Abstract
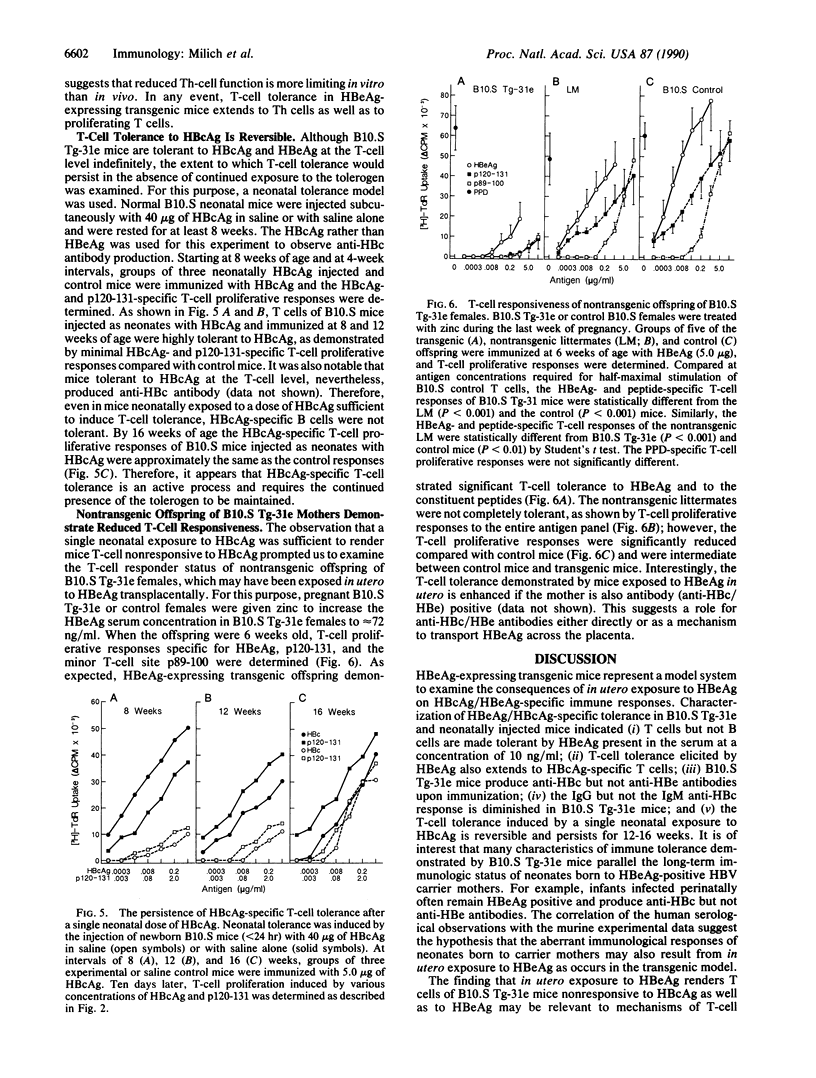
Infants born to hepatitis B virus carrier mothers, who express a secreted form of the nucleocapsid antigen designated HBeAg, invariably become persistently infected. To investigate the role of immunologic tolerance mechanisms in chronic infection of the newborn, we have generated HBeAg-expressing transgenic mice. HBeAg-expressing transgenic mice were tolerant to both HBeAg and the nonsecreted nucleocapsid (hepatitis B cor antigen/HBcAg) at the T-cell level. Transgenic mice did not produce antibody to HBeAg but did produce anti-HBc antibody in vivo and in vitro. The coexistence of tolerance to HBc/HBe T-cell determinants and anti-HBc antibody production in vivo parallels the immunologic status of neonates born to carrier mothers. It was also demonstrated that the maintenance of T-cell tolerance to HBcAg/HBeAg required the continued presence of the tolerogen and in its absence persisted for less than 16 weeks. The reversibility of T-cell tolerance to HBcAg/HBeAg may explain the inverse correlation between age of infection and rates of viral persistence. These observations suggest that a function of the HBeAg may be to induce immunologic tolerance in utero. Expression of HBeAg may represent a viral strategy to guarantee persistence after perinatal infection.
Full text
PDF
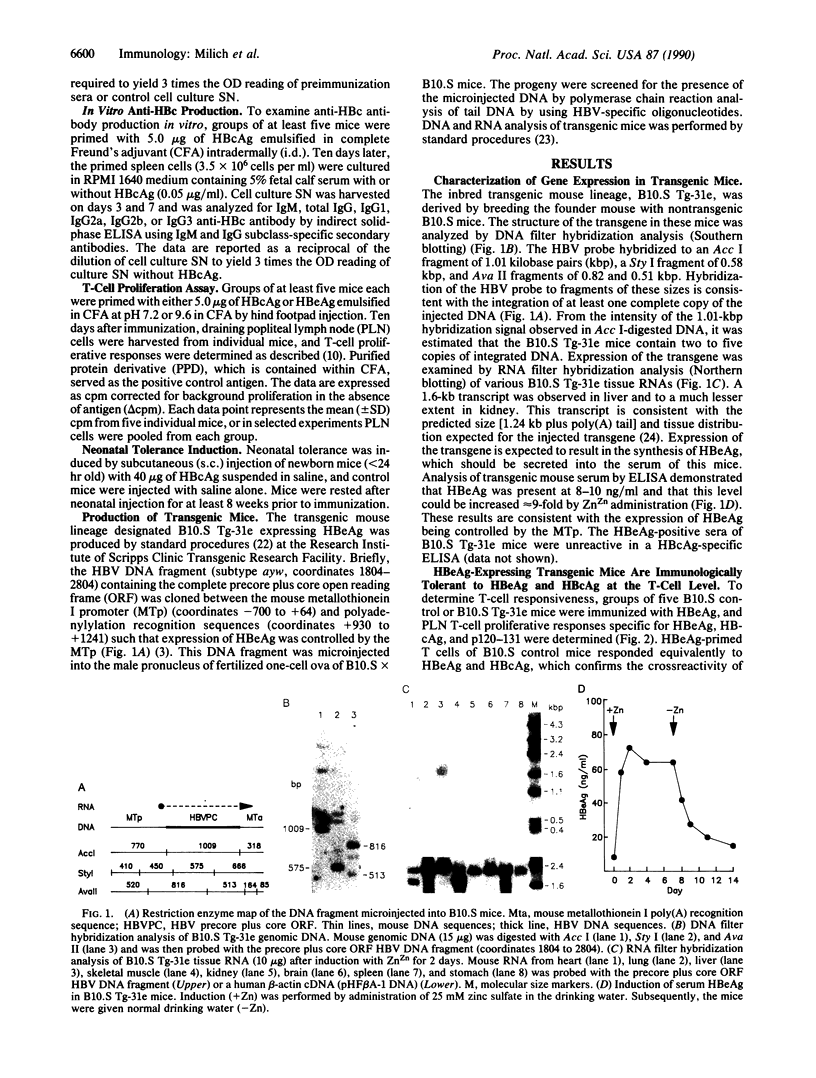
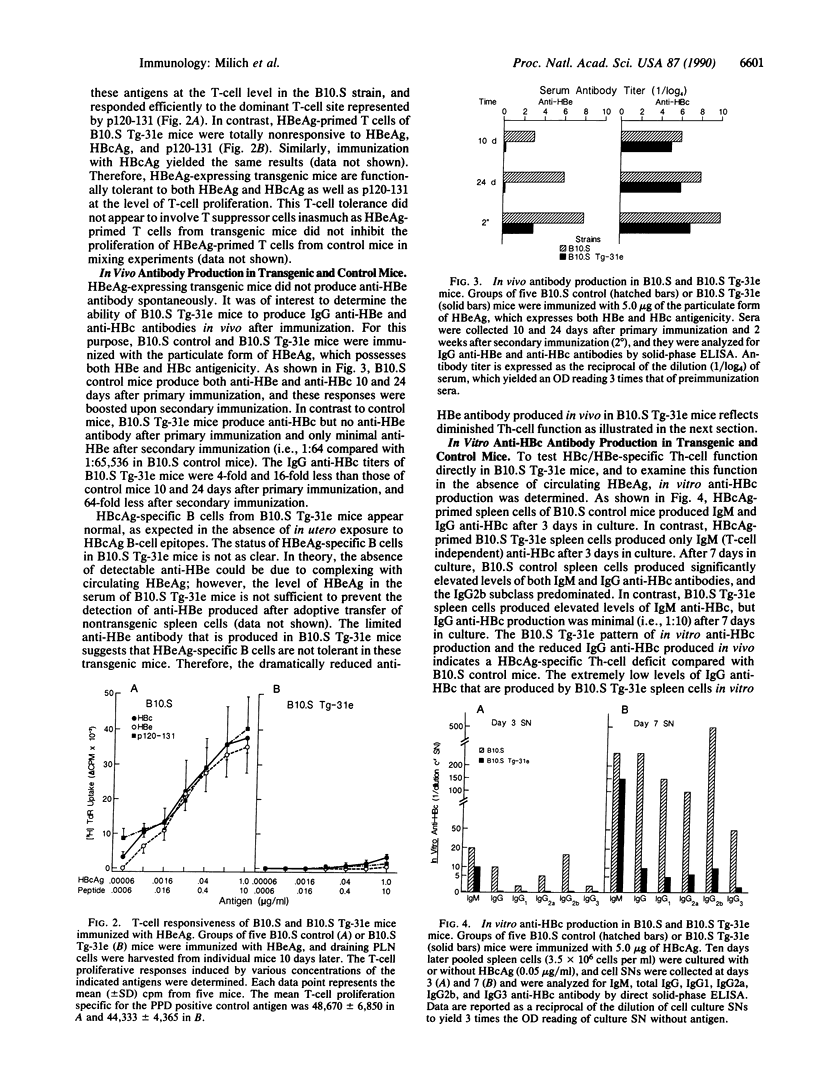

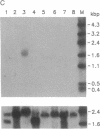
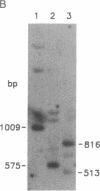
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldershvile J., Frösner G. G., Nielsen J. O., Hardt F., Deinhardt F., Skinhøj P. Hepatitis B e antigen and antibody measured by radioimmunoassay in acute hepatitis B surface antigen-positive hepatitis. J Infect Dis. 1980 Mar;141(3):293–298. doi: 10.1093/infdis/141.3.293. [DOI] [PubMed] [Google Scholar]
- Arakawa K., Tsuda F., Takahashi K., Ise I., Naito S., Kosugi E., Miyakawa Y., Mayumi M. Maternofetal transmission of IgG-bound hepatitis B e antigen. Pediatr Res. 1982 Mar;16(3):247–250. doi: 10.1203/00006450-198203000-00017. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Hwang L. Y. Postnatal infectivity of hepatitis B surface antigen-carrier mothers. J Infect Dis. 1983 Feb;147(2):185–190. doi: 10.1093/infdis/147.2.185. [DOI] [PubMed] [Google Scholar]
- Beasley R. P., Trepo C., Stevens C. E., Szmuness W. The e antigen and vertical transmission of hepatitis B surface antigen. Am J Epidemiol. 1977 Feb;105(2):94–98. doi: 10.1093/oxfordjournals.aje.a112370. [DOI] [PubMed] [Google Scholar]
- Biswas S. C., Gupta I., Ganguly N. K., Chawla Y., Dilawari J. B. Prevalence of hepatitis B surface antigen in pregnant mothers and its perinatal transmission. Trans R Soc Trop Med Hyg. 1989 Sep-Oct;83(5):698–700. doi: 10.1016/0035-9203(89)90401-x. [DOI] [PubMed] [Google Scholar]
- Chang C., Enders G., Sprengel R., Peters N., Varmus H. E., Ganem D. Expression of the precore region of an avian hepatitis B virus is not required for viral replication. J Virol. 1987 Oct;61(10):3322–3325. doi: 10.1128/jvi.61.10.3322-3325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudeau A., Yvonnet B., Lesage G., Barin F., Denis F., Coursaget P., Chiron J. P., Diop Mar I. Lack of anti-HBc IgM in neonates with HBsAg carrier mothers argues against transplacental transmission of hepatitis B virus infection. Lancet. 1983 Nov 12;2(8359):1103–1104. doi: 10.1016/s0140-6736(83)90625-6. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Lee S. D., Lo K. J., Tsai Y. T., Wu J. C. Maternal hepatitis B virus DNA in mother-infant transmission. Lancet. 1989 Apr 1;1(8640):719–719. doi: 10.1016/s0140-6736(89)92227-7. [DOI] [PubMed] [Google Scholar]
- Lee S. D., Lo K. J., Wu J. C., Tsai Y. T., Wang J. Y., Ting L. P., Tong M. J. Prevention of maternal-infant hepatitis B virus transmission by immunization: the role of serum hepatitis B virus DNA. Hepatology. 1986 May-Jun;6(3):369–373. doi: 10.1002/hep.1840060306. [DOI] [PubMed] [Google Scholar]
- Li L., Sheng M. H., Tong S. P., Chen H. Z., Wen Y. M. Transplacental transmission of hepatitis B virus. Lancet. 1986 Oct 11;2(8511):872–872. doi: 10.1016/s0140-6736(86)92916-8. [DOI] [PubMed] [Google Scholar]
- McLachlan A., Milich D. R., Raney A. K., Riggs M. G., Hughes J. L., Sorge J., Chisari F. V. Expression of hepatitis B virus surface and core antigens: influences of pre-S and precore sequences. J Virol. 1987 Mar;61(3):683–692. doi: 10.1128/jvi.61.3.683-692.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milich D. R., Jones J. E., McLachlan A., Houghten R., Thornton G. B., Hughes J. L. Distinction between immunogenicity and tolerogenicity among HBcAg T cell determinants. Influence of peptide-MHC interaction. J Immunol. 1989 Nov 15;143(10):3148–3156. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Moriarty A., Thornton G. B. Immune response to hepatitis B virus core antigen (HBcAg): localization of T cell recognition sites within HBcAg/HBeAg. J Immunol. 1987 Aug 15;139(4):1223–1231. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Stahl S., Wingfield P., Thornton G. B., Hughes J. L., Jones J. E. Comparative immunogenicity of hepatitis B virus core and E antigens. J Immunol. 1988 Nov 15;141(10):3617–3624. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science. 1986 Dec 12;234(4782):1398–1401. doi: 10.1126/science.3491425. [DOI] [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Mondelli M., Vergani G. M., Alberti A., Vergani D., Portmann B., Eddleston A. L., Williams R. Specificity of T lymphocyte cytotoxicity to autologous hepatocytes in chronic hepatitis B virus infection: evidence that T cells are directed against HBV core antigen expressed on hepatocytes. J Immunol. 1982 Dec;129(6):2773–2778. [PubMed] [Google Scholar]
- Ohto H., Lin H. H., Kawana T., Etoh T., Tohyama H. Intrauterine transmission of hepatitis B virus is closely related to placental leakage. J Med Virol. 1987 Jan;21(1):1–6. doi: 10.1002/jmv.1890210102. [DOI] [PubMed] [Google Scholar]
- Ou J. H., Laub O., Rutter W. J. Hepatitis B virus gene function: the precore region targets the core antigen to cellular membranes and causes the secretion of the e antigen. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1578–1582. doi: 10.1073/pnas.83.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli M., Waters J., Lever A., Iwarson S., Gerety R., Thomas H. C. Cytotoxic T-cell responses to the nucleocapsid proteins of HBV in chronic hepatitis. Evidence that antibody modulation may cause protracted infection. J Hepatol. 1987 Feb;4(1):15–21. doi: 10.1016/s0168-8278(87)80004-1. [DOI] [PubMed] [Google Scholar]
- Poovorawan Y., Sanpavat S., Pongpunlert W., Chumdermpadetsuk S., Sentrakul P., Safary A. Protective efficacy of a recombinant DNA hepatitis B vaccine in neonates of HBe antigen-positive mothers. JAMA. 1989 Jun 9;261(22):3278–3281. [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlicht H. J., Salfeld J., Schaller H. The duck hepatitis B virus pre-C region encodes a signal sequence which is essential for synthesis and secretion of processed core proteins but not for virus formation. J Virol. 1987 Dec;61(12):3701–3709. doi: 10.1128/jvi.61.12.3701-3709.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl S., MacKay P., Magazin M., Bruce S. A., Murray K. Hepatitis B virus core antigen: synthesis in Escherichia coli and application in diagnosis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1606–1610. doi: 10.1073/pnas.79.5.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Ou J. H., Masiarz F. R., Rutter W. J. A signal peptide encoded within the precore region of hepatitis B virus directs the secretion of a heterogeneous population of e antigens in Xenopus oocytes. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8405–8409. doi: 10.1073/pnas.85.22.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. E., Beasley R. P., Tsui J., Lee W. C. Vertical transmission of hepatitis B antigen in Taiwan. N Engl J Med. 1975 Apr 10;292(15):771–774. doi: 10.1056/NEJM197504102921503. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Machida A., Funatsu G., Nomura M., Usuda S., Aoyagi S., Tachibana K., Miyamoto H., Imai M., Nakamura T. Immunochemical structure of hepatitis B e antigen in the serum. J Immunol. 1983 Jun;130(6):2903–2907. [PubMed] [Google Scholar]
- Thomas H. C., Jacyna M., Waters J., Main J. Virus-host interaction in chronic hepatitis B virus infection. Semin Liver Dis. 1988 Nov;8(4):342–349. doi: 10.1055/s-2008-1040555. [DOI] [PubMed] [Google Scholar]