Abstract
Background:
Frailty confers risk for surgical morbidity and mortality. Whether patient-reported measures of health, well-being, or quality of life respond differently to surgery in non-frail and frail individuals is unknown.
Methods:
Older adults with severe aortic stenosis presenting for surgery were assessed for frailty using Cardiovascular Health Study Criteria. Patient-reported measures of functional capacity (Duke Activity Status Index [DASI]), physical and mental health (Medical Outcomes Study Short Form-Physical and Mental Component Scales [SF-12 PCS and SF-12 MCS, respectively]), well-being (linear analogue self-assessment [LASA]), and quality of life (LASA) were administered before and 3 months after surgery.
Results:
Of 103 participants (mean age of 80.6 years), 54 were frail. Frail participants had lower baseline DASI, SF-12 PCS, SF-12 MCS, physical well-being, and quality of life scores than non-frail participants. At follow-up, frail participants showed significant improvement in physical function, with DASI and SF-12 PCS scores improving by 50% and 14%, respectively. Non-frail subjects did not significantly improve in these measures. SF-12 MCS scores also improved to a greater extent in frail compared to non-frail participants (3.6 vs < 1 point). Furthermore, the frail participants improved to a greater extent than non-frail participants in physical well-being (21.6 vs 7.1 points) and quality of life measures (25.1 vs 8.7 points).
Conclusions:
Frailty is prevalent in older adults with severe aortic stenosis and is associated with poor physical and mental function, physical well-being, and quality of life. In response to surgery, frail participants exhibited greater improvement in these patient-centered outcomes than non-frail peers.
Keywords: Aging, Resilience, Physical function, Healthspan, Patient-reported outcomes
Cardiovascular disease (CVD) remains the leading cause of death in the United States. The number of operations and procedures for CVD increased by 28% between 2000 and 2010 (1) and will further escalate in concert with population aging. Increased mortality and morbidity risk is an inherent challenge of operative decisions for older adults with CVD. An improved understanding of the association between surgical risk and surgical benefit has the potential to optimize medical management decisions.
Frailty is a geriatric syndrome resulting from declines across multiple physiological systems (2). Frailty is a predictor of operative complications, prolonged hospital stay, disability, institutionalization, and mortality in older patients (3–6). Moreover, when phenotypically characterized using Cardiovascular Health Study (CHS) criteria (7), frailty improves the predictive power of conventional risk models, including the American Society of Anesthesiologists score, Lee score, and the Eagle score for adverse health outcomes (3).
Frailty is prominent in the context of CVD (8). Older adults with either subclinical signs or overt CVD may exhibit up to 7.5-fold higher prevalence of frailty than those without CVD (9). Following surgery for CVD, frail older adults experienced greater postoperative complications, prolonged length of stay and in-hospital mortality, discharge to an institution, and reduced midterm survival (10). Thus, frailty is prevalent in older adults with CVD and can be used to identify those at higher risk for poor surgical outcomes.
Inherent risks of invasive interventions must be weighed against potential benefits including improvements in symptom burden, physical and mental health, and quality of life. In the context of CVD, recent studies have demonstrated improvements in health status and quality of life outcomes following surgery (11,12). However, differences in patient-centered outcome measures between lower-risk and higher-risk participants were not interrogated. Given the decisive impact on risk for adverse health outcomes, there is a pressing need to better understand how frailty status affects patient-centered outcomes.
Herein, we assessed the prevalence of frailty in older adults presenting for surgical valve replacement due to severe aortic stenosis, a prevalent age-associated CVD (13).We prospectively ascertained physical capacity, physical and mental function, five domains of well-being, and overall quality of life through self-report at baseline and follow-up. Our objectives were to determine the extent to which surgery affected measures of physical and mental health and quality of life, and examine how changes in these patient-centered outcomes compared between non-frail and frail study participants.
Methods
Study Protocol and Participants
The Mayo Clinic Institutional Review Board approved this study and informed consent was obtained from each participant. A convenience sample of patients aged 65 years and older diagnosed with severe aortic stenosis and scheduled to undergo transcatheter (TAVR) or surgical aortic valve replacement (SAVR) was recruited at Mayo Clinic in Rochester, MN as previously described (14). Before surgery, participant demographic characteristics, medical and surgical history, New York Heart Association (NYHA) classification, and Society of Thoracic Surgery (STS) risk index, were ascertained by interview, physical exam, and electronic medical record review.
Frailty Assessment
Frailty was assessed before surgery using CHS criteria; weak grip strength by electronic dynamometer, slow walk speed assessed by a handheld ultrasonic monitor, self-report of low endurance and energy on the Center for Epidemiological Studies Depression Scale, unintended weight loss greater than or equal to 10 lbs. in the prior year, and low physical activity by the Physical Activity Scale for the Elderly (7). Participants with three or more criteria were classified as frail.
Adverse Health Outcomes
Following surgery, participants were monitored for adverse events using the Society of Thoracic Surgeons National Database, which includes infection, reoperation, neurologic, pulmonary, renal, and vascular events. Rehospitalizations and deaths were monitored for 1 year.
Patient-Centered Outcome Measures
Patient-reported outcome measures were completed prior to surgery and at 3 months after hospital discharge. Functional capacity was assessed using the 12-item Duke Activity Status Index (DASI) (15). Physical and mental function were measured using the Physical Component Scale (PCS) and the Mental Component Scale (MCS) from the 12-item short form general health survey (SF-12) (16). Linear analogue self-assessment scales—from 0 (“as bad as it can be”) to 10 (“as good as it can be”)—of mental, physical, emotional, and spiritual well-being, level of social activity, and overall quality of life during the past week were also administered (17). Scores were linearly transformed to a 0–100 point scale for analysis.
Statistical Analyses
Continuous variables were summarized as mean ± standard deviation (SD) and the Student t test was used to test for differences between frail and non-frail groups. Categorical variables were summarized as N (%) and the chi-square test was used to compare between frail and non-frail groups. Logistic regression was used to calculate odd’s ratios and confidence intervals for adverse events, rehospitalization, and death.
Results
Subject Characteristics
Demographic and clinical characteristics of 103 study participants (42 women, 61 men) with severe aortic stenosis are presented in Table 1.
Table 1.
Study Sample Demographic Characteristics, Frailty Measures, Cardiovascular Parameters, and Comorbid Conditions, Stratified by Frailty Status
| All Subjects (n = 103) | Non-Frail (n = 49) | Frail (n = 54) | p-value | |
|---|---|---|---|---|
| Demographics | Mean (SD) or Number (%) | |||
| Age (y) | 80.6 ± 7.4 | 78.3 ± 8.0 | 82.8 ± 6.3 | .002a |
| Female | 42 (41%) | 12 (25%) | 30 (56%) | .003b |
| Body mass index (kg/m2) | 30.5 ± 6.5 | 29.6 ± 5.0 | 31.4 ± 7.5 | .159a |
| TAVR procedure | 61 (59%) | 19 (39%) | 42 (78%) | <.001b |
| SAVR procedure | 42 (41%) | 30 (61%) | 12 (22%) | <.001b |
| Frailty parameters | ||||
| CHS frailty score | 2.4 ± 1.4 | 1.1 ± 0.7 | 3.6 ± 0.7 | <.001a |
| Gait speed (m/s) | 0.9 ± 0.3 | 1.1 ± 0.2 | 0.7 ± 0.2 | <.001a |
| Grip strength (kg) | 27.2 ± 9.4 | 32.2 ± 8.8 | 22.5 ± 7.5 | <.001a |
| CVD measures | ||||
| Mean gradient (mmHg) | 49 ± 10.6 | 48.6 ± 9.8 | 49.6 ± 11.4 | .640a |
| Aortic valve area (cm2) | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.8 ± 0.2 | <.001a |
| Ejection fraction (%) | 58.3 ± 12.8 | 60.1 ± 11.9 | 56.6 ± 13.5 | .174a |
| B-natriuretic peptide (pg/m) | 1872 ± 1945 | 1145 ± 1326 | 2474 ± 2172 | .001a |
| STS score (%) | 7.2 ± 3.9 | 5.4 ± 3.1 | 8.6 ± 3.9 | <.001a |
| NYHA score | 2.8 ± 0.7 | 2.4 ± 0.7 | 3.1 ± 0.6 | <.001a |
| Co-morbidities | ||||
| Diabetes | 30 (29%) | 13 (27%) | 17 (32%) | .737b |
| Hypertension | 93 (90%) | 44 (90%) | 49 (91%) | .999b |
| Hyperlipidemia | 71 (69%) | 36 (74%) | 35 (65%) | .463b |
| Atrial fibrillation or flutter | 32 (31%) | 9 (18%) | 23 (43%) | .015b |
| Previous PCA | 38 (37%) | 16 (33%) | 22 (41%) | .519b |
| Previous CABG | 31 (30%) | 14 (29%) | 17 (32%) | .915b |
| Previous pacemaker | 9 (9%) | 3 (6%) | 6 (11%) | .585b |
| Previous stroke | 9 (9%) | 3 (6%) | 6 (11%) | .585b |
| PVD | 45 (44%) | 14 (29%) | 31 (57%) | .006b |
| Pulmonary disease | 45 (45%) | 17 (35%) | 28 (52%) | .120b |
Note: CABG = coronary artery bypass graft; CHS = Cardiovascular Health Study; CVD = Cardiovascular disease; NYHA = New York Heart Association; PCA = percutaneous coronary angioplasty; PVD = peripheral vascular disease; SAVR = Surgical Aortic Valve Replacement; STS = Society of Thoracic Surgeons; TAVR = Transcatheter Aortic Valve Replacement.
aUnpaired t test. bPearson’s Chi-squared test.
Frailty in Older Adults With Severe Aortic Stenosis
Fifty-four study participants (52%) were frail. Frail participants were slightly older, more likely to be female, and had significantly slower walking speed (0.71 vs 1.06 m/s, p < .001) and 43% weaker grip strength (22.5 vs 32.2 kg, p < .001) than non-frail participants (Table 1).
Frailty, Disease Status, and Comorbid Conditions
Parameters of aortic stenosis severity, including mean gradient and ejection fraction, did not differ between frail and non-frail participants. However, aortic valve area was 14% smaller in frail individuals (0.77 vs 0.90, p < .001). Frail participants had higher NYHA and STS scores (both p < .001), with the latter predictor of mortality exceeding 8.6%. Coexisting conditions and prior procedural interventions were prevalent in both frail and non-frail study participants. Prior atrial fibrillation or flutter and peripheral vascular disease were more prevalent in frail than non-frail participants (Table 1).
Postoperative Adverse Events
The incidence of monitored adverse events did not significantly differ between frail (67%) and non-frail (55%) study participants during the hospital stay (p = .23). However, a greater percentage of frail (37%) compared to non-frail (18%) participants were rehospitalized one or more times (OR = 2.61, 95% CI, 1.08–6.75, p = .038). One-year all-cause mortality following hospital discharge trended to be higher in frail (13%) compared to non-frail (4.3%) individuals (OR = 3.35, 95% CI = 0.76–23.3, p = .144).
Changes in Patient-Centered Outcomes Following Aortic Valve Replacement
Eighty participants (78%) completed both baseline and follow-up patient-reported outcome measures. There were no significant differences in baseline scores between responders and non-responders in any patient-reported outcome measure (data not shown).
Self-Reported Physical Function
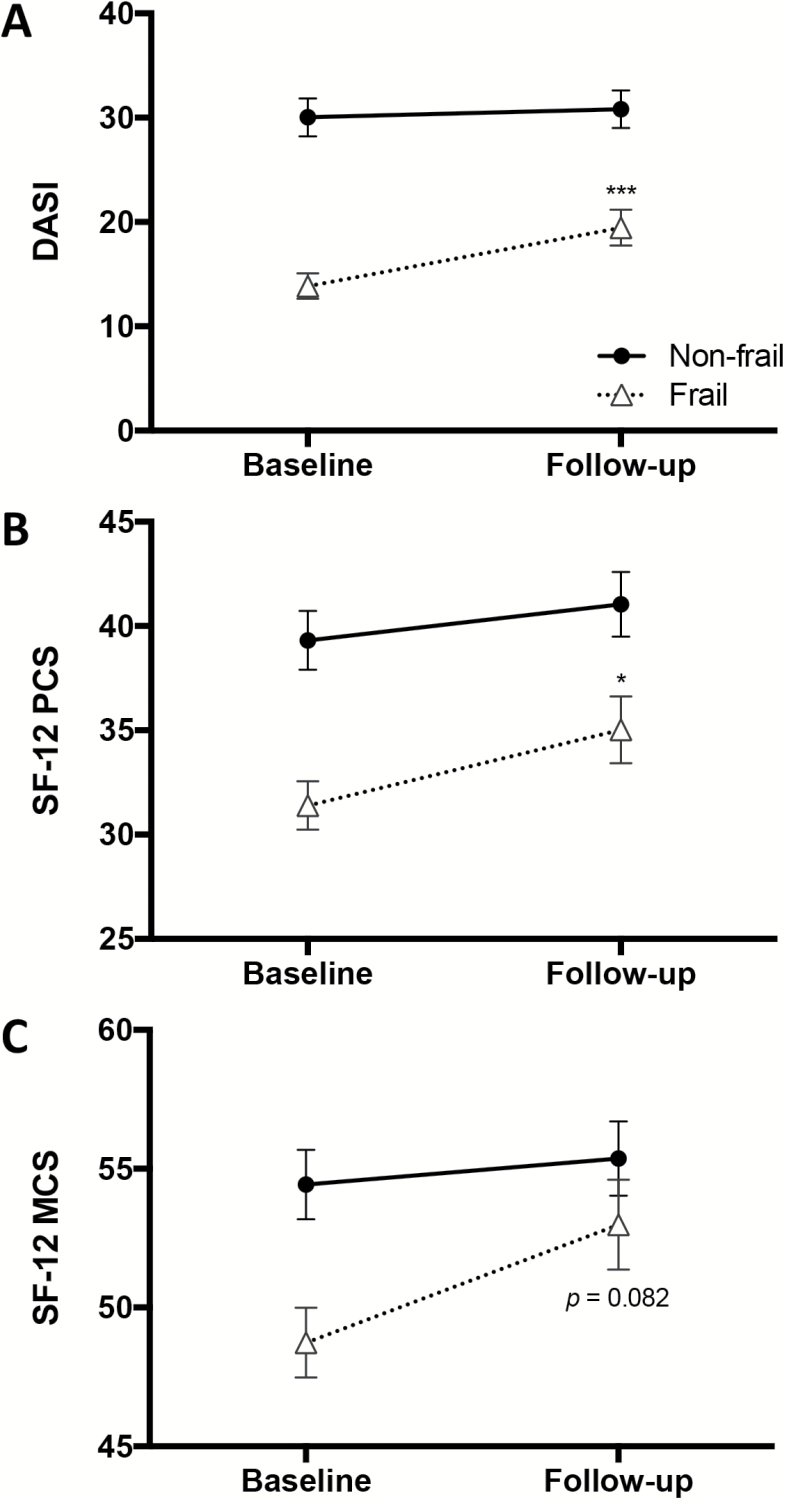
At baseline, non-frail participants had significantly higher functional capacity by DASI (30.3 vs 12.9, p < .001) and physical function by SF-12 PCS (38.9 vs 30.6, p < .001) than frail participants. However, non-frail participants failed to improve in either of these measures at 3 months following surgery. In contrast, frail participants exhibited significantly improved DASI (6.5 points, or 50% improvement) and SF-12 PCS (4.4 points, or 14% improvement) scores at 3 months post-discharge (both p < .05) (Figure 1A and B).
Figure 1.
Self-reported functional capacity, physical function, and mental function in older adults with severe aortic stenosis. (A) Functional capacity was assessed prior to and 3-months after surgery using the Duke Activity Scale Index (DASI). (B) Self-reported physical function was measured using the Medical Outcomes Survey SF-12 Physical Component Scale (PCS). (C) Self-reported mental function was determined by the SF-12 Mental Component Scale (MCS). * and *** denote a significant change from baseline by paired t test at a significance of p < .05 and < .001, respectively.
Self-Reported Mental Function
Non-frail participants had significantly higher mental health by SF-12 MCS than frail peers at baseline (54.9 vs 49.4, p = .005). Three months after surgery, scores for the non-frail group improved less than a point (p = .776). On the other hand, scores for the frail group trended towards improvement (49.4–53.0, p = .082) (Figure 1C).
Self-Reported Well-Being and Quality of Life
At baseline, non-frail participants had significantly higher physical well-being (p < .001), social activity (p = .029), and quality of life (p < .001) than their frail peers (Table 2). When combined, study participants demonstrated significant improvements in all measures of well-being, social activity, and overall quality of life after surgery. Further analysis revealed that frail participants improved to a greater extent than non-frail participants in physical well-being (21.6 vs 7.1 points, p = .004) and quality of life (25.1 vs 8.7 points, p < .001) (Table 2). As a result, no differences were observed between non-frail and frail individuals in any measure of well-being or quality of life at follow-up (Table 2).
Table 2.
Measures of Well-Being and Quality of Life Before and After Surgery in Non-Frail and Frail Older Adults With Severe Aortic Stenosis
| Non-Frail Subjects (n = 38) | Frail Subjects (n = 37) | p-valuea | |
|---|---|---|---|
| Baseline | |||
| Physical well-being | 70.8 ± 22.2 | 52.2 ± 22.7 | <.001 |
| Mental well-being | 75.8 ± 24.9 | 78.6 ± 19.7 | .584 |
| Emotional well-being | 79.2 ± 21.2 | 73.2 ± 21.2 | .227 |
| Spiritual well-being | 83.9 ± 17.9 | 83.8 ± 16.9 | .968 |
| Level of social activity | 73.4 ± 21.2 | 60.8 ± 27.5 | .029 |
| Quality of life | 72.1 ± 19.3 | 52.7 ± 21.6 | <.001 |
| 3-months after surgery | |||
| Physical well-being | 77.9 ± 17.1 | 73.8 ± 18.9 | .327 |
| Mental well-being | 86.3 ± 15.0 | 87.0 ± 14.7 | .836 |
| Emotional well-being | 84.2 ± 18.4 | 82.7 ± 15.4 | .702 |
| Spiritual well-being | 88.2 ± 15.0 | 87.8 ± 16.2 | .930 |
| Level of social activity | 81.1 ± 17.8 | 75.1 ± 22.6 | .211 |
| Quality of life | 80.8 ± 15.8 | 77.8 ± 17.7 | .448 |
| Change from baseline | |||
| Physical well-being | 7.1 ± 19.7* | 21.6 ± 22.8** | .004 |
| Mental well-being | 10.5 ± 21.8** | 8.4 ± 21.3* | .667 |
| Emotional well-being | 5.0 ± 16.2 | 9.5 ± 24.1* | .350 |
| Spiritual well-being | 4.2 ± 15.7 | 4.1 ± 18.3 | .968 |
| Level of social activity | 7.6 ± 19.7* | 14.3 ± 31.2** | .269 |
| Quality of life | 8.7 ± 15.1** | 25.1 ± 23.9** | <.001 |
Note: For within group comparisons, * and **denote a significant difference between baseline and 3-months after surgery by paired t test at a significance of p < .05 and < .01, respectively.
aBetween group comparisons by unpaired t test.
Discussion
Our study reveals the robust influence of frailty on patient-centered measures of physical and mental health, well-being, and quality of life in older adults with CVD. Potential gains in these patient-centered outcomes are important to be weighed against the anticipated risks associated with surgery in vulnerable older adults. This is particularly true for severe aortic stenosis, a condition for which SAVR and TAVR confer a significant survival benefit over standard therapy.
To our knowledge, our study is the first to examine the extent to which patient-centered outcomes compare between frail and non-frail persons. Baseline functional capacity, physical function, and physical well-being were significantly compromised in frail older adults with severe aortic stenosis. Despite this, frail participants improved to a greater extent than non-frail peers in DASI, SF-12 PCS, and physical well-being scores in response to SAVR and TAVR. Improvements in these parameters were clinically meaningful (18–20). Quality of life prior to surgery bordered the threshold of 50 among frail participants, a score below which interventions are warranted (21,22). However, this important measure increased an impressive 22 points following surgery, a change that far exceeds a clinically important difference. These data suggest that compromised physical health, well-being, and quality of life can be markedly and meaningfully improved by surgery in higher risk older adults. Additional work is needed to understand the durability of these improvements in frail and non-frail older adults with distinct forms of CVD and other age-related conditions.
Our data add to the existing evidence that CHS frailty criteria can be used to identify higher risk surgical candidates (3,10). Despite generally comparable age, disease severity, cardiac function, and comorbid disease burden, rehospitalizations and death were twice as common in frail compared to non-frail older adults receiving SAVR or TAVR. Opportunities to minimize risk and optimize outcomes among vulnerable older adults include “prehabilitation” strategies (eg, exercise, diet, drugs) to optimize resilience (23), and innovative transitional care plans to minimize surgical complications and adverse events. Indeed, more research is needed in these domains.
How chronological age, disease severity, comorbid conditions, and frailty individually and collectively influence patient-centered outcomes of health and quality of life is not well understood. Correspondingly, how these variables are affected by a very specific disease-modifying intervention, TAVR or SAVR, is also not clear. It is plausible that surgery enhanced physiological integrity, or strengthened the “weakest link” of frail participants, to attenuate frailty and improve patient-centered outcomes. A weakness of the study is that we did not reassess frailty in participants at follow-up. Considerable work is needed to disentangle the basic biology underlying compromised resilience and the mechanisms by which targeted interventions bestow multi-system benefit.
In summary, frailty is prevalent in older adults with severe aortic stenosis and is associated with increased adverse outcomes and mortality risk following SAVR and TAVR. Frail participants reported substantially lower functional capacity, physical function, social activity, and quality of life than non-frail participants prior to surgery. However, frail older adults exhibited more robust improvements in important patient-centered outcomes after surgery than non-frail counterparts. Our data further support frailty measures and patient-centered outcomes as tools to guide clinical decision-making. Additional research is needed to understand the impact of frailty-mitigating interventions on surgical, medical, and patient-centered outcomes in vulnerable older adults.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (NIH) (grant number AG53832 to NKL) the Hoeft family, and the Pritzker Foundation. This study was also supported by the Mayo Clinical Center for Clinical and Translational Science, grant number UL1 TR000135 from the National Center for Advancing Translational Science, a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Acknowledgments
We are grateful to the women and men who participated in this study.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. ; Writing Group Members; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi:10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 2. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi:10.1111/j.1532-5415.2006.00745 [DOI] [PubMed] [Google Scholar]
- 3. Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Sur. 2010;210:901–908. doi:10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 4. Ensrud Ke ESKTBC et al. Comparison of 2 frailty indexes for prediction of falls, disability, fractures, and death in older women. Arch Int Med. 2008;168:382–389. doi:10.1001/archinternmed.2007.113 [DOI] [PubMed] [Google Scholar]
- 5. Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr. 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 7. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–156. [DOI] [PubMed] [Google Scholar]
- 8. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. 2009;103:1616–1621. doi:10.1016/j.amjcard.2009.01.375. [DOI] [PubMed] [Google Scholar]
- 9. Newman AB, Gottdiener JS, McBurnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–166. [DOI] [PubMed] [Google Scholar]
- 10. Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi:10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 11. Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation. 2011;124:1964–1972. doi:10.1161/CIRCULATIONAHA.111.040022 [DOI] [PubMed] [Google Scholar]
- 12. Osnabrugge RL, Arnold SV, Reynolds MR, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv. 2015;8:315–323. doi:10.1016/j.jcin.2014.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Supino PG, Borer JS, Preibisz J, Bornstein A. The epidemiology of valvular heart disease: a growing public health problem. Heart Fail Clin. 2006;2:379–393. doi:10.1016/j.hfc.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 14. Schafer MJ, Atkinson EJ, Vanderboom PM, et al. Quantification of GDF11 and Myostatin in human aging and cardiovascular disease. Cell Metab. 2016;23:1207–1215. doi:10.1016/j.cmet.2016.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 16. Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 17. Singh JA, Satele D, Pattabasavaiah S, Buckner JC, Sloan JA. Normative data and clinically significant effect sizes for single-item numerical linear analogue self-assessment (LASA) scales. Health Qual Life Outcomes. 2014;12:187. doi:10.1186/s12955-014-0187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grodin JL, Hammadah M, Fan Y, Hazen SL, Tang WH. Prognostic value of estimating functional capacity with the use of the duke activity status index in stable patients with chronic heart failure. J Card Fail. 2015;21:44–50. doi:10.1016/j.cardfail.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wyrwich KW, Spertus JA, Kroenke K, Tierney WM, Babu AN, Wolinsky FD. Clinically important differences in health status for patients with heart disease: an expert consensus panel report. American Heart J. 2004;147:615–622. [DOI] [PubMed] [Google Scholar]
- 20. Sloan J, Symonds T, Vargas-Chanes D, Fridley B. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Information J. 2003;37:23–31. [Google Scholar]
- 21. Huschka MM, Mandrekar SJ, Schaefer PL, Jett JR, Sloan JA. A pooled analysis of quality of life measures and adverse events data in north central cancer treatment group lung cancer clinical trials. Cancer. 2007;109:787–795. doi:10.1002/cncr.22444. [DOI] [PubMed] [Google Scholar]
- 22. Butt Z, Wagner LI, Beaumont JL, et al. Use of a single-item screening tool to detect clinically significant fatigue, pain, distress, and anorexia in ambulatory cancer practice. J Pain Symptom Manage. 2008;35:20–30. [DOI] [PubMed] [Google Scholar]
- 23. Huffman DM, Schafer MJ, LeBrasseur NK. Energetic interventions for healthspan and resiliency with aging. Exp Gerontol. 2016;86:73–83. doi:10.1016/j.exger.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]