Abstract
Background:
With aging, health deficits accumulate: people with few deficits for their age are fit, and those with more are frail. Despite recent reports of improved health in old age, how deficit accumulation is changing is not clear. Our objectives were to evaluate changes over 30 years in the degree of deficit accumulation and in the relationship between frailty and mortality in older adults.
Methods:
We analyzed data from two population based, prospective longitudinal cohorts, assembled in 1971–1972 and 2000–2001, respectively. Residents of Gothenburg Sweden, systematically drawn from the Swedish population registry. The 1901–1902 cohort (N = 973) had a response rate of 84.8%; the 1930 cohort (N = 500) had a response rate of 65.1%. A frailty index using 36 deficits was calculated using data from physical examinations, assessments of physical activity, daily, sensory and social function, and laboratory tests. We evaluated mortality over 12.5 years in relation to the frailty index.
Results:
Mean frailty levels were the same ( = 0.20, p = .37) in the 1901–1902 cohort as in the 1930 cohort. Although the frailty index was linked to the risk of death in both cohorts, the hazards ratio decreased from 1.67 per 0.1 increment in the frailty index for the first cohort to 1.32 for the second cohort (interaction term p = .005).
Discussion:
Although frailty was as common at age 70 as before, its lethality appears to be less. Just why this is so should be explored further.
Keywords: Frail older adults, Frailty index, Cohort effects, Deficit accumulation, Mortality
Over the last century, life expectancy in higher income countries has increased by approximately 30 years, accompanying lower infant mortality, less mortality from infectious diseases and a longer life span, both from birth and from age 65 years (1), as well as decline in chronic disabilities (2,3). With this, many age-associated chronic illnesses and health problems have become more common (1,4,5). Even so, several reports suggest that, during the last decades, functional ability (6), cognitive function (6,7), and lung function (8) have improved among older people. Individual conditions vary (9) [eg, less hypertension (10) and hypercholesterolemia (11), more obesity (12), and diabetes mellitus (13)] as do results between studies (14). Results can vary within studies—for example, in England, the extent of improvement in the health of older adults depends on which aspect of health is measured: cognition, disability, or health attitude (15). All this challenges summary statements about population health (16,17).
One summary health measure is a frailty index, which for any individual is the fraction of health deficits that they have, in relation to the number of health deficits evaluated (18). (eg, in a study that evaluated 50 health deficits, someone with 10 deficits present would have a frailty index score of 10/50 = 0.20.) Frailty itself is a state of increased risk which reflects multisystem physiological changes (19,20). Accumulated health deficits put older people at risk for adverse health outcomes, including death, disability, dependency, falls, and need for long-term care (20,21). Frailty has been related to mortality in both general population (22–24) and clinical studies (25–27). Effects can be far-reaching: a frailty index predicted 25-year mortality in 70-year-olds examined in 1971–1972 (5).
Although mortality increases as deficits accumulate, their lethality can vary. In China, for example, any given level of deficit accumulation is more lethal than in high income countries (18,28). We wondered therefore whether within a country this might vary over time. Improved perinatal care, early childhood education, working conditions, antibiotics, and lifelong health care could favor later-born cohorts; alternately, more sedentary lifestyles might make them more vulnerable.
To understand how frailty and its lethality might be changing, we compared frailty indices and their relation to medium term mortality (12.5 years) between two cohorts of 70-year-olds born 1901–1902 and 1930. We have reported that worse lung function and lower cognitive performance was related to a higher mortality in the cohort born 1901–1902 but not in the one born in 1930 (7,8). Based on these findings, we hypothesized that the later-born birth cohort should sustain deficits better than the earlier-born cohort.
Methods
The two H-70 samples were systematically drawn from the Swedish population registry.
Birth Cohort 1901–1902: In 1971–1972, 70-year-olds living in Gothenburg and born between July 1, 1901 and June 30, 1902 with birth dates ending with two, five, or eight were invited to participate (N = 973; response rate 84.8%) (29). Participants differed from nonparticipants in that male responders had a higher mean income compared to nonresponders. In women, responders more often had received in-patient care than had nonresponders.
Birth Cohort 1930: In 2000–2001, 70-year-olds born in 1930 on Days 3, 6, 12, 18, 21, 24, or 30 of each month were invited (30). The response rate was 65.1% (N = 500). Participants were more often women and showed less 3-year-mortality and less psychiatric illness in the Swedish Hospital Discharge Registry than did nonparticipants.
Examinations took place at a geriatric outpatient clinic or in the participant’s residence. Physical, neuropsychiatric, and neuropsychological examinations, assessments of physical activity, daily, sensory and social functioning, laboratory tests, ECG, and biochemical measures were recorded.
Measures
We included 38 deficits in the frailty index (Table 1). Deficits must be health- or age-related, and together should cover a range of systems; individually they should be neither too rare (<1%) nor too common (>80%) (31). We included only items which were comparable between cohorts and only individuals with ≥80% of responses on all deficits. All items were dichotomized and recoded so that “1” indicates the presence of a deficit and “0” indicates its absence.
Table 1.
Variables Included in the Index
| Prevalence % Cohort 1901–1902 | Prevalence % Cohort 1930 | ||
|---|---|---|---|
| 1 | Are you capable of washing yourself? | 4.06 | 3.10 |
| 2 | Do you feel lonely? | 6.41 | 2.90 |
| 3 | Daily contact with other people through meetings, phone contacts, emails, etc. | 15.32 | 43.75 |
| 4 | Feeling well | 34.40 | 21.58 |
| 5 | Feeling tired | 21.97 | 36.67 |
| 6 | Feeling frozen | 6.74 | 20.13 |
| 7 | Appetite problems | 7.98 | 4.03 |
| 8 | Calf pain when walking | 8.29 | 7.77 |
| 9 | Swollen legs or wrists | 20.00 | 32.84 |
| 10 | Have you experienced chest pain? | 21.35 | 31.09 |
| 11 | Have you experienced chest tightness? | 18.17 | 25.69 |
| 12 | Do you feel exhaustion or feelings of weakness (born 1971–1972) or out of breath (born 1930) after mild physical activity? | 3.23 | 26.32 |
| 13 | Wheezing sound from chest | 13.28 | 22.90 |
| 14 | Back pain | 27.67 | 43.62 |
| 15 | Joint problems | 21.45 | 25.68 |
| 16 | Are you capable of rising from a chair? | 9.53 | 6.44 |
| 17 | Are you capable of rising from bed? | 7.88 | 2.44 |
| 18 | Are you capable of moving around? | 8.29 | 8.20 |
| 19 | Dyspnea | 26.38 | 3.80 |
| 20 | Systolic murmur | 30.98 | 7.59 |
| 21 | Auscultation findings from lungs | 16.06 | 4.43 |
| 22 | Tremor | 12.24 | 3.62 |
| 23 | Systolic blood pressure > 140mm Hg | 88.50 | 80.92 |
| 24 | Diastolic blood pressure > 90mm Hg | 65.70 | 37.53 |
| 25 | Diabetes mellitus | 5.60 | 10.69 |
| 26 | Angina pectoris | 10.36 | 33.75 |
| 27 | Have you had a myocardial infarction? | 4.66 | 7.34 |
| 28 | Have you had a peptic ulcer? | 16.89 | 13.38 |
| 29 | Do you have rheumatoid arthritis? | 4.46 | 2.55 |
| 30 | Have you had cancer? | 7.25 | 14.62 |
| 31 | Treatment in a hospital during the last 10 (born 1901–1902) or 8 (born 1930) years? | 52.19 | 44.89 |
| 32 | Hemoglobin <120 for women, <130 for men | 4.67 | 2.77 |
| 33 | BMI (<20 or >30) | 19.38 | 24.16 |
| 34 | Waist circumference >89cm for women >97cm for men | 25.91 | 24.47 |
| 35 | Do you have your own teeth left? | 75.85 | 24.35 |
| 36 | Are you able to chew food? | 12.85 | 6.21 |
| 37 | Dryness of mouth | 20.83 | 32.68 |
| 38 | Pain from mouth or teeth | 16.29 | 3.27 |
Dates of death over 12.5 years were obtained from the Swedish Population Registry. This is a national register covering all people living in Sweden and Swedish citizens living abroad.
Analyses
We used simple summary statistics and t-tests to examine baseline differences. Cox regression and Kaplan–Meier curves were used to analyze the relationship between frailty index and mortality. Two Cox models were fitted using the entire sample. One employed the frailty index as a continuous variable. In the second, the frailty index was divided into four previously employed frailty classes, defined as none (0–0.12), vulnerable (0.13–0.22), mild-moderate (0.23–0.39), and severe (>0.40) (32). Adjustments for sex and cohort were included in both models and interactions between sex and frailty index as well as cohort and frailty index were included and kept in the model only if significant. The assumption of proportional hazards was checked with simulation methods and negative log-log plots from Kaplan–Meier analysis. All analyses were performed with SAS 9.3.
Results
The prevalence of each deficit used to calculate the frailty indices are shown in Table 1. Both frailty indices showed a skewed distribution with a long right tail, and a submaximal limit. After excluding those with missing data on frailty (8 in the first cohort and 13 in the second), 1,442 individuals (965 from the first cohort and 477 from the second cohort) were available for all subsequent analyses. The mean value of the frailty index was the same (0.20; t-test = 0.89, df = 1,440, p = .37; Table 2) in both cohorts and both frailty indices had similar standard deviations. A Kolmogorov–Smirnov test for the equality of distributions could not reject the null hypothesis of equal distributions (KS = 1.00, p = .27). Mortality over the 12.5-year-follow-up was significantly higher in the 1901–1902 cohort compared to the 1930 cohort (50.0% vs 27.9 %; p < .001).
Table 2.
Characteristics of the Frailty Indexes and Cohorts
| 1971–1972 (n = 965) | 2000–2003 (n = 477) | |
|---|---|---|
| Mean (SD) | 0.20 (0.10) | 0.20 (0.11) |
| Median | 0.18 | 0.18 |
| Range | 0–0.58 | 0–0.66 |
| % died within 12.5 years | 49.9 | 27.9 |
| % female | 53.7 | 52.4 |
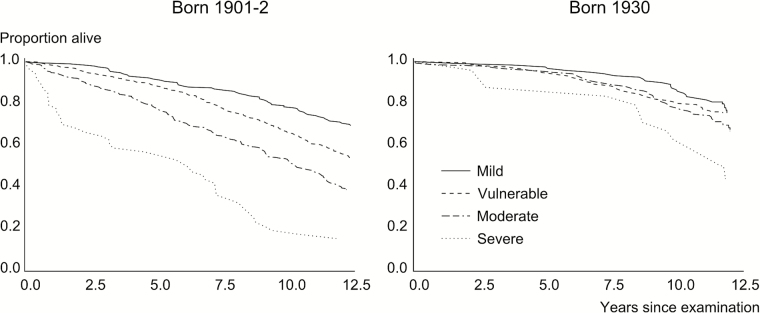
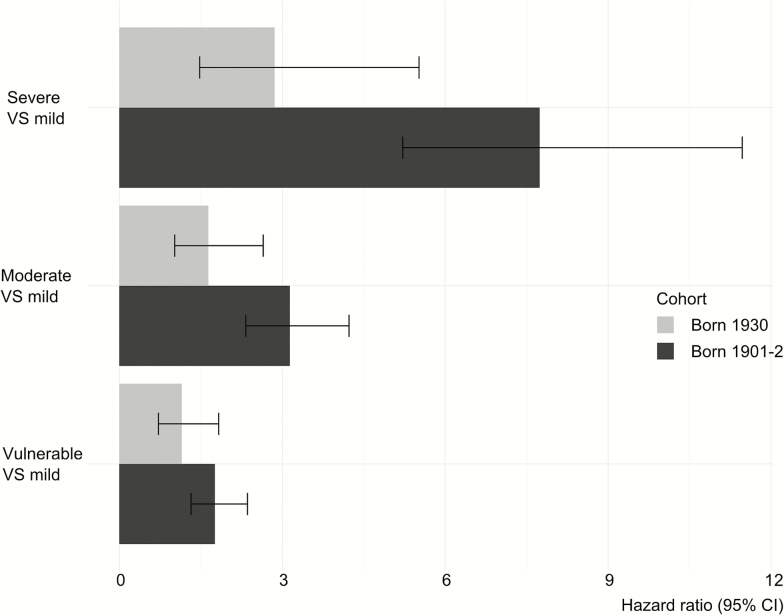
Frailty as a continuous measure (the first model) was related to mortality in both cohorts (Table 3), but was less associated with mortality in the 1930 cohort than in the 1901–1902 cohort (HR 1.68 for a 0.1 increase in the frailty index for the first cohort and 1.32 for the second cohort; interaction effect between cohort and frailty: p = .005). For the second model with frailty as a categorical variable (Table 3), the frailty index was also less associated with mortality in cohort 1930 than in cohort 1901–1902 (Figures 1 and 2). In both models, women had a lower mortality rate than did men. There was no interaction between gender and frailty in any of the models, indicating that gender did not modify the association between the frailty index and mortality. The assumption of proportional hazards was validated for both models.
Table 3.
Cox Regression Relating Survival to Frailty (FI): Model 1 and Model 2
| Model 1 (Continuous Frailty) | Model 2 (Categorical Frailty) | |||||
|---|---|---|---|---|---|---|
| Variable | Log HR (SE) | P > x2 | HR (95% CI) | Log HR (SE) | P > x2 | HR (95% CI) |
| FI | 5.10 (0.43) | <.001 | ||||
| FI × Cohort 2 | −2.36 | .005 | ||||
| FI ∆ 0.1 for cohort 1 | 0.51 (0.04) | <.001 | 1.67 (1.53, 1.81) | |||
| FI ∆ 0.1 for cohort 2 | 0.27 (0.07) | <.001 | 1.32 (1.14, 1.52) | |||
| FI (vulnerable vs mild) × Cohort 2 | −0.43 (0.28) | .13 | ||||
| FI for cohort 1 (vulnerable vs mild) | 0.57 (0.15) | <.001 | 1.76 (1.32, 2.36) | |||
| FI for cohort 2 (vulnerable vs mild) | 0.14 (0.24) | .56 | 1.15 (0.72, 1.83) | |||
| FI (moderate vs mild) * Cohort 2 | −0.65 (0.29) | .02 | ||||
| FI for cohort 1 (moderate vs mild) | 1.14 (0.15) | <.001 | 3.14 (2.33, 4.23) | |||
| FI for cohort 2 (moderate vs mild) | 0.50 (0.24) | .04 | 1.64 (1.02, 2.65) | |||
| FI (severe vs mild) × Cohort 2 | −0.99 (0.39) | .01 | ||||
| FI for cohort 1 (severe vs mild) | 2.05 (0.20) | <.001 | 7.74 (5.22, 11.47) | |||
| FI for cohort 2 (severe vs mild) | 1.05 (0.36) | .002 | 2.86 (1.48, 5.52) | |||
| Sex (female) | −0.82 (0.08) | <.001 | 0.44 (0.38, 0.52) | −0.81 (0.08) | <.001 | 0.46 (0.38, 0.52) |
| Cohort (2) FI | −0.78 (0.10) | <.001 | 0.46 (0.38, 0.56) | −0.97 (0.17) | <.001 | 0.38 (0.27, 0.53) |
Note: For frailty (FI) as a continuous measure the incremental change is 0.1 units. In Model 1, frailty is centered at the mean value and cohort mortality is assessed at that value. For Model 2, cohort mortality is assessed at mild frailty levels. The categories of frailty were based on four previously decided frailty classes, defined as mild (0–0.12), vulnerable (0.13–0.22), moderate (0.23–0.39), and severe (>0.40).
Figure 1.
Survival curves based on the level of frailty.
Figure 2.
Bar graph of the hazards ratios.
Discussion
Overview
Increasing values of a frailty index calculated from 38 health deficits were associated with mortality in Swedish population-based samples of 70-year-olds born 1901–1902 and 1930. Although the mean frailty index scores were the same, their lethality was lower in the 1930 cohort than in the cohort born in 1901–1902. This suggests that present cohorts of older Swedish adults might better be able to tolerate health deficits than were their immediate ancestors. This result is important in light of increasing survival, and thus increasing numbers of older people world-wide.
Possible Sources of Cohort Differences
Several factors might contribute to these cohort differences. Unknown and unmeasured effect modifiers across the life-span likely operate. Given their more favorable life circumstances, present birth cohorts of individuals in the oldest ages are a less selected surviving population than earlier-born cohorts. Of those born 1901–1902, only 60% of women and 51% of men survived to age 70. In contrast, of those born in 1930, 78% of women and 66% of men were alive at age 70. These numbers reflect both higher childhood mortality (87% of women born in 1901 lived until age 5 compared with 93% of the later-born cohort) and a higher mortality across the lifespan in those born 1901–1902 compared with those born 1930.
Logically, the presence of any health deficit reflects damage that has gone unremoved or unrepaired (18). More favorable developmental conditions might mean that later-born cohorts can better withstand later onset cognitive and physical deficits (33). The 1901–1902 cohort probably experienced more factors that reduced damage, or diminished their ability to withstand deficits, such as worse working and housing conditions. Likewise, more effective health care represents a form of better repair capability.
The fewer deficits and less lethality of frailty in the later-born cohort could also reflect that the frequency of underlying deficits differed, even if mean values were the same. For example, symptoms such as shortness of breath when walking, angina pectoris, stomach aches and headaches increased, whereas high blood pressure, lung auscultation abnormalities, and mouth/tooth pain decreased (data not shown). A shift to less lethal diseases might reflect more effective prevention or therapies. In this way, increased life expectancy can be coupled with more chronic disease (1,34). In the Framingham study, a frailty index calculated for different birth cohorts showed different associations with mortality for those items which had improved when compared with those which had not changed or worsened. Deficits which had worsened had little or no impact on mortality risk, whereas those which had improved predicted long term survival and those which showed no change predicted short term survival (35). The current report makes clear that any frailty index value needs to be interpreted in the context in which it is found.
Strengths and Limitations
The strengths include the use of similar designs and questionnaires during three decades, and the large time interval between the examinations. Limitations are first, that differences in participation rate between the cohorts could lead to different participation biases in the two surveys, so that relatively fewer healthy individuals in the second cohort might have participated than in the first one. This should however not influence the association between frailty and mortality, but might influence differences in mean frailty index between cohorts. Second, despite similar methodologies, there were differences between the examinations regarding formulations of questions, examiners and other methodological factors. For example, some questions were answered during an interview in the first examination and in the later examinations these questions were answered using a self-administered questionnaire (Table 1, the prevalence columns). It shows that individual items vary between the two FIs. That is commonly the case, and indeed we see similar mean FI/age values even comparing surveys that use only self-report to those that use largely examination/test measures. Third, comparison of two cohorts might not solely reveal cohort effects; differences might also be due to period effects.
Frailty and Aging: Conceptualization
Our data contribute to the debate over frailty operationalization, joining reports of frailty operationalized as deficit accumulation, from the level of cellular biomarkers (36) to common clinical and laboratory tests (37,38), to clinically evident deficits (39). Proponents of frailty as a definable syndrome/phenotype argue that the frailty index, in its indifference to its constituent items, does not allow for the elucidation of mechanisms and physiological etiology (19). This argument turns on what is meant by a mechanism and whether there is likely to be a mechanism for aging or whether it arises from interactions of many deficits resulting in physiological dysregulation across multiple systems components (24,36,40–43). Note that another H70 report of secular changes in frailty found that the proportion of people who were phenotypcially frail had declined between the two cohorts (44). Given that so much age-related damage arises in that context (18,43) we believe that environmental change (health care, social circumstances) is about all that could reasonably have changed in the interval. How this is related to changes in resilience on aging (45,46) remains to be seen. Even so, that study included only three of the five items needed to operationalize the frailty phenotype, and did not report mortality differences by degree of frailty. Although frailty phenotype reports often are operationalized differently than in the original report, a systematic review found that results vary depending on which items are used and are least stable when fewer than five items are employed (47).
Conclusions
The association between frailty and mortality was weaker in the 2000s than in the 1970s. This shows that the effect of the frailty index on mortality is relative, depending on the historical period in which the data were collected. Unmeasured effects from early- and mid-life, differences in physical reserve and repair between cohorts, and qualitative differences in the composition of the index probably may explain some of this difference. In a world where the number of people reaching old age is increasing, our findings have useful and positive public health implications, including for managing frailty as a long term condition (48). At a minimum, it appears that elderly people today have greater opportunities for health in old age, despite the presence of frailty.
Funding
K.B., E.J., H.F., and I.S. was supported by The Swedish Research Council (11267, 825-2007-7462, 825-2012-5041, 2013–8717, 2015–02830), Swedish Council for Working Life and Social Research (2001–2835, 2004-0145, 2006-0596, 2008-1111, 2010-0870, 2013-1202, 2013–2496, AGECAP 2013–2300, Epilife 2006-1506), Swedish Brain Power, The Alzheimer’s Association Zenith Award (ZEN-01-3151) The Alzheimer’s Association Stephanie B. Overstreet Scholars (IIRG-00-2159), Sahlgrenska University Hospital, The Bank of Sweden Tercentenary Foundation, Konung Gustaf V:s och Drottning Victorias Frimurarestiftelse, Eivind och Elsa K:son Sylvans stiftelse, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, Stiftelsen Professor Bror Gadelius’ Minnesfond. K.R. receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research. A.M. receives support from the Canadian Institutes of Health Research operating fund grant (MOP 115006).
Conflict of Interest
All authors have completed the ICMJE uniform disclosure at www.icmje.org/coi_disclosure.pdf and declare: K.B., E.J., I.S., and H.F. have nothing to disclose. K.R. and A.M. have previously applied unsuccessfully to various commercial schemes which have been reported in previous publications.
Acknowledgments
Author contributions: K.B. and E.J. did all statistical analysis and wrote the manuscript. I.S., K.R., A.M., and H.F. interpreted the results and contributed to review and revision of the manuscript. All authors read the manuscript and contributed to the final version. All authors had full access to all of the data in the study and take responsibility for the integrity and the accuracy of the data analysis. Ethical approval: The Ethics Review Board in Gothenburg approved the study. All participants gave their written informed consent. Transparency: The lead author (K.B. and E.J.) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. Data sharing: Patient level data and statistical code are available from I.S. at ingmar.skoog@neuro.gu.se.
References
- 1. Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet. 2009;374 (9696):1196–1208. doi:10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Manton KG, Gu X, Lamb VL. Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Natl Acad Sci U S A. 2006;103:18374–18379. doi:10.1073/pnas.0608483103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Manton KG. Recent declines in chronic disability in the elderly U.S. population: risk factors and future dynamics. Annu Rev Public Health. 2008;29:91–113. doi:10.1146/annurev.publhealth.29.020907.090812 [DOI] [PubMed] [Google Scholar]
- 4. Shamliyan T, Talley KM, Ramakrishnan R, et al. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12(2):719–736. doi:10.1016/j.arr.2012.03.001 [DOI] [PubMed] [Google Scholar]
- 5. Rockwood K, Mitnitski A, Song X, et al. Long term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc. 2006;54(6):975–979. doi:10.1111/j.1532-5415.2006.00738.x [DOI] [PubMed] [Google Scholar]
- 6. Christensen K, Thinggaard M, Oksuzyan A, et al. Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet. 2013;382:1507–1513. doi:10.1016/S0140-6736(13)60777-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sacuiu S, Gustafson D, Sjögren M, et al. Secular changes in cognitive predictors of dementia and mortality in 70-year-olds. Neurology. 2010;75:779–785. doi:10.1212/WNL.0b013e3181f0737c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lak VW, Skoog I, Guo X. Secular trends in lung function and its relation to survival in Swedish 75 year olds 1976–2006. Age Ageing. 2012;41:735–740. doi:10.1093/ageing/afs098 [DOI] [PubMed] [Google Scholar]
- 9. Global Burden of Disease Study 2013 Collaborators. Global regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi:10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Danaei G, Finucane MM, Lin JK, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5· 4 million participants. Lancet. 2011;377(9765):568–577. doi:10.1016/S0140-6736(10)62036-3 [DOI] [PubMed] [Google Scholar]
- 11. Farzadfar F, Finucane MM, Danaei G et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3· 0 million participants. Lancet. 2011;377(9765):578–586. doi:10.1016/S0140-6736(10)62038-7 [DOI] [PubMed] [Google Scholar]
- 12. Ford ES, Maynard LM, Li C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA. 2014;312:1151–1153. doi:10.1001/jama.2014.8362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhi X, Joas E, Waern M, et al. Prevalence of cardiovascular disorders and risk factors in two 75-year-old birth cohorts examined in 1976–1977 and 2005–2006. Aging Clin Exp Res. 2013;25:377–383. doi:10.1007/s40520-013-0058-1 [DOI] [PubMed] [Google Scholar]
- 14. Wu YT, Fratiglioni L, Matthews FE, Lobo A, Bretler MM, Skoog I, Brayne C. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15:116–124. doi:10.1016/S1474-4422(15)00092-7 [DOI] [PubMed] [Google Scholar]
- 15. Jagger C, Matthews FE, Wohland P, et al. , A comparison of health expectancies over two decades in England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2016;387(10020):779–786. doi:10.1016/S0140-6736(15)00947-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rockwood K. What can we expect of health in old age? Lancet. 2016;387(10020):730–731. doi:10.1016/S0140-6736(15)01022-3 [DOI] [PubMed] [Google Scholar]
- 17. Freedman VA, Crimmins E, Schoeni RF, et al. Resolving inconsistencies in trends in old-age disability: report from a technical working group. Demography. 2004;41:417–441. [DOI] [PubMed] [Google Scholar]
- 18. Mitnitski A, Rockwood K. Aging as a process of deficit accumulation: its utility and origin. Interdiscip Top Gerontol. 2015;40:85–98. doi:10.1159/000364933 [DOI] [PubMed] [Google Scholar]
- 19. Walston JD, Bandeen-Roche K. Frailty: a tale of two concepts. BMC Med. 2015;13:185. doi:10.1186/s12916-015-0420-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet. 2013;381(9868):752–762. Erratum in Lancet 2013;382(9901). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc. 2005;53:2184–2189. doi:10.1111/j.1532-5415.2005.00506.x [DOI] [PubMed] [Google Scholar]
- 22. Saum KU, Dieffenbach AK, Muller H, et al. Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol. 2014;29(3):171–179. [DOI] [PubMed] [Google Scholar]
- 23. Clegg A, Bates C, Young J, et al. Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Ageing. 2016;45:353–360. doi:10.1016/S0140-6736(12)62167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mekli K, Marshall A, Nazroo J, Vanhoutte B, Pendleton N. Genetic variant of Interleukin-18 gene is associated with the Frailty Index in the English Longitudinal Study of Ageing. Age Ageing. 2015;44:938–942. doi:10.1093/ageing/afv122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kelaiditi E, Andrieu S, Cantet C, Vellas B, Cesari M; ICTUS/DSA Group Frailty index and incident mortality, hospitalization, and institutionalization in Alzheimer’s disease: data from the ICTUS Study. J Gerontol A Biol Sci Med Sci. 2016;71:543–548. doi:10.1093/gerona/glv137 [DOI] [PubMed] [Google Scholar]
- 26. Pajewski NM, Williamson JD, Applegate WB, et al. ; SPRINT Study Research Group. Characterizing frailty status in the systolic blood pressure intervention trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi:10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zeng A, Song X, Dong J, et al. Mortality in relation to frailty in patients admitted to a specialized geriatric intensive care unit. J Gerontol A Biol Sci Med Sci. 2015;70(12):1586–1594. doi:10.1093/gerona/glv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shi J, Song X, Yu P, et al. Analysis of frailty and survival from late middle age in the Beijing Longitudinal Study of Aging. BMC Geriatr. 2011;11:17. doi:10.1186/1471-2318-11-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rinder L, Roupe S, Steen B, et al. Seventy‐year‐old People in Gothenburg. A population study in an industrialized Swedish city. Acta Med Scand. 1975;198(1–6):397–407. [DOI] [PubMed] [Google Scholar]
- 30. Beckman N, Waern M, Gustafson D, Skoog I. Secular trends in self reported sexual activity and satisfaction in Swedish 70 year olds: cross sectional survey of four populations, 1971–2001. BMJ. 2008;337:a279. doi:10.1136/bmj.a279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Ger Med. 2011;27(1):17. doi:10.1016/j.cger.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 32. Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ: Can Med Assoc J. 2005;173(5):489–495. doi:10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- 34. Parker M, Schon P, Lagergren M, et al. Functional ability in the elderly Swedish population from 1980 to 2005. Eur J Ageing. 2008;5(4):299–309. doi:10.1007/s10433-008-0096-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kulminski AM, Arbeev KG, Ukraintseva SV, et al. Changes in health status among participants of the Framingham Heart Study from the 1960s to the 1990s: application of an index of cumulative deficits. Ann Epidemiol. 2008;18(9):696–701. doi:10.1016/j.annepidem.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mitnitski A, Collerton J, Martin-Ruiz C, et al. Age-related frailty and its association with biological markers of ageing. BMC Med. 2015;13:161. doi:10.1186/s12916-015-0400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Med. 2014;12:171. doi:10.1186/s12916-014-0171-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Blodgett JM, Theou O, Howlett SE, Wu FC, Rockwood K. A frailty index based on laboratory deficits in community-dwelling men predicted their risk of adverse health outcomes. Age Ageing. 2016. doi:10.1093/ageing/afw054 [DOI] [PubMed] [Google Scholar]
- 39. Warwick J, Falaschetti E, Rockwood K, et al. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78. doi:10.1186/s12916-015-0328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim S, Jazwinski SM. Quantitative measures of healthy aging and biological age. Healthy Aging Res. 2015;4: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pincus Z. Ageing: a stretch in time. Nature. 2016;530(7588):37–38. [DOI] [PubMed] [Google Scholar]
- 42. Taneja S, Mitnitski A, Rockwood K, Rutenberg A. Dynamical network model for age-related health deficits and mortality. Phys Rev E. 2016;93:022309. doi:10.1103/PhysRevE.93.022309 [DOI] [PubMed] [Google Scholar]
- 43. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi:10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hörder H, Skoog I, Johansson L, Falk H, Frändin K. Secular trends in frailty: a comparative study of 75-year olds born in 1911–12 and 1930. Age Ageing. 2015;44:817–822. doi:10.1093/ageing/afv084 [DOI] [PubMed] [Google Scholar]
- 45. Whitson HE, Duan-Porter W, Schmader KE, Morey MC, Cohen HJ, Colón-Emeric CS. Physical resilience in older adults: systematic review and development of an emerging construct. J Gerontol A Biol Sci Med Sci. 2016;71:489–495. doi:10.1093/gerona/glv202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ukraintseva S, Yashin AI, Arbeev KG. Resilience versus robustness in aging. J Gerontol A Biol Sci Med Sci. 2016. doi:10.1093/gerona/glw083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Theou O, Cann L, Blodgett J, Wallace LM, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev. 2015;21:78–94. doi:10.1016/j.arr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 48. Harrison JK, Clegg A, Conroy SP, Young J. Managing frailty as a long-term condition. Age Ageing. 2015;44:732–735. doi:10.1093/ageing/afv085 [DOI] [PubMed] [Google Scholar]