There is increasing interest in the mechanisms underlying the rapid antidepressant actions of the N-methyl-D-aspartate receptor (NMDAR) antagonist ketamine (1). In contrast to classical antidepressant treatments where efficacy builds over weeks, ketamine’s actions can be seen within hours after intravenous administration, or even within minutes following intranasal administration (2), and can last for several days after a single administration. In their functional magnetic resonance imaging study of anesthetized monkeys, Lv et al. (3) found that intramuscular administration of ketamine 18 hours before the functional magnetic resonance imaging scan reduced the correlations of spontaneous blood oxygen level–dependent (BOLD) signal fluctuations within the medial prefrontal cortex (mPFC) circuits associated with reward, schematically illustrated in Figure 1A in dark blue. This pattern was of particular interest, as mPFC circuits are often dysregulated in patients with major depressive disorder. In particular, depressed patients show overactivation of the ventral mPFC subgenual cortex, Brodmann area (BA) 25 (4), while increased activation of the dorsomedial anterior cingulate cortex (BA24) to fearful faces correlates with subsequent ketamine antidepressant response [reviewed in (2)]. Thus, the finding that ketamine reduced correlated activity in these circuits in monkeys suggests it may normalize brain activity in patients with depression.
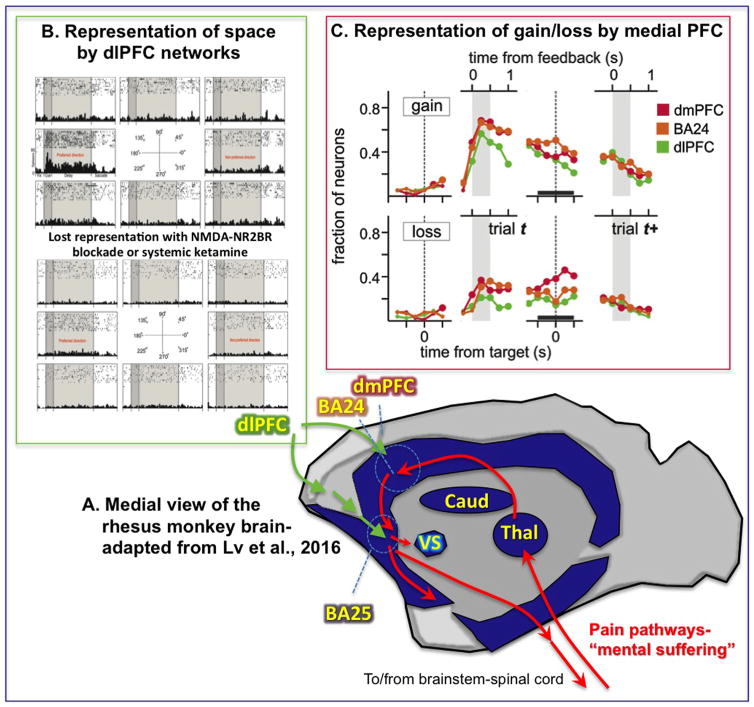
Figure 1.
(A) Schematic illustration of the results of Lv et al. (3) showing reduced functional connectivity (dark blue) in monkey medial prefrontal cortex (PFC) and subcortical structures 18 hours following ketamine. Anatomical pathways mediating the emotional aspects of pain are shown in red, with Brodmann area (BA) 24, dorsomedial PFC (dmPFC), and BA25 outlined; the dorsolateral PFC (dlPFC) (not shown, as it is on the lateral surface) connects to BA25 via rostral medial PFC. (B) Effects of N-methyl-D-aspartate (NMDA) receptor blockade on dlPFC delay cell firing, where iontophoresis of an NMDA receptor-NR2B antagonist causes complete loss of the representation of visual space. (C) Time course of neural signals related to gains and losses in dmPFC, dlPFC, and the anterior cingulate cortex (BA24). Shown are the fractions of neurons with significant gain- or loss-related activity within each region at different time lags from the time of target or feedback onset. Caud, caudate; Thal, thalamus; VS, ventral striatum. [(B) Adapted from Wang et al. (9). (C) Adapted from Seo and Lee (8)].
Lv et al. (3) applied a graph-theoretic analysis framework to characterize ketamine-induced changes in large-scale functional network architecture. Nodes in the network were defined as parcellated brain regions, including cortical areas and subcortical structures. Links between nodes were defined by the strength of functional connectivity, which is measured as the correlation of the BOLD signal time series, spatially averaged within each parcel. Network measures revealed that ketamine induced changes in the topology of the small-world architecture, with reduced clustering and efficiency. Each node in the network was characterized by its nodal strength, which is the mean value of functional connectivity to all other nodes. Most affected nodes showed reductions in nodal strength; key brain regions with reduced nodal strength include orbitofrontal cortex, anterior cingulate cortex (BA24), subgenual cortex (BA25), insular cortex, and thalamus.
Interestingly, these findings of an overall reduction in functional connectivity contrast with a prior study that found an overall increase in voxelwise nodal strength in healthy humans during acute subanesthetic administration of ketamine (5). This suggests that ketamine triggers complex, time-dependent processes mediating long-lasting network reconfigurations that continue even after the drug is gone. These differences also highlight difficulties in the interpretation of functional connectivity as communication between regions. Functional connectivity is simply statistical correlation and should be distinguished from the strength of signal transfer between regions, which has been termed effective connectivity. For example, ketamine may induce synchronized slow, burst-mode oscillations in thalamocortical circuits where most neurons fire together (6). Increased functional connectivity during ketamine may therefore reflect a state of abnormal synchronization of cortical areas with little information transfer between them, particularly in circuits that rely heavily on NMDAR synaptic connections (6).
The results of Lv et al. (3) suggest that the rhesus monkey can be used to more deeply explore the mechanism of ketamine’s antidepressant actions. The connections and functions of the primate PFC have been studied extensively, providing a rich database for exploration. The PFC expands greatly over brain evolution, consistent with its fundamental role in generating representations in the absence of sensory stimulation, capabilities that rely on NMDAR synapses (e.g., Figure 1B). The primate PFC is topographically organized, whereby the dorsolateral PFC (dlPFC) regions mediate cognitive functions based on the external sensory world, and orbital and medial PFC regions mediate internal states and emotion [reviewed in (2)]. Although Lv et al. (3) describe these as reward circuits, they are also the circuits that mediate responses to pain and aversive stimulation, which may explain their overactivity in patients with depression.
Particularly relevant to the current discussion, the mPFC is thought to be a key aspect of the pathways that mediate the emotional suffering aspects of a painful event, and ascending pain pathways target these mPFC areas in primates (schematically illustrated as red arrows in Figure 1A). Thus, projections from the spinal cord relay through the medial thalamus to the insular cortex and anterior cingulate (BA24), which then, in turn, project to the subgenual PFC (BA25) [reviewed in (2)]. BA24 and BA25 have been described as the PFC’s visceral motor system [reviewed in (2)], as they provide the main output from the PFC to subcortical structures controlling emotional state, including projections to the amygdala, ventral striatum, subthalamic nucleus, hypothalamus, and brainstem. Activation of these pathways may maintain the brain in a primitive state, e.g., inducing mental paralysis through activation of the subthalamic nucleus and the periaqueductal gray. BA25 is greatly enriched in serotonin transporters and is a target of deep brain stimulation for the treatment of intractable depression, where electrical inactivation of this region can produce an immediate lifting of mood (4). Conversely, recordings from the dorsal aspects of BA25 in monkeys find neurons that respond to an aversive air puff (7). Similarly, the anterior cingulate has been a target of surgical approaches for treating intractable pain [reviewed in (2)].
Most interestingly, neurons in the mPFC show persistent signatures of loss in monkeys playing a token-based decision-making task with a computer that is designed to simulate competitive social interactions, e.g., similar to rock-paper-scissors (8). This paradigm is a useful tool for probing brain response to aversive experience in an ethical manner, as it is a mild event but still sufficient to influence subsequent behavioral choices. During this token-based decision-making task, we found persistent neural activity related to gains and losses in multiple regions of the primate PFC (8). These persistent outcome signals were more prevalent in the mPFC areas than in the dlPFC (Figure 1C). Moreover, neurons with persistent gain signals were found more frequently in the dorsal anterior cingulate cortex, whereas neurons persistently encoding loss signals were more frequent in the dorsomedial PFC just above it, suggesting an anatomical gradient of gain versus loss signals in the primate mPFC. A similar dorsal to ventral gradient has been reported in BA25 (7) in regard to sensory-driven responses to aversive versus appetitive stimuli (although their longer term persistent responses have not been tested and will be a subject for future research). Thus, neurons in mPFC circuits generate persistent representations of aversive experience, which in humans may contribute to the symptoms of depression.
We have wondered if these persistent representations of loss may require NMDAR synaptic excitation, based on parallel recordings from the dlPFC (9). The principal sulcal region of the primate dlPFC receives visuospatial projections from the parietal association cortex, and so-called delay cells in this area are able to generate persistent representations of visual space in the absence of sensory stimulation, e.g., during the delay epoch of a spatial working memory task (9). Representations arise from recurrent excitatory pyramidal cell networks that interconnect via synapses on dendritic spines. These synapses contain NMDAR with NR2B subunits, and blocking these receptors with either local iontophoresis of an NMDAR antagonist or by systemic administration of ketamine reduces the firing of delay cells and erodes their representation of visual space (Figure 1B) (9). Interestingly, these higher cognitive circuits are little altered by alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor blockade, receptors that have more rapid kinetics and are typically concentrated in sensorimotor and hippocampal circuits (9). Indeed, the sensorimotor neurons within layer V of the dlPFC rely on both NMDAR and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor and show increased firing during ketamine (9), suggesting that increases in the BOLD response and/or functional connectivity during ketamine administration in human imaging studies (5) may reflect a distorted brain state where representational circuits are silenced but sensorimotor circuits are hyperactive.
We have hypothesized that ketamine’s rapid antidepressant actions may involve immediate actions in mPFC circuits, reducing NMDAR-mediated representations of pain or loss by neurons in BA24, dorsomedial PFC, and/or BA25 (2). We hope to test this hypothesis in monkeys performing the token-based decision-making task, but the current imaging data from Lv et al. (3) lend initial support to this view. This hypothesis would account for the ultra-rapid effects of intranasal administered ketamine in patients, where initial antidepressant actions can be seen within minutes (2,10), reminiscent of the ultra-rapid effects of deep brain stimulation (4). Longer term, more stable anti-depressant actions may arise from spine growth in higher PFC areas [e.g., as suggested by rodent models (1)], allowing higher circuits to better regulate medial affective PFC circuits. For example, the dlPFC may help to regulate emotional circuits through direct projections to BA24 and dorsomedial PFC and through indirect projections to BA25 via rostral mPFC areas 10m and 32 [schematically illustrated in Figure 1A; reviewed in (2)]. This may contribute to a sustained reduction in mPFC activity 18 hours following ketamine administration, as seen in Lv et al. (3). In humans, these top-down actions may reside particularly in the left dlPFC, a focus of transcranial magnetic stimulation therapy (2). Thus, understanding how aversive experience is represented and regulated in higher primate circuits may provide insights into the mental aspects of depression and strategies for its treatment.
Acknowledgments
This commentary was supported by Grant No. DP1AG047744 to AFTA.
Footnotes
Disclosures
AFTA and Yale University receive royalties from the sales of Intuniv (extended release guanfacine) in the United States from Shire Pharmaceuticals but not from generic formulations. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: The path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opler LA, Opler MG, Arnsten AFT. Ameliorating treatment-refractory depression with intranasal ketamine: Potential NMDA receptor actions in the pain circuitry representing mental anguish. CNS Spectr. 2015;26:1–11. doi: 10.1017/S1092852914000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lv Q, Yang L, Li G, Wang Z, Shen Z, Yu W, et al. Large-scale persistent network reconfiguration induced by ketamine in anesthetized monkeys: Relevance to mood disorders. Biol Psychiatry. 2016;79:765–775. doi: 10.1016/j.biopsych.2015.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 5.Driesen NR, McCarthy G, Bhagwagar Z, Bloch M, Calhoun V, D’Souza DC, et al. Relationship of resting brain hyperconnectivity and schizophrenia-like symptoms produced by the NMDA receptor antagonist ketamine in humans. Mol Psychiatry. 2013;18:1199–1204. doi: 10.1038/mp.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick DA, Bal T. Sleep and arousal: Thalamocortical mechanisms. Annu Rev Neurosci. 1997;20:185–215. doi: 10.1146/annurev.neuro.20.1.185. [DOI] [PubMed] [Google Scholar]
- 7.Monosov IE, Hikosaka O. Regionally distinct processing of rewards and punishments by the primate ventromedial prefrontal cortex. J Neurosci. 2012;32:10318–10330. doi: 10.1523/JNEUROSCI.1801-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seo H, Lee D. Behavioral and neural changes after gains and losses of conditioned reinforcers. J Neurosci. 2009;29:3627–3641. doi: 10.1523/JNEUROSCI.4726-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Yang Y, Wang CJ, Gamo NJ, Jin LE, Mazer JA, et al. NMDA receptors subserve working memory persistent neuronal firing in dorsolateral prefrontal cortex. Neuron. 2013;77:736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapidus KA, Levitch CF, Perez AM, Brallier JW, Parides MK, Soleimani L, et al. A randomized controlled trial of intranasal ketamine in major depressive disorder. Biol Psychiatry. 2014;76:970–976. doi: 10.1016/j.biopsych.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]