Abstract
Introduction
Skin fragility and recurrent wounds are hallmarks of hereditary epidermolysis bullosa (EB). Treatment options to accelerate wound healing are urgently needed. Oleogel-S10 contains a betulin-rich triterpene extract from birch bark. In this study, we tested the wound healing properties of topical Oleogel-S10 in patients with dystrophic EB.
Methods
We conducted an open, blindly evaluated, controlled, prospective phase II pilot trial in patients with dystrophic EB (EudraCT number 2010-019945-24). Healing of wounds treated with and without topical Oleogel-S10 was compared. Primary efficacy variable was faster reepithelialization as determined by 2 blinded experts. The main secondary outcome variable of the study was percentage of wound epithelialization.
Results
Twelve wound pairs of 10 patients with dystrophic EB were evaluated. In 5 of 12 cases, both blinded reviewers considered epithelialization of the intervention wounds as superior. In 3 cases, only one reviewer considered Oleogel-S10 as superior and the other one as equal to control. Measurements of wound size showed a trend towards accelerated wound healing with the intervention but without reaching statistical significance.
Conclusion
Our results indicate a potential for faster reepithelialization of wounds in patients with dystrophic EB when treated with Oleogel-S10 but larger studies are needed to confirm significance.
1. Introduction
Epidermolysis bullosa (EB) comprises a heterogeneous group of inherited skin diseases characterized by skin fragility leading to recurrent wounds [1, 2]. Cutaneous and extracutaneous manifestations can result in severe morbidity and a reduced life expectancy [3–6]. Particularly in the generalized dystrophic EB subtypes, there is a significant risk of developing aggressive squamous cell carcinomas, with an increased incidence of metastases and death [7].
Up to now, no causal therapy in the sense of prevention of blister formation or improvement of skin stability is available. Treatment of EB mostly remains symptomatic [8] with optimal wound care and protection of the skin being the core therapeutic strategies. Wound care in these patients usually follows the “wound bed preparation model” [9–11], including the whole patient centered concerns [12], as well as local wound factors such as reduction of bacterial load and choice of optimal atraumatic dressings. Additional means to accelerate wound healing are urgently needed [13].
Birch (Betula species) is a medical plant. Its bark has been used as a natural remedy for skin diseases and wound care for centuries [14, 15]. Oleogel-S10 is a semisolid gel, containing 10% triterpene dry extract (TE) from Betulae cortex (birch bark) and refined sunflower oil (SFO) without the need for further excipients [16].
Both components have a low potential for allergic sensitization and seem therefore suitable for use on wounds. To our knowledge, over the course of 10 years, there have been 2 cases of contact sensitization towards the triterpene extract, one of them being published [17]. Both patients known to us showed only a local skin reaction. No anaphylaxis has been reported.
Refined SFO is also considered to have a very low potential for sensitization and is therefore often used as a moisturizer in little children [18]. In an older study by Halsey et al., SFO did not provoke a reaction when tested in sunflower seed allergic patients [19].
Triterpenes were shown to enhance epidermal barrier recovery and to stimulate wound healing [20–24]. In 2014, Ebeling et al. published results around the molecular mechanism of the effects of birch bark on keratinocytes [25]. The authors showed significantly accelerated reepithelialization in a porcine ex vivo wound healing model when treating the wound with Oleogel-S10 as compared to sunflower oil alone or the oil in combination with a gelling agent (ethyl cellulose). In the same model, this treatment led to an improvement of barrier regeneration. Various mediators involved in the inflammatory phase of wound healing were positively modulated, among them COX-2 and IL-6, the latter being known for playing an important role in wound healing and epidermal barrier repair [26, 27]. In addition, in vitro and in vivo studies suggest that betulin has anticarcinogenic properties and induces apoptosis in different tumor cells including human squamous cell carcinoma (SCC) cells [28–31].
The ideal formulation of Oleogel-S10 has further been examined by Steinbrenner et al. [32]. The group tested the effect of different oils on wound healing when used alone or in combination with TE. The majority of oils seemed to hinder wound healing to some extent, SFO being among the least impairing oils when used alone. However, when using SFO with TE as in Oleogel-S10, wound healing was significantly improved as compared to both sunflower oil (SFO) and SFO with ethyl cellulose for an improved viscosity.
The effects of Oleogel-S10 have already been investigated in vivo on different types of wounds where this treatment seemed to promote wound healing and was very well tolerated [33–35]. Additionally, a very recent randomized controlled trial found enhanced epithelialization of split-thickness skin graft donor site wounds after treatment with Oleogel-S10 [22]. In 2016, Oleogel-S10 was approved in the European Union as a new medicine for the treatment of partial thickness wounds in adults (http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/003938/human_med_001956.jsp).
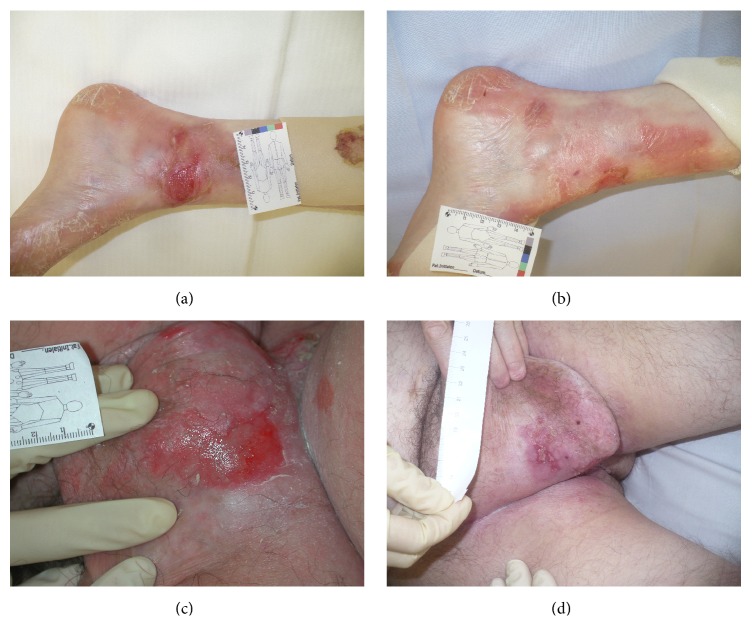
The results of these different reports were in keeping with our own experiences from using Oleogel-S10 for wound care of few individual patients suffering from different subtypes of EB outside of a study setting (Figure 1).
Figure 1.
Healing of chronic wound on the ankle of a 12-year-old suffering from RDEB after 5 weeks of treatment with Oleogel-S10 (a-b). Healing of chronic wound in the groin of a 50-year-old male after only 6 days of treatment with Oleogel-S10 (c-d).
In the present study, we investigated the effect of Oleogel-S10 on wound healing in patients with dystrophic EB. We hypothesized that Oleogel-S10 in combination with standard nonadhesive wound dressings would lead to faster wound healing as opposed to the use of standard wound care alone. Furthermore, we assessed the feasibility of conducting a larger trial in this patient group.
2. Methods
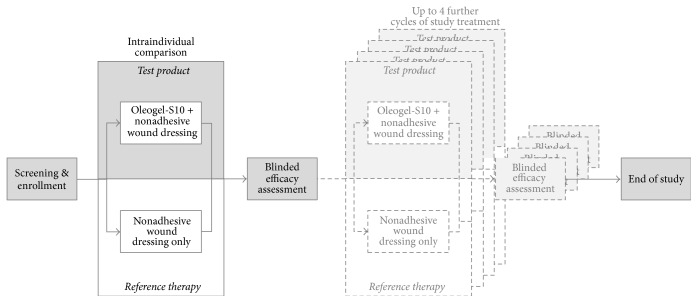
This study was designed as an open-label, prospective, controlled, blindly evaluated, monocentric phase II pilot trial to compare intraindividually the efficacy and tolerance of Oleogel-S10 in combination with nonadhesive wound dressing versus nonadhesive wound dressing alone (Figure 2). The intraindividual comparison was chosen to minimize the influence of age, health status, medications, and other potentially confounding factors.
Figure 2.
Study design.
The study (EudraCT number 2010-019945-24) was conducted in compliance with IEC, informed consent regulations, and ICH and GCP guidelines and was approved by the ethics committee of the University of Freiburg.
2.1. Patient Eligibility Criteria
Patients of any age beyond infancy and with an immunochemically and/or genetically proven diagnosis of hereditary EB and at least 1 wound between 10 cm2 and 200 cm2 (alternatively 2 comparable lesions of at least 5 cm2 each) were recruited for this study. We differentiated wounds that had appeared less than 6 weeks earlier and those with no tendency to heal for at least 6 weeks. Children were deliberately included in this study, as they represent a large proportion of our patients and optimized wound healing would be of particular interest to them.
Patients were excluded from this study if they had been on a systemic treatment with corticosteroids within the last 30 days or if they had taken any investigational drugs within 3 months before screening. They were also excluded if they suffered from uncontrolled diabetes mellitus, diabetic ulcers, or other diseases or conditions that could interfere with the assessment of safety, tolerance, or efficacy of Oleogel-S10 during the study.
Patients or their legal representatives had to provide written informed consent before participation in the study. For this proof-of-concept study, we aimed to include 10 patients.
2.2. Intervention
Patients of all ages presenting at the EB-Centre Freiburg for regular follow-up visits were screened according to the study protocol. If the investigator identified a skin lesion (10 cm2 to 200 cm2) eligible for study treatment, it was divided into two halves which were then allocated to either the intervention or the treatment arm. This method ensured a comparison between wound areas in a similar anatomic location and within identical wound healing phases. Alternatively, if there were two comparable lesions (≥5 cm2 each) of similar size and shape, these were selected and considered one wound pair. Wounds located at sites of major trauma (e.g., elbows, knees, and buttocks) and circumferential wounds were not included.
One half of the wound was chosen at random to be treated with Oleogel-S10 (applied approximately at 1 mm thickness) and covered by a nonadhesive wound dressing (Mepilex Transfer®, Mölnlycke Health Care, Sweden); the unwounded skin next to this half as well as the border between the wound halves was marked to ensure that Oleogel-S10 was always applied to the same part of the wound. In other prior studies, it had been shown that the product did not spread to other areas than the ones it was applied to. Each wound half was measured and the data were entered into a wound surface-measuring program developed at our wound care clinic (University of Freiburg, Department of Dermatology) to ensure a comparable initial wound size. To serve as control, the other half of the lesion was covered with a nonadhesive wound dressing only (Mepilex Transfer, reference therapy). If the investigator had identified two comparable wounds, these were treated correspondingly. The intervention and control treatment were applied in an open-label fashion.
Dressings were changed based on the study flow chart every 24 to 48 hours until the end of treatment at day 14. In case of delayed wound healing (wound present > 6 weeks at initiation of study treatment), the treatment period was prolonged until day 28.
2.3. Outcome Assessment
Before the start of treatment and at every dressing change, photographs of the respective wounds were taken. We used a Canon camera, EOS Digital SLR series, and our in-house wound measuring system, which allowed outer tracing of wound borders and calculation of wound surface.
The images were uploaded into the electronic Case Report Form (eCRF), cropped to remove pen markings at the treatment side, and coded for blinding. The (open) wound surface area (in cm2) was recorded on day 0 and at each dressing change until the end of the treatment period at day 14/day 28. These data enabled us to calculate the percentage of reepithelialization. Two independent experts evaluated treatment efficacy in a blinded fashion based on comparing chronological series of cropped wound photographs taken before the start of treatment, during wound dressing changes, and at the end of treatment on day 14/day 28 and choosing the series with signs of better reepithelialization. Remote photographic analysis has been shown to be a reliable method of wound evaluation [36].
The degree of reepithelialization was calculated as the ratio of reepithelialized wound area relative to the initial wound area.
At each dressing change, severity of touch sensitivity and itch of each treatment area were examined and rated on a visual analog scale from 1 to 10. Accordingly, patients or their legal representatives were asked to provide feedback on tolerability and efficacy of the intervention and control treatment. The amount of exudate was rated as low, middle, or strong.
2.4. Primary Outcome
All wound photographs were summarized within chronological series of even orientation and size by patient and wound. The 2 blinded experts were asked to evaluate reepithelialization of the wound pairs for each series and to decide which part of the wound reepithelialized faster than the other or if there was an equal degree of reepithelialization in both parts of the wound pair.
2.5. Secondary Outcomes
Secondary efficacy variables of the study were percentage of wound epithelialization as measured and documented at each dressing change. The degree of reepithelialization was calculated for each wound as the ratio of reepithelialized area to initial open wound area. Wounds were considered closed if they were at least 95% epithelialized.
Additionally, we evaluated the extent of touch sensitivity, itch, exudation, and the assessment of efficacy and tolerance given by the investigators and patients or their legal representatives.
Secondary safety variables of the study were all adverse events, treatment-related or not, graded according to the NCI-CTC grading system on a 4-point scale (mild, moderate, severe, and life-threatening) and assessment of tolerability by the investigators and patients and/or their legal representatives.
2.6. Statistical Considerations
A sample size calculation was not performed, as the planned number of patients eligible for enrollment into this pilot study was limited. Statistical analyses on the primary outcome parameter of faster reepithelialization were performed using a two-sided exact binomial test with a significance level of p = 0.05 against the null hypothesis of no difference between treatments. For the secondary outcome variable of the amount of reepithelialization, we applied Wilcoxon signed-rank test.
3. Results
3.1. Patient Data
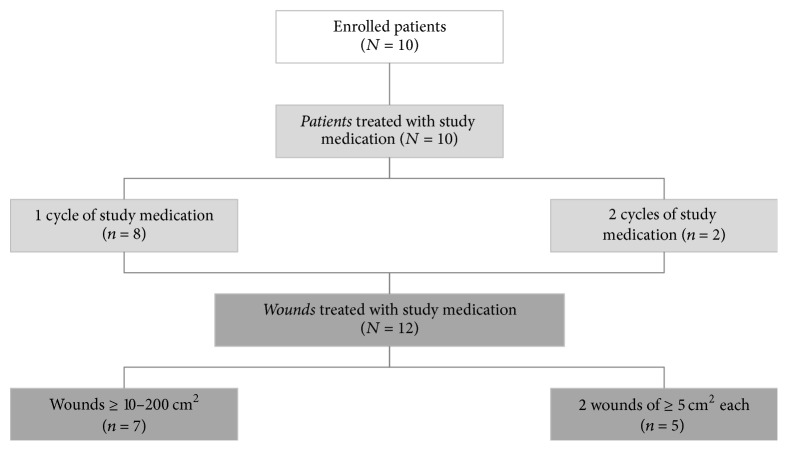
10 patients were enrolled in the study. As 2 patients participated in a second cycle of treatment, 12 wound pairs were evaluated. None of the patients was lost to follow-up or dropped out (Figure 3): all patients who qualified for the inclusion criteria suffered from dystrophic EB, 9 patients had recessive dystrophic EB (RDEB), one had intermediate generalized RDEB, 8 had severe generalized RDEB, and 1 had localized dominant dystrophic EB. Expression of collagen VII and/or mutations of COL7A1 were determined as summarized in Table 1. The median age of patients was 20 years (range: 6–48 years). No infant screened during the study period showed large enough wounds enabling participation.
Figure 3.
Disposition of patients.
Table 1.
Patient data.
| Number | Age (years) | Gender | Type of EB | Collagen VII staining (IFM) |
Mutation cDNA |
Mutation protein | Skin involvement |
|---|---|---|---|---|---|---|---|
| 1 | 6 | M | RDEB (SG) |
Negative | c.3141delG homozygous |
p.Cys1048 Alafs∗61 |
Generalized blistering, scarring, mutilations |
| 2 | 10 | F | RDEB (SG) |
Not done | c.4590delA homozygous |
p.Gly1531 Glufs∗179 |
Generalized blistering, scarring, mutilations |
| 3 | 16 | F | RDEB (SG) |
Not done | c.4590delA homozygous |
p.Gly1531 Glufs∗179 |
Generalized blistering, scarring, mutilations |
| 4 | 9 | M | RDEB (SG) |
Not done | c.1934delC homozygous |
p.Pro645 Glnfs∗45 |
Generalized blistering, scarring, ++pseudo synechiae |
| 5 | 36 | M | RDEB (SG) |
Negative | 425A>G homozygous |
p.? Altered splicing |
Generalized blistering, scarring, mutilations |
| 6 | 47 | F | RDEB (GI) |
Reduced | c.[3832-2A>G]; [4039G>T] |
p.[?]; [G1347W] |
Generalized blistering, scarring, mild proximal pseudo synechiae |
| 7 | 21 | M | DDEB (loc.) |
Normal | c.7868G>T | p.Gly2623Val | Blistering on lower legs, nail dystrophy |
| 8 | 28 | M | RDEB (SG) |
Not done | Not done | Not done | Generalized blistering, scarring, mutilations |
| 9 | 20 | M | RDEB (SG) |
Not done | c.[2212_2215dup]; [7621C>T] |
p.Glu739 GlyfsX2 |
Generalized blistering, scarring, mutilations |
| 10 | 17 | M | RDEB (SG) |
Negative | c.[425A>G]; [1837C>T] |
p.[?]; [Arg613X] | Generalized blistering, scarring, mutilations |
EB: epidermolysis bullosa; DDEB: dominant dystrophic EB; RDEB: recessive dystrophic EB; SG: severe generalized; GI: generalized intermediate; loc.: localized; IFM: immunofluorescence mapping.
3.2. Wounds
Nine of 12 wound pairs (75.0%) analyzed in the study were present in less than 6 weeks. 3 of 12 wound pairs (25%) were chronic, showing no tendency to heal for more than 6 weeks. The mean wound size was 17.5 cm2 in the intervention group (7.3–45 cm2) versus 17.7 cm2 in the control group (6.3–45 cm2). In 7 cases, large wounds were chosen and divided into two halves for comparative treatment. In the other 5 cases, 2 comparable wounds were identified, where one was treated with Oleogel-S10 and the other with nonadhesive wound dressing alone (Table 2). All patients followed the study protocol except for one patient, whose wound was additionally treated with an antiseptic gel (polyhexanide 0.04%, Lavasept®) on both areas for the duration of 3 dressing changes towards the end of the study due to a suspected superinfection.
Table 2.
Wound characteristics.
| Patient data | Wound characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Number | Age (years) | Gender | Type of EB | Wound number |
Type | Location | Age of wound |
| 1 | 6 | M | RDEB (SG) |
1 | Large divided | Lower extremity | Recent |
| 2 | 10 | F | RDEB (SG) |
1 | Large divided | Lower extremity | Recent |
| 3 | 16 | F | RDEB (SG) |
1 | Large divided | Upper extremity | Recent |
| 4 | 9 | M | RDEB (SG) |
1 | Large divided | Lower extremity | Chronic |
| 5 | 36 | M | RDEB (SG) |
1 | Large divided | Upper extremity | Recent |
| 6 | 47 | F | RDEB (GI) |
1 | Separate | Trunk | Chronic |
| 7 | 21 | M | DDEB (loc.) |
1 | Large divided | Lower extremity | Recent |
| 8 | 28 | M | RDEB (SG) |
1 | Separate | Trunk | Chronic |
| 9 | 20 | M | RDEB (SG) |
1 | Separate | Trunk | Recent |
| 9 | 20 | M | RDEB (SG) |
2 | Separate | Trunk | Recent |
| 10 | 17 | M | RDEB (SG) |
1 | Large divided | Trunk | Recent |
| 10 | 17 | M | RDEB (SG) |
2 | Large divided | Lower extremity | Recent |
EB: epidermolysis bullosa; DDEB: dominant dystrophic EB; RDEB: recessive dystrophic EB; SG: severe generalized; GI: generalized intermediate; loc.: localized; recent: less than 6 weeks; chronic: longer than 6 weeks.
3.3. Reepithelialization
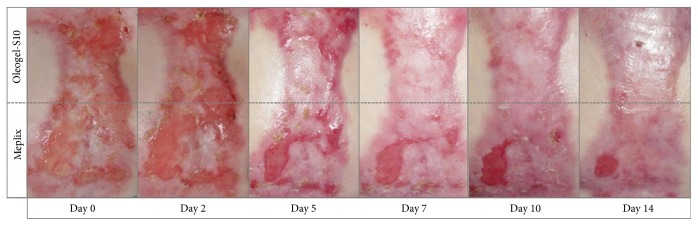
In 5 of 12 cases (41%), the intervention wound was rated as better epithelialized unanimously by both independent reviewers. In another 3 of 12 cases, 1 reviewer rated epithelialization of the intervention wound as superior whereas the other expert considered the wounds as healing equally well. In the remaining 4 cases, either epithelialization was considered as equal or the result was controversial (Table 3). Thus, the comparison of unanimous “winners” was 5 versus 0 (p = 0.063, binomial test) in favor of Oleogel-S10 compared to standard-of-care treated wound halves.
Table 3.
Results of blinded efficacy evaluation by patient and wound.
| Number | Reviewer 1 | Reviewer 2 | Unanimous “winner” | |
|---|---|---|---|---|
| Oleogel-S10 | Wound dressing | |||
| 1 | Control | Oleogel-S10 | — | — |
| 2 | Oleogel-S10 | Equal | — | — |
| 3 | Oleogel-S10 | Oleogel-S10 | 1 | — |
| 4 | Oleogel-S10 | Equal | — | — |
| 5 | Equal | Equal | — | — |
| 6 | Oleogel-S10 | Oleogel-S10 | 1 | — |
| 7 | Equal | Equal | — | — |
| 8 | Oleogel-S10 | Control | — | — |
| 9 (1) | Oleogel-S10 | Oleogel-S10 | 1 | — |
| 9 (2) | Equal | Oleogel-S10 | — | — |
| 10 (1) | Oleogel-S10 | Oleogel-S10 | 1 | — |
| 10 (2) | Oleogel-S10 | Oleogel-S10 | 1 | — |
Control = nonadhesive wound dressing.
3.4. Secondary Outcomes
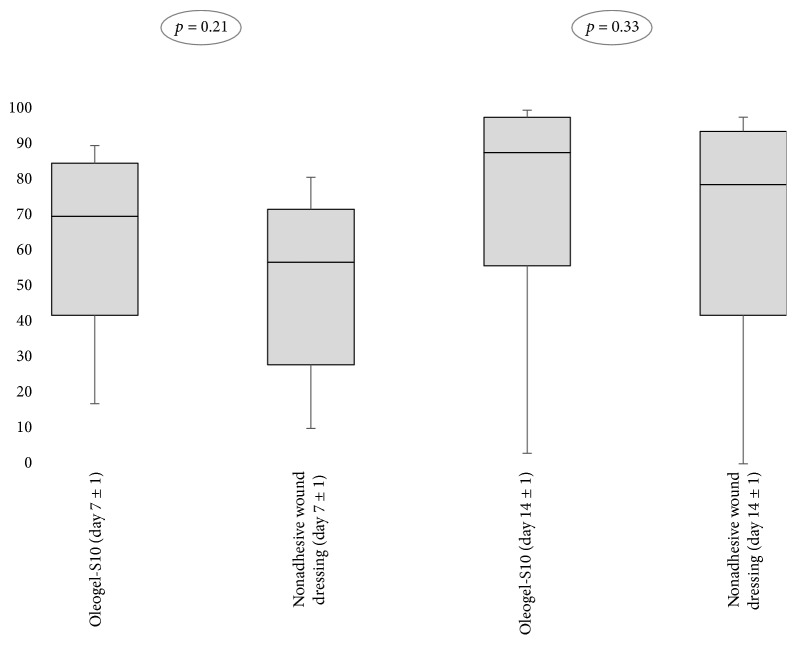
Measurement of wound sizes suggested a trend towards faster closure of wounds that had been treated with Oleogel-S10 both on day 7 and on day 14. On day 7 (±1), the median epithelialization of the initial wound surface was 69.7% (intervention) versus 57.4% (control) and on day 14 was 87.7% (intervention) versus 79.2% (control) (p = 0.21 on day 7; p = 0.33 on day 14, Wilcoxon test, Figure 4).
Figure 4.
Percentage of wound area with a newly formed epithelial layer on day 7 ± 1 and day 14 ± 1.
Wound closure with at least 95% epithelialization was reached in 5 of 12 wounds in the Oleogel-S10 group versus 2 of 12 in the control group. The mean time for wound closure was 10.5 days in the intervention group versus 14 days in the control group (Figure 5).
Figure 5.
Complete wound closure of the intervention wound at day 13 of treatment.
As can be expected in EB patients, we experienced retraumatization of wounds with an increase of wound size ≥5% compared with the preceding measurement in 7 of 12 wound pairs. In 3 wound pairs, it occurred on both sides, the intervention and the control wound. Additionally, it occurred in another 3 intervention and 1 control wound halves. As this made the evaluation of reepithelialization more difficult, we looked at the epithelialization of all wounds at one follow-up prior to retrauma and compared the mean percentage of epithelialization at this point, which was 78% for Oleogel-S10 versus 73% for the control. There was an advance of epithelialization by ≥10% in 5 of 12 cases for Oleogel-S10 as opposed to 2 of 12 cases for the control.
Patients' assessment of tolerability of Oleogel-S10 and standard treatment was considered as good (97.4%) or acceptable (2.6%) in the intervention and as good in 100% of the control group. Patients reported treatment with Oleogel-S10 more frequently as efficient (75.3%) than with standard dressings alone (53.3%). The application of Oleogel-S10 was easy. The substance was tolerated very well without any complaints of stinging or burning.
We did not find any relevant differences in the perception of touch sensitivity, itch, and amount of exudate between intervention and control arms.
4. Discussion
In this phase II pilot study, we investigated the effects of Oleogel-S10 on wounds of patients suffering from dystrophic EB. Both the evaluation of two blinded experts and the percentage of epithelialization measured by the investigators were consistent and suggested a trend towards faster healing of wounds when treated with Oleogel-S10 combined with nonadhesive dressings versus standard wound care with the dressings alone. The number of healed wounds (≥95% epithelialization) was higher and the mean time needed for wound closure was shorter in the intervention than in the control group. Likely owing to the relatively low number of 10 patients, however, these results did not reach statistical significance.
The results of this pilot trial showed feasibility of study procedures and call for larger randomized controlled trials to confirm the promising effects of Oleogel-S10 application in EB wounds.
Recruitment of patients was achieved within the scheduled period and patients were compliant, cooperative, and motivated to fulfil this study. Also, the duration of the study between 2 and 4 weeks was feasible for all patients. None of them dropped out or was lost to follow-up, which proved the good tolerability of the product and the great need for new therapeutic measures to treat wounds in patients with EB. However, healing of large wounds exceeded the anticipated 4 weeks, which led to a rather low number of fully epithelialized wounds after the completed study period of 2 and 4 weeks.
Tolerability of the study medication was very good. We did not experience any local or generalized irritability or contact sensitization towards either of the two components of Oleogel-S10. Certainly, any medically active ingredient has the potential for allergic sensitization, especially when applied onto open wounds. Therefore, in patients with known sunflower seed allergy, Oleogel-S10 should be used with caution.
According to the study protocol, we included the first 10 patients willing to participate in the study. As the majority of patients who present at our center suffer from dystrophic EB, we included only this EB subtype in our study. This allowed a more homogenous study population sharing characteristic traits of this rare disorder. However, it is possible that our results are not applicable to other EB types.
Dystrophic EB causes major morbidity for the affected individuals leading to multiple secondary complications such as anemia and deficiencies of iron, zinc, vitamins, and micronutrients that impair wound healing. Therefore, the intraindividual control, comparing wounds or wound halves of the same individual, was a suitable approach, eliminating confounding factors.
When planning the study, we chose to compare Oleogel-S10 to standard treatment instead of using the vehicle, respectively, placebo, as control. Apart from betulin extract, the only other ingredient of Oleogel-S10 is sunflower oil (SFO) which, other than Oleogel-S10, does not stay in the allocated wound half due to its liquid consistency. Also, in recent studies, a significantly better effect on wound healing of Oleogel-S10 as opposed to both SFO and SFO with ethyl cellulose has been demonstrated [32]. The use of pure SFO has, as have the large majority of pure oils, even shown a mild negative effect on wound healing as opposed to control making pure SFO a less promising candidate as control than standard wound care.
5. Conclusion
Oleogel-S10 is a topical gel made of pure sunflower oil and a 10% triterpene extract from birch bark. We used Oleogel-S10 on acute and chronic wounds (>6 weeks) in 10 patients with dystrophic EB.
The results of the present study were promising and provided reason for further investigations of the effects of Oleogel-S10. Larger studies using Oleogel-S10 for wounds of patients with all different forms of EB are needed to clarify the effectiveness and safety in EB in general.
Concurrent to additional clinical studies with Oleogel-S10, further insights into the anticarcinogenic effects of betulin on human SCC cells, ideally coming from EB patients, are needed. The anticarcinogenic effect, if proven to be real, in combination with wound healing effects would make Oleogel-S10 an ideal substance to use in EB wounds at early stages, especially in severe generalized RDEB, the subtype with the highest number of aggressive and metastasizing SCCs.
The above-mentioned additional studies would help to identify the optimal indications for use of Oleogel-S10 and to clarify whether it can stand up to its promises.
Acknowledgments
This study was sponsored by Birken AG, Niefern-Öschelbronn. The company also provided the study formulation. The authors are grateful to Sandra Löwe who helped with the medical writing, Regina Eickhoff from Alcedis, Rolf Fimmers (statistical advice), and the two blinded reviewers, Oliver Rennekampff und Peter Vogt (MHH). And finally, thanks are due to the patients without whom this study would not have been possible. This study (EudraCT no. 2010-019945-24) was conducted in compliance with IEC, informed consent regulations, and ICH and GCP guidelines and was approved by the ethics committee of the University of Freiburg.
Abbreviations
- EB:
Epidermolysis bullosa
- RDEB:
Recessive dystrophic EB
- DDEB:
Dominant dystrophic EB
- SG:
Severe generalized
- GI:
Generalized intermediate
- TE:
Triterpene extract.
Conflicts of Interest
After completion of the study, A. Schwieger-Briel has functioned as blinded expert for further studies with the test substance and has been a medical advisor for an EMA hearing in London. C. Schempp has conducted studies in cooperation with Birken GmbH. He and H. Schumann were consultants of Birken GmbH. C. Has and D. Kiritsi have no conflicts of interest to report.
References
- 1.Fine J.-D., Bruckner-Tuderman L., Eady R. A. J., et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. Journal of the American Academy of Dermatology. 2014;70(6):1103–1126. doi: 10.1016/j.jaad.2014.01.903. [DOI] [PubMed] [Google Scholar]
- 2.Has C., Bruckner-Tuderman L. The genetics of skin fragility. Annual Review of Genomics and Human Genetics. 2014;15:245–268. doi: 10.1146/annurev-genom-090413-025540. [DOI] [PubMed] [Google Scholar]
- 3.Bolling M. C., Veenstra M. J., Jonkman M. F., et al. Lethal acantholytic epidermolysis bullosa due to a novel homozygous deletion in DSP: Expanding the phenotype and implications for desmoplakin function in skin and heart. British Journal of Dermatology. 2010;162(6):1388–1394. doi: 10.1111/j.1365-2133.2010.09668.x. [DOI] [PubMed] [Google Scholar]
- 4.Yuen W. Y., Duipmans J. C., Molenbuur B., Herpertz I., Mandema J. M., Jonkman M. F. Long-term follow-up of patients with Herlitz-type junctional epidermolysis bullosa. British Journal of Dermatology. 2012;167(2):374–382. doi: 10.1111/j.1365-2133.2012.10997.x. [DOI] [PubMed] [Google Scholar]
- 5.Pigors M., Kiritsi D., Krümpelmann S., et al. Lack of plakoglobin leads to lethal congenital epidermolysis bullosa: a novel clinico-genetic entity. Human Molecular Genetics. 2011;20(9):1811–1819. doi: 10.1093/hmg/ddr064.ddr064 [DOI] [PubMed] [Google Scholar]
- 6.Has C., Spartà G., Kiritsi D., et al. Integrin α 3 mutations with kidney, lung, and skin disease. New England Journal of Medicine. 2012;366(16):1508–1514. doi: 10.1056/NEJMoa1110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fine J.-D., Johnson L. B., Weiner M., Li K.-P., Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986–2006. Journal of the American Academy of Dermatology. 2009;60(2):203–211. doi: 10.1016/j.jaad.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Hsu C.-K., Wang S.-P., Lee J. Y.-Y., McGrath J. A. Treatment of hereditary epidermolysis bullosa: updates and future prospects. American Journal of Clinical Dermatology. 2014;15(1):1–6. doi: 10.1007/s40257-013-0059-z. [DOI] [PubMed] [Google Scholar]
- 9.Sibbald R. G., Goodman L., Woo et al. K. Y. Special considerations in wound bed preparation: an update. Advances in Skin and Wound Care. 2011;24(9):415–436. doi: 10.1097/01.ASW.0000405216.27050.97. [DOI] [PubMed] [Google Scholar]
- 10.Pope E., Lara-Corrales I., Mellerio J., et al. A consensus approach to wound care in epidermolysis bullosa. Journal of the American Academy of Dermatology. 2012;67(5):904–917. doi: 10.1016/j.jaad.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope E., Lara-Corrales I., Mellerio J. E., Martinez A. E., Sibbald C., Sibbald R. G. Epidermolysis bullosa and chronic wounds: a model for Wound Bed Preparation of fragile skin. Advances in Skin and Wound Care. 2013;26(4):177–188. doi: 10.1097/01.ASW.0000428864.72412.b7. [DOI] [PubMed] [Google Scholar]
- 12.Denyer J. E. Wound management for children with epidermolysis bullosa. Dermatologic Clinics. 2010;28(2):257–264. doi: 10.1016/j.det.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Davila-Seijo P., Hernández-Martín Á., Morcillo-Makow E., Rajan C., García-Doval I. Current dystrophic epidermolysis bullosa research does not match research needs perceived by patients and clinicians. Journal of the American Academy of Dermatology. 2014;71(5):1008–1011. doi: 10.1016/j.jaad.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Hiller K., Melzig M. F. Lexikon der Arzneipflanzen und Drogen. 2nd. Heidelberg, Germany: Spektrum Akademischer; 2010. [DOI] [Google Scholar]
- 15.Krasutsky P. A. Birch bark research and development. Natural Product Reports. 2006;23(6):919–942. doi: 10.1039/b606816b. [DOI] [PubMed] [Google Scholar]
- 16.Laszczyk M., Jäger S., Simon-Haarhaus B., Scheffler A., Schempp C. M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (Betulae cortex) Planta Medica. 2006;72(15):1389–1395. doi: 10.1055/s-2006-951723. [DOI] [PubMed] [Google Scholar]
- 17.Meyer-Hoffert U., Brasch J. Allergic contact dermatitis caused by betulin-containing triterpene extract from the outer bark of birch (Betula alba) Contact Dermatitis. 2013;68(6):382–383. doi: 10.1111/cod.12048. [DOI] [PubMed] [Google Scholar]
- 18.Kanti V., Günther M., Stroux A., et al. Influence of sunflower seed oil or baby lotion on the skin barrier function of newborns: a pilot study. Journal of Cosmetic Dermatology. 2017 doi: 10.1111/jocd.12302. [DOI] [PubMed] [Google Scholar]
- 19.Halsey A. B., Martin M. E., Ruff M. E., Jacobs F. O., Jacobs R. L. Sunflower oil is not allergenic to sunflower seed-sensitive patients. The Journal of Allergy and Clinical Immunology. 1986;78(3):408–410. doi: 10.1016/0091-6749(86)90025-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee H. K., Nam G. W., Kim S. H., Lee S. H. Phytocomponents of triterpenoids, oleanolic acid and ursolic acid, regulated differently the processing of epidermal keratinocytes via PPAR-α pathway. Experimental Dermatology. 2006;15(1):66–73. doi: 10.1111/j.0906-6705.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 21.Harish B. G., Krishna V., Santosh Kumar H. S., Khadeer Ahamed B. M., Sharath R., Kumara Swamy H. M. Wound healing activity and docking of glycogen-synthase-kinase-3-β-protein with isolated triterpenoid lupeol in rats. Phytomedicine. 2008;15(9):763–767. doi: 10.1016/j.phymed.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 22.Metelmann H.-R., Brandner J. M., Schumann H., et al. Accelerated reepithelialization by triterpenes: proof of concept in the healing of surgical skin lesions. Skin Pharmacology and Physiology. 2015;28(1):1–11. doi: 10.1159/000357501. [DOI] [PubMed] [Google Scholar]
- 23.Agra L. C., Ferro J. N. S., Barbosa F. T., Barreto E. Triterpenes with healing activity: A systematic review. Journal of Dermatological Treatment. 2015;26(5):465–470. doi: 10.3109/09546634.2015.1021663. [DOI] [PubMed] [Google Scholar]
- 24.Woelfle U., Laszczyk M. N., Kraus M., et al. Triterpenes promote keratinocyte differentiation in vitro, Ex vivo and in vivo: a role for the transient receptor potential canonical (subtype) 6. Journal of Investigative Dermatology. 2010;130(1):113–123. doi: 10.1038/jid.2009.248. [DOI] [PubMed] [Google Scholar]
- 25.Ebeling S., Naumann K., Pollok S., et al. From a traditional medicinal plant to a rational drug: understanding the clinically proven wound healing efficacy of birch bark extract. PLoS ONE. 2014;9(1) doi: 10.1371/journal.pone.0086147.e86147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Z.-Q., Kondo T., Ishida Y., Takayasu T., Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. Journal of Leukocyte Biology. 2003;73(6):713–721. doi: 10.1189/jlb.0802397. [DOI] [PubMed] [Google Scholar]
- 27.Gallucci R. M., Simeonova P. P., Matheson et al. J. M. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. The FASEB Journal. 2000;14(15):2525–2531. doi: 10.1096/fj.00-0073com. [DOI] [PubMed] [Google Scholar]
- 28.Kessler J. H., Mullauer F. B., de Roo G. M., Medema J. P. Broad in vitro efficacy of plant-derived betulinic acid against cell lines derived from the most prevalent human cancer types. Cancer Letters. 2007;251(1):132–145. doi: 10.1016/j.canlet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 29.Thurnher D., Turhani D., Pelzmann M., et al. Betulinic acid: a new cytotoxic compound against malignant head and neck cancer cells. Head and Neck. 2003;25(9):732–740. doi: 10.1002/hed.10231. [DOI] [PubMed] [Google Scholar]
- 30.Król S. K., Kiełbus M., Rivero-Müller A., Stepulak A. Comprehensive review on betulin as a potent anticancer agent. BioMed Research International. 2015;2015:2015. doi: 10.1155/2015/584189.584189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drag-Zalesińska M., Wysocka T., Borska S., et al. The new esters derivatives of betulin and betulinic acid in epidermoid squamous carcinoma treatment - in vitro studies. Biomedicine and Pharmacotherapy. 2015;72:91–97. doi: 10.1016/j.biopha.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Steinbrenner I., Houdek P., Pollok S., Brandner J. M., Daniels R. Influence of the oil phase and topical formulation on the wound healing ability of a birch bark dry extract. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0155582.e0155582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schempp C., Huyke C. Behandlung von Verbrennungen 2. Grades mit Birkencreme. Der Merkurstab. 2005;5:p. 402. [Google Scholar]
- 34.Weckesser S., Laszczyk M. N., Müller M. L., Schempp C. M., Schumann H. Topical treatment of necrotising herpes zoster with betulin from birch bark. Forschende Komplementarmedizin. 2010;17(5):271–273. doi: 10.1159/000320592. [DOI] [PubMed] [Google Scholar]
- 35.Metelmann H. R., Brandner J., Schumann H., Bross F., Hoffmann M., Podmelle F. Accelerating the aesthetic benefit of wound healing by triterpene. Journal of Cranio-Maxillofacial Surgery. 2012;40(5):e150–e154. doi: 10.1016/j.jcms.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Rennekampff H.-O., Fimmers R., Metelmann H.-R., Schumann H., Tenenhaus M. Reliability of photographic analysis of wound epithelialization assessed in human skin graft donor sites and epidermolysis bullosa wounds. Trials. 2015;16(1, article 235) doi: 10.1186/s13063-015-0742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]