Abstract
Purpose
We hypothesized that intermittent anticoagulation based on daily rhythm monitoring using the novel oral anticoagulants (NOACs) is feasible and safe among patients with paroxysmal atrial fibrillation (AF).
Methods
Patients with paroxysmal AF and ≥1 risk factors for stroke were randomized to either intermittent or continuous anticoagulation. Those in the intermittent group were instructed to transmit a daily ECG using an iPhone-based rhythm monitoring device. If AF was detected, patients received one of the NOACs for 48 hours -1 week. Patients who failed to transmit an ECG for 3 consecutive days or more than 7 days total were crossed over to continuous anticoagulation. Patients in the continuous group received one of the NOACs.
Results
Fifty-eight patients were randomized to either intermittent (n=29) or continuous anticoagulation (n=29). Over a median follow-up of 20 months, 20 patients in the intermittent group failed to submit a daily ECG at least once (median 3 failed submissions). Four patients (14%) crossed over to continuous anticoagulation due to failure to submit an ECG for 3 consecutive days. One stroke (continuous group) occurred during the study. Major bleeding occurred in 2 patients in the continuous and one patient in the intermittent group, after crossing over to continuous anticoagulation. In a pre-specified per-protocol analysis, gastrointestinal bleeding was more frequent in the continuous group (16% vs. 0%; p=0.047).
Conclusions
Intermittent anticoagulation based on daily rhythm monitoring is feasible and may decrease bleeding in low-risk patients with paroxysmal AF. A larger trial, adequately powered to detect clinical outcomes, is warranted.
Keywords: atrial fibrillation, anticoagulation, remote monitoring, bleeding
Introduction
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia and is associated with increased cardiovascular morbidity and mortality [1, 2]. Anticoagulation with either warfarin or one of the novel oral anticoagulants (NOACs) is indicated for patients with AF with prior stroke, transient ischemic attack or a CHA2DS2-VASc (Congestive heart failure/left ventricular dysfunction, Hypertension, Age ≥75 [doubled], Stroke [doubled] – Vascular disease, Age 65-74, Sex category [female]) score of 2 or greater [3]. Multiple randomized studies and meta-analyses have shown that the NOACs decrease thromboembolic events and mortality, compared to warfarin [4-8]. Nonetheless, the bleeding risk associated with the NOACs is not negligible, with rates of major bleeding for dabigatran and rivaroxaban being similar to those seen with warfarin [5, 7]. In addition, these agents are associated with increased risk of gastrointestinal bleeding [9, 8]. As such, the risk of thromboembolism should be balanced against the risk of bleeding [3]. As patient preferences regarding the relative importance of preventing strokes and avoiding bleeding vary widely [10], alternative approaches to long-term anticoagulation need to be considered.
Thrombus formation in the left atrium is thought to represent the main source of thromboembolic events in patients with AF [11]. Consistent with recent evidence suggesting that paroxysmal AF may be associated with a lower incidence of stroke compared to persistent AF [12-15], we hypothesized that in a selected group of patients with paroxysmal AF and a low risk of stroke, maintaining therapeutic anticoagulation only during episodes of AF (either symptomatic or asymptomatic) may provide the benefits of continuous anticoagulation in terms of stroke prevention, while decreasing the risk of bleeding complications. This approach requires an oral anticoagulant with rapid onset of anticoagulation (a NOAC), as well as remote rhythm monitoring. Although implantable devices offer continuous rhythm monitoring, their use is limited to patients with an indication for a dual-chamber pacemaker or defibrillator. Insertable cardiac monitors represent an emerging alternative approach for continuous monitoring, and although they can accurately detect AF [16], they may be associated with higher risk of complications [17] and increased cost, thus decreasing patient acceptability. A new, inexpensive and convenient iPhone-based rhythm monitoring device is currently available and can detect AF with high sensitivity and specificity [18]. In this pilot study, we tested the feasibility and preliminary safety of intermittent anticoagulation based on daily iPhone-based rhythm monitoring among patients with paroxysmal AF and one or more additional risk factors for stroke.
Methods
This was a prospective open-label randomized controlled pilot study. Patients with paroxysmal AF, documented by ECG, implantable device electrogram or Holter monitor, within 6 months of randomization on 2 separate occasions, at least 1 day apart, were eligible for enrollment. In addition, patients were required to have at least one additional risk factor for stroke, including left ventricular ejection fraction ≤40% or symptomatic heart failure, age ≥75, diabetes, hypertension, or age ≥65 with documented coronary artery disease. Patients were excluded if they had any of the following: prior stroke or transient ischemic attack, prosthetic valve or hemodynamically significant valve disease, reversible causes of AF, severe renal impairment (estimated creatinine clearance 30 mL/min or less), active liver disease, anemia (hemoglobin less than 10g/dL) or thrombocytopenia (platelets less than 100 × 109/L) and pregnancy or nursing. Patients were enrolled from November 2013 until June 2015. In December 2014, after the new guidelines for AF were published [3], the Data Safety Monitoring Board recommended that the protocol be amended to include only patients with a CHA2DS2-VASc score of 1, to comply with the new guidelines. Women with one additional risk factor could be included in the study. Patients who were randomized to intermittent anticoagulation who subsequently met a new exclusion criterion were informed of the new guidelines and were crossed over to the continuous anticoagulation arm unless the patient refused to do so, in which case, the patient remained in the intermittent anticoagulation group and continued to be followed for clinical events as per the study protocol.
Following enrollment, patients were randomly assigned in a 1:1 ratio to either intermittent or continuous anticoagulation. Patients in the continuous anticoagulation group received one of the NOACs, with the choice of the NOAC left to the discretion of the referring physician. Patients who were previously on warfarin were started on one of the NOACs after their INR fell to about 2.0 [3]. Those in the intermittent anticoagulation group were provided with an iPhone-based rhythm monitoring device and were instructed to transmit a daily 30-second ECG rhythm strip at approximately the same time of the day, as well as when experiencing symptoms of AF. If AF was detected, as confirmed by one of the investigators, the patients were instructed to start anticoagulation immediately. By doing so, patients started anticoagulation within 24 hours after the onset of AF. To increase compliance of the participants with daily rhythm monitoring, an automatic daily reminder was programmed through their iPhones. In addition, patients who did not transmit their rhythm for 2 consecutive days, received a reminder call from one of the investigators. When AF was detected based on rhythm monitoring, patients received anticoagulation for 48 hours to 1 week, according to a pre-specified algorithm, depending on the duration of the AF episode, to account for atrial stunning [19-21]. Specifically, for episodes lasting less than 48 hours, anticoagulation was continued for 48 hours, while for episodes lasting 2 days to 1 week, anticoagulation was continued for 1 week (Figure 1). Patients who failed to transmit an ECG for 3 consecutive days, despite measures to ensure compliance, or failed to transmit an ECG for a total of more than 7 days regardless of time span, were crossed over to the continuous anticoagulation arm. Patients who developed persistent AF, defined as AF duration greater than 7 days or requiring cardioversion [3], were also crossed over to the continuous anticoagulation arm. The percentage of participants who crossed over to continuous anticoagulation was used as a measure of adherence to the protocol of daily rhythm monitoring.
Figure 1. Pre-specified algorithm for intermittent anticoagulation based on the duration of each atrial fibrillation (AF) episode.

The primary feasibility endpoint of the study was adherence to the pre-specified protocol of intermittent anticoagulation. Primary efficacy and safety endpoints included stroke or systemic embolism and major bleeding, respectively. Secondary endpoints included death; the composite of death or stroke or systemic embolism; gastrointestinal bleeding and the composite of major or gastrointestinal bleeding. Major bleeding was defined according to the ISTH criteria as clinically overt bleeding accompanied by a decrease in the hemoglobin level of at least 2 g/dL or transfusion of at least 2 units of packed red cells, occurring at a critical site, or resulting in death or permanent disability. All endpoints were adjudicated by an independent committee, the members of which were not be involved in patient care and were unaware of the treatment assignments. The Institutional Review Board at the University of Oklahoma Health Sciences Center approved the study prior to its initiation and informed consent was obtained from each patient.
Statistical analysis
Continuous data are presented as mean ± standard deviation or median and interquartile range (IQR), as appropriate. Categorical data are presented as percentages. Means were compared using a two sample t-test. Proportions were compared using a Chi-square test, Fisher's exact test, when more than 25% of expected frequency counts were less than five or any zero counts were observed in a category, or a Cochran-Armitage trend test for ordered categories. The time-to-major bleeding and time-to- gastrointestinal bleeding distributions were estimated for each therapy group using the Kaplan- Meier method and 18-month event rates for each group were derived. The time-to- thromboembolic event distributions and survival distributions were estimated in a similar manner. Time-to-event distributions were compared between the 2 groups using a log- rank test. The cumulative incidence of crossing over from intermittent to continuous therapy due to poor adherence was estimated while accounting for death and crossing over due to AF as competing events [22]. Analyses reflect an intent-to-treat paradigm in which all data were analyzed according to randomized treatment assignment; per-protocol analyses were also performed, where data were analyzed up to the point in time when the patient on intermittent therapy crossed over to continuous therapy. A 2-stage monitoring approach was used to monitor major cardiovascular event rates, in each arm separately, as a guide to identify unacceptable levels of risk using a Simon 2-stage design [23]. The sample size of this pilot study was driven by feasibility considerations with the intent to estimate protocol adherence and to derive preliminary estimates of stroke, death and bleeding event rates. Statistical significance was declared at p<0.05.
Results
Of 88 potentially eligible patients screened, 58 patients were enrolled in the study (Figure 2). Among the 58 patients, 29 (50%) were randomized to intermittent anticoagulation and 29 (50%) were randomized to continuous anticoagulation. The baseline characteristics of the patients were balanced between the 2 groups (Table 1). In the intermittent group, 19 (65%) patients were prescribed apixaban, 8 (28%) were prescribed rivaroxaban and 2 (7%) were prescribed dabigatran. In the continuous group, apixaban, rivaroxaban and dabigatran were prescribed in 20 (69%), 7 (24%) and 2 (7%) patients, respectively. Sixteen (55%) and 14 (48%) patients in the intermittent and the continuous group, respectively, were taking an antiarrhythmic drug at the time of enrollment, including amiodarone (2 intermittent and 4 continuous) and dronedarone (1 intermittent). Five (18%) patients in each group had undergone a catheter ablation for AF at least 6 months prior to enrollment.
Figure 2. Enrollment, randomization, follow-up and attrition of patients.
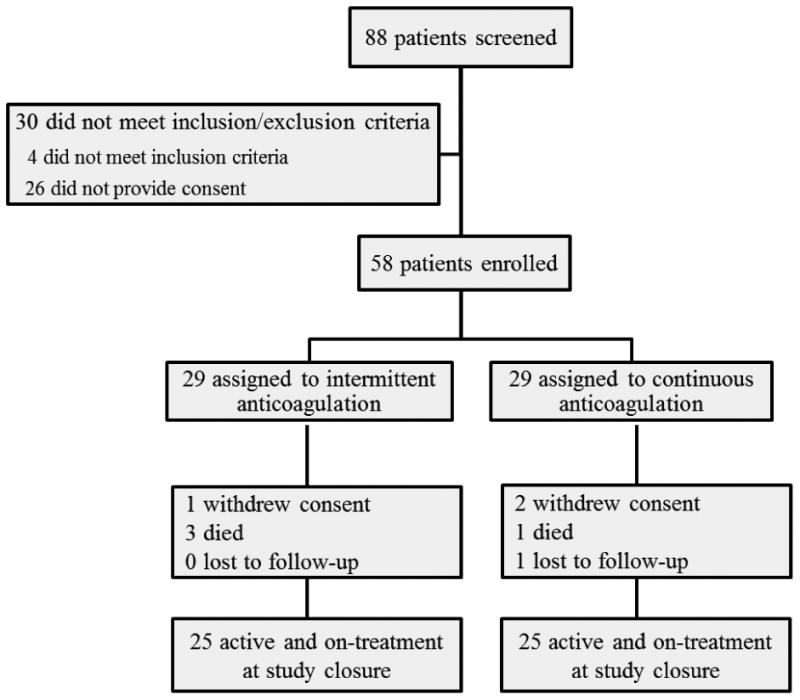
Table 1. Baseline characteristics of the patients in the randomized intervention groups.
| Baseline Characteristic | Intermittent Anticoagulation (n=29) | Continuous Anticoagulation (n=29) | P-value | ||
|---|---|---|---|---|---|
| Age (years) | 62.5±12.2 | 60.9±14.4 | 0.66 | ||
| Male | 17 | 59% | 16 | 55% | 0.79 |
| Body mass index (kg/m2) | 33.0±8.2 | 32.3±8.4 | 0.77 | ||
| Systolic Blood Pressure (mmHg) | 120.9±12.9 | 125.8±20.9 | 0.29 | ||
| Diastolic Blood Pressure (mmHg) | 72.9±8.3 | 74.3±11.4 | 0.57 | ||
| Heart rate (beats per minute) | 70.1±13.9 | 67.5±11.0 | 0.42 | ||
| Years with atrial fibrillation | 0.74 | ||||
| <1 year | 5 | 17% | 6 | 21% | |
| ≥1 year | 24 | 83% | 23 | 79% | |
| Congestive heart failure | 2 | 7% | 7 | 24% | 0.14 |
| Coronary artery disease | 7 | 25% | 10 | 36% | 0.38 |
| Diabetes | 10 | 34% | 8 | 28% | 0.57 |
| Sleep Apnea | 4 | 14% | 10 | 36% | 0.06 |
| Hypertension | 26 | 90% | 24 | 83% | 0.71 |
| CHADS2 Score | 0.86 | ||||
| 0-1 | 15 | 52% | 13 | 45% | |
| 2 | 11 | 38% | 13 | 45% | |
| ≥3 | 3 | 10% | 3 | 10% | |
| Mean (±SD) | 1.6±0.7 | 1.6±0.9 | 0.87 | ||
| CHA2DS2-VASc Score | 0.59 | ||||
| 0-1 | 7 | 24% | 3 | 10% | |
| 2 | 9 | 31% | 13 | 45% | |
| ≥3 | 13 | 45% | 13 | 45% | |
| Mean (±SD) | 2.5±1.2 | 2.7±1.3 | 0.60 | ||
| HAS-BLED Score | 0.54 | ||||
| 0-2 | 21 | 72% | 23 | 79% | |
| ≥3 | 8 | 28% | 6 | 21% | |
| Mean (±SD) | 1.9±0.9 | 1.6±1.1 | 0.24 | ||
| Smoking Status | 0.59 | ||||
| Current | 3 | 10% | 5 | 17% | |
| Former | 12 | 41% | 9 | 31% | |
| Never | 14 | 48% | 15 | 52% | |
| Employment status | 0.81 | ||||
| Disabled, do not work | 2 | 7% | 3 | 10% | |
| Full-time employed | 15 | 52% | 16 | 55% | |
| Homemaker | 2 | 7% | 3 | 10% | |
| Retired | 10 | 34% | 7 | 24% | |
| Education status | 0.86 | ||||
| < High School | 1 | 3% | 1 | 3% | |
| High School/GED | 6 | 21% | 5 | 17% | |
| Vo-tech/College | 22 | 76% | 23 | 79% | |
| Antithrombotic or anticoagulant medications at randomization* | |||||
| Warfarin | 3 | 10% | 4 | 14% | 1.0 |
| Aspirin | 13 | 45% | 9 | 31% | 0.28 |
| Clopidogrel | 2 | 7% | 0 | 0% | 1.0 |
| Apixaban | 4 | 14% | 5 | 17% | 1.0 |
| Dabigatran | 2 | 7% | 2 | 7% | 1.0 |
| Rivaroxaban | 7 | 24% | 6 | 21% | 0.75 |
| None | 4 | 14% | 7 | 24% | 0.32 |
| Creatinine (mg/dL) | 1.2±0.8 | 1.0±0.3 | 0.19 | ||
| Hemoglobin (g/dL) | 13.3±1.9 | 13.6±1.7 | 0.51 | ||
Patients could select multiple agents and therefore, the counts do not sum to the total number of patients.
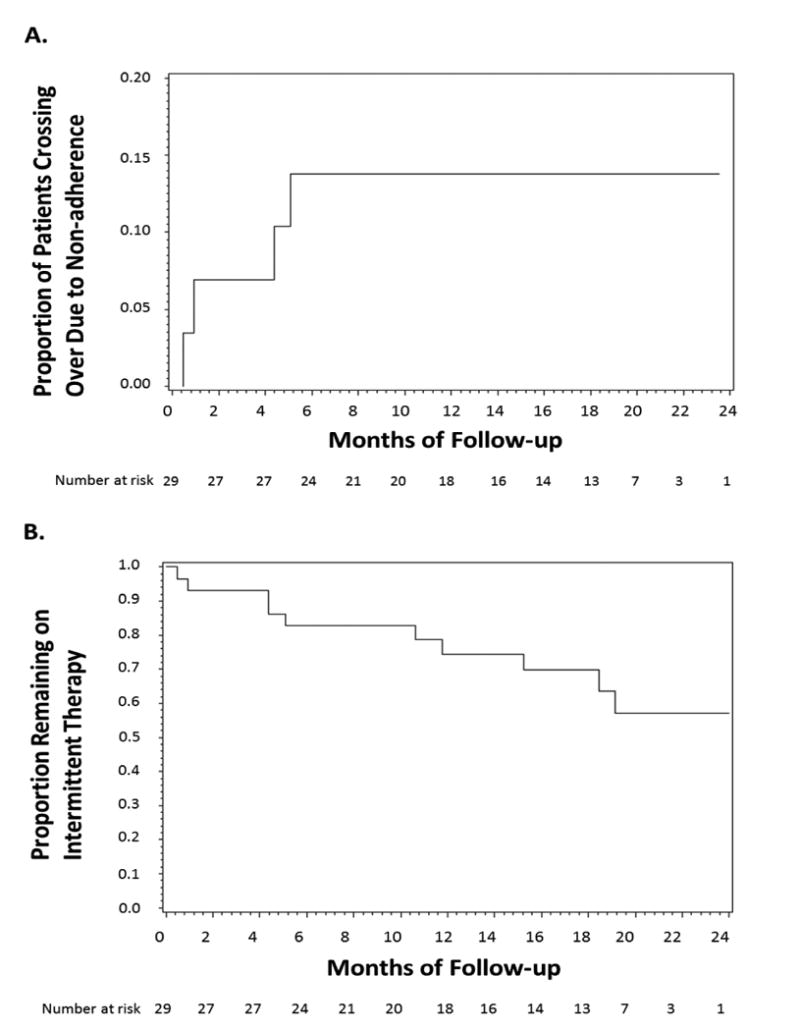
During a median follow up of 20 months (interquartile range 13 to 22 months), among the 29 patients randomized to intermittent anticoagulation, 4 (14%) crossed over to continuous anticoagulation due to failure to submit rhythm strips. Six additional patients crossed over because due to development of persistent AF. Following the Data and Safety Board's recommendation to change eligibility criteria, no patients agreed to be crossed over from intermittent to continuous therapy. The cumulative incidence of crossing over due to failure to submit the rhythm strips for 3 consecutive days is summarized in Figure 3A. The time-to-crossover distribution for all patients assigned to intermittent therapy, regardless of the reason for crossing over, is summarized in Figure 3B. Among the 29 patients assigned to the intermittent arm, 20 patients failed to submit rhythm strips a total of 81 times over 410 person-months of follow-up completed prior to crossing over to the continuous therapy arm. The resulting rate is 1 failed submission per 5 person-months of follow-up. Among the 29 patients, the number of failed submissions ranged from 0 to 7 with a median value of 3 failed submissions (IQR: 0 to 5 submissions).
Figure 3. Cumulative incidence of crossover to continuous anticoagulation due to non-adherence (A) and time-to-crossover distribution regardless of the reason for crossover (B) in patients initially assigned to intermittent anticoagulation.
A total of 160 AF episodes were detected through the submitted rhythm strips in 18 of 29 patients in the intermittent arm during 478 person-months of follow-up, resulting in an AF rate of approximately 1 episode per 2.7 person-months of follow-up. Among the 29 patients assigned to the intermittent group, the number of AF episodes ranged from 0 to 49 with a median value of 1 episode (IQR: 0 to 5 episodes). Among the 18 patients experiencing at least one episode, the number of AF episodes ranged from 1 to 49 with a median value of 4 episodes (IQR 1 to 16). Of the 160 detected episodes, 138 (86%) lasted <48 hours and 16 (10%) episodes lasted 48 hours-1 week (12 lasted <72 hours and all lasted <96 hours). Six episodes (one in each patient) lasted >1 week and resulted in crossing over to the continuous arm due to development of persistent AF, according to study protocol. Of those, 4 patients were cardioverted to sinus rhythm and all 6 remained in the continuous group for the remainder of the study. In all patients, anticoagulation was started within 1 hour of confirmation of the presence of AF by one of the investigators. In 5 patients in the intermittent group, the presence of implantable devices allowed us to compare their true AF burden, with the AF detection rate obtained from non-invasive daily rhythm monitoring. One patient had a lot of short episodes (approximately 25 episodes per month, corresponding to high AF density [24]), many of which were not picked up by daily rhythm monitoring, which provided an AF rate of approximately 1 episode per month. In the rest of the patients with infrequent, more prolonged episodes, the AF burden obtained by the 2 methods was comparable, ranging from 0 to 1 episode per 1.3 months by continuous monitoring through the implantable device vs. 0 to 1 episode per 1.8 months by daily rhythm monitoring. Concordance and discordance rates between iPhone- and device-based AF detection are summarized in Table 2.
Table 2. Concordance and discordance rates between iPhone- and device-based AF detection in patients with implantable devices.
| Patient | Concordant | Discordant | Total | ||||
|---|---|---|---|---|---|---|---|
| Both AF | Both SR | Concordance rate | iPhone AF/device SR | iPhone SR/device AF | Discordance rate | ||
| #2 | 11 | 548 | 98.9% | 2 | 4 | 1.1% | 565 |
| #8 | 7 | 364 | 99.7% | 1 | 0 | 0.3% | 372 |
| #22 | 13 | 684 | 99.3% | 0 | 5 | 0.7% | 702 |
| #32 | 0 | 582 | 100% | 0 | 0 | 0% | 582 |
| #47 | 17 | 328 | 70.7% | 0 | 143 | 29.3% | 488 |
| Total | 48 | 2,506 | 94.3% | 3 | 152 | 5.7% | 2,709 |
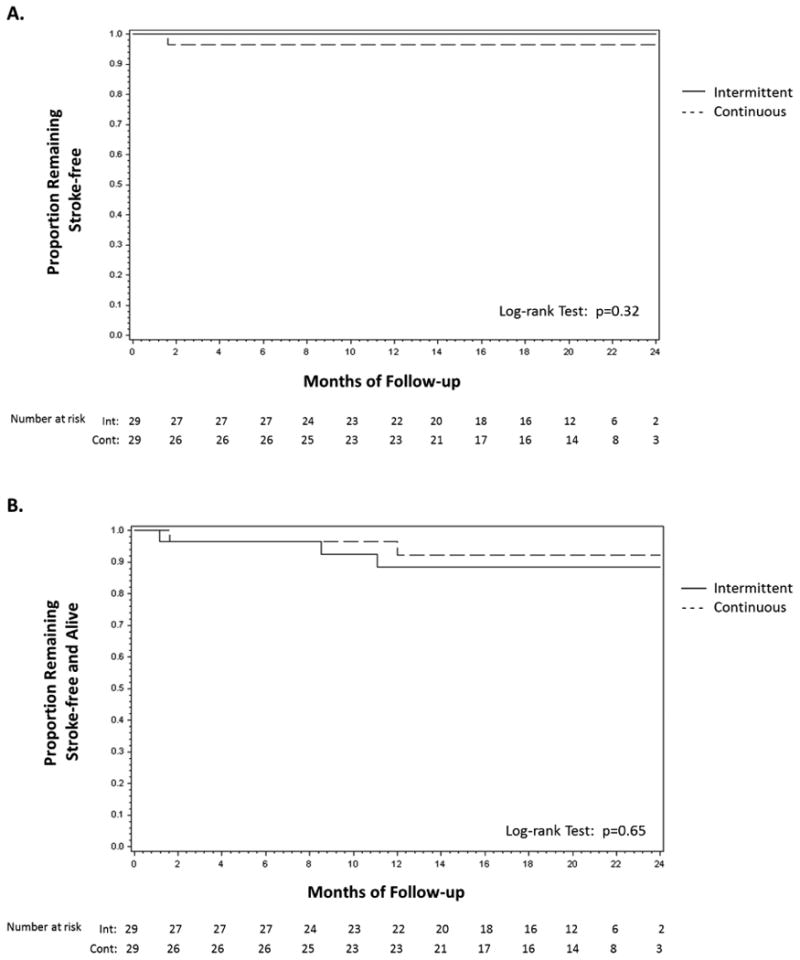
Three patients withdrew consent (1 in the intermittent and 2 in the continuous group) and 1 patient was lost to follow-up (continuous group) during the course of the study (Figure 1). Three non-cardiac deaths (1 in the continuous and 2 in the intermittent group), one cardiovascular death (cardiogenic shock in the intermittent group) and one stroke (continuous group) occurred during the study. No strokes occurred in the intermittent anticoagulation group. Of note, the patient who had a stroke was found to have significant ipsilateral carotid artery stenosis and underwent carotid artery stenting. Under the intent-to-treat analysis, the rates of stroke or systemic embolism did not differ significantly between the intervention groups (p=0.32) (Figure 3A). Likewise, the rates of death or stroke (composite endpoint) were not significantly different between the groups (p=0.65) (Figure 3B).
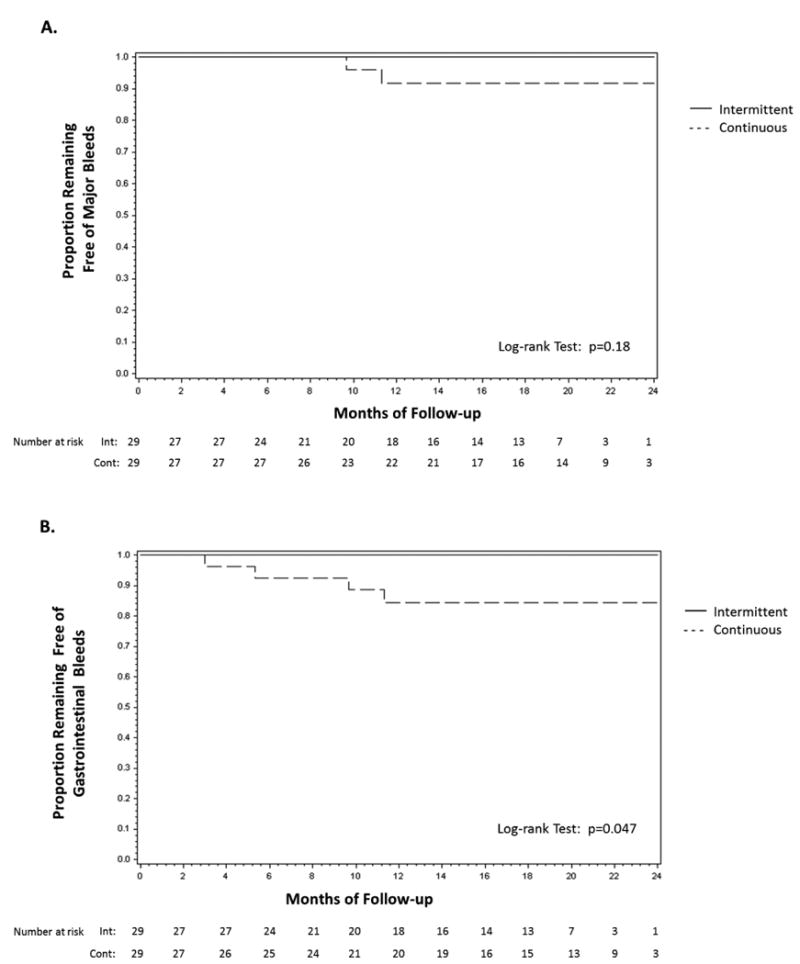
Major bleeding occurred in 2 patients in the continuous group and one patient in the intermittent group, after crossing over to continuous anticoagulation. All major bleeding events were due to gastrointestinal bleeding. There was no difference in the major bleeding-free probability between the 2 groups (p=0.61 in the intent-to-treat analysis and p=0.18 in the per-protocol analysis; Figure 5A) Seven gastrointestinal bleeding events occurred in 5 patients (4 in the continuous group and 1 in the intermittent group after crossing over to continuous group). In the intent-to-treat analysis, the 18-month estimated gastrointestinal bleeding-free probability was 96% (95% CI: 76% to 99%) in the intermittent group and 84% (95% CI: 63% to 94%) in the continuous group (p=0.17). In a pre-specified per-protocol analysis, gastrointestinal bleeding was more frequent in the continuous group compared to the intermittent group (18-month estimated gastrointestinal bleeding-free probability 84% [95% CI 63% to 94%] vs. 100%, respectively; p=0.047) (Figure 5B).
Figure 5. Time-to-event distribution for the endpoints of major bleeding (A) and gastrointestinal bleeding (B) in the randomized intervention groups (per-protocol analysis).
Discussion
In this study, a strategy of intermittent anticoagulation only during paroxysmal episodes of AF, based on daily rhythm monitoring using an iPhone-based device, was feasible among patients with paroxysmal AF and relatively low risk of stroke. Our study confirms the results of the recently published REACT.COM study, which showed that targeted anticoagulation based on rhythm monitoring using an insertable cardiac monitor is feasible [25], and expands them in the sense that it is the first study to use a non-invasive monitoring device to guide anticoagulation therapy. Patients who may mostly benefit from this strategy include those with low risk of thromboembolic events (specifically those with a CHA2DS2-VASc score of 1), and/or those with a relatively high bleeding risk, in whom the current practice is to err on the side of withholding anticoagulation. In addition, this approach may be very attractive to the small percentage of patients who would not be willing to consider any anticoagulation therapy even it was 100% effective in preventing strokes, because of concerns of bleeding complications [10]. Such patient-tailored anticoagulation therapy for AF is supported by the current guidelines, which recommend a discussion of the risks of stroke and bleeding and the individual patient's preferences before initiating anticoagulation therapy [3]. As a result of the pilot nature of our study and small sample size, our study was not powered to detect clinically important differences of modest size in stroke or major bleeding. Nonetheless, in a pre-specified per-protocol analysis, there was a significant reduction of gastrointestinal bleeding in the intermittent group, without an increase in the stroke rate. Few patients developed gastrointestinal bleeding (4 in the continuous arm and none in the intermittent arm prior to crossing over) and therefore, this result must be interpreted cautiously. An increased risk of bleeding on the continuous arm is in line with recent data showing that NOACs increase gastrointestinal bleeding rates [9, 8]. In essence, the concept of intermittent anticoagulation attempts to decrease the risk of bleeding while maintaining stroke prevention. In other words, it aims to maximize the risk/benefit ratio of anticoagulation. Our study by no means implies that this is the case, but it does prove that the approach is feasible and provides the basis for the design of a larger trial, adequately powered to detect clinically significant outcomes.
Adherence to the protocol of intermittent anticoagulation may represent a challenge, given that daily monitoring is required, but it is an essential component of this approach. The process of daily rhythm monitoring using a smartphone-based device to guide anticoagulation therapy requires adequate patient motivation, thus patient selection is extremely important. It has been shown that patients' attitudes towards anticoagulation vary widely, with some patients refusing anticoagulation because of the increased bleeding risk [10]. Thus, some patients with AF would be highly motivated and would be willing to adhere to a protocol of daily rhythm monitoring, in order to avoid continuous anticoagulation. In a non-selected group of patients, such as ours, adherence to the protocol of daily rhythm monitoring was very good, with only 4 of 29 (14%) crossing over to continuous anticoagulation due to non-adherence to the protocol of daily rhythm monitoring and only 1 failed submission per 5 person-months of follow-up. In addition, as shown in Figure 2A, those who did not adhere to the protocol and had to be crossed over to the continuous arm, did so early in the study, and no late crossovers were observed, suggesting that non-adherence was due to intrinsic patient characteristics (making them prone to non-compliance to any measure), rather than the burden of the daily rhythm monitoring process. A run-in phase would have likely reduced the incidence of non-adherence. On the other hand, daily physician interpretation of the transmitted ECG, the workflow, and the information technology associated with this approach is not trivial. Thus, improvement in the AF detection algorithm to achieve almost 100% sensitivity is imperative for the success of future studies using this approach. This will allow the investigators to review only those recordings that are read by the device as AF, in order to decrease the burden of having to review each and every transmitted recording.
Although the association between AF and stroke has been established in multiple epidemiological studies, the exact mechanism of stroke remains poorly understood [11]. Multiple studies have shown a correlation between thromboembolic risk and asymptomatic AF episodes of variable duration detected through implantable devices [26-29], even though a temporal association of an incident stroke with an antecedent AF episode was frequently absent in the aforementioned studies [30, 31]. This apparent discrepancy can be explained in part by the understanding that thrombus formation during an AF episode and thrombus embolization causing a stroke, may be temporally distinct events. The former may occur during an AF episode, which is associated with prothrombotic activation, endothelial dysfunction and inflammation [32], while the thrombus may remain stable for months before it finally embolizes [33]. In addition, it has been proposed that AF burden may play a significant role in patients with low risk of stroke (low CHA2DS2-VASc score), whereas in patients with a high risk of stroke, the influence of AF burden on stroke diminishes [34, 35]. This notion is consistent with recent evidence indicating that paroxysmal AF may be associated with a lower stroke risk compared to persistent [12-15]. Moreover, alternative mechanisms beyond AF, including other potential sources of cardiac emboli, as well as atherothrombotic mechanisms, may be the actual cause of stroke in a significant proportion of patients with AF [36]. Importantly, the proportion of alternative mechanisms of stroke increased with increasing CHA2DS2-VASc score [36]. Although the optimal duration of AF that merits anticoagulation has not been established, the available evidence suggests that recurrent episodes of AF lasting more than a few hours do carry an increased risk of stroke, with the patients' other thromboembolic risk factors acting as modifiers of this risk [37, 34].
In our study, the AF burden was relatively low. The AF detection rate was approximately 1 episode per 3 person-months of follow-up and 11 patients had no AF episodes detected. Although these numbers likely underestimate the patients' true AF burden, these data are consistent with the results of studies in patients with symptomatic paroxysmal AF who also had an indication for a standard dual-chamber pacemaker, which showed that most patients have no more than a few episodes per month, while a significant number of patients do not experience any episode during short-term follow-up [38, 39]. Data from a small subgroup of patients with implantable devices support the notion that in the presence of low AF density (infrequent episodes), the relative benefit of continuous monitoring over intermittent monitoring in representing the true AF burden is attenuated [24]. The recently published REACT.COM study explored the concept of intermittent anticoagulation in 59 patients with paroxysmal or persistent AF who had an insertable loop recorder. NOACs were initiated for 30 days for any AF episode lasting ≥1 hour based on daily loop recorder transmissions [25]. This study showed a 98% compliance with daily transmissions and a 94% reduction in anticoagulation utilization with the use of targeted rhythm-guided anticoagulation therapy, establishing the feasibility of this approach. Consistent with prior studies including patients with implantable devices [38, 39], REACT.COM demonstrated that long periods of sinus rhythm in patients with paroxysmal AF are not uncommon, forming the basis for intermittent anticoagulation to decrease exposure to anticoagulants. Importantly, recent evidence suggests that these patients with rare, short lived episodes (low AF burden) may have a lower risk of stroke compared with those with long frequent episodes (high AF burden), especially in the presence of only mild comorbidities [37].
Our findings contrast with the results of a recent randomized trial of remote monitoring to guide initiation and discontinuation of anticoagulation in patients with previously implanted defibrillators or cardiac desynchronization devices (IMPACT study), which failed to show a positive result [40]. There are significant differences between the 2 studies in terms of study design and population included, which might explain the contrasting results. In the IMPACT study, continuation of AF for 24 to 48 hours (for CHADS2 score 3-4 and 1-2, respectively) was required before initiation of anticoagulation, whereas in our study, anticoagulation was initiated immediately after AF was identified. In addition, the anticoagulant used in the majority of the patients (>80%) in the IMPACT study was warfarin, which in contrast to the NOACs used in our study, requires a few days to reach therapeutic levels. Finally, the patients included in the IMPACT study had on average a higher stroke risk (median CHA2DS2-VASc score of 4) compared to our patient population, supporting the notion that the temporal dissociation of AF with stroke may have resulted from more advanced atrial myopathy in this patient population with heart failure.
Limitations
The small sample size of this pilot study does not allow definitive conclusions to be drawn. However, the study was primarily designed to show feasibility of intermittent anticoagulation and derive preliminary estimates for power calculations for a larger, more definitive study. Nonetheless, these results suggest that this approach may decrease the rates of bleeding complications associated with the use of NOACs. It should be noted that intermittent anticoagulation would work only if the major source of thromboembolism is related to AF, i.e. in the absence of significant comorbidities [37]. The protocol of anticoagulation in the intermittent group was based on extrapolations from previous studies examining the duration of atrial stunning in relation to the duration of the AF episode [19-21]. Specifically, these demonstrated that recovery of the left atrial mechanical function occurred within 3 minutes for AF episodes lasting 15 minutes, within 24 hours for AF episodes lasting <2 weeks and within 1 week for AF episodes lasting 2-6 weeks. A systematic study of the optimal intermittent anticoagulation protocol was out of the scope of this pilot study. A more conservative approach would be to provide anticoagulation for a longer period of time (e.g. 3-4 weeks), irrespective of the duration of the AF episode. However, this approach would result in a longer exposure to anticoagulants, thus negating the primary benefit of intermittent anticoagulation, which is to prevent bleeding. Although more frequent rhythm monitoring (e.g. twice or thrice a day) would have resulted in identification of shorter episodes of AF (less than 12 hours and 8 hours, respectively), adherence to the monitoring protocol may have been significantly reduced [41]. Therefore, we opted to simplify the protocol to include once daily monitoring rather than multiple times a day, in order to maximize adherence. Importantly, the protocol was designed to ensure initiation of anticoagulation within 24 hours of AF onset, which is well within the 48-hour window currently accepted as the minimum duration required for thrombus formation [3]. Our study underestimated the true AF burden of these patients due to the intermittent nature of AF monitoring, corroborated by the disparity between iPhone-based and device-based AF detection in patients with implantable devices, especially in the presence of frequent, short episodes. Continuous monitoring through an implantable device is superior to intermittent non-invasive monitoring in defining the AF burden [24]. An alternative approach would be to guide anticoagulation therapy using an insertable cardiac monitor [25]. However, rhythm monitoring through an inexpensive and convenient iPhone-based device greatly expands the generalizability and decreases the cost of this approach.
Conclusions
These data suggest that intermittent anticoagulation using an iPhone-based rhythm monitoring device is feasible and may be associated with decreased bleeding risk without apparent increase in stroke in low-risk patients with paroxysmal AF. Further investigation with a larger trial, adequately powered to establish non-inferiority of intermittent anticoagulation compared to continuous anticoagulation in reducing the risk of stroke or death and superiority in reducing the risk of major bleeding, is warranted.
Figure 4. Time-to-event distribution for the endpoints of stroke or systemic embolism (A) and death or stroke (B) in the randomized intervention groups (intention-to-treat analysis).
Acknowledgments
Funded by the American Heart Association #13CRP16860078 to Stavros Stavrakis and in part by the National Institutes of Health, National Institute of General Medical Sciences (#U54GM104938) to the University of Oklahoma Health Sciences Center
Footnotes
Disclosures: The authors report no relationships that could be construed as a conflict of interest
References
- 1.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285(18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 5.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 7.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–91. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 8.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383(9921):955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 9.Loffredo L, Perri L, Violi F. Impact of new oral anticoagulants on gastrointestinal bleeding in atrial fibrillation: A meta-analysis of interventional trials. Dig Liver Dis. 2015;47(5):429–31. doi: 10.1016/j.dld.2015.01.159. [DOI] [PubMed] [Google Scholar]
- 10.Lahaye S, Regpala S, Lacombe S, Sharma M, Gibbens S, Ball D, et al. Evaluation of patients' attitudes towards stroke prevention and bleeding risk in atrial fibrillation. Thromb Haemost. 2014;111(3):465–73. doi: 10.1160/TH13-05-0424. [DOI] [PubMed] [Google Scholar]
- 11.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, et al. Evaluating the Atrial Myopathy Underlying Atrial Fibrillation: Identifying the Arrhythmogenic and Thrombogenic Substrate. Circulation. 2015;132(4):278–91. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, et al. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34(31):2464–71. doi: 10.1093/eurheartj/eht135. [DOI] [PubMed] [Google Scholar]
- 13.Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders P, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591–602. doi: 10.1093/eurheartj/ehw007. [DOI] [PubMed] [Google Scholar]
- 14.Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36(5):288–96. doi: 10.1093/eurheartj/ehu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takabayashi K, Hamatani Y, Yamashita Y, Takagi D, Unoki T, Ishii M, et al. Incidence of Stroke or Systemic Embolism in Paroxysmal Versus Sustained Atrial Fibrillation: The Fushimi Atrial Fibrillation Registry. Stroke. 2015;46(12):3354–61. doi: 10.1161/STROKEAHA.115.010947. [DOI] [PubMed] [Google Scholar]
- 16.Sanders P, Purerfellner H, Pokushalov E, Sarkar S, Di Bacco M, Maus B, et al. Performance of a new atrial fibrillation detection algorithm in a miniaturized insertable cardiac monitor: Results from the Reveal LINQ Usability Study. Heart Rhythm. 2016;13(7):1425–30. doi: 10.1016/j.hrthm.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Gunda S, Reddy YM, Pillarisetti J, Koripalli S, Jeffery C, Swope J, et al. Initial real world experience with a novel insertable (Reveal LinQ(@Medtronic)) compared to the conventional (Reveal XT(@Medtronic)) implantable loop recorder at a tertiary care center - Points to ponder! Int J Cardiol. 2015;191:58–63. doi: 10.1016/j.ijcard.2015.04.241. [DOI] [PubMed] [Google Scholar]
- 18.Lowres N, Neubeck L, Salkeld G, Krass I, McLachlan AJ, Redfern J, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost. 2014;111(6):1167–76. doi: 10.1160/TH14-03-0231. [DOI] [PubMed] [Google Scholar]
- 19.Daoud EG, Marcovitz P, Knight BP, Goyal R, Man KC, Strickberger SA, et al. Short-term effect of atrial fibrillation on atrial contractile function in humans. Circulation. 1999;99(23):3024–7. doi: 10.1161/01.cir.99.23.3024. [DOI] [PubMed] [Google Scholar]
- 20.Manning WJ, Silverman DI, Katz SE, Riley MF, Come PC, Doherty RM, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. 1994;23(7):1535–40. doi: 10.1016/0735-1097(94)90652-1. [DOI] [PubMed] [Google Scholar]
- 21.Takami M, Suzuki M, Sugi K, Ikeda T. Time course for resolution of left atrial appendage stunning after catheter ablation of chronic atrial flutter. J Am Coll Cardiol. 2003;41(12):2207–11. doi: 10.1016/s0735-1097(03)00496-0. [DOI] [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 24.Charitos EI, Ziegler PD, Stierle U, Robinson DR, Graf B, Sievers HH, et al. Atrial fibrillation burden estimates derived from intermittent rhythm monitoring are unreliable estimates of the true atrial fibrillation burden. Pacing Clin Electrophysiol. 2014;37(9):1210–8. doi: 10.1111/pace.12389. [DOI] [PubMed] [Google Scholar]
- 25.Passman R, Leong-Sit P, Andrei AC, Huskin A, Tomson TT, Bernstein R, et al. Targeted Anticoagulation for Atrial Fibrillation Guided by Continuous Rhythm Assessment With an Insertable Cardiac Monitor: The Rhythm Evaluation for Anticoagulation With Continuous Monitoring (REACT.COM) Pilot Study. J Cardiovasc Electrophysiol. 2016;27(3):264–70. doi: 10.1111/jce.12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boriani G, Glotzer TV, Santini M, West TM, De Melis M, Sepsi M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices) Eur Heart J. 2014;35(8):508–16. doi: 10.1093/eurheartj/eht491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46(10):1913–20. doi: 10.1016/j.jacc.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 28.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2(5):474–80. doi: 10.1161/CIRCEP.109.849638. [DOI] [PubMed] [Google Scholar]
- 29.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366(2):120–9. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 30.Brambatti M, Connolly SJ, Gold MR, Morillo CA, Capucci A, Muto C, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014;129(21):2094–9. doi: 10.1161/CIRCULATIONAHA.113.007825. [DOI] [PubMed] [Google Scholar]
- 31.Daoud EG, Glotzer TV, Wyse DG, Ezekowitz MD, Hilker C, Koehler J, et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011;8(9):1416–23. doi: 10.1016/j.hrthm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 32.Lim HS, Willoughby SR, Schultz C, Gan C, Alasady M, Lau DH, et al. Effect of atrial fibrillation on atrial thrombogenesis in humans: impact of rate and rhythm. J Am Coll Cardiol. 2013;61(8):852–60. doi: 10.1016/j.jacc.2012.11.046. [DOI] [PubMed] [Google Scholar]
- 33.Larsen JA, McPherson DD, Kadish AH, Goldberger JJ. Course of intraatrial thrombi resolution using transesophageal echocardiography. Echocardiography. 2003;20(2):121–8. doi: 10.1046/j.1540-8175.2003.03011.x. [DOI] [PubMed] [Google Scholar]
- 34.Zimetbaum P, Waks JW, Ellis ER, Glotzer TV, Passman RS. Role of atrial fibrillation burden in assessing thromboembolic risk. Circ Arrhythm Electrophysiol. 2014;7(6):1223–9. doi: 10.1161/CIRCEP.114.001356. [DOI] [PubMed] [Google Scholar]
- 35.Botto GL, Padeletti L, Santini M, Capucci A, Gulizia M, Zolezzi F, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009;20(3):241–8. doi: 10.1111/j.1540-8167.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 36.Cha MJ, Kim YD, Nam HS, Kim J, Lee DH, Heo JH. Stroke mechanism in patients with non-valvular atrial fibrillation according to the CHADS2 and CHA2 DS2 -VASc scores. Eur J Neurol. 2012;19(3):473–9. doi: 10.1111/j.1468-1331.2011.03547.x. [DOI] [PubMed] [Google Scholar]
- 37.Reiffel JA. If it were only that simple. Eur Heart J. 2016;37(20):1603–5. doi: 10.1093/eurheartj/ehw014. [DOI] [PubMed] [Google Scholar]
- 38.Lee MA, Weachter R, Pollak S, Kremers MS, Naik AM, Silverman R, et al. The effect of atrial pacing therapies on atrial tachyarrhythmia burden and frequency: results of a randomized trial in patients with bradycardia and atrial tachyarrhythmias. J Am Coll Cardiol. 2003;41(11):1926–32. doi: 10.1016/s0735-1097(03)00426-1. [DOI] [PubMed] [Google Scholar]
- 39.Quirino G, Giammaria M, Corbucci G, Pistelli P, Turri E, Mazza A, et al. Diagnosis of paroxysmal atrial fibrillation in patients with implanted pacemakers: relationship to symptoms and other variables. Pacing Clin Electrophysiol. 2009;32(1):91–8. doi: 10.1111/j.1540-8159.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 40.Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36(26):1660–8. doi: 10.1093/eurheartj/ehv115. [DOI] [PubMed] [Google Scholar]
- 41.Laliberte F, Nelson WW, Lefebvre P, Schein JR, Rondeau-Leclaire J, Duh MS. Impact of daily dosing frequency on adherence to chronic medications among nonvalvular atrial fibrillation patients. Adv Ther. 2012;29(8):675–90. doi: 10.1007/s12325-012-0040-x. [DOI] [PubMed] [Google Scholar]


