Abstract
Objective
The aim was to identify differences in survival based on type of heart disease while awaiting orthotopic heart transplantation (OHT).
Background
Restrictive cardiomyopathy (RCM), congenital heart disease (CHD), and hypertrophic cardiomyopathy (HCM) patients may be at a disadvantage while awaiting OHT since they often are poor candidates for mechanical circulatory support and/or inotropes.
Methods
We included all adults in the Scientific Registry of Transplant Recipients database awaiting OHT from 2004–2014 and evaluated outcomes based on type of heart disease. The primary endpoint was time to all-cause mortality censored at last patient follow-up and time of transplantation. Multivariable Cox proportional hazards models were performed to evaluate survival by type of cardiomyopathy.
Results
There were 14447 DCM, 823 RCM, 11799 ischemic cardiomyopathy (ICM), 602 HCM, 964 CHD, 584 valvular disease, and 1528 “other” (including 1216 for re-transplantation). During median follow-up of 3.7 months, 4943 died (1253 F, 3690 M). After adjusting for possible confounding variables including age, renal function, inotropes, mechanical ventilation and mechanical circulatory support, the adjusted hazard ratio (aHR) by diagnoses relative to DCM were RCM aHR 1.70 (1.43–2.02), ICM aHR 1.10 (1.03–1.18), HCM aHR 1.23 (0.98–1.54), valvular disease aHR 1.30 (1.07–1.57), CHD aHR 1.37 (1.17–1.61) and “Other” aHR 1.51 (1.34–1.69). Sex was a significant modifier of mortality for ICM, RCM and “other” (P<0.05 for interaction).
Conclusion
In the United States, patients with RCM, CHD and prior heart transplantation had a higher risk of death awaiting OHT than patients with a DCM, ICM, HCM and valvular heart disease.
Keywords: heart failure, cardiomyopathy, hypertrophic cardiomyopathy, congenital heart disease, cardiac amyloidosis and heart transplantation
There are few studies comparing the survival of different types of heart disease. Prognosis and optimal timing to waitlist advanced heart failure patients for orthotopic heart transplantation (OHT) is especially important among cohorts not easily rescued with inotropes or mechanical circulatory support. Studies have shown that congenital heart disease (CHD) patients have higher 2 months mortality on the waiting list after multivariate analysis compared to non-CHD patients1 and no survival benefit with ventricular assist device (VAD) support.2 Restrictive cardiomyopathy (RCM) patients may also be at a disadvantage3 since VAD support is often not possible with small ventricular cavities. In addition, there is concern that hypertrophic cardiomyopathy (HCM) patients may have a poor prognosis and may not qualify for high priority transplantation based on our current allocation system.4 In one national study analyzing survival among patients removed from the heart transplant waiting list, HCM and RCM were among the highest predictors of death.5
The goal of this study is to evaluate whether the type of heart disease affects mortality while awaiting OHT. Since OHT is a competing outcome, differences in rate of transplantation, UNOS Status at time of transplantation, and usage of VAD support will also be evaluated. The cohort includes all adult patients registered on the national heart transplant waitlist between January 1, 2004–September 3, 2014.
Methods
Scientific Registry of Transplant Recipients (SRTR)
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR database includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN), and has been described elsewhere. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. Human error collecting data is minimized by edit checks, validation of data at time of entry and internal verification when there are outliers.6
Patient Population and UNOS Status
We included all adult patients in the SRTR database placed on the waiting list for heart transplantation from January 1, 2004–September 3, 2014. Patients less than 18 years of age were excluded since United Network for Organ Sharing (UNOS) criteria for listing pediatric patients differed from adults.7
Primary diagnosis was the principle explanatory variable and categorized based on definitions in the SRTR database: Dilated cardiomyopathy (DCM), RCM (idiopathic, amyloid, endocardial fibrosis, sarcoidosis, radiation/chemotherapy induced heart disease, and other), ischemic cardiomyopathy (ICM), HCM, valvular heart disease, CHD, and “other” (1216 prior heart transplantations and 312 “other”).
UNOS Status was at time of listing for heart transplantation. UNOS Status 1A (high priority) includes patients requiring an intra-aortic balloon pump (IABP), extracorporeal membrane oxygenator (ECMO), total artificial heart (TAH), VADs with device complications, VADs without complications for a total of 30 days, continuous mechanical ventilation, multiple inotropes or a single high dose inotrope with continuous hemodynamic monitoring. UNOS Status 1B is defined as a patient not meeting criteria for UNOS Status 1A but still requiring continuous intravenous inotrope support or VAD support. Finally, UNOS Status 2 is for all other OHT active candidates.
Outcome Measures
The primary endpoint was all-cause mortality assessed as a right-censored time to death with follow-up censored at the time of heart transplantation. SRTR mortality data is maintained by the transplant centers and verified with the complete Social Security Death Master File, which is recently available through a specific waiver granted to the SRTR. We also evaluated the cumulative incidence of time to transplant based on primary diagnosis, censored at the time of last patient follow up and death.
Statistical Analysis
Baseline characteristics at time of listing for OHT were stratified by type of heart disease. Continuous variables were expressed as means +/− standard deviations. Categorical variables were expressed as number of patients with frequency except absolute values were not provided if patient number <10 to protect the identity of the cohort as per SRTR policy. Waitlist survival analysis based on type of heart disease was performed using the Kaplan-Meier method with censoring for OHT. The primary analysis was based on Intent-to-Treat such that deaths following removal from the waiting list were included in the primary analysis. Multivariable Cox proportional hazard models were created to compare type of heart disease to DCM in order to evaluate differences in survival. The proportional hazards assumption for Cox models was evaluated using the proportionality test in PROC PHREG in SAS. No deviations from the assumptions were noted in the analyses. Model 1 was adjusted for the following characteristics at time of listing: age, sex, race (White, Black, Hispanic, Other), body mass index (BMI), insurance (private, medicare, medicaid, other), ABO blood type, era (January 1, 2004–March 31, 2008; April 1, 2008–Sept 3, 2014 to account for the FDA approval of LVAD devices in 2008 that could be implanted in smaller patients), history of tobacco, diabetes mellitus, malignancy, hypertension, prior cerebral vascular accident (CVA), peripheral vascular disease (PVD), implantable cardioverter defibrillator (ICD), dialysis, estimated glomerular filtration rate (GFR), serum albumin, pulmonary artery pressure (PAP) mean, cardiac index (CI), mechanical ventilation, ECMO, IABP, inotropes, LVAD, RVAD +/− LVAD or unspecified mechanical circulatory support (MCS), and TAH. Model 2 included UNOS Status at time of listing and all variables in Model 1 except mechanical ventilation, IABP, ECMO, LVAD, RVAD, TAH and inotropes given the fact that these are collinear variables that define the different UNOS tiers and highly correlate with UNOS Status. Multiple imputation was used for missing data but variables with a high proportion of missingness were excluded from the model including peak oxygen consumption (65% missingness) and antiarrythmics (21% missingness). For multiple imputation we assumed data were missing at random. We used the SAS procedure Proc MI and generated five models with imputed data followed by use of Proc MIANALYZE to evaluate parameter estimates and measures of variability. As a sensitivity analysis, we generated models excluding patients that were on VADs at the time of listing to evaluate whether the association of primary diagnoses with outcomes was similar to the model with entire study population. All analyses were performed using SAS (v.9.4., Cary, NC). A p-value ≤0.05 was considered statistically significant.
Results
Study Population
Baseline characteristics of 30747 adult HF patients (25% women) awaiting OHT are shown in Table 1. This cohort included 14447 patients with DCM, 823 with RCM, 11799 with ICM, 602 with HCM, 964 with CHD, 584 with valvular disease, and 1528 “other”. DCM patients represented the largest subgroup (47% of cohort) with a mean age of 49 years old. Compared to the other subgroups, DCM had one of the highest usages of inotropes, ICDs and permanent mechanical circulatory support (LVAD, RVAD +/− LVAD, and TAH), and very few patients requiring mechanical ventilation or dialysis. RCM patients represented 3% of the SRTR cohort with a mean age of 53 years old. RCM patients had the highest percent of prior malignancy, slightly more patients than most other groups requiring dialysis and few patients receiving temporary (IABP and ECMO), permanent mechanical circulatory support, or mechanical ventilation. ICM represented 38% of the cohort. Patients were older than the other subgroups with a mean age of 58 years old and predominately men. ICM had the highest frequency of diabetes mellitus, peripheral vascular disease, and usage of permanent mechanical circulatory support. ICM patients also had a high usage of ICDs and few requiring mechanical ventilation. HCM patients represented 2% of the cohort with a mean age 46 years old. HCM patients were predominately White, had the highest percent of ICD usage, and relatively few patients with usage of mechanical circulatory support (temporary or permanent), inotropes, dialysis or mechanical ventilation. Valvular cardiomyopathy patients represented the smallest subgroup (2% of cohort) with a mean age 54 years old. Valvular patients had among the highest usage of antiarrythmics and inotropes, and a relatively low frequency of mechanical circulatory support (temporary or permanent), dialysis or mechanical ventilation. CHD represented 3% of the entire cohort, had the youngest patients with a mean age of 36 years old, and the lowest BMI. CHD patients were predominately White, few Blacks, the highest percent of Medicaid patients, and the lowest percent with diabetes mellitus, hypertension, peripheral vascular disease, malignancy and usage of either mechanical circulatory support (temporary and permanent) or inotropes. “Other” CM included mostly patients listed for re-transplantation (1216 with prior OHT and 312 other) and represented 5% of the entire cohort. In this group patients were relatively young with a mean age of 45 years old, predominately White, few with ICDs, relatively poor renal function, and highest percentage of temporary mechanical circulatory support (13%), dialysis (9%) and mechanical ventilatory support (10%).
Table 1.
Baseline Characteristics
| DCM (N=14447) | RCM (N=823) | ICM (N=11799) | HCM (N=602) | Valvular (N=584) | CHD (N=964) | Other (N=1528) | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Females | 4562 (32) | 291 (35) | 1556 (13) | 231 (38) | 208 (36) | 385 (40) | 535 (35) |
|
| |||||||
| Age, (yrs) | 49+/−13 | 53+/−12 | 58+/−8 | 46+/−14 | 54+/−11 | 36+/−12 | 45+/−15 |
|
| |||||||
| Race | |||||||
| White | 8190 (57) | 600 (73) | 9380 (80) | 489 (81) | 396 (68) | 795 (82) | 1101 (72) |
| Black | 4461 (31) | 166 (20) | 1210 (10) | 52 (9) | 105 (18) | 82 (9) | 247 (16) |
| Hispanic | 1260 (9) | 36 (4) | 757 (6) | 35 (6) | 51 (9) | 55 (6) | 125 (8) |
| Other | 536 (4) | 21 (3) | 452 (4) | 26 (4) | 32 (5) | 32 (3) | 55 (4) |
|
| |||||||
| Insurance | |||||||
| Private | 7658 (53) | 590 (72) | 5994 (51) | 411 (68) | 327 (56) | 584 (61) | 877 (57) |
| Medicare | 3826 (26) | 157 (19) | 4142 (35) | 110 (18) | 170 (29) | 152 (16) | 400 (26) |
| Medicaid | 2106 (15) | 40 (5) | 1028 (9) | 56 (9) | 63 (11) | 185 (19) | 188 (12) |
| Other | 857 (6) | 36 (4) | 635 (5) | 25 (4) | 24 (4) | 43 (4) | 63 (4) |
|
| |||||||
| BMI, kg/m2 | |||||||
| 14–19 | 734 (5) | 46 (6) | 266 (2) | 35 (6) | 46 (8) | 138 (14) | 97 (6) |
| 20–24 | 3842 (27) | 262 (32) | 2715 (23) | 179 (30) | 197 (34) | 347 (36) | 465 (30) |
| 25–29 | 4877 (34) | 305 (37) | 4727 (40) | 207 (34) | 233 (40) | 269 (28) | 527 (34) |
| 30–34 | 3457 (24) | 167 (20) | 3100 (26) | 142 (24) | 83 (14) | 163 (17) | 316 (21) |
| 35–40 | 1514 (10) | 43 (5) | 974 (8) | 38 (6) | 25 (4) | 45 (5) | 118 (8) |
|
| |||||||
| UNOS Status | |||||||
| 1A | 3319 (23) | 145 (18) | 2539 (22) | 100 (17) | 115 (20) | 107 (11) | 420 (27) |
| 1B | 6089 (42) | 212 (26) | 4175 (35) | 164 (27) | 198 (34) | 259 (27) | 331 (22) |
| 2 | 4421 (31) | 439 (53) | 4544 (39) | 330 (55) | 258 (44) | 569 (59) | 727 (48) |
| Inactive | 625 (4) | 27 (3) | 541 (5) | * | 13 (2) | 29 (3) | 50 (3) |
|
| |||||||
| ABO blood type | |||||||
| O | 6685 (46) | 349 (42) | 4769 (40) | 270 (45) | 264 (45) | 447 (46) | 678 (44) |
| A | 5073 (35) | 314 (38) | 4963 (42) | 232 (39) | 209 (36) | 367 (38) | 601 (39) |
| B | 2050 (14) | 124 (15) | 1503 (13) | 77 (17) | 81 (14) | 117 (12) | 180 (12) |
| AB | 639 (4) | 36 (4) | 564 (5) | 23 (4) | 30 (5) | 33 (3) | 69 (5) |
|
| |||||||
| Era | |||||||
| Jan1,‘04–Mar31,‘08 | 4729 (33) | 218 (26) | 4431 (38) | 195 (32) | 245 (42) | 339 (35) | 523 (34) |
| Apr1,‘08–Sept3,‘14 | 9718 (67) | 605 (74) | 7368 (62) | 407 (68) | 339 (58) | 625 (65) | 1005 (66) |
|
| |||||||
| Tobacco usage | 5672 (39) | 253 (31) | 7356 (62) | 204 (34) | 226 (39) | 177 (18) | 371 (24) |
|
| |||||||
| Diabetes mellitus | 3274 (23) | 113 (14) | 4518 (38) | 47 (8) | 98 (17) | 67 (7) | 308 (20) |
|
| |||||||
| Hypertension | 5572 (39) | 251 (31) | 5962 (51) | 180 (30) | 203 (35) | 213 (22) | 661 (43) |
|
| |||||||
| Malignancy | 1090 (8) | 109 (13) | 664 (6) | * | 25 (4) | 22 (2) | 128 (8) |
|
| |||||||
| Prior CVA | 655 (5) | 26 (3) | 619 (5) | 29 (5) | 30 (5) | 55 (6) | 66 (4) |
|
| |||||||
| PVD | 218 (2) | 14 (2) | 574 (5) | * | * | 17 (2) | 38 (2) |
|
| |||||||
| Dialysis at listing | 353 (2) | 31 (4) | 305 (3) | 15 (2) | 18 (3) | 17 (2) | 143 (9) |
|
| |||||||
| eGFR, ml/min/1.73m2 | 72+/−30 | 66 +/−28 | 66 +/−26 | 72+/−28 | 65+/−30 | 84+/−35 | 59+/−31 |
|
| |||||||
| Serum albumin, g/dl | 3.6+/−0.7 | 3.8+/−0.7 | 3.7+/−0.7 | 3.9+/−0.7 | 3.8+/−0.7 | 3.9+/−0.8 | 3.6+/−0.7 |
|
| |||||||
| Mean PAP, mm Hg | 30.8 +/−9.7 | 29.7 +/−8.6 | 29.8 +/−10.0 | 30.6 +/−9.7 | 30.6 +/−9.6 | 26.4+/−10.9 | 26.1+/−8.4 |
|
| |||||||
| CI, L/min/m2 | 2.1 +/−0.7 | 2.1 +/−0.6 | 2.2 +/−0.6 | 2.1+/−0.6 | 2.3 +/−0.7 | 2.3 +/−0.7 | 2.2 +/−0.7 |
|
| |||||||
| Pk VO2, ml/kg/min | 12.0 +/−3.5 | 12.0 +/−3.5 | 11.7 +/−3.0 | 11.8 +/−3.5 | 11.3+/−3.3 | 13.0 +/−3.6 | 12.2 +/−4.2 |
|
| |||||||
| Antiarrythmics | 4350 (30) | 182 (22) | 3800 (32) | 176 (29) | 203 (35) | 274 (28) | 259 (17) |
|
| |||||||
| ICD | 11112 (77) | 378 (46) | 8879 (75) | 480 (80) | 405 (69) | 450 (47) | 418 (27) |
|
| |||||||
| Inotropes | 5201 (36) | 210 (26) | 3449 (29) | 157 (26) | 183 (31) | 226 (23) | 407 (27) |
|
| |||||||
| LVAD | 2652 (18) | 31 (4) | 2314 (20) | 22 (4) | 46 (8) | 30 (3) | 63 (4) |
|
| |||||||
| RVAD +/− LVAD Or unspecified | 675 (5) | 16 (2) | 542 (5) | 11 (2) | 25 (4) | 20 (2) | 118 (8) |
|
| |||||||
| TAH or | 69 (<1) | * | 38 (<1) | * | * | * | 16 (1) |
|
| |||||||
| IABP | 670 (5) | 28 (3) | 714 (6) | 12 (2) | 14 (2) | 12 (1) | 94 (6) |
|
| |||||||
| ECMO | 88 (1) | * | 99 (1) | * | * | 17 (18) | 103 (7) |
|
| |||||||
| Ventilator | 316 (2) | 12 (2) | 394 (3) | 10 (2) | 20 (3) | 26 (27) | 150 (10) |
Values are means +/− standard deviations or n (%).
Number <10 patients
BMI=body mass index, CHD=congenital heart disease, CI=cardiac index, CVA=cerebral vascular accident, IABP=intra-aortic balloon pump, DCM=dilated cardiomyopathy, eGFR=estimated glomerular filtration rate, ECMO=extracorporeal membrane oxygenation, HCM=hypertrophic cardiomyopathy, ICD=implantable cardioverter defibrillator, ICM=ischemic cardiomyopathy, LVAD=left ventricular assist device, PAP=pulmonary artery pressure, Pk VO2 peak oxygen consumption, PVD= peripheral vascular disease, RCM=restrictive cardiomyopathy, RVAD=right ventricular assist device, TAH=total artificial heart, UNOS= United Network for Organ Sharing
UNOS Status at time of listing varied among the different heart diseases (See Table 1). The majority of DCM, ICM and valvular heart disease patients were initially listed at the highest priority (UNOS Status 1A and 1B). In contrast, the majority of RCM, HCM, and CHD patients were initially listed at the lowest priority (UNOS Status 2). Those patients with “other” heart disease had about 50% of the patients listed as UNOS Status 1 and 50% listed as UNOS Status 2.
Wait-List Mortality
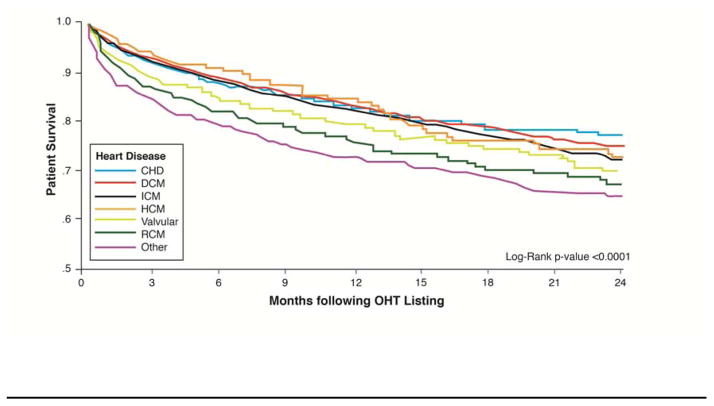
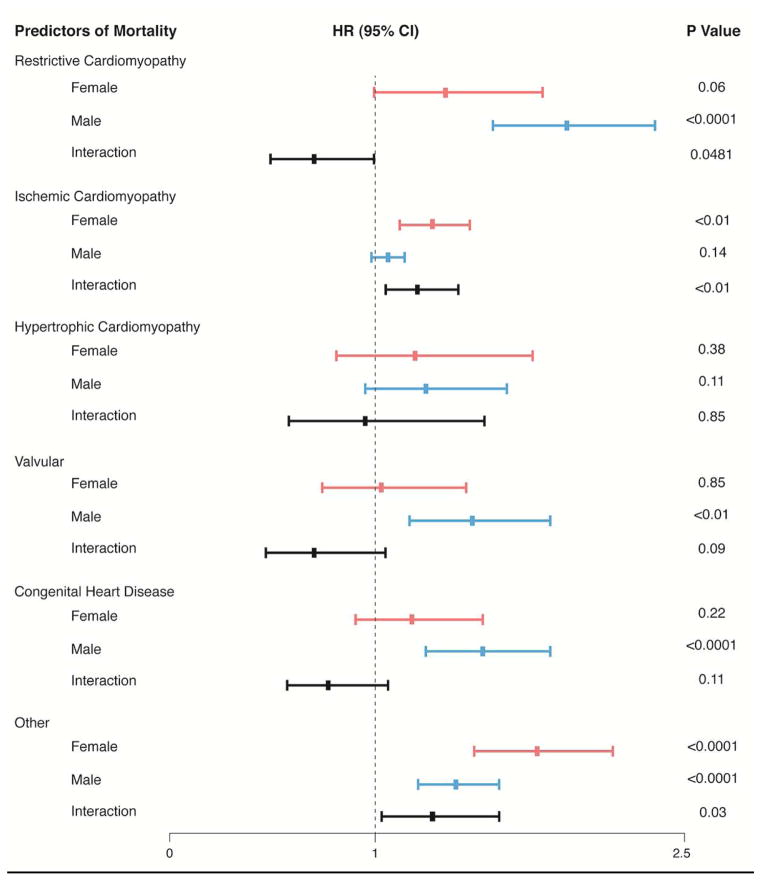
There were 4943 people who died (1253 women, 3690 men) during a median follow-up of 3.7 months (IQR, 0.9–12.8 mo). RCM, valvular, and “other” had the worse survival compared to all other types of heart disease listed for OHT (see Figure 1). HCM patients awaiting OHT had the best survival for the first 12 months and a survival similar to ICM at 24 months by univariate analysis. After adjusting for multiple confounding variables, RCM compared to DCM had the highest risk for mortality followed by “other”, CHD, and then valvular disease. ICM and HCM had a better survival than the above-mentioned heart diseases but a higher risk of death than DCM after multivariate analysis (See Table 2). The risk of death for RCM (HR 1.70, 95% CI 1.43–2.02, p <0.0001) and “Other” (HR 1.51, 95% CI 1.34–1.69, p <0.0001) when compared with that of DCM was higher than IABP (HR 1.43, 95% CI 1.28–1.60, p <0.0001), inotrope (HR 1.38, 95% CI 1.29–1.47, p <0.0001), and mechanical ventilation (HR 1.42, 95% CI 1.24–1.62, p <0.0001) but lower than ECMO (HR 1.95, 95% CI 1.61–2.36, p <0.0001; See Supplementary Table 1). As a sensitivity analysis we excluded patients with VADs and qualitative results were similar to Table 2. There was an interaction between sex and type of cardiomyopathy (see Figure 2) with women at higher risk of death compared to men for ICM (P<0.01) and “other” (P=0.03) and lower risk of death compared to men for RCM (P=0.048).
Figure 1. Survival Differences Awaiting OHT Stratified by Type of Heart Disease.
Kaplan-Meier survival curves while awaiting OHT according to type of heart disease with censoring at time of heart transplantation.
CHD=congenital heart disease, DCM=dilated cardiomyopathy, HCM=hypertrophic cardiomyopathy, ICM=ischemic cardiomyopathy, RCM=restrictive cardiomyopathy, valvular=valvular heart disease, and Other=(1216 prior heart transplants and 312 “other”)
Table 2.
Multivariate Analysis
| Unadjusted HR (95% CI) | Adjusted Model 1 HR (95% CI) | Adjusted Model 2 HR (95% CI) | |
|---|---|---|---|
| DCM | --- | --- | --- |
| RCM | 1.50 (1.27–1.77) * | 1.70 (1.43–2.02) * | 1.80 (1.51–2.13) * |
| ICM | 1.17 (1.10–1.24) * | 1.10 (1.03–1.18) * | 1.13 (1.05–1.21) * |
| HCM | 0.99 (0.79–1.24) | 1.23 (0.98–1.54) | 1.28 (1.02–1.60) * |
| Valvular | 1.33 (1.10–1.60) * | 1.30 (1.07–1.57) * | 1.35 (1.12–1.63) * |
| CHD | 0.96 (0.83–1.12) | 1.37 (1.17–1.61) * | 1.50 (1.28–1.76) * |
| Other | 1.78 (1.60–1.98) * | 1.51 (1.34–1.69) * | 1.64 (1.46–1.84) * |
Values are Hazard ratio (95% Confidence Interval), Reference = DCM, and
P<0.05
CHD=congenital heart disease, DCM=dilated cardiomyopathy, HCM=hypertrophic cardiomyopathy, ICM=ischemic cardiomyopathy, RCM=restrictive cardiomyopathy
Model 1 includes sex, age, race, insurance, BMI, ABO blood type, hypertension, Era, tobacco usage, diabetes mellitus, malignancy, prior CVA, PVD, dialysis, estimated GFR, albumin, PAP mean, CI, ICD, mechanical ventilation, IABP, ECMO, VAD, TAH, inotropes and excludes UNOS Status
Model 2 includes sex, age, race, insurance, BMI, ABO blood type, hypertension, Era, tobacco usage, diabetes mellitus, malignancy, prior CVA, PVD, dialysis, estimated GFR, albumin, PAP mean, CI, ICD, and UNOS Status
Figure 2. Sex Differences in Survival Based on Type of Heart Disease.
The risk of being female or male with a given type of heart disease while awaiting heart transplantation was assessed by multivariate Cox proportional hazard analyses adjusting for sex, age, race, insurance, body mass index, ABO blood type, hypertension, Era, tobacco usage, diabetes mellitus, malignancy, prior cerebral vascular accident, peripheral vascular disease, dialysis, estimated glomerular filtration rate, albumin, mean pulmonary arterial pressure, cardiac index, implantable cardioverter defibrillator, mechanical ventilation, intra-aortic balloon pump, extracorporeal membrane oxygenation, ventricular assist device, total artificial heart, and inotropes. The analyses also included possible interactions between female sex and type of heart disease.
CHD=congenital heart disease, DCM=dilated cardiomyopathy, HCM=hypertrophic cardiomyopathy, ICM=ischemic cardiomyopathy, RCM=restrictive cardiomyopathy, valvular=valvular heart disease, and Other=(1216 prior heart transplants and 312 “other”)
Heart Transplantation
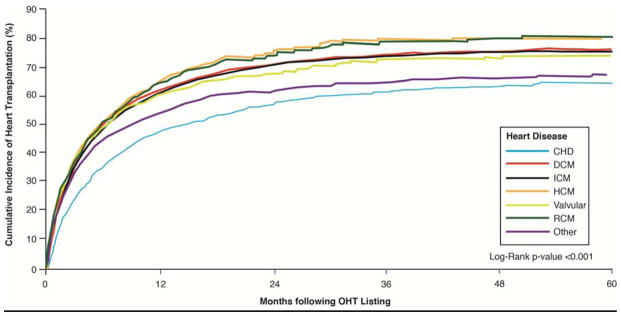
The rate of heart transplantation was highest among RCM and HCM and lowest among CHD and “Other” types of heart disease (See Figure 3). More than 50% of the study cohort underwent OHT at conclusion of the study (See Table 3 for data stratified by heart disease). Among the different heart diseases, CHD patients were less likely to undergo transplantation than the other subgroups. Most patients at time of transplant were UNOS Status 1A or 1B. Permanent mechanical circulatory support (VAD or TAH) at time of transplant was most common in patients with DCM and ICM and least likely in patients with CHD and RCM. ECMO was rarely used to bridge patients to transplantation but more likely to be used among those with CHD and “Other.” Few patients had mechanical ventilation at time of transplant with CHD and “Other” more likely than DCM, RCM, ICM, HCM, and valvular disease.
Figure 3. Time to Heart Transplantation Stratified by Type of Heart Disease.
Time to heart transplantation among patients on the waiting list with data stratified by type of heart disease.
CHD=congenital heart disease, DCM=dilated cardiomyopathy, HCM=hypertrophic cardiomyopathy, ICM=ischemic cardiomyopathy, RCM=restrictive cardiomyopathy, valvular=valvular heart disease, and Other=(1216 prior heart transplants and 312 “other”)
Table 3.
Characteristics at Time of Heart Transplantation
| DCM | RCM | ICM | HCM | Valvular | CHD | Other | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| OHT | 9616 (67) | 540 (66) | 7758 (66) | 422 (70) | 377 (65) | 554 (57) | 1101 (72) |
|
| |||||||
| UNOS Status | |||||||
| 1A | 5272 (55) | 261 (48) | 3866 (50) | 200 (47) | 174 (46) | 236 (43) | 507 (46) |
| 1B | 3578 (37) | 203 (38) | 2941 (38) | 154 (36) | 145 (38) | 230 (42) | 392 (36) |
| 2 | 766 (8) | 76 (14) | 951 (12) | 68 (16) | 58 (15) | 87 (16) | 202 (18) |
|
| |||||||
| LVAD, n | 3036 (32) | 42 (8) | 2413 (31) | 46 (11) | 53 (14) | 28 (5) | 132 (12) |
|
| |||||||
| RVAD +/−LVAD Or unspecified | 786 (8) | 15 (3) | 544 (7) | 18 (4) | 16 (4) | 19 (3) | 102 (9) |
|
| |||||||
| TAH | 125 (1) | * | 60 (1) | * | * | * | 16 (1) |
|
| |||||||
| ECMO | 60 (1) | * | 46 (1) | * | * | 11 (2) | 30 (3) |
|
| |||||||
| Ventilator | 163 (2) | * | 175 (2) | * | * | 20 (4) | 59 (5) |
Values are n (%).
Number <10 patients
CHD=congenital heart disease, DCM=dilated cardiomyopathy, ECMO=extracorporeal membrane oxygenation, HCM=hypertrophic cardiomyopathy, ICM=ischemic cardiomyopathy, LVAD=left ventricular assist device, OHT=orthotopic heart transplantation, RCM=restrictive cardiomyopathy, RVAD=right ventricular assist device, TAH=total artificial heart, UNOS= United Network for Organ Sharing
Discussion
In a large, national transplant registry we found differences in adult survival based on type of heart disease while awaiting cardiac transplantation. After adjustment for possible confounding variables, restrictive cardiomyopathy (RCM), congenital heart disease (CHD), and “Other” were associated with the highest risk of death compared to dilated cardiomyopathy (DCM). HCM had a better prognosis but still worse than DCM and ICM. There was an interaction between sex and type of heart disease with women compared to men at higher risk of death with ICM and “other” and lower risk of death with RCM.
Our research is the first to document differences in adult outcome based on type of cardiomyopathy while awaiting heart transplantation. The results for adults with RCM are similar to pediatrics.8 Poor survival among patients with heart diseases like RCM, CHD, and “other” may be due to differences in the underlying disease or our ability to rescue patients with inotropes and permanent mechanical circulatory support while they wait for transplantation. Rate of heart transplantation may also have contributed to the higher mortality rate among CHD and “Other” but not for RCM.
The current heart transplant allocation system is based on severity of illness defined by usage of mechanical circulatory support, inotropes, and mechanical ventilation. Patients who do not meet the standard criteria can be listed as high priority by exception if deemed appropriate by their transplant center and accepted by the UNOS regional board. However, medical providers have little evidence based knowledge to request an exception based on type of cardiomyopathy and even less information regarding the risk factors for mortality based on type of heart disease. Without this knowledge, the optimal timing to request high priority UNOS Status remains unknown.
RCM comprises a broad category defined in SRTR database as idiopathic, amyloidosis, endocardial fibrosis, sarcoidosis, radiation/chemotherapy induced heart disease, and other. Prognosis is likely to be different for each of these subgroups and dependent on the underlying cause. However, very limited survival data based on heart disease exists. In one single center study evaluating only patients with light chain cardiac amyloidosis (N=31), 35% of the patients died while awaiting OHT.9 Data for patients with cardiac sarcoidosis is limited and confounded by the fact that many are diagnosed after OHT by histologic confirmation of the explanted heart.10–12 Few other studies regarding outcome for type of heart disease are available, yet another SRTR analysis noted findings similar to ours. By univariate analysis, re-transplantation had a higher six months waitlist mortality than RCM among patients listed between 2010–2011.13
Re-transplantation includes patients with allograft vasculopathy, rejection and unknown causes for allograft failure. In our analysis, this cohort had a poor survival while awaiting OHT, a slower rate of transplantation and appeared very ill at time of listing (high frequency of ECMO, mechanical ventilation, dialysis, intra-aortic balloon pump compared to other subgroups). They were also less likely than the other subgroups to have a prophylactic ICD. There are many possible reasons for the findings and yet limited data to support any hypothesis. The degree of illness at time of listing may reflect the fact that there are no guidelines defining which candidate should be considered for re-transplantation and when to re-list. Lack of prophylactic ICDs may be due to few heart transplant patients qualifying for these devices at time of listing since our current guidelines for prophylactic ICDs are based on “native hearts” with moderate to severe systolic dysfunction.14 Finally, the slow rate of transplantation in this subgroup may have been due to sensitization (suspected but not proved) and/or failure to meet criteria for high priority status even when ill.
CHD has been known to be a high risk group while awaiting heart transplantation. Studies have shown patients with CHD are more likely than non-CHD patients to be listed at lower status, less likely to receive mechanical circulatory support at time of listing, less likely to have an ICD and less likely to undergo heart transplantation.15 Waiting times are significantly longer for CHD compared with non-CHD which may be related to lower UNOS Status at time of listing and/or sensitization due to blood transfusions during prior surgeries.15–17 Poor prognosis on the heart transplant waiting list and slower rates for heart transplantation are therefore not unexpected.
HCM patients awaiting OHT have a better outcome than most other types of cardiomyopathy including RCM which is contrary to the popular belief that this is a disadvantaged group with our current allocation system.4 HCM was once considered a rare disease with a high mortality rate but now is known to be one of the most common congenital heart diseases with an adult annual mortality rate of about 0.5% and a “burned out“ phase occurring in 3% of the HCM population. The risk of sudden death is greatest in patients < 30 years of age and is reduced with usage of implantable cardioverter defibrillators.18 Since our HCM cohort had a mean age of 46+/−14 years, they were at lower risk of sudden death. This risk was further reduced with 80% of the cohort having an ICD at time of listing.
Limitations
The validity of the information is dependent on accuracy upon data entry. To reduce human error, SRTR data is assessed by edit checks, validation of data at time of entry and internal verification when there are outliers.6 Despite these attempts, critical data like the type of heart disease may be inaccurate due to misdiagnosis, difficulty with classification when more than one diagnosis is known, and failure to standardize the criteria for each subgroup. Misdiagnosis is unlikely to occur with coronary artery disease or congenital heart disease. However, it is possible to occur with HCM since thickening of the ventricle may be from hypertension or infiltrative diseases. Hypertensive heart disease is more common among Blacks and women,19, 20 yet in our cohort the HCM subgroup was predominately men (62% men, 38% women) and few were Black (9%) making it less likely that a substantial number of patients with hypertensive heart disease were misdiagnosed with HCM. Infiltrative diseases like RCM could be mislabeled as HCM. However, the baseline characteristics including age for RCM and HCM were very different as well as outcome suggesting no significant overlap between the groups. The RCM group may have been mislabeled in the idiopathic and “other” categories but unlikely for the diagnosis of amyloidosis, endocardial fibrosis, sarcoidosis, and radiation/chemotherapy induced heart disease. Standardizing the criteria for all heart disease and requesting pathology data post transplantation will improve accuracy in the future.
Conclusions
Our research demonstrates a higher mortality rate in the United States for patients awaiting OHT based on etiology of heart disease and gender. Patients with RCM, CHD and prior heart transplantation had a higher risk of death than patients with DCM, ICM, HCM and valvular heart disease. Our current heart allocation system does not address these differences. A revised heart allocation scheme has been proposed that will recognize these subgroups and should account for the disadvantaged.
CLINICAL PERSPECTIVE.
For patients awaiting heart transplantation, there are differences in survival based on type of heart disease. Patients with restrictive cardiomyopathy, congenital heart disease, and prior transplantation have a higher risk of death than those with dilated cardiomyopathy, ischemic cardiomyopathy, hypertrophic cardiomyopathy and valvular disease.
TRANSLATIONAL OUTLOOK.
Our data supports a change in the allocation system to prioritize restrictive cardiomyopathy, congenital heart disease and prior heart transplantation over the other types of heart disease. More research is needed to further define the risk factors for these subgroups.
Acknowledgments
Financial Support: Supported by the National Heart, Lung and Blood Institute of the National Institute of Health under Award Number R56HL125420-01A1.
Abbreviations List
- BMI
Body mass index
- CI
Cardiac index
- CHD
Congenital heart disease
- CVA
Cerebral vascular accident
- DCM
Dilated cardiomyiopathy
- ECMO
Extracorporeal membrane oxygenation
- GFR
glomerular filtration rate
- HCM
Hypertrophic cardiomyopathy
- HRSA
Health Resources and Services Administration
- IABP
Intra-aortic balloon pump (IABP)
- ICD
implantable cardioverter defibrillator
- ICM
Ischemic cardiomyopathy
- MCS
Mechanical circulatory support
- OHT
Orthotopic heart transplantation
- OPTN
Organ Procurement and Transplantation Network
- PAP
Pulmonary artery pressure
- PVD
peripheral vascular disease
- RCM
Restrictive cardiomyopathy
- SRTR
Scientific Registry of Transplant Recipients
- TAH
Total artificial heart
- VAD
Ventricular assist device
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the united states, 1999–2008. Am J Transplant. 2010;10:1035–1046. doi: 10.1111/j.1600-6143.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 2.Davies RR, Russo MJ, Yang J, Quaegebeur JM, Mosca RS, Chen JM. Listing and transplanting adults with congenital heart disease. Circulation. 2011;123:759–767. doi: 10.1161/CIRCULATIONAHA.110.960260. [DOI] [PubMed] [Google Scholar]
- 3.Kobashigawa JA. Time for change in united states donor heart allocation policy. JACC Heart Fail. 2014;2:178–179. doi: 10.1016/j.jchf.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Kobashigawa JA, Johnson M, Rogers J, Vega JD, Colvin-Adams M, Edwards L, Meyer D, Luu M, Reinsmoen N, Dipchand AI, Feldman D, Kormos R, Mancini D, Webber S. Report from a forum on us heart allocation policy. Am J Transplant. 2015;15:55–63. doi: 10.1111/ajt.13033. [DOI] [PubMed] [Google Scholar]
- 5.VanderPluym C, Graham DA, Almond CS, Blume ED, Milliren CE, Singh TP. Survival in patients removed from the heart transplant waiting list before receiving a transplant. J Heart Lung Transplant. 2014;33:261–269. doi: 10.1016/j.healun.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, Kasiske BL. Scientific registry of transplant recipients: Collecting, analyzing, and reporting data on transplantation in the united states. Transplant Rev (Orlando) 2013;27:50–56. doi: 10.1016/j.trre.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 7. [accessed on july 13, 2013];Optn policy 3.7. Available at: Http://optn.Transplant.Hrsa.Gov/policiesandbylaws2/policies/pdfs/policy_9.Pdf.
- 8.Webber SA, Lipshultz SE, Sleeper LA, Lu M, Wilkinson JD, Addonizio LJ, Canter CE, Colan SD, Everitt MD, Jefferies JL, Kantor PF, Lamour JM, Margossian R, Pahl E, Rusconi PG, Towbin JA. Outcomes of restrictive cardiomyopathy in childhood and the influence of phenotype: A report from the pediatric cardiomyopathy registry. Circulation. 2012;126:1237–1244. doi: 10.1161/CIRCULATIONAHA.112.104638. [DOI] [PubMed] [Google Scholar]
- 9.Gray Gilstrap L, Niehaus E, Malhotra R, Ton VK, Watts J, Seldin DC, Madsen JC, Semigran MJ. Predictors of survival to orthotopic heart transplant in patients with light chain amyloidosis. J Heart Lung Transplant. 2014;33:149–156. doi: 10.1016/j.healun.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akashi H, Kato TS, Takayama H, Naka Y, Farr M, Mancini D, Schulze PC. Outcome of patients with cardiac sarcoidosis undergoing cardiac transplantation--single-center retrospective analysis. J Cardiol. 2012;60:407–410. doi: 10.1016/j.jjcc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Kandolin R, Lehtonen J, Airaksinen J, Vihinen T, Miettinen H, Ylitalo K, Kaikkonen K, Tuohinen S, Haataja P, Kerola T, Kokkonen J, Pelkonen M, Pietila-Effati P, Utrianen S, Kupari M. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 12.Perkel D, Czer LS, Morrissey RP, Ruzza A, Rafiei M, Awad M, Patel J, Kobashigawa JA. Heart transplantation for end-stage heart failure due to cardiac sarcoidosis. Transplant Proc. 2013;45:2384–2386. doi: 10.1016/j.transproceed.2013.02.116. [DOI] [PubMed] [Google Scholar]
- 13.Meyer DM, Rogers JG, Edwards LB, Callahan ER, Webber SA, Johnson MR, Vega JD, Zucker MJ, Cleveland JC., Jr The future direction of the adult heart allocation system in the united states. Am J Transplant. 2015;15:44–54. doi: 10.1111/ajt.13030. [DOI] [PubMed] [Google Scholar]
- 14.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. Circulation. 2013;128:e240–327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 15.Everitt MD, Donaldson AE, Stehlik J, Kaza AK, Budge D, Alharethi R, Bullock EA, Kfoury AG, Yetman AT. Would access to device therapies improve transplant outcomes for adults with congenital heart disease? Analysis of the united network for organ sharing (unos) J Heart Lung Transplant. 2011;30:395–401. doi: 10.1016/j.healun.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Patel ND, Weiss ES, Allen JG, Russell SD, Shah AS, Vricella LA, Conte JV. Heart transplantation for adults with congenital heart disease: Analysis of the united network for organ sharing database. Ann Thorac Surg. 2009;88:814–821. doi: 10.1016/j.athoracsur.2009.04.071. discussion 821-812. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg SW, Fisher SA, Wehman B, Mehra MR. Adults with congenital heart disease and heart transplantation: Optimizing outcomes. J Heart Lung Transplant. 2014;33:873–877. doi: 10.1016/j.healun.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: Present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83–99. doi: 10.1016/j.jacc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Krim SR, Vivo RP, Krim NR, Qian F, Cox M, Ventura H, Hernandez AF, Bhatt DL, Fonarow GC. Racial/ethnic differences in b-type natriuretic peptide levels and their association with care and outcomes among patients hospitalized with heart failure: Findings from get with the guidelines-heart failure. JACC Heart Fail. 2013;1:345–352. doi: 10.1016/j.jchf.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Hsich EM, Grau-Sepulveda MV, Hernandez AF, Eapen ZJ, Xian Y, Schwamm LH, Bhatt DL, Fonarow GC. Relationship between sex, ejection fraction, and b-type natriuretic peptide levels in patients hospitalized with heart failure and associations with inhospital outcomes: Findings from the get with the guideline-heart failure registry. Am Heart J. 2013;166:1063–1071. e1063. doi: 10.1016/j.ahj.2013.08.029. [DOI] [PubMed] [Google Scholar]