Abstract
Sophora davidii (Franch.) Kom. ex Pavol is an important medicinal plant and a feeding scrub with ecological value. The effects of different gamma irradiation doses (20–140 Kr) on seed germination and seedling morphology were investigated in S. davidii, and intersimple sequence repeat (ISSR) markers were used to identify the DNA polymorphism among mutants. Significant variations were observed for seed germination, stem diameter, and number of branches per plant. The improved agronomic traits, such as stem diameter and number of branches per plant, were recorded at 80 Kr dose and 20 Kr dose for seed germination. ISSR analysis generated in total 183 scorable fragments, of which 94 (51.37%) were polymorphic. The percentage of polymorphism ranged from 14.29 to 93.33 with an average of 45.69%. Jaccard's coefficients of dissimilarity varied from 0.6885 to 1.000, indicative of the level of genetic variation among the mutants. The constructed dendrogram grouped the entities into five clusters. Consequently, it was concluded that gamma rays irradiation of seeds generates a sufficient number of induced mutations and that ISSR analysis offered a useful molecular marker for the identification of mutants.
1. Introduction
Sophora davidii (Franch.) Kom. ex Pavol is an important leguminous scrub which has significant economic values and has been widely used as fodder, as herbal medicine, and as a nectar source [1, 2]. It is also a kind of pioneer plant in karst rocky desertification areas of southwestern China for eco-economical consideration. The reason for its application in restoring vegetation and ecological engineering is simply that this species could tolerate extremely dry environments, with deeper root systems, smaller leaf sizes, and greater accumulating ability of soluble sugars in leaves [3]. S. davidii has drawn wide attention of many scientists due to its high adaptability among diverse native species in karst rocky desertification areas. However, complex local microclimates and habitat fragmentation in karst areas have made this species suffer serious risk of genetic diversity reduction. So, species conservation and improvement are the major problems for its reasonable utilization. Induced mutation has been an important technique of cultivars improvement and has been highly effective in enhancing natural genetic resources especially for unusual plants and in the selection of mutants with desirable agronomic characteristics [4]. And more than 2,700 varieties derived via mutations of 170 different species throughout the world have been released officially [5].
Induced mutations contribute to genetic variability mainly by increasing DNA polymorphism [6]. A great number of DNA markers have been broadly used to estimate genetic diversity, genetic stability, the genetic characterization, and the identification and screening of mutant plants, such as maize [7], soybean [8], faba bean [9], potato [10], Curcuma alismatifolia Gagnep. [11], and Acorus calamus L. [12].
However, molecular characterization of mutation events induced by irradiation treatments in Sophora is limited. The aim of our study was to develop a suitable technique for mutation breeding of S. davidii cultivars using gamma radiation. For this purpose, we investigated the effect of different doses (20–140 Kr) of gamma irradiation on seed germination and seedling morphological traits of S. davidii and identified DNA polymorphism among the mutants through intersimple sequence repeat (ISSR) marker analysis.
2. Materials and Methods
2.1. Plant Source, Irradiation, and Morphology Study
The seeds of Sophora davidii (Franch.) Kom. ex Pavol were collected in October 2013 from a karst mountain region (105°38′N, 25°55′E) in Guanling Town, Guizhou, China. All seeds were dried under sunshine and then stored in a cool, dry location. Prior to radiation, the moisture content of all seeds was equilibrated by placing them inside paper bags stored in a calcium oxide-dried container.
Then, the seeds were irradiated with different doses (20, 40, 60, 80, 100, 120, and 140 Kr) of 60Co-γ in October 2013 at the Institute of Biological and Nuclear Technology, Guizhou Province Academy of Agricultural Sciences, China.
Immediately after radiation, 200 treated seeds were sown at a depth of 1 cm with a mixed medium of river sand, red soil, and farm yard manure at the ratio of 3 : 2 : 1 in plastic pots (23 × 27 cm, 6 cm in height). After a month of germination, the number of germinated seeds was counted and expressed as percentage of the total number of seeds planted. Ten seedlings from each treatment were transplanted to experimental field. Morphological traits, including leaf length, leaf width, plant height, branch length, stem diameter, and number of bunches per plant, were recorded two months later after transplantation.
2.2. DNA Extraction and ISSR-PCR Amplification
The fresh leaf material was harvested from the three-month-old plants treated with gamma rays. DNA was extracted using a modified CTAB method [13] and dissolved in 1x TE butter for subsequent use. ISSR-PCR amplifications were performed in a GeneAmp PCR System 9700 DNA Thermal Cycler (PerkinElmer, USA) with cycling profiles: 4 min at 94°C, followed by 35 cycles of 30 s at 94°C, 30 s at 50°C, and 1 min at 72°C and ending with 10 min at 72°C. 50 primers (Biotechnology Laboratory, Inner Mongolia Agriculture University) were screened initially to identify well-amplified polymorphic bands among the populations. Of the 50 primers tested, 20 produced bright, clear, and reproducible bands.
PCR was carried out in a total volume of 20 µL, which included 50 ng of template DNA, 2 µL 10x PCR buffer (Mg2+ Plus), 2 µL MgCl2 (25 mM), 1.0 µL dNTPs mixture (2.5 mM), 1.0 U of Taq polymerase (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China), and 0.4 μM primers (Shanghai Sangon Biological Engineering Technology and Service Co. Ltd., Shanghai, China). Amplification products, along with a GeneRuler100 ladder (Fermentas UAB, Inc.), were separated via electrophoresis on 1.5% (w/v) agarose gels with 0.5x TBE buffer at 120 V for 3-4 h, stained with ethidium bromide (0.1 mg/µL). They were then photographed with an Epson digital still AF camera. Negative controls, which lacked template DNA, were also included in each PCR set to test for possible contamination.
2.3. Data Statistical Analysis
Data of seed germination and morphological traits were analyzed using one-way ANOVA with SPSS software package (17.0). Data of ISSR marker analysis were scored as discrete variables, using “1” for presence and “0” for absence of bands for each of the primer pairs. The faint and unclear bands were not considered for data scoring. The binary data generated were used to estimate levels of polymorphism by dividing the polymorphic bands by the total number of scored bands. A dendrogram based on Jaccard's similarity coefficients was constructed by using Unweighted Pair Group Method with Arithmetic Mean (UPGMA) with the SHAN module of NTSYS-PC 2.0 to show phenetic representation of genetic relationships as revealed by the similarity coefficient.
3. Results
3.1. Seed Germination and Seedling Morphological Traits in Gamma Rays Treated Plants
In this study, we measured the effect of different doses (control, 20, 40, 60, 80, 100, 120, and 140 Kr) of gamma irradiation on seed germination and seedling morphological traits. The results (mean ± standard deviation) are given in Table 1. Repeated measures ANOVA revealed a significant positive influence of the gamma irradiation on seed germination, stem diameter, and number of branches per plant (P < 0.05).
Table 1.
Effects of gamma radiation on seed germination percentage and morphological traits of S. davidii at seedling stage.
| Dose | SG (%) | LL (cm) | LW (cm) | SH (cm) | BL (cm) | SD (mm) | NB |
|---|---|---|---|---|---|---|---|
| Control | 40.00 ± 1.00b | 1.48 ± 0.36a | 0.69 ± 0.17a | 27.11 ± 3.31a | 11.56 ± 1.89a | 2.88 ± 0.32c | 9.67 ± 1.53ab |
| 20 Kr | 45.67 ± 3.21a | 1.48 ± 0.32a | 0.67 ± 0.18a | 27.07 ± 4.16a | 10.42 ± 2.55a | 3.11 ± 0.32c | 9.33 ± 2.08ab |
| 40 Kr | 39.67 ± 2.52b | 1.49 ± 0.25a | 0.70 ± 0.12a | 25.43 ± 5.89a | 10.41 ± 2.33a | 3.33 ± 0.28bc | 10.33 ± 1.52ab |
| 60 Kr | 33.67 ± 1.15c | 1.42 ± 0.29a | 0.61 ± 0.19a | 23.10 ± 4.39a | 10.09 ± 4.02a | 3.86 ± 0.29ab | 9.67 ± 1.15ab |
| 80 Kr | 31.33 ± 1.15c | 1.36 ± 0.34a | 0.55 ± 0.21a | 23.21 ± 4.63a | 9.46 ± 2.52a | 3.93 ± 0.47a | 12.00 ± 2.65a |
| 100 Kr | 22.67 ± 2.31d | 1.31 ± 0.30a | 0.51 ± 0.20a | 22.85 ± 4.48a | 8.52 ± 2.81a | 3.23 ± 0.21c | 9.67 ± 1.15ab |
| 120 Kr | 20.00 ± 2.00d | 1.33 ± 0.31a | 0.47 ± 0.19a | 19.84 ± 5.24a | 7.70 ± 2.92a | 2.79 ± 0.29c | 7.33 ± 0.58b |
| 140 Kr | 20.33 ± 1.53d | 1.23 ± 0.30a | 0.46 ± 0.13a | 19.54 ± 4.53a | 7.93 ± 2.81a | 2.99 ± 0.33c | 8.33 ± 2.08b |
Each value was expressed as mean ± standard deviation. SG, seed germination; LL, leaf length; LW, leaf width; SH, seedling height; BL, branch length; SD, stem diameter; NB, number of branches per plant. Within columns, means ± standard deviation followed by the same letter are not significantly different at P = 0.05.
Seeds untreated with gamma rays showed higher germination rate than the irradiation treatment except for the 20 Kr dose treatment with germination percentage of 45%. In the treated seeds, the germination decreased significantly (P < 0.05) as the irradiation doses of gamma rays increased to 120 Kr, while the 140 Kr treatment showed an increased germination of 20.33%. Leaf length and leaf width ranged from 1.23 to 1.49 cm and from 0.46 to 0.70 cm, respectively. The maximum of leaf length (1.49 cm) and the maximum of leaf width (0.72 cm) were obtained at plants treated with 40 Kr dose, and the minimum of leaf length (1.23 cm) and the minimum of leaf width (0.46 cm) occurred at 140 Kr dose in gamma rays treated plants.
Only the seedling height showed a constant decline as the irradiation doses increased, though not reaching the significant level. Similar to the effect on germination, the branch length decreased gradually as the irradiation dose increased up to 120 Kr and showed a slight rise at 140 Kr treatment. The maximum stem diameter (3.93 mm) and number of branches per plant (12) occurred at 80 Kr dose treatment, while the minimum stem diameter (2.79 mm) and number of branches per plant (7.33) were observed at 120 Kr dose treatment.
3.2. ISSR Marker Analysis in Gamma Rays Treated Plants
In our research, the differences among the gamma irradiation mutants were examined by using ISSR marker. 20 primers were selected for amplification and data scoring (Table 2); the results were analyzed for identifying genetic diversity in gamma rays treated plants.
Table 2.
List of primers, number of amplified products, polymorphic bands, and polymorphism percentage.
| Number | Primers | Sequence (5′–3′) | TAP | NPB | PPB (%) |
|---|---|---|---|---|---|
| 1 | UBC857 | AGAGAGAGAGAGAGAGYG | 17 | 12 | 70.59 |
| 2 | UBC818 | CACACACACACACACAG | 7 | 2 | 28.57 |
| 3 | UBC850 | GTGTGTGTGTGTGTGTYC | 9 | 4 | 44.44 |
| 4 | UBC840 | GAGAGAGAGAGAGAGAYT | 10 | 2 | 20.00 |
| 5 | UBC812 | GAGAGAGAGAGAGAGAA | 7 | 1 | 14.29 |
| 6 | ISSR04 | GAGAGAGAGAGAGAGACT | 10 | 2 | 20.00 |
| 7 | ISSR11 | ACACACACACACACACTG | 11 | 8 | 72.73 |
| 8 | ISSR21 | GAGAGAGAGAGAGAGATG | 7 | 2 | 28.57 |
| 9 | ISSR25 | ACACACACACACACACCT | 10 | 4 | 40.00 |
| 10 | ISSR24 | ACACACACACACACACCA | 13 | 7 | 53.85 |
| 11 | ISSR36 | GCGTCTCTCTCTCTCTC | 8 | 2 | 25.00 |
| 12 | ISSR47 | AGAAGAAGAAGAAGAAGAAGA | 7 | 4 | 57.14 |
| 13 | ISSR02 | AGAGAGAGAGAGAGAGCC | 12 | 10 | 83.33 |
| 14 | ISSR05 | GAGAGAGAGAGAGAGACC | 7 | 5 | 71.43 |
| 15 | ISSR06 | GAGAGAGAGAGAGAGACG | 12 | 9 | 75.00 |
| 16 | ISSR08 | GTGTGTGTGTGTGTGTCC | 15 | 14 | 93.33 |
| 17 | ISSR12 | CCCTCCCTCCCTCCCT | 3 | 1 | 33.33 |
| 18 | ISSR14 | CTTCACTTCACTTCA | 7 | 3 | 42.86 |
| 19 | ISSR10 | ACACACACACACACACTA | 4 | 1 | 25.00 |
| 20 | ISSR15 | GGAGAGGAGAGGAGA | 7 | 1 | 14.29 |
|
| |||||
| Total | 183 | 94 | 913.75 | ||
|
| |||||
| Mean | 9.15 | 4.7 | 45.69 | ||
TAP, total amplified products; NPB, number of polymorphic bands; PPB, percentage of polymorphic bands.
A total of 183 bands were scored, of which 94 were polymorphic. The number of bands generated per primer varied from 3 to 17 and a minimum of 3 bands was generated by the primer ISSR12, while the maximum of 17 bands was scored with UBC857 followed by ISSR08 which produced 15 bands (Table 2). The percentage of polymorphism was ranged from 14.29 to 93.33%, with an average polymorphism percentage of 45.69%. Primer ISSR08 produced relatively high number of bands and the vast majority of these bands were polymorphic, showing 93.33% of polymorphism (about 14 polymorphic bands), while UBC812 and ISSR15 produced relatively low number of bands and showed the lowest percentage of polymorphism of 14.29% (only 1 polymorphic band).
The genetic similarity based on ISSR data ranged from 0.6885 to 1.000, with an overall mean of 0.7884 (Table 3). All the treatments revealed the maximum genetic diversity with combination of control. The dendrogram obtained from UPGMA analysis of genetic similarity based on the ISSR marker is presented in Figure 1. The dendrogram shows the formation of five main groups of mutants. The dendrogram indicated five distinct clusters: the first cluster comprised three treatments, namely, control, 40 Kr, and 80 Kr, the second cluster comprised two treatments, namely, 120 Kr and 140 Kr, and the other three clusters included 100 Kr, 60 Kr, and 20 Kr, respectively, indicating their higher genetic distinctness from other treatments of gamma rays. According to the dendrogram obtained, 20 Kr, 60 Kr, and 100 Kr were more distant to control than to other treatments.
Table 3.
Distance matrix based on Jaccard's coefficients.
| Control | 20 Kr | 40 Kr | 60 Kr | 80 Kr | 100 Kr | 120 Kr | 140 Kr | |
|---|---|---|---|---|---|---|---|---|
| Control | 1.0000 | |||||||
| 20 Kr | 0.7596 | 1.0000 | ||||||
| 40 Kr | 0.8579 | 0.7814 | 1.0000 | |||||
| 60 Kr | 0.7377 | 0.7486 | 0.8033 | 1.0000 | ||||
| 80 Kr | 0.8361 | 0.7705 | 0.8251 | 0.8251 | 1.0000 | |||
| 100 Kr | 0.7814 | 0.7596 | 0.8033 | 0.7486 | 0.8142 | 1.0000 | ||
| 120 Kr | 0.8087 | 0.6885 | 0.8197 | 0.7432 | 0.8306 | 0.7650 | 1.0000 | |
| 140 Kr | 0.8415 | 0.7541 | 0.8087 | 0.7869 | 0.7978 | 0.7541 | 0.8251 | 1.0000 |
Figure 1.
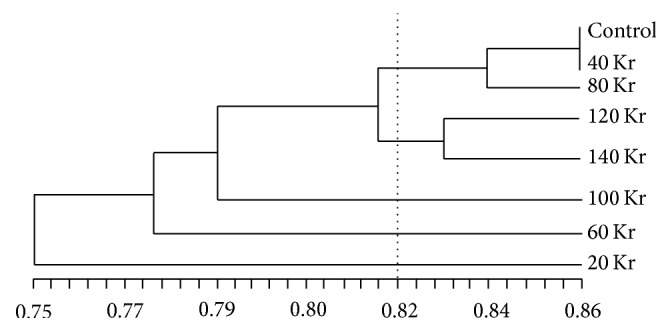
Dendrogram representing the morphological variation among seven irradiated mutants (20, 40, 60, 80, 100, 120, and 140 Kr) and the control based on similarity coefficients of ISSR data.
4. Discussion
4.1. Germination and Morphological Traits in the Gamma Rays Treated Plants
Many researchers have reported that mutagens, including chemical and physical mutagens, can interact with cellular molecules, particularly water, to produce free radicals (H, OH). These free radicals could combine to form toxic substances, such as hydrogen peroxide (H2O2), which indirectly lead to the destruction of cells [4]. This indirect effect is especially significant in vegetative cells, as the cytoplasm contains about 80% of water [14]. Finally, the excessive radicals resulted in damage or modification of important cell components and affected the morphology, biochemistry, and physiology of plants. The degree of this injury depends on the sensitivity of radiating material. In this study, germination percentage of the irradiated seedlings decreased significantly with a steady trend as the irradiation doses increased, showing relative sensitivity to irradiation. This reduction/stimulation in seed germination, where the hypothetic origins can accelerate cell division rates [15], might have been due to the effect of mutagens on meristematic tissues of the seed [6]. Our result agreed with researches reported by [16–18]. The decrease in germination at high doses of the mutagens may be attributed to disturbances at cellular level (caused at either physiological level or physical level) including chromosomal damage [6]. And it was reported that higher exposures are usually inhibitor on seed germination of Gymnosperm and Angiosperm, while lower exposures are inducer on seed germination [19–21]. This could be the reason why the abnormal performance of S. davidii seed germination occurred at 20 Kr and 140 Kr (a slight rise occurred at 20 Kr dose treatment and 140 Kr treatment).
Many researchers reported that gamma rays treated plants can change the vegetative traits, rhizome characteristics, and flowering development and its maturity in either a positive direction or a negative direction [4, 6, 22–24]. In this study, significant variations were observed in stem diameter and number of branches per plant after gamma irradiation. But the variation was not proportional to the change in dosages and was not in a definite pattern. High doses of ionizing radiation have been reported to damage macromolecular cellular components such as cell walls, membranes, and DNA [25]. So we supposed these changes to happen because of the effects of excessive irradiation on cells. However, a small tendency towards a decrease in leaf size was observed in mutagenised seedlings, though the differences were not significant. The leaf length and leaf width decreased as the radiation doses increased, especially in irradiated plants treated with doses from 40 Kr to 140 Kr. Similar decreases have been reported by Taheri et al. [4] and Tangpong et al. [23]. Same trends were also detected in seedling height and branch length. Taheri et al. [4] have reported similar decreases in seeding height of mutagenised populations in Curcuma alismatifolia Gagnep. Such effects are known to arise due to chromosomal aberrations in addition to genetic mutations [4]. It is a fact that the cells which have relatively more chromosomal damage at high irradiation exposures are at a disadvantage due to diplontic section, as these cells cannot compete well with the normal cells and are thus prevented from making any further contribution [6].
That is, this progressive reduction in S. davidii morphological traits can be interpreted as the interference on normal mitosis and frequent occurrence of mitotic aberrations. Moreover, some researchers thought that the inhibition of assimilation rate and consequent change in the nutrient level of plant and the inactivation of vital enzymes especially those associated with respiration account for this. In addition, mutagenic effects such as auxin destruction, inhibition of auxin synthesis, failure of assimilatory mechanism, and changes in the specific activity of enzymes can also cause growth reductions [26]. Since no major reduction of parameters was found in our study, we propose that this is favorable for breeding attempts, especially with 40 Kr and 80 Kr irradiation doses.
4.2. ISSR Marker Analysis in the Gamma Rays Treated Plants
Mutation breeding is based on induction of mutation, genetic diversity evaluation, and screening and molecular identification of desired character. During this process, molecular markers have made great contributions in the genetic characterization, screening of mutant plants, and the molecular identification of specific agronomic trait. Among various molecular markers, ISSR is easy to apply, highly informative, reliable, repeatable, and inexpensive [27, 28] and is a practicable method for the assessment of genetic diversity studies, especially for plants with no or little specific primers released. In present research, amplifications were successfully performed for all the 20 ISSR primers assayed. 183 scorable fragments, in total, were generated, of which 94 (51.37%) were found to be polymorphic (the average polymorphism percentage per primer was 45.69%). It is indicated that gamma rays irradiation is an effective method of mutant induction, which has been proven previously [4, 29, 30], and ISSR is of relatively high discriminative power of mutants or mutant loci (or the genetic diversity between them), corresponding to similar studies [4, 29–32]. The genetic similarity of seedlings among different doses treatments ranged from 0.6885 to 1.000, with an average genetic similarity of 0.7884. It is directly revealed that DNA changes had happened to these seedlings and the dendrogram, showing the formation of five main groups of mutants, indicated that the effects of different irradiation dosages on seedlings are far from each other. This result was in accordance with studies in lily [32], banana [31], Jatropha curcas L. [6], and sugar beet [33]. So, it can be concluded that gamma ray treatment was an effective way for mutation induction in S. davidii and the mutants were successfully identified through ISSR analysis.
Acknowledgments
This work was funded through projects of the National Natural Science Foundation of China (31260572 and 71263012), the Science and Technology Innovation Talent Team Construction of Guizhou Province ([2016]5617), the Talent Team of Guizhou Province (2016GZ13881), and the National Key Research Plan (2016YFC0502603).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Tanaka T., Ito T., Iinuma M., Ohyama M., Ichise M., Tateishi Y. Stilbene oligomers in roots of Sophora davidii. Phytochemistry. 2000;53(8):1009–1014. doi: 10.1016/S0031-9422(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 2.Qi X., Wang K., Zhang C. Effectiveness of ecological restoration projects in a karst region of southwest China assessed using vegetation succession mapping. Ecological Engineering. 2013;54:245–253. doi: 10.1016/j.ecoleng.2013.01.002. [DOI] [Google Scholar]
- 3.Wu X., Liu H., Huang X., Zhou T. Human driving forces: analysis of rocky desertification in karst region in Guanling County, Guizhou Province. Chinese Geographical Science. 2011;21(5):600–608. doi: 10.1007/s11769-011-0496-7. [DOI] [Google Scholar]
- 4.Taheri S., Abdullah T. L., Ahmad Z., Abdullah N. A. P. Effect of acute gamma irradiation on Curcuma alismatifolia varieties and detection of DNA polymorphism through SSR marker. BioMed Research International. 2014;2014:245–256. doi: 10.1155/2014/631813.631813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo X. M., Tinker N. A., Jiang Y., Xuan P., Zhang H. Q., Zhou Y. H. Suitable dose of 60Co γ-ray for mutation in Roegneria seeds. Journal of Radioanalytical and Nuclear Chemistry. 2013;295(2):1129–1134. doi: 10.1007/s10967-012-1888-6. [DOI] [Google Scholar]
- 6.Dhakshanamoorthy D., Selvaraj R., Chidambaram A. L. A. Induced mutagenesis in Jatropha curcas L. using gamma rays and detection of DNA polymorphism through RAPD marker. Comptes Rendus—Biologies. 2011;334(1):24–30. doi: 10.1016/j.crvi.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Rustikawati R., Suprijono E., Romeida A., Herison C., Sutjahjo S. H. Identification of M4 gamma irradiated maize mutant based on RAPD markers. Agrivita. 2012;34(2):161–165. doi: 10.17503/Agrivita-2012-34-2-p161-165. [DOI] [Google Scholar]
- 8.Mudibu J., Nkongolo K. K. C., Mehes-Smith M., Kalonji-Mbuyi A. Genetic analysis of a soybean genetic pool using ISSR marker: effect of gamma radiation on genetic variability. International Journal of Plant Breeding & Genetics. 2011;5:235–245. [Google Scholar]
- 9.Mejri S., Mabrouk Y., Voisin M., et al. Variation in quantitative characters of faba bean after seed irradiation and associated molecular changes. African Journal of Biotechnology. 2014;11(34):8383–8390. [Google Scholar]
- 10.Afrasiab H., Iqbal J. Genetic analysis of somaclonal variants and induced mutants of Potato (Solanum tuberosum L.) CV. diamant using RAPD markers. Pakistan Journal of Botany. 2012;44:215–220. [Google Scholar]
- 11.Taheri S., Abdullah T. L., Abdullah N. A. P., Ahmad Z. Use of intersimple sequence repeat assay for detection of DNA polymorphism induced by gamma rays in Curcuma alismatifolia. HortScience. 2013;48:1346–1351. [Google Scholar]
- 12.Lee J.-H., Han T.-H. Selection of mutants obtained by gamma ray irradiation and analysis of genetic variation using RAPD markers in Acorus calamus L. Horticulture Environment and Biotechnology. 2014;55(3):207–212. doi: 10.1007/s13580-014-0701-6. [DOI] [Google Scholar]
- 13.Zhang L., Li Q.-J., Li H.-T., Chen J., Li D.-Z. Genetic diversity and geographic differentiation in Tacca chantrieri (Taccaceae): an autonomous selfing plant with showy floral display. Annals of Botany. 2006;98(2):449–457. doi: 10.1093/aob/mcl123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kovács E., Keresztes Á. Effect of gamma and UV-B/C radiation on plant cells. Micron. 2002;33(2):199–210. doi: 10.1016/S0968-4328(01)00012-9. [DOI] [PubMed] [Google Scholar]
- 15.Zaka R., Chenal C., Misset M. T. Effects of low doses of short-term gamma irradiation on growth and development through two generations of Pisum sativum. Science of the Total Environment. 2004;320(2-3):121–129. doi: 10.1016/j.scitotenv.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Gaur S., Singh M., Rathore N., Bhati P. S., Kumar D. radiobiological responses of Cowpea. Proceedings of the National Symposium on Arid Legumes, for Food Nutrition Security and Promotion of Trade; 2002; Hisar India. pp. 75–78. [Google Scholar]
- 17.Toker C., Uzun B., Canci H., Oncu Ceylan F. Effects of gamma irradiation on the shoot length of Cicer seeds. Radiation Physics and Chemistry. 2005;73(6):365–367. doi: 10.1016/j.radphyschem.2005.03.011. [DOI] [Google Scholar]
- 18.Melki M., Marouani A. Effects of gamma rays irradiation on seed germination and growth of hard wheat. Environmental Chemistry Letters. 2010;8(4):307–310. doi: 10.1007/s10311-009-0222-1. [DOI] [Google Scholar]
- 19.Saric M. R., Curic R., Ceric I., Hadzijev D. Effect of gamma radiation of some varieties of wheat seed on the morphological characteristics of the seedlings. Proceedings of the Symposium on the Effects of Ionizing Radiations on Seeds and Their Significance for Crop Improvement; 1961; Karlsruhe, Germany. pp. 103–116. [Google Scholar]
- 20.Thapa C. B. Effect of acute exposure of gamma rays on seed germination and seedling growth of Pinus kesiya gord and P. wallichiana A.B. Jacks. Our Nature. 2004;2(1):13–17. doi: 10.3126/on.v2i1.318. [DOI] [Google Scholar]
- 21.Taylor F. G., Jr. Some effects of acute gamma radiation in giant Sequoia seedlings. Radiation Botany. 1968;8(1):67–70. doi: 10.1016/S0033-7560(68)80068-7. [DOI] [Google Scholar]
- 22.Abdullah T. L., Endan J., Nazir B. M. Changes in flower development, chlorophyll mutation and alteration in plant morphology of Curcuma alismatifolia by gamma irradiation. American Journal of Applied Sciences. 2009;6(7):1436–1439. doi: 10.3844/ajassp.2009.1436.1439. [DOI] [Google Scholar]
- 23.Tangpong P., Taychasinpitak T., Jompuk C., Jompuk P. Effects of acute and chronic gamma irradiations on in vitro culture of Anubias congensis N.E. Brown. Kasetsart Journal. 2009;43:449–457. [Google Scholar]
- 24.Ambavane A. R., Sawardekar S. V., Sawantdesai S. A., Gokhale N. B. Studies on mutagenic effectiveness and efficiency of gamma rays and its effect on quantitative traits in finger millet (Eleusine coracana L. Gaertn) Journal of Radiation Research and Applied Sciences. 2015;8(1):120–125. doi: 10.1016/j.jrras.2014.12.004. [DOI] [Google Scholar]
- 25.Wi S. G., Chung B. Y., Kim J. H., et al. Ultrastructural changes of cell organelles in Arabidopsis stems after gamma irradation. Journal of Plant Biology. 2005;48(2):195–200. doi: 10.1007/BF03030408. [DOI] [Google Scholar]
- 26.Hegde R. V. Studies on Induced Mutagenesis and In Vitro Regeneration in Turmeric (Curcuma longa L) Dharwad, India: University of Agricultural Sciences Dharwad; 2006. [Google Scholar]
- 27.Reddy M. P., Sarla N., Siddiq E. A. Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica. 2002;128(1):9–17. doi: 10.1023/A:1020691618797. [DOI] [Google Scholar]
- 28.Semagn K., Rnstad B., Ndjiondjop M. N. An overview of molecular marker methods for plants. African Journal of Biotechnology. 2006;5:2540–2568. [Google Scholar]
- 29.Pestanana R. K. N., Amorim E. P., Ferreira C. F., et al. Agronomic and molecular characterization of gamma ray induced banana (Musa sp.) mutants using a multivariate statistical algorithm. Euphytica. 2011;178(2):151–158. doi: 10.1007/s10681-010-0329-2. [DOI] [Google Scholar]
- 30.Oražem P., Štajner N., Bohanec B. Effect of X-ray irradiation on olive shoot culture evaluated by morphological measurements, nuclear DNA content and SSR and AFLP markers. Trees—Structure and Function. 2013;27(6):1587–1595. doi: 10.1007/s00468-013-0906-9. [DOI] [Google Scholar]
- 31.Khatri A., Bibi S., Dahot M. U., Khan I. A., Nizamani G. S. In vitro mutagenesis in banana and variant screening through ISSR. Pakistan Journal of Botany. 2011;43:2427–2431. [Google Scholar]
- 32.Xi M., Sun L., Qiu S., Liu J., Xu J., Shi J. In vitro mutagenesis and identification of mutants via ISSR in lily (Lilium longiflorum) Plant Cell Reports. 2012;31(6):1043–1051. doi: 10.1007/s00299-011-1222-8. [DOI] [PubMed] [Google Scholar]
- 33.Sen A., Alikamanoglu S. Analysis of drought-tolerant sugar beet (Beta vulgaris L.) mutants induced with gamma radiation using SDS-PAGE and ISSR markers. Mutation Research—Fundamental and Molecular Mechanisms of Mutagenesis. 2012;738-739(1):38–44. doi: 10.1016/j.mrfmmm.2012.08.003. [DOI] [PubMed] [Google Scholar]


