Abstract
Rats are a commonly used model for aging studies, and a frailty assessment tool for rats would be of considerable value. There has been a recent focus on the development of preclinical models of frailty in mice. A mouse clinical frailty index (FI) was developed based on clinical frailty assessment tools. This FI measures the accumulation of clinically evident health-related deficits in mice. This paper aimed to develop a rat clinical FI. Male Fischer 344 rats were aged from 6 to 9 months (n = 12), and from 13 to 21 months (n = 41). A FI comprised of 27 health-related deficits was developed from a review of the literature and consultation with a veterinarian. Deficits were scored 0 if absent, 0.5 if mild, or 1 if severe. A FI score was determined for each rat every 3–4 months, and for the older group mortality was assessed up to 21 months. Mean FI scores significantly increased at each time point for the older rats. A high FI score measured at both 17 months of age and terminally was also associated with decreased probability of survival as assessed with Kaplan–Meier curves. The rat clinical FI has significant value for use in aging and interventional studies.
Keywords: Deficit accumulation, Preclinical models, Frailty index
Frailty is a state of high vulnerability for adverse health outcomes (1). It is highly prevalent in the older population and is associated with increased risk of hospitalization, institutionalization, and mortality (1–3). Studies of potential frailty mechanisms and interventions to prevent or delay frailty are limited by the logistical and ethical issues of undertaking studies in a population that is highly dependent, potentially vulnerable, and extremely heterogeneous (4,5).
The two most common ways of assessing frailty clinically are the frailty index (FI) and the frailty phenotype assessment (6,7). The FI measures the accumulated health deficits in a patient (8,9). Health deficits include symptoms, comorbidities, abnormal laboratory results, self-reported functional declines, or problems with activities of daily living (9,10). A FI score is determined by summing the number of health-related deficits a person displays and dividing by the maximum number of possible deficits, to give a continuous variable between 0 and 1, whereby a larger number indicates a person is more frail (7,9). The frailty phenotype assesses a person as frail based on decreased grip strength, walking speed, activity, endurance, and unintentional weight loss. A person is classified as either prefrail or frail based on their performance in these variables (6). The assessment of frailty with each of these clinical tools is associated with adverse outcomes including mortality (11,12).
Recently, there has been a focus on developing preclinical models of frailty in naturally aging mice based on these clinical assessment tools (13,14). Parks and colleagues developed a mouse FI based on activity levels, hemodynamic measures, body composition, and metabolic status (15). This was associated with age-related adverse cardiac outcomes, but included invasive and time consuming assessments (15). Whitehead and colleagues continued this work and developed a 31-item mouse clinical FI based on clinically apparent health deficits (16). They showed that the FI increased with age at a similar rate to that seen in human studies. The mouse clinical FI has been used to assess frailty interventions (17) and the effect of frailty on outcomes (18,19). Liu and colleagues developed a mouse frailty phenotype assessment based on a mouse’s performance in four functional assessments (20). This assessment has been used to assess an exercise intervention on frailty (21). However, the time consuming nature of the functional assessments and the need for specialized equipment may limit its use in aging and interventional studies. Each of these mouse frailty assessments could be adapted for use in other mouse strains as well as other species (14).
Rats, in particular Fischer 344 rats, are a commonly used model for aging studies (22). However, as of yet, there is no tool for assessing frailty in rats. The aim of the current study was to develop a rat clinical FI in Fischer 344 rats based on the mouse clinical FI (16). We also aimed to validate the tool by looking at the association of the rat clinical FI scores with mortality.
Methods
Animals
Male Fischer 344 rats purchased from Harlan Laboratories (Maine) were aged from middle age (13 months, n = 41) into old age (21 months) in a longitudinal study. A second cohort of young rats were aged from 6 months (n = 12) to 9 months, to serve as young adult comparators. Rats were fed ad libitum and housed individually on a 12-hour light/dark cycle. All experiments were approved by the Dalhousie University Committee on Laboratory Animals and performed in accordance with guidelines published by the Canadian Council on Animal Care.
Food was weighed twice weekly to assess changes in food intake. Biweekly measurements were summed and divided by 7 to calculate daily food intake. The day of death for a rat was determined as the day it was found dead or determined to be moribund by a veterinarian and euthanized. A terminal FI was assessed for each rat on the day of euthanasia, if possible.
Development of a 27-Item Clinical FI for Rats
A 27-item FI was developed based upon the mouse clinical FI (16). The index included assessment of health deficits across the integument, musculoskeletal system, ocular/nasal systems, digestive/urogenital systems, respiratory system as well as assessment of discomfort, body weight, temperature, and food intake. Clinical signs/deficits were selected through a review of the literature on age-related changes in the F344 rat and through consultations with a veterinarian (Table 1). Young rats were observed in comparison to older rats to refine assessment techniques.
Table 1.
Clinical Signs of Deterioration in Aging Fischer 344 rats
| System/variable | Description | Reference |
|---|---|---|
| Alopecia | Acquired hair loss due to inflammation, endocrine disorder, or idiopathic disease. | (41) |
| Skin lesions/dermatitis | Excessive scratching, self-mutilation, or skin conditions leading to open sores on the body. | (32,42) |
| Coat condition | Ungroomed appearance: fur appears ruffled and matted. | (31,32) |
| Tumours | Presence of neoplastic growths. | (31,42) |
| Distended abdomen | Asymmetric/enlarged abdomen. May be due to neoplastic growths, organ enlargement or peritoneal fluid accumulation. | (31,43) |
| Hunched posture | Presence of hunched posture (head down, feet together); reduced mobility. | (32,41) |
| Body condition | Visual signs of emaciation or obesity. Based on the amount of flesh covering the vertebral column and dorsal pelvis. | (25) |
| Gait disorders | Abnormal locomotion: slow movement, lack of coordination, stumbling, falling, or limping. | (32,44) |
| Tremor | Involuntary shaking at rest. | (45,46) |
| Hearing loss | Impaired acoustic startle reflex; associated with loss of hearing sensitivity. | (43) |
| Cataracts | Opaque spot in the center of the eye; clouding of the lens. | (43,44) |
| Chromodacryorrhea | Porphyrin staining around the eyes/nose. | (31) |
| Exophthalmos | Abnormal protrusion of the eye. | (47) |
| Microphthalmos | Abnormally small eye. Sunken in appearance. | (48) |
| Corneal opacity | Cornea appears white or clouded. | (41,43) |
| Head tilt | Abnormal/asymmetric head position associated with a central nervous system disturbance. | (31,43) |
| Malocclusion | Abnormal occlusion due to uneven or overgrown incisors. | (31) |
| Diarrhea | Increased frequency and decreased consistency of bowel movements. Fecal smearing in cage. | (31,43) |
| Jaundice | Yellowing of the feet, nose, ears and tail associated with accumulation of bilirubin. | (43) |
| Penile/vaginal prolapse | Tissue protruding from the penis or vagina. | (43) |
| Rectal prolapse | Tissue protruding from the rectum. | (43) |
| Breathing rate/depth | Bradypnea, tachypnea, dyspnea, or snuffling. | (31) |
| Piloerection | Fur standing on end | (32) |
| Unusual sounds | Acute vocalization in response to touch. | (32) |
| Body weight | Increase or decrease in body weight | (25,44) |
| Food intake | Decrease in daily food intake | (25) |
| Body temperature | Increase or decrease in body temperature. | (49) |
To complete the clinical FI, rats were moved to an assessment room in the animal care facility and allowed 5 minutes to acclimatize to their new surroundings. Animals were then briefly observed in their home cage prior to undergoing examination for clinical signs of deterioration as described in Table 1. Details of the assessment methods for each item of the FI are described in Supplementary Table 1. As outlined in (16), each deficit was scored as follows: 0 if there was no sign of a deficit, 0.5 if there was a mild deficit, and 1 if there was a severe deficit. Table 2 shows the Rat Frailty Assessment Form developed for this study, based on the mouse FI published by Whitehead and colleagues (16) and refined by Feridooni and colleagues (23). Deficits in body weight (g), daily food intake (g), and body surface temperature (°C) were scored based on the number of standard deviations from mean reference values calculated for young adult rats (6 months) in the current study. Reference values (mean ± SD) calculated for daily food intake, weight, and temperature for 6 month old rats were 17.7 ± 1.6 g, 371.8 ± 30.1 g, and 26.3 ± 0.4 °C, respectively. A score of 0 was given if the deficit was less than 1 SD from the reference mean, a score of 0.5 was given for 1–2 SDs difference, and a score of 1 was given for greater than 2 SDs difference. Frailty assessments were completed at 6 and 9 months for the young rats. For 36 of the old rats, frailty assessments were completed at 13, 17, and 21 months, provided the mouse survived to the time point. For the remaining 5 old rats, frailty was only evaluated at 21 months.
Table 2.
27-Item Index to Assess Frailty in Fischer 344 Rats
| Rat #:_________________ | Date of Birth:_____________ | |||
| Body Weight (g):__________________ | Body Surface Temperature (°C):______________________ | |||
| Daily food intake (g):________________ | Sex: F M | |||
| Rating: 0 = absent 0.5 = mild 1 = severe | ||||
| Integument | Comments: | |||
| 1. Alopecia | 0 | 0.5 | 1 | ________________________ |
| 2. Dermatitis | 0 | 0.5 | 1 | ________________________ |
| 3. Coat condition | 0 | 0.5 | 1 | ________________________ |
| Physical/Musculoskeletal | ||||
| 4. Tumours | 0 | 0.5 | 1 | ________________________ |
| 5. Distended abdomen | 0 | 0.5 | 1 | ________________________ |
| 6. Hunched posture | 0 | 0.5 | 1 | ________________________ |
| 7. Body condition score | 0 | 0.5 | 1 | ________________________ |
| 8. Gait disorder | 0 | 0.5 | 1 | ________________________ |
| 9. Tremor | 0 | 0.5 | 1 | ________________________ |
| Vestibulocochlear/Auditory | ||||
| 10. Hearing loss | 0 | 0.5 | 1 | ________________________ |
| Ocular/Nasal | ||||
| 11. Cataracts | 0 | 0.5 | 1 | ________________________ |
| 12. Chromodacryorrhea/porphyrin | 0 | 0.5 | 1 | ________________________ |
| 13. Exophthalmos | 0 | 0.5 | 1 | ________________________ |
| 14. Microphthalmos | 0 | 0.5 | 1 | ________________________ |
| 15. Corneal opacity | 0 | 0.5 | 1 | ________________________ |
| Neurological | ||||
| 16. Head tilt | 0 | 0.5 | 1 | ________________________ |
| Digestive/Urogenital | ||||
| 17. Malocclusion | 0 | 0.5 | 1 | ________________________ |
| 18. Diarrhea | 0 | 0.5 | 1 | ________________________ |
| 19. Jaundice | 0 | 0.5 | 1 | ________________________ |
| 20. Penile/vaginal prolapse | 0 | 0.5 | 1 | ________________________ |
| 21. Rectal prolapse | 0 | 0.5 | 1 | ________________________ |
| Respiratory | ||||
| 22. Breathing rate/depth | 0 | 0.5 | 1 | ________________________ |
| Pain/Discomfort | ||||
| 23. Piloerection | 0 | 0.5 | 1 | ________________________ |
| 24. Unusual sounds | 0 | 0.5 | 1 | ________________________ |
| Other | ||||
| 25. Body weight score | ________ | |||
| 26. Temperature score | ________ | |||
| 27. Food intake score | ________ | |||
| TOTAL SCORE =________ | TOTAL SCORE/MAX SCORE =________ | |||
© Susan E. Howlett, 2016
Statistics
Data are presented as mean ± standard error of mean unless otherwise specified. One-way repeated measures analysis of variance with Bonferroni post hoc testing was used to compare mean FI scores between age groups. To investigate the upper limit of frailty in this study, the range of FI scores, and the 99th percentile FI score (24) were calculated for each age group. Rat ages were normalized to a 90% mortality rate at 900 days (25) to allow comparison with previously published mouse and human FI data that were normalized in the same way (16). All FI scores were plotted against normalized age and fitted with an exponential growth curve (2 parameters). The natural log of all FI scores was also plotted against normalized age and fitted with a linear polynomial equation. Survival data was illustrated using a Kaplan–Meier survival curve and analyzed using the log-rank test. For the survival analysis, the continuous FI scores were dichotomously stratified by < or > 0.21 (26,27). p values <0.05 were considered significant. Statistical analysis was completed using SPSS 22 and Sigma Plot 11.0.
Results
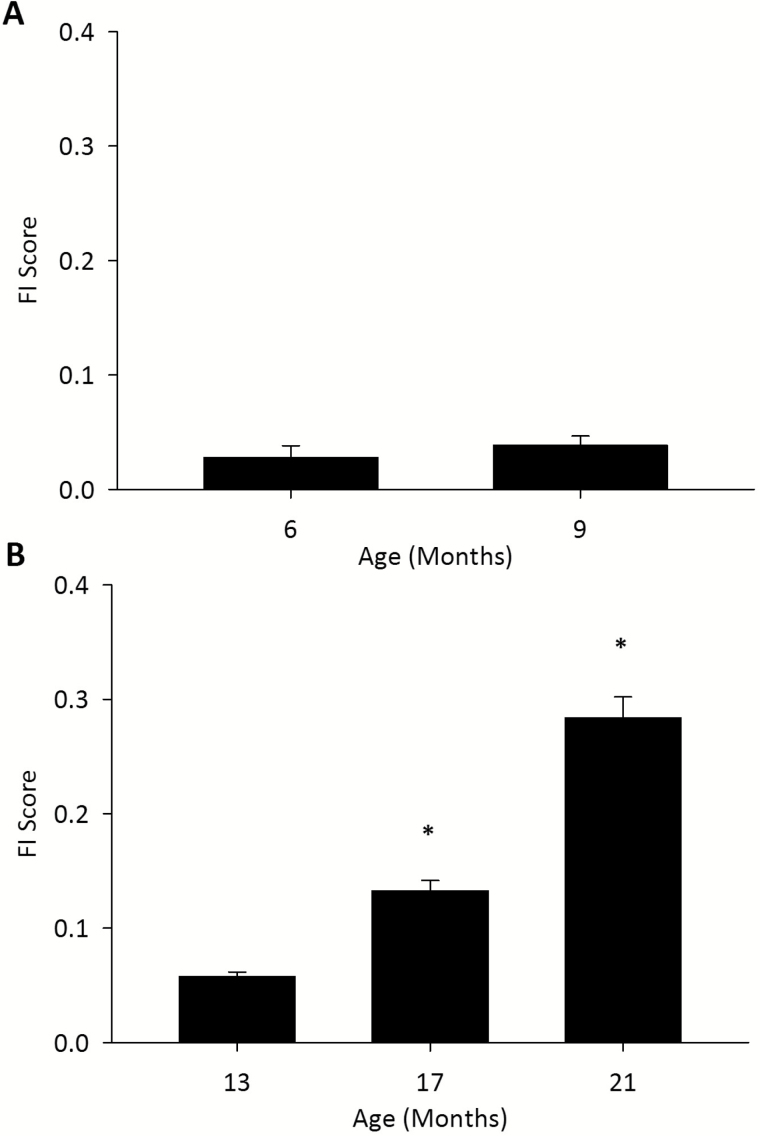
Mean FI scores did not significantly change in young rats from 6 to 9 months (p = 0.27) but increased in old rats (p < 0.0001; Figure 1 and Table 3). Bonferroni post hoc testing showed that there was a significant difference between all 3 time points for the older rats (Figure 1). The mean FI for terminal FI scores was 0.31 ± 0.02 (Table 3). The range of FI scores also increased with age in the older rats, as did the 99th percentile FI score (Table 3). Supplementary Table 2 shows the scoring of the individual deficits in the FI at the different assessment time points. The proportion of rats scored 0.5 or 1 increased for each deficit over time (Supplementary Table 2).
Figure 1.
Mean frailty index (FI) scores for young (A) and old (B) rats at various ages. One-way repeated measures ANOVA determined a significant difference across all time points for the old rats. ANOVA, analysis of variance.
Table 3.
Summary Statistics for Frailty Index Scores for Younger and Older Rats
| Younger rats | Older rats | |||||
|---|---|---|---|---|---|---|
| 6 Months | 9 Months | 13 Months | 17 Months | 21 Months | Terminal | |
| N | 12 | 12 | 36 | 33 | 23 | 18 |
| Mean FI score | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.00 | 0.13 ± 0.01 | 0.21 ± 0.01 | 0.31 ± 0.02 |
| FI score range | 0.00–0.11 | 0.00–0.11 | 0.02–0.11 | 0.04–0.30 | 0.11–0.35 | 0.19–0.40 |
| 99th Percentile of FI score | 0.11 | 0.11 | 0.11 | 0.28 | 0.34 | 0.40 |
Abbreviation: FI, frailty index.
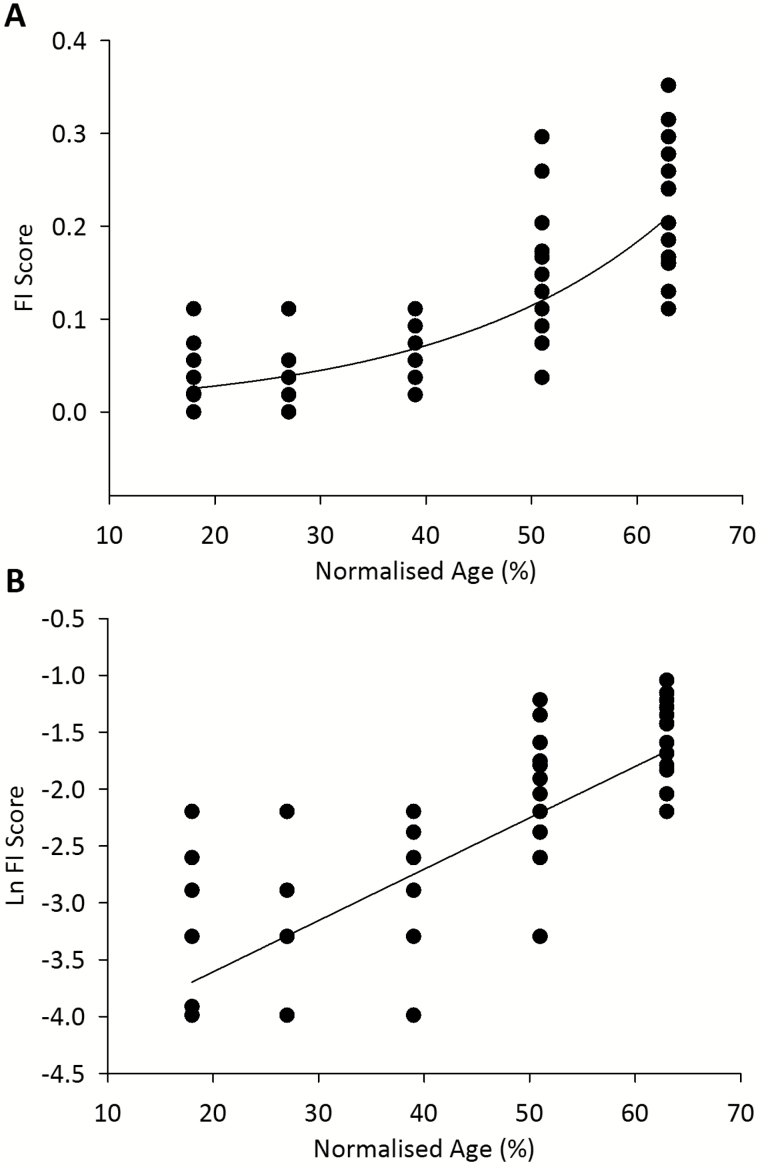
Rat ages were normalized to a 90% mortality rate at 900 days (25) to allow comparison with previously published mouse and human FI data that were normalized in the same way (16). Figure 2A shows all of the individual rat frailty scores plotted against normalized age, fitted with an exponential curve (r2 = 0.64, p < 0.0001, n = 116). Figure 2B shows the natural logarithm of FI score plotted as a function of normalized rat age. The slope of the line, which represents the rate of deficit accumulation (16,28) was 0.045 (p < 0.0001).
Figure 2.
Individual FI scores for older rats fitted with an exponential curve (r2 = 0.64, p < 0.0001, n = 116) (A). The natural logarithm of FI versus time fitted with a straight line (slope = 0.045, p < 0.0001, n = 116) (B). All rat ages are normalized to the 90% mortality rate at 900 days.
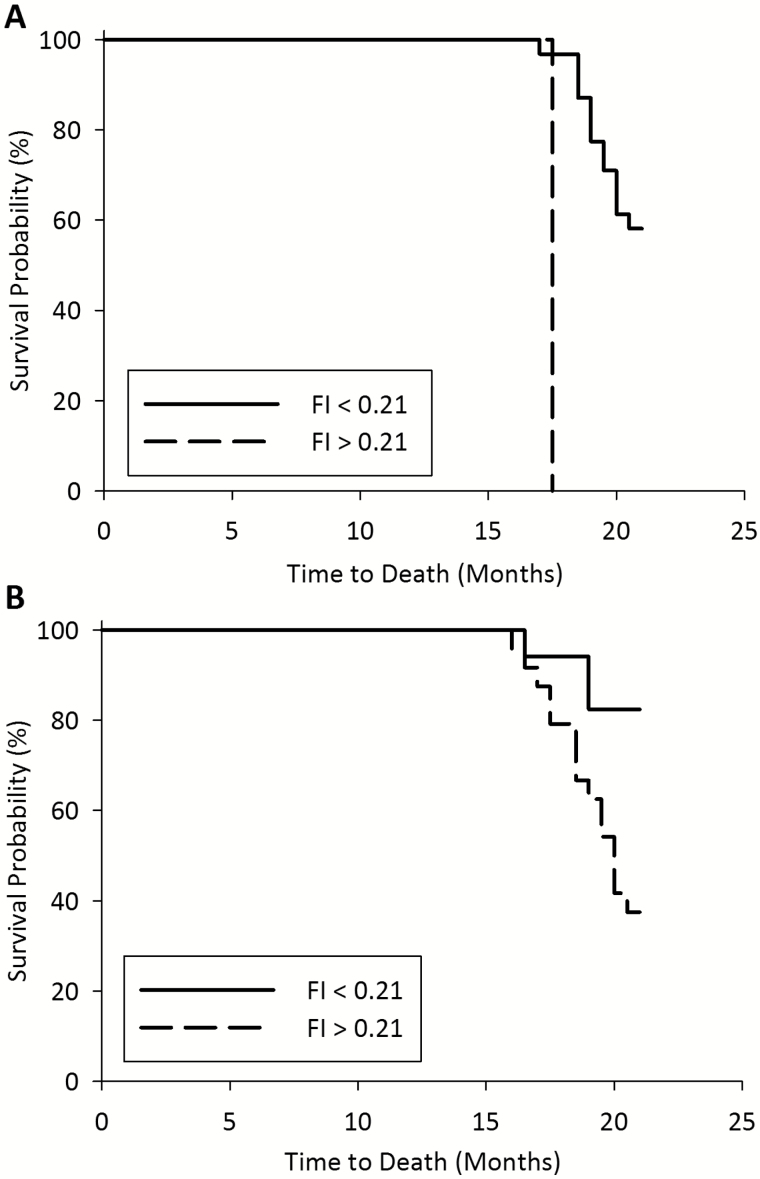
Of the 41 older rats, 18 (44%) died before 21 months of age, and of these, 3 died before 17 months of age. Figure 3 shows that a high FI score is associated with an increased probability of mortality when assessed with Kaplan–Meier curves in rats with FI scores completed at 17 months (panel A) and 21 months (panel B). Log-rank tests showed that for frailty assessments completed at both 17 months (n = 33 rats, 8 excluded for missing FI scores or mortality before this time point) and terminally/at 21 months (n = 41 rats), there was a significant difference between the survival curves for FI >0.21 and FI <0.21 (p < 0.0001 and p = 0.007, respectively). For both time points a greater proportion of rats with higher FI scores, compared to lower scores, died before 21 months. Of the rats assessed for frailty at 17 months, 2 rats had FI scores >0.21 and both rats (100%) died before 21 months of age, while 31 rats had FI scores <0.21 and 13 (41.9%) of these died before 21 months (Figure 3A). Of the rats assessed for frailty terminally or at 21 months, 24 rats had FI scores >0.21 and 15 (62.5%) died before 21 months, while 17 mice had FI scores <0.21 and 3 (17.6%) of these died before 21 months (Figure 3B).
Figure 3.
Kaplan–Meier curves for 21 month survival probability stratified by frailty index (< or > 0.21), for mice assessed for FI at age 17 months (A) or mice assessed for FI terminally (B). Log-rank analysis showed a significant difference between the curves for both time points (A, p < 0.0001; B, p < 0.01). A, n = 33 (8 excluded for missing FI scores or mortality before this time point); B, n = 41.
Discussion
This study is the first to develop a frailty assessment tool for use in rats. We successfully developed a rat clinical FI based on deficit accumulation across a range of systems. We then used this tool to assess frailty in rats, and found a significant increase in mean FI scores as the rats aged. We also further validated the FI by looking at the association with mortality and found that high FI scores increased the risk of death.
An exponential increase in mean FI scores with age, and an increase in the variability of FI scores with age has been seen in FI studies of humans, mice (16,23) and now rats. In the current study, the rate of deficit accumulation as determined from a graph of the natural log of FI plotted against normalized age was 0.045. A previous study found the rate of accumulation to be 0.038 in mice and 0.034 in humans (16). This may suggest that rats accumulate deficits at a higher rate than mice or humans but would require further studies. Interestingly, in the current study, our maximum FI score was 0.40, which is close to the maximum FI scores seen in previous clinical FI studies in mice (16,23). Also of interest, clinical studies have clearly shown that there is a submaximal limit to frailty in humans (29) and animal model data strongly suggest that such a limit is also present in mice (16,23) and now in rats (our data). Furthermore, we saw an association between high FI score and mortality risk in the current study. The association of FI and mortality has not yet been explored in mice but has been clearly shown in clinical studies (2). The quantification of the accumulation of health deficits as a model of frailty appears to be conserved across species. This provides evidence for the value of the rat FI tool, but also for the FI as a concept.
It will be interesting for further studies to explore the FI in other mouse and rat strains, as well as other species (14). As the mouse clinical FI was adapted in the current study to be suitable for Fischer 344 rats, each of these FIs could be adapted for use in other strains or species with adjustment or replacement of the deficit items for strain- or species-specific deficits. For example, as the Fischer 344 are an albino rat strain, some deficits such as chromodacryorrhea may not be as clearly identifiable in nonalbino rat strains such as Long–Evans rats. Additionally, some deficits that were not applicable for Fischer 344 rats may be applicable for other strains or species. For example, vision loss was used as a deficit in the mouse clinical FI, but light-induced retinal degeneration is universal among albino rats exposed to fluorescent or incandescent light (30).
There are several other health-related deficits that could be incorporated into the rat clinical FI for future studies. Whisker loss (31), splayed posture (32), increased nail length (32), and decreased muscle tone (32,33) are also seen in aging rats and could easily be assessed. There has been some criticism of the mouse clinical FI for the lack of assessment of cognitive deficits (34). It may be possible to include hopping or placing reflex assessment (33,35,36) as measures of cognitive deficits in the rat clinical FI.
There are some limitations to the current study, and more studies should be undertaken to further validate this tool. The rats in the current study were only aged until 21 months, and as the 50% mortality age of Fischer 344 rats is 26 months (25), or 23 months for singly housed rats (37) it would be interesting to look at the changes in frailty, and the associations with mortality in even older animals. The current study also only used one assessor for the FI, which ensures the data is consistent across individual rats and time points. However, it would be interesting in future studies to look at the inter-rater reliability of the rat clinical FI, as has been done for the mouse clinical FI (23,38). The current study looks at the relationship between rat FI and mortality. Although this is an important outcome, it would also be interesting to look at the association of the rat FI with other adverse outcomes such as functional decline. It would also be possible to develop a rat phenotype assessment, based on the mouse phenotype assessment (20) and look at the association between frailty in the rat measured with these two tools. Finally, the current study used a cut point of 0.21 for the FI for the Kaplan–Meier analysis, as has been used in previous clinical studies (26,27). Although cut points are frequently used for clinical FI studies, the cut points for use in preclinical studies may still require optimization. As the rate of deficit accumulation appeared to be higher in rats, this may mean that a lower cut point may be appropriate when dichotomising rat FI data.
As with the mouse models of frailty, the rat clinical FI will have great value in preclinical studies of frailty mechanisms, interventions, and outcomes. Preclinical models of frailty are an increasingly important area as evidenced by several recent reviews (14,34,39,40). The mouse clinical FI has been used to show that dietary and pharmaceutical interventions can attenuate frailty (17), as well as in studies looking at frailty as a geriatric outcome (18). Given that rats are commonly preferred for behavioral studies, and are a commonly used aging model (22), the rat clinical FI could be an important tool for use in aging studies.
We have developed a convenient and relevant rat clinical FI assessment tool to assess frailty in aging Fischer 344 rats. The rat clinical FI is an important contribution to the growing area of preclinical models of frailty.
Supplementary Material
Supplementary data are available at The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences online.
Funding
SEH is supported by a Canadian Institute for Health Research grant (MOP 126018).
Supplementary Material
References
- 1. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 2. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x [DOI] [PubMed] [Google Scholar]
- 3. Rockwood K, Howlett SE, MacKnight C, et al. Prevalence, attributes, and outcomes of fitness and frailty in community-dwelling older adults: report from the Canadian study of health and aging. J Gerontol A Biol Sci Med Sci. 2004;59:1310–1317. doi:10.1093/gerona/59.12.1310 [DOI] [PubMed] [Google Scholar]
- 4. Harris R, Dyson E. Recruitment of frail older people to research: lessons learnt through experience. J Adv Nurs. 2001;36:643–651. doi:10.1046/j.1365-2648.2001.02029.x [DOI] [PubMed] [Google Scholar]
- 5. Hilmer SN, Gnjidic D, Abernethy DR. Pharmacoepidemiology in the postmarketing assessment of the safety and efficacy of drugs in older adults. J Gerontol A Biol Sci Med Sci. 2012;67:181–188. doi: 10.1093/gerona/glr066 [DOI] [PubMed] [Google Scholar]
- 6. Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi:10.1093/gerona/56.3.M146 [DOI] [PubMed] [Google Scholar]
- 7. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Scientific World J. 2001;1:323–336. doi: 10.1100/tsw.2001.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitnitski AB, Mogilner AJ, MacKnight C, Rockwood K. The mortality rate as a function of accumulated deficits in a frailty index. Mech Ageing Dev. 2002;123:1457–1460. doi:10.1016/S0047-6374(02)00082-9 [DOI] [PubMed] [Google Scholar]
- 9. Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. J Gerontol A Biol Sci Med Sci. 2007;62:722–727. doi:10.1093/gerona/62.7.722 [DOI] [PubMed] [Google Scholar]
- 10. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi: 10.1186/s12916-014-0171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc. 2013;61:1537–1551. doi: 10.1111/jgs.12420 [DOI] [PubMed] [Google Scholar]
- 12. Woo J, Leung J, Morley JE. Comparison of frailty indicators based on clinical phenotype and the multiple deficit approach in predicting mortality and physical limitation. J Am Geriatr Soc. 2012;60:1478–1486. doi: 10.1111/j.1532-5415.2012.04074.x [DOI] [PubMed] [Google Scholar]
- 13. Howlett SE, Rockwood K. Ageing: Develop models of frailty. Nature. 2014;512:253. doi: 10.1038/512253d [DOI] [PubMed] [Google Scholar]
- 14. Howlett S. Assessment of frailty in animal models. In: Theou O, Rockwood K, eds. Frailty in Aging Biological, Clinical and Social Implications. Basel: Karger; 2015: 15–25. [Google Scholar]
- 15. Parks RJ, Fares E, Macdonald JK, et al. A procedure for creating a frailty index based on deficit accumulation in aging mice. J Gerontol A Biol Sci Med Sci. 2012;67:217–227. doi: 10.1093/gerona/glr193 [DOI] [PubMed] [Google Scholar]
- 16. Whitehead JC, Hildebrand BA, Sun M, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci. 2014;69:621–632. doi: 10.1093/gerona/glt136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kane AE, Hilmer SN, Boyer D, et al. Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2016;71:333–339. doi: 10.1093/gerona/glu315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huizer-Pajkos A, Kane AE, Howlett SE, et al. Adverse Geriatric outcomes secondary to polypharmacy in a mouse model: the influence of aging. J Gerontol A Biol Sci Med Sci. 2016;71:571–577. doi: 10.1093/gerona/glv046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kane AE, Mitchell SJ, Mach J, et al. Acetaminophen hepatotoxicity in mice: effect of age, frailty and exposure type. Exp Gerontol. 2016;73:95–106. doi: 10.1016/j.exger.2015.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu H, Graber TG, Ferguson-Stegall L, Thompson LV. Clinically relevant frailty index for mice. J Gerontol A Biol Sci Med Sci. 2014;69:1485–1491. doi: 10.1093/gerona/glt188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Graber TG, Ferguson-Stegall L, Liu H, Thompson LV. Voluntary aerobic exercise reverses frailty in old mice. J Gerontol A Biol Sci Med Sci. 2015;70:1045–1058. doi: 10.1093/gerona/glu163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mitchell SJ, Scheibye-Knudsen M, Longo DL, de Cabo R. Animal models of aging research: implications for human aging and age-related diseases. Annu Rev Anim Biosci. 2015;3:283–303. doi: 10.1146/annurev-animal-022114-110829 [DOI] [PubMed] [Google Scholar]
- 23. Feridooni HA, Sun MH, Rockwood K, Howlett SE. Reliability of a frailty index based on the clinical assessment of health deficits in male C57BL/6J mice. J Gerontol A Biol Sci Med Sci. 2015;70:686–693. doi: 10.1093/gerona/glu161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8:24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi:10.1093/gerona/54.11.B492 [DOI] [PubMed] [Google Scholar]
- 26. Rockwood K, Song X, Mitnitski AB. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;138(8):487–94. doi:10.1503/cmaj.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch Gerontol Geriatr. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 28. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi: 10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 29. Rockwood K, Mitnitski A. Limits to deficit accumulation in elderly people. Mech Ageing Dev. 2006;127:494–496. doi: 10.1016/j.mad.2006.01.002 [DOI] [PubMed] [Google Scholar]
- 30. Williams RA, Howard AG, Williams TP. Retinal damage in pigmented and albino rats exposed to low levels of cyclic light following a single mydriatic treatment. Curr Eye Res. 1985;4:97–102. doi:10.3109/02713688508999974 [DOI] [PubMed] [Google Scholar]
- 31. Kahn CM, Line S, Co M. The Merck Veterinary Manual. 10th Edition Kenilworth, NJ: Merck; 2010. [Google Scholar]
- 32. Phillips PM, Jarema KA, Kurtz DM, MacPhail RC. An observational assessment method for aging laboratory rats. J Am Assoc Lab Anim Sci. 2010;49:792–799. [PMC free article] [PubMed] [Google Scholar]
- 33. Marshall JF. Sensorimotor disturbances in the aging rodent. J Gerontol. 1982;37:548–554. doi:10.1093/geronj/37.5.548 [DOI] [PubMed] [Google Scholar]
- 34. Seldeen KL, Pang M, Troen BR. Mouse models of frailty: an emerging field. Curr Osteoporos Rep. 2015;13:280–286. doi: 10.1007/s11914-015-0283-y [DOI] [PubMed] [Google Scholar]
- 35. Wallace JE, Krauter EE, Campbell BA. Motor and reflexive behavior in the aging rat. J Gerontol. 1980;35:364–370. doi:10.1093/geronj/35.3.364 [DOI] [PubMed] [Google Scholar]
- 36. Altun M, Bergman E, Edström E, Johnson H, Ulfhake B. Behavioral impairments of the aging rat. Physiol Behav. 2007;92:911–923. doi: 10.1016/j.physbeh.2007.06.017 [DOI] [PubMed] [Google Scholar]
- 37. Yu BP, Masoro EJ, Murata I, Bertrand HA, Lynd FT. Life span study of SPF Fischer 344 male rats fed ad libitum or restricted diets: longevity, growth, lean body mass and disease. J Gerontol. 1982;37:130–141. doi:10.1093/geronj/37.2.130 [DOI] [PubMed] [Google Scholar]
- 38. Kane AE, Hilmer SN, Huizer-Pajkos A, et al. Factors that impact on interrater reliability of the mouse clinical frailty index. J Gerontol A Biol Sci Med Sci. 2015;70:694–695. doi: 10.1093/gerona/glv032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kane A, Hilmer S, Mach J, Mitchell S, de Cabo R, Howlett S. Animal models of frailty: current applications in clinical research. Clin Interv Aging. 2016;11:1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohler MJ, Fain MJ, Wertheimer AM, Najafi B, Nikolich-Žugich J. The frailty syndrome: clinical measurements and basic underpinnings in humans and animals. Exp Gerontol. 2014;54:6–13. doi: 10.1016/j.exger.2014.01.024 [DOI] [PubMed] [Google Scholar]
- 41. Lewis SM, Ullrey DE, Barnard DE, Knapka JJ. Chapter 9. Nutrition. In: The Laboratory Rat. 2nd Edition Burlington, MA: Elsevier Inc; 2006. 219–301. [Google Scholar]
- 42. Jacomelli M, Pitozzi V, Zaid M, et al. Dietary extra-virgin olive oil rich in phenolic antioxidants and the aging process: long-term effects in the rat. J Nutr Biochem. 2010;21:290–296. doi: 10.1016/j.jnutbio.2008.12.014 [DOI] [PubMed] [Google Scholar]
- 43. Sharp P, Villiano J. The Laboratory Rat, Second Edition Boca Raton, FL: CRC Press; 2012. [Google Scholar]
- 44. Nadon NL. Gerontology and age-associated lesions. In: Suckow MA, Weisbroth SH, Franklin CL, eds. The Laboratory Rat. Burlington, MA: Elsevier Inc; 2006. 761–772. [Google Scholar]
- 45. Deacon RM. Housing, husbandry and handling of rodents for behavioral experiments. Nat Protoc. 2006;1:936–946. doi: 10.1038/nprot.2006.120 [DOI] [PubMed] [Google Scholar]
- 46. Marshall J. The effect of ageing upon physiological tremor. J Neurol Neurosurg Psychiatry. 1961;24:14–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rothwell TL, Everitt AV. Exophthalmos in ageing rats with Harderian gland disease. Lab Anim. 1986;20:97–100. [DOI] [PubMed] [Google Scholar]
- 48. Cook C. Eye and ear. In: Jones T, Mohr U, Hunt R, eds. Springer: Berlin Heidlberg; 1991. [Google Scholar]
- 49. Keil G, Cummings E, de Magalhães JP. Being cool: how body temperature influences ageing and longevity. Biogerontology. 2015;16:383–397. doi: 10.1007/s10522-015-9571-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.