Abstract
Background:
Due to the recent emerging information on the antioxidant properties of soy products, substitution of soy milk for milk in the diet has been proposed by some nutritionists. We aimed to compare four distinct antioxidant measuring methods in the evaluation of antioxidant properties of industrial ultra-high temperature (UHT) milk, UHT soy milk, and their fermented products by Lactobacillus plantarum A7.
Materials and Methods:
Ascorbate auto-oxidation inhibition assay, 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical scavenging method, hydrogen peroxide neutralization assay and reducing activity test were compared for the homogeneity and accuracy of the results.
Results:
The results obtained by the four tested methods did not completely match with each other. The results of the DPPH assay and the reducing activity were more coordinated than the other methods. By the use of these methods, the antioxidant capability of UHT soy milk was measured more than UHT milk (33.51 ± 6.00% and 945 ± 56 μM cysteine compared to 8.70 ± 3.20% and 795 ± 82 μM cysteine). The negative effect of fermentation on the antioxidant potential of UHT soy milk was revealed as ascorbate auto-oxidation inhibition assay, DPPH method and reducing activity tests ended to approximately 52%, 58%, and 80% reduction in antioxidant potential of UHT soy milk, respectively.
Conclusions:
The antioxidative properties of UHT soy milk could not be solely due to its phenolic components. Peptides and amino acids derived from thermal processing in soy milk probably have a main role in its antioxidant activity, which should be studied in the future.
Keywords: Antioxidative properties, Lactobacillus plantarum A7, soy milk, ultra-high temperature milk
Introduction
Antioxidant property is defined as the ability of compounds to inhibit or suppress the process of oxidation by scavenging the free radicals and/or reactive oxygen species (ROS).[1] Free radicals and ROS are common by-products of oxidation produced in the body as part of normal cell function, and overproduction can cause oxidative damage to DNA and other biomolecules, which could result in cell death and oxidative stress.[2] Continued oxidative stress, in turn, can lead to chronic inflammation resulting in the development of many chronic and degenerative disorders.[2,3] Dietary source of antioxidants, instead, can reduce the oxidative damage caused by the overproduction of free radicals and decrease the risk of related diseases.[4] Soy beans and soy-derived products are known to possess antioxidant properties. In particular, soy milk contains a diverse group of phenolic components, namely isoflavonoids that can provide health benefits.[5,6,7] Recent studies have indicated that the antioxidant activity of fermented soy milk with lactic acid bacteria is significantly stronger than nonfermented soy milk.[5,8,9] Apart from the traditional usage of soy milk and soy milk fermented products in specific geographies, recently, as a result of the associated benefits, their consumption in other regions is being recommended by health advisories.[10,11,12,13,14,15,16] Being rich in phenolic antioxidants, soy milk may be a healthier alternative to milk for individuals suffering from inflammatory diseases. However, milk in itself is a good source of antioxidant activity; hydrolyzed milk proteins and free peptides have been extensively studied and introduced as a powerful source of antioxidants. In particular, a succession of some amino acids including histidine and/or hydrophobic amino acids have been mainly considered in this regard.[17]
Several methodologies have been explained for the measurement of antioxidative properties of foods.[18,19,20,21,22,23,24] However, different antioxidant measurement methods may result in varied responses in the quantity and even the quality of antioxidant characteristic of the same food sample.[1,25]
Two studies have recently been conducted by the Food Security Research Center in Isfahan University of Medical Science, Iran, in type 2 diabetes patients with nephropathy to examine the effects of the consumption of soy milk over cow’s milk with respect to inflammatory markers and blood pressure.[26,27] The results showed that soy milk consumption had no significant effect on the tested inflammatory and oxidative markers. However, soy milk significantly reduced the blood pressure of the participants. In the current study, we looked at two different types of commercial milk and soy milk products. There is little information regarding the antioxidant properties of ultra-high temperature (UHT) milk and UHT soy milk and their fermented products that are commercially available. Hence, the present study was directed to compare the antioxidant properties of the aforementioned products, which are routinely used by the public. In our study, the antioxidant properties of UHT milk and UHT soy milk and their fermented products were derived through four distinct analytical experiments: (1) Inhibition of ascorbate auto-oxidation; (2) scavenging effect of 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical; (3) the scavenging activity of hydrogen peroxide (H2O2); and (4) the reducing activity.
Materials and Methods
UHT sterilized milk (1.5% fat, Tehran Mihan Food Dairy and Ice Cream Industries Group, Tehran, Iran), and UHT sterilized whole soy milk (1% fat, Maxsoy, Soyasun Co., Ltd., Tehran, Iran) were used in the study. As declared on the food labels of each product, the content composition in each 100 ml of UHT milk was as follows: Protein, 3.3 g; carbohydrate, 4.9 g; fat, 1.5 g; total mineral, 0.6 g; calcium, 100 mg; phosphorus, 120 mg and 100 ml of UHT soy milk contained protein, 2.5 g; carbohydrate, 5.5 g; fat, 1.0 g; calcium, 40 mg; and sodium, 40 mg. In addition, proximate analysis of the samples was performed regarding fat and carbohydrate content of each type of UHT milk. The Gerber method and Folch method were adopted for the determination of the fat content in the UHT milk and soy milk samples, respectively. The Fehling method was used for carbohydrate measurements of the UHT milk while, for the UHT soy milk, the correction factor corresponding to sucrose was considered.
Lactobacillus plantarum A7 was obtained from the culture collection of the food microbiology laboratory of Isfahan University of Medical Science. The microorganism was activated in de Man-Rogosa-Sharpe (MRS) broth at 37°C for 24 h. The fresh culture (2%v/v) was adjusted in optical density of 1.2 at 620 nm, containing approximately 108 bacterial cell/ml and was used for UHT milk and UHT soy milk inoculation. Fermentation was carried out in a 500 ml Erlenmeyer flask (WT-Binder, Germany) incubated at 37°C in aerobic condition for 48 h. Sampling was performed in time intervals of 3, 5, 7, 15, and 37 h. Serial dilution was prepared using sterilized normal saline. Viable counts of fermented UHT milk and UHT soy milk was monitored by plate count agar on MRS-Agar (Merck, Germany) during the incubation period. The residual of each sample was used for measuring pH (Hanna Instruments, Italy).
Measurement of antioxidant properties
-
Ascorbate autoxidation inhibition assay was adapted from Wang et al. and Rekha and Vijayalakshmi first described by Mishra and Kovachich.[23,25,28] In brief, 0.1 ml of sample or distilled water which served as the control was mixed with an ascorbate solution containing 0.1 ml of 5.0 mM ascorbate (Merck, Germany) in phosphate buffer 9.8 ml of 0.2 M at pH 7.0. After being placed at 37°C for 10 min, the absorbance of the resultant mixture was measured at 265 nm (Jenway Scientific Instruments, England). The ascorbate autoxidation inhibition rate of the sample was then calculated according to the following equation:
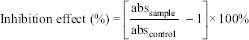
-
The free radical scavenging activity of all samples was measured by DPPH method of Devi et al. (2009) previously described by Chen and Ho with minor modifications.[29,30] In brief, 0.2 ml of each sample was added to 3.8 ml DPPH (Sigma-Aldrich, Germany) ethanol solution (final concentration was 0.1 mM) in a test tube. The mixture was shaken vigorously for 1 min by vortexing and left to stand at room temperature in the dark for 30 min. Thereafter, the absorbance of the sample (A sample) was measured using the ultraviolet-visible spectrophotometer at 517 nm against ethanol blank. A negative control (A control) was taken after adding DPPH solution to 0.2 ml of the respective extraction solvent. The percent of DPPH discoloration of the sample was calculated according to the following equation:
Percent discoloration: (1 – [Asample/Acontrol]) × 100
-
The H2O2 scavenging activity was measured by the method of Wang et al. described by Pick and Keisari with minor modification.[25,31] In brief, 50 μl of the sample or distilled water (control) was mixed with 50 μl of 5 mM H2O2 solution (Merck, Germany) and incubated at room temperature for 20 min. It was then supplemented with 100 ml of horseradish peroxidase-phenol red (Merck, Germany) solution (100 mM phosphate buffer containing horseradish peroxidase. 300 μg/ml and phenol red 4.5 mM). After another 10 min of incubation, the sample absorbance at 610 nm was monitored by an automated microplate reader (microReader 4 plus - Hyperion, USA, MD 6258). The scavenging effect was calculated according to the following equation:
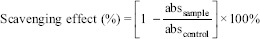
The reducing activity of samples was determined as described by Wang et al., Rekha and Vijayalakshmi and Oyaizu.[24,25,28] A sample or distilled water (control) (0.5 ml) was mixed with 1.0% potassium ferricyanide (0.5 ml, Merck, Germany) and sodium phosphate buffer (0.5 ml, 0.02 M, pH 7). The mixture was incubated at 50°C for 20 min and then 10% trichloroacetic acid (0.5 ml, Merck, Germany) was added. The mixture was then centrifuged at 780 g for 5 min. The upper layer (1.5 ml) was mixed with 0.1% ferrichloride (0.2 ml, Merck, Germany), and the absorbance was measured at 700 nm. The higher the absorbance of the given mixture, the higher was the reducing activity expressed as μmol of cysteine (Merck, Germany). The cysteine reducing activity data corresponded to the absorbance at 700 nm and has been illustrated in Figure 1. The standard equation was obtained as Y = 0.0022X −0.0064, which was validated by R2 = 0.98.
Figure 1.
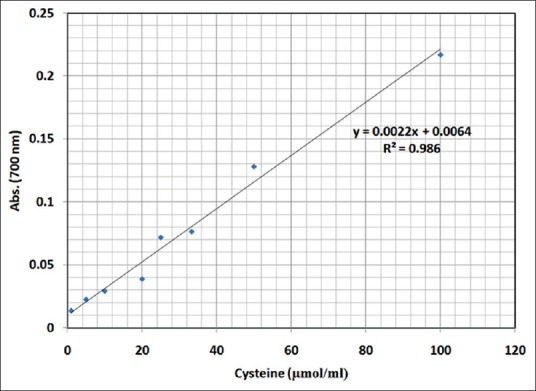
Standard curve of cysteine reducing activity
Each experiment was independently repeated in triplicate, and each measurement was at least replicated twice. The mean values of at least six data obtained plus standard deviation was used to express the result of each experiment. All the data was analyzed using a one-way ANOVA, and the comparison of the means was tested by Tukey’s test (SPSS, Version 16.0, Chicago, SPSS Inc.). The significance of the different results were considered as α = 5%.
Results
The growth curve of L. plantarum in UHT-treated milk and UHT soy milk is shown in Figure 2. The corresponding changes in pH are illustrated in Figure 3. At the first sampling time (3 h of fermentation), the pH value in both tested media was measured at about 6.5 and about 108 bacterial cells existed in each ml of the given samples. However as shown in the figure, the growth rate of L. plantarum rapidly increased in UHT soy milk, reached to a peak and drastically decreased when it was compared with UHT milk fermented counterpart which revealed a slower but more stable viable cell counts. After 37 h fermentation, the pH of UHT-treated soy milk was reduced to 3.8 whereas fermented UHT milk showed the pH value of 4.55.
Figure 2.
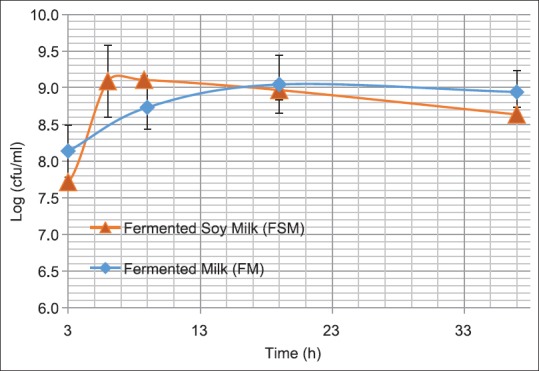
Lactobacillus plantarum A7 growth curves in ultra-high temperature milk and soy milk during 37 h aerobic incubation at 37°C
Figure 3.
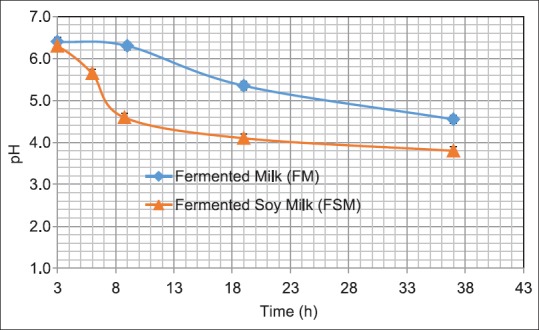
Changes in pH during Lactobacillus plantarum growth in ultra-high temperature milk and soy milk
For antioxidant activity test, the samples of fermented UHT milk were selected at the end of fermentation (37 h incubation). Under these conditions, even though the pH dropped to about 4.5, the bacterial population did not change compared with its maximum. For UHT soy milk samples, the fermented samples were used after 6–8 h of fermentation, at which, pH value was about 4.6 ± 0.1 and the population of live bacteria was at the maximum.
Antioxidant properties of the examined samples were measured as the ascorbate auto-oxidation inhibition effect, DPPH free radical scavenging effect, scavenging activity of H2O2 and measuring the reducing activity. These have been tabulated in Table 1.
Table 1.
The results of antioxidant properties of commercial UHT milk, soy milk and their fermented counterparts*
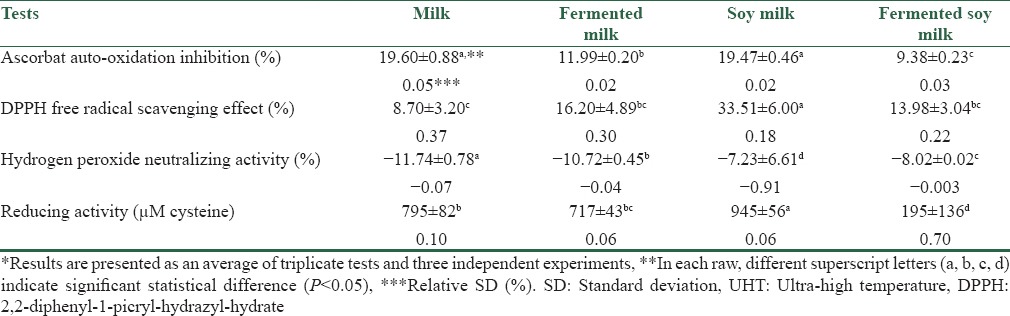
The results of ascorbate auto-oxidation inhibition properties of the tested samples showed no significant difference (P > 0.05) between UHT soy milk and UHT milk, they both presented with more inhibition activity than their fermented counterparts [Table 1]. By using the DPPH free radical scavenging assay, a significant statistical difference (P < 0.05) was revealed among the products. The most antioxidant activity was seen from UHT soy milk. Nevertheless, as mentioned, fermentation resulted in an increase in antioxidant capability of UHT milk but decreased in this property in UHT soy milk.
Based on the results obtained from the third experiment, the significant statistical difference (P < 0.05) was observed between the four trails [Table 1]. However, none of the tested samples exhibited any efficiency regarding this property as H2O2 neutralizing effect was measured in a negative amount for both fermented and nonfermented samples. Meaning that in all the samples, H2O2 was measured in higher concentration than that of distilled water as the control sample. Because UHT milk and soy milk tested in this study were in tetra-pack packaging, the presence of H2O2 in nonfermented samples was probably due to the discharge through the aseptic packaging process in the plant. Nevertheless, H2O2 concentration was reduced in UHT milk and increased in UHT soy milk by the effect of fermentation. Reduction of the H2O2 content in the fermented UHT milk could be due to its degradation during long fermentation time period, and its increase in fermented UHT soy milk might be as a result of its accumulation in the course of the short fermentation process. Regarding the reducing activity test, the significant statistical difference (P < 0.05) was observed between the average results of the four products. In line with DPPH test, UHT soy milk was shown to have more reducing potential compared to UHT milk. In addition, no increase in reducing activity appeared to be generated by fermentation either in UHT milk or UHT soy milk.
Discussion
Nondairy lactobacilli, particularly probiotic species that have been isolated from the digestive system, generally present a slow growth and activity in milk, which has been referred to their exotrophic nature for peptide and free amino acids.[32] Rapid growth upon drastic pH decline by L. plantarum in UHT soy milk in this study can be attributed to the presence of more available peptides in UHT soy milk than UHT milk.
It should be noted that the utilized samples in this project were the commercially available UHT sterilized packs and the severe thermal treatment tolerated by the samples might have provided the possibility for creating more heat induced peptides. This may explain the higher growth rate and activity of the human isolated L. plantarum used in this study compared to other studies that have used autoclaved milk or soy milk for this purpose.[32]
The results of the ascorbate auto-oxidation inhibition effect contradict the previous reports by Wang et al. and Rekha and Vijayalakshmi in that soy milk fermentation using pure or mixed Lactobacillus cultures provide a more ascorbate auto-oxidation inhibition property.[25,28] It should be mentioned that in the present study, the value of inhibition effect of commercial UHT soy milk obtained was 19% in comparison with 9% reported for home-made soy milk by Wang et al. and 4.3% reported by Rekha and Vijayalakshmi. In the latter study, the liberation of aglycones form of genistein and daidzein by the catalytic function of β-glucosidase during fermentation and the presence of intracellular antioxidants of the starter organism accounted for the increase in the inhibition of ascorbate autoxidation found in fermented soy milk.[28] Phenolic compounds have also been considered as the main factor in creating antioxidant properties in the study of Wang et al. It could be suggested that a considerable part of the measured antioxidant property in the present study are attributed to the processing of soy milk and the released free peptides. Because L. plantarum presented superior growth in UHT soy milk than UHT milk, an instantaneous decrease in the oxidation inhibition revealed in fermented UHT soy milk is justifiable.
The DPPH scavenging effect has been used in several studies for evaluating antioxidant properties of soy products. It was reported that using this test, the antioxidant activity of various soy product extracts ranged from 41.6% to 81.6%. The lowest values were attributed to soy sauce (55%) and soy milk (41.6%). Several processing steps in the production line which adversely affect the total phenolic compounds in the manufactured soy products presumed, by the authors, to be the reasons for this wide range.[30] Hubert et al. indicated that among the antioxidant compounds in soy germ extract, tocopherols have the highest inclination to react with free radical of DPPH and thus, destruction of tocopherols during fermentation was declared as the reason for the reduction of antioxidant capability of soy product after fermentation.[33] DPPH radical scavenging activities of milk-kefir and soy milk-kefir have been investigated by Liu et al., and their results concur with the result of the present study. Soy milk showed a higher antioxidative activity than milk, however, notably, the tested products in their study were not the UHT commercial types.[34] Regarding the increase in the antioxidant capability of milk after fermentation which was observed in this experiment, the results are in agreement with the results of other studies; different bioactive components with proteinaceous nature, which is derived from milk proteins during fermentation by lactic culture, were discussed to be responsible for this property including casein hydrolysate and some peptides derived from the pepsinic hydrolysate of casein,[34] peptides released due to proteolysis and bacterial growth and histidine and some hydrophobic amino acids.[17,35] Growth and activity of the proteolytic bacterium, L. plantarum A7, in the present study, probably caused the increase in this property in UHT milk after fermentation.
Based on the results of human study, which was conducted in the Isfahan University of Medical Sciences, inflammatory markers were not significantly modified in the patients that received UHT-soy milk that was used in this study compared to caw’s milk.[27] However, those patients showed a decrease in the systolic blood pressure, which might be because of heat-induced peptides in the UHT-soy milk.[26]
In the third experiment, the observed result was in agreement with the results of Wang et al. in that fermentation had a negative impact on the soy milk H2O2 neutralizing property.[25] Because there is no other report in the literature concerning the effectiveness of the given experiment as a means of antioxidant property measure, it could be concluded that this method cannot be regarded as a proper way of assessing antioxidant properties when similar food types are under investigation.
In the fourth experiment, infirmity of fermentation in the development of antioxidant property of both UHT milk and UHT soy milk contradicted with previous studies in that reducing activity was found to be increased after soy milk and/or milk fermentation.[8] Similar results by Hubert et al. have been reported on the changes in reducing ability of soy germ extracts. They indicated that lactic acid bacteria fermentation significantly decreased the reducing power of soy bean products. Such reduction was attributed to the loss of phytosterol content during the incubation period.[33]
It should be mentioned that there is a great difference among studies regarding the reported range of reducing activity presented based on μM of cysteine as an antioxidant index. In the present study, the reducing capability of UHT soy milk was measured as 945 cysteine μM which declined to about 195 cysteine μM as a result of fermentation. Reducing the power of soy milk and its fermented product was previously determined as 604 cysteine μM and 699 cysteine μM, respectively.[34] In addition, data for milk was reported to be 500–759 cysteine μM by the same authors. Instead, using the same experimental protocol, the reducing activity of soy milk or its fermented derivatives were measured as about 0.8–11 cysteine μM by other researchers.[25,28] The difference in soy composition, as well as their thermal treatment, could be the reason for such differences. Moreover, the methodology of this test may partly be responsible for the varied results. Incomplete precipitation of proteinaceous material in the tested samples can be a source of error having a great impact on the absorption data and the final results of spectrophotometric measurements.[36]
Among four experiments conducted in the present study, the results of the DPPH assay was in agreement with the reducing activity test in two points: Higher antioxidant capability of UHT soy milk than UHT milk and reduction in the antioxidant capability of UHT soy milk by fermentation. However the reducing activity was considered a less reliable method, partly due to some stages of the experiment, which make it a less reproducible method. For instance, difficulty in the protein precipitation step for both UHT milk and UHT soy milk could be a source of error in the spectrophotometric measurements.
Furthermore, in view of changes in antioxidant properties after fermentation in UHT milk, the reducing activity assay was in agreement with the method of ascorbate auto-oxidation inhibition effect. In the latter experiment, the conditions for oxidation of ascorbic acid added to the food sample is provided, and the rate of prevention from its oxidation is considered as a measure of antioxidant activity. Because no difference was observed between the antioxidant activity of UHT milk and UHT soy milk in this test, it could be concluded that the presence of soya polyunsaturated fatty acids and their susceptibility to the provided oxidation conditions in this test may partly contribute as a source of error in this test and thus this method may not be appropriate for food samples, which contain chimerical compounds susceptible to oxidation. However, this method was regarded as a simple, cheap, and accessible among the tested methods in the present study.
Comparison of four distinct methods in evaluating the antioxidant properties of the commercial products that are available for the public could be regarded as a strength point of this study. In addition, the link between the results of this study on the in vitro properties of examined soy milk and milk and the results of a human study in which the same articles were consumed as by the subjects makes this study different from the previous ones. On the other hand, lack of proteolysis measurement, as well as the lack of complementary examination of peptides produced in the examined products are the limitation of this study which could be considered in the future studies in the field.
Conclusion
Four proposed methods for antioxidant activity measurement were used to compare this property among UHT milk, UHT soy milk, and their fermented products. The employed experiments did not support each other on all the obtained results. UHT soy milk was found to have more antioxidant property than UHT milk by a couple of methods. However, it is unlikely that such superiority rooted from just the phenolic components as after fermentation, the antioxidant property decreased. Heat-induced peptides were suggested to have an adverse effect in this property as they can be affected by lactic cultures, leading to reduction in the antioxidant value of the soy milk substrate. Rapid growth of lactic culture in UHT soy milk supported this proposition. Further research with UHT soy milk and the fermented product is needed to justify the influence of the antioxidant capacity on different human disorders.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors would like to express their appreciation to Mr. Yahay for his collaboration and assistance. In addition, the Food Security Research Center was kindly appreciated for providing the facility for the experiments.
References
- 1.Müller L, Fröhlich K, Böhm V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011;129:139–48. [Google Scholar]
- 2.Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 3.Jacob RA. Nutrition, health and antioxidants. Inform. 1994;5:1271–5. [Google Scholar]
- 4.Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agric Food Chem. 1999;47:1460–6. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- 5.Murakami H, Terao J, Matsushita S, Asakawa T. Antioxidative stability of tempeh [fermented soybean food] and liberation of isoflavones by fermentation. Agric Biol Chem Tokyo. 1984;48:2971–75. [Google Scholar]
- 6.Drumm TD, Gray JI, Hosfield GL. Variability in the saccharide, protein, phenolic acid and saponin contents of four market classes of edible dry beans. J Sci Food Agric. 1990;51:285–97. [Google Scholar]
- 7.Wang HJ, Murphy PA. Isoflavone content in commercial soybean foods. J Agric Food Chem. 1994;42:1666–73. [Google Scholar]
- 8.Berghofer E, Grzeskowiak B, Mundigler N, Sentall W, Walcak J. Antioxidative properties of faba bean-, soybean- and oat tempeh. Int J Food Sci Nutr. 1998;49:45–54. [Google Scholar]
- 9.Pyo YH, Lee TC. The potential antioxidant capacity and angiotensin I-converting enzyme inhibitory activity of Monascus-fermented soybean extracts: Evaluation of Monascus-fermented soybean extracts as multifunctional food additives. J Food Sci. 2007;72:S218–23. doi: 10.1111/j.1750-3841.2007.00312.x. [DOI] [PubMed] [Google Scholar]
- 10.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Dietary soya intake alters plasma antioxidant status and lipid peroxidation in postmenopausal women with the metabolic syndrome. Br J Nutr. 2007;98:807–13. doi: 10.1017/S0007114507746871. [DOI] [PubMed] [Google Scholar]
- 11.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Hu FB, Willett WC. Soy consumption, markers of inflammation, and endothelial function: A cross-over study in postmenopausal women with the metabolic syndrome. Diabetes Care. 2007;30:967–73. doi: 10.2337/dc06-2126. [DOI] [PubMed] [Google Scholar]
- 12.Azadbakht L, Kimiagar M, Mehrabi Y, Esmaillzadeh A, Padyab M, Hu FB, et al. Soy inclusion in the diet improves features of the metabolic syndrome: A randomized crossover study in postmenopausal women. Am J Clin Nutr. 2007;85:735–41. doi: 10.1093/ajcn/85.3.735. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy A, Brown JE, Hawdon A, Faughnan MS, King LJ, Millward J, et al. Factors affecting the bioavailability of soy isoflavones in humans after ingestion of physiologically relevant levels from different soy foods. J Nutr. 2006;136:45–51. doi: 10.1093/jn/136.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Franke AA, Ashburn LA, Kakazu K, Suzuki S, Wilkens LR, Halm BM. Apparent bioavailability of isoflavones after intake of liquid and solid soya foods. Br J Nutr. 2009;102:1203–10. doi: 10.1017/S000711450937169X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nhan S, Anderson KE, Nagamani M, Grady JJ, Lu LJ. Effect of a soymilk supplement containing isoflavones on urinary F2 isoprostane levels in premenopausal women. Nutr Cancer. 2005;53:73–81. doi: 10.1207/s15327914nc5301_9. [DOI] [PubMed] [Google Scholar]
- 16.Reinwald S, Akabas SR, Weaver CM. Whole versus the piecemeal approach to evaluating soy. J Nutr. 2010;140:2335S–43S. doi: 10.3945/jn.110.124925. [DOI] [PubMed] [Google Scholar]
- 17.Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J Nutr Biochem. 2000;11:128–31. doi: 10.1016/s0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 18.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–56. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 19.Mathew S, Abraham TE. In vitro antioxidant activity and scavenging effects of Cinnamomum verum leaf extract assayed by different methodologies. Food Chem Toxicol. 2006;44:198–206. doi: 10.1016/j.fct.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Sharififar F, Moshafi M, Mansouri S, Khodashenas M, Khoshnoodi M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control. 2007;18:800–5. [Google Scholar]
- 21.Abdille MH, Singh R, Jayaprakasha G, Jena B. Antioxidant activity of the extracts from Dillenia indica fruits. Food Chem. 2005;90:891–6. [Google Scholar]
- 22.Burits M, Asres K, Bucar F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. Phytother Res. 2001;15:103–8. doi: 10.1002/ptr.691. [DOI] [PubMed] [Google Scholar]
- 23.Mishra OP, Kovachich GB. Inhibition of the autoxidation of ascorbate and norepinephrine by extracts of Clostridium butyricum, Megasphaera elsdenii and Escherichia coli. Life Sci. 1984;35:849–54. doi: 10.1016/0024-3205(84)90410-7. [DOI] [PubMed] [Google Scholar]
- 24.Oyaizu M. Antioxidative activities of browning reaction prepared from glucosamine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 25.Wang YC, Yu RC, Chou CC. Antioxidative activities of soymilk fermented with lactic acid bacteria and bifidobacteria. Food Microbiol. 2006;23:128–35. doi: 10.1016/j.fm.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 26.Miraghajani MS, Najafabadi MM, Surkan PJ, Esmaillzadeh A, Mirlohi M, Azadbakht L. Soy milk consumption and blood pressure among type 2 diabetic patients with nephropathy. J Ren Nutr. 2013;23:277–82.e1. doi: 10.1053/j.jrn.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Miraghajani MS, Esmaillzadeh A, Najafabadi MM, Mirlohi M, Azadbakht L. Soy milk consumption, inflammation, coagulation, and oxidative stress among type 2 diabetic patients with nephropathy. Diabetes Care. 2012;35:1981–5. doi: 10.2337/dc12-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rekha CR, Vijayalakshmi G. Biomolecules and nutritional quality of soymilk fermented with probiotic yeast and bacteria. Appl Biochem Biotechnol. 2008;151:452–63. doi: 10.1007/s12010-008-8213-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black teas. J Food Lipids. 1995;2:35–46. [Google Scholar]
- 30.Devi M, Gondi M, Sakthivelu G, Giridhar P, Rajasekaran T, Ravishankar G. Functional attributes of soybean seeds and products, with reference to isoflavone content and antioxidant activity. Food Chem. 2009;114:771–6. [Google Scholar]
- 31.Pick E, Keisari Y. A simple colorimetric method for the measurement of hydrogen peroxide produced by cells in culture. J Immunol Methods. 1980;38:161–70. doi: 10.1016/0022-1759(80)90340-3. [DOI] [PubMed] [Google Scholar]
- 32.Shihata A, Shah N. Proteolytic profiles of yogurt and probiotic bacteria. Int Dairy J. 2000;10:401–8. [Google Scholar]
- 33.Hubert J, Berger M, Nepveu F, Paul F, Daydé J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008;109:709–21. doi: 10.1016/j.foodchem.2007.12.081. [DOI] [PubMed] [Google Scholar]
- 34.Liu JR, Chen MJ, Lin CW. Antimutagenic and antioxidant properties of milk-kefir and soymilk-kefir. J Agric Food Chem. 2005;53:2467–74. doi: 10.1021/jf048934k. [DOI] [PubMed] [Google Scholar]
- 35.Virtanen T, Pihlanto A, Akkanen S, Korhonen H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J Appl Microbiol. 2007;102:106–15. doi: 10.1111/j.1365-2672.2006.03072.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Lindmark-Månsson H, Gorton L, Åkesson B. Antioxidant capacity of bovine milk as assayed by spectrophotometric and amperometric methods. Int Dairy J. 2003;13:927–35. [Google Scholar]


