Abstract
Background:
In this randomized, double-blinded case–control study, we investigated the intravenous effects of ephedrine or phenylephrine on prevention of post–spinal hypotension in elective lower abdominal surgery under spinal anesthesia.
Materials and Methods:
One hundred and thirty-five patients, American Society of Anesthesiologists physical status I or II candidate for elective lower abdominal surgery under spinal anesthesia were randomized to three groups (45 each). According to their allocated group, patients received either ephedrine 2.5 mg (E group), phenylephrine (P group) 25 mic as vasopressor or the same volume of saline normal as placebo (S group) immediately after the spinal anesthesia. hemodynamic parameters, and complications were recorded.
Results:
Patients’ demographics were similar in all the groups. The mean systolic blood pressure (SBP), diastolic blood pressure (DBP), and MAP and also heart rate were similar over time for groups E and P (P > 0.05). The incidence of reactive hypertension was more in group E than group P and placebo (P < 0.05). The incidence of nausea and vomiting were significantly lower in groups E and P in comparison with placebo (P < 0.05).
Conclusion:
Although the mean fall of SBP and DBP were significantly less in groups E and P compared with placebo but we did not find significant differences in prophylactic use of ephedrine or phenylephrine for prevention of post–spinal hypotension in elective lower abdominal surgery. Vasopressors infusion have added benefit of lower incidence of nausea and vomiting.
Keywords: Elective lower abdominal surgery, ephedrine, phenylephrinr, postspinal hypotension
Introduction
Nowadays spinal anesthesia is widely used for elective lower abdominal surgeries.[1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21] It is frequently accompanied by hypotension, which may be defined in absolute terms as a systolic blood pressure (SBP) less than 90 or 100 mmHg or in relative terms as a percentage (20% fall from baseline).[1] The severity of this hypotension depends on the height of the block, the position of the patient, and the volume status of them.[1] But the chance of most serious complication such as postspinal hypotension is a major limitation of this technique.[2,3] The incidence of hypotension can be as high as 70%–80% when pharmacological prophylaxis is not used.[4,5,6] Several drugs and methods have been used to prevent or reduce this serious complication but till date, no single drug or method completely prevents hypotension without any adverse effects.[7,8] Different vasopressors are commonly used at present with varying degrees of success.[9,10]
Materials and Methods
This is a randomized double-blind clinical trial, approved by Research Committee of School of Medicine, Isfahan University of Medical Sciences in 2012.
Prior to this study, all patients signed an informed written consent. The present clinical trial was carried out on 135 people, divided into three groups of 45 patients.
The participants were in the age range of 18–65 years, with American Society of Anesthesiologists (ASA) physical status I–II (ASA I: Normal healthy patient, ASA II: Patient with mild systemic disease; no functional limitation) and were a candidate for elective lower abdominal surgery under spinal anesthesia.
On arrival to the operating room all patients had a wide bore 18 G intravenous (IV) catheter, patients had one blood pressure and heart rate (HR) reading record, while lying comfortable in the bed in supine position before induction of spinal block.
Monitoring was standard and included non-invasive blood pressure, continuous electrocardiography, and pulse oximetry.
The participants were randomly allocated to one of three groups (with 45 patients in each group, respectively) using sealed envelopes that contained a computer-generated randomization code.
The sample size was estimated based on a power calculation, which showed that at least 42 patients per group were necessary to achieve 80% power to detect a 20% difference between the groups. We recruited 45 patients per group to compensate for any exclusion. Patients were excluded from the study if any changes in anesthesia plan and also surgical plan were needed.
One of the investigators who was not related to data collection, monitoring, or conduct of anesthesia, prepared ephedrine (2.5 mg/mL), or phenylephrine (25 mcg/mL), or placebo in a 2 mL syringe as per randomization number. The patients were preloaded with 10 mL/kg of crystalloid (Ringer Lactate) before the induction of spinal anesthesia. Patients received Ringer Lactate at a rate of 10 mL/kg/h during the procedure.
Subarachnoid block was performed with all patients in the sitting position. After skin preparation and infiltration with 2% Lidocaine, a 23 G Quincke’s needle was inserted at L3–L4 vertebral interspace and once free flow of cerebrospinal fluid was obtained, 3 mL of hyperbaric bupivacaine 0.5% (15 mg) was injected intrathecally. Patients were then immediately turned supine.
Immediately following spinal block, patients received a 1 mL bolus of the study drug (ephedrine = 2.5 mg or phenyephrine 25 mcg or placebo) and thereafter another 5 mg bolus dose of ephedrine if the blood pressure dropped 10% below the baseline and repeated as necessary.
The block height was assessed by response to cold sensation using alcohol swab and also bilateral loss of pinprick discrimination every 3 min until maximum block was achieved. Surgery was started as soon as upper level of sensory block reached T8.
Oxygen 8 L/min was administered via a simple facemask throughout the operation. SBP, diastolic blood pressure (DBP), MAP, and HR was measured at 5-min intervals beginning immediately after spinal injection until 30 min and then at 15 min intervals thereafter until the end of the surgery.
Bradycardia (HR less than 60 beats/min) if associated with hypotension was treated with 0.5 mg IV atropine. A backup plan was designed anticipating some critical events. These situations allowed the anesthesiologists to adopt any measure to manage all events.
The data were recorded by the anesthetist conducting the spinal anesthesia. Nausea and vomiting were scored on a scale of 0-2 (0 = none, 1 = nausea without vomiting, 2 = vomiting). The maximum nausea and vomiting score during the operation and also in 2, 6, and 24 postoperatively were noted. At the end of operation the total dose of vasopressor was noted.
The primary outcome of the study was defined as the incidence of hypotension. Secondary outcomes included changes in blood pressure and HR, the incidence of bradycardia (HR <60 bpm), spinal injection to hypotension interval, amount of rescue ephedrine administered, nausea, and vomiting.
If the severity of nausea, as reported by patients, was assessed by anesthetist nurse who was unaware of the study on operation bed and also in recovery room by 100 mm Visual Analog Scale (VAS) and defined as severe if exceeded 30 mm. In case of vomiting or severe nausea, during operation or in recovery room, metoclopramide (0.15 mg/kg body weight, IV) were administered. SBP, DBP, HR and O2 saturation of patients were recorded at the admission to operating room (baseline), immediately after anesthesia (displayed as time 0), every 5–30 min and then every 15 min till 60 min and then at 2, 6, and 24 h after spinal injection. Time interval between the spinal injection and the occurrence of hypotension, prolongation of hypotension, and the amount of rescue ephedrine administered were recorded.
Statistical analysis was done using SPSS software (version 20). Data were presented as mean ± SD unless mentioned otherwise. Analysis of mean fall of SBP in each groups were done by an independent sample t test. Demographic data (mean ± SD) were compared between three groups by a one-way analysis of variance (ANOVA) test. Outcome measures were compared by number needed to treat (NNT), proportion, and Chi-square tests as required. For all quantitative characteristics 95% confidence intervals were given.
Results
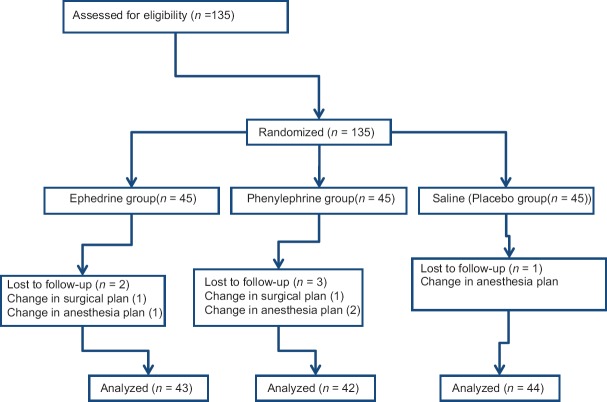
A total of 135 patients selected for this study and were randomly divided into three groups of 45 patients each. The flowchart of randomized patients was shown in [Figure 1].
Figure 1.
The flowchart of randomized patients
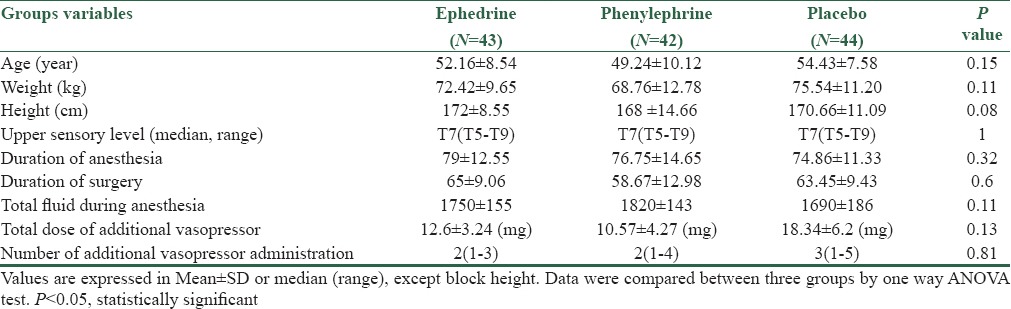
The three groups were comparable with respect to gender, age, body weight, height, operation type and time, and block height [Table 1].
Table 1.
Patients characteristics and intraoperative variables in three groups
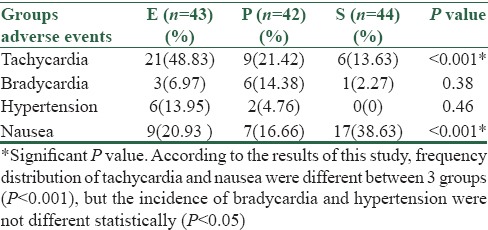
Statistically significant tachycardia was seen in group Eehedrine than the other two groups (P < 0.05) [Table 2]. In placebo group, patients suffered more nausea and vomiting and it was statistically significant (P < 0.05) [Table 2].
Table 2.
Adverse events in three groups
The differences observed in baseline heart rate, systolic, diastolic, and mean blood pressures between three groups were statistically insignificant [Table 3]. There was higher incidence of bradycardia in patients receiving phenylephrine than those receiving ephedrine or placebo (p) [Table 2]. The difference in mean heart rate, SBP, DBP, and MAP compared between two groups (E and P) immediately after spinal anesthesia, at 5, 10, 15, 45, 60 min and also 2, 6, and 24 postoperatively were not statistically significant (P > 0.05) [Figure 2].
Table 3.
Comparison of baseline heart rate, systolic, diastolic and mean blood pressure in all groups
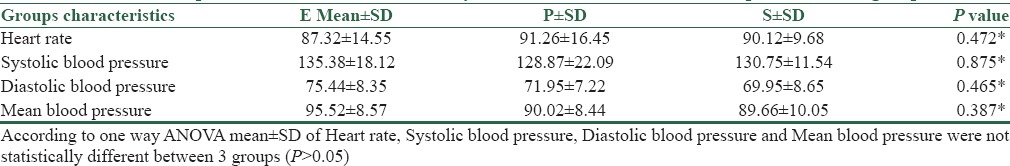
Figure 2.
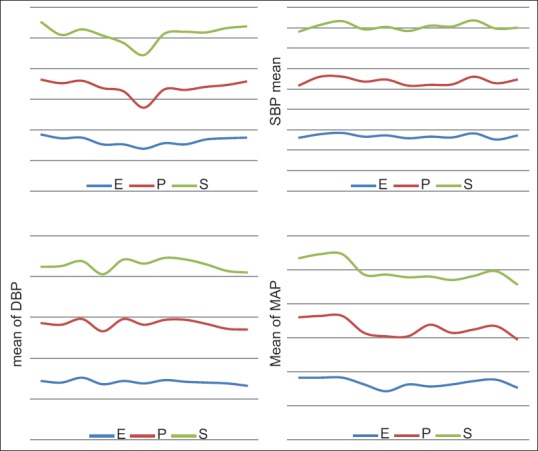
The trend of SBP, DBP, MAP, and HR are shown during the intervention between three groups. SBP (systolic blood pressure), DBP (diastolic blood pressure), MAP, HR (heart rate)
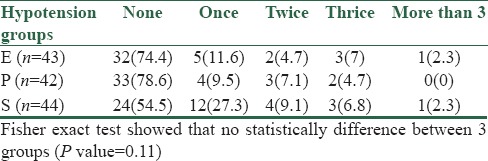
Overall, 11/43 (25.58%) patients in the E group and 9/42 (21.42%) patients in P group, and 20/44 (45.45%) in the placebo group had one or more episodes of hypotension and required one or more boluses of vasopressor [Table 4]. The number of rescue doses required in the placebo group was more than the other two groups and was statistically significant (P < 0.05: Significant).
Table 4.
Incidence of hypotension in three groups
Discussion
The most important physiological response to spinal anesthesia involves cardiovascular system and overall incidence of hypotension during spinal anesthesia is 70%–80%.[1,2,3,4,5,6]
In this study, all patients in the three groups were comparable with respect to age, gender, body weight, high, operation duration, and ASA status. The difference observed in baseline parameters, that is, pulse, systolic, diastolic, and mean arterial pressures between three groups was statistically insignificant, respectively.
In this study, there was a higher incidence of bradycardia in patients receiving phenylephrine than those receiving ephedrine or placebo (p). This is expected to be due to increase in blood pressure with an α-agonist may lead to reactive bradycardia (baroreceptor reflex). However, this was responsive to atropine without adverse consequences. Atropine was required in 6 of 42 patients in group P compared with 3 of 43 patients in group E. There was no significant difference in maximum recorded HR between groups E and P in comparison with the placebo group.
The results of this study were in accordance with the study of other investigators in which they reported higher incidence of bradycardia in patients receiving phenylephrine as compared with patients receiving ephedrine for prevention of hypotension during spinal anesthesia for cesarean section.[10,11,12]
We confirmed in this study that there was no significant difference between ephedrine and phenylephrine in their efficacy for prevention of hypotension following spinal anesthesia in patients undergoing lower abdominal surgeries in the range of doses that have been studied [Table 4].
Chandrakala et al. compared the effectiveness and the side effects of vasopressors, ephedrine, and phenylephrine, administered for management of hypotension during elective cesarean section under spinal anesthesia and they found no significant difference, similar to our findings. However, the study suggests that phenylephrine may be more appropriate vasopressor when considering maternal well-being.[13]
Our study is not consistent with the work of resent researchers whom studied on ephedrine and phenylephrine for prevention of hypotension during spinal block for cesarean section and effects on fetus and they concluded that ephedrine was more effective than phenylephrine in the prevention of hypotension.[14,15,16,17] This may have been because more dose of phenylephrine was used in their study as compared with this study and on the other hand they studied only on cesarean section, which needs different management than our study.
Conclusion
Prevention and management of hypotension during spinal anesthesia continues to be controversial. Although fluid preloading is frequently employed in an attempt to prevent post–spinal hypotension, vasopressors are often required and have been shown to be more effective at limiting this complication than other treatment. There is an abundance of evidence to suggest that phenylephrine is as good as ephedrine for maintaining blood pressure and a more liberal use of this drug is justified. A dose–response study to find equipotent dose of the two vasopressors is required. Further work is required to determine the optimal therapy for prevention of hypotension during spinal anesthesia, especially in high-risk patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We express our gratitude to all those who gave us the possibility to complete this research. Furthermore, our special thanks are extended to the stuff of post-anesthesia care unit and the surgery ward for their assistance with the collection of our data.
References
- 1.Das S, Mukhopadhyay S, Mandal M, Mandal S, Basu SR. A comparative study of infusions of phenylephrine, ephedrine and phenylephrine plus ephedrine on maternal haemodynamics in elective caesarean section. Indian J Anaesth. 2011;55:578–83. doi: 10.4103/0019-5049.90612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saravanan S, Kocarev M, Wilson RC, Watkins E, Columb MO, Lyons G. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in Caesarean section. Br J Anaesth. 2006;96:95–9. doi: 10.1093/bja/aei265. [DOI] [PubMed] [Google Scholar]
- 3.Stewart A, Fernando R, McDonald S, Hignett R, Jones T, Columb M. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg. 2010;111:1230–7. doi: 10.1213/ANE.0b013e3181f2eae1. [DOI] [PubMed] [Google Scholar]
- 4.Ansari T, Hashem MM, Hassan AA, Gamassy A, Saleh A. Comparison between two phenylephrine infusion rates with moderate co-loading for the prevention of spinal anaeshtesia-induced hypotension during elective caesarean section. Middle East J Anesthesiol. 2011;21:361–6. [PubMed] [Google Scholar]
- 5.Loubert C. Fluid and vasopressor management for Cesarean delivery under spinal anesthesia: Continuing professional development. Can J Anaesth. 2012;59:604–19. doi: 10.1007/s12630-012-9705-9. [DOI] [PubMed] [Google Scholar]
- 6.Habib AS. A review of the impact of phenylephrine administration on maternal hemodynamics and maternal and neonatal outcomes in women undergoing cesarean delivery under spinal anesthesia. Anesth Analg. 2012;114:377–90. doi: 10.1213/ANE.0b013e3182373a3e. [DOI] [PubMed] [Google Scholar]
- 7.Jabalameli M, Safavi M, Honarmand A, Saryazdi H, Moradi D, Kashefi P. The comparison of intraincisional injection tramadol, pethidine and bupivacaine on postcesarean section pain relief under spinal anesthesia. Adv Biomed Res. 2012;1:53. doi: 10.4103/2277-9175.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalili G, Janghorbani M, Saryazdi H, Emaminejad A. Effect of preemptive and preventive acetaminophen on postoperative pain score: A randomized, double-blind trial of patients undergoing lower extremity surgery. J Clin Anesth. 2013;25:188–92. doi: 10.1016/j.jclinane.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Khalili G, Janghorbani M, Sajedi P, Ahmadi G. Effects of adjunct intrathecal magnesium sulfate to bupivacaine for spinal anesthesia: A randomized, double-blind trial in patients undergoing lower extremity surgery. J Anesth. 2011;25:892–7. doi: 10.1007/s00540-011-1227-z. [DOI] [PubMed] [Google Scholar]
- 10.Yoon HJ, Cho HJ, Lee IH, Jee YS, Kim SM. Comparison of hemodynamic changes between phenylephrine and combined phenylephrine and glycopyrrolate groups after spinal anesthesia for cesarean delivery. Korean J Anesthesiol. 2012;62:35–9. doi: 10.4097/kjae.2012.62.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park YH, Ryu T, Hong SW, Kwak KH, Kim SO. The effect of the intravenous phenylephrine on the level of spinal anesthesia. Korean J Anesthesiol. 2011;61:372–6. doi: 10.4097/kjae.2011.61.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhardwaj N, Jain K, Arora S, Bharti N. A comparison of three vasopressors for tight control of maternal blood pressure during cesarean section under spinal anesthesia: Effect on maternal and fetal outcome. J Anaesthesiol Clin Pharmacol. 2013;29:26–31. doi: 10.4103/0970-9185.105789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunda CP, Malinowski J, Tegginmath A, Suryanarayana VG, Chandra SB. Vasopressor choice for hypotension in elective Cesarean section: Ephedrine or phenylephrine? Arch Med Sci. 2010;6:257–63. doi: 10.5114/aoms.2010.13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kol IO, Kaygusuz K, Gursoy S, Cetin A, Kahramanoglu Z, Ozkan F, et al. The effects of intravenous ephedrine during spinal anesthesia for cesarean delivery: A randomized controlled trial. J Korean Med Sci. 2009;24:883–8. doi: 10.3346/jkms.2009.24.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jabalameli M, Soltani HA, Hashemi J, Behdad S, Soleimani B. Prevention of post-spinal hypotension using crystalloid, colloid and ephedrine with three different combinations: A double blind randomized study. Adv Biomed Res. 2012;1:36. doi: 10.4103/2277-9175.100129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shim KS, Kim EJ, Lee JH, Lee SG, Ban JS, Min BW. Combined spinal-epidural anesthesia for cesarean section in a patient with Moyamoya disease -A case report- Korean J Anesthesiol. 2010;59(Suppl):S150–3. doi: 10.4097/kjae.2010.59.S.S150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Acar NS, Uzman S, Toptas M, Vahapoglu A, Akkoc I, Dinc SC. Spinal anesthesia with hyperbaric bupivacaine: A comparison of hypertensive and normotensive patients. Med Sci Monit. 2013;19:1109–13. doi: 10.12659/MSM.889412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kweon TD, Kim SY, Cho SA, Kim JH, Kang YR, Shin YS. Heart rate variability as a predictor of hypotension after spinal anesthesia in hypertensive patients. Korean J Anesthesiol. 2013;65:317–21. doi: 10.4097/kjae.2013.65.4.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehdi Fathi, Farnad Imani, Marjan Joudi, Vahid Goodarzi. Comparison Between the Effects of Ringer’s Lactate and Hydroxyethyl Starch on Hemodynamic Parameters After Spinal Anesthesia: A Randomized Clinical Trial. Anesth Pain Med. 2013;2:127–33. doi: 10.5812/aapm.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jabalameli M, Safavi M, Honarmand A, Saryazdi H, Moradi D, Kashefi P. The comparison of intraincisional injection tramadol, pethidine and bupivacaine on postcesarean section pain relief under spinal anesthesia. Adv Biomed Res. 2012;1:53. doi: 10.4103/2277-9175.100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heidari SM, Soltani H, Hashemi SJ, Talakoub R, Soleimani B. Comparative study of two anesthesia methods according to postoperative complications and one month mortality rate in the candidates of hip surgery. J Res Med Sci. 2011;16:323–30. [PMC free article] [PubMed] [Google Scholar]