Abstract
This work, for the first time, investigated the diversity of endophytic fungi harbored in the xylem and phloem of the root of Sophora tonkinensis Gapnep from three geographic localities with emphasis on the influence of the tissue type and geographic locality on endophytic fungal communities and their potential as biocontrol agents against phytopathogens of Panax notoginseng. A total of 655 fungal strains representing 47 taxa were isolated. Forty‐two taxa (89.4%) were identified but not five taxa (10.6%) according to morphology and molecular phylogenetics. Out of identifiable taxa, the majority of endophyte taxa were Ascomycota (76.6%), followed by Basidiomycota (8.5%) and Zygomycota (4.3%). The alpha‐diversity indices indicated that the species diversity of endophytic fungal community harbored in the root of S. tonkinensis was very high. The colonization and species diversity of endophytic fungal communities were significantly influenced by the geographic locality but not tissue type. The geographic locality and tissue type had great effects on the species composition of endophytic fungal communities. Forty‐seven respective strains were challenged by three fungal phytopathogens of P. notoginseng and six strains exhibited significant inhibitory activity. It was noteworthy that endophytic Rhexocercosporidium sp. and F. solani strongly inhibited pathogenic F. solani and other fungal phytopathogens of P. notoginseng.
Keywords: endophytic fungal communities, geographic locality, inhibitory activity, tissue type
1. INTRODUCTION
Endophytes are microorganisms that reside within internal tissues of living plants without visibly harming the host plant (Clay, 1992; Hyde & Soytong, 2008; Schulz & Boyle, 2005). Endophytes, mainly represented by both endophytic fungi and endophytic bacteria, have great promise with diverse potential for exploitation (Staniek, Woerdenbag, & Kayser, 2008; Strobel, Daisy, Castillo, & Harper, 2004). Recently, enormous biological diversity (Li, Zhao, Liu, & Xu, 2010; Tejesvi, Kajula, Mattila, & Pirttilä, 2011) coupled with capability to biosynthesize bioactive secondary metabolites (Aly, Debbab, Kjer, & Proksch, 2010; Chandra, 2012) and tremendous potential as biocontrol agents (Mejia et al., 2008; Zhang et al., 2014) have provided the impetus for several investigations on endophytic fungi.
The diversity of endophytic fungal communities in the tissues of plants aboveground as well as belowground, including stems, leaves, and/or roots is very high (Kusari, Kusari, Spiteller, & Kayser, 2013; Qadri, Rajput, Abdin, Vishwakarma, & Riyaz‐Ul‐Hassan, 2014). The colonization frequency (CF), species diversity, and species composition of endophytic fungal communities are affected by season, tissue type, geographic location (Mishra et al., 2012), tissue age (Nascimento et al., 2015), and host (Gonzalez and Tello, 2011; Kernaghan & Patriquin, 2015). Many endophytic fungi have the potential to synthesize secondary metabolites with various bioactivities (Kusari, Hertweck, & Spiteller, 2012), including cytotoxicity (Xu, Espinosa‐Artiles, Liu, Arnold, & Gunatilaka, 2013), antimicrobial activity (Li, Jiang, Guo, Zhang, & Che, 2008), anti‐ HIV‐1 activity (Li et al., 2008), insecticidal activity (Sappapan et al., 2008), and antioxidant activity (Wang et al., 2006), which may directly or indirectly be used as therapeutic agents against numerous diseases. Occasionally, endophytic fungi that produce host plant secondary metabolites (Kusari, Lamshoeft, Zuehlke, & Spiteller, 2008; Stierle, Strobel, & Stierle, 1993) with therapeutic value or potential have been discovered. Endophytic fungi have recently been considered an important resource for screening biocontrol agents to suppress insects and pathogens attacking plants including the host (Kusari et al., 2013; Mejia et al., 2008) and other plants (Bailey et al., 2008; Waweru, Losenge, Kahangi, Dubois, & Coyne, 2013), promoting host plant growth (Chen et al., 2010; Silva, Tozzi, Terrasan, & Bettiol, 2012).
Sophora tonkinensis Gapnep, is a well‐known medicinal plant of China that grows in an area of karst topography near the Tropic of Cancer, is mainly distributed in Guangxi province, and oddly is found in Guangdong province as well as Yunnan province (Wang, Xie, Fan, & Liu, 2011). The chemical constituents (Wang et al., 2011), including primarily flavonoids, alkaloids, polysaccharides, and saponins, have been isolated from the root of S. tonkinensis, and have pharmacological activities (Commission, 2015b; Wang et al., 2011) such as antitumor activity, antimicrobial activity, antiinflammation, antiarrhythmia, antihypertension, hepatoprotection, and immune stimulation. The crude extracts from the root of S. tonkinensis have been effectively applied to control symptoms on Panax notoginseng, a famous traditional Chinese herb with a long history in China as a valuable cardiovascular remedy (Commission, 2015a; Li, Xie, Fan, & Wang, 2011). These symptoms, namely black spots caused by Alternaria panax Whetz (Wei & Chen, 1992), anthracnose by Colletotrichum gloeosporioides (Wei, Chen, & Wu, 1989), and root rot by Fusarium solani (Miao et al., 2006), seriously affect the quality and yield of P. notoginseng in the geo‐authentic‐producing areas. During the course of plant–endophyte coevolution, it might be possible for endophytes to assist the plant in chemical defense by producing bioactive secondary metabolites according to the theories of “mosaic effect” and “acquired immune systems” (Carroll, 1991; Rodriguez, Redman, & Henson, 2004). Hence, several members of endophytic fungi harbored in the root of S. tonkinensis may have an antagonistic activity against three fungal phytopathogens of P. notoginseng. Therefore, we selected this plant to isolate endophytic fungi. Most of the studies on endophytic fungi have been carried out in tropical, subtropical, temperate, and boreal regions, but there are only a few studies that have been carried out near the Tropic of Cancer, and overall, no major studies exist on endophytic fungi harbored in medicinal plant from karst topography near the Tropic of Cancer in Guangxi province of China.
The aim of this study was to isolate and identify endophytic fungi harbored in the root of S. tonkinensis, characterize the diversity of endophytic fungal communities, investigate the influence of the tissue type and geographic locality on the colonization, species diversity, and species composition of endophytic fungal communities, and further screen them for potential as biocontrol agents against three phytopathogens of P. notoginseng cultivated in China. The findings will not only enrich the knowledge of endophytic fungi from S. tonkinensis but also benefit the development of organic cultivation techniques for P. notoginseng in China. To the best of our knowledge, this report is the first to describe the diversity, phylogeny, and communities of endophytic fungi harbored in the root of S. tonkinensis, and assess their potential as biocontrol agents against phytopathogens of P. notoginseng.
2. MATERIALS AND METHODS
2.1. Sample collection from selected sites
In 2014, healthy plants of S. tonkinensis were collected in three periods from three different localities of traditional geo‐authentic‐producing areas (Wang et al., 2011) in Guangxi province of south China: Tiandeng county (T), where S. tonkinensis grows as a natural part of an intact shrub forest; Jingxi county (J), where S. tonkinensis grows in the rock crack in limestone mountainous areas; and Guangxi university (G), where S. tonkinensis is cultivated in a medicinal herb garden. Details of three sampling localities and dates were given in Table 1. These plants were carefully up‐rooted with the help of a spade, placed in jute bags, labeled, immediately transported to the laboratory, and processed within 24 hr of collection. Import of the plant material from Tiandeng county and Jingxi county was allowed according to the permission of the Department of Forestry of Guangxi province, Guangxi, China, and that from Guangxi university was allowed according to the permission of the College of Agriculture, Guangxi University, Guangxi, China.
Table 1.
Location characteristics and sampling dates in this work
| Sampling locations | Sampling dates | Geographic coordinates | Altitude (m) | Annual rainfall (mm) | Mean temperature (°C) |
|---|---|---|---|---|---|
| G: medicinal herb garden, Guangxi university | 04.02.2014 | 23°07′8′′N, | 76 | 1309.7 | 21.8 |
| 15.05.2014 | 108°17′28′′E | ||||
| 02.10.2014 | |||||
| T: Hongkui village, Tiandeng town, Tiandeng county | 04.02.2014 | 23°06′36′′N, | 437.1 | 1409.3 | 20.7 |
| 15.05.2014 | 107°08′20′′E | ||||
| 02.10.2014 | |||||
| J: Chengliang village, Xinjing town, Jingxi county | 04.02.2014 | 23°08′49′′N, | 850 | 1634.2 | 19.5 |
| 15.05.2014 | 106°25′26′′E | ||||
| 02.10.2014 |
2.2. Isolation of endophytic fungi
Root samples (diameter, 1–2 cm) were excised from the plant and cut into segments (length, 5–7 cm). For the surface sterilization and isolation of endophytic fungi, we established the optimum procedures according to previously described methods (Kusari et al., 2013; Tejesvi et al., 2011). The root segments were thoroughly washed in running tap water for 30 min and rinsed with double‐distilled water for 10 min. Next, the samples were sterilized with 75% ethanol for 1 min, sodium hypochlorite containing 1% available chlorine for 2 min, and 75% ethanol for 30 s. Finally, these surface‐sterilized samples were rinsed three times with sterile, double‐distilled water to remove excess surface sterilants, blotted on a sterile filter paper, and dried under aseptic conditions. To ensure the isolation of endophytic fungi, the epidermis and ends of each root segment were removed. The xylem (X) and phloem (P) were separated from the remaining part of each root segment (R) and transversely cut into 1‐cm‐long pieces, respectively, which were individually placed in Petri dishes (9 cm in diameter) containing potato dextrose agar (PDA) with chloramphenicol to eliminate any bacterial growth. The dissection of the roots was showed in Figure 1 . The Petri dishes with three pieces per dish were incubated at 28°C and checked daily for fungal growth for up to 6 weeks. Each colony which emerged from the segments was transferred to an antibiotic‐free PDA medium. Purification was carried out by cutting a small piece of media with mycelia at the edge of a colony and then transplanted on to new medium plates.
Figure 1.
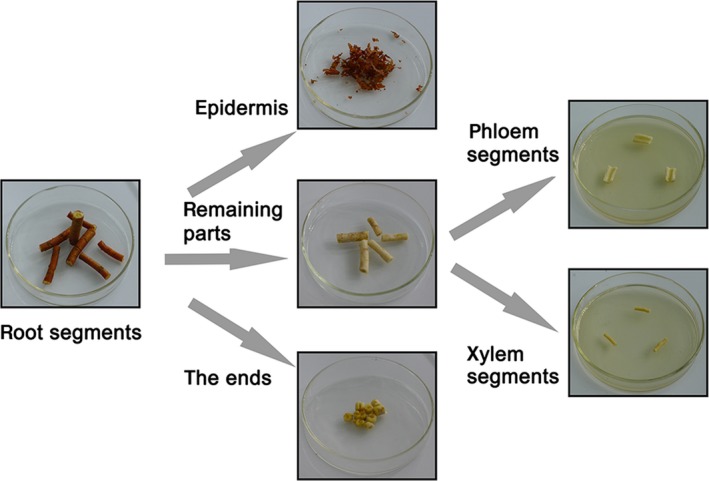
The dissection of the roots in this work
2.3. Storage of the purified endophytic fungi
Every purified endophytic fungus sample received a specific code number according to its origin (e.g., TRXY‐1 or TRXY‐2, from the xylem of the root collected from Tiandeng county, and TRPH‐1 TRPH‐2, from the phloem of the root collected from Tiandeng county). All endophytic fungi were deposited at the College of Agriculture, Guangxi University, Guangxi, China. For short‐term storage, they were cultured on PDA at 28°C for 3–10 days and maintained at 4°C (up to 3 months); for long‐term storage, they were preserved with spores or mycelia in 25% (v/v) glycerol at −80°C.
2.4. Genomic DNA extraction, PCR amplification, and sequencing
Endophytic fungi were cultured on sterilized cellophane stuck on PDA at 28°C for 3–10 days. Fresh cultures were harvested, and the genomic DNA was extracted following the previously described protocol (Guo, Hyde, & Liew, 2000). The ITS regions, including ITS1, 5.8S, and ITS2 regions of rDNA, were amplified with universal primer pairs ITS1 and ITS4 (White, Bruns, Lee, & Taylor, 1990). Amplification was performed in a 50‐μl reaction volume, which contained 5 μl of PCR buffer (10×), 4 μl of dNTP Mixture (each 2.5 mmol/L), 1 μl of each primer (20 pmol), 2.5 μl of template DNA (100 ng), 0.5 μl (2.5 U) of Taq DNA polymerase (TAKARA BIO INC., Japan, cat. no. R001A), and 36 μl of sterile double‐distilled water. A negative control using sterile double‐distilled water instead of template DNA was included in the amplification process. The thermal cycling program was as follows: 3 min of initial denaturation at 95°C, followed by 30 cycles of 30 s denaturation at 94°C, 30 s primer annealing at 55°C, 60 s extension at 72°C, and a final 10 min extension at 72°C. Next, 3 μl of PCR products from each PCR reaction was checked by electrophoresis on 1% (w/v) agarose gels containing SYBR Green I nucleic acid gel stain at 90 V (5 V cm−1) for 1.5 hr in TBE buffer (1×) and visualized under 300‐nm UV light. PCR products were purified using PCR Cleanup Filter Plates (MultiScreen® PCRμ96; Millipore, USA) according to the manufacturer's protocol. Purified PCR products were directly sequenced with primer pairs as mentioned above in an ABI 3730‐XL DNA sequencer (Applied Biosystems, USA).
2.5. Fungal identification
The taxonomic identification of endophytic fungi isolated was based on morphology and molecular phylogenetics including phylogenetic position and similarity to reference sequences of the GenBank (Guo et al., 2000; Rivera‐Orduna, Suarez‐Sanchez, Flores‐Bustamante, Gracida‐Rodriguez, & Flores‐Cotera, 2011). When the isolates did not produce spores on PDA medium, sterile mycelia were cultured on quarter‐strength PDA medium containing sterilized fragments of the roots of S. tonkinensis to promote sporulation. All endophytic fungi were classified as different morphotypes according to their morphological characters. The ITS regions (ITS1‐5.8S rDNA‐ITS2) were amplified and sequenced for all of the morphotypes. Consequently, each sequence from different morphotypes as query sequence was matched against ITS sequences available in the GenBank by BLASTn search to obtain similar sequences. The most similar reference sequences with query sequence were used for phylogenetic analysis along with selected taxonomic reference sequences using MEGA version 6.0. In order to avoid using mis‐annotated sequences, the closest related strains with the most similar reference sequences from published references were deposited original cultures that were identified based on morphology and ITS sequencing. Similarities among sequences were calculated using the MatGAT v.2.01 software. The sequence was accepted at genus level when the similarity between a query sequence and a phylogenetically related reference sequence was higher than 95%, and the sequence was considered to be conspecific when that was higher than those within same genus. The strain with an ITS sequence showing a divergence greater than 5% with any entry at GenBank was considered as unidentified. These thresholds have been previously employed in other endophyte‐related studies to identify fungal taxa (Gonzalez & Luisa, 2011; Kusari et al., 2013; Sanchez, Bills, & Zabalgogeazcoa, 2008).
2.6. Fungal culture and extraction
Every selected endophytic fungus was cultured on a Petri dish containing PDA at 28°C for 5–10 days. The culture materials from each Petri dish were cut into small pieces and transferred to a 2‐L Erlenmeyer flask containing sterile solid medium, which included 400 g of potato, 20 g of dextrose, and 20 g of sucrose at 28°C for 30–40 days. The culture materials from the Erlenmeyer flask were successively extracted with methanol to yield crude extracts.
2.7. Preparation of crude extracts from the root of S. tonkinensis
The dried root of S. tonkinensis was pulverized and soaked in 1,000 ml of methanol for 2 weeks at room temperature. The organic solvent was filtered through a filter paper and evaporated to dryness under vacuum to afford crude extracts.
2.8. In vitro antagonistic assays of endophytes against fungal phytopathogens
In order to screen antagonistic fungi against phytopathogens of P. notoginseng, we took three fungal phytopathogens including A. panax (L), C. gloeosporioides (T), and F. solani (F) in P. notoginseng from the Institute of Plant Pathology, College of Agriculture, Guangxi University. The in vitro antagonistic activity of endophytic fungi against three fungal phytopathogens of P. notoginseng was tested using the coculture method established earlier (Kusari et al., 2013; Zhang et al., 2014). One mycelial plug (6 mm diameter) of each 10‐day‐old endophytic fungus was placed at the center of the dish containing approximately 25 ml of PDA, yielding a final depth of 4 mm. Three mycelial plugs (6 mm diameter) from three 3‐day‐old fungal phytopathogens were symmetrically placed 3 cm from the endophytic inoculant to establish a coculture as the coculture treatment. The fungal phytopathogens alone were symmetrically placed 3 cm from the center of the dish containing PDA with the crude extracts from the root of S. tonkinensis at the concentration of 2 mg/ml as the ecological treatment, and that without crude extracts as the growth control. All treatments and controls were run in duplicates. The cultures were incubated at 28°C. The colony growth radius of each fungal phytopathogen between its inoculation and the center of the dish in the treatment was measured when the fungal growth in the growth control had completely reached the center of the Petri dishes. The average radius of each fungal phytopathogen in the treatment was recorded as R 1, and that in the growth control was recorded as R 2. The inhibition percentage of the growth of the fungal phytopathogen in the endophyte‐phytopathogen antagonism was calculated with the help of the modified formula as mentioned below:
Fungal strains with great inhibitions against three fungal phytopathogens in the above test were selected for further testing antifungal activity of their crude extracts using the mycelial growth method (Rabea, Badawy, Steurbaut, & Stevens, 2009; Tian et al., 2015). Every selected strain with antagonistic activity was cultured and extracted to yield crude extracts as mentioned above. All crude extracts were dissolved in 1% (v/v) dimethyl sulfoxide (DMSO). Appropriate volumes of the solutions of each crude extract were incorporated into the PDA medium and poured into the Petri dishes to obtain final concentrations ranging from 2 to 8 mg/ml according to the concentration of carbendazim wettable powders used in field, and each concentration was tested in triplicate. The positive control with carbendazim wettable powders was treated in the same way. The growth control without drug was maintained with 1% DMSO mixed with PDA medium. Every mycelial plug (6 mm diameter) from each 3‐day‐old fungal phytopathogen was, respectively, placed at the center of the Petri dishes. The cultures were incubated at 28°C, and the colony growth diameter of each fungal phytopathogen was measured when the fungal growth in the growth control had completely covered the Petri dishes. The radial growth of each fungal phytopathogen in the treatment measured by removing 6 mm from the growth diameter was recorded as D 1, and that in the growth control was recorded as D 2. The inhibition percentage of mycelial growth was calculated with the help of the modified formula as follows:
2.9. Statistical analysis
The colonization frequency (CF) was expressed in percentages and calculated as the number of segments colonized by a single endophyte divided by the total number of segments examined ×100 (Mishra et al., 2012). The percentage of species composition (S i) was calculated as the number of taxa that belong to a specific phylum, class, or order divided by the total number of taxa in the sample (Botella and Diez, 2011). The relative species frequency (P i) was calculated as the number of isolates that belong to taxon i divided by the total number of isolates in the sample (Kusari et al., 2013). The fungal dominance was determined by Camargo's index (1/s), where S (Species richness) is the number of fungal taxa. A species was defined as dominant if P i >1/s. The alpha‐diversity indices such as Species richness (S), Shannon‐Wiener index (H′), and Simpson's diversity index (1‐D) were assessed for the species diversity of the endophytic fungal communities harbored in the root of S. tonkinensis. The beta‐diversity indices such as Jaccard's index and Sorensen's index were calculated to compare the similarity of endophytic fungal communities regarding species composition between two localities or two tissues. The influences of geographic locality on CF, Shannon‐Wiener index, and Simpson's diversity index were analyzed by One‐Way ANOVA. The influences of tissue type on CF, Shannon‐Wiener index, and Simpson's diversity index were examined using analysis of t tests. The inhibition percentage of crude extracts from endophytic fungi against three phytopathogens was subjected to ANOVA to analyze their antifungal properties. The software “R version 3.2.3” was used for all the statistical analyses.
3. RESULTS
3.1. Fungal identification
A total of 655 endophytic fungal isolates were isolated from 3,740 tissue segments (1,870 xylem segments and 1,870 phloem segments from the root of S. tonkinensis) of 48 plant individuals. Among these, 470 isolates produced spores (conidia or sexual spores) on PDA medium. Furthermore, 118 isolates sporulated and were identified by morphology after inducing sporulation. All isolates were classified as 102 morphotypes according to morphological characters and subsequently identified as 47 fungal taxa according to phylogenetic analyses based on the ITS region sequence. Out of 47 taxa, 33 taxa (588 isolates) were identified to the species level, followed by seven taxa (46 isolates) to the genus level, one taxon (four isolates) to the order level, one taxon (one isolate) to the subclass level, and five unidentifiable taxa (16 isolates). The sequences from respective strains of 47 taxa in this work were deposited in the Genbank database. Out of 47 closest related strains, 39 strains were from published references but not eight strains. Details of these strains are summarized in Table 2.
Table 2.
Summary of the endophytic fungi isolated from the root of S. tonkinensis with their respective strain numbers, GenBank accession numbers, and closest affiliations of the representative isolates in the GenBank according to ITS analysis
| Strain number | Accession number | Closest related strain (accession number) | Similaritya (%) | Reference |
|---|---|---|---|---|
| TRXY‐73 | KP204265 | Cladosporium perangustum (HM148139) | 99.5 | Bensch, K., et al. 2010 |
| TRXY‐75 | KP204270 | Cladosporium sp. (KF367474) | 100 | Oliveira, B.R., et al. 2013 |
| TRXY‐5 | KP204304 | Phoma herbarum (KJ188712) | 99.8 | Luo, J., et al. 2014 |
| TRXY‐18‐1 | KP204290 | Phoma sp. (KC928322) | 100 | Choi, I.Y., et al. 2014 |
| GRPH‐2‐1 | KP204295 | Epicoccum sp. (FJ176473) | 99.6 | Qi, F.H., et al. 2009 |
| TRPH‐24 | KP204307 | Alternaria alternata (KJ957793) | 100 | Choi, M.S., et al. 2014 |
| TRPH‐85 | KP204310 | Pleosporales sp. (GQ254682) | 99.8 | Unpublished |
| TRXY‐70 | KP204312 | Rhytidhysteron sp. (GU199428) | 99 | Sakalidis, M.L., et al. 2011 |
| TRXY‐42‐2 | KP204314 | Trichosporon asahii (AF455425) | 100 | Buzina, W., et al. 2003 |
| TRXY‐69 | KP204316 | Fomitopsis sp. (FJ372676) | 99.3 | Rungjindamai, N., et al. 2009 |
| TRXY‐13‐2 | KP204317 | Schizophyllum commune (KF679517) | 100 | Chan, J.F., et al. 2014 |
| TRXY‐41‐2 | KP204318 | Exobasidiomycetidae sp. (DQ682574) | 100 | Aime, M.C. 2007 |
| GRXY‐7‐1 | KP204319 | Mortierella alpina (KC018229) | 99.8 | Wagner, L., et al. 2013 |
| TRPH‐18‐1 | KP204320 | Mucor circinelloides (JN205949) | 99.8 | Walther, G., et al. 2013 |
| TRXY‐32‐2 | KP204332 | Aureobasidium pullulans (JF439462) | 99.8 | Han, G., et al. 2011 |
| TRPH‐105 | KP204334 | Rhexocercosporidium sp. (EU543257) | 99.3 | Unpublished |
| TRPH‐87 | KP204336 | Rhexocercosporidium sp. (EU543257) | 100 | Unpublished |
| TRXY‐50 | KP204340 | Phialophora mustea (JN123359) | 99.6 | Ban, Y., et al. 2012 |
| TRPH‐68 | KP204345 | Cryptosporiopsis radicicola (GU062273) | 99.6 | Arhipova, N., et al. 2011 |
| TRPH‐73 | KP204347 | Talaromyces funiculosus (GU183120) | 100 | Wicklow, D.T., et al. 2009 |
| TRXY‐29 | KP204349 | Talaromyces pinophilus (JX684010) | 100 | Barnes, C.W., et al. 2012 |
| JXRXY‐11 | KP204350 | Talaromyces verruculosus (HQ607791) | 99.6 | Rodrigues, A., et al. 2011 |
| GRPH‐5‐2 | KP204353 | Penicillium spinulosum (KF646101) | 100 | Menkis, A., et al. 2014 |
| JXRXY‐26 | KP204356 | Penicillium toxicarium (EF198650) | 100 | Serra, R., et al. 2008 |
| TRXY‐33‐2 | KP204359 | Penicillium sclerotiorum (AY373931) | 99.5 | Haugland, R.A., et al. 2004 |
| TRPH‐107 | KP204360 | Penicillium coffeae (AY742702) | 98.5 | Peterson, S.W., et al. 2005 |
| TRPH‐62 | KP204371 | Sagenomella sp. (EU140821) | 99.8 | Unpublished |
| TRXY‐26 | KP204372 | Aspergillus flavus (KJ775476) | 99.8 | Visagie, C.M., 2014 |
| JXRPH‐20 | KP204373 | Aspergillus versicolor (AJ937752) | 100 | Fomicheva, G.M., 2006 |
| JXRXY‐5 | KP204375 | Lasiodiplodia theobromae (HM466953) | 100 | Sulaiman, R., et al. 2012 |
| TRPH‐22‐1 | KP204385 | Chaetomium aureum (KF156298) | 99.8 | Stenstrom, E., et al. 2013 |
| JXRPH‐21‐2 | KP204387 | Phialocephala humicola (AB671503) | 100 | Kiyuna, T., et al. 2012 |
| JXRPH‐23 | KP204388 | Chaetosphaeria sp. (HQ630994) | 98.3 | Shrestha, P., et al. 2011 |
| TRPH‐35 | KP204396 | Colletotrichum simmondsii (JN121206) | 99.6 | Faedda, R., et al. 2011 |
| TRXY‐63 | KP204398 | Purpureocillium lilacinum (EU553316) | 99.6 | Inglis, P.W. and Tigano, M.S. 2006 |
| TRPH‐89 | KP204400 | Trichoderma asperellum (GU198311) | 100 | Samuels, G.J., et al. 2010 |
| TRPH‐13 | KP204401 | Trichoderma sp. (KF367564) | 99.8 | Oliveira, B.R., et al. 2013 |
| JXRPH‐2‐1 | KP204402 | Hypocrea nigricans (JN943371) | 100 | Schoch, C.L., et al. 2012 |
| GRPH‐0 | KP204404 | Myrothecium verrucaria (HQ608048) | 99.0 | Rodrigues, A., et al. 2011 |
| TRXY‐58 | KP204405 | Metarhizium anisopliae (FJ545312) | 99.6 | Freed, S., et al. 2011 |
| TRXY‐34‐1 | KP204422 | Fusarium solani (AB498917) | 100 | Hamada, N., et al. 2010 |
| GRXY‐1 | KP204407 | Fusarium oxysporum (KJ909935) | 100 | Garibaldi, A., et al. 2014 |
| TRXY‐60 | KU862685 | Pleosporales sp. (JN116643) | 92.7 | Supaphon, P., et al. 2014 |
| TRXY‐56‐1 | KU862686 | Trichosporon asahii (KM982986) | 92.2 | Unpublished |
| TRPH‐94 | KU862687 | Dothideomycetes sp. (JQ905832) | 91.4 | Unpublished |
| JXRPH‐21‐1 | KU862688 | Nectria haematococca (JX868649) | 81.5 | Unpublished |
| JXRPH‐24 | KT935174 | Fusarium solani (EU982942) | 80.9 | Unpublished |
The similitude percentages based on ITS sequence between respective strains and closest related strains were calculated using MatGAT v. 2.01 software.
3.2. Phylogeny and fungal diversity analysis
A total of 84 ITS sequences of respective strains including 42 identifiable taxa isolated from the root of S. tonkinensis, 41 closely related strains, and one external reference strain retrieved from GenBank, were employed to construct the phylogenetic tree using the software of MEGA version 6.0. The respective strains with strong inhibitory activity against fungal phytopathogens were marked with a triangle (▲). For the closely related strains, the numbers behind the scientific name were the accession number of GenBank. The evolutionary history was inferred using the neighbor‐joining method. The optimal tree with the sum of branch length = 4.62712089 is shown. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown above the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the p‐distance method and are in the units of the number of base differences per site. All ambiguous positions were removed for each sequence pair. There were a total of 728 positions in the final dataset. The phylogenetic tree displayed diverse taxonomic affinities among identifiable taxa (Figure 2). All taxa were distributed in three clusters, namely phylum Ascomycota within four classes represented by 13 orders, phylum Basidiomycota with three classes represented by three orders, and phylum Zygomycota represented by two orders.
Figure 2.
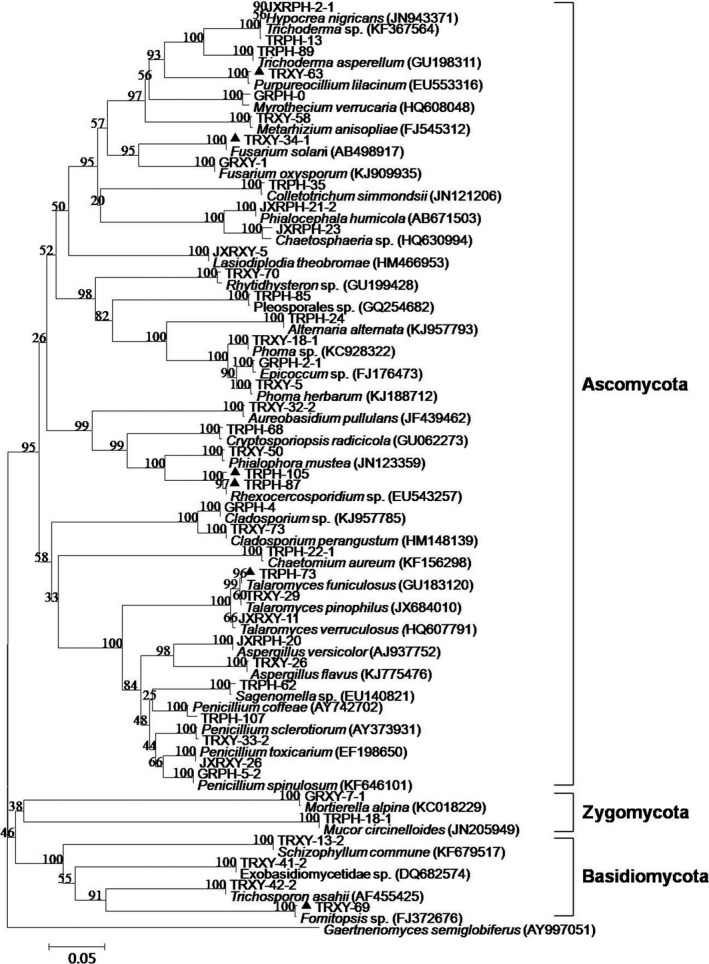
Phylogenetic tree of identifiable endophytes harbored in the root of S. tonkinensis based on ITS sequences using the software of MEGA version 6.0
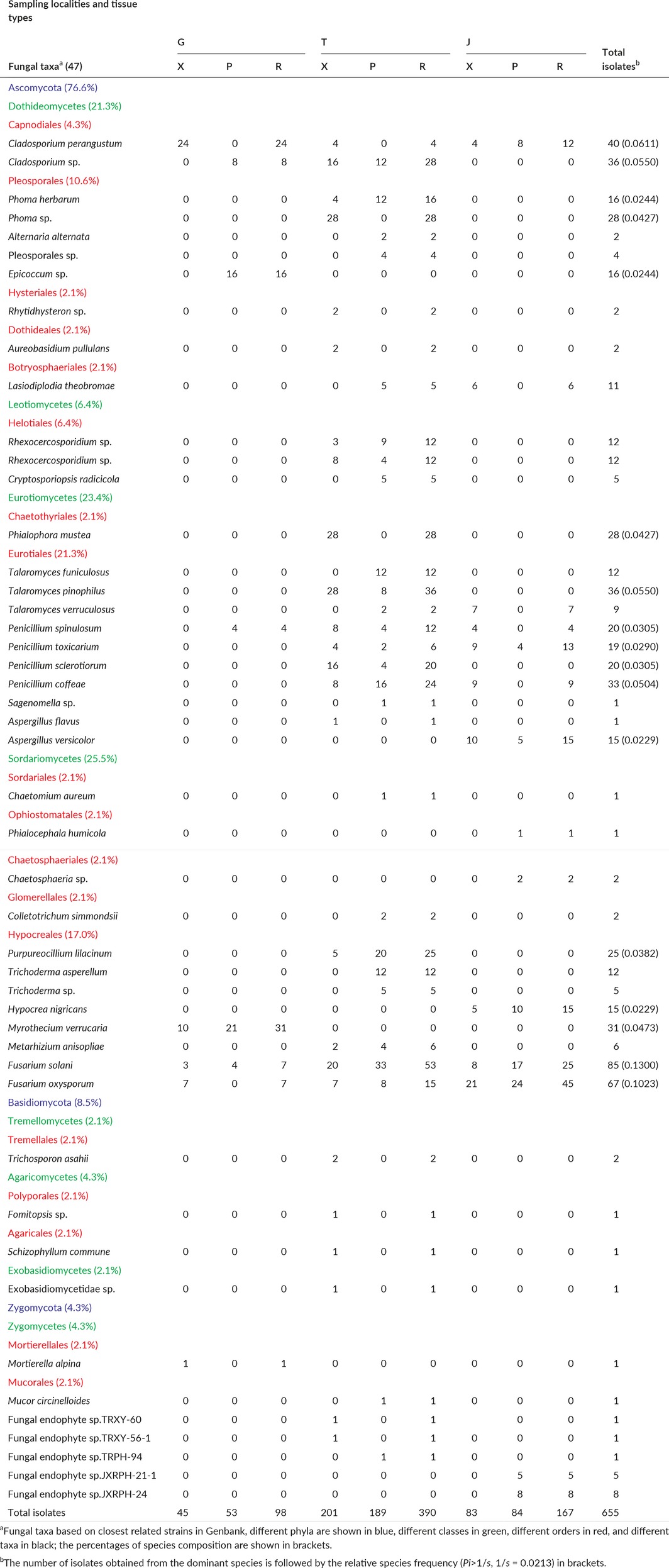
To characterize the colonization, species diversity, and species composition of endophytic fungal community in the root of S. tonkinensis, we calculated the total colonization frequency (CF, 17%), alpha‐diversity indices such as species richness (S, 47), Shannon‐Wiener index (H′, 3.2356), and Simpson's diversity index (1‐D, 0.9458), and the percentage of species composition (S i) (Table 3). The species richness consisted of frequent species (29 species, 61.7%) and rare species (18 species, 38.3%) with singleton and doubleton isolates (Table 3 ), indicating that frequent species were dominant in this community. The alpha‐diversity indices showed that the species diversity was rather high in this community. According to the percentages of species composition in this community, the most abundant phylum, by far, was Ascomycota (36 taxa, 76.6%) with abundant class Sordariomycetes (12 taxa, 25.5%), Eurotiomycetes (11 taxa, 23.4%), and Dothideomycetes (10 taxa, 21.3%), represented by particular abundant order Eurotiales (10 taxa, 21.3%), Hypocreales (8 taxa, 17%), and Pleosporales (5 taxa, 10.6%). However, rare species belonged to the phylum Basidiomycota (4 taxa, 8.5%) and Zygomycota (2 taxa, 4.3%). There were 17 taxa obtained in this community that could be considered as dominant species (Table 3). The dominant species F. solani (0.1300) and F. oxysporum (0.1023) were isolated from the root of S. tonkinensis from three localities of Guangxi province. The next dominant species were C. perangustum (0.0611), T. pinophilus (0.0550), Cladosporium sp. (0.0550), P. coffeae (0.0504), M. verrucaria (0.0473), and a collective group of species (0.0229 to 0.0427).
Table 3.
Summary of the endophytic fungi isolated from the xylem and phloem of the root of S. tonkinensis from three sampling localities with their taxa and the number of isolates from each taxon
3.3. The influences of geographic locality and tissue type on endophytic fungal communities
The influences of geographic locality and tissue type on the colonization, species diversity, and species composition of the endophytic fungal communities were investigated. The xylem and phloem of the root had no significant influence on the CF (Sig = 0.886, 0.886 >0.05), Shannon‐Wiener index (Sig = 0.941, 0.941 >0.05), and Simpson's diversity index (Sig = 0.383, 0.383 >0.05) according to t test, but geographic locality significantly affected these as displayed in Table 4. These results indicated that the colonization and species diversity of these communities were significantly influenced by geographic locality but not tissue type.
Table 4.
The influences of geographic locality on CF, Shannon‐Wiener index, and Simpson's diversity index
| Geographic localitya | CF% | Shannon‐Wiener index (H′) | Simpson's diversity index (1‐D) |
|---|---|---|---|
| G | 8.0000 ± 1.3528 a | 1.7170 ± 0.1226 a | 0.7894 ± 0.0248 a |
| T | 31.0667 ± 1.8148 b | 3.0090 ± 0.1003 b | 0.9367 ± 0.0070 b |
| J | 13.3333 ± 1.4572 c | 2.2279 ± 0.0717 c | 0.8621 ± 0.0053 c |
CF, colonization frequency
Data were analyzed by one‐way ANOVA followed by LSD test; results are expressed as the mean ± SD. (n = 3); and results followed by different letters are significantly different according to LSD test (p < .05).
Sorenson's and Jaccard's similarity indices of the endophytic fungal communities between two tissues or two localities were rather low, as exhibited in Table 5. The most dominant species was also diverse in different tissue types or geographic localities—that is, F. oxysporum in the xylem, F. solani in the phloem, M. verrucaria in Guangxi University, F. solani in Tiandeng county, and F. oxysporum in Jingxi county (Table 3). In addition, 16 fungal taxa exclusively colonized the phloem of the root, and 12 fungal taxa were only isolated from the xylem of that, providing clear evidence for tissue specificity (Table 3). These results revealed that geographic locality and tissue type had great effects on the species composition of these communities.
Table 5.
Sorenson's and Jaccard's similarity indices of endophytic fungi communities between two tissues or two localities
| Tissues | Localities | |||
|---|---|---|---|---|
| Similarity indicesa | X and P | G and T | G and J | T and J |
| Sorenson's index (QS) | 0.36 | 0.16 | 0.24 | 0.25 |
| Jaccard's index (JS) | 0.22 | 0.09 | 0.14 | 0.15 |
Both indices range from 0 (no overlap between communities) to 1 (total overlap between communities).
3.4. In vitro antagonistic assays of endophytic fungi against fungal phytopathogens of P. notoginseng
All respective strains from 47 taxa isolated from the root of S. tonkinensis were screened for antagonistic activity against three fungal phytopathogens of P. notoginseng using the coculture method. In the ecological treatment, the crude extract from the root of S. tonkinensis at the concentration of 2 mg/ml showed 58% inhibition against F. solani, 59% inhibition against C. gloeosporioides, and 68% inhibition against A. panax (Figure 3 ). In coculture, 24 strains showed 50% or more inhibition against F. solani (15 strains), C. gloeosporioides (22 strains), and A. panax (12 strains), respectively (Table 6).
Figure 3.
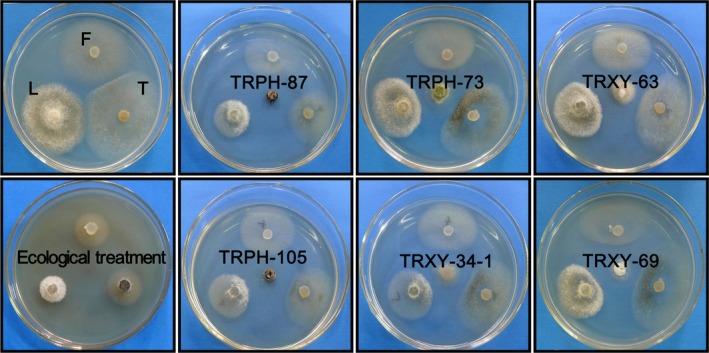
The coculture interactions between endophytic fungi strains and three fungal phytopathogens on PDA
Table 6.
Growth inhibition of fungal phytopathogens antagonized by endophytic fungi isolated from the root of S. tonkinensis on PDA using the coculture method
| Endophytic fungi strain number | Growth inhibition of fungal phytopathogensa | ||
|---|---|---|---|
| F. solani | C. gloeosporioides | A. panax | |
| TRXY‐75 | +++ | +++ | + |
| TRXY‐5 | +++ | ++++ | ++ |
| TRXY‐18‐1 | +++ | ++++ | ++++ |
| GRPH‐2‐1 | ++ | +++ | + |
| TRPH‐24 | ++ | +++ | + |
| TRXY‐69 | ++++ | ++++ | ++++ |
| TRXY‐13‐2 | +++ | +++ | ++ |
| GRXY‐7‐1 | ++ | +++ | + |
| TRPH‐18‐1 | + | +++ | + |
| TRPH‐105 | ++++ | ++++ | ++++++ |
| TRPH‐87 | ++++ | ++++ | ++++ |
| TRPH‐68 | + | +++++ | ++ |
| TRPH‐73 | ++++ | ++++ | ++++ |
| TRXY‐29 | ++ | +++ | ++ |
| JXRXY‐26 | +++ | ++ | ++ |
| TRXY‐33‐2 | ++++ | ++++ | +++ |
| TRXY‐26 | ++++ | +++ | ++++ |
| JXRXY‐5 | +++ | + | +++ |
| TRPH‐22‐1 | ++ | ++++ | ++++ |
| TRXY‐63 | ++++ | ++++ | ++++ |
| TRPH‐89 | ++ | ++++ | ++++ |
| TRPH‐13 | + | +++ | ++ |
| TRXY‐34‐1 | ++++ | +++++ | ++++ |
| GRXY‐1 | +++ | +++ | ++ |
Symbols represent inhibition percentage in different ranges: +, <40%; ++, 40%–49%; +++, 50%–59%; ++++, 60%–69%; +++++,70%–79%; ++++++, >80%.
Six endophytic strains with more than 60% inhibition against all of three fungal phytopathogens were selected to further test the antifungal activity of their crude extracts using the mycelial growth method (Table 7, Figure 3, Figure 4). In the test plates, mycelial growth inhibition, including no growth, only growth on the mycelial plug and growth on the medium, was observed. The colonial morphology was changed in the plates with crude extracts from different strains. Crude extracts from strains TRPH‐73, TRPH‐105, and TRPH‐87 exhibited more than 90% inhibition against all of three fungal phytopathogens even at the low concentration of 2 mg/ml. The most susceptible phytopathogen was C. gloeosporioides whose mycelial growth was completely inhibited by the crude extracts of strains TRPH‐73, TRPH‐87, and TRPH‐105, even at the low concentration of 2 mg/ml, and by the crude extracts of strains TRXY‐34‐1, TRXY‐69, and TRXY‐63 at the concentration of 8 mg/ml. The six strains showed significant antifungal activity against three fungal phytopathogens, based on that of carbendazim wettable powders, which were widely applied to control fungal phytopathogens of P. notoginseng using the concentration range of 2–8 mg/ml. Particularly, the antifungal activity of the crude extracts from strains TRPH‐73, TRPH‐105, TRPH‐87, and TRXY‐34‐1 was more than that of carbendazim wettable powders against A. panax and almost equal to that of carbendazim wettable powders against C. gloeosporioides. The inhibitory activity of the crude extracts from strains TRPH‐73 and TRPH‐105 was also equal to that of carbendazim wettable powders against F. solani.
Table 7.
Percent of inhibitory activity on mycelial growth of fungal phytopathogens produced by the crude extracts of six endophytic strains from the root of S. tonkinensis on PDA
| Fungal phytopathogensb | ||||
|---|---|---|---|---|
| Treatmenta | Concentration mg/ml | F. solani | C. gloeosporioides | A. panax |
| Carbendazim wettable powders | 2 | 100.00 ± 0.00 | 100.00 ± 0.00 | 93.17 ± 0.35 |
| 4 | 100.00 ± 0.00 | 100.00 ± 0.00 | 94.17 ± 0.47 | |
| 8 | 100.00 ± 0.00 | 100.00 ± 0.00 | 96.10 ± 0.36 | |
| Crude extracts of strain TRPH‐73 | 2 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00c |
| 4 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00c | |
| 8 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00c | |
| Crude extracts of strain TRPH‐105 | 2 | 90.10 ± 0.36c | 100.00 ± 0.00 | 96.20 ± 0.44c |
| 4 | 99.9 ± 0.10 | 100.00 ± 0.00 | 97.23 ± 0.49c | |
| 8 | 99.83 ± 0.21 | 100.00 ± 0.00 | 99.03 ± 0.25c | |
| Crude extracts of strain TRPH‐87 | 2 | 93.93 ± 0.40c | 100.00 ± 0.00 | 99.20 ± 0.44c |
| 4 | 94.20 ± 0.44c | 100.00 ± 0.00 | 99.03 ± 0.15c | |
| 8 | 94.06 ± 0.40c | 100.00 ± 0.00 | 100.00 ± 0.00c | |
| Crude extracts of strain TRXY‐34‐1 | 2 | 68.10 ± 0.66c | 94.07 ± 0.40c | 93.13 ± 0.42 |
| 4 | 91.10 ± 0.36c | 97.17 ± 0.38c | 94.17 ± 0.47 | |
| 8 | 94.90 ± 0.46c | 100.00 ± 0.00 | 100.00 ± 0.00c | |
| Crude extracts of strain TRXY‐69 | 2 | 88.10 ± 0.36c | 96.13 ± 0.32c | 78.03 ± 0.45c |
| 4 | 95.27 ± 0.71c | 99.10 ± 0.36c | 78.07 ± 0.21c | |
| 8 | 95.23 ± 0.31c | 100.00 ± 0.00 | 78.20 ± 0.41c | |
| Crude extracts of strain TRXY‐63 | 2 | 78.10 ± 0.32c | 94.27 ± 0.21c | 67.93 ± 0.40c |
| 4 | 85.43 ± 0.25c | 96.53 ± 0.31c | 80.30 ± 0.40c | |
| 8 | 91.50 ± 0.20c | 100.00 ± 0.00 | 84.30 ± 0.56c | |
Carbendazim wettable powders containing 50% carbendazim.
Results are expressed as the mean ± S.D. (n = 3); data were analyzed by one‐way ANOVA followed by LSD test.
Significant difference between each treatment and the positive control at the same concentration are shown as p < .05.
Figure 4.

The mycelial growth of fungal phytopathogens on PDA‐containing drug. Notes: growth control (columns: 1, 5, 9, 13), treated dishes (columns: 2–4, 6–8, 10–12, 14–16)
4. DISCUSSION
Morphological characteristics and ITS sequences analysis have been employed for the identification of endophytic fungi in this work. However, this work still failed to identify some taxa based on >5% divergences of ITS sequences and no spores. These unidentifiable taxa require the analysis of other gene markers to provide better taxonomic resolution. Many other markers which have been used for fungal identification are 28S rDNA gene, cytochrome c oxidase subunit I, and beta‐tubulin 2 gene (Liu, Xu, & Guo, 2007; Rivera‐Orduna et al., 2011; Robideau et al., 2011). Some endophytic fungi belonging to unidentifiable taxa may represent novel species. The taxonomic novelty of endophytic fungi may also correspond to chemical novelty of their secondary metabolites (Kumar et al., 2013). Furthermore, these endophytic fungi have not been explored for their natural products. Thus, these organisms will be given priority to isolate and characterize novel molecules from their secondary metabolites.
The total CF of endophytic fungi with 17% in the root of S. tonkinensis was much lower than that with the range of 33% to 53% in the roots of other medicinal plants (Jin et al., 2013; Kharwar, Verma, Strobel, & Ezra, 2008; Mishra et al., 2016). Two reasons may account for the high CF in the roots of medicinal plants in previous reports. One likely reason is that the soil fungi and rhizospheric fungi are so prevalent and diversified to easily establish an endophytic relationship with the roots (Ghimire, Charlton, Bell, Krishnamurthy, & Craven, 2011). The other reason is that roots as important sources of easily accessible substrate may provide a relatively stable environment favoring many fungal survival and coexistence (Angelini et al., 2012; Garbeva, Veen, & Elsas, 2004). However, the low CF in the root of S. tonkinensis may be associated with the presence of antimicrobial chemicals as mentioned above that may have suppressed the growth of some endophytic fungi.
In the root of S. tonkinensis, the majority of endophyte taxa were Ascomycota, a finding that was in agreement with that of previous reports (Qadri et al., 2014; Rivera‐Orduna et al., 2011; Vieira et al., 2014). The low proportion of the phylum Basidiomycota and Zygomycota were consistent with that reported in other studies (Gonzalez & Luisa, 2011; Sánchez, Bills, Acuña, & Zabalgogeazcoa, 2010; Tejesvi et al., 2011). However, recent papers have suggested that Basidiomycota constitute an important component of certain endophytic communities (Pinruan et al., 2012; Rungjindamai, Pinruan, Hattori, & Choeyklin, 2008). The abundant classes Sordariomycetes, Eurotiomycetes, and Dothideomycetes, were similar to that of endophytic fungal community associated with ferns in Costa Rica (Del Olmo‐Ruiz & Arnold, 1991) and Huperzia serrata in China (Xiong et al., 2015). The abundant orders Eurotiales, Hypocreales, and Pleosporales, were in line with that of endophytic fungal community in Ficus tree (Solis, Edison Dela Cruz, Schnittler, & Unterseher, 2013a), Annona squamosa (Lin et al., 2010), and Stellera chamaejasme L. (Jin et al., 2013), respectively. The species F. solani, F. oxysporum, C. perangustum, Cladosporium sp., T. pinophilus, P. coffeae, and M. verrucaria were dominant in this work, possibly due to the high spore production of these fungi and their cosmopolitan nature, which statistically increases their chance to become established as endophytes, as indicated in previous studies (Mishra et al., 2012; Raviraja, 2005; Schulthess & Faeth, 1998). In addition, based on the “balanced antagonisms” hypothesis (Schulz, Haas, Junker, Andree, & Schobert, 2015; Schulz, Rommert, Dammann, Aust, & Strack, 1999), they as dominant species might not only secrete toxic metabolites to inhibit microbial competitors (Breinholt et al., 1997; Lee & Lee, 2012; Zhai et al., 2015) but also possess the ability to resist the attack of the host alkaloids with antitumor and antifungal activities (Liu et al., 2014; Yang, Zhao, & Ju, 2008).
The alpha‐diversity indices indicated that the species diversity of the endophytic fungal community harbored in the root of S. tonkinensis from three localities of Guangxi province was very high, showing a similarity to that in other plant hosts (Garcia, Rhoden, Rubin Filho, Nakamura, & Pamphile, 2012; Li et al., 2010). Furthermore, this community was dominated by frequent species, following the same pattern as those in other plant hosts (Gonzalez & Luisa, 2011; Kusari et al., 2013). The rare species usually were recognized as the result of unstable associations that possibly only occurred when a given plant and fungal phenotype were confronted (Joshee, Paulus, Park, & Johnston, 2009; Orlandelli, Alberto, Rubin Filho, & Pamphile, 2012). However, 38.3% rare species in this work imply that some members of these fungi are host‐specific and occupy specific ecological niche in this community (Yuan et al., 2011).
Geographic locality significantly affected the colonization, species diversity, and species composition of endophytic fungal communities harboring the root of S. tonkinensis, possibly because ecological environment primarily including temperature, rainfall, altitude, and geographic coordinates are diverse in three geographic localities as mentioned above. In different ecosystems, the fungi are subjected to different selection pressures (Goere & Bucak, 2007; Petrini, Sieber, Toti, & Viret, 1993). Furthermore, in order to adapt to the ecological environment, a plant may produce several toxic metabolites toward which biotransformation abilities of many endophytic fungi to a certain extent decide the colonization range of their hosts (Saunders & Kohn, 2009; Wang & Dai, 2011; Zikmundova, Drandarov, Bigler, Hesse, & Werner, 2002). These lead to the establishment of a quite specific endophytic fungal community at each geographic locality, as reported previously (Goere & Bucak, 2007; Hoffman & Arnold, 2008).
Tissue type, including root, stem, bark, twig, leaf, and seed, influenced the colonization, species diversity, and species composition of endophytic fungi communities as indicated in previous reports (Gonzalez & Luisa, 2011; Mishra et al., 2012; Raviraja, 2005). However, there were few works about endophytic fungi communities in the xylem and the phloem of the root tissue in previous studies. In this work, results showed that the xylem and phloem of the root influenced the species composition of the endophytic fungi communities but not the colonization and species diversity of that. The striking difference in the species composition of fungal communities between the xylem and phloem may be due to tissue specificity as reported in other tissues (Mishra et al., 2012; Raviraja, 2005). These tissues may represent two distinct microenvironments including toxic metabolites, oxygen, nutrition, anatomy, and endophytic bacteria consequently shaping their difference in species composition (Huang, Cai, Hyde, Corke, & Sun, 2008; Qadri et al., 2014; Schulz et al., 2015). Further work is needed to investigate the reasons for similarity in the colonization and species diversity of fungal communities between the xylem and the phloem.
This work also demonstrated that geographic locality affected the endophytic fungi communities harbored in the root of S. tonkinensis more strongly than the tissue type, a finding that was not in agreement with a previous report (Mishra et al., 2012).
Some endophytic fungi with strongly antimicrobial activities as biological agents are of increasing public interest (Bailey et al., 2008; Rubini et al., 2005). Because the crude extracts from the roots of S. tonkinensis were effectively used to control symptoms on P. notoginseng cultivated in Guangxi province, we attempted to screen antagonistic fungi from endophytic fungi isolated from them against three fungal phytopathogens of P. notoginseng.
The results that 24 strains showed 50% or more inhibition against three fungal phytopathogens of P. notoginseng, suggested that it is possible to effectively screen potential biocontrol agents against fungal phytopathogens of P. notoginseng from the root of S. tonkinensis. Furthermore, the endophytic fungi and the host plant exerting similar antifungal activities proved that endophytic fungi may assist the host plant in chemical defense.
The antifungal activity of the crude extracts from six strains was more than or almost equal to that of carbendazim wettable powders against three fungal phytopathogens of P. notoginseng in vitro, therefore, future investigations will be conducted to study their potential as biocontrol agents on an agronomic scale.
It was noteworthy that there was a few works in the antagonistic activity and compounds of Rhexocercosporidium species in previous study. Therefore, the strains Rhexocercosporidium sp TRPH‐87 and Rhexocercosporidium sp TRPH‐105 probably produce new natural compounds with antifungal activity, and the isolation and characterization of the active substance from them are in progress.
The result that the endophytic strain F. solani TRX‐34‐1 strongly inhibited pathogenic F. solani compelled reconsidering whether F. solani TRX‐34‐1 was capable of producing associated plant secondary metabolites as a result of horizontal gene transfer (Gogarten & Townsend, 2005) from host plant to endophytic fungus during the course of evolution. In this work, F. solani TRX‐34‐1 is likely a nonpathogenic strain based on its antagonistic activity against three fungal phytopathogens of P. notoginseng. Thus, key research on the mode of action of F. solani TRX‐34‐1 against phytopathogens of P. notoginseng by several methods is progress.
In conclusion, endophytic fungal communities harbored in the roots of S. tonkinensis with high diversity were affected by geographic locality more strongly than tissue type, and they have great promise not only as potential sources of bioactive secondary metabolites, but also as biocontrol agents against fungal phytopathogens of P. notoginseng and possibly other pathogens.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENT
This work was supported by the Science and Technology Major Project of Guangxi, China (14124002‐1 and 1598005‐15), the Natural Science Foundation of Guangxi, China (2015GXNSFAA139091), and the Science and Technology Project of Baise city, Guangxi, China (20141201).
Yao YQ, Lan F, Qiao YM, Wei JG, Huang RS, and Li LB. Endophytic fungi harbored in the root of Sophora tonkinensis Gapnep: Diversity and biocontrol potential against phytopathogens. MicrobiologyOpen. 2017;6:e437 https://doi.org/10.1002/mbo3.437
Contributor Information
Rong Shao Huang, Email: hrs17252@gxu.edu.cn.
Liang Bo Li, Email: llb100@126.com.
REFERENCES
- Aime, M. C. , Posada, F. , Peterson, S. W. , Rehner, S. A. , & Vega, F. E. (2007). Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycological Research, 111, 748–757. [DOI] [PubMed] [Google Scholar]
- Aly, A. H. , Debbab, A. , Kjer, J. , & Proksch, P. (2010). Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Diversity, 41(1), 1–16. [Google Scholar]
- Angelini, P. , Rubini, A. , Gigante, D. , Reale, L. , Pagiotti, R. , & Venanzoni, R. (2012). The endophytic fungal communities associated with the leaves and roots of the common reed (Phragmites australis) in Lake Trasimeno (Perugia, Italy) in declining and healthy stands. Fungal Ecology, 5(6), 683–693. [Google Scholar]
- Arhipova, N. , Gaitnieks, T. , Donis, J. , Stenlid, J. , & Vasaitis, R. (2011). Decay, yield loss and associate fungi in stands of grey alder (Alnus incana) in Latvia. Forestry, 84(4), 337–348. [Google Scholar]
- Bailey, B. A. , Bae, H. , Strem, M. D. , Crozier, J. , Thomas, S. E. , Samuels, G. J. , … Holmes, K. A. (2008). Antibiosis, mycoparasitism, and colonization success for endophytic Trichoderma isolates with biological control potential in Theobroma cacao . Biological Control, 46(1), 24–35. [Google Scholar]
- Ban, Y. , Tang, M. , Chen, H. , Xu, Z. , Zhang, H. , & Yang, Y. (2012). The Response of Dark Septate Endophytes (DSE) to Heavy Metals in Pure Culture. PLoS ONE, 7(10), E47968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, C. W. , Ordonez, M. E. , & Salazar, A. (2012). Identification and evaluation of some fungi with cellulase activity isolated in Ecuador. Rev Ecuat Med Cienc Biol, 33, 65–81. [Google Scholar]
- Bensch, K. , Groenewald, J. Z. , Dijksterhuis, M. , Starink‐Willemse, M. , Andersen, B. , Summerell, B. A. , … Crous, P. W. (2010). Species and ecological diversity within the Cladosporium cladosporioides complex (Davidiellaceae, Capnodiales). Studies in Mycology, 67(67), 1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botella, L. , & Diez, J. J. (2011). Phylogenic diversity of fungal endophytes in Spanish stands of Pinus halepensis . Fungal Diversity, 47(1), 9–18. [Google Scholar]
- Breinholt, J. , Ludvigsen, S. , Rassing, B. R. , Rosendahl, C. N. , Nielsen, S. E. , & Olsen, C. E. (1997). Oxysporidinone: A Novel, Antifungal N‐Methyl‐4‐hydroxy‐2‐pyridone from Fusarium oxysporum. Journal of Natural Products, 60(1), 33–35. [DOI] [PubMed] [Google Scholar]
- Buzina, W. , Braun, H. , Freudenschuss, K. , Lackner, A. , Habermann, W. , & Stammberger, H. (2003). Fungal biodiversity–as found in nasal mucus. Medical Mycology, 41(2), 149–161. [DOI] [PubMed] [Google Scholar]
- Carroll, G. C. (1991). Beyond Pest Deterrence—Alternative Strategies and Hidden Costs of Endophytic Mutualisms in Vascular Plants In Andrews J. H., & Hirano S. S. (Eds.), Microbial Ecology of Leaves (pp. 358–375). New York: Springer New York. [Google Scholar]
- Chan, J. F. , Teng, J. L. , Li, I. W. , Wong, S. C. , Leung, S. S. , Ho, P. O. , … Yuen, K. Y. (2014). Fatal Empyema Thoracis Caused by Schizophyllum commune with Cross‐Reactive Cryptococcal Antigenemia. Journal of Clinical Microbiology, 52(2), 683–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra, S. (2012). Endophytic fungi: Novel sources of anticancer lead molecules. Applied Microbiology and Biotechnology, 95(1), 47–59. [DOI] [PubMed] [Google Scholar]
- Chen, X. M. , Dong, H. L. , Hu, K. X. , Sun, Z. R. , Chen, J. , & Guo, S. X. (2010). Diversity and Antimicrobial and Plant‐Growth‐Promoting Activities of Endophytic Fungi in Dendrobium loddigesii Rolfe. Journal of Plant Growth Regulation, 29(3), 328–337. [Google Scholar]
- Choi, L. Y. , Cho, S. E. , Park, J. H. , & Shin, H. D. (2014a). First Report of Leaf Spot Caused by a Phoma sp. on Schisandra chinensis in Korea. Plant Disease, 98(1), 157. [DOI] [PubMed] [Google Scholar]
- Choi, M. S. , Eo, J. K. , & Eom, A. H. (2014b). Diversity of endophytic fungi isolated from korean ginseng leaves. Mycobiology, 42(2), 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay, K. (1992). Fungal endophytes of plants: Biological and chemical diversity. Natural Toxins, 1(3), 147–149. [DOI] [PubMed] [Google Scholar]
- Commission, C. P. (2015a). Chinese Pharmacopoeia (p. 11). Bei Jing: China Medical Science Press. [Google Scholar]
- Commission, C. P. (2015b). Chinese Pharmacopoeia (p. 27). Bei Jing: China Medical Science Press. [Google Scholar]
- Del Olmo‐Ruiz, M. , & Arnold, A. E. (2014). Interarmual variation and host affiliations of endophytic fungi associated with ferns at La Selva, Costa Rica. Mycologia, 106(1), 8–21. [DOI] [PubMed] [Google Scholar]
- Faedda, R. , Agosteo, G.E. , Schena, L. , Mosca, S. , Frisullo, S. , Magnanodi San Lio, G. , & Cacciola, S.O. (2011). olletotrichum clavatum sp. nov. identified as the causal agent of olive anthracnose in Italy. Phytopathologia Mediterranea, 50(2), 283–302. [Google Scholar]
- Fomicheva, G. M. , Vasilenko, O. V. , & Marfenina, O. E. (2006). Comparative morphological, ecological, and molecular studies of Aspergillus versicolor (Vuill.) tiraboschi strains isolated from different ecotopes. Microbiology, 75(2), 186–191. [PubMed] [Google Scholar]
- Freed, S. , Jin, F.‐L. , & Ren, S. X. (2011). Determination of genetic variability among the isolates of Metarhizium anisopliae var. anisopliae from different geographical origins. World Journal of Microbiology and Biotechnology, 27(2), 359–370. [Google Scholar]
- Garbeva, P. , Veen, J. A. , & Elsas, J. D. (2004). Microbial diversity in soil: Selection of the microbial populations by plant and soil type and implementations for disease suppressivenss. Annual Review of Phytopathology, 42, 243–270. [DOI] [PubMed] [Google Scholar]
- Garcia, A. , Rhoden, S. A. , Rubin Filho, C. J. , Nakamura, C. V. , & Pamphile, J. A . (2012). Diversity of foliar endophytic fungi from the medicinal plant Sapindus saponaria L. and their localization by scanning electron microscopy. Biological Research, 45 (2), 139–148. [DOI] [PubMed] [Google Scholar]
- Garibaldi, A. , Pensa, P. , Bertetti, D. , Ortu, G. , & Gullino, M. L. (2014). First Report of Dry and Soft Rot of Cereus marginatus var. cristata Caused by Fusarium oxysporum in Italy. Plant Disease, 98(10), 1441. [DOI] [PubMed] [Google Scholar]
- Ghimire, S. R. , Charlton, N. D. , Bell, J. D. , Krishnamurthy, Y. L. , & Craven, K. D. (2011). Biodiversity of fungal endophyte communities inhabiting switchgrass (Panicum virgatum L.) growing in the native tallgrass prairie of northern Oklahoma. Fungal Diversity, 47(1), 19–27. [Google Scholar]
- Goere, M. E. , & Bucak, C. (2007). Geographical and seasonal influences on the distribution of fungal endophytes in Laurus nobilis . Forest Pathology, 37(4), 281–288. [Google Scholar]
- Gogarten, J. P. , & Townsend, J. P. (2005). Horizontal gene transfer, genome innovation and evolution. Nature Reviews Microbiology, 3(9), 679–687. [DOI] [PubMed] [Google Scholar]
- Gonzalez, V. , & Tello, M. L (2011). The endophytic mycota associated with Vitis vinifera in central Spain. Fungal Diversity, 47(1), 29–42. [Google Scholar]
- Guo, L. D. , Hyde, K. D. , & Liew, E. C. Y. (2011a). Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytologist, 147(3), 617–630. [DOI] [PubMed] [Google Scholar]
- Hamada, N. , & Abe, N. (2010). Comparison of fungi found in bathrooms and sinks. Biocontrol Science, 15(2), 51–56. [DOI] [PubMed] [Google Scholar]
- Han, G. , Feng, X. , & Tian, X. (2011). Isolation and evaluation of terrestrial fungi with algicidal ability from Zijin Mountain, Nanjing, China.. Journal of Microbiology, 49(4), 562–567. [DOI] [PubMed] [Google Scholar]
- Haugland, R. A. , Varma, M. , Wymer, L. J. , & Vesper, S. J. (2004). Quantitative PCR analysis of selected Aspergillus, Penicillium and Paecilomyces species. Systematic & Applied Microbiology, 27(2), 198–210. [DOI] [PubMed] [Google Scholar]
- Hoffman, M. T. , & Arnold, A. E. (2008). Geographic locality and host identity shape fungal endophyte communities in cupressaceous trees. Mycological Research, 112, 331–344. [DOI] [PubMed] [Google Scholar]
- Huang, W. Y. , Cai, Y. Z. , Hyde, K. D. , Corke, H. , & Sun, M. (2008). Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Diversity, 33, 61–75. [Google Scholar]
- Hyde, K. D. , & Soytong, K. (2008). The fungal endophyte dilemma. Fungal Diversity, 33, 163–173. [Google Scholar]
- Inglis, P. W. , & Tigano, M. S. (2006). Identification and taxonomy of some entomopathogenic Paecilomyces spp. (Ascomycota) isolates using rDNA‐ITS Sequences. Genet. Mol. Biol., 29(1), 132–136. [Google Scholar]
- Jin, H. , Yan, Z. , Liu, Q. , Yang, X. , Chen, J. , & Qin, B . (2013). Diversity and dynamics of fungal endophytes in leaves, stems and roots of Stellera chamaejasme L. in northwestern China. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology, 104 (6), 949–963. [DOI] [PubMed] [Google Scholar]
- Joshee, S. , Paulus, B. C. , Park, D. , & Johnston, P. R. (2009). Diversity and distribution of fungal foliar endophytes in New Zealand Podocarpaceae. Mycological Research, 113(9), 1003–1015. [DOI] [PubMed] [Google Scholar]
- Kernaghan, G. , & Patriquin, G. (2015). Diversity and host preference of fungi co‐inhabiting Cenococcum mycorrhizae . Fungal Ecology, 17, 84–95. [Google Scholar]
- Kharwar, R. N. , Verma, V. C. , Strobel, G. , & Ezra, D. (2008). The endophytic fungal complex of Catharanthus roseus (L.) G. Don. Current Science, 95(2), 228–233. [Google Scholar]
- Kiyuna, T. , An, K. , Kigawa, R. , Sano, C. , Miura, S. , & Sugiyama, J. (2012). Bristle‐like fungal colonizers on the stone walls of the Kitora and Takamatsuzuka Tumuli are identified as Kendrickiella phycomyces. Mycoscience, 53, 446–459. [Google Scholar]
- Kumar, M. , Qadri, M. , Sharma, P. R. , Kumar, A. , Andotra, S. S. , Kaur, T. , … Shah, B. A. (2013). Tubulin Inhibitors from an Endophytic Fungus Isolated from Cedrus deodara . Journal of Natural Products, 76(2), 194–199. [DOI] [PubMed] [Google Scholar]
- Kusari, S. , Hertweck, C. , & Spiteller, M. (2012). Chemical ecology of endophytic fungi: Origins of secondary metabolites. Chemistry & Biology, 19(7), 792–798. [DOI] [PubMed] [Google Scholar]
- Kusari, P. , Kusari, S. , Spiteller, M. , & Kayser, O. (2013). Endophytic fungi harbored in Cannabis sativa L.: Diversity and potential as biocontrol agents against host plant‐specific phytopathogens. Fungal Diversity, 60(1), 137–151. [Google Scholar]
- Kusari, S. , Lamshoeft, M. , Zuehlke, S. , & Spiteller, M. (2008). An endophytic fungus from Hypericum perforatum that produces hypericin. Journal of Natural Products, 71(2), 159–162. [DOI] [PubMed] [Google Scholar]
- Lee, H.‐S. , & Lee, C. (2012). Structural Analysis of a New Cytotoxic Demethylated Analogue of Neo‐N‐methylsansalvamide with a Different Peptide Sequence Produced by Fusarium solani Isolated from Potato. Journal of Agricultural and Food Chemistry, 60(17), 4342–4347. [DOI] [PubMed] [Google Scholar]
- Li, E. , Jiang, L. , Guo, L. , Zhang, H. , & Che, Y. (2008). Pestalachlorides A‐C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta . Bioorganic & Medicinal Chemistry, 16(17), 7894–7899. [DOI] [PubMed] [Google Scholar]
- Li, E. , Tian, R. , Liu, S. , Chen, X. , Guo, L. , & Che, Y. (2013b). Pestalotheols A‐D, bioactive metabolites from the plant endophytic fungus Pestalotiopsis theae . Journal of Natural Products, 71(4), 664–668. [DOI] [PubMed] [Google Scholar]
- Li, M. , Xie, X. F. , Fan, C. Y. , & Wang, J. K. (2011a). Panax notoginseng In Geoherbs Chinese. (Ed.), Peng C (pp. 3149–3184). Bei Jing: China Press of Traditional Chinese Medicine. [Google Scholar]
- Li, H.‐Y. , Zhao, C.‐A. , Liu, C.‐J. , & Xu, X.‐F. (2011b). Endophytic Fungi Diversity of Aquatic/Riparian Plants and Their Antifungal Activity In Vitro. Journal of Microbiology, 48(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Lin, X. , Huang, Y. J. , Zheng, Z. H. , Su, W. J. , Qian, X. M. , & Shen, Y. M. (2010). Endophytes from the pharmaceutical plant, Annona squamosa: Isolation, bioactivity, identification and diversity of its polyketide synthase gene. Fungal Diversity, 41(1), 41–51. [Google Scholar]
- Liu, A.‐R. , Xu, T. , & Guo, L.‐D. (2007). Molecular and morphological description of Pestalotiopsis hainanensis sp nov., a new endophyte from a tropical region of China. Fungal Diversity, 24, 23–36. [Google Scholar]
- Liu, Y. , Xu, Y. , Ji, W. , Li, X. , Sun, B. , Gao, Q. , & Su, C. (2014). Anti‐tumor activities of matrine and oxymatrine: Literature review. Tumor Biology, 35(6), 5111–5119. [DOI] [PubMed] [Google Scholar]
- Luo, J. , Walsh, E. , Naik, A. , Zhuang, W. , Zhang, K. , Cai, L. , & Zhang, N. (2014). Temperate pine barrens and tropical rain forests are both rich in undescribed fungi. PLoS ONE, 9(7), e103753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia, L. C. , Rojas, E. I. , Maynard, Z. , Van Bael, S. , Arnold, A. E. , Hebbar, P. , … Herre, E. A. (2008). Endophytic fungi as biocontrol agents of Theobroma cacao pathogens. Biological Control, 46(1), 4–14. [Google Scholar]
- Menkis, A. , Ihrmark, K. , Stenlid, J. , & Vasaitis, R. (2014). Root‐Associated Fungi of Rosa rugosa Grown on the Frontal Dunes of the Baltic Sea Coast in Lithuania. Microbial Ecology, 67(4), 769–774. [DOI] [PubMed] [Google Scholar]
- Miao, Z. Q. , Li, S. D. , Liu, X. Z. , Chen, Y. J. , Li, Y. H. , Wang, Y. , … Zhang, K. Q. (2006). The causal microorganisms of root rot disease in Panax notoginseng . Scientia Agricultura Sinica, 39(7), 1371–1378. [Google Scholar]
- Mishra, A. , Gond, S. K. , Kumar, A. , Sharma, V. K. , Verma, S. K. , Kharwar, R. N. , & Sieber, T. N. (2012). Season and Tissue Type Affect Fungal Endophyte Communities of the Indian Medicinal Plant Tinospora cordifolia More Strongly than Geographic Location. Microbial Ecology, 64(2), 388–398. [DOI] [PubMed] [Google Scholar]
- Mishra, V. K. , Singh, G. , Passari, A. K. , Yadav, M. K. , Gupta, V. K. , & Singh, B. P. (2016). Distribution and antimicrobial potential of endophytic fungi associated with ethnomedicinal plant Melastoma malabathricum L. Journal of Environmental Biology, 37(2), 229–237. [PubMed] [Google Scholar]
- Nascimento, T. L. , Oki, Y. , Lima, D. M. M. , Almeida‐Cortez, J. S. , Fernandes, G. W. , & Souza‐Motta, C. M. (2015). Biodiversity of endophytic fungi in different leaf ages of Calotropis procera and their antimicrobial activity. Fungal Ecology, 14, 79–86. [Google Scholar]
- Oliveira, B. R. , Barreto Crespo, M. T. , San Romao, M. V. , Benoliel, M. J. , Samson, R. A. , & Pereira, V. J. (2013a). New insights concerning the occurrence of fungi in water sources and their potential pathogenicity. Water Research, 47(16), 6338–47. [DOI] [PubMed] [Google Scholar]
- Oliveira, B. R. , Barreto Crespo, M. T. , San Romao, M. V. , Benoliel, M. J. , Samson, R. A. , & Pereira, V. J. (2013b). New insights concerning the occurrence of fungi in water sources and their potential pathogenicity. Water Research, 47(16), 6338–47. [DOI] [PubMed] [Google Scholar]
- Orlandelli, R. C. , Alberto, R. N. , Rubin Filho, C. J. , & Pamphile, J. A . (2012). Diversity of endophytic fungal community associated with Piper hispidum (Piperaceae) leaves. Genetics and Molecular Research, 11 (2), 1575–1585. [DOI] [PubMed] [Google Scholar]
- Peterson, S. W. , Vega, F. E. , Posada, F. , & Nagai, C. (2005). Penicillium coffeae, a new endophytic species isolated from a coffee plant and its phylogenetic relationship to P. fellutanum, P. thiersii and P. brocae based on parsimony analysis of multilocus DNA sequences. Mycologia, 97(3), 659–666. [DOI] [PubMed] [Google Scholar]
- Petrini, O. , Sieber, T. N. , Toti, L. , & Viret, O. (1993). Ecology, metabolite production, and substrate utilization in endophytic fungi. Natural Toxins, 1(3), 185–196. [DOI] [PubMed] [Google Scholar]
- Pinruan, U. , Rungjindamai, N. , Choeyklin, R. , Lumyong, S. , Hyde, K. D. , & Jones, E. B. G. (2010). Occurrence and diversity of basidiomycetous endophytes from the oil palm, Elaeis guineensis in Thailand. Fungal Diversity, 41(1), 71–88. [Google Scholar]
- Qadri, M. , Rajput, R. , Abdin, M. Z. , Vishwakarma, R. A. , & Riyaz‐Ul‐Hassan, S. (2014). Diversity, Molecular Phylogeny, and Bioactive Potential of Fungal Endophytes Associated with the Himalayan Blue Pine (Pinus wallichiana). Microbial Ecology, 67(4), 877–887. [DOI] [PubMed] [Google Scholar]
- Qi, F. H. , Jing, T. Z. , Wang, Z. X. , & Zhan, Y. G. (2009). Fungal endophytes from Acer ginnala Maxim: isolation, identification and their yield of gallic acid. Letters in Applied Microbiology, 49(1), 98–104. [DOI] [PubMed] [Google Scholar]
- Rabea, E. I. , Badawy, M. E. I. , Steurbaut, W. , & Stevens, C. V. (2009). In vitro assessment of N‐(benzyl)chitosan derivatives against some plant pathogenic bacteria and fungi. European Polymer Journal, 45(1), 237–245. [Google Scholar]
- Raviraja, N. S. (2005). Fungal endophytes in five medicinal plant species from Kudremukh Range, Western Ghats of India. Journal of Basic Microbiology, 45(3), 230–235. [DOI] [PubMed] [Google Scholar]
- Rivera‐Orduna, F. N. , Suarez‐Sanchez, R. A. , Flores‐Bustamante, Z. R. , Gracida‐Rodriguez, J. N. , & Flores‐Cotera, L. B. (2011). Diversity of endophytic fungi of Taxus globosa (Mexican yew). Fungal Diversity, 47(1), 65–74. [Google Scholar]
- Robideau, G. P. , de Cock, A. W. A. M. , Coffey, M. D. , Voglmayr, H. , Brouwer, H. , Bala, K. , … Levesque, C. A. (2011). DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Molecular Ecology Resources, 11(6), 1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, A. , Mueller, U. G. , Ishak, H. D. , Bacci, M. Jr , & Pagnocca, F. C. (2011a). Ecology of microfungal communities in gardens of fungus‐growing ants (Hymenoptera: Formicidae): a year‐long survey of three species of attine ants in Central Texas. FEMS Microbiology Ecology, 78(2), 244–255. [DOI] [PubMed] [Google Scholar]
- Rodrigues, A. , Mueller, U. G. , Ishak, H. D. , Bacci, M. Jr , & Pagnocca, F. C. (2011b). Ecology of microfungal communities in gardens of fungus‐growing ants (Hymenoptera: Formicidae): a year‐long survey of three species of attine ants in Central Texas. FEMS Microbiology, 78(2), 244–255. [DOI] [PubMed] [Google Scholar]
- Rodriguez, R. J. , Redman, R. S. , & Henson, J. M. (2004). The Role of Fungal Symbioses in the Adaptation of Plants to High Stress Environments. Mitigation & Adaptation Strategies for Global Change, 9(3), 261–272. [Google Scholar]
- Rubini, M. R. , Silva‐Ribeiro, R. T. , Pomella, A. W. V. , Maki, C. S. , Araujo, W. L. , dos Santos, D. R. , & Azevedo, J. L. (2005). Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of Witches’ Broom Disease. International Journal of Biological Sciences, 1(1), 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rungjindamai, N. , Pinruan, U. , Choeyklin, R. , Hattori, T. , & Jones, G. (2009). Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Diversity, 33, 139–161. [Google Scholar]
- Rungjindamai, N. , Pinruan, U. , Hattori, T. , & Choeyklin, R. (2008). Molecular characterization of basidiomycetous endophytes isolated from leaves, rachis and petioles of the oil palm, Elaeis guineensis . Thailand. Fungal Diversity, 33(12), 139–161. [Google Scholar]
- Sakalidis, M. L. , Hardy, G. E. , & Burgess, T. I. (2011). Endophytes as potential pathogens of the baobab species Adansonia gregorii: a focus on the Botryosphaeriaceae. Fungal Ecology, 4(1), 1–14. [Google Scholar]
- Samuels, G. J. , Ismaiel, A. , Bon, M. C. , De Respinis, S. , & Petrini, O. (2010). Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia, 102(4), 944–966. [DOI] [PubMed] [Google Scholar]
- Sánchez Márquez, S. , Bills, G. F. , Acuña, D. L. , & Zabalgogeazcoa, I. (2010). Endophytic mycobiota of leaves and roots of the grass Holcus lanatus . Fungal Diversity, 41(1), 115–123. [Google Scholar]
- Sanchez Marquez, S. , Bills, G. F. , & Zabalgogeazcoa, I. (2008). Diversity and structure of the fungal endophytic assemblages from two sympatric coastal grasses. Fungal Diversity, 33, 87–100. [Google Scholar]
- Sappapan, R. , Sommit, D. , Ngamrojanavanich, N. , Pengpreecha, S. , Wiyakrutta, S. , Sriubolmas, N. , & Pudhom, K. (2008). 11‐hydroxymonocerin from the plant endophytic fungus Exserohilum rostratum . Journal of Natural Products, 71(9), 1657–1659. [DOI] [PubMed] [Google Scholar]
- Saunders, M. , & Kohn, L. M. (2009). Evidence for alteration of fungal endophyte community assembly by host defense compounds. New Phytologist, 182, 229–238. [DOI] [PubMed] [Google Scholar]
- Schoch, C. L. , Seifert, K. A. , Huhndorf, S. , Robert, V. , Spouge, J. L. , Levesque, C. A. , & Chen, W. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Science, 109(16), 6241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulthess, F. M. , & Faeth, S. H. (1998). Distribution, abundances, and associations of the endophytic fungal community of Arizona fescue (Festuca arizonica). Mycologia, 90(4), 569–578. [Google Scholar]
- Schulz, B. , & Boyle, C. (2005). The endophytic continuum. Mycological Research, 109, 661–686. [DOI] [PubMed] [Google Scholar]
- Schulz, B. , Haas, S. , Junker, C. , Andree, N. , & Schobert, M. (2015). Fungal endophytes are involved in multiple balanced antagonisms. Current Science, 109(1), 39–45. [Google Scholar]
- Schulz, B. , Rommert, A. K. , Dammann, U. , Aust, H. J. , & Strack, D. (1999). The endophyte‐host interaction: A balanced antagonism? Mycological Research, 103, 1275–1283. [Google Scholar]
- Serra, R. , Peterson, S. , & Venancio, A. (2008). Multilocus sequence identification of Penicillium species in cork bark during plank preparation for the manufacture of stoppers. Research in Microbiology, 159(3), 178–186. [DOI] [PubMed] [Google Scholar]
- Shrestha, P. , Szaro, T. M. , Bruns, T. D. , & Taylor, J. W. (2011). Systematic search for cultivatable fungi that best deconstruct cell walls of Miscanthus and sugarcane in the field. Applied & Environmental Microbiology, 77(15), 5490–5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva, H. S. A. , Tozzi, J. P. L. , Terrasan, C. R. F. , & Bettiol, W. (2012). Endophytic microorganisms from coffee tissues as plant growth promoters and biocontrol agents of coffee leaf rust. Biological Control, 63(1), 62–67. [Google Scholar]
- Solis, M. J. L. , Edison Dela Cruz, T. , Schnittler, M. , & Unterseher, M. (2016). The diverse community of leaf‐inhabiting fungal endophytes from Philippine natural forests reflects phylogenetic patterns of their host plant species Ficus benjamina . F. Elastica and F. Religiosa. Mycoscience, 57(2), 96–106. [Google Scholar]
- Staniek, A. , Woerdenbag, H. J. , & Kayser, O. (2008). Endophytes: Exploiting biodiversity for the improvement of natural product‐based drug discovery. Journal of Plant Interactions, 3(2), 75–93. [Google Scholar]
- Stenstrom, E. , Ndobe, N.E. , Jonsson, M. , Stenlid, J. , & Menkis, A. (2014). Root‐associated fungi of healthy‐looking Pinus sylvestris and Picea abies seedlings in Swedish forest nurseries. Scandinavian Journal of Forest Research, 29(1), 12–21. [Google Scholar]
- Stierle, A. , Strobel, G. , & Stierle, D. (1993). Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew . Science, 260, 214–216. [DOI] [PubMed] [Google Scholar]
- Strobel, G. , Daisy, B. , Castillo, U. , & Harper, J. (2004). Natural products from endophytic microorganisms. Journal of Natural Products, 67(2), 257–268. [DOI] [PubMed] [Google Scholar]
- Sulaiman, R. , Thanarajoo, S. S. , Kadir, J. , & Vadamalai, G. (2012). First Report of Lasiodiplodia theobromae Causing Stem Canker of Jatropha curcas in Malaysia. Plant Disease, 96(5), 767. [DOI] [PubMed] [Google Scholar]
- Supaphon, P. , Phongpaichit, S. , Rukachaisirikul, V. , & Sakayaroj, J. (2014). Diversity and antimicrobial activity of endophytic fungi isolated from the seagrass Enhalus acoroides . Indian Journal of Geo‐Marine Sciences, 43(5), 785–797. [Google Scholar]
- Tejesvi, M. V. , Kajula, M. , Mattila, S. , & Pirttilä, A. M. (2011). Bioactivity and genetic diversity of endophytic fungi in Rhododendron tomentosum Harmaja. Fungal Diversity, 47(1), 97–107. [Google Scholar]
- Tian, J. , Zeng, X. , Lu, A. , Zhu, A. , Peng, X. , & Wang, Y. (2015). Perillaldehyde, a potential preservative agent in foods: assessment of antifungal activity against microbial spoilage of cherry tomatoes. Lwt‐Food Science and Technology, 60(1), 63–70. [Google Scholar]
- Vieira, M. L. A. , Hughes, A. F. S. , Gil, V. B. , Vaz, A. B. M. , Alves, T. M. A. , Zani, C. L. , … Rosa, L. H. (2012). Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae). Canadian Journal of Microbiology, 58(1), 54–66. [DOI] [PubMed] [Google Scholar]
- Visagie, C. M. , Houbraken, J. , Frisvad, J. C. , Hong, S. B. , Klaassen, C. H. , Perrone, G. , … Samson, R. A. (2014). Identification and nomenclature of the genus Penicilliu . Studies in Mycology, 78, 343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, L. , Stielow, B. , Hoffmann, K. , Petkovits, T. , Papp, T. , Vagvolgyi, C. , … Voigt, K. (2013). A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA. Persoonia, 30, 77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, G. , Pawlowska, J. , Alastruey‐Izquierdo, A. , Wrzosek, M. , Rodriguez‐Tudela, J. L. , … de Hoog, G. S. (2013). DNA barcoding in Mucorales: an inventory of biodiversity. Persoonia, 30, 11–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , & Dai, C. C. (2011). Endophytes: A potential resource for biosynthesis, biotransformation, and biodegradation. Annals of Microbiology, 61(2), 207–215. [Google Scholar]
- Wang, S. , Li, X.‐M. , Teuscher, F. , Li, D.‐L. , Diesel, A. , Ebel, R. , … Wang, B.‐G. (2006). Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata . Journal of Natural Products, 69(11), 1622–1625. [DOI] [PubMed] [Google Scholar]
- Wang, J. K. , Xie, X. F. , Fan, C. Y. , & Liu, M. (2011). Sophorae tonkinensis radix et rhizoma In Geoherbs Chinese. (Ed.), Peng C (pp. 3305–3320). Bei Jing: China Press of Traditional Chinese Medicine. [Google Scholar]
- Waweru, B. W. , Losenge, T. , Kahangi, E. M. , Dubois, T. , & Coyne, D. (2013). Potential biological control of lesion nematodes on banana using Kenyan strains of endophytic Fusarium oxysporum . Nematology, 15, 101–107. [Google Scholar]
- Wei, J. G. , & Chen, Y. X. (1992). Preliminary investigation on black spot disease of Panax notoginseng in Guangxi. Journal of Chinese Medicinal Materials, 15(1), 7–8. [Google Scholar]
- Wei, J. G. , Chen, Y. X. , & Wu, J. H. (1989). Biological characteristics of Colletotrichum gloeosporioides isolated from Panax notoginseng anthracnose. Journal of Guangxi Agricultural University, 8(1), 25–33. [Google Scholar]
- White, T. J. , Bruns, T. , Lee, S. , & Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In Innis M. A., Sninsky D. H., & White T. J. (Eds.), PCR Protocols (pp. 315–322). London: Academic. [Google Scholar]
- Wicklow, D. T. , & Poling, S. M. (2009). Antimicrobial activity of pyrrocidines from Acremonium zeae against endophytes and pathogens of maize. Phytopathology, 99(1), 109–115. [DOI] [PubMed] [Google Scholar]
- Xiong, Z.‐Q. , Yang, Y.‐Y. , Liu, Q.‐X. , Sun, C.‐C. , Jin, Y. , & Wang, Y. (2015). Endophytes in the plant Huperzia serrata: Fungal diversity and discovery of a new pentapeptide. Archives of Microbiology, 197(3), 411–418. [DOI] [PubMed] [Google Scholar]
- Xu, Y.‐M. , Espinosa‐Artiles, P. , Liu, M. X. , Arnold, A. E. , & Gunatilaka, A. A. L. (2013). Secoemestrin D, a Cytotoxic Epitetrathiodioxopiperizine, and Emericellenes A‐E, Five Sesterterpenoids from Emericella sp AST0036, a Fungal Endophyte of Astragalus lentiginosus . Journal of Natural Products, 76(12), 2330–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. Y. , Zhao, B. G. , & Ju, Y. W. (2008). Antifungal activities and synergetic tests of matrine and oxymatrine to some tree pathogens. Journal of Nanjing Forestry University, 32(2), 79–82. [Google Scholar]
- Yuan, Z.‐L. , Su, Z.‐Z. , Mao, L.‐J. , Peng, Y.‐Q. , Yang, G.‐M. , Lin, F.‐C. , & Zhang, C.‐L. (2011). Distinctive Endophytic Fungal Assemblage in Stems of Wild Rice (Oryza granulata) in China with Special Reference to Two Species of Muscodor (Xylariaceae). Journal of Microbiology, 49(1), 15–23. [DOI] [PubMed] [Google Scholar]
- Zhai, M.‐M. , Niu, H.‐T. , Li, J. , Xiao, H. , Shi, Y.‐P. , Di, D.‐L. , … Wu, Q.‐X. (2015). Talaromycolides A‐C, Novel Phenyl‐Substituted Phthalides Isolated from the Green Chinese Onion‐Derived Fungus Talaromyces pinophilus AF‐02. Journal of Agricultural and Food Chemistry, 63(43), 9558–9564. [DOI] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, J. , Yang, L. , Zhang, L. , Jiang, D. , Chen, W. , & Li, G. (2014). Diversity and biocontrol potential of endophytic fungi in Brassica napus . Biological Control, 72, 98–108. [Google Scholar]
- Zikmundova, M. , Drandarov, K. , Bigler, L. , Hesse, M. , & Werner, C. (2002). Biotransformation of 2‐Benzoxazolinone and 2‐Hydroxy‐1,4‐Benzoxazin‐3‐one by Endophytic Fungi Isolated from Aphelandra tetragona . Applied and Environmental Microbiology, 68(10), 4863–4870. [DOI] [PMC free article] [PubMed] [Google Scholar]


