Abstract
Background:
The contribution of cardiovascular dysfunction to frailty in older adults is uncertain. This study aimed to define the relationship between frailty and cardiovascular structure and function, and determine whether these associations are independent of coexisting abnormalities in other organ systems.
Methods:
We studied 3,991 older adults (mean age 75.6±5.0 years; 59% female) from the Atherosclerosis Risk in Communities (ARIC) Study in whom the following six organ systems were uniformly assessed: cardiac (by echocardiography), vascular (by ankle-brachial-index and pulse-wave-velocity), pulmonary (by spirometry), renal (by estimated glomerular filtration rate), hematologic (by hemoglobin), and adipose (by body mass index and bioimpedance). Frailty was defined by the presence of ≥3 of the following: low strength, low energy, slowed motor performance, low physical activity, or unintentional weight loss.
Results:
Two hundred eleven (5.3%) participants were frail. In multivariable analyses adjusted for demographics, diabetes, hypertension, and measures of other organ system function, frailty was independently and additively associated with left ventricular hypertrophy (odds ratio [OR] = 1.72; 95% confidence interval [CI] = 1.30–2.40), reduced global longitudinal strain (reflecting systolic function; OR = 1.68; 95% CI = 1.16–2.44), and greater left atrial volume index (reflecting diastolic function; OR = 1.60; 95% CI = 1.13–2.27), which together demonstrated the greatest association with frailty (OR = 2.10; 95% CI = 1.57–2.82) of the systems studied. Lower magnitude associations were observed for vascular and pulmonary abnormalities, anemia, and impaired renal function. Cardiovascular abnormalities remained associated with frailty after excluding participants with prevalent cardiovascular disease.
Conclusions:
Abnormalities of cardiac structure and function are independently associated with frailty, and together show the greatest association with frailty among the organ systems studied.
Keywords: Frailty, Echocardiography, Systolic function, Elderly
Frailty is a state of increased vulnerability to stressors resulting from multisystem dysregulation that accompanies aging (1–3), and is associated with a higher risk of impaired physical functioning, institutionalization, and mortality among older adults (1,2). Cardiovascular diseases are associated with a higher prevalence of, and an increased risk for, frailty (4,5). Cardiovascular dysfunction may therefore play an important role in the development of frailty. However, the extent to which alterations in cardiovascular structure and function relate to frailty remains unclear. Higher left ventricular (LV) mass and surrogates of atherosclerosis have been related to frailty in some populations (6–8) but not in others (9–11), while studies using conventional echocardiography have not consistently observed worse LV function in frail individuals (6,10,12). Furthermore, impairments in multiple other organ systems are related to frailty, including the pulmonary, renal, and hematopoietic systems, and adiposity (13–15). Given that multiple comorbidities often coexist and overlap in older adults, it is unknown whether cardiovascular dysfunction is associated with frailty after accounting for coexisting alterations in other organ systems. This study used cross-sectional data from a large community-based sample of older adults in the Atherosclerosis Risk in Communities (ARIC) study at the fifth study visit to address the hypothesis that abnormalities of cardiovascular structure and function are associated with frailty in older adults independent of coexisting abnormalities in the pulmonary, renal, hematologic, and adipose systems.
Methods
Study Population
The study design and procedures of the ARIC study have been previously described in detail (16). ARIC is an ongoing, prospective observational study that originally enrolled 15,792 participants aged 45–64 years recruited between 1987 and 1989 (visit 1) from four communities in the United States: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland. In this analysis, 6,538 surviving participants attending Visit 5 (2011–2013) were evaluated. We excluded those whose race was neither black nor white (n = 18) or with missing data at Visit 5 for echocardiography (n = 491), ankle-brachial index (ABI; n = 457), pulse wave velocity (PWV; n = 541), or spirometry (n = 1,040), resulting in 3,991 individuals included in the present analysis. Institutional review boards from each site approved the study, and informed consent was obtained from all participants.
Definition of Frailty
Frailty was defined using a validated measure (17), that was based on available data and previous work in other cohorts (1,6,18), and consisted of five binary criteria: (a) Low energy expenditure, based on gender-specific 20th percentile rank of the Baecke leisure sports activity index; (b) Low walking speed, based on the bottom gender- and height-specific quintile of time to walk 4 m; (c) Lack of energy (exhaustion), defined as present if the participant responded “some of the time” or “most of the time” to either of the following questions: “I felt everything I did was an effort” or “I could not get going”; (d) Low grip strength: based on the bottom gender- and body mass index-specific grip strength quintile; (e) Unintentional weight loss, defined as 10% unintentional weight loss between Visits 4 (1996–1998) and 5 or body mass index <18.5kg/m2 at Visit 5. Frailty was defined by the presence of at least three of these criteria.
Assessment of Cardiovascular Parameters
The echocardiographic protocol, including reproducibility metrics, has been previously described in detail (19). All studies were digitally acquired on Philips IE33 machines and quantitative analysis was performed by a central echocardiography reading center that was blinded to clinical information. Cutpoints for defining abnormal values for echocardiographic measures were based on sex-specific 95th percentile limits derived from a subgroup of healthy participants who underwent echocardiography at ARIC Visit 5 and had no prevalent cardiac disease (prior heart failure hospitalization or self-report; coronary heart disease, including history of myocardial infarction or regional wall motion abnormality on echocardiography; atrial fibrillation; or moderate or greater valvular disease) or cardiovascular risk factors (hypertension, active smoking, diabetes, body mass index ≥30 or <18.5kg/m2, QRS duration ≥120 msec, or estimated glomerular filtration rate <60mL/min/1.73 m2 at Visit 5). LV mass was indexed to body surface area and LV hypertrophy was defined as LV mass index >105g/m2 in men and >89g/m2 in women. Left atrial volume was indexed to body surface area and was considered abnormal if >34mL/m2 in men and >32mL/m2 in women. Septal E/E′ was considered abnormal if >15 in men and >17 in women. LV ejection fraction was considered abnormal if <59% in men and <58% in women. Global longitudinal strain (GLS) was derived from speckle-tracking echocardiography and was considered abnormal if >−15% in men and women.
Carotid-femoral PWV was measured using a ColinVP-1000 plus system (Omron Co., Komaki, Japan) and was considered abnormal if >1,300cm/s (20). ABI was considered abnormal if <0.9 (21).
Assessment of Noncardiovascular Parameters
Abnormalities in four noncardiovascular physiological systems were defined as follows: pulmonary: percent of predicted forced expiratory volume in 1 second (FEV1) <80% or percent of predicted forced vital capacity (FVC) <80% as assessed by spirometry (22); renal: estimated glomerular filtration rate <60mL/min/1.73 m (2) using the CKD-Epi equation (23); hematologic: anemia (hemoglobin <13g/dL in men and <12g/dL in women) (24); adipose: obesity (body mass index ≥ 30kg/m2) or body fat percentage >25% in men and >35% in women as assessed by bioelectric impedance (Tanita Body Composition Analyzer, TBF-300A) (25). All of the above cardiovascular and noncardiovascular parameters were obtained at Visit 5.
Assessment of Covariates
Hypertension was defined as a self-report of antihypertensive medication use or blood pressure ≥140/90 mmHg. Diabetes mellitus was defined as fasting glucose ≥126mg/dL, nonfasting glucose ≥200mg/dL or a self-report of anti-diabetic medication. Coronary artery disease was defined as previous myocardial infarction or coronary intervention. Heart failure was defined based on adjudicated heart failure hospitalization since 2005 or hospitalization with a heart failure ICD code prior to 2005 (26). Overt peripheral artery disease was defined as a self-report of physician diagnosis or a history of claudication.
Statistical Analysis
Continuous variables are expressed as mean ± standard deviation for normally distributed data or median (25th, 75th percentiles) for non-normally distributed data. Categorical variables are expressed as number of subjects and proportions. Differences in clinical and demographic features between frail and nonfrail groups were evaluated by unpaired t-test and chi-square test.
Differences in measures of the six studied systems (cardiac, vascular, pulmonary, renal, hematologic, and adipose) between frail and nonfrail participants were first assessed by linear or logistic regression adjusting for age, sex, race, and field center. This analysis included measures of the following three domains of the cardiac system: LV structure, LV systolic function, and LV diastolic function. The presence of abnormal LV structure was represented by LV hypertrophy. Within the LV systolic and LV diastolic function domains, multivariable logistic regression was employed to identify the measure that differed most significantly between the frail and nonfrail groups, and identified GLS and LAVI, respectively. As a result, cardiac abnormality was defined as the presence of abnormal LV structure (LV hypertrophy), LV systolic function (represented by abnormal GLS), or LV diastolic function (represented by abnormal LAVI). Abnormality of other organ systems for which there were multiple measures was defined by the presence of an abnormality of any component measure that also showed an independent association with frailty after adjusting for age, sex, race, and field center: Abnormal vascular function was defined as abnormal PWV or ABI; Abnormal pulmonary function was defined as abnormal percent FEV1 or FVC; Abnormal adiposity was defined as a body mass index ≥30kg/m2.
To identify organ systems for which abnormal function was independently associated with frailty, a multivariable logistic regression model was constructed including dichotomous variables representing abnormal function of each organ system (cardiac, vascular, and each noncardiovascular system) in addition to age, sex, race, field center, hypertension, and diabetes. In an additional analysis, component measures of cardiac dysfunction (LV hypertrophy, abnormal GLS, abnormal LAVI) were entered together in this model in place of a single dichotomous variable for cardiac dysfunction to assess the independent association of components cardiac impairment with frailty. The likelihood ratio test was used to assess interactions between the studied variables and frailty by gender, by race, and by the presence of cardiovascular diseases (coronary artery disease, heart failure, atrial fibrillation, peripheral artery disease, and previous stroke). To assess for the potential impact of Visit 5 nonattendance, we also determined the association of the studied variables with frailty in inverse probability of attrition weighted models. Finally, to determine whether a graded relationship exists between number of frailty criteria and cardiovascular and noncardiovascular organ dysfunction, we performed parallel analyses dividing the study sample into three groups: robust, if no frailty criteria were present (n = 1,932); prefrail, if one or two frailty criteria were present (n = 1,848); and frail, if three or more criteria were present (n = 211).
A two sided p-value <.05 was considered significant. Statistical analysis was performed using Stata software Version 13.1 (Stata Corp LP, College Station, TX).
Results
The mean age was 75.6±5.0 years, 59.1% were female, 23.4% were black, and 5.3% were frail. Frail participants were more likely to be older, female, had higher pulse pressure and heart rate, and had higher prevalence of cardiovascular comorbidities and cardiovascular disease (Table 1).
Table 1.
Clinical and Demographic Characteristics of Studied Participants
| Variable | Nonfrail (n = 3,780) | Frail (n = 211) | p Value |
|---|---|---|---|
| Female, n (%) | 2,210 (58) | 148 (70) | <.001 |
| Age, years | 75.5±4.9 | 78.6±5.5 | <.001 |
| Black, n (%) | 875 (23) | 59 (28) | .11 |
| Systolic blood pressure, mmHg | 130±17 | 131±20 | .27 |
| Diastolic blood pressure, mmHg | 67±10 | 65±11 | .003 |
| Pulse pressure, mmHg | 63±14 | 67±16 | <.001 |
| Heart rate, bpm | 62±10 | 66±11 | <.001 |
| Hypertension, n (%) | 3,074 (81) | 194 (92) | <.001 |
| Diabetes mellitus, n (%) | 1,089 (29) | 99 (47) | <.001 |
| Smokers, n (%) | 224 (6) | 12 (6) | .85 |
| Cardiovascular diseases | |||
| Coronary artery disease, n (%) | 465 (12) | 36 (17) | .042 |
| Previous myocardial infarction, n (%) | 233 (6) | 23 (11) | .006 |
| Atrial fibrillation, n (%) | 246 (7) | 35 (17) | <.001 |
| Heart failure, n (%) | 121 (3) | 31 (15) | <.001 |
| Previous stroke, n (%) | 92 (2) | 14 (7) | <.001 |
| Peripheral artery disease, n (%) | 157 (4) | 22 (10) | <.001 |
Note: Continuous variables are presented as mean ± standard deviation.
Frail participants had higher prevalence of LV hypertrophy and demonstrated worse LV systolic function based on GLS, although the prevalence of abnormal LV ejection fraction did not differ between groups (Table 2). LV diastolic function was also worse based on multiple measures, with the greatest between group differences noted for LAVI. Frailty was associated with worse vascular function, reflected in a higher prevalence of both abnormal ABI and PWV. Frail participants were also more likely to have worse lung function, as evidenced by higher prevalence of abnormal FEV1 and FVC, worse renal function, and anemia. Obesity was more prevalent in the frail, although no differences in percentage of body fat were noted.
Table 2.
Cardiac and Noncardiac Physiological Measures of Nonfrail and Frail Participants
| Nonfrail (n = 3,780) | Frail (n = 211) | p Value* | |
|---|---|---|---|
| Cardiac | |||
| LV structure | |||
| LVMI, g/m2 | 78±19 | 88±25 | <.001 |
| LV hypertrophy, n (%) | 597 (16) | 70 (33) | <.001 |
| LV systolic function | |||
| Ejection fraction, % | 65.5±6.4 | 64.1±8.0 | .004 |
| Abnormal ejection fraction, n (%) | 374 (10) | 25 (12) | .18 |
| GLS, % | −18.0±2.5 | −17.3±3.0 | <.001 |
| Abnormal GLS, n (%) | 448 (12) | 47 (22) | <.001 |
| LV diastolic function | |||
| Septal E/E′ | 12.1±4.3 | 13.5±5.2 | .001 |
| Abnormal septal E/E′, n (%) | 480 (13) | 46 (22) | .003 |
| LAVI, mL/m2 | 25.7±8.7 | 30.3±16.0 | <.001 |
| Abnormal LAVI, n (%) | 561 (15) | 64 (30) | <.001 |
| Vascular | |||
| Pulse wave velocity, cm/s | 1,159±330 | 1,272±470 | .015 |
| Abnormal pulse wave velocity, n (%) | 1,011 (27) | 87 (41) | .012 |
| Ankle-brachial index | 1.2±0.1 | 1.1±0.2 | .020 |
| Abnormal ankle-brachial index, n (%) | 231 (6) | 28 (13) | .030 |
| Pulmonary | |||
| % predicted FEV1 | 95.0±21.2 | 90.7±21.9 | <.001 |
| Abnormal % predicted FEV1, n (%) | 822 (22) | 57 (27) | .007 |
| % predicted FVC | 96.5±20.4 | 90.6±24.5 | <.001 |
| Abnormal % predicted FVC, n (%) | 612 (16) | 59 (28) | <.001 |
| Renal | |||
| Glomerular filtration rate, mL/min/1.73 m2 | 71.2±16.5 | 62.6±20.6 | <.001 |
| Abnormal glomerular filtration rate, n (%) | 932 (25) | 88 (42) | <.001 |
| Hematologic | |||
| Hemoglobin, g/dL | 13.4±1.5 | 12.8±1.5 | <.001 |
| Anemia, n (%) | 692 (18) | 69 (33) | <.001 |
| Adipose | |||
| Body mass index, kg/m2 | 28.3±5.1 | 29.1±6.5 | .001 |
| Obesity, n (%) | 1,212 (32) | 81 (38) | .005 |
| % Fat mass | 34.7±8.7 | 35.0±9.5 | .38 |
| Abnormal fat mass, n (%) | 2,625 (69) | 124 (59) | .30 |
Notes: Continuous variables are presented as mean ± standard deviation. LV hypertrophy was defined as LVMI >105g/m2 for M and >89g/m2 for W. Values of LV ejection fraction <59% for M and <58% for W; GLS > −15% for both sexes; septal E/E′ >15 for M and >17 for W; LAVI > 34mL/m2 for M and >32mL/m2 for W; pulse wave velocity >1,300cm/s; ankle-brachial index <0.9; % predicted FEV1<80%; % predicted FVC <80%; glomerular filtration rate <60mL/min/1.73 m2; and fat mass >25% for M and >35% for W were considered abnormal. Anemia was defined as hemoglobin <13g/dL for M and <12g/dL for W and obesity was defined as body mass index ≥30kg/m2. E/E′ = early transmitral inflow to early mitral relaxation velocity ratio; GLS = global longitudinal strain; FEV1 = forced expiratory volume in 1s; FVC = forced vital capacity; LAVI = left atrial volume index; LV = left ventricular; LVMI = LV mass index; M = men; W = women.
*p Values were adjusted for age, sex, race, and field center.
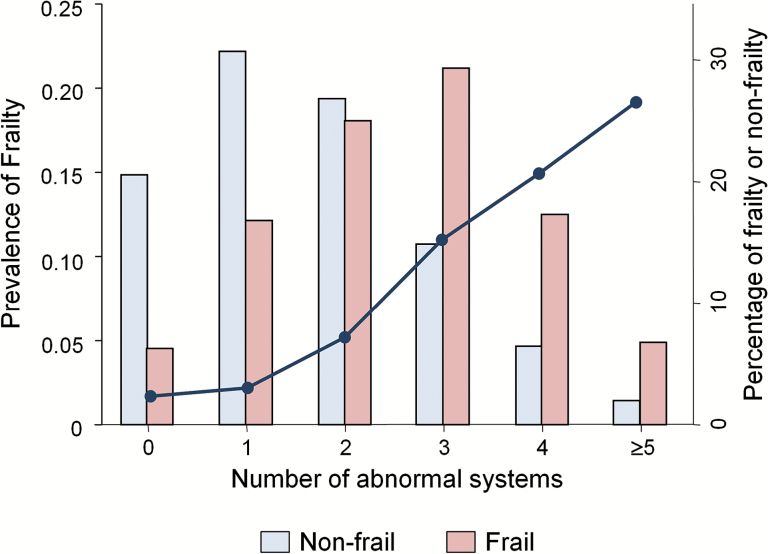
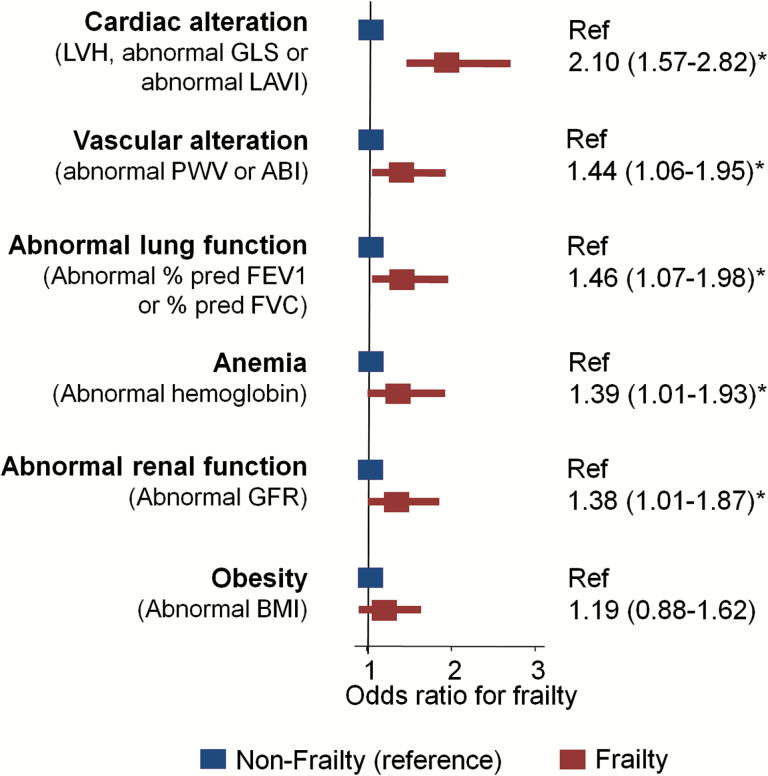
The prevalence of frailty was directly related to the number of abnormal organ systems (p for trend < 0.001; Figure 1). In multivariable logistic regression analysis adjusting for abnormalities in the six studied organ systems, demographic variables, diabetes, and hypertension, abnormality of each organ system except obesity was associated with frailty (Figure 2). Notably, cardiac abnormalities showed the greatest association with frailty (odds ratio [OR] = 2.10; 95% confidence interval [CI] = 1.57–2.82), while associations of lower magnitude were observed for vascular (OR = 1.44; 95% CI = 1.06–1.95) and pulmonary (OR = 1.46; 95% CI = 1.07–1.98) abnormalities, anemia (OR = 1.39; 95% CI = 1.01–1.93), and impaired renal function (OR = 1.38; 95% CI = 1.01–1.87). In addition, analysis including individual component measures of cardiac dysfunction (LV hypertrophy, abnormal GLS, and abnormal LAVI) demonstrated an independent association of each of these components with frailty (Table 3).
Figure 1.
Frailty and the number of abnormal systems. Prevalence of frailty (dark blue line) by the number of abnormal systems and frequency distribution (bars) of nonfrailty and frailty according to the number of abnormal systems. A trend test showed a direct relationship between frailty prevalence and the number of impaired systems (p < .001).
Figure 2.
Logistic regression analysis between frailty status and systems abnormalities. Binary logistic regression analysis between systems abnormalities and frailty status adjusted for age, sex, race, field center, hypertension, diabetes, and all systems abnormalities simultaneously. LVH was defined as left ventricular mass index >105g/m2 in men or >89g/m2 in women. Values of GLS >−15% for both sexes; LAVI >34mL/m2 for men and >32mL/m2 for women; PWV >1,300cm/s; ABI <0.9; % predicted FEV1 <80%; % predicted FVC <80%; and GFR <60mL/min/1.73 m2 were considered abnormal. Anemia was defined as hemoglobin <13g/dL for men and <12g/dL for women and obesity was defined as BMI ≥30kg/m2. ABI = ankle-brachial index; BMI = body mass index; FEV1 = forced expiratory volume in 1s; FVC = forced vital capacity; GFR = glomerular filtration rate; GLS = global longitudinal strain; LAVI = left atrial volume index; LVH = left ventricular hypertrophy; PWV = pulse wave velocity. *p < .05.
Table 3.
Multivariable Logistic Regression Analyses Between Frailty and Abnormal Cardiac and Components
| Variable | OR [95% CI] | p Value |
|---|---|---|
| Left ventricular hypertrophy | 1.72 [1.30–2.40] | .002 |
| Abnormal global longitudinal strain | 1.68 [1.16–2.44] | .006 |
| Abnormal left atrial volume index | 1.60 [1.13–2.27] | .008 |
Note: The dependent variable used in the models was: non-frailty (0); frailty (1). The model included left ventricular hypertrophy, abnormal global longitudinal strain, abnormal left atrial volume index, age, sex, race, field center, hypertension, diabetes, abnormal lung function, impaired renal function, anemia, obesity, and vascular abnormalities (composite of abnormal ankle-brachial index or pulse wave velocity) as independent variables. CI = confidence interval; OR = odds ratio.
In multivariable-adjusted analyses stratified by sex, race, and the presence of cardiovascular diseases, results were similar to those reported in the whole sample (Supplementary Tables 1–3). Frailty was associated with higher odds of anemia in men, of abnormal lung function in blacks, and of impaired renal function and obesity in participants with cardiovascular diseases. To account for potential bias due to nonattendance at Visit 5 of surviving cohort participants, multivariable analysis with inverse probability of attendance weights did not meaningfully alter the associations between frailty and system abnormalities (Supplementary Table 4). The association of frailty with LV hypertrophy or cardiac abnormalities did not significantly change when using the cutoff points recommended by the American Society of Echocardiography for the diagnosis of LV hypertrophy (LV mass index >115g/m2 in men and >95g/m2 in women (27); Supplementary Table 5) or when indexing LV mass and left atrial volume by height2.7, rather than by body surface area, to define LV hypertrophy and abnormal LAVI (Supplementary Table 6).
Adjusted multinomial logistic regression analysis was also performed to assess for a graded relationship between organ system dysfunction and number of frailty criteria (reflected in three participant groups: robust: 0 criteria; prefrail: 1–2 criteria; and frail: ≥3 criteria; Supplementary Tables 7 and 8). Compared to robust participants as reference, cardiac and vascular abnormalities were associated with frailty, but not with prefrailty (Supplementary Figure 1). In contrast, abnormal lung function, anemia and obesity were related to both prefrailty and frailty in a graded fashion.
Discussion
This study has three major novel findings. First, frail subjects have a higher prevalence of abnormal cardiac and vascular physiological measures when compared to nonfrail and prefrail individuals, after accounting for impairments in pulmonary, renal, hematologic, and adipose systems. These findings persisted in analyses restricted to participants without overt cardiovascular diseases. Second, multiple cardiac abnormalities, including impaired measures of LV systolic and diastolic function and LV hypertrophy are independently and additively associated with frailty. Third, cardiac abnormalities show the greatest association with frailty of the organ systems studied, while lower magnitude associations were seen for vascular and pulmonary abnormalities, anemia, and impaired renal function. Together, these findings demonstrate that cardiovascular abnormalities are independently associated with frailty, particularly impairments in cardiac function and structure, and raise the hypothesis that cardiovascular dysfunction may contribute to the pathophysiology of this syndrome.
Frailty results from multi-system dysregulation that accompanies aging (1,3), but the contribution of cardiac impairments to frailty in older adults is unclear. Previous studies suggest that frailty is associated with higher LV mass and left atrial volume, although these findings have been inconsistent (6,10). Furthermore, none of these reports accounted for impairments in other organ systems, which may be a relevant limitation as multiple comorbidities usually coexist in older adults and concomitant impairments in noncardiac systems may confound the association between cardiac alterations and frailty. In the present study, LV mass and the prevalence of LV hypertrophy were higher in frail participants compared to those without frailty. These findings are in accordance with data from the Cardiovascular Health Study (6), which showed greater LV mass in frail participants. Importantly, LV hypertrophy remained significantly associated with frailty in our study after accounting for measures of vascular, respiratory, renal, hematopoietic function, adiposity, and measures of LV function.
We also found that frailty was associated with impairments in both LV systolic and diastolic function, even after adjusting for measures of other organ systems. Previous studies have not found an association between frailty and impaired LV systolic function assessed by LV ejection fraction (6,10,12), similar to our finding. However, we found that GLS was significantly worse in frail participants. GLS is a novel measure of systolic function that appears more sensitive and prognostic than LV ejection fraction (28), particularly when ejection fraction is grossly preserved (19). Therefore, our results suggest that LV ejection fraction may not be the optimal measure to detect impairments in systolic function across the frailty spectrum. Our analysis also showed that frail participants had worse LV diastolic function, as demonstrated by higher E/E′ ratio and a higher LAVI, indicating that abnormalities in multiple components of cardiac function are related to frailty. It is noteworthy that in our sample the presence of cardiac abnormalities was independently associated with frailty even in the absence of overt cardiovascular diseases. Our finding that frail individuals have a higher rate of subclinical cardiac functional and structural alterations may help explain the increased risk of incident heart failure associated with frailty (29).
Previous reports show inconsistent results regarding the relationship between markers of vascular impairment and frailty in general population samples (6–9,11). In the present study, vascular dysfunction, defined as abnormal PWV or ABI, was independently related to frailty in analyses adjusted for demographic factors and abnormalities in other systems. The mechanisms relating vascular damage to frailty are not known. One possible explanation is that these measures may reflect more widespread dysfunction of several vascular beds and may be coupled with end-organ damage and consequent impairments in physiologic reserve.
The prevalence of frailty is known to increase directly with the number of abnormal physiological systems (30), a finding that was reproduced in our study. However, the magnitude of association between cardiac and vascular abnormalities with frailty relative to and accounting for abnormalities in other organ systems remains unknown. We found that cardiac abnormalities have the greatest association with frailty when compared to vascular, pulmonary, renal, hematologic, and adipose abnormalities. This finding suggests that cardiac dysfunction may be an important contributor to the frailty syndrome. Although causality cannot be established in this cross-sectional analysis, this association also raises the question of whether pharmacological strategies aiming to improve cardiac remodeling and dysfunction would exert beneficial effects on the frailty phenotype. Although the available literature on this topic is very scarce, a previous observational study suggested that the use of angiotensin receptor blockers was associated with lower prevalence of frailty (31), particularly in women, indicating that this may be a promising field to be explored in future studies.
Our study is one of the first, to our knowledge, to evaluate relationship of abnormalities of cardiac structure, systolic function, and diastolic function to frailty—independent of other physiological abnormalities across multiple systems—in a large community-based sample of older adults. However, some limitations of this analysis should be noted. First, as this is an observational and cross-sectional study, the temporal relationship between frailty status and cardiovascular phenotype cannot be determined, and the observed associations cannot be considered to be causal. Second, it is possible that attrition before Visit 5 may have introduced bias in our analysis. However, our results did not significantly change after applying inverse-probability-of-attrition weights to multivariable analyses. Third, the characterization of the hematopoietic and renal systems based on quantitative measures was less comprehensive than the cardiovascular system, which may have decreased our sensitivity to detect dysfunction in these systems. Fourth, it is unknown whether our frail participants would keep this diagnosis according to other proposed criteria. However, we defined frailty based on a well validated and widely used definition (1,5). Fifth, even though there was no significant interaction between frailty and abnormal cardiac characteristics according to race, the association between cardiac alterations and frailty was not statistically significant in blacks. Finally, the generalizability of our findings to younger samples or samples outside the general community setting is unknown.
Conclusions
Abnormalities of cardiac structure, systolic function, and diastolic function are independently associated with frailty in older adults after accounting for impairments in vascular, pulmonary, renal, hematologic, and adipose systems, even in the absence of overt cardiovascular diseases. Cardiac abnormalities show the greatest association with frailty among the organ systems studied. Future prospective studies are necessary to determine whether cardiac abnormalities contribute to the frailty syndrome in older adults.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute (NHLBI) contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). This work was also supported by NHLBI (K08-HL-116792 to A.M.S.), American Heart Association (14CRP20380422 to A.M.S.), NHLBI cooperative agreement (NHLBI-HC-11-08 to S.D.S.), and the Brazilian National Council for Scientific and Technological Development Grant (249481/2013–8 to W.N.J.).
Conflict of Interest
Dr Shah reports receiving research support from Novartis, Gilead, and Actelion.
Supplementary Material
Acknowledgments
The authors wish to thank the staff and participants of the ARIC study for their important contributions. Drs Nadruz Jr and Shah had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong JJ, Mitnitski A, Launer LJ, White LR, Rockwood K. Frailty in the Honolulu-Asia Aging Study: deficit accumulation in a male cohort followed to 90% mortality. J Gerontol A Biol Sci Med Sci. 2015;70:125–131. doi:10.1093/gerona/glu089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi:10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
- 4. Bandeen-Roche K, Seplaki CL, Huang J, et al. Frailty in older adults: a nationally representative profile in the United States. J Gerontol A Biol Sci Med Sci. 2015;70:1427–1434. doi:10.1093/gerona/glv133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014;63:747–762. doi:10.1016/j.jacc.2013.09.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Newman AB, Gottdiener JS, Mcburnie MA, et al. ; Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi:10.1093/gerona/56.3.M158 [DOI] [PubMed] [Google Scholar]
- 7. Singh S, Bailey KR, Noheria A, Kullo IJ. Frailty across the spectrum of ankle-brachial index. Angiology. 2012;63:229–236. doi:10.1177/0003319711413457 [DOI] [PubMed] [Google Scholar]
- 8. Avila-Funes JA, Meillon C, González-Colaço Harmand M, Tzourio C, Dartigues JF, Amieva H. Association between frailty and carotid central structure changes: the Three-City Study. J Am Geriatr Soc. 2014;62:1906–1911. doi:10.1111/jgs.13062 [DOI] [PubMed] [Google Scholar]
- 9. Lee JS, Auyeung TW, Leung J, Kwok T, Leung PC, Woo J. Physical frailty in older adults is associated with metabolic and atherosclerotic risk factors and cognitive impairment independent of muscle mass. J Nutr Health Aging. 2011;15:857–862. doi:10.1007/s12603-011-0134-1 [DOI] [PubMed] [Google Scholar]
- 10. Gharacholou SM, Tashiro T, Cha SS, Scott CG, Takahashi PY, Pellikka PA. Echocardiographic indices associated with frailty in adults ≥65 years. Am J Cardiol. 2015;116:1591–1595. doi:10.1016/j.amjcard.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 11. Hwang AC, Liu LK, Lee WJ, et al. Association of frailty and cardiometabolic risk among community-dwelling middle-aged and older people: Results from the I-Lan Longitudinal Aging Study. Rejuvenation Res. 2015;18:564–572. doi:10.1089/rej.2015.1699 [DOI] [PubMed] [Google Scholar]
- 12. Frisoli A, Jr, Ingham SJ, Paes ÂT, et al. Frailty predictors and outcomes among older patients with cardiovascular disease: data from Fragicor. Arch Gerontol Geriatr. 2015;61:1–7. doi:10.1016/j.archger.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 13. Chaves PH, Semba RD, Leng SX, et al. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women’s Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. doi:10.1093/gerona/60.6.729 [DOI] [PubMed] [Google Scholar]
- 14. Ramsay SE, Arianayagam DS, Whincup PH, et al. Cardiovascular risk profile and frailty in a population-based study of older British men. Heart. 2015;101:616–622. doi:10.1136/heartjnl-2014-306472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stenholm S, Strandberg TE, Pitkälä K, Sainio P, Heliövaara M, Koskinen S. Midlife obesity and risk of frailty in old age during a 22-year follow-up in men and women: the Mini-Finland Follow-up Survey. J Gerontol A Biol Sci Med Sci. 2014;69:73–78. doi:10.1093/gerona/glt052 [DOI] [PubMed] [Google Scholar]
- 16. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687–702. doi:10.1093/oxfordjournals.aje.a115184 [PubMed] [Google Scholar]
- 17. Kucharska-Newton AM, Palta P, Burgard S, et al. Operationalizing frailty in the Atherosclerosis Risk in Communities (ARIC) Study cohort. J Gerontol A Biol Sci Med Sci 2016. doi:10.1093/gerona/glw144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bandeen-Roche K, Xue QL, Ferrucci L, et al. Phenotype of frailty: characterization in the women’s health and aging studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. [DOI] [PubMed] [Google Scholar]
- 19. Shah AM, Cheng S, Skali H, et al. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–181. doi:10.1161/CIRCIMAGING.113.000736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alecu C, Labat C, Kearney-Schwartz A, et al. Reference values of aortic pulse wave velocity in the elderly. J Hypertens. 2008;26:2207–2212. doi:10.1097/HJH.0b013e32830e4932 [DOI] [PubMed] [Google Scholar]
- 21. Zheng ZJ, Sharrett AR, Chambless LE, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125. doi:10.1016/S0021-9150(97)06089-9 [DOI] [PubMed] [Google Scholar]
- 22. Li J, Agarwal SK, Alonso A, et al. Airflow obstruction, lung function, and incidence of atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2014;129:971–980. doi:10.1161/CIRCULATIONAHA.113.004050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarnak MJ, Tighiouart H, Manjunath G, et al. Anemia as a risk factor for cardiovascular disease in The Atherosclerosis Risk in Communities (ARIC) study. J Am Coll Cardiol. 2002;40:27–33. doi:10.1016/S0735-1097(02)01938-1 [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi:10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22:1164–1171. [DOI] [PubMed] [Google Scholar]
- 26. Folsom AR, Yamagishi K, Hozawa A, Chambless LE; Atherosclerosis Risk in Communities Study Investigators Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi:10.1161/CIRCHEARTFAILURE.108.794933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1.e14–39.e14. doi:10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 28. Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi:10.1136/heartjnl-2014-305538 [DOI] [PubMed] [Google Scholar]
- 29. Khan H, Kalogeropoulos AP, Georgiopoulou VV, et al. Frailty and risk for heart failure in older adults: the health, aging, and body composition study. Am Heart J. 2013;166:887–894. doi:10.1016/j.ahj.2013.07.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi:10.1093/gerona/glp076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pozos-López T, Navarrete-Reyes AP, Ávila-Funes JA. Is frailty associated with cardiovascular drug use? J Am Geriatr Soc. 2011;59:1977–1979. doi:10.1111/j.1532-5415.2011.03610_12.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.