Abstract
Introduction
The role of atrial fibrillation (AF) substrates is unclear in patients with paroxysmal AF (PAF) that recurs after pulmonary vein isolation (PVI). We hypothesized that patients with recurrent post-ablation (redo) PAF despite PVI have electrical substrates marked by rotors and focal sources, and structural substrates that resemble persistent AF more than patients with (de novo) PAF at first ablation.
Methods
In 175 patients at 11 centers, we compared AF substrates in both atria using 64 pole-basket catheters and phase mapping, and indices of anatomical remodeling between patients with de novo or redo PAF and first ablation for persistent AF.
Results
Sources were seen in all patients. More patients with de novo PAF (78.0%) had sources near PVs than patients with redo PAF (47.4%, p=0.005) or persistent AF (46.9%, p=0.001). The total number of sources per patient (p=0.444), and number of non-PV sources (p=0.701) were similar between groups, indicating that redo PAF patients had residual non-PV sources after elimination of PV sources by prior PVI. Structurally, left atrial size did not separate de novo from redo PAF (49.5±9.5 vs. 49.0±7.1mm, p=0.956) but was larger in patients with persistent AF (55.2±8.4mm, p=0.001).
Conclusions
Patients with paroxysmal AF despite prior PVI show electrical substrates that resemble persistent AF more closely than patients with paroxysmal AF at first ablation. Notably, these subgroups of paroxysmal AF are indistinguishable by structural indices. These data motivate studies of trigger versus substrate mechanisms for patients with recurrent paroxysmal AF after PVI.
Keywords: atrial fibrillation, ablation, sources
Introduction
Pulmonary vein isolation (PVI) is central to the ablation of paroxysmal and persistent AF, yet its results remain suboptimal even in recent clinical trials (1–4). An increasingly recognized fact is that PAF patients may do well after PVI even when the PVs have reconnected (5,6), suggesting that lesions sets interrupted other mechanisms. Indeed, studies suggest that paroxysmal AF is a heterogeneous population that may overlap with persistent AF (7), which may have substrate mechanisms remote from the PVs. However, these mechanisms are yet unidentified in paroxysmal AF.
We hypothesized that patients with recurrent paroxysmal AF after prior PVI are more likely to have substrates remote from the PVs, and more closely resemble patients with persistent AF, than patients with paroxysmal AF at their first PVI procedure. This may follow for several reasons. First, patients with recurrent PAF may have peri-PV mechanisms not eliminated at initial ablation. Second, it may be artificial to ‘dichotomize’ populations with paroxysmal and persistent AF given their overlap in true AF burden (7), left atrial size and atrial structural abnormalities (8). Third, recent mapping of paroxysmal AF shows substrates in the form of rotors and focal sources remote from the PVs where targeted ablation can eliminate AF acutely and long term in many centers (9–12) with different techniques (13). Moreover, AF sources have now been demonstrated in human optical mapping studies (14) with many similarities to these clinical studies.
We tested our hypothesis by examining electrical substrates of rotors or focal sources, and structural substrates by echocardiography, in patients with paroxysmal AF at first ablation, recurrent paroxysmal AF despite prior PVI and first-time persistent AF ablation in an 11-center study prospective observational study.
Methods
Enrolment at Contributing Centers
Between 2012 and 2014 we enrolled 175 patients undergoing AF ablation for routine indications at 11 centers in the United States (Table 1). All studies and data analysis were carried out with local institutional review board approval and patients provided written consent for data collection.
Table 1.
Contributing Centers
| Stanford Medical Center, Palo Alto, CA |
| Arizona Heart Rhythm Center, Phoenix, AZ |
| Baylor University Medical Center, Dallas, TX |
| Duke University Medical Center, Durham, NC |
| Intermountain Medical Center, Salt Lake City, UT |
| Ohio State University Wexner Medical Center, Columbus, OH |
| University of California San Diego Medical Center, San Diego, CA |
| San Diego Veterans Affairs Medical Center, CA |
| Massachusetts General Hospital, Boston, MA |
| Central Baptist Hospital, Lexington, KY |
| Indiana University Health University Hospital, Indianapolis, IN |
Patient Classification
We classified the 175 patients prospectively into de novo PAF, redo PAF and persistent AF. Paroxysmal AF was defined as AF that terminates spontaneously or with intervention within 7 day of onset, while persistent AF was defined as continuous AF that is sustained >7 days (15). The ‘de novo’ PAF group consisted of patients presenting for their first catheter ablation of AF (n= 48). ‘Redo’ PAF patients had recurrent PAF despite one prior PVI (n=31) and persistent AF patients were presenting for their first ablation for persistent AF (n=96). We excluded those presenting for redo persistent AF ablation.
Electrophysiological Study
Patients discontinued anti-arrhythmic medications for >5 half-lives except amiodarone which was stopped as early as possible prior to the procedure. During the procedure, heparin was used to maintain activated clotting time >350 seconds. A 64-pole basket catheter was advanced to right atrium (RA), then trans-septally to left atrium (LA), with attention paid to basket location to prevent errors such as inadvertent ventricular placement as noted in some recent studies (16). Our practice is to move the basket during successive FIRM map epochs to ensure basket coverage of the majority of the atrial surface (Figure 1b). Electrodes were referenced to electroanatomic shells (NavX, St Jude Medical, or Carto, Biosense-Webster). In both NavX and Carto shells, distances were estimated based on the fixed distance between electrodes along basket splines, and cartographic rulers on the atrial shell when available.
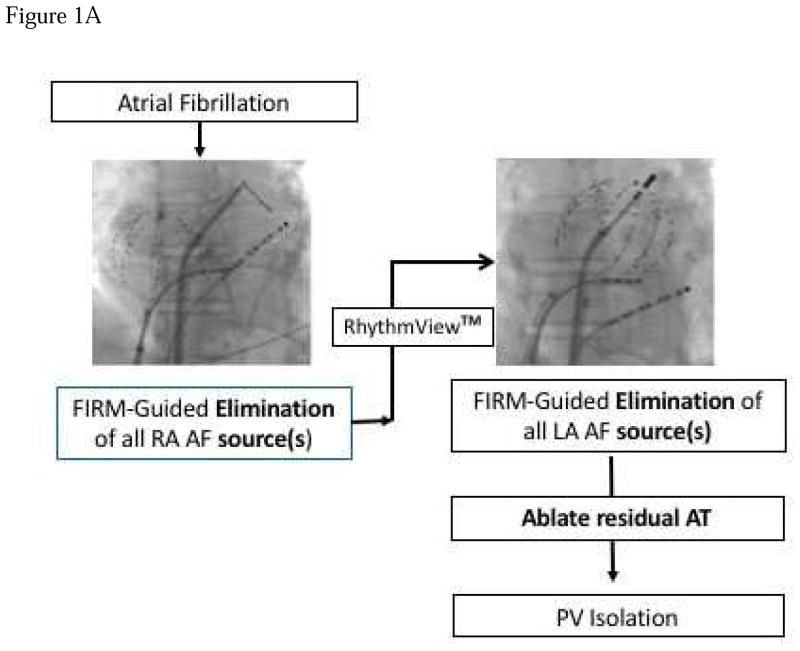
Figure 1. Technique for FIRM Guided Ablation.
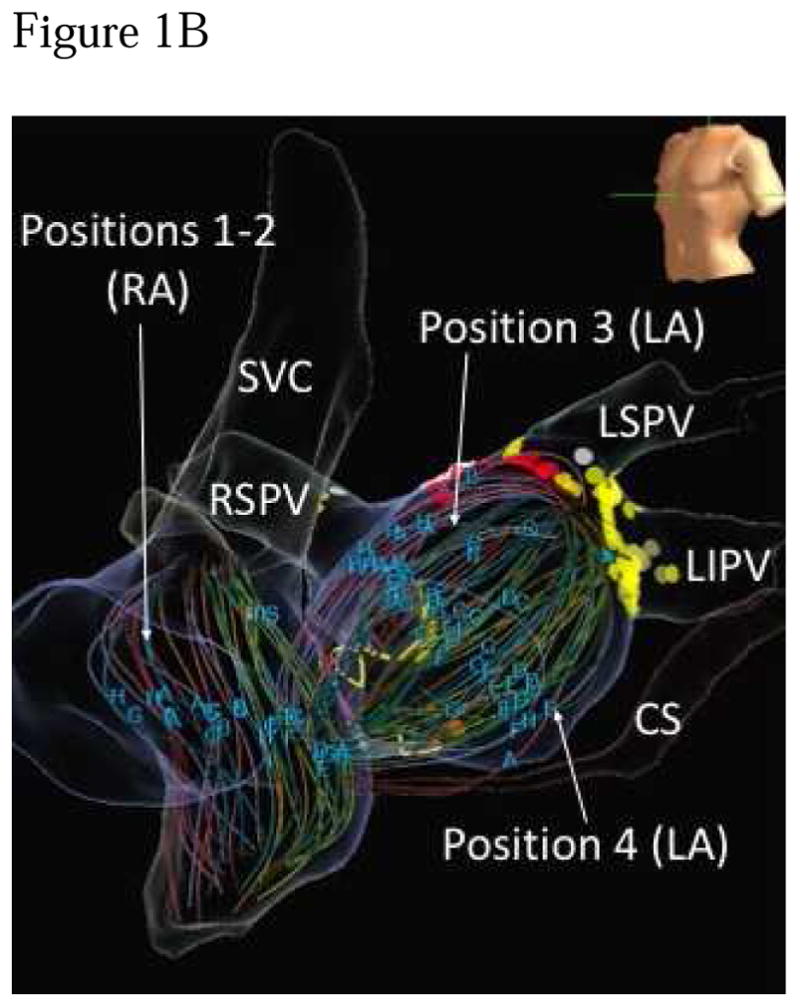
A) Typical atrial basket placement is indicated in the fluoroscopic images. Sequential atrial basket recordings were taken with source elimination prior to PVI. B) Multiple basket positions used per FIRM-mapping epoch to minimize unmapped atrial regions. Positions 1–2 are in the right atrium, positions 3–4 are in the LA. SVC – superior vena cava, RSPV – right superior pulmonary vein, RIPV right inferior pulmonary vein, LSPV – left superior pulmonary vein, LIPV – left inferior pulmonary vein.
FIRM mapping of AF Substrate
FIRM maps identify electrical rotors as phase singularities with surrounding disorganization. Rotors were considered AF sources if stable within <1 electrode for multiple recording epochs over 2–5 minutes. Focal AF sources were defined as origins with centrifugal activation and breakdown into meandering wavelets.
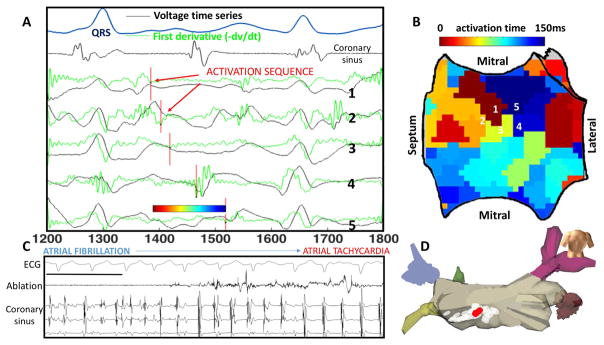
FIRM mapping uses algorithms to map propagation sequences from observed AF activations. For unipolar deflections that are non-complex, mapping can be straightforward. Figure 2A depicts activation mapping from a basket catheter where points of maximum negative dV/dt (green line) indicate each AF activation cycle. These activations identify a counterclockwise rotational circuit in the inferior left atrium (shown in isochrones panel B and on electroanatomic map panel D) where ablation terminated AF to AT (shown, panel C). Conversely, in cases where multiple AF deflections are seen for any cycle, classical rules often mis-identify signals within repolarization which are by definition far-field (17). In such cases, FIRM determines local activation by analyzing variations in signal morphology between each basket electrode and neighboring channels. A sawtooth-shaped wave of normalized amplitude is obtained from action potential duration (18) and conduction restitution data (19) and rate adapted to estimate phase to identify rotors. If no signal is detected, that region of the atria remains black on FIRM maps. FIRM identified rotors show many similarities to micro-reentrant AF drivers found in optical mapping of AF in human atria(14), with early data showing concordance between FIRM mapped and optically mapped AF sources in human hearts (20).
Figure 2. Rotational Activity at FIRM-mapped Rotor, where Ablation terminated AF to Atrial Tachycardia.
In this 61 year old man with persistent AF, unipolar AF electrograms confirm rotation around rotor core. A) Electrograms (unipoles) and dV/dt (first derivative, green) shown, with one cycle annotated (red lines). B) Left atrial shell with activation map from the annotated cycle, demonstrating earliest-latest interaction in a rotational pattern. C) Termination panel shows abrupt termination of AF to organized AT with ablation at this site, prior to any PVI. Black bar represents 1000ms. D) Location on LA posterior wall of termination site (red) during ablation of rotor area (white).
Characterizing substrate by Functional mapping
We described functional substrate in each patient prospectively using the following criteria: Source number – the total number of sources reported by FIRM mapping, whether or not ablated. Source location was further sub-categorized into: PV vs. non-PV location, those within 1cm of a PV where considered ‘PV’, all others being remote from the PVs and right vs. left atrial. These were identified prospectively at each case. Reproducibility of this assignment by individual operators has recently been reported to be good with kappa = 0.89 (21). These data were collected prospectively by each investigator during each case, then collated retrospectively blinded to patient group.
Characterizing the anatomical substrate
We measured left atrial size on 2D echo, a well validated index of left atrial structural remodeling, in all patients pre-ablation. Additional imaging tests such as late-gadolinium enhanced MRI were not in widespread use at the time of the study.
FIRM-Guided Ablation
Ablation commenced with FIRM-guided radiofrequency ablation for AF, using 3.5 mm (Biosense-Webster) or 4mm (St Jude Medical) tipped irrigated catheters or a non-irrigated catheter (Boston Scientific) in some patients with heart failure. Ablation was delivered to the organized domain of rotors (areas of 2–3 cm2, similar to AF driver areas in human optical mapping (14)). Ablation typically commenced in right atria then proceeded to left atrial sites, and was repeated to eliminate rotors on remapping (Figure 1A). Rotors and focal sources were not ablated if near sensitive structures such as the phrenic nerve.
Pulmonary Vein Isolation
Ablation was performed to isolate left and right pulmonary veins in pairs, with verification of pulmonary vein entrance block using a circular mapping catheter. Ablation was avoided near sensitive structures such as the esophagus or phrenic nerve. The ablation protocol was FIRM first, until no more sources, then PVI. If AF sources (identified prospectively) fell within the operator’s planned PVI lesion set, this sequence could be changed. At redo-ablation, if veins were reconnected they were re-isolated.
Statistical analysis
Continuous variables with a normal distribution are presented as mean ± standard deviation (SD) unless otherwise noted and evaluated with one-way ANOVA, Bonferroni and Tukey post-hoc tests where indicated. Counts of sources and CHADS2VASc scores are reported with medians and quartiles and compared among groups with Kruskal-Wallis tests. Nominal variables are reported as counts and percentages and evaluated with chi-square tests. Logistic regression was used to evaluate the group difference in the presence of PV sources controlling for site differences. Probabilities below 0.05 were considered significant.
Results
A total of 175 patients were included in the present study; Table 2 summarizes patient characteristics.
Table 2.
Population Demographics
| De Novo Paroxysmal AF (n=41) | Redo Paroxysmal AF (n=38) | Persistent AF (n=96) | |
|---|---|---|---|
| Age (years) | 58.8±12.5* | 61.3±9.5 | 63.5±10.1 |
| LA diameter (mm) | 49.5±9.5 | 49.0±7.1 | 56.1±8.0** |
| LVEF (%) | 57.6±9.7 | 56.9±7.9 | 56.4±10.3 |
| CHADS2VASc | 1 (0–2) | 1 (0–2) | 1 (0–2) |
p< 0.05 vs. persistent AF,
p=0.001 using three group ANOVA with post-hoc pairwise comparisons.
Number of sources
There were 144 total FIRM identified rotors/sources in the de novo PAF group compared to 129 in the redo PAF and 304 in the persistent AF group. This corresponded to roughly equal numbers of sources per patient of 3 (2–5) in the de novo PAF group, 3 (2–4) in the redo PAF group and 3 (2–4) in the persistent group (p=0.444). Overall, 91% were rotors and 9% focal sources.
Source locations
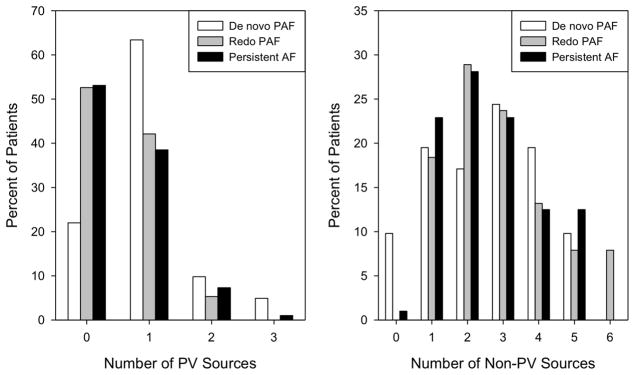
Only 54.3% of patients had any sources within 1cm of PVs. The presence of any PV-localized sources differed by group (p=0.002). More patients with PAF at first ablation (78.0%) showed 1 or more PV sources than patients with redo PAF (47.4%, p=0.005) and persistent AF (46.9%, p=0.001). Group difference in the presence of any PV sources remained significant (p=0.008) in a logistic regression model controlling for study site differences. When analyzed as a count, the number of PV-localized sources per patient showed a similar pattern of differences (p=0.002). By contrast, there was no difference among the groups in the number of non-PV sources (p=0.701).
The distributions of PV and non-PV sources by group are illustrated in Figure 3. In patients with redo PAF, sources near PVs lay near gaps in prior PV isolation lesion sets or just outside prior lesion sets, such that electrograms were detected to yield sources (for example, see case in Figure 2). Figure 4 illustrates rotor sites near and remote from PVs with direct termination to sinus rhythm during ablation.
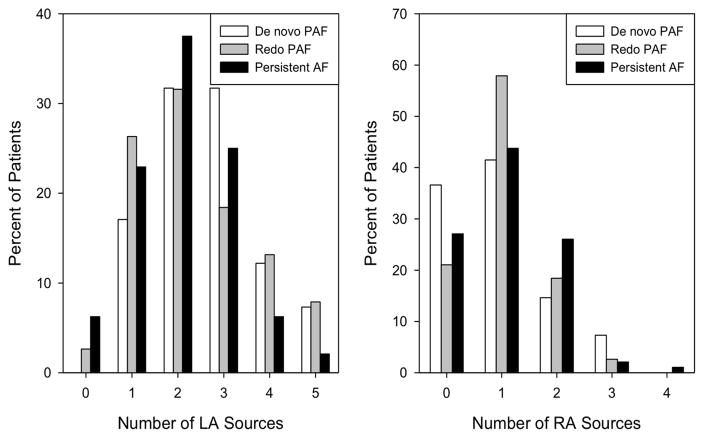
Figure 3. Higher number of PV sources in de novo PAF.
(Left) The bar chart shows a higher percentage of patients with PV rotors/sources in de novo PAF compared to redo PAF and persistent AF (p=0.002). Differences in the number of non-PV sources (right) were not significant (p=0.701).
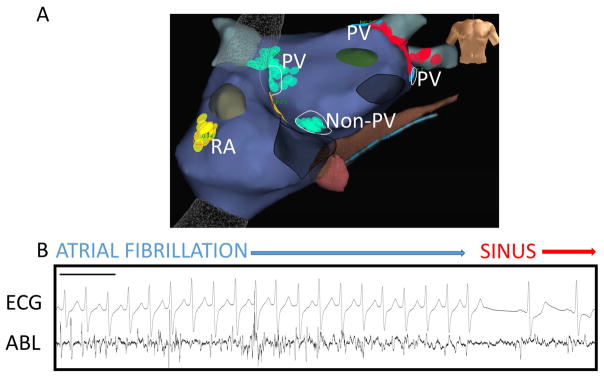
Figure 4. Ablation of AF sources adjacent and remote to PVs causing acute termination to sinus rhythm.
A) Antero-posterior bi-atrial NavX map with RA, non-PV and PV LA sources labelled in a patient at redo ablation for PAF. B) AF signals on ablation catheter at FIRM-mapped rotor adjacent to prior left superior PVI lesion set, where AF was terminated by FIRM-guided ablation. Black bar represents 1000ms. ABL – ablation catheter.
There was a non-significant trend towards a difference among the groups in the number of LA sources (p=0.070) with medians of 3 (2–3) for de novo PAF and 2 (1–3) for both redo PAF and persistent AF (Figure 5).
Figure 5. Non-significant differences in RA and LA sources.
There was a trend towards a difference among the groups in the number of LA sources (p=0.070) which was somewhat higher in de novo PAF than in the other groups. There was no group difference in the number of RA sources (p=0.541).
Structural Remodeling
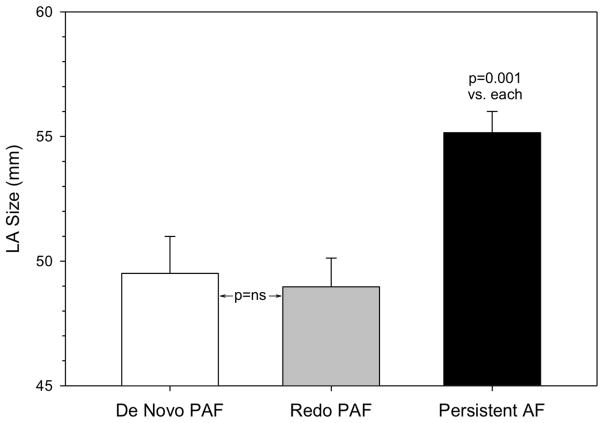
There was a significant difference among groups in left atrial size (p<0.001). LA size did not separate de novo from redo PAF (49.5±9.5 vs. 49.0±7.1mm respectively, p=0.956) but was significantly larger in patients with persistent AF (55.2±8.4mm, p=0.001 vs. each using three group ANOVA with post-hoc pairwise comparisons). Thus despite the electrical similarities between redo PAF and persistent AF, they were structurally distinct using traditional indices (Figure 6). The CHADS2VASc score did not differentiate among the 3 groups (p=0.681) with a median of 1 (0–2) in each group. Likewise, LVEF did not differentiate among the 3 groups (p=0.777, using three group ANOVA with post-hoc pairwise comparisons, see table 2).
Figure 6. LA size did not separate de novo from redo PAF.
LA size was higher only in the persistent AF group, despite the electrophysiological differences in this study. (Mean + SEM).
Acute Impact of Ablation
The acute impact of ablation is summarized in table 3. Acute termination was seen in 83 patients, of which 41% were to atrial tachycardia and 59% to sinus rhythm directly. Figure 2 shows an example of a phase-identified AF rotor, in which good unipolar signal quality enabled confirmation of FIRM-mapped rotors by showing rotational activation using traditional analysis of minimum dV/dt (green line), in a patient with persistent AF. Targeted ablation of this rotor in the inferior left atrium (panel D) terminated AF to an AT prior to PVI. Figure 4 shows another patient with abrupt termination to sinus rhythm during ablation of a rotor adjacent to the pulmonary veins.
Table 3.
Acute impact of ablation
| Total cases with terminations during ablation | Cases terminating to SR | Cases terminating to AT | |
|---|---|---|---|
| De Novo PAF (n=41) | 27(65%) | 18 | 9 |
| Redo PAF (n=38) | 24(64%) | 15 | 9 |
| Persistent AF (n=96) | 32(33%) | 16 | 16 |
Discussion
This study uses mapping of functional AF substrates in patients at 11 U.S. centers to show that patients with PAF despite prior PVI (“redo PAF”) are more similar electrophysiologically to patients with persistent AF than those with de novo PAF at first ablation. Compared to patients with de novo PAF, those with redo PAF had rotor distributions away from the PVs in the left atrium and in right atrium, more like persistent AF. Notably, this electrophysiological difference was not reflected in structural remodeling, since patients with de novo and redo PAF had similar left atrial dimensions that were each lower than in patients with persistent AF. These data suggest the possibility of identifying a priori the 35–50% of patients with paroxysmal AF who may not be arrhythmia free after a single PVI. Mechanistically, these data motivate studies to examine the role of trigger versus substrate ablation in patients at repeat ablation procedures.
Role of Rotors and Focal Sources in Human AF
Evidence continues to mount that human AF is sustained by localized rotors and focal sources. The characteristics of rotors on FIRM mapping are similar to those from optical mapping in human atria, that show stable endocardial rotors in ~2 cm2 areas where ablation can acutely terminate AF, yet are unstable and transient on the epicardium (14). These data may help reconcile differences between endocardial FIRM mapping and less stable rotors on ECGI mapping (13). Early data show concordance between concurrent FIRM and optically mapped AF in human hearts (20).
FIRM guided ablation (FIRM plus PVI) has been reported in many patients with many results in large series consistent with the original CONFIRM trial (10,11,22), (12). However, not all studies support the rotor mechanism. Some studies suggest that rotors do not exist, but typically used empirical rules with technical errors such as reported cycle lengths of 250–500ms in AF (rates 2–4Hz) that are less consistent with AF, and the use of Shannon entropy to unipolar signals although it is likely valid only for bipolar signals (16). The recent OASIS trial initially reported FIRM+PVI success of 52% in 40 patients (23) but has subsequently been retracted due to non-randomization issues which may also impact outcome comparisons between each group. Even despite this, those authors’ very recently reported 20% 1 year success from PVAI alone in similar persistent AF patients (24) suggests that FIRM substantially improves the results of PVAI at that center. Direct comparisons of FIRM+PVI to PVI alone (akin to the STAR-AF2 trial) are warranted and ongoing. Interestingly, other disappointing studies show a substantial acute termination rate of persistent AF to atrial tachycardia (>30%) with FIRM-guided ablation alone (25), supporting the presence of sources mechanistically. Large multicenter randomized trials are ongoing to test these questions.
Shared and Discordant Mechanisms in Paroxysmal and Persistent AF
This study defines potential mechanisms that may separate paroxysmal AF patients with and without AF recurrence after index PVI, that may outline a spectrum for AF phenotypes, from de novo PAF to redo PAF then to persistent AF. Of particular note is the electrical but not anatomical separation of de novo from redo PAF, which shared AF source distributions and characteristics with persistent AF.
Despite effective antral ablation at index ablation of paroxysmal AF, up to 70% of patients may have reconnection at repeat electrophysiological study 3 months later (6), which could be due to gaps in radiofrequency ablation lines, or incomplete circumferential contact with balloon based technologies. While PV reconnection may occur in patients with or without clinical AF recurrence, few studies have examined if substrates may differ between such groups. Other candidate differences in substrate between groups include fibrosis and MRI-identified scar, that are the subject of intense investigation.
Structure Function Dissociation in AF Progression
Structurally, there was no difference in LA size between de novo and redo PAF despite differences in atrial source numbers and atrial distributions. In contrast, persistent AF showed a significantly larger LA size. This structure-function dissociation is supported by recent data with a similar mix of AF phenotypes (26) and ‘lone’ PAF patients compared to controls (27). Atrial MRI imaging using delayed enhancement MRI also reveals a wide spectrum of fibrotic substrates which do not conform to current classification schemes based on arbitrary time cut-offs. It is hoped such delineation of functional substrates will allow for patient-tailored risk stratification to improve outcomes (28).
Future Directions
These data contribute to the discussion on how to improve the results from PV antral isolation. This is particularly timely since recent trials show that empiric linear ablation or ablation of complex fractionated may not improve the results of PV isolation in persistent AF patients (1,29,30), and success in PAF is limited to ~65% at 1 year and ~50 % at 2 years with PVI by cryoballoon or radiofrequency energy using force-sensing catheters (one or more procedures permitted in blanking period) (31). While more durable PVI may further enhance outcomes, the present data demonstrate the presence of AF substrates that are often remote from the PVs or empirical line sites – such as in the right atrium – that could be targeted for ablation. In a retrospective analysis of the CONFIRM trial, the presence of rotors that were not directly or inadvertently targeted by ablation portended a worse arrhythmia-free prognosis (32).
Limitations
This study has a number of limitations. Ideally, it would have been useful to FIRM map all patients with paroxysmal AF at first ablation, only perform PVI then assess what substrates were present on a repeat ablation. Such a trial is planned. More generally, the current study did not prospectively randomize substrates to ablation/non-ablation; such a study however is also underway. Echocardiographic indices of structure are increasingly being replaced by MRI which is more widely available now, and neither left atrial volume data nor CT data were universally available from all sites. Echocardiographic data were reported from clinical records at each site, not from a core lab. Further electrophysiological characterization with voltage or fractionation indices would be attenuated by prior ablation in redo PAF, and was not performed.
Conclusions
Across 11 US centers, we found that PAF patients at repeat ablation are electrically more similar to patients with persistent AF with numerous extra-PV sources than to PAF patients at their first ablation, despite similar left atrial size. Our findings motivate studies to further define ‘functional substrate’, and motivate trials to test the benefit of substrate ablation over repeat PVI in patients at repeat procedures. These findings may also help improve knowledge of AF progression and improve clinical outcomes.
Clinical Perspectives.
Medical Knowledge – this study advances the increasing literature highlighting heterogeneity within patients with paroxysmal AF, suggesting that those with recurrent AF may overlap with persistent AF patients.
Translational Outlook.
The scientific field continues to find growing evidence of organization within fibrillating atria, with a hierarchy of spatial sites identified by the methods in this study and corroborated by others that may guide ablation to improve outcomes in persistent AF. By showing similarities between patients with paroxysmal AF who recurred despite prior PVI and those with persistent AF, these data start to define a functional spectrum of atrial fibrillation which may improve the current binary classification of AF based upon detected duration. We believe this will allow for more tailored, patient specific therapies to help understand and treat this disease.
Acknowledgments
Funding Sources:
Dr Zaman is the recipient of a Fulbright British Heart Foundation Scholarship 2015–2016 (68150918) and British Heart Foundation Travel Grant 2014–2015 (FS/14/46/30907), Dr Baykaner is the recipient of the Josephson and Wellens Heart Rhythm Society Fellowship 2015–2016, Dr Schricker was the recipient of an ACC/Merck fellowship 2012–2013. Dr Narayan reports funding from NIH (R01 HL83359; R01 HL 122384; K24 HL103800).
We are grateful to Dr Vivek Reddy and Mount Sinai School of Medicine, NY for contributing to this study.
Abbreviations
- AF
atrial fibrillation
- AT
atrial tachycardia
- CHADSVASc
Congestive heart failure, Hypertension, Age, Diabetes, Stroke, Vascular risk factors, Age, Sex
- FIRM
focal impulse and rotor mapping
- LA
left atrial
- PAF
paroxysmal atrial fibrillation
- PVI
pulmonary vein isolation
- RA
right atrium
- Redo PAF
recurrent post-ablation paroxysmal atrial fibrillation
Footnotes
Disclosures:
J.A. Zaman: Meeting travel - Atricure, Medtronic, Inc.
T. Baykaner: None.
P. Clopton: None.
V. Swarup: Consulting Fees/Honoraria; Biosense Webster, Inc. I - Research Grants; Medtronic, Inc., Boston Scientific Corp., St. Jude Medical, Biotronik.
R.C. Kowal: Consulting Fees/Honoraria; Medtronic, Inc.
J.P. Daubert: Consulting Fees/Honoraria; Medtronic, Inc., St. Jude Medical, Boston Scientific Corp., Sorin Group, CardioFocus, Inc. Equity Interests/Stock Options – Public; Biosense Webster, Inc. Research Grants; Boston Scientific Corp., Biosense Webster, Inc., Medtronic, Inc., Gilead Sciences, Inc. Fellowship Support; Medtronic, Inc., Boston Scientific Corp., Biotronik, St. Jude Medical, Biosense Webster, Inc., Bard Electrophysiology.
J.D. Day: None.
J.D. Hummel: Consulting Fees/Honoraria; Biosense Webster, Inc.
A.A. Schricker: Consulting Fees/Honoraria; Abbott EP.
D.E. Krummen: Consulting Fees/Honoraria; Topera Medical, Pacific Blue Innovations. Research Grants; National Institutes for Health. Fellowship Support; Boston Scientific Corp., Medtronic, Inc., St. Jude Medical, Biotronik, Biosense Webster, Inc.
M. Mansour: Consulting Fees/Honoraria; Biosense Webster, Inc., St. Jude Medical, Sentreheart. Research Grants; Biosense Webster, Inc., St. Jude Medical, Boston Scientific Corp.
G. F. Tomassoni: Consulting Fees/Honoraria; Stereotaxis, Inc., Topera Medical, St. Jude Medical. Speaker’s Bureau; Zoll Medical Corporation, Topera Medical. Speaker’s Bureau; Pfizer, Inc., St. Jude Medical. Officer, Trustee, Director, Committee Chair, or Any Other Fiduciary Role; Stereotaxis, Inc.
K.R. Wheelan: Consulting Fees/Honoraria; Medtronic, Inc. Equity Interests/Stock Options – Public; Medtronic, Inc. Fellowship Support; St. Jude Medical, Boston Scientific Corp., Medtronic, Inc.
M. Viswanathan: Consulting Fees/Honoraria; Biosense Webster, Inc.
S. Park: Consulting Fees/Honoraria; Medtronic, Inc.
P.J. Wang: Consulting Fees/Honoraria; Medtronic, Inc. Research Grants; Medtronic, Inc. Fellowship Support; Medtronic, Inc.
S.M. Narayan: Consulting Fees/Honoraria; Medtronic, Inc., St. Jude Medical, Biotronik, Boston Scientific Corp. Equity Interests/Stock Options – Non-Public; Topera Medical. Ownership/Partnership/Principal; Topera Medical. Research Grants; National Institutes for Health. Intellectual Property Rights; University of California Regents.
J.M. Miller: Consulting Fees/Honoraria; Topera Medical, Medtronic, Inc., Boston Scientific Corp., St. Jude Medical, Biosense Webster, Inc. Fellowship Support; Medtronic, Inc., Boston Scientific Corp., St. Jude Medical, Biosense Webster, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Verma A, Jiang C, Betts TR, et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N Engl J Med. 2015;372(19):1812–22. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 2.Natale A, Reddy VY, Monir G, et al. Paroxysmal AF catheter ablation with a contact force sensing catheter: results of the prospective, multicenter SMART-AF trial. J Am Coll Cardiol. 2014;64(7):647–56. doi: 10.1016/j.jacc.2014.04.072. [DOI] [PubMed] [Google Scholar]
- 3.Calkins H. Demonstrating the Value of Contact Force Sensing More Difficult Than Meets the Eye. Circulation. 2015:901–4. doi: 10.1161/CIRCULATIONAHA.115.018354. [DOI] [PubMed] [Google Scholar]
- 4.Dukkipati SR, Cuoco F, Kutinsky I, et al. Pulmonary Vein Isolation Using the Visually Guided Laser Balloon. J Am Coll Cardiol. 2015;66(12):1350–60. doi: 10.1016/j.jacc.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Jiang R-H, Po SS, Tung R, et al. Incidence of pulmonary vein conduction recovery in patients without clinical recurrence after ablation of paroxysmal atrial fibrillation: mechanistic implications. Heart Rhythm. 2014;11(6):969–76. doi: 10.1016/j.hrthm.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Kuck K-H, Hoffmann BA, Ernst S, et al. Impact of Complete Versus Incomplete Circumferential Lines Around the Pulmonary Veins During Catheter Ablation of Paroxysmal Atrial Fibrillation Circ Arrhythmia. Electrophysiol. 2016;9(1):e003337. doi: 10.1161/CIRCEP.115.003337. [DOI] [PubMed] [Google Scholar]
- 7.Charitos EI, Pürerfellner H, Glotzer TV, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol. 2014;63(25 Pt A):2840–8. doi: 10.1016/j.jacc.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 8.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119(13):1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller JM. Treatment of Atrial Fibrillation by the Ablation of Localized Sources. J Am Coll Cardiol. 2012;60(x):628–36. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomassoni G, Duggal S, Muir M, et al. Long-term Follow-up of FIRM-guided Ablation of Atrial Fibrillation: A Single-center Experience. J Innov Card Rhythm Manag. 2015;6(October):2145–51. [Google Scholar]
- 11.Rashid H, Sweeney A. Approaches for Focal Impulse and Rotor Mapping in Complex Patients3: A US Private Practice Perspective. J Innov Card Rhythm Manag. 2015;6(November):2193–8. doi: 10.19102/icrm.2015.061104. [DOI] [Google Scholar]
- 12.Miller JM, Kowal RC, Swarup V, et al. Initial Independent Outcomes from Focal Impulse and Rotor Modulation Ablation for Atrial Fibrillation: Multicenter FIRM Registry. J Cardiovasc Electrophysiol. 2014;25:921–9. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haissaguerre M, Hocini M, Denis A, et al. Driver domains in persistent atrial fibrillation. Circulation. 2014;130(7):530–8. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 14.Hansen BJ, Zhao J, Csepe Ta, et al. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36(35):2390–401. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64(21):2246–80. doi: 10.1016/j.jacc.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 16.Jalife J, Filgueiras-Rama D, Berenfeld O. Letter by Jalife et al Regarding Article, “Quantitative Analysis of Localized Sources Identified by Focal Impulse and Rotor Modulation Mapping in Atrial Fibrillation” Nothing. Circ Arrhythmia Electrophysiol. 2015;8:1296–8. doi: 10.1080/13518040701205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayan SM, Wright M, Derval N, et al. Classifying fractionated electrograms in human atrial fibrillation using monophasic action potentials and activation mapping: evidence for localized drivers, rate acceleration, and nonlocal signal etiologies. Heart Rhythm. 2011;8(2):244–53. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Narayan SM, Krummen DE, Enyeart MW, Rappel W-J. Computational mapping identifies localized mechanisms for ablation of atrial fibrillation. PLoS One. 2012;7(9):e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123(25):2922–30. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen BJ, Briggs C, Moore BT, et al. Abstract 18402: Human Atrial Fibrillation Drivers Seen Simultaneously by Focal Impulse and Rotor Mapping and High-resolution Optical Mapping. Circulation. 2015;132(Suppl_3):A18402. [Google Scholar]
- 21.Lalani GG, Coysh T, Baykaner T, et al. Organized Sources Are Spatially Conserved in Recurrent Compared to Pre-Ablation Atrial Fibrillation: Further Evidence for Non-Random Electrical Substrates. J Cardiovasc Electrophysiol. 2016 doi: 10.1111/jce.12964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sommer P, Kircher S, Rolf S, John S, Arya A. Successful Repeat Catheter Ablation of Recurrent Longstanding Persistent Atrial Fibrillation with Rotor Elimination as the Procedural Endpoint3: A Case Series Short title3: Rotor elimination as endpoint for AF ablations. J Cardiovasc Electrophysiol. 2015;27(3):274–80. doi: 10.1111/jce.12874.This. [DOI] [PubMed] [Google Scholar]
- 23.Natale A, Mohanty S, Gianni C, et al. Heart Rhythm Society Scientific Sessions. San Francisco: Elsevier; 2016. LBCT02-01/LBCT02-01 Impact of rotor ablation in non-paroxysmal AF patients: results from a randomized trial (OASIS) [Google Scholar]
- 24.Bai R, Di Biase L, Prasant Mohanty, et al. Proven isolation of the pulmonary vein antrum with or without left atrial posterior wall isolation in patients with persistent atrial fibrillation. Hear Rhythm. 2016;13(1):132–40. doi: 10.1016/j.hrthm.2015.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Gianni C, Mohanty S, Di Biase L, et al. Acute and early outcomes of FIRM-guided rotors-only ablation in patients with non-paroxysmal atrial fibrillation. Hear Rhythm. 2015 doi: 10.1016/j.hrthm.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 26.Maury P, Thomson E, Rollin A, et al. Lack of Correlations between Electrophysiological and Anatomical-Mechanical Atrial Remodeling in Patients with Atrial Fibrillation. Pacing Clin Electrophysiol. 2015 doi: 10.1111/pace.12598. [DOI] [PubMed] [Google Scholar]
- 27.Stiles MK, John B, Wong CX, et al. Paroxysmal lone atrial fibrillation is associated with an abnormal atrial substrate: characterizing the “second factor”. J Am Coll Cardiol. 2009;53(14):1182–91. doi: 10.1016/j.jacc.2008.11.054. [DOI] [PubMed] [Google Scholar]
- 28.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. Jama. 2014;311(5):498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 29.Wong KCK, Paisey JR, Sopher M, et al. No Benefit OF Complex Fractionated Atrial Electrogram (CFAE) Ablation in Addition to Circumferential Pulmonary Vein Ablation and Linear Ablation3: BOCA Study. Circ Arrhythmia Electrophysiol. 2015;8(6):1316–24. doi: 10.1161/CIRCEP.114.002504. [DOI] [PubMed] [Google Scholar]
- 30.Vogler J, Willems S, Sultan A, et al. Pulmonary Vein Isolation Versus Defragmentation: The CHASE-AF Clinical Trial. J Am Coll Cardiol. 2015;66(24):2743–52. doi: 10.1016/j.jacc.2015.09.088. [DOI] [PubMed] [Google Scholar]
- 31.Kuck K-H, Brugada J, Fürnkranz A, et al. Cryoballoon or Radiofrequency Ablation for Paroxysmal Atrial Fibrillation. N Engl J Med. 2014 doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 32.Narayan SM, Krummen DE, Clopton P, Shivkumar K, Miller JM. Direct Or Coincidental Elimination of Stable Rotors or Focal Sources May Explain Successful Atrial Fibrillation Ablation: On-Treatment Analysis of the CONFIRM (CONventional ablation for AF with or without Focal Impulse and Rotor Modulation) Trial. J Am Coll Cardiol. 2013;62(2):138–47. doi: 10.1016/j.jacc.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]