine (20 mg/kg) was used as a positive control. All these behavioral tests were conducted 30 minutes after the injection. All data were expressed as mean ± SEM.
To exclude the possibility that the reduced immobility in these tests may be induced by an increase in spontaneous activity (Bourin et al., 2001), naive mice treated as in the above schedule were exposed to the open-field apparatus for 5 minutes. Results showed that there was no difference in the number of squares an animal crossed between areas of the center and periphery in all groups (Figure 1C), and ANOVA showed no significant effects for Z-guggulsterone treatment (10 or 30 mg/kg, i.p., 30 minutes) on peripheral [F(3, 36) = 0.07, P = .70], central [F(3, 36) = 0.22, P = .92], and total activity [F(3, 36) = 0.02, P = .46]. These data indicated that the reduction of immobility observed in the TST and FST after Z-guggulsterone treatment was not due to the change in locomotor activity.
Chronic Z-Guggulsterone Treatment Reverses the CUS-Induced Depressive Symptoms of Mice
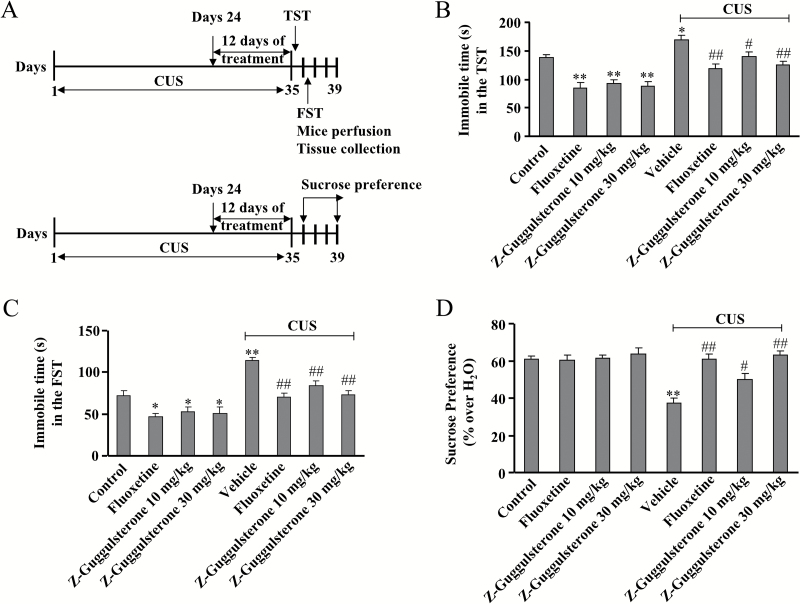
To further characterize the antidepressant effect of Z-guggulsterone, we employed CUS, which is currently regarded as one of the most predictive animal models of depression. In the TST, the 2-way ANOVA indicated significant effects for stress [F(1, 72) = 56.85, P<.001] and treatment [F(3, 72) = 21.84, P<.001], but there was no significant stress ×treatment interaction [F(3, 72) = 0.41, P = .75] (Figure 2B). CUS treatment robustly increased the immobile time of mice in the TST (n = 10, P<.05 vs control group), and this increase was substantially reversed by 12 days of Z-guggulsterone (10, 30 mg/kg) treatment, similar to fluoxetine treatment (n = 10, P<.05, P<.01 vs vehicle + CUS group) (Figure 2B). Z-guggulsterone treatment also reduced the immobile duration of naive mice in the TST (n = 10, P<.01 vs control group) (Figure 2B).
Figure 2.
Effects of Z-guggulsterone on chronic unpredictable stress (CUS)-induced behavioral abnormalities. (A) The schematic diagram for the experimental timeline and tissue preparation in the present study. (B-C) Quantitative analysis showing the inhibitory effect of Z-guggulsterone (10 or 30 mg/kg) on CUS-induced increase in the immobile time in the TST (A, n = 10, *P<.05, **P<.01 vs control; #P<.05, ##P<.01 vs vehicle + CUS) and FST (B, n = 10, *P<.05, **P<.01 vs control; ##P<.01 vs vehicle + CUS). (D) Quantitative analysis showing the inhibitory effect of Z-guggulsterone (10 or 30 mg/kg) on CUS-induced decrease in sucrose intake (n = 10, **P<.01 vs control; #P<.05, ##P<.01 vs vehicle + CUS). The fluoxetine administration (20 mg/kg) was used as a positive control, and all data were shown as mean ± SEM.
In the FST, 2-way ANOVA indicated significant effects for stress [F(1, 72) = 55.23, P<.001] and treatment [F(3, 72) = 15.99, P<.001], but not for stress ×treatment interaction [F(3, 72) = 1.32, P = .27] (Figure 2C). CUS treatment robustly increased the immobile time of mice in the FST (n = 10, P<.01 vs control group), and this increase was reversed by 12 days of Z-guggulsterone (10, 30 mg/kg) treatment (n = 10, P<.01 vs vehicle + CUS group) (Figure 2C). In addition, Z-guggulsterone also reduced the immobile duration of naive mice in the FST (n = 10, P<.05 vs control group) (Figure 2C).
We then evaluated the antidepressant effect of Z-guggulsterone using the sucrose preference experiment. After a daily exposure to a group of unpredictable stresses for 23 days, the CUS-treated mice received 12 days of treatment of Z-guggulsterone, fluoxetine, or vehicle, and behavioral tests were then performed. The Z-guggulsterone (10, 30 mg/kg) or fluoxetine (20 mg/kg) was administrated i.p.. Two-way ANOVA indicated significant effects for stress [F(1, 72)=22.26, P<.001], treatment [F(3, 72) = 11.66, P<.001], and stress × treatment interaction [F(1, 72) = 9.06, P<.001] (Figure 2D). The CUS treatment induced a significant decrease in sucrose consumption compared with the control group (n = 10, P<.01 vs control group) (Figure 2D). While Z-guggulsterone produced no significant effects in naive mice, 12 days of Z-guggulsterone (10, 30 mg/kg) treatment in CUS-stimulated mice caused a significant increase in sucrose intake (n = 10, P<.05, P<.01 vs vehicle + CUS group) (Figure 2D). Taken together, these results suggest that Z-guggulsterone is able to reverse the CUS-induced depressive symptoms in mice.
Z-Guggulsterone Attenuates the CUS-Induced Impairment of the CREB-BDNF Signal
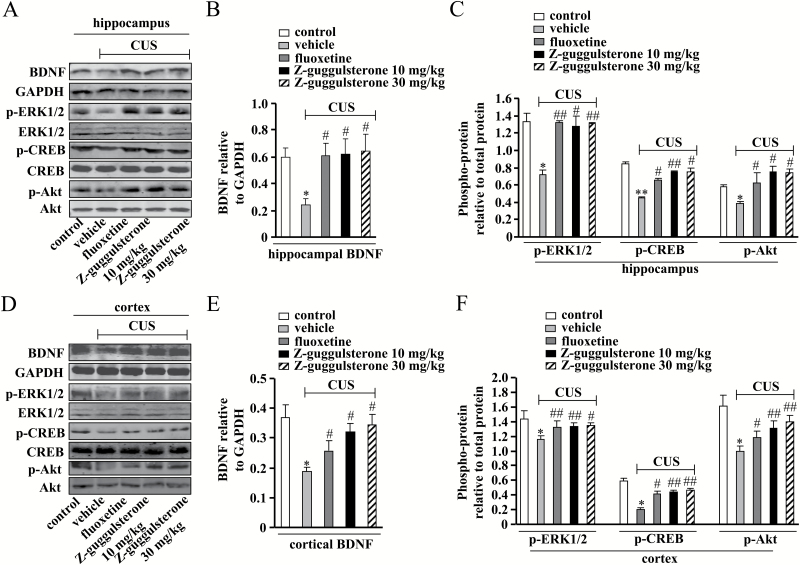
Since one of our previous studies showed that Z-guggulsterone can affect the CREB-BDNF signaling pathway in a memory-deficient model induced by scopolamine (Chen et al., 2016), we then investigated whether Z-guggulsterone could improve depressive symptoms via activating the BDNF signal in CUS models. Adult normal mice received daily injections of Z-guggulsterone (10, 30 mg/kg) for 12 days, and the protein expression levels of BDNF in the hippocampus and mPFC were detected. Two-way ANOVA revealed significant effects for stress [F(1, 42) = 6.99, P<.05] and treatment [F(3, 42) = 6.03, P<.01], but not for stress × treatment interaction [F(3, 42)=2.24, P=.10] in the hippocampus (Figure 3B). The protein expression level of BDNF was significantly downregulated by CUS treatment in the hippocampus (n = 5, P<.05 vs control group), and this downregulation was reversed by Z-guggulsterone treatment at doses of 10 and 30 mg/kg (n = 5, P<.05 vs vehicle + CUS group) (Figure 3A-B). In the mPFC, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 51.43, P<.01] and treatment [F(3, 42) = 3.02, P<.05], but not for stress × treatment interaction [F(3, 42) = 0.06, P=.98] (Figure 3E). The CUS treatment reduced the protein expression level of BDNF in the mPFC, and this reduction was attenuated by Z-guggulsterone treatment at doses of 10 and 30 mg/kg (n = 5, P<.05 vs control or vehicle + CUS group) (Figure 3D-E).
Figure 3.
Effects of Z-guggulsterone on chronic unpredictable stress (CUS)-induced impairments of the hippocampal and cortical cAMP response element binding protein- brain-derived neurotrophic factor (CREB-BDNF) signals. (A) Representative images showing the restoration effects of Z-guggulsterone (10 or mg/kg) on CUS-induced decreases in the protein expression level of BDNF as well as the phosphorylation levels of extracellular-signal related kinase 1/2 (ERK1/2), CREB, and protein kinase B (Akt) in mice hippocampus. (B) Quantitative analysis showing the restoration effect of Z-guggulsterone on CUS-induced decrease in hippocampal BDNF protein expression (n=5, *P<.05 vs control; #P<.05 vs vehicle + CUS). (C) Quantitative analysis showing the restoration effects of Z-guggulsterone on CUS-induced decreases in hippocampal ERK1/2, CREB, and Akt phosphorylation levels (n = 5, *P<.05, **P<.01 vs control; #P<.05, ##P<.01 vs vehicle + CUS). (D) Representative images showing the restoration effects of Z-guggulsterone (10, 30 mg/kg) on CUS-induced decreases in the protein expression level of BDNF as well as the phosphorylation levels of ERK1/2, CREB, and Akt in medial prefrontal cortex (mPFC). (E) Quantitative analysis showing the restoration effect of Z-guggulsterone on CUS-induced decrease in cortical BDNF protein expression (n = 5, *P<.05 vs control; #P<.05 vs vehicle + CUS). (F) Quantitative analysis showing the restoration effects of Z-guggulsterone on CUS-induced decreases in cortical ERK1/2, CREB, and Akt phosphorylation levels (n = 5, *P<.05 vs control; #P<.05, ##P<.01 vs vehicle + CUS). The fluoxetine administration (20 mg/kg) was used as a positive control, and all data were shown as mean ± SEM.
Accordingly, the phosphorylation levels of ERK1/2, CREB, and Akt in the mice hippocampus (n = 5, P<.05, P<.01 vs control; Figure 3A,C) and mPFC (n = 5, P<.05 vs control group; Figure 3D,F) were also reduced by CUS treatment, and these reductions were substantially reversed by Z-guggulsterone treatment (10, 30 mg/kg, P<.05, P<.01 vs vehicle + CUS group; Figure 3A,C, D,F). For hippocampal phospho-ERK1/2, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 24.41, P<.05], treatment [F(3, 42) = 11.16, P<.01], and stress × treatment interaction [F(3, 42) = 4.40] (Figure 3C). For hippocampal phospho-CREB, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 33.76, P<.01] and treatment [F(3, 42) = 3.47, P<.05], but not for stress × treatment interaction [F(3, 42) = 0.96] (Figure 3C). For hippocampal phospho-Akt, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 12.14, P<.05], treatment [F(3, 42) = 22.99, P<.01], and stress × treatment interaction [F(3, 42) = 11.43] (Figure 3C). For cortical phospho-ERK1/2, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 63.06, P<.001] and treatment [F(3, 42) = 5.71, P<.05], but not for stress × treatment interaction [F(3, 42) = 0.59, p = 0.62] (Figure 3F). For cortical phospho-CREB, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 76.68, P<.001] and treatment [F(3, 42) = 5.29, P<.01], but not for stress × treatment interaction [F(3, 42) = 2.07, P = .12] (Figure 3F). For cortical phospho-Akt, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 89.10, P<.001] and treatment [F(3, 42) = 3.12, P<.05], but not for stress × treatment interaction [F(3, 42) = 1.91, P = .15] (Figure 3F). Taken together, these data indicated that the Z-guggulsterone-induced restoration of BDNF protein expression in the hippocampus and cortex was tightly associated with the activation of the signal molecules upstream of the BDNF signaling pathway.
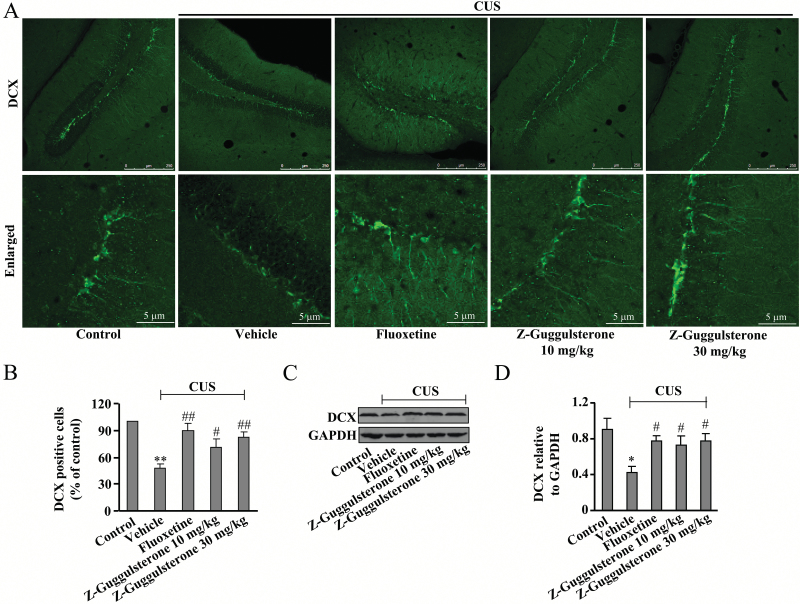
Z-Guggulsterone Restores the CUS-Induced Impairment of the Hippocampal Neurogenesis
Since hippocampal neurogenesis is closely implicated in the pathogenesis of major depression and can be modulated by CREB-BDNF signals (Kotani et al., 2008; Lee et al., 2013), we then explored whether Z-guggulsterone could affect the process of hippocampal neurogenesis in CUS-treated mice. Results showed that the CUS treatment induced a significant decrease in the number of doublecortin (DCX)-positive cells in the hippocampus (n = 5, P<.01 vs control; Figure 4A-B), and the DCX protein expression levels were inhibited by CUS treatment (n = 5, P<.05 vs control; Figure 4C-D). Chronic treatment of mice with Z-guggulsterone (10 or 30 mg/kg) substantially reversed the CUS-induced decreases in the number of DCX-positive cells (P<.05, P<.01 vs vehicle + CUS group; Figure 4A-B) as well as the DCX protein expression level (P<.05 vs vehicle + CUS group; Figure 4C-D). For DCX-positive cells, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 75.16, P<.001] and treatment [F(3, 42) = 7.04, P<.001], but not for stress × treatment interaction [F(3, 42) = 1.43, P = .25] (Figure 4B). For DCX protein expression levels, 2-way ANOVA revealed significant effects for stress [F(1, 42) = 92.50, P<.001] and treatment [F(3, 42) = 11.29, P<.001] treatment, but not for stress × treatment interaction [F(3, 42) = 0.11, P = .95] (Figure 4D).
Figure 4.
Effects of Z-guggulsterone on chronic unpredictable stress (CUS)-induced impairments of the hippocampal neurogenesis. (A) Representative images showing the restoration effect of Z-guggulsterone (10 or 30 mg/kg) on CUS-induced decrease in hippocampal doublecortin (DCX)+ cells. (B) Quantitative analysis showing the restoration effect of Z-guggulsterone on CUS-induced decrease in hippocampal DCX+ cells (n = 5, **P<.01 vs control; #P<.05, ##P<.01 vs vehicle + CUS). (C) Representative images showing the restoration effect of Z-guggulsterone on CUS-induced decrease in the protein expression level of hippocampal DCX. (D) Quantitative analysis showing the restoration effect of Z-guggulsterone on CUS-induced decrease in hippocampal DCX protein expression (n = 5, *P<.05 vs control; #P<.05 vs vehicle + CUS). The fluoxetine administration (20 mg/kg) was used as a positive control, and all data were shown as mean ± SEM.
Blockade of the BDNF Signal Prevents the Antidepressant-Like Effects of Z-Guggulsterone
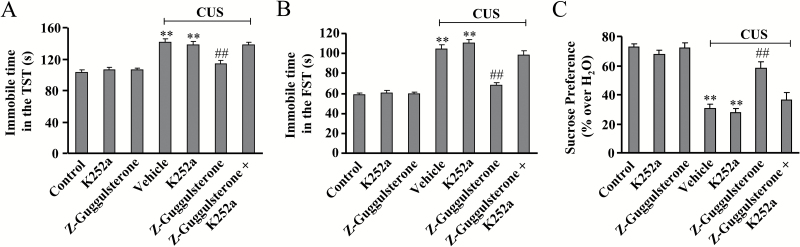
To further investigate the potential role of BDNF in the antidepressive effects of Z-guggulsterone, a potent inhibitor of BDNF receptor, K252a (Tapley et al., 1992; Yan et al., 2010), was employed in this study. The CUS-treated mice were co-injected with Z-guggulsterone (30 mg/kg) and K252a (25 μg/kg) for 12 days, with behavioral tests performed 2 hours after the last injection. Results showed that K252a co-administration almost completely reversed the CUS-induced increase in the immobile time in the experiments of TST (P<.01 vs control or vehicle + CUS group; Figure 5A) and FST (P<.01 vs control or vehicle + CUS group; Figure 5B). For TST, ANOVA revealed significant main effects for K252a [F(1, 36) = 4.82, P<.05] and Z-guggulsterone [F(1, 36) = 6.95, P<.05] with a significant K252a × Z-guggulsterone interaction [F(1, 36) = 8.33, P<.01] (Figure 5A). For FST, ANOVA revealed significant main effects for K252a [F(1, 36)=20.01, P<.001] and Z-guggulsterone [F(1, 36) = 30.80, P<.001] with a significant K252a × Z-guggulsterone interaction [F(1, 36)=11.40, P<.01] (Figure 5B). In the experiment of sucrose preference, K252a co-administration (25 μg/kg) also reversed the CUS-induced decrease in sucrose intake (Figure 5C, P<.01 vs control or vehicle + CUS group), and ANOVA revealed significant main effects for K252a [F(1, 36) = 13.12, P<.001] and Z-guggulsterone [F(1, 36) = 8.93, P<.01] with a significant K252a × Z-guggulsterone interaction [F(1, 36) = 5.50, P<.05] (Figure 5C).
Figure 5.
Effects of the inhibitor of brain-derived neurotrophic factor (BDNF), K252a, on Z-guggulsterone-mediated restoration of behavioral abnormalities induced by chronic unpredictable stress (CUS). (A) Quantitative analysis showing that K252a co-administration (25 μg/kg) attenuated the restoration effect of Z-guggulsterone (30 mg/kg) on CUS-induced increase in the immobile time in the tail suspension test (TST) (n = 10, **P<.01 vs control; ##P<.01 vs vehicle + CUS). (B) Quantitative analysis showing that K252a co-administration (25 μg/kg) attenuated the restoration effect of Z-guggulsterone (30 mg/kg) on CUS-induced increase in the immobile time in the forced swim test (FST) (n = 10; **P<.01 vs control; ##P<.01 vs vehicle + CUS). (C) Quantitative analysis showing that K252a co-administration (25 μg/kg) attenuated the restoration effect of Z-guggulsterone (30 mg/kg) on CUS-induced decrease in sucrose intake (n = 10; **P<.01 vs control; ##P<.01 vs vehicle + CUS). All data were shown as mean ± SEM.
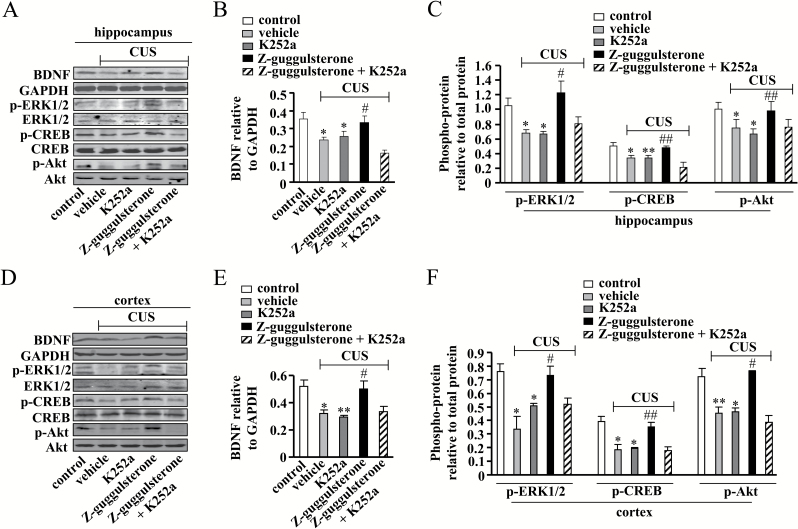
The Z-guggulsterone-induced increases in the protein expression level of BDNF (P<.05 vs control or vehicle + CUS group; Figure 6A-B) as well as the phosphorylation levels of ERK1/2 (P<.05 vs control or vehicle + CUS group; Figure 6A, C), CREB (P<.05 or P<.01 vs control or vehicle + CUS group; Figure 6A, C), and Akt (P<.05 or P<.01 vs control or vehicle + CUS group; Figure 6A, C) in the hippocampus of stressed mice were blocked by K252a co-administration (25 μg/kg). For hippocampal BDNF, 2-way ANOVA revealed significant effects for K252a [F(1, 16) = 24.79, P<.001] and K252a × Z-guggulsterone interaction [F(1, 16) = 44.79, P<.001] (Figure 6B). For hippocampal phospho-ERK1/2, 2-way ANOVA revealed significant effects for K252a [F(1, 16) = 39.35, P<.001], Z-guggulsterone [F(1, 16)=81.39, P<.001], and K252a × Z-guggulsterone interaction [F(1, 16)=36.54] (Figure 6C). For hippocampal phospho-CREB, 2-way ANOVA revealed significant effects for K252a [F(1, 16)=29.07, P<.001] and K252a × Z-guggulsterone interaction [F(1, 16)=29.19] (Figure 6C). For hippocampal phospho-Akt, 2-way ANOVA revealed significant effects for K252a [F(1, 16) = 6.68, P<.05] and Z-guggulsterone [F(1, 16) = 7.08, P<.05] (Figure 6C).
Figure 6.
Effects of K252a on Z-guggulsterone-mediated restoration of the impairments of the hippocampal and cortical cAMP response element binding protein- brain-derived neurotrophic factor (CREB-BDNF) signals induced by chronic unpredictable stress (CUS). (A) Representative images showing that K252a co-administration (25 μg/kg) attenuated the restoration effect of Z-guggulsterone (30 mg/kg) on CUS-induced decreases in the protein expression level of BDNF as well as the phosphorylation levels of extracellular-signal related kinase 1/2 (ERK1/2), CREB, and protein kinase B (Akt) in the hippocampus. (B) Quantitative analysis that K252a co-administration attenuated the restoration effect of Z-guggulsterone on CUS-induced decrease in hippocampal BDNF protein expression (n = 5; *P<.05 vs control; #P<.05 vs vehicle + CUS). (C) Quantitative analysis that K252a co-administration attenuated the restoration effects of Z-guggulsterone on CUS-induced decreases in hippocampal ERK1/2, CREB, and Akt phosphorylation levels (n = 5; *P<.05, **P<.01 vs control; #P<.05, ##P<.01 vs vehicle + CUS). (D) Representative images showing that K252a co-administration (25 μg/kg) attenuated the restoration effect of Z-guggulsterone (30 mg/kg) on CUS-induced decreases in the protein expression level of BDNF as well as the phosphorylation levels of ERK1/2, CREB, and Akt in mouse medial prefrontal cortex (mPFC). (E) Quantitative analysis that K252a co-administration attenuated the restoration effect of Z-guggulsterone on CUS-induced decrease in cortical BDNF protein expression (n = 5; *P<.05, **P<.01 vs control; #P<.05 vs vehicle + CUS). (F) Quantitative analysis that K252a co-administration attenuated the restoration effect of Z-guggulsterone on CUS-induced decreases in cortical ERK1/2, CREB, and Akt phosphorylation levels (n = 5; *P<.05, **P<.01 vs control; #P<.05, ##P<.01 vs vehicle + CUS). All data were shown as mean ± SEM.
In the mPFC region of CUS-treated mice, K252a co-administration (25 μg/kg) also abolished the restoration effects of Z-guggulsterone on the protein expression level of BDNF (P<.05 or P<.01 vs control; P<.05 vs vehicle + CUS group; Figure 6D-E) as well as the phosphorylation levels of ERK1/2 (P<.05 vs control; P<.05 vs vehicle + CUS group), CREB (P<.05 vs control; P<.01 vs vehicle + CUS group), and Akt (P<.05 or P<.01 vs control; P<.05 vs vehicle + CUS group) (Figure 6D, F). For cortical BDNF, 2-way ANOVA revealed significant effects for K252a [F(1, 16) = 24.53, P<.001], Z-guggulsterone [F(1, 16)=30.20, P<.001] and K252a × Z-guggulsterone interaction [F(1, 16) = 12.61, P<.01] (Figure 6E). For cortical phospho-ERK1/2, 2-way ANOVA revealed significant effects for Z-guggulsterone [F(1, 16)=32.26, P<.001] and K252a×Z-guggulsterone interaction [F(1, 16) = 30.83, P<.001] (Figure 6F). For cortical phospho-CREB, 2-way ANOVA revealed significant effects for K252a [F(1, 16) = 30.28, P<.001], Z-guggulsterone [F(1, 16) = 22.87, P<.001], and K252a × Z-guggulsterone interaction [F(1, 16) = 36.56, P<.001] (Figure 6F). For cortical phospho-Akt, 2-way ANOVA revealed significant effects for K252a [F(1, 16)=76.59, P<.001] and Z-guggulsterone [F(1, 16)=29.79, P<.001] and K252a×Z-guggulsterone interaction [F(1, 16) = 91.34, P<.001] (Figure 6F).
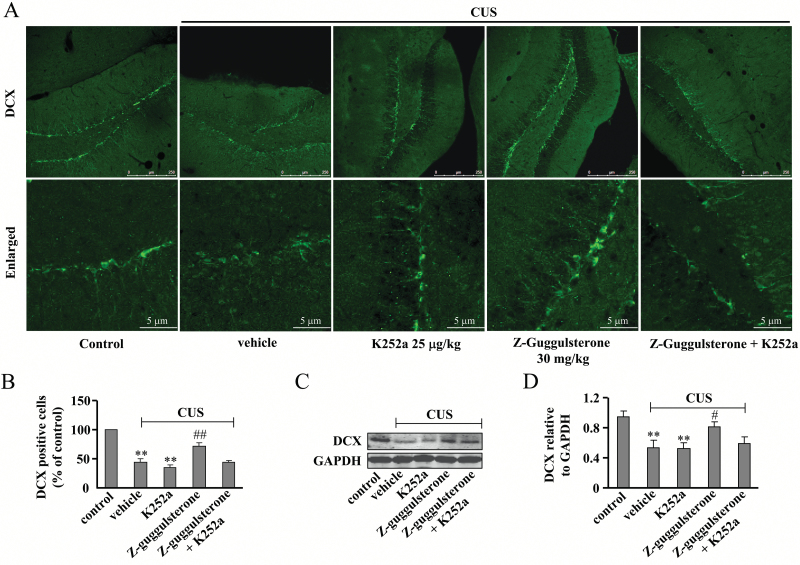
Immunofluorescence studies showed that the BDNF signal was essential for the effect of Z-guggulsterone on hippocampal neurogenesis, as K252a co-administration (25 μg/kg) was found to block the increases in the hippocampal neurogenesis (P<.01 vs control or vehicle + CUS group; Figure 7A-B) and DCX protein expression level (P<.01 vs control; P<.05 vs vehicle + CUS group; Figure 7C-D) after Z-guggulsterone treatment. For DCX-positive cells, 2-way ANOVA revealed significant effects for K252a [F(1, 16) = 9.86, P<.01] and Z-guggulsterone [F(1, 16) = 9.19, P<.01] (Figure 7B). For DCX protein levels, 2-way ANOVA revealed significant effects for K252a [F(1, 16)=6.03, P<.05], Z-guggulsterone [F(1, 16)=12.50, P<.01], and K252a × Z-guggulsterone interaction [F(1, 16) = 5.43, P<.05] (Figure 7D).
Figure 7.
Effects of K252a on Z-guggulsterone-mediated restoration of the impairment of hippocampal neurogenesis induced by chronic unpredictable stress (CUS). (A) Representative images showing that K252a co-administration (25 μg/kg) attenuated the restoration effect of Z-guggulsterone (30 mg/kg) on CUS-induced decrease in hippocampal doublecortin (DCX)+ cells. (B) Quantitative analysis showing that K252a co-administration attenuated the restoration effect of Z-guggulsterone on CUS-induced decrease in hippocampal DCX+ cells (n = 5; **P<.01 vs control; ##P<.01 vs vehicle + CUS). (C) Representative images showing that K252a co-administration (25 μg/kg) attenuated the restoration effect of Z-guggulsterone (30 mg/kg) on CUS-induced decrease in the protein expression level of hippocampal DCX. (D) Quantitative analysis showing that K252a co-administration attenuated the restoration effect of Z-guggulsterone on CUS-induced decrease in the protein expression level of hippocampal DCX (n = 5; **P<.01 vs control; #P<.05 vs vehicle + CUS). All data were shown as mean ± SEM.
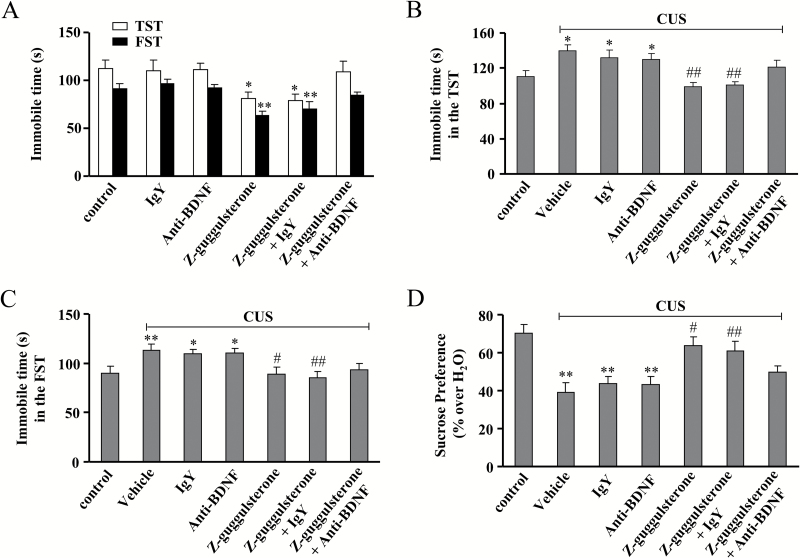
Furthermore, the anti-BDNF antibody was used to block the BDNF signal. Mice were first infused with anti-BDNF antibody for 3 days and then treated with Z-guggulsterone (30 mg/kg), followed by behavioral experiments. As shown in Figure 8A, anti-BDNF or IgY infusion alone produced no significant effects on the immobile time in the TST and FST. However, anti-BDNF infusion significantly blocked the antidepressant effect of Z-guggulsterone in the TST and FST (n = 10, P<.05 or P<.01 vs control or IgY alone-treated group) (Figure 8A). The CUS-treated mice were co-treated with Z-guggulsterone and anti-BDNF antibody for 12 days and behavioral tests were then performed. Results showed that anti-BDNF infusion abolished the antidepressant effects of Z-guggulsterone in the TST (n = 10, P<.05 vs control; P<.01 vs vehicle + CUS or IgY + CUS; Figure 8B), FST (n = 10, P<.05, P<.01 vs control; P<.05, P<.01 vs vehicle + CUS or IgY + CUS; Figure 8C), and sucrose preference experiment (n = 10, P<.01 vs control; P<.05, P<.01 vs vehicle + CUS or IgY + CUS; Figure 8D). For TST, 2-way ANOVA revealed significant effects for Z-guggulsterone treatment [F(2, 54) = 22.05, P<.001] but not for anti-BDNF treatment [F(2, 54)=0.92] and Z-guggulsterone × anti-BDNF interaction [F(2, 54)=2.79] (Figure 8B). For FST, 2-way ANOVA revealed significant effects for Z-guggulsterone treatment [F(2, 54)=19.78, P<.001] but not for anti-BDNF treatment [F(2, 54) = 0.32] and Z-guggulsterone × anti-BDNF interaction [F(2, 54) = 0.21] (Figure 8C). For sucrose preference, 2-way ANOVA revealed significant effects for Z-guggulsterone treatment [F(2, 54)=19.21, P<.001] but not for anti-BDNF treatment [F(2, 54)=1.02] and Z-guggulsterone × anti-BDNF interaction [F(2, 54) = 2.01] (Figure 8D).
Figure 8.
Effects of brain-derived neurotrophic factor (BDNF) antibody on the antidepressant-like effect of Z-guggulsterone. (A) Preinfusion of anti-BDNF antibody blocked the Z-guggulsterone-induced decrease in the immobile time of C57BL/6J mice in the tail suspension test (TST) and forced swim test (FST) (n = 10, *P<.05, **P<.01 vs control or IgY alone-treated group). (B-C) Preinfusion of anti-BDNF antibody prevented the Z-guggulsterone-induced decrease in the immobile time of C57BL/GJ mice in the TST (B, n = 10, *P<.05 vs control, ##P<.01 vs vehicle, vehicle + CUS, or IgY + CUS) and FST (C, n = 10, *P<.05 or **P<.01 vs control, #P<.05 or ##P<.01 vs vehicle + CUS or IgY + CUS). (D) CUS-treated mice were co-treated with Z-guggulsterone and anti-BDNF antibody for 12 days. CUS + Z-guggulsterone + anti-BDNF mice displayed significantly lower sucrose consumption than CUS + Z-guggulsterone + IgY mice (n = 10, **P<.01 vs control, #P<.05 or ##P<.01 vs vehicle + CUS or IgY + CUS). All results were expressed as means ± SEM.
Serotonin Depletion Does Not Alter the Antidepressant-Like Effects of Z-Guggulsterone
Since the monoaminergic system, especially the serotonin system, is also closely involved in the etiology of depression (Berton and Nestler, 2006; Dell’Osso et al., 2016), we then assessed whether the 5-hydroxytryptamine is associated with the antidepressant effect of Z-guggulsterone through depletion of serotonin using the tryptophan hydroxylase inhibitor PCPA (Coryell et al., 2009). The CUS-treated mice were first co-injected with Z-guggulsterone (30 mg/kg) and PCPA (300 mg/kg) for 12 consecutive days and then the experiments of TST, FST, and sucrose preference were performed to evaluate the behavioral changes in C57BL/6J mice. As shown in supplementary Figure 2A-C, Z-guggulsterone administration (30 mg/kg) markedly reversed the increases in the immobile time in the experiment of TST (n = 10, P<.01 vs vehicle + CUS; supplementary Figure 2A) and FST (n = 10, P<.01 vs vehicle + CUS; supplementary Figure 2B) induced by CUS, and also reversed the decrease in sucrose intake in the experiment of sucrose preference (n = 10, P<.01 vs vehicle + CUS; supplementary Figure 2C). PCPA (300 mg/kg) treatment showed no significant effect on the increase in the immobile time in the TST (n = 10, P<.05 vs PCPA + CUS; supplementary Figure 2A), FST (n = 10, P<.01 vs PCPA + CUS group; supplementary Figure 2B), and sucrose intake (n = 10, P<.01 vs PCPA + CUS group; supplementary Figure 2C) induced by CUS. These data suggest that the monoaminergic system is not involved in the antidepressant-like effects of Z-guggulsterone. For TST, 2-way ANOVA revealed significant effects for Z-guggulsterone [F(1, 36) = 13.19, P<.001] but not for PCPA [F(1, 36)=0.055] and PCPA × Z-guggulsterone interaction [F(1, 36)=1.50, P=.23] (supplementary Figure 2A). For FST, 2-way ANOVA revealed significant effects for Z-guggulsterone [F(1, 36) = 62.26, P<.001] but not for PCPA [F(1, 36)=1.04, P=.31] and PCPA × Z-guggulsterone interaction [F(1, 36)=0.41, P=.53] (supplementary Figure 2A). For sucrose preference, 2-way ANOVA revealed significant effects for Z-guggulsterone [F(1, 36) = 183.30, P<.001] but not for PCPA [F(1, 36)=1.13] and PCPA × Z-guggulsterone interaction [F(1, 36) = 0.61] (Supplementary Figure 2A). These data suggest that the monoaminergic system is not involved in the antidepressive effects of Z-guggulsterone.
Discussion
One of the major findings in the present study is the identification of the antidepressant-like effect of Z-guggulsterone, an active compound extracted from the gum resin of the tree Commiphora mukul, in a mice depression model induced by CUS. This effect was similar to fluoxetine and mediated by the activation of BDNF signaling pathway but not the 5-hydroxytryptaminergic system.
Commiphora mukul is a popular herb that has been used for a long time in Ayurvedic medicine to treat various disorders, such as obesity, arthritis, and some lipid metabolism disorders, and most of its effects are being confirmed by modern scientific research. For example, Commiphora mukul has been shown to reduce high cholesterol via lowering harmful low-density lipoproteins and elevating the beneficial high-density lipoproteins in animals (Singh et al., 1994; Shields and Moranville, 2005; Sharma et al., 2009). Commiphora mukul is also demonstrated to prevent inflammation (Tariq et al., 1986; Song et al., 2010; Huang et al., 2016a) and stroke (Adams et al., 2002) and improve the function of the immune system (Mencarelli et al., 2009). However, to date, the potential application of Commiphora mukul in depression therapy is largely unknown, though a study by Kalshetti et al. (2015) has already reported an antidepresant-like activity of Commiphora mukul in olfactory bulbectomized rats. Our study provides direct evidence to show that Z-guggulsterone, an active ingredient extracted from Commiphora mukul, displays significant antidepressant-like activity in a chronic depression model, extending the role of Z-guggulsterone beyond a metabolic regulator to a potential modulator of major depression.
Acute injection of Z-guggulsterone at doses of 10 and 30 mg/kg in mice produced significant reductions in the immobile time in TST and FST, and these effects were thought not to be induced by the change in locomotor activity, as Z-guggulsterone at both doses did not result in a significant change in locomotor activity. Furthermore, chronic daily injections of Z-guggulsterone (12 days) markedly improved the behavioral impairments induced by CUS in the experiments of TST, FST, and sucrose preference. More importantly, the antidepressant-like effects of Z-guggulsterone were similar to that of fluoxetine. Together with the fact that Commiphora mukul is widely used to treat human disorders (Tariq et al., 1986; Singh et al., 1994; Adams et al., 2002; Shields and Moranville, 2005; Mencarelli et al., 2009; Song et al., 2010), our data indicate that Z-guggulsterone may be a new compound with potential antidepressant-like activities.
Chronic stress has been confirmed to impair hippocampal neurogenesis by numerous studies (Danzer, 2012). In this study, the CUS-induced impairment of hippocampal neurogenesis was largely reversed by chronic Z-guggulsterone treatment, which was reflected by the increase in DCX-positive cells and DCX protein expression levels after Z-guggulsterone treatment. Our results also showed that Z-guggulsterone upregulated the BDNF protein expression levels in both hippocampus and mPFC of stressed mice. BDNF is known to play an important role in adult hippocampal neurogenesis. Knockout of the brain BDNF would make rodents more susceptible to chronic stresses via attenuation of hippocampal neurogenesis (Gao and Chen, 2009; Burke et al., 2013). Overexpression of BDNF in mouse hippocampal astrocytes has been reported to promote local neurogenesis and elicits anxiolytic-like activities (Quesseveur et al., 2013). Chronic treatment with most clinical-available antidepressants can increase the BDNF protein levels in different regions (Buttenschøn et al., 2015; Hisaoka-Nakashima et al., 2016). More and more experimental compounds are also being confirmed as capable of regulating depressive progresses via activating the BDNF signaling pathway (Osborn et al., 2013; Yang et al., 2015). More importantly, the hippocampal BDNF and other neurotrophic factors have been shown to be reduced in patients with bipolar disorder and major depression (Polyakova et al., 2015; Vinberg et al., 2015). Therefore, increasing BDNF protein expression might be a common pathway for antidepressants to exert their therapeutic actions, and searching agents that can increase BDNF protein expression is beneficial for the development of new antidepressants. Here, our data showed that Z-guggulsterone reversed the CUS-induced decrease in the protein expression levels of hippocampal and cortical BDNF in parallel to the upregulation of hippocampal neurogenesis, suggesting that BDNF is critical for the antidepressant-like activity of Z-guggulsterone. This finding was supported by the K252a co-administration experiment, which showed that the antidepressant-like effects of Z-guggulsterone on mice behaviors and hippocampal neurogenesis were blocked by co-treatment of mice with K252a, a potent inhibitor of the BDNF receptor. This result was to some extent in accordance with one of our recent studies, which showed that Z-guggulsterone attenuates the scopolamine-induced memory impairments through activation of the BDNF signaling pathway (Chen et al., 2016).
Our studies also answered how Z-guggulsterone augments BDNF protein expression in stressed mice. CREB is a regulator upstream of the BDNF signaling pathway (Lv et al., 2013). Increasing evidence shows that the level of phospho-CREB is tightly associated with the formation and/or development of major depression. Phospho-CREB is found to be downregulated in postmortem brain of suicide victims with a history of depression (Pandey et al., 2007). CREB is also considered a molecular marker for the response of patients with major depression to antidepressants, as major classes of clinical antidepressants have been reported to increase the transcriptional activity of CREB in several brain regions, including the hippocampus and mPFC (Gass and Riva, 2007; Castren and Rantamaki, 2010). In the present study, chronic treatment of mice with Z-guggulsterone upregulated the levels of phospho-CREB in both hippocampus and mPFC of stressed mice, to the basal level of vehicle-treated mice, suggesting that the CREB may initiate the transcription and expression of BDNF in Z-guggulsterone-treated mice, thereby improving depressive symptoms. We also examined the activity of ERK/12 and Akt, whose activation is usually considered an intracellular signaling mechanism downstream of the BDNF signal mediating antidepressant efficacy in depressed humans and animals (Gass and Riva, 2007; Castren and Rantamaki, 2010; Liu et al., 2015). Results showed that the activities of ERK1/2 and Akt, as evaluated by the anti-phospho-ERK1/2 and anti-phospho-Akt specific antibody, were much higher in Z-guggulsterone-treated mice than that in stressed mice. These data further strengthened the importance of the CREB-BDNF signaling pathway in the antidepressant-like effects of Z-guggulsterone.
How exactly Z-guggulsterone affects the CREB-BDNF signaling pathway in stressed mice remains unknown. The hypercholesterol and hyperlipidemic status has recently been shown to decrease the expression of brain BDNF gene and protein (Kaczmarczyk et al., 2013; Huang et al., 2016a), and guggulsterone has hypolipidemic effects in the central nervous system (Urizar and Moore, 2003). Thus, Z-guggulsterone may activate the CREB-BDNF signaling pathway in CUS-treated mice through reduction of brain lipid. The proinflammatory response also mediates the formation and/or development of major depression (Lisi et al., 2013; Couch et al., 2016), and proinflammatory cytokines have been reported to impair the brain BDNF signaling pathway (Calabrese et al., 2014; Daniele et al., 2015). Given the fact that Z-guggulsterone can attenuate the neuroinflammation-mediated behavioral abnormalities in the FST and TST (Huang et al., 2016a), we speculated that the proinflammatory mechanism may be involved in the antidepressant-like effects of Z-guggulsterone. More studies should be done to test this hypothesis. Z-guggulsterone is considered a functional inhibitor of farnesoid X receptor (FXR) (Urizar et al., 2002), and FXR has recently been reported to inhibit the process of liver autophagy through antagonizing the CREB function (Seok et al., 2014). Therefore, there exists a possibility that Z-guggulsterone restores the function of the CREB-BDNF signaling pathway, likely through inhibition of the neuronal FXR. If this hypothesis is the case, we may find out the true target for Z-guggulsterone in depression therapy. It is worth mentioning that the functional FXR in brain neurons has just been identified by our studies (Huang et al., 2016b). That study may provide a molecular basis for the effect of Z-guggulsterone on major depression.
Besides the BDNF and neurogenesis impairment hypothesis, the dysfunction of the monoamine system is widely accepted to contribute to the formation and/or development of major depression, and most of the current available antidepressants such as the selective serotonin reuptake inhibitors have been reported to exert their therapeutic effects though balancing the monoamine signal (Stewart et al., 2014; Barth et al., 2016). However, in this study, we found that depleting the 5-hydroxytryptamine by PCPA did not abolish the antidepressant-like effect of Z-guggulsterone in the experiments of TST, FST, and sucrose preference, indicating that the molecular mechanism for Z-guggulsterone in depression therapy may be distinct from the conventional antidepressants.
Collectively, our results showed that Z-guggulsterone exerts antidepressant-like effects through promotion of the BDNF signaling pathway, providing a new insight into the pharmacological role of Z-guggulsterone in the central nervous system and shedding light on the development of new antidepressants.
Supplementary Material
Supplementary data are available at International Journal of Neuropsychopharmacology online.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by the Natural Science Foundation of China (no. 81571323), the Natural Science Foundation of Jiangsu Province (no. BK20141240), and the Science and Technology Project of Nantong City (no. MS12015050) to Dr. Chao Huang.
References
- Adams JD, Jr, Klaidman LK, Mishra L, Singh BB. (2002) Effects of guggul in a rat model of stroke. Altern Ther Health Med 8:20–21. [PubMed] [Google Scholar]
- Barth M, Kriston L, Klostermann S, Barbui C, Cipriani A, Linde K. (2016) Efficacy of selective serotonin reuptake inhibitors and adverse events: meta-regression and mediation analysis of placebo-controlled trials. Br J Psychiatry 208:114−–119. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. (2006) New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 7:137–151. [DOI] [PubMed] [Google Scholar]
- Bourin M, Fiocco AJ, Clenet F. (2001) How valuable are animal models in defining antidepressant activity? Hum Psychopharmacol 16:9–21. [DOI] [PubMed] [Google Scholar]
- Burke TF, Advani T, Adachi M, Monteggia LM, Hensler JG. (2013) Sensitivity of hippocampal 5-HT1A receptors to mild stress in BDNF-deficient mice. Int J Neuropsychopharmacol 16:631−–645. [DOI] [PubMed] [Google Scholar]
- Buttenschøn HN, Foldager L, Elfving B, Poulsen PH, Uher R, Mors O. (2015) Neurotrophic factors in depression in response to treatment. J Affect Disord 183:287–294. [DOI] [PubMed] [Google Scholar]
- Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R. (2014) Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front Cell Neurosci 8:430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castren E, Rantamaki T. (2010) The role of BDNF and its receptors in depression and antidepressant drug action: reactivation of developmental plasticity. Dev Neurobiol 70:289–297. [DOI] [PubMed] [Google Scholar]
- Chen Z, Huang C, Ding W. (2016) Z-Guggulsterone improves the scopolamine-induced memory impairments through enhancement of the BDNF signal in C57BL/6J mice. Neurochem Res 41:3322–3332. [DOI] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu ZQ, Light AR, Langbehn DR, Wemmie JA. (2009) Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci 29:5381–5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch Y, Trofimov A, Markova N, Nikolenko V, Steinbusch HW, Chekhonin V, Schroeter C, Lesch KP, Anthony DC, Strekalova T. (2016) Low-dose lipopolysaccharide (LPS) inhibits aggressive and augments depressive behaviours in a chronic mild stress model in mice. J Neuroinflammation 13:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele S, Da Pozzo E, Zappelli E, Martini C. (2015) Trazodone treatment protects neuronal-like cells from inflammatory insult by inhibiting NF-κB, p38 and JNK. Cell Signal 27:1609−–1629. [DOI] [PubMed] [Google Scholar]
- Danzer SC. (2012) Depression, stress, epilepsy and adult neurogenesis. Exp Neurol 233:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell’Osso L, Carmassi C, Mucci F, Marazziti D. (2016) Depression, serotonin and tryptophan. Curr Pharm Des 22:949−–954. [DOI] [PubMed] [Google Scholar]
- Fabbri C, Marsano A, Balestri M, De Ronchi D, Serretti A. (2013) Clinical features and drug induced side effects in early versus late antidepressant responders. J Psychiatr Res 47:1309−–1318. [DOI] [PubMed] [Google Scholar]
- Fava M. (2010) Switching treatments for complicated depression. J Clin Psychiatry 71:e04. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen J. (2009) Conditional knockout of brain-derived neurotrophic factor in the hippocampus increases death of adult-born immature neurons following traumatic brain injury. J Neurotrauma 26:1325–1335. [DOI] [PubMed] [Google Scholar]
- Gass P, Riva MA. (2007) CREB, neurogenesis and depression. Bioessays 29:957–961. [DOI] [PubMed] [Google Scholar]
- Hisaoka-Nakashima K, Kajitani N, Kaneko M, Shigetou T, Kasai M, Matsumoto C, Yokoe T, Azuma H, Takebayashi M, Morioka N, Nakata Y. (2016) Amitriptyline induces brain-derived neurotrophic factor (BDNF) mRNA expression through ERK-dependent modulation of multiple BDNF mRNA variants in primary cultured rat cortical astrocytes and microglia. Brain Res 1634:57–67. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang J, Hu W, Wang C, Lu X, Tong L, Wu F, Zhang W. (2016b) Identification of functional farnesoid X receptors in brain neurons. FEBS Lett 590:3233–3242. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang J, Lu X, Hu W, Wu F, Jiang B, Ling Y, Yang R, Zhang W. (2016a) Z-guggulsterone negatively controls microglia-mediated neuroinflammation via blocking IκB-α-NF-κB signals. Neurosci Lett 619:34−–42. [DOI] [PubMed] [Google Scholar]
- Huang YN, Lin CI, Liao H, Liu CY, Chen YH, Chiu WC, Lin SH. (2016) Cholesterol overload induces apoptosis in SH-SY5Y human neuroblastoma cells through the up regulation of flotillin-2 in the lipid raft and the activation of BDNF/Trkb signaling. Neuroscience 328:201–209. [DOI] [PubMed] [Google Scholar]
- Jiang B, Huang C, Chen XF, Tong LJ, Zhang W. (2015) Tetramethylpyrazine produces antidepressant-like effects in mice through promotion of BDNF signaling pathway. Int J Neuropsychopharmacol 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarczyk MM, Machaj AS, Chiu GS, Lawson MA, Gainey SJ, York JM, Meling DD, Martin SA, Kwakwa KA, Newman AF, Woods JA, Kelley KW, Wang Y, Miller MJ, Freund GG. (2013) Methylphenidate prevents high-fat diet (HFD)-induced learning/memory impairment in juvenile mice. Psychoneuroendocrinology 38:1553–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalshetti PB, Alluri R, Thakurdesai PA. (2015) Antidepressant effects of standardized extract of Commiphora mukul Engl. in olfactory bulbectomized rats. Braz arch biol technol 58:41–48. [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. (2008) Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact 175:227−–230. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim J, Jang S, Oh S. (2013) Administration of phytoceramide enhances memory and upregulates the expression of pCREB and BDNF in hippocampus of mice. Biomol Ther (Seoul) 21:229−–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Luo Y, Zhang R, Shi H, Zhu W, Shi J. (2015) Neuropeptide trefoil factor 3 reverses depressive-like behaviors by activation of BDNF-ERK-CREB signaling in olfactory bulbectomized rats. Int J Mol Sci 16:28386−–28400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisi L, Camardese G, Treglia M, Tringali G, Carrozza C, Janiri L, Dello Russo C, Navarra P. (2013) Monocytes from depressed patients display an altered pattern of response to endotoxin challenge. PLoS One 8:e52585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Kay JC, Shen S, Qiao LY. (2015) Endogenous BDNF augments NMDA receptor phosphorylation in the spinal cord via PLCγ, PKC, and PI3K/Akt pathways during colitis. J Neuroinflammation 12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Xin Y, Zhou W, Qiu Z. (2013) The epigenetic switches for neural development and psychiatric disorders. J Genet Genomics 40:339–346. [DOI] [PubMed] [Google Scholar]
- Mencarelli A, Renga B, Palladino G, Distrutti E, Fiorucci S. (2009) The plant sterol guggulsterone attenuates inflammation and immune dysfunction in murine models of inflammatory bowel disease. Biochem Pharmacol 78:1214–1223. [DOI] [PubMed] [Google Scholar]
- Mizuki D, Matsumoto K, Tanaka K, Thi Le X, Fujiwara H, Ishikawa T, Higuchi Y. (2014) Antidepressant-like effect of Butea superba in mice exposed to chronic mild stress and its possible mechanism of action. J Ethnopharmacol 156:16−–25. [DOI] [PubMed] [Google Scholar]
- Moreno ML, Vanderhasselt MA, Carvalho AF, Moffa AH, Lotufo PA, Benseñor IM, Brunoni AR. (2015) Effects of acute transcranial direct current stimulation in hot and cold working memory tasks in healthy and depressed subjects. Neurosci Lett 591:126−–131. [DOI] [PubMed] [Google Scholar]
- Osborn M, Rustom N, Clarke M, Litteljohn D, Rudyk C, Anisman H, Hayley S. (2013) Antidepressant-like effects of erythropoietin: a focus on behavioural and hippocampal processes. PLoS One 8:e72813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Ren X, Rizavi HS, Roberts RC, Conley RR. (2007) Cyclic AMP response element-binding protein in post-mortem brain of teenage suicide victims: specific decrease in the prefrontal cortex but not the hippocampus. Int J Neuropsychopharmacol 10:621−–629. [DOI] [PubMed] [Google Scholar]
- Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. (2015) BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord 174:432–440. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. (1977) Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther 229:327–336. [PubMed] [Google Scholar]
- Quesseveur G, David DJ, Gaillard MC, Pla P, Wu MV, Nguyen HT, Nicolas V, Auregan G, David I, Dranovsky A, Hantraye P, Hen R, Gardier AM, Déglon N, Guiard BP. (2013) BDNF overexpression in mouse hippocampal astrocytes promotes local neurogenesis and elicits anxiolytic-like activities. Transl Psychiatry 3:e253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter SH, Zeuch B, Lankisch K, Gass P, Durstewitz D, Vollmayr B. (2013) Where have I been? Where should I go? Spatial working memory on a radial arm maze in a rat model of depression. PLoS One 8:e62458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F. (2015) Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145:43−–57. [DOI] [PubMed] [Google Scholar]
- Saxena G, Singh SP, Pal R, Singh S, Pratap R, Nath C. (2007) Gugulipid, an extract of Commiphora whighitii with lipid-lowering properties, has protective effects against streptozotocin-induced memory deficits in mice. Pharmacol Biochem Behav 86:797−–805. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Murrough JW, Iosifescu DV. (2016) Ketamine for treatment-resistant depression: recent developments and clinical applications. Evid Based Ment Health 19:35−–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok S, Fu T, Choi SE, Li Y, Zhu R, Kumar S, Sun X, Yoon G, Kang Y, Zhong W, Ma J, Kemper B, Kemper JK. (2014) Transcriptional regulation of autophagy by an FXR-CREB axis. Nature 516:108−–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma B, Salunke R, Srivastava S, Majumder C, Roy P. (2009) Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem Toxicol 47:2631–2639. [DOI] [PubMed] [Google Scholar]
- Shields KM, Moranville MP. (2005) Guggul for hypercholesterolemia. Am J Health Syst Pharm 62:1012–1014. [DOI] [PubMed] [Google Scholar]
- Singh RB, Niaz MA, Ghosh S. (1994) Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc Drugs Ther 8:659–664. [DOI] [PubMed] [Google Scholar]
- Song JJ, Kwon SK, Cho CG, Park SW, Chae SW. (2010) Guggulsterone suppresses LPS induced inflammation of human middle ear epithelial cells (HMEEC). Int J Pediatr Otorhinolaryngol 74:1384–1387. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacol (Berl) 85:367–370. [DOI] [PubMed] [Google Scholar]
- Stewart JW, McGrath PJ, Blondeau C, Deliyannides DA, Hellerstein D, Norris S, Amat J, Pilowsky DJ, Tessier P, Laberge L, O’shea D, Chen Y, Withers A, Ber geron R, Blier P. (2014) Combination antidepressant therapy for major depressive disorder: speed and probability of remission. J Psychiatr Res 52:7−–14. [DOI] [PubMed] [Google Scholar]
- Tapley P, Lamballe F, Barbacid M. (1992) K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene 7:371–381. [PubMed] [Google Scholar]
- Tariq M, Ageel AM, Al-Yahya MA, Mossa JS, Al-Said MS, Parmar NS. (1986) Anti-inflammatory activity of Commiphora molmol. Agents Actions 17:381–382. [DOI] [PubMed] [Google Scholar]
- Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. (2002) A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296:1703–1706. [DOI] [PubMed] [Google Scholar]
- Urizar NL, Moore DD. (2003) GUGULIPID: a natural cholesterol- lowering agent. Annu Rev Nutr 23:303–313. [DOI] [PubMed] [Google Scholar]
- Vinberg M, Miskowiak K, Hoejman P, Pedersen M, Kessing LV. (2015) The effect of recombinant erythropoietin on plasma brain derived neurotrophic factor levels inpatients with affective disorders: a randomised controlled study. PLoS One 10:e0127629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Pan J, Sun J, Ding L, Ruan L, Reed M, Yu X, Klabnik J, Lin D, Li J, Chen L, Zhang C, Zhang H, O’Donnell JM. (2015) Inhibition of phosphodiesterase 2 reverses impaired cognition and neuronal remodeling caused bychronic stress. Neurobiol Aging 36:955−–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HC, Qu HD, Sun LR, Li SJ, Cao X, Fang YY, Jie W, Bean JC, Wu WK, Zhu XH, Gao TM. (2010) Fuzi polysaccharide-1 produces antidepressant-like effects in mice. Int J Neuropsychopharmacol 13:623–633. [DOI] [PubMed] [Google Scholar]
- Yang LP, Jiang FJ, Wu GS, Deng K, Wen M, Zhou X, Hong X, Zhu MX, Luo HR. (2015) acute treatment with a novel trpc4/c5 channel inhibitor produces antidepressant and anxiolytic-like effects in mice. PLoS One 10:e0136255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Wang X, Cong Y, Deng Y, Xu Y, Chen A, Yin Y. (2014) Effects of bile acids and the bile acid receptor FXR agonist on the respiratory rhythm in the in vitro brainstem medulla slice of neonatal Sprague-Dawley rats. PLoS One 9:e112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.